Abstract
Background
High intake of ultra-processed foods (UPFs) is associated with increased risk of chronic disease; thus, it is important to understand how UPFs influence diet quality early in life.
Objectives
We describe complementary foods and beverages (CFBs) according to the Nova Classification System of Food Processing for infants and toddlers in the United States and estimate how Nova groups and subgroups contribute to energy and select nutrients and food groups.
Methods
We used day 1 24-h recall from infants and toddlers aged 6–23 mo from the cross-sectional, nationally representative 2013–18 National Health and Nutrition Examination Survey (n = 1140). We estimated contributions of Nova groups and subgroups to energy and select nutrients and food groups consumed as CFBs (excluding human milk and formula) using the population ratio with weighted survey commands in SAS.
Results
For infants and toddlers in the United States, 42 ± 0.9% (mean ± standard error of the mean) of energy intake from CFBs came from unprocessed/minimally processed foods (U/MPFs) and 45 ± 0.8% from UPFs. U/MPFs contributed most to nutrient intakes (except iron, zinc, and sodium); ≥20% of all selected nutrients was from UPFs. UPFs contributed most to iron (75 ± 1.0%) and zinc (48 ± 1.3%); breakfast cereals were the top source. Most fruit, vegetables, and dairy were from U/MPFs. More than 80% of total grains, whole grains, refined grains, and added sugars were UPFs.
Conclusions
U/MPFs support healthy dietary intake of infants and toddlers in the United States, whereas UPFs contribute meaningfully to nutrients and food groups to be encouraged (iron, zinc, and whole grains), as well as some that should be limited (added sugars and sodium). More research is needed to better understand the utility and sensitivities of using Nova for providing dietary guidance for infants and toddlers in the United States.
Keywords: birth to 24-mo, food processing, national nutrition surveillance, dietary guidance, dietary patterns
Introduction
Choices of complementary foods and beverages (CFB) for infants (6–11 mo) and toddlers (12–23 mo) prime taste preferences and shape dietary patterns into adolescence and adulthood [1]. Infants are weaned from breast milk or formula by the introduction of CFB. CFBs help meet changing nutrient and food group needs and thus are often fortified with essential vitamins and minerals (e.g., iron-fortified infant cereals) [2]. The landscape of CFB is changing over time as more convenience foods are available in the marketplace, such as bars, pouches, and ready-to-eat meals [3,4]. These options are increasingly attractive to parents in the United States, given the declining amount of time and skills available for food preparation [5]. However, many of the emerging CFBs may be considered “ultra-processed” according to the Nova Classification System of Food Processing [6] because of formulations high in added sugars, oils, fats, and salt as well as food additives (e.g., colorings, emulsifiers) used to improve the sensory, nutrition, and texture properties of these products [[7], [8], [9]]. High intake of ultra-processed foods (UPFs) is associated with increased risk of chronic disease outcomes in adult life [[10], [11], [12], [13]]. Food choices early in life, including UPF intake, can influence dietary intake across the lifespan and are associated with health outcomes into adulthood [[14], [15], [16]]. Therefore, our objective was to describe how Nova groups and subgroups contribute to total energy intake from CFBs consumed by infants and toddlers aged 6–23 mo in the United States, as well as to select nutrients and food groups that are highlighted as important public health considerations by the 2020–2025 United States Dietary Guideline for Americans (DGA) [1].
Methods
Study design, population, and dietary assessment
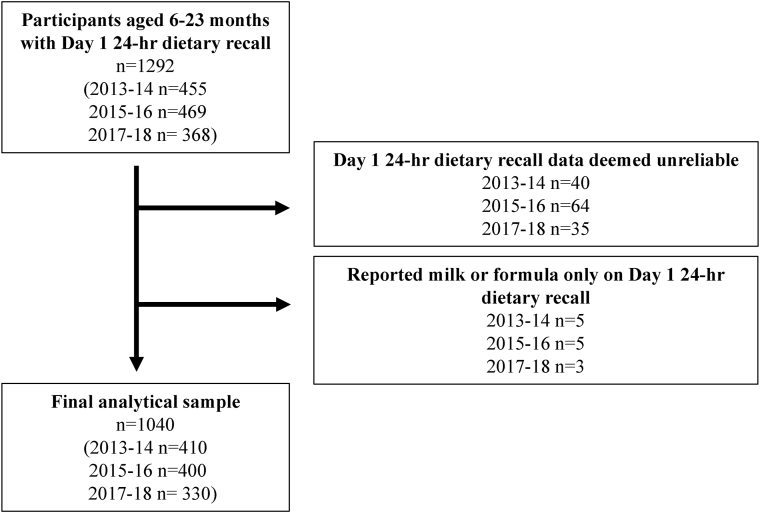
Methods used are described in detail in Supplementary Material. We used data from participants aged 6–23 mo (n = 1140) from the cross-sectional 2013–14, 2015–16, and 2017–18 National Health and Nutrition Examination Survey (NHANES) [17,18]; see Figure 1. The United States' National Center for Health Statistics (NCHS) research ethics review board approves all protocols for NHANES. Written consent is provided by a parent or guardian for infants and toddlers. Trained interviewers administer 24-h recalls using the United States Department of Agriculture (USDA)’s computer-assisted Automated Multiple-Pass Method (AMPM) [19]. For children <5 y, a proxy reported the child’s intake for the previous day.
FIGURE 1.
Participant flowchart for final analytical sample.
