Abstract
Background
β-carotene oxygenase 1 (BCO1) and β-carotene oxygenase 2 (BCO2) are responsible for the cleavage of carotenoids in mammals. Objective: The goals of this study were to (1) establish the relative contribution of each enzyme on lycopene accumulation in mice and (2) examine the role of lycopene on gene expression in the gut of wild type (WT) mice.
Methods
We utilized male and female WT, Bco1-/-, Bco2-/-, and Bco1-/-Bco2-/- double knockout (DKO) mice. We gavaged the mice with either 1 mg of lycopene resuspended in cottonseed oil or vehicle as a control group daily for 2 wk. In a second study, we evaluated the effect of dietary vitamin A on lycopene absorption and intestinal gene expression by RT-PCR. We also quantified lycopene concentration isomer distribution by high performance liquid chromatography.
Results
Of the 11 tissues measured, the liver accounted for 94 to 98% of the lycopene content across genotypes. We did not observe sex differences between genotypes, although hepatic lycopene levels in Bco1-/- mice were approximately half in comparison to the other genotypes; Bco1-/- verses Bco2-/- (P < 0.0001), DKO mice (P < 0.001), WT (ns). Analyses of mitochondrial lycopene content revealed a 3- to 5-fold enrichment compared with total hepatic content (P < 0.05) in all genotypes and sexes. In our second study, WT mice fed a vitamin A-deficient diet (VAD) accumulated greater amounts of lycopene in the liver than those fed a vitamin A-sufficient diet (VAS) (P < 0.01). These changes were accompanied by an upregulation of the vitamin A-responsive transcription factor intestine specific homeobox (ISX) in mice fed VAD + lycopene and VAS + lycopene diets compared with VAD control-fed mice (P < 0.05).
Conclusions
Our data suggest that BCO2 is the primary lycopene cleavage enzyme in mice. Lycopene concentration was enriched in the mitochondria of hepatocytes independently of genotype, and lycopene stimulated vitamin A signaling in WT mice.
Keywords: absorption, scavenger receptor class B type 1, retinoic acid, biodistribution
Introduction
Lycopene is one of the most abundant carotenoids found in the human diet and tissues. Over the past decades, the antioxidant properties of lycopene have led researchers to explore its potential in the treatment and prevention of chronic diseases such as cardiovascular disease, non-alcoholic fatty liver disease, and prostate cancer [1]. However, the mechanisms that regulate lycopene cleavage and accumulation in tissues remain relatively unstudied, as most research has been focused on its biological and physiochemical properties [1]. Although the metabolism of lycopene is not fully elucidated, metabolites called lycopenoids have been identified in foods and tissues but are difficult to study as they are only found in very low concentrations and are likely metabolized rapidly in tissues [[2], [3], [4], [5], [6]].
It is well established that 2 enzymes, β-carotene oxygenase 1 (BCO1) and β-carotene oxygenase 2 (BCO2), are responsible for mammalian cleavage of most carotenoids, including lycopene [[7], [8], [9]]. BCO1 is a cytosolic enzyme that symmetrically cleaves carotenoids such as β-carotene at the 15, 15’double bond to form vitamin A [10]. BCO2 cleaves carotenoids asymmetrically between the carbons in position 9’,10’, is localized in the mitochondria, and has broader substrate specificity than BCO1 [11,12].
Carlo dela Seña et al. reported that lycopene could be cleaved in vitro using recombinant human BCO1 [13] but not by chicken BCO2 [14], suggesting BCO1 is the main lycopene cleaving enzyme. In contrast, we showed substantially lower hepatic lycopene accumulation in Bco1 -/- mice compared with wild type (WT) mice, suggesting that in vitro enzymatic activities might differ from animal studies [15].
The goal of this study was to probe which enzyme cleaves lycopene in vivo through evaluation of the impact of ablation of one or both carotenoid cleaving enzymes and to study the influence that sex has on lycopene accumulation. For this work, we cross-bred C57BL/6J WT mice with Bco1-/- and Bco2-/- mice for 10 generations to ensure similar genetic backgrounds of all genotypes. Mice lacking both BCOs (double knockout, DKO) were also derived following backcrossing. Both males and females were utilized as previous research has indicated that sex has an impact on carotenoid accumulation, with females tending to accumulate more carotenoids and be more sensitive to the bioactivity of carotenoids [16]. We dosed these mice with lycopene and evaluated the accumulation of lycopene in tissues, plasma, and isolated hepatic mitochondria.
In a second study, we evaluated the influence that dietary lycopene has on the expression of intestine specific homeobox (ISX), a vitamin A-sensitive gene implicated in the uptake of dietary lipids, including cycled carotenoids [17]. ISX expression is upregulated through retinoic acid stimulation of retinoic acid receptors (RARs). ISX then downregulates scavenger receptor class B type 1 (SRB1), a protein that helps facilitate the uptake of carotenoids in the gut, and it also downregulates BCO1 [17]. The study's second aim was to explore whether SRB1 facilitates the uptake of lycopene from the gastrointestinal tract and, to elucidate whether lycopene, through this ISX/SRB1 axis, suppresses intestinal SRB1 and BCO1 as retinoic acid does, therefore, impacting the uptake and accumulation of lycopene in the body.
Methods
Mice breeding
The University of Illinois Institutional Animal Care and Use Committee reviewed and approved the experimental procedures used in these studies. Breeding colonies of C57BL/6J WT, Bco1-/-, Bco2-/-, and DKO mice were maintained at the Animal Care Facility. Mouse colonies were backcrossed for 10 generations with C57BL/6J mice in order to ensure mice were in the same genetic background, as done in the past [18]. Genotyping was routinely performed using DNA isolated from the tail snips at 10 d of age. DNA samples were isolated using the Extract-N-Amp Tissue PCR kits (Sigma-Aldrich, St. Louis, Missouri).
Lycopene oil dilution and resuspension
The lycopene oil used to gavage the mice was Redivivo lycopene oil 10% fluid suspension and was a gift from DSM, Basel, Switzerland. We prepared a working solution containing 1 mg lycopene/0.2 mL of oil using cottonseed oil. We aliquoted the working solution into 2 mL Eppendorf tubes, flushed them with argon, and immediately sealed them with parafilm. Working solutions were stored at 4 °C for a maximum of 2 wks until used for gavage.
Animal trials
For the first study, we utilized male (n = 4-7/group) and female (n = 4-6/group) WT, Bco1-/-, Bco2-/-, and DKO mice. After weaning, mice continued on a chow diet (Teklad Global 18% protein rodent diet 2918; Envigo, Indianapolis, Indiana) for 1 wk before being placed on a semi-purified powder AIN-93G diet containing cottonseed oil as a source of fat to minimize carotenoid contamination in their feed. (Supplemental Table 1). Mice remained on this diet for the entire duration of the study. We provided at least 5 g of diet/mouse/d to ensure mice had constant access to food. After 2 wks, mice were gavaged daily with 1 mg lycopene suspended in 0.2 mL of cottonseed oil. A subset of WT mice was gavaged with 0.2 mL of cottonseed oil alone to serve as a control. We measured body weight gain once mice started on the AIN-93G diet. After 2 wks of daily gavage, mice were fasted overnight and killed for tissue and blood collection.
In a second study, we utilized WT male and female mice (n = 3-6 per sex, n = 6-8 per group). After weaning, mice continued on a chow diet (Teklad Global 18% protein rodent diet 2918; Envigo) for 1 wk and then were switched to a vitamin A-deficient (VAD) AIN-93G diet (Supplemental Table 2) supplemented with either 100 mg of lycopene/kg diet or the same diet without lycopene. Lycopene was added to the diets in the form of 10% lycopene beadlets (Redivivo DSM). The VAD diet without lycopene contained the same amount of control beadlets (Redivivo DSM). A third subset of mice received an AIN-93G vitamin A-sufficient diet (VAS) (4 IU/kg) supplemented with 100 mg of lycopene/kg diet in the form of beadlets. After 4 wks on these diets, mice were fasted overnight and killed for tissue and blood collection.
Necropsy
Mice were injected intraperitoneally with a dose of a mixture containing ketamine/xylazine (87mg/kg ketamine, 13mg/kg xylazine) to anesthetize. Once mice were unresponsive, the blood was collected via cardiac puncture followed by cervical dislocation. Serum was separated from blood by centrifuging (2400 × g for 15 min, 4 °C) and stored at -80 °C until analysis. The liver, duodenum, gonadal white adipose tissue (gWAT), kidneys, spleen, heart, adrenals, prostate, seminal vesicles, testes, and ovaries were harvested. For study 2, the jejunum, duodenum, and ileum were harvested.
For mRNA expression analyses, a portion of the gWAT, duodenum, jejunum, ileum, the dorsal lobe of the prostate, and the caudal lobe of the liver were preserved in RNAlater (Thermo Fisher Scientific, Waltham, MA), following manufacturer’s instructions.
Carotenoid extraction and HPLC analysis
Tissue lycopene was extracted, and concentrations and isomers were analyzed by HPLC using a well-established method from our lab [19,20] utilizing approximately 0.05 to 0.1 g of tissue or 200 μl of serum. For the adrenals, prostate, and ovaries, tissues were pooled for n = 1 per sex/genotype. Samples were minced with scissors in a 30 mL screw crap glass centrifuge tube. 0.5 mL of ethanol with 0.1% butylated hydroxytoluene (BHT) was added to prevent oxidation, after which 1 mL of saturated potassium hydroxide (KOH) was added and vortexed for saponification. Samples were placed in a water bath for 30 min, receiving a vigorous vortex every 10 min. After 30 min, they were removed from the bath and received 2 mL of double distilled water. Six mL of hexane was then added, and the samples were vortexed before centrifugation for 5 min to create layer separation. The top hexane layer was transferred to a 13 × 100 mm test tube. This step was repeated twice more, after which the samples were dried down in an automatic speedvac (Eppendorf, Hamburg, Germany). Samples were transferred into 1.5 mL Eppendorf tubes (Eppendorf) through further rinsing with hexane. They were then completely dried down in the Eppendorf tubes, covered with argon, and parafilmed for storage at -20 °C until use (used no more than 48 h later). The samples were reconstituted in a mix of methyl tert-Butyl Ether, methanol, and ammonia acetate (Thermo Fisher Scientific) and injected into the HPLC for analysis. Lycopene was identified using commercially available standards and comparing their spectra and elution times to the sample. Isomers were characterized by relative retention times and spectral characteristics.
All HPLC reagents utilized for lycopene analyses were HPLC grade. Ethanol was obtained from Decon Laboratories (King of Prussia, PA). Butylated hydroxytoluene was purchased from Sigma-Aldrich. All other chemicals utilized are ACS grade and were purchased from Thermo Fischer Scientific.
Lipid analyses
Hepatic lipids were extracted using the Folch method, with small modifications [21]. Total cholesterol was measured using the total cholesteryl and cholesteryl ester colorimetric/fluorometric assay kit (BioVision, Milpitas, CA), following the manufacturer’s instructions.
RNA isolation
We isolated mRNA from approximately 50 mg of tissue using the RNA isolation kit (Thermo Fisher Scientific). The tissues were homogenized in 1mL of TRIzol Reagent (Thermo Fisher Scientific) with a sonicator, and the isolation was carried out following the manufacturer’s instructions. The concentration and purity of the RNA were measured with a Nano-drop 2000 spectrophotometer (Thermo Fisher Scientific).