Nova contribution to energy, nutrient, and food group intakes
Each food or beverage reported for infants and toddlers was classified according to the Nova Classification System of Food Processing [6]. Nova defines UPFs and beverages based on the perceived extent and purpose of physical, biological, and chemical food processing techniques. Nova classifies foods into the following 4 main groups: Group 1 includes unprocessed/minimally processed foods (U/MPFs), Group 2 includes processed culinary ingredients, Group 3 includes processed foods, and Group 4 includes UPFs. These 4 groups are then broken down into 41 food-based subgroups, which we aggregated because of the age of our population and sample size (Supplemental Table 1). A more detailed description of each Nova group and subgroup is available in the Supplemental Methods and described previously [6], as well as a description of how to apply Nova to NHANES data [20].
We estimated the contribution of Nova groups and subgroups to energy from CFB as well as to the following select nutrients and food groups (all excluding human milk and formula): iron, vitamin D, zinc, vitamin B12, choline, potassium, vitamin A, vitamin C, calcium, fiber, total fruit, whole fruit, total vegetables, total grains, whole grains, total dairy, and total protein foods. These nutrients and food groups were selected because they are identified as either dietary components that are of public health concern or foods or nutrients that should be encouraged when introducing CFPs according to the 2020–25 DGA [1]. We also estimated contributions of each Nova group and subgroup to sodium, added sugars, refined grains, and solid fats. Foods high in sodium should be limited, whereas added sugars should be avoided for this age group, according to the DGAs [1]. There are no constraints or recommendations for solid fats, although there is no room for extra calories from these foods in the dietary patterns of infants and toddlers beyond what is naturally occurring within the foods included in the recommended dietary patterns [1]. Further, dietary intake early in life can prime taste buds into adulthood [1]. Therefore, solid fats may be of interest, even if not identified as a dietary constituent of concern for this life stage.
Statistical analysis
We used SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) weighted survey commands to account for the complex survey design of NHANES. All analyses were weighted using day 1 dietary recall survey weights to account for oversampling, non-response, and post-stratification. We combined 3 cycles of NHANES (2013–14, 2015–16, and 2017–18) to obtain adequate sample sizes for reliable estimates. We estimated percent of energy, nutrient, or food group intake from CFBs for each main Nova group and subgroup via the population ratio method [21] using the ratio statement in the proc survey means. We calculated percent of energy, nutrient, or food group from each Nova group and subgroup by the following equation: e.g., % iron from UPFs = [population sum of iron intake (g) from UPFs as CFBs]/[population sum of total iron intake (g) from all CFB]. We then stratified intake from the 4 main Nova groups by sex (male, female), age (6–11 mo, 12–23 mo), feeding status (consumed human milk but no formula, or consumed formula but no human milk), race/Hispanic origin [Hispanic, non-Hispanic (NH) White, NH Black, NH Asian], head of household education level (less than high school, high school plus some college, college graduate) and poverty to income ratio (≤1.85 and >1.85 used for Women, Infants, and Children program eligibility [22]). We identified differences via pairwise comparisons within strata and corrected for multiple comparisons via the Bonferroni method by dividing all 2-sided P values by 4 (P < 0.0125) because there are 4 Nova groups assessed within each stratum.
Results
Contribution of Nova groups to energy intake from CFBs
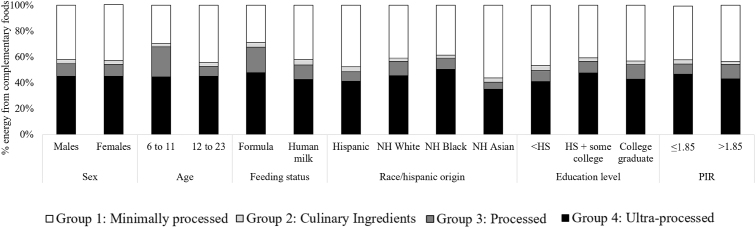
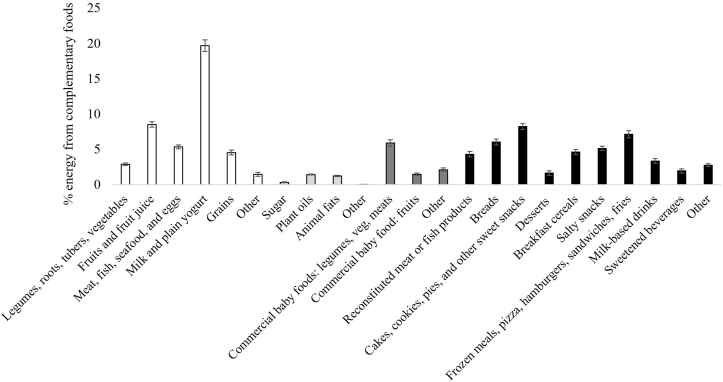
Our sample is nationally representative of infants and toddlers aged 6–23 mo in the United States (Table 1). For energy intake from CFBs, 42 ± 0.9% was from U/MPFs, 3 ± 0.2% from processed culinary ingredients, 9 ± 0.6% from processed foods, and 45 ± 0.8% from UPFs (Supplemental Table 2). Those aged 6–11 mo consumed 15 ± 2.0% less energy from U/MPFs (P < 0.0001), 16 ± 2.0% more energy from processed foods (P < 0.0001), and no difference in UPFs compared to those aged 12–23 mo. Those fed formula consumed 13 ± 3.0% less energy from U/MPFs (P < 0.0001), 8 ± 2.0% more energy from processed foods (P < 0.0001), and no difference in UPFs compared to those fed human milk. Individuals who were reported as NH Black or White had higher UPF and lower U/MPF intakes than those who reported as NH Asian or Hispanic. Energy intake was lowest from UPFs (35 ± 2.5% energy) and highest from U/MPFs (56 ± 3.3% energy) among individuals that were reported as NH Asian compared to all other race/Hispanic origin groups. There were few notable differences in energy distribution based on the head of the household’s education level, poverty income ratio, or sex (Figure 2 and Supplemental Table 2). For the total sample, the highest contributing UPF subgroup to energy intake from CFBs were cakes, cookies, pies, and other sweet snacks (8 ± 0.4%); frozen meals, pizza, hamburgers, sandwiches, and fries (7 ± 0.5%); and bread (6 ± 0.4%) (Figure 3 and Supplemental Table 3).
TABLE 1.
Demographic characteristics of infants and toddlers from a nationally representative United States sample
| Characteristic | Infants aged 6–11 mo | Toddlers aged 12–23 mo | Aged 6–23 mo |
|---|---|---|---|
| Total | 510 | 630 | 1140 |
| Sex, (%) | |||
| Male | 265 (55) | 346 (56) | 611 (55) |
| Female | 245 (45) | 284 (44) | 529 (45) |
| Race and Hispanic origin, (%) | |||
| Non-Hispanic White | 192 (52) | 210 (50) | 402 (50) |
| Hispanic | 177 (25) | 184 (27) | 361 (26) |
| Non-Hispanic Black | 82 (13) | 136 (12) | 218 (12) |
| Non-Hispanic Asian | 21 (3) | 38 (4) | 59 (4) |
| PIR, (%) | |||
| ≤1.85 | 275 (48) | 326 (46) | 601 (46) |
| >1.85 | 191 (45) | 243 (46) | 434 (46) |
| Head of household education, (%) | |||
| <High school | 100 (16) | 133 (18) | 233 (17) |
| High school + some college | 285 (54) | 335 (52) | 620 (53) |
| College graduate and above | 102 (25) | 138 (26) | 240 (26) |
| Feeding status, (%) | |||
| Formula | 350 (64) | 59 (9) | 409 (28) |
| Human milk | 102 (24) | 61 (10) | 163 (14) |
An unweighted number of participants (weighted column percentage; unknown, unspecified, or subgroups with small sample sizes are not listed; thus, columns may not add ≤100%). Data source: United States CDC/NCHS, NHANES 2013–2018. Demographic information on infants and toddlers was provided by a parent or guardian during the household interview portion of data collection [[48], [49], [50]]. All estimates are deemed reliable according to the NCHS data standards, i.e., a relative SE (calculated by dividing the SE of the estimate by the estimate itself and multiplying by 100) <30% [51], unless otherwise noted. We also required reliable estimates to have ≥30 reports of each food or beverage (on the food code level) per stratum estimate. Participants were excluded from stratified analyses if the stratified covariate of interest had missing data. Formula-fed is defined as consumed formula, and no human milk, and human milk-fed is defined as consumed human milk and no formula. PIR, Poverty Income Ratio.
FIGURE 2.
Percent of energy from complementary foods and beverages consumed by infants and toddlers aged 6–23 mo old in the United States classified according to the Nova Classification System of Food Processing, stratified by demographic characteristics. Data are shown as means ± SEM (n = 1140) using the population ratio method multiplied by 100; e.g., percentage of energy from UPFs = (population sum of kcal from UPFs)/(population sum of total kcal from all complementary foods and beverages, excluding human milk and formula). Data source: United States CDC/NCHS, NHANES 2013–2018. Corresponding summary data and statistical comparisons are shown in Supplementary Table 2. All estimates are deemed reliable according to the NCHS data standards, i.e., a relative SE (calculated by dividing the SE of the estimate by the estimate itself and multiplying by 100) <30% [51], unless otherwise noted in Supplementary Table 2. We also required reliable estimates to have ≥30 reports of each food or beverage (on the food code level) per stratum estimate. Participants were excluded from stratified analyses if the stratified covariate of interest had missing data. Formula-fed is defined as consumed formula, and no human milk, and human milk-fed is defined as consumed human milk and no formula. Those who consumed both or neither are not presented because of the inadequate sample sizes. HS, High school education; NH, non-Hispanic; PIR, Poverty Income Ratio; UPF, ultra-processed food.
FIGURE 3.
Percent of energy from complementary foods and beverages consumed by infants and toddlers aged 6 to 23 mo old in the United States classified according to subgroups of the Nova Classification System of Food Processing. Data are shown as means ± SEM (n = 1140) using the population ratio method multiplied by 100; e.g., percentage of energy from ‘Breads’ = (population sum of kcal from ‘Breads’)/(population sum of total kcal from all complementary foods and beverages, excluding human milk and formula). Data source: United States CDC/NCHS, NHANES 2013–2018. Corresponding summary data are available in Supplementary Table 3, as well as results stratified by age group. All estimates are deemed reliable according to the NCHS data standards, i.e., a relative SE (calculated by dividing the SE of the estimate by the estimate itself and multiplying by 100) <30% [51], unless otherwise noted in Supplementary Table 3. We also required reliable estimates to have ≥30 reports of each food or beverage (on the food code level) per stratum estimate. Participants were excluded from stratified analyses if the stratified covariate of interest had missing data.