Real-time PCR Analyses
Real-time PCRs were conducted using either TaqMan Fast Advanced Reaction Mix (Applied Biosystems, Foster City, CA) or PowerUp SYBR Green Master Mix (Applied Biosystems). Primers were purchased from Integrated DNA Technologies (Coralville, IA): intestine specific homeobox (ISX; 5'-ATCTGGGCTTGTCCTTCTCC-3' and 5'-TTTTCTCTTCTTGGGGCTGA-3'), Scavenger receptor type B, class 1 (SRB1; 5'-CTCATCAAGCAGCAGGTGCTCA-3' and 5'- GAGGATTCGGGTGTCATGAA-3'), BCO1 (Bco1; 5'-CGGAAGTATGTGGCGGTAAA-3'), BCO2 (Bco2; 5'-GCACATCCTCATTACGACCC3' and 5'-CCCCGGGCTCTTTCTTTTT-3'), mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 5'-TTGGCATTGTGGAAGGGCTCAT-3' and 5'-GATGACCTTGCCCACAGCCTT-3') mouse α-tubulin (5'-CAGGGCTTCTTGGTTTTCC-3' and 5'-GGTGGTGTGGGTGGTGAG-3'). Probes were purchased from Thermo Fisher Scientific: (SRB1; Mm00450234). Gene expression analysis was conducted with the StepOnePlus Real-Time PCR System, and the calculations were made utilizing either GAPDH (study 1) or α-tubulin as a housekeeping gene (study 2). RNA expression levels were determined using the Pfaffl method as done in the past [22].
Mitochondrial purification
Hepatic mitochondria were isolated using established protocols, with small modifications [23]. Briefly, approximately 400 mg of the liver was placed in a Dounce homogenizer with 3 mL of the homogenization buffer (75mM sucrose, 225mM mannitol, 5mM Tris-HCL pH 7.4, phenylmethylsulfonyl fluoride (PMSF), 1x protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and ground over ice exactly 80 times. We kept an aliquot as total liver homogenate while we employed the remaining homogenate (∼2.25 mL) for mitochondrial purification. First, the homogenate was centrifuged at 2,800 × g for 5 min at 4° C twice to pellet undigested tissues and cells. The supernatant was centrifuged at 10,500 × g for 5 min at 4° C, and the pellet was then resuspended in 0.3 mL of the homogenization buffer and centrifuged at 10,500 × g for 10 min at 4° C. The supernatant was discarded, and the pellet left comprised the mitochondrial fraction. This fraction was resuspended in 0.2 mL homogenization buffer and stored at -80°C. The purity of the isolation was confirmed through a western blot analysis. Mitochondrial purity was estimated using western blot for translocase of outer mitochondria membrane 20 (TOM20) and GAPDH as mitochondrial and cytosolic markers, respectively [23].
Western blot
The western blot methods, including protein quantification, electrophoresis, and detection method, were the same as those previously detailed in Coronel et al. [16]. The total protein quantification was performed using the bicinchoninic acid assay, following the manufacturer’s protocol (Thermo Fisher Scientific). Between 20 and 40 μg of protein was loaded onto an SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA). After blocking with fat-free milk powder (5% w/v), the membrane was washed with Tris-buffered saline (15 mM NaCl and mM Tris/HCL, pH 7.5) and 1% triton-100 (TBS-T). After washing, the membranes were incubated overnight with the antibody of interest. The primary antibodies and their dilutions are as follows: GAPDH, 1:1000 (Invitrogen #PA1-988), and TOM20, 1:1000 (Cell Signaling #42406). Secondary antibodies that were either infrared fluorescent-labeled (Li-CorBioscience, Lincoln, NE) or HRP-conjugated were prepared in TBS-T and 5% fat-free milk powder and incubated at room temperature for 1 h. These immunoblots were developed with the ECL system (Thermo Fisher Scientific), and the band intensities were visualized through ImageQuant LAS 4000 (GE Healthcare) [16].
Statistical Analysis
GraphPad Prism software (GraphPad Software, Inc.) was utilized to perform statistical analysis. We performed a 4 × 2 multifactorial 2-way analysis of variance (ANOVA) to explore interactions between genotypes and sex. No sex differences within genotypes were found for any tissue, so we continued our analyses comparing the effect of genotype on lycopene levels by one-way ANOVA, utilizing Tukey’s post-hoc test. When assumptions of ANOVA were violated, the data either underwent a transformation or a non-parametric test was conducted. For analyzing mouse weight gain, repeated measurement analysis was used. During study 2 gene analysis, a 2-way ANOVA was utilized for the comparison of treatment groups. For all analyses, a P value of <0.05 was considered statistically significant.
Results
Study 1
Mice body and tissue weights
There was a sex effect in mouse weight across all genotypes, with males having higher final weights than females (P = 0.0012, P = 0.033, P = 0.0024, P < 0.0001, P < 0.0001) (Figure 1). Additionally, when examining the genotype effect among each sex, the WT (vehicle) females weighed significantly less than the female WT, Bco2-/-, and DKO mice (P = 0.024, P = 0.0001, P = 0.039). When analyzed for genotype effect regardless of sex, Bco2-/- mice had a significantly higher weight than the Bco1-/- mice (P = 0.001). Regarding tissue weights, the Bco2-/- mice had a greater gWAT tissue weight than the Bco1-/- mice (P < 0.01). In the Bco2-/- and DKO groups, the males had a significantly higher gWAT tissue weight than the females (P < 0.001). However, in the rest of the tissues measured, there were no further sex effects, and there were only genotype effects seen when males and females were analyzed separately (Supplementary Tables 3, 4). These results did not differ when the tissue weights were analyzed as a percent of the total body weight (Supplementary Tables 5, 6).
FIGURE 1.