Contribution of Nova groups to nutrient intake of CFBs
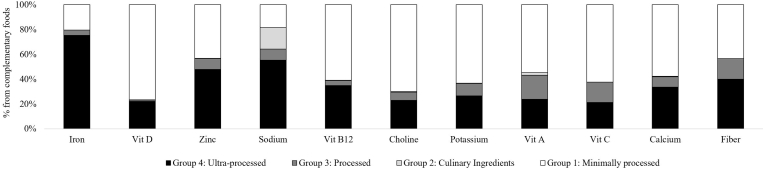
U/MPFs contributed most to nutrients that should be encouraged [except for iron (20 ± 0.9%) and zinc (43 ± 1.4%)] according to the 2020–25 DGA, and least to sodium (18 ± 0.7%) (Figure 4 and Supplemental Table 4). The majority of vitamin D (77 ± 1.8%), vitamin B12 (61 ± 2.0%), choline (70 ± 1.2%), potassium (63 ± 1.0%), vitamin A (55 ± 1.5%), vitamin C (62 ± 2.0%), and calcium (58 ± 1.7%) were from U/MPFs.
FIGURE 4.
Nutrient intake from complementary foods and beverages consumed by infants and toddlers aged 6–23 mo old in the United States according to the Nova Classification System of Food Processing. Data are shown as means ± SEM (n = 1140) using the population ratio method multiplied by 100; e.g., percentage of iron from UPFs = (population sum of mg of iron from UPFs)/(population sum of total mg iron from all complementary foods and beverages, excluding human milk and formula). Data source: United States CDC/NCHS, NHANES 2013–2018. Corresponding summary data are available in Supplementary Table 4. All estimates are deemed reliable according to the NCHS data standards, i.e., a relative SE (calculated by dividing the SE of the estimate by the estimate itself and multiplying by 100) <30% [51], unless otherwise noted in Supplementary Table 4. We also required reliable estimates to have ≥30 reports of each food or beverage (on the food code level) per estimate. UPF, ultra-processed food, Vit, vitamin.
At least 20% of intake from all selected nutrients was from UPFs. UPFs contributed most to iron (75 ± 1.0%) and zinc (48 ± 1.3%), both of which breakfast cereals were a top source (45 ± 2.2% of iron and 23 ± 1.5% of zinc). UPFs also contributed the most to sodium (55 ± 1.2%). U/MPFs (43 ± 1.4%) and UPFs (40 ± 1.2%) contributed similarly to fiber. Unprocessed/minimally proessed fruit and fruit juice (19 ± 0.9%), Unprocessed/minimally processed legumes, roots, tubers, and vegetables (16 ± 0.8%), as well as ultra-processed breakfast cereals (9 ± 0.6%) and ultra-processed bread (9 ± 0.7%) were top contributors to fiber. Culinary ingredients and processed food contributed <20% to all nutrients.
Contribution of Nova groups to food group intake of CFBs
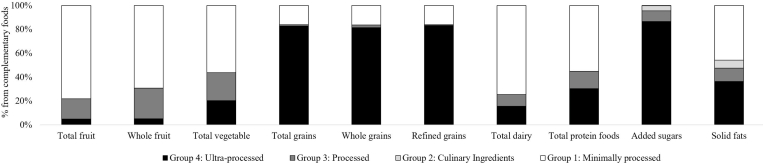
Total fruit (78 ± 1.4%), whole fruit (69 ± 2.0%), total vegetable (56 ± 2.0%), and total dairy (74 ± 1.6%) were largely from U/MPFs (Figure 5 and Supplemental Table 4). UPFs were the largest contributor to the intake of total grains (83 ± 1.1%), refined grains (83 ± 1.2%), whole grains (81 ± 2.3%), and added sugars (87 ± 1.2%). Ultra-processed breakfast cereals contributed modestly to refined grains (5 ± 0.6%) and added sugars (7 ± 0.8%), and almost half of whole grains (41 ± 2.6%). Ultra-processed bread contributed ∼25% of whole and refined grains and 9 ± 0.7% of fiber. Cakes, cookies, pies, and other sweet snacks (27 ± 1.4%) and sweetened beverages (20 ± 2.7%) were top contributors to added sugars intake. Frozen meals, pizza, hamburgers, sandwiches, and fries (11 ± 0.9%) and reconstituted meat and fish products (10 ± 0.8%) contributed most to sodium intake. About half (55 ± 2.3%) of total protein foods were U/MPFs, and 30 ± 1.7% were UPFs. Unprocessed/minimally processed meats, fish, seafood, and eggs contributed to 47 ± 2.2% of total protein foods, whereas ultra-processed reconstituted meat or fish products contributed 23 ± 1.6%. About half (46 ± 1.5%) of solid fats were from U/MPFs, and 36 ± 1.3% were from UPFs. Unprocessed/minimally processed milk and plain yogurt contributed 40 ± 1.4% of solid fats, with reconstituted meat and fish products; cakes, cookies, pies, and other sweet snacks; and frozen meals, pizza, hamburgers, sandwiches, and fries contributed ∼8% each (Supplemental Appendix).
FIGURE 5.
Food group intake from complementary foods and beverages consumed by infants and toddlers aged 6–23 mo old in the United States according to the Nova Classification System of Food Processing. Data are shown as means ± SEM (n = 1140) using the population ratio method multiplied by 100; e.g., percentage of added sugars from UPFs = (population sum of g of added sugars from UPFs)/(population sum of total g of added sugars from all complementary foods and beverages, excluding human milk and formula). Data source: United States CDC/NCHS, NHANES 2013–2018. Corresponding summary data are available in Supplementary Table 4. All estimates are deemed reliable according to the NCHS data standards, i.e., a relative SE (calculated by dividing the SE of the estimate by the estimate itself and multiplying by 100) <30% [51], unless otherwise noted. We also required reliable estimates to have ≥30 reports of each food or beverage (on the food code level) per estimate. UPF, ultra-processed food.