Body weight of mice (WT (vehicle), WT, Bco1-/-, Bco2-/-, DKO) over the treatment period of 28 d. The weights were tracked starting when mice were placed on the AIN-93G diet at 4 wk of age (day zero in the figure). Days 0 to 14, they were fed a powdered diet, and from days 14 to 28, they also received a daily oral gavage (start of gavage indicated by arrow). Males weighed significantly more than females on the final treatment day in all genotypes (WT (vehicle), WT, Bco1-/-, Bco2-/-, DKO) (P = 0.0012, P = 0.03, P = 0.0024, P < 0.0001, P < 0.0001). Female WT vehicle mice weighed significantly less than female WT, Bco2-/-, and DKO mice (P = 0.02, P = 0.0001, P = 0.04). The Bco2-/- mice had a significantly higher weight than the Bco1-/- mice (P = 0.001) when the sexes were combined. There was no genotype effect among male mice. One-way and two-way ANOVAs were used to measure the significance between sexes and genotypes. N = 4-7 per group. Error bars represent SEM.
Impact of genotype on tissue lycopene accumulation
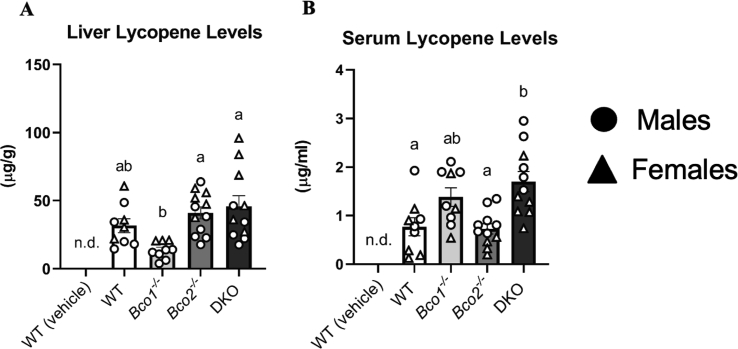
In the liver, which accounted for 94 to 98% of total measured lycopene, the Bco1-/- mice exhibited less lycopene accumulation than the Bco2-/- (P < 0.0001) or the DKO group (P < 0.001) (Figure 2A). No differences were observed between WT and Bco1-/- mice. Bco2-/- mice also accumulated more splenic lycopene than the Bco1-/- mice (P < 0.001).
FIGURE 2.
Lycopene accumulation in mouse liver and serum. (A) Hepatic lycopene accumulation in mice (WT (vehicle), WT, Bco1-/-, Bco2-/-, DKO). The Bco2-/- and DKO mice accumulated significantly more lycopene than the Bco1-/- mice. (B) Serum lycopene accumulation in mice. The DKO mice accumulated significantly more lycopene than the Bco1-/- and WT mice. There was no significant sex effect. One-way ANOVA was used to analyze for statistical significance. N = 9-12 per group. For each gene, bars not sharing the same letter are significantly different (P < 0.05). Circles represent males, triangles females. n.d. = not detected. Error bars represent SEM.
In the serum and the rest of the extrahepatic tissues measured, Bco1-/- mice tended to have a higher accumulation of lycopene than Bco2-/- mice. In the serum, the Bco2-/- and WT groups accumulated less lycopene than the DKO group (P < 0.0001) (P < 0.01) (Figure 2B).
The Bco2-/- group accumulated less lycopene than the DKO group in the heart (P < 0.0001), kidneys (P < 0.0001), duodenum (P < 0.01), gonadal adipose (P < 0.01), and testes (P < 0.001) (Table 1). Compared to the Bco1-/- mice, the Bco2-/- group accumulated significantly less lycopene in the kidneys (P < 0.001). The pooled tissues also exhibited a similar extrahepatic genotype accumulation trend, with DKO mice accumulating the most lycopene, followed by the Bco1-/- group and then the Bco2-/- group (Table 1). The WT mice accumulated less lycopene than the Bco2-/- mice in the spleen (P < 0.001) and heart (P < 0.001) and less than the DKO mice in the kidneys (P < 0.01) and heart (P < 0.01). Additionally, the WT mice accumulated less than the Bco1-/- mice in the heart (P < 0.001).
TABLE 1.
Lycopene accumulation in tissues.
| Tissues | Genotype P value | WT (vehicle) | WT | (Lycopene μg/g; μg/ml) |
DKO | |
|---|---|---|---|---|---|---|
| Bco1-/- | Bco2-/- | |||||
| Kidney | 0.0001 | n.d. | .23±.033ab | .32±.034ac | .23±.014b | .46±.037c |
| Heart | 0.0001 | n.d. | .15±.023a | .38±.031bc | .32±.012b | .58±.047c |
| Duodenum | 0.0101 | n.d. | 5.2±.871ab | 4.5±1.41a | 2.9±.341a | 7.0±1.40b |
| Spleen | 0.0001 | n.d. | .89±.264a | .90±.078ab | 2.0±.268b | 1.8±.220b |
| Adipose | 0.0088 | n.d. | .37±.064ab | .66±.105a | .36±.048b | .82±.149a |
| Testes | 0.0001 | n.d. | 1.2±.223a | 2.1±.273a | 3.8±.150a | 12.0±.323b |
| Seminal v esicles | 0.3327 | n.d. | .14±.077 | .11±.013 | .14±.011 | .22±.061 |
| Adrenals | NA | n.d. | 3.6 | 8.9 | 3.8 | 10.7 |
| Prostate | NA | n.d. | .34 | .53 | .30 | .80 |
| Ovaries | NA | n.d. | 2.3 | 3.1 | .95 | 9.7 |
One-way ANOVA was used to analyze for statistical significance. N = 9-12 per group (N = 4-7 for testes and seminal vesicles). Adrenals were pooled (n = 2), as well as prostate and ovaries (n = 1). Tissues not sharing the same letter in a row are significantly different (P < 0.05). NA = not applicable. n.d.= not detected.
Lycopene accumulates in the mitochondria
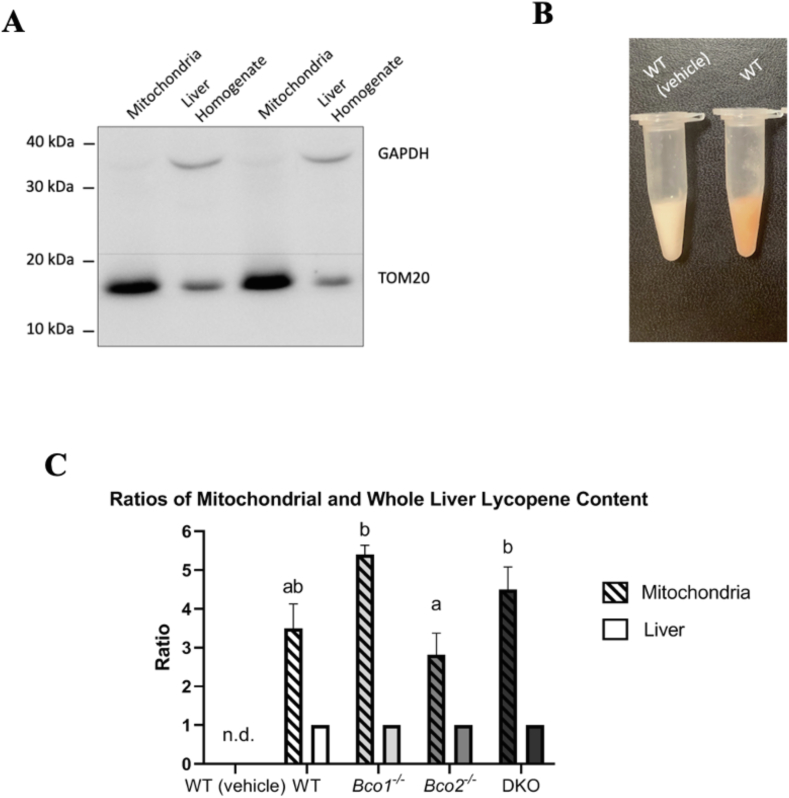
BCO2 is localized in the inner membrane of the mitochondria, and previous studies show that cycled carotenoids can accumulate in this organelle [11,24]. To examine whether lycopene can also accumulate in the mitochondria, we isolated hepatic mitochondria from WT, Bco1-/-, Bco2-/-, and DKO mice (Figure 3A). Lycopene accumulation was obvious comparing the color of isolated mitochondria samples from WT mice dosed with vehicle and lycopene (Figure 3B). HPLC analysis showed mitochondria are enriched with lycopene in comparison to liver homogenates, independently of the genotype (Figure 3C).
FIGURE 3.
Mitochondrial and whole liver lycopene accumulation. (A) Western blot of liver homogenate and purified mitochondrial fractions. The cytosolic housekeeping gene GAPDH was detected in the liver homogenate only, and the mitochondrial protein TOM20 showed stronger bands in the purified mitochondrial samples. (B) Lycopene accumulation in the mitochondria of a WT mouse dosed with lycopene. (C) Ratios generated from the accumulation of lycopene (μg/g protein) in mitochondrial fractions compared to the whole liver homogenate. In all genotypes, the ratio of mitochondria levels of lycopene compared to liver levels was enhanced. The Bco2-/- mice had a smaller ratio difference than the Bco2-/- mice (P < 0.004) and the DKO mice (P < 0.04). One-way ANOVA was utilized, n = 9-12 per group. Bars not sharing the same letter are significantly different (P < 0.05). Error bars represent SEM.
We also evaluated the whole liver and mitochondrial lycopene isomeric profile. First, however, to gain better context, we examined the isomer configuration in the gavage dose of lycopene, the serum, and the liver. The dose of lycopene that was given to the mice via gavage contained 84% all-trans, 10% 5 cis, and 6% other cis isomers. Tissues contained a substantially lower percentage of trans isomers than the dose. For a WT mouse dosed with lycopene, as an example, the serum profile was 13% all-trans, 55% 5 cis, and 32% other cis, whereas the liver contained 29% all-trans, 41% 5 cis, and 30% other cis (Figure 4 A-C). For the whole liver and mitochondrial evaluation, the ratio of all-trans, 5-cis, and other cis lycopene isomers between the homogenate and mitochondria remained largely unaltered across all genotypes (Figure 4D and E). However, Bco1-/- mice had a reduction in all-trans isomers in comparison to the other genotypes. (Figure 4D and E). This reduction resulted in an increase in cis isomers across the whole liver and mitochondria, including 5 cis.
FIGURE 4.
HPLC chromatograms of the dose of lycopene given, a liver and serum sample, and the mitochondria and liver homogenate isomers. Retention times for lycopene isomers HPLC chromatograms (measured at 470 nm) beginning at 19 and ending at 30 min. The number (1) indicates other cis peaks, while (2) and (3) are all-trans and 5 cis, respectively. (A) The isomeric profile of the dose gavaged to the mice was 84% all-trans, 10% 5 cis, and 6% other cis.(B)(C) In the serum of a WT mouse dosed with lycopene, there was 13% all-trans, 55% 5 cis, and 32% other cis, whereas the liver contained 29% all-trans, 41% 5 cis, and 30% other cis. (D)(E) When comparing the isomers in the mitochondria-enriched samples to the liver homogenate samples, there was no difference seen for any genotypes. However, both the mitochondria and liver homogenate samples from Bco1-/- mice had significantly lower levels of all-trans and higher levels of 5 cis and other cis (P < 0.05). One-way ANOVA was utilized, n = 9-12 per group. Bars not sharing the same letter are significantly different (P < 0.05). Error bars represent SEM.