Discussion
There is scientific debate about the utility of classifying foods and beverages according to the Nova Classification System of Food Processing for dietary guidance [23,24]. In this analysis of a nationally representative sample of the United States, we described dietary intake of infants and toddlers aged 6–23 mo using Nova, as this age group is often under-researched. Our results indicate that infants and toddlers in the United States consume ∼45% of energy from CFBs as UPFs, ≥15% less than older age groups [25,26]. U/MPFs contributed ∼40% of energy from CFBs, as well as the majority of most nutrient and food group intakes encouraged by the 2020–2025 DGA [1]. However, ≥20% of commonly under-consumed nutrients came from UPFs, particularly iron and zinc, for which ultra-processed breakfast cereals were the top source. UPFs were also top contributors of sodium and added sugars. Previous research shows that higher UPF intake was associated with increased added sugars intake, but no trend was found for sodium, zinc, or iron for children and adolescents aged 2–18 y [25]. Our results indicate that U/MPFs positively influence dietary intake of infants and toddlers in the United States, and UPFs contribute meaningfully to foods and nutrients that should be encouraged, as well as some to be limited [1].
Food-based dietary guidance in some countries, such as Brazil [27] and Canada [28], recommend that U/MPFs should be the basis of a healthy dietary pattern. Our results support this notion for infants and toddlers because a high proportion of foods and nutrients to encourage came from U/MPFs. However, our results also suggest that some UPFs have an important role in the dietary intake of infants and toddlers. For example, breakfast cereals, including infant cereals, are classified as UPFs by Nova because these often include additives that improve texture, flavor, or mouthfeel, such as artificial flavors, maltodextrin, and emulsifiers. Breakfast cereals are micronutrient-fortified, affordable, or subsidized for most income levels [[29], [30], [31]], and formulations have decreased in added sugars and increased in whole grains and fiber over time [32]. Breakfast cereal consumers are also more likely to co-consume dairy, leading to higher intakes of calcium and vitamin D [30]. Research from The Feeding Infants and Toddlers study showed that infant cereals were the top source of dietary iron among infants aged 6–12 mo and that infants and toddlers who consumed cereal also consumed more iron than those who did not [33]. In our study, ultra-processed breakfast cereals were top sources of iron, zinc, B12, and whole grains and contributed <7% of total added sugars to dietary intake of infants and toddlers in the United States. Guidance on food processing should consider that some UPFs, and processed foods, more generally, provide defense against micronutrient deficiencies in the United States [29,34].
“Every bite counts” [1], particularly for infants and toddlers who consume small quantities of foods and beverages but have high nutrient requirements during rapid growth and development. Thus, there is little room for calories from added sugars or solid fats that displace intakes of other food groups needed to meet intake targets. The 2020–2025 DGA recommends for those younger than 2 y old avoid foods and beverages with added sugars and limit foods and beverages that are higher in sodium [1]. The AHA recommendations align with this in that children <2 y old avoid added sugars intake [35]. Our results show that UPFs contributed >60% of sodium, >85% of added sugars, and >35% of solid fats. Cakes, cookies, pies, other sweet snacks, milk-based drinks, and sweetened beverages were top sources of added sugars, consistent with previous analyses [36]. Reduction of these types of UPFs may reduce sodium, added sugars, and solid fats for infants and toddlers. Higher intakes of UPFs are also associated with lower diet quality [37], higher phthalates/bisphenols exposure [38], and increased EI [39] in older age groups. However, many of these foods are ready-to-eat or ready-to-heat UPF products that are largely consumed for convenience, taste, and cost [40]. More research is needed into how UPF subtypes contribute to dietary intake and health in earlier life stages.
Types and amounts of food processing, formulation, and fortification differ across the globe. Our results are specific to the United States, which may differ from other countries where infants and toddlers are fed more often at home, with less processed or pre-packaged foods. For example, in countries such as Colombia or Italy, <20% of preschoolers’ dietary intake is from UPFs, compared to 40–60% in the UK and the United States [41,42]. There does not seem to be a comparison of Nova subgroups across countries, to the best of our knowledge, which could further inform the potential application of Nova to varying countries’ food systems. For example, UPFs such as commercial bread, ready-to-eat breakfast cereals, commercial infant foods, or infant formulas may be more prevalent in dietary patterns of children in the UK and the United States, contributing to the higher percentage of energy intake from UPFs in those countries as well as a higher percent of micronutrients. For example, The Feeding Infants and Toddlers study showed that infants and toddlers in the United States fed commercially prepared infant foods had higher diet quality than those who were not, including more nutrients consumed directly from the infant foods but also the quality of CFBs [43]. Minimally processed replacements could potentially bridge nutrient shortfalls by reducing these foods, but these replacements would not be systematically fortified. Further, a lack of cooking skills and meal preparation time is associated with a higher intake of UPFs [44,45]. Thus, additional meal preparation skills and time would be needed, which are scarce in the United States [5].
Our results highlight that a balanced perspective is needed on this topic, particularly for vulnerable populations like infants and toddlers [46]. As shown in our analysis and others [25,26], some UPFs are high in sodium, added sugars, and solid fats, but some are important sources of iron, zinc, and whole grains. Many UPFs and other processed foods are fortified in the United States food supply, which has resulted in successful public health advances [47]. Beyond nutrient contributions and safety of the food supply, it is important to consider the industrial and technological revolution that ushered in food processing and enabled people (specifically women) to be less burdened from the time and labor-intensive tasks of preparing daily meals and future food reserves [48]. Currently, it is impractical to expect United States parents, guardians, or care providers to prepare more home-cooked meals from scratch because of increasing time demands [49]. Thus, caution is warranted in stigmatizing UPFs, as some UPFs (e.g., commercial infant foods, fortified infant cereals) and formulations (e.g., infant formula) are of particular importance to this vulnerable population [46]. Perhaps a pathway forward includes reformulations to expand healthy and U/MPF options or providing point-of-sale guidance for co-consumption with prepared options (e.g., how to pair a ready-to-heat meal with a fresh or frozen vegetable) to guide those with time and economic constraints.
We combined 3 cycles of nationally representative data on dietary intakes in the United States, which used AMPM, the reference method for self-reported dietary recalls. However, self-reported dietary intakes are subject to documented measurement errors [50], and proxy reporters may not know all foods and beverages infants and toddlers consume, resulting in under or over-reporting [51]. We used 1 d of dietary recalls, which may overlook foods or beverages that are infrequently consumed but is sufficient to describe population-level intake means [52]. Our objective was to describe intake from CFB, excluding contributions from human milk and formula intake, which circumvented challenges of estimating total energy intake in this age group. Lastly, the AMPM method was not developed to probe the degree of processing, so post hoc classification of foods may lead to categorization errors [20]. Therefore, we used a recommended standardized method of estimating Nova groups in NHANES, developed by the Nova research group, making our results comparable to previous research [25,26]. A novelty of our paper is that we adapted this method to also estimate percent contributions of nutrients and food groups to further describe how Nova groups influence dietary intake of infants and toddlers.
In conclusion, foods that are classified as unprocessed/minimally processed according to Nova are top sources of nutrients and food groups that are encouraged for infants and toddlers in the United States. Foods classified as ultra-processed are top sources of sodium and added sugars but also contribute meaningfully to nutrients and food groups that are under-consumed, particularly iron, zinc, and whole grains, mainly via consumption of ready-to-eat breakfast cereals.
Author Contributions
The authors’ responsibilities were as follows – LEO, KAH: conceptualized, designed, and conducted the research; LEO: analyzed data with assistance from Lisa Kahle at IMS and drafted the initial manuscript; LEO: has primary responsibility for the final content. All authors critically reviewed and revised, and all authors read and approved the final manuscript.
Conflict of Interest
LEO is PI on a grant administered by the USDA’s National Institute of Food and Agriculture (USDA-NIFA #2022-07671) that coordinated a workshop to establish a research roadmap for the future of food processing, processed food, and human health research. This was a public-private initiative; thus, the grant included scientists from ADM with special input from General Mills and academic institutions. The findings in this article of those of the authors and do not necessarily represent the official position of the NCI, NIH, ARS, or USDA. All other authors report no conflicts of interest.
Funding
The authors reported no funding received for this study.
Data Availability
Data from the NHANES are publicly available through the United States CDC's NCHS:https://www.cdc.gov/nchs/nhanes/index.htm. The code used to classify all foods and beverages from these data according to the Nova is currently available upon request.
Acknowledgments
We thank Lisa Kahle at IMS, Inc. for her contributions to programming the analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.06.020.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.United States Department of Agriculture, Department of Health and Human Services . 2020. Dietary guidelines for Americans.https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf Available from: [Google Scholar]
- 2.English L.K., Obbagy J.E., Wong Y.P., Butte N.F., Dewey K.G., Fox M.K., et al. Types and amounts of complementary foods and beverages consumed and growth, size, and body composition: a systematic review. Am. J. Clin. Nutr. 2019;109(Suppl 7) doi: 10.1093/ajcn/nqy281. 956S–77S, [DOI] [PubMed] [Google Scholar]
- 3.Koletzko B., Bührer C., Ensenauer R., Jochum F., Kalhoff H., Lawrenz B., et al. Complementary foods in baby food pouches: position statement from the Nutrition Commission of the German Society for Pediatrics and Adolescent Medicine (DGKJ, e.V.) Mol. Cell. Pediatr. 2019;6(1):2. doi: 10.1186/s40348-019-0089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia A.L., Curtin L., Ronquillo J.D., Parrett A., Wright C.M. Changes in the UK baby food market surveyed in 2013 and 2019: the rise of baby snacks and sweet/savoury foods. Arch. Dis. Child. 2020;105(12):1162–1166. doi: 10.1136/archdischild-2020-318845. [DOI] [PubMed] [Google Scholar]