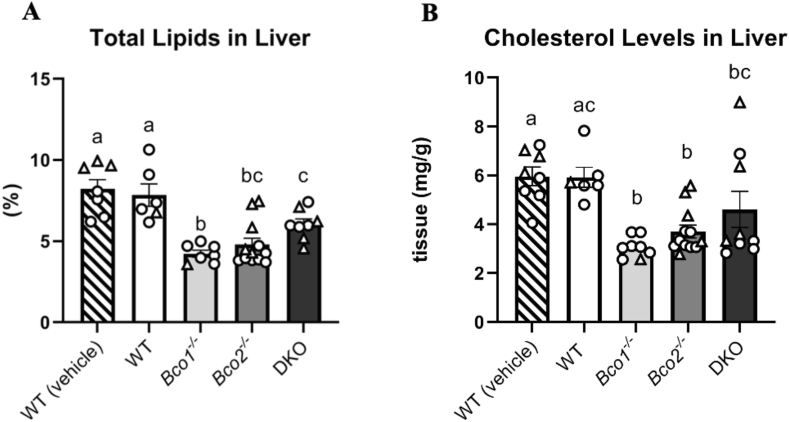
Total liver lipids and cholesterol
Previous research has shown the ablation of BCO1 or BCO2 can lead to alterations in hepatic lipid content [11,[25], [26], [27]]. Both groups of WT mice displayed greater total lipid level content than mice lacking one or both BCOs (P < 0.01) (Figure 5A). Additionally, the DKO mice had a higher percentage of lipids than the Bco1-/- mice (P < 0.05).
FIGURE 5.
Total hepatic lipid and cholesterol levels. (A) Percentage of lipids in livers of mice (WT (vehicle), WT, Bco1-/-, Bco2-/-, DKO). The WT vehicle group had significantly higher lipid levels than the knockout groups (P < 0.0001, P < 0.0001, P = 0.01), as did the WT group (P < 0.0001, P = 0.0002, P = 0.05). Additionally, the DKO mice had a higher percentage of lipids than the Bco1-/- mice (P = 0.046). (B) Cholesterol levels in the livers of mice (WT (vehicle), WT, Bco1-/-, Bco2-/-, DKO) showed that the WT vehicle group had significantly higher levels than the knockout groups (P < 0.0001, P = 0.002, P = 0.05), and the WT group had higher levels than the single KO groups (P = 0.0001, P = 0.005) Results were calculated through two-way ANOVA. N = 10-12 for both A and B. No sex effect was seen. For each gene, bars not sharing the same letter are significantly different (P <0.05). Circles represent males, triangles females. Error bars represent SEM.
Hepatic total cholesterol concentrations mirrored the results described in our total lipid analysis, showing that the WT vehicle mice had greater cholesterol levels than the knockout mice (P < 0.05), and the WT mice dosed with lycopene had greater levels than the single knockout mice (P < 0.01) (Figure 5B).
RNA expression levels
Portions of the liver, duodenum, gWAT, and prostate were used for RNA isolation and RT-PCRs for relevant genes related to lycopene metabolism (gWAT and prostate data not shown). Lycopene dosing in the control group did not enhance the expression of any tested genes in the liver. The absence of either BCO1 or BCO2 did not result in an upregulation in the expression of the alternate cleavage enzyme, and in the Bco1-/- mice, hepatic BCO2 expression was significantly downregulated compared to the WT groups (P < 0.01) (Figure 6A).
FIGURE 6.
Gene expression of Bco1, Bco2, ISX, and SRB1 in the liver and duodenum of different mouse genotypes. (A) In the liver, BCO1 expression in the Bco2-/- group was significantly downregulated compared to the WT groups. Similarly, the BCO2 expression in the Bco1-/- group was also downregulated compared to the WT groups. (B) In the duodenum, the WT group dosed with lycopene exhibited a significant downregulation of SRB1 compared to the WT control. DKO mice had downregulated ISX expression compared to WT groups. n = 9-12 per group, one-way ANOVA was used to analyze statistical significance. For each gene, bars not sharing the same letter are significantly different (P < 0.05). Error bars represent SEM.
In the duodenum, the WT mice dosed with lycopene exhibited a significant downregulation of SRB1 expression compared with the WT vehicle. Additionally, ISX expression in DKO mice was downregulated compared with the WT groups (P < 0.01) (Figure 6B).
Study 2
Lycopene accumulation in VAS and VAD mice
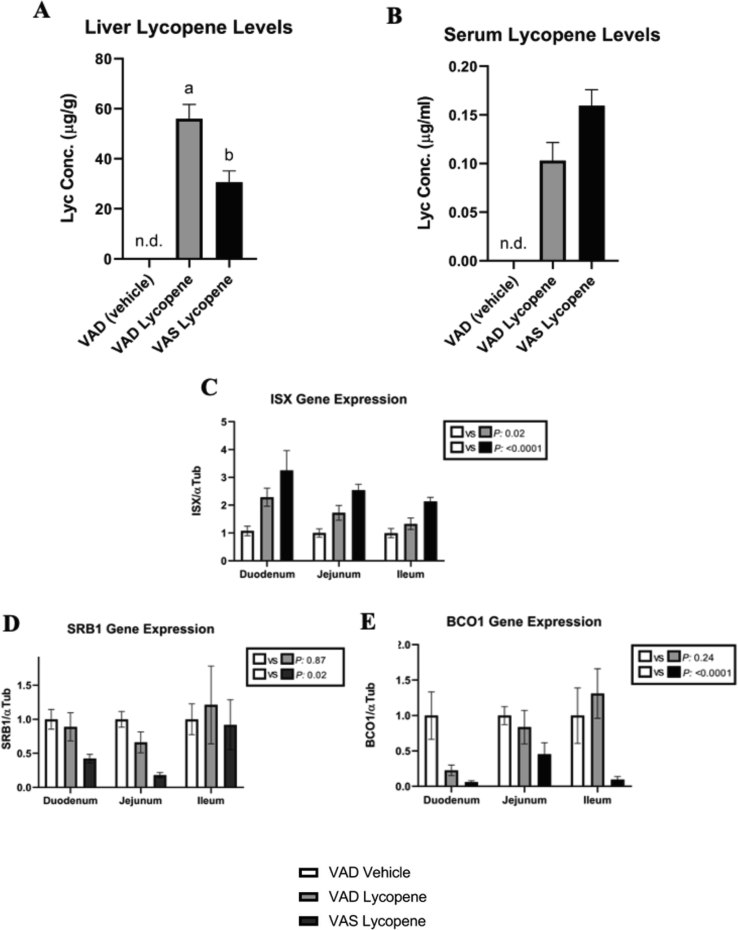
To evaluate the effect of vitamin A status on lycopene accumulation, we measured lycopene in the liver and serum in WT mice fed either VAS or VAD diets for 4 wks. Hepatic lycopene was higher in VAD mice than in VAS mice (Figure 7A, P < 0.05), while we did not observe significant differences in plasma lycopene levels between these groups (Figure 7B).
FIGURE 7.
Lycopene concentration levels in the liver and serum of mice supplemented with lycopene on VAS and VAD diets and gene expression levels of the genes ISX, SRB1, and BCO1 in VAS, VAD, and VAD (vehicle) mice. (A) In the liver, the VAD mice had significantly greater levels of lycopene than the VAS mice (P < 0.05). (B) In the serum, no significant difference was seen between the treatment groups. (C) Both the VAD and VAS mice had higher ISX expression than the VAD vehicle mice. (D)(E) VAS mice had lower SRB1 and BCO1 expression than the VAD vehicle mice. n = 7-9 per group, two-way ANOVA was used to analyze statistical significance. For each tissue, bars not sharing the same letter are significantly different (P < 0.05). For each gene expression comparison, a value of P < 0.05 is significant. Error bars represent SEM.
Gene expression
Next, we evaluated gene expression between groups at the level of the duodenum, jejunum, and ileum. In comparison to mice fed a VAD diet without lycopene, mice fed either VAD or VAS diets supplemented with lycopene showed an upregulation of the transcription factor ISX (P = 0.02) and (P < 0.0001), respectively (Figure 7C). We also evaluated the expression of 2 target genes of ISX: SRB1 and BCO1. Only those mice fed VAS presented lower SRB1 and BCO1 expression (P = 0.02) and (P < 0.0001) compared with WT mice fed a VAD vehicle diet (Figure 7D and E).
Discussion
We have had a long-time interest in lycopene metabolism and tissue deposition using rodent, ferret, and gerbil models [1,6,19,[28], [29], [30]]. With the availability of transgenic mouse models with an ablated expression of single or both carotenoid cleavage enzymes, it is possible to investigate which enzyme might be the main contributor to lycopene cleavage. Our previous publications with Bco1-/- and Bco2-/- mice strongly suggested that the primary enzyme responsible for the initial cleavage of lycopene was BCO2 [15,29,31]. The genetic background of the WT and transgenic BCO/DKO mice were not identical for those studies, however. We overcame this limitation in our current study, allowing us to compare lycopene distribution more accurately in mice.
In humans, BCO1 and BCO2 are expressed in the liver, kidney, adrenal glands, testes, retina, and epithelial cells of the small intestines and stomach [32]. Based on data from the Human Protein Atlas (proteinatlas.org) [33], the tissues with the greatest levels of BCO1 and BCO2 are the liver, intestines, and eyes [34]. Additionally, our study found that the liver accounted for 94 to 98% of the total lycopene measured across genotypes. Because of the high lycopene liver stores and high hepatic expression of BCOs, we inferred that the data derived from this organ would inform us about which enzyme was most important for the cleavage of lycopene. The results reported in this manuscript support our notion that BCO2 is the most important cleavage enzyme for lycopene based on the substantial differences in lycopene accumulation in the liver. Bco1-/- mice had significantly lower hepatic and total body lycopene concentrations than all other genotypes. Although serum and many extrahepatic tissues did not follow this trend, these tissues were minor accumulation sites for lycopene under our experimental conditions.
It was also found that lycopene preferentially accumulated in the mitochondria, as observed for other carotenoids in the past [11,24]. Previously, mice fed high levels of lutein resulted in impaired mitochondrial function and accumulation of uncharacterized lutein isomers in this organelle, probably due to the capacity of carotenoids to act as electron scavengers in mice [11]. Whereas we did not explore whether lycopene accumulation resulted in the impairment of mitochondrial function, the presence of a comparable isomeric profile between mitochondria and total liver homogenate suggests that this carotenoid did not affect the respiratory chain.
SRB1 is a protein that has been shown to be involved in the uptake of carotenoids [35,36], and additionally, the transcriptional factor ISX has been shown to be involved in the regulation of SRB1 expression. Lobo et al. found that retinoic acid-induced ISX expression via RARs, which in turn downregulated SRB1 and BCO1 [16]. Just as BCO1 cleaves β-carotene to form retinoids, we have hypothesized that lycopene is cleaved into derivatives known as lycopenoids [6]. It is believed that BCO1-derived lycopenoids could bind and serve as agonists/antagonists for RARs and retinoid x receptors (RXRs) [13,37]. Results from study 1 demonstrated that in the duodenum, where this ISX/SRB1 axis exists, the WT group dosed with lycopene had a significant decrease in SRB1 expression compared with the WT control group. Thus, lycopene, like dietary beta carotene [16], might suppress intestinal SRB1. Therefore, study 2 utilized VAS and VAD diets to further explore this axis and to determine if SRB1 is involved in the uptake of lycopene.