- 5.Smith L.P., Ng S.W., Popkin B.M. Trends in US home food preparation and consumption: analysis of national nutrition surveys and time use studies from 1965-1966 to 2007-2008. Nutr. J. 2013;12:45. doi: 10.1186/1475-2891-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro C.A., Cannon G., Levy R.B., Moubarac J.C., Louzada M.L., Rauber F., et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–941. doi: 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexy U., Dilger J.J., Koch S. Commercial complementary food in Germany: A 2020 market survey. Nutrients. 2022;14(18) doi: 10.3390/nu14183762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunacci K.A., Salmon L., McCann J., Gribble K., Fleming C.A.K. The big squeeze: a product content and labelling analysis of ready-to-use complementary infant food pouches in Australia. BMC Public Health. 2023;23(1):656. doi: 10.1186/s12889-023-15492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauregard J.L., Bates M., Cogswell M.E., Nelson J.M., Hamner H.C. Nutrient content of squeeze pouch foods for infants and toddlers sold in the United States in 2015. Nutrients. 2019;11(7) doi: 10.3390/nu11071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juul F., Martinez-Steele E., Parekh N., Monteiro C.A., Chang V.W. Ultra-processed food consumption and excess weight among US adults. Br. J. Nutr. 2018;120(1):90–100. doi: 10.1017/S0007114518001046. [DOI] [PubMed] [Google Scholar]
- 11.Kim H., Hu E.A., Rebholz C.M. Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (Nhanes III, 1988-1994) Public Health Nutr. 2019;22(10):1777–1785. doi: 10.1017/S1368980018003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srour B., Fezeu L.K., Kesse-Guyot E., Allès B., Méjean C., Andrianasolo R.M., et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Sante) BMJ. 2019;365:l1451. doi: 10.1136/bmj.l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srour B., Kordahi M.C., Bonazzi E., Deschasaux-Tanguy M., Touvier M., Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 2022;7(12):1128–1140. doi: 10.1016/S2468-1253(22)00169-8. [DOI] [PubMed] [Google Scholar]
- 14.Vedovato G.M., Vilela S., Severo M., Rodrigues S., Lopes C., Oliveira A. Ultra-processed food consumption, appetitive traits and BMI in children: a prospective study. Br. J. Nutr. 2021;125(12):1427–1436. doi: 10.1017/S0007114520003712. [DOI] [PubMed] [Google Scholar]
- 15.Costa C.D.S., Assunção M.C.F., Loret de Mola C., Cardoso J.S., Matijasevich A., Barros A.J.D., et al. Role of ultra-processed food in fat mass index between 6 and 11 years of age: a cohort study. Int. J. Epidemiol. 2021;50(1):256–265. doi: 10.1093/ije/dyaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa C.D.S., Buffarini R., Flores T.R., Neri D., Freitas Silveira M., Monteiro C.A. Consumption of ultra-processed foods and growth outcomes in early childhood: 2015 Pelotas Birth Cohort. Br. J. Nutr. 2022:1–8. doi: 10.1017/S0007114522002926. [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics, Centers for Disease Control and Prevention National health and nutrition examination survey. https://www.cdc.gov/nchs/nhanes/index.htm Available from:
- 18.National Center for Health Statistics, Centers for Disease Control and Prevention NHANES survey methods and analytical guidelines. https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#sample-design Available from:
- 19.Moshfegh A.J., Rhodes D.G., Baer D.J., Murayi T., Clemens J.C., Rumpler W.V., et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am. J. Clin. Nutr. 2008;88(2):324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 20.Martinez Steele E., O'Connor L.E., Juul F., Khandpur N., Galastri Baraldi L., Montiero C.A., Parekh N., et al. Identifying and estimating ultraprocessed food intake in the US NHANES according to the NOVA classification system of food processing. J. Nutr. 2023;153(1):225–241. doi: 10.1016/j.tjnut.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krebs-Smith S.M., Kott P.S., Guenther P.M. Mean proportion and population proportion: two answers to the same question? J. Am. Diet. Assoc. 1989;89(5):671–676. doi: 10.1016/S0002-8223(21)02224-0. [DOI] [PubMed] [Google Scholar]
- 22.Food and Nutrition Service USDoA. WIC 2021-2022 Income eligibility guidelines.
- 23.Monteiro C.A., Astrup A. Does the concept of “ultra-processed foods” help inform dietary guidelines, beyond conventional classification systems? YES. Am. J. Clin. Nutr. 2022;116(6):1476–1481. doi: 10.1093/ajcn/nqac122. [DOI] [PubMed] [Google Scholar]
- 24.Astrup A., Monteiro C.A. Does the concept of “ultra-processed foods” help inform dietary guidelines, beyond conventional classification systems? NO. Am J Clin Nutr. 2022;116(6):1482–1488. doi: 10.1093/ajcn/nqac123. [DOI] [PubMed] [Google Scholar]
- 25.Wang L., Martínez Steele E., Du M., Pomeranz J.L., O’Connor L.E., Herrick K.A., et al. Trends in consumption of ultraprocessed foods among US youths aged 2-19 years. JAMA. 2021;326(6):519–530. doi: 10.1001/jama.2021.10238. 1999-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juul F., Parekh N., Martinez-Steele E., Monteiro C.A., Chang V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022;115(1):211–221. doi: 10.1093/ajcn/nqab305. [DOI] [PubMed] [Google Scholar]
- 27.Brazilian Ministry of Health . 2015. Dietary guidelines for the Brazilian population.https://bvsms.saude.gov.br/bvs/publicacoes/dietary_guidelines_brazilian_population.pdf Available from: [Google Scholar]
- 28.Canada’s dietary guidelines Available from: https://food-guide.canada.ca/sites/default/files/artifact-pdf/CDG-EN-2018.pdf. (Accessed 29 December 2022).
- 29.Berner L.A., Keast D.R., Bailey R.L., Dwyer J.T. Fortified foods are major contributors to nutrient intakes in diets of US children and adolescents. J. Acad. Nutr. Diet. 2014;114(7):1009–1022.e8. doi: 10.1016/j.jand.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Smith J.D., Zhu Y., Vanage V., Jain N., Holschuh N., Hermetet Agler A. Association between ready-to-eat cereal consumption and nutrient intake, nutritional adequacy, and diet quality among infants, toddlers, and children in the national health and nutrition examination survey 2015-2016. Nutrients. 2019;11(9) doi: 10.3390/nu11091989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Affenito S.G., Thompson D., Dorazio A., Albertson A.M., Loew A., Holschuh N.M. Ready-to-eat cereal consumption and the School Breakfast Program: relationship to nutrient intake and weight. J. Sch. Health. 2013;83(1):28–35. doi: 10.1111/j.1746-1561.2012.00744.x. [DOI] [PubMed] [Google Scholar]
- 32.Thomas R.G., Pehrsson P.R., Ahuja J.K.C., Smieja E., Miller K.B. Recent Trends in Ready-to-eat Breakfast Cereals in the U.S, Procedia. Food Sci. 2013;2:20–26. [Google Scholar]
- 33.Finn K., Callen C., Bhatia J., Reidy K., Bechard L.J., Carvalho R. Importance of dietary sources of iron in infants and toddlers: lessons from the FITS study. Nutrients. 2017;9(7) doi: 10.3390/nu9070733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver C.M., Dwyer J., Fulgoni V.L., 3rd, King J.C., Leveille G.A., MacDonald R.S., et al. Processed foods: contributions to nutrition. Am. J. Clin. Nutr. 2014;99(6):1525–1542. doi: 10.3945/ajcn.114.089284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vos M.B., Kaar J.L., Welsh J.A., Van Horn L.V., Feig D.I., Anderson C.A.M., et al. Added sugars and cardiovascular disease risk in children: A scientific statement from the American Heart Association. Circulation. 2017;135(19):e1017. doi: 10.1161/CIR.0000000000000439. 34–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrick K.A., Fryar C.D., Hamner H.C., Park S., Ogden C.L. Added sugars intake among US infants and toddlers. J. Acad. Nutr. Diet. 2020;120(1):23–32. doi: 10.1016/j.jand.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Steele E.M., Li Y., Karageorgou D., Micha R., Monteiro C.A., et al. Consumption of ultraprocessed foods and diet quality among U.S. Children and adults. Am. J. Prev. Med. 2022;62(2):252–264. doi: 10.1016/j.amepre.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckley J.P., Kim H., Wong E., Rebholz C.M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013-2014. Environ. Int. 2019;131:105057. doi: 10.1016/j.envint.2019.105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall K.D., Ayuketah A., Brychta R., Cai H., Cassimatis T., Chen K.Y., et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake, Cell. Metab. 2019;30(1):67–77.e3. doi: 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.International Food Information Council Food and Health Survey. 2022. https://foodinsight.org/2022-food-and-health-survey/
- 41.Neri D., Steele E.M., Khandpur N., Cediel G., Zapata M.E., Rauber F., et al. Ultraprocessed food consumption and dietary nutrient profiles associated with obesity: A multicountry study of children and adolescents. Obes. Rev. 2022;23(Suppl 1) doi: 10.1111/obr.13387. [DOI] [PubMed] [Google Scholar]
- 42.Mertens E., Colizzi C., Peñalvo J.L. Ultra-processed food consumption in adults across Europe. Eur. J. Nutr. 2022;61(3):1521–1539. doi: 10.1007/s00394-021-02733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reidy K.C., Bailey R.L., Deming D.M., O’Neill L., Carr B.T., Lesniauskas R., et al. Food consumption patterns and micronutrient density of complementary foods consumed by infants fed commercially prepared baby foods. Nutr. Today. 2018;53(2):68–78. doi: 10.1097/NT.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam M.C.L., Adams J. Association between home food preparation skills and behaviour, and consumption of ultra-processed foods: cross-sectional analysis of the UK National Diet and nutrition survey (2008-2009) Int. J. Behav. Nutr. Phys. Act. 2017;14(1):68. doi: 10.1186/s12966-017-0524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djupegot I.L., Nenseth C.B., Bere E., Bjørnarå H.B.T., Helland S.H., Øverby N.C., et al. The association between time scarcity, sociodemographic correlates and consumption of ultra-processed foods among parents in Norway: a cross-sectional study. BMC Public Health. 2017;17(1):447. doi: 10.1186/s12889-017-4408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibney M.J. Ultra-processed foods: definitions and policy issues. Curr. Dev. Nutr. 2019;3(2):nzy077. doi: 10.1093/cdn/nzy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention (CDC) Spina bifida and anencephaly before and after folic acid mandate--United States, 1995-1996 and 1999-2000, M.M.W.R . Morb. Mortal. Wkly. Rep. 2004;53(17):362–365. [PubMed] [Google Scholar]
- 48.Greenwood J. The MIT Press; Cambridge, MA, USA: 2019. Evolving households: the imprint of Technology on life. [Google Scholar]
- 49.Forde C.G., Decker E.A. The importance of food processing and eating behavior in promoting healthy and sustainable diets. Annu. Rev. Nutr. 2022;42:377–399. doi: 10.1146/annurev-nutr-062220-030123. [DOI] [PubMed] [Google Scholar]
- 50.Subar A.F., Freedman L.S., Tooze J.A., Kirkpatrick S.I., Boushey C., Neuhouser M.L., et al. Addressing current criticism regarding the value of self-report dietary data. J. Nutr. 2015;145(12):2639–2645. doi: 10.3945/jn.115.219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burrows T.L., Martin R.J., Collins C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J. Am. Diet. Assoc. 2010;110(10):1501–1510. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Kirkpatrick S.I., Guenther P.M., Subar A.F., Krebs-Smith S.M., Herrick K.A., Freedman L.S., et al. Using short-term dietary intake data to address research questions related to usual dietary intake among populations and subpopulations: assumptions, statistical techniques, and considerations. J. Acad. Nutr. Diet. 2022;122(7):1246–1262. doi: 10.1016/j.jand.2022.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the NHANES are publicly available through the United States CDC's NCHS:https://www.cdc.gov/nchs/nhanes/index.htm. The code used to classify all foods and beverages from these data according to the Nova is currently available upon request.