In study 2, VAD mice supplemented with lycopene had significantly greater lycopene accumulation in the liver than VAS, lycopene-supplemented mice. This suggests that SRB1 plays a role in facilitating the uptake of lycopene. Gene expression results in the 3 intestinal sections demonstrated that lycopene-fed VAD and VAS mice expressed significantly higher levels of ISX than the VAD vehicle mice. The opposite effect was seen in SRB1 and BCO1 expression, where VAS mice expressed significantly fewer levels of both genes than the VAD vehicle mice. These results suggest that lycopene, or its metabolites, might act similarly to retinoic acid on RAR receptors and influence the ISX/SRB1 axis, as some studies have speculated [37,38]. Lycopenoids may act as agonists or antagonists for other nuclear receptors, but there is still much we do not know about the effect of lycopenoids on the body. These metabolites are difficult to study due to their short half-life, lack of commercially available standards, and because they are only found in very low concentrations in tissues [2,4,5].
Previous studies conducted by our laboratory explored the bioavailability/accumulation of all-trans and cis isomers of lycopene. Common findings from these studies and the current results showed that whereas the dose of lycopene that was provided was largely in all-trans form (84% in the current study), tissue and serum lycopene were mostly in cis forms. It is estimated that 90% of the lycopene humans receive from foods is in the all-trans form [5]. However, a study from our laboratory [39] where humans were provided with a single dose of 82% all-trans 13-C lycopene revealed that cis isomers of lycopene increase over time in body tissues, leading to a shorter half-life of the all-trans isomer (5.3 versus 8.8 h) and a shorter time to reach maximal plasma concentrations (28 versus 48 h) [39]. Results from a ferret study by Hu et al. suggested that ferret BCO2 does not cleave all-trans lycopene efficiently or at all and that based on the Km value when examining the BCO2 interaction with the 5-cis lycopene and all-trans substrates, cis lycopene was preferentially cleaved by BCO2 [40]. However, our results revealed that the isomer composition across all tissues, except for the duodenum, showed a lower percentage of all-trans isomers than either 5 cis or other cis. Additionally, in the liver, isolated hepatic mitochondria, kidneys, spleen, adipose, and testes, the Bco1-/- mice had significantly fewer percentages of all-trans isomers than the other groups, suggesting that BCO2 prefers an all-trans substrate. The differences in results between these 2 studies could be due to interspecific differences between ferrets and mice.
Another observation we had was that our gene expression results for BCO1 and BCO2 in the liver revealed that the ablation of one enzyme did not enhance the expression of the other enzyme but rather resulted in a significant downregulation of the other enzyme. Coronel et al. found that BCO1 over-expression in adipocytes of Bco1-/- mice also failed to alter BCO2 expression in the adipose tissue [16].
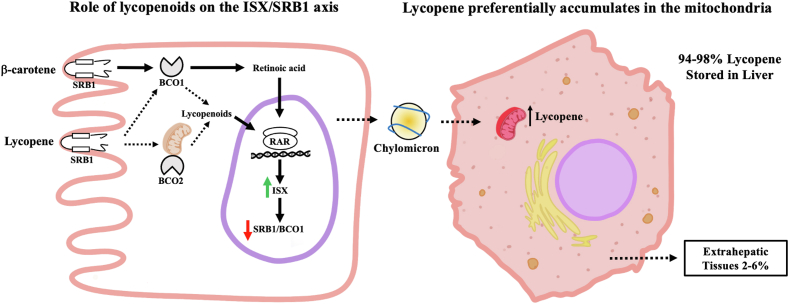
A strength of this work is the use of transgenic mouse models lacking one or both lycopene cleaving enzymes, which has not been done in previous research and has been highly beneficial for creating a complete picture of the impact of one or both cleavage enzymes on lycopene metabolism and accumulation. The DKO mice provided valuable insight into lycopene metabolism when normal metabolic pathways are disrupted, specifically in studying lycopene bioaccumulation and mRNA expression levels. Figure 8 illustrates some of the major themes from this study, including the role of lycopenoids on the ISX/SRB1 axis and lycopene’s preferential accumulation in mitochondria. Although the results from study 1 indicate that BCO2 is the primary cleavage enzyme of lycopene, there may be some tissue specificity in this regard. Future research at the tissue level would be valuable. Additionally, the evaluation of various dosing amounts would be interesting as well.
FIGURE 8.
Role of dietary lycopene on the intestinal ISX/SRB1/BCO1 axis and preferential lycopene accumulation in hepatic tissue. The left panel compares the impact of BCO1 and BCO2 on the metabolism of newly absorbed β-carotene or lycopene in the intestine and the subsequent influence of their degradation products (retinoic acid from β-carotene or lycopenoids from lycopene) on the ISX/SRB1/BCO1 axis. Retinoic acid and lycopenoids down-regulate carotenoid absorption and conversion of β-carotene to vitamin A through this axis. The right panel highlights the preferential accumulation of lycopene in the liver (94-98% of lycopene measured across all genotypes), especially in the hepatic mitochondria.
Study 2 was a novel study design aimed at examining lycopene’s potential influence on the ISX/SRB1 axis in vivo. It clearly showed that lycopene (or more likely its metabolites) has an influence; however, it is difficult to study what specific metabolites are involved as they are only found in trace amounts in the body, disappear rapidly, and there are no available commercial standards. Additionally, this study suggests but does not prove, that lycopene is acting through the RAR receptor, as we have hypothesized.
It is clear from these results that the metabolism of lycopene is a complex process, and there are many factors to consider when trying to elucidate this process. Lycopene tissue accumulation is a product of the uptake, metabolism, storage, and transport out of tissues, and these factors are made more complex by the differential activity of BCO1 and BCO2 in various tissues. Although there is progress being made in understanding the bioactivity of lycopene, additional research is needed to address the gaps of knowledge when it comes to lycopene’s metabolism and also whether intact lycopene or its metabolites attenuate risk of chronic diseases.
In conclusion, our results support the hypothesis that BCO2 is the most important cleavage enzyme of lycopene in the liver, the tissue where most of the lycopene accumulated across mouse genotypes (94-98% of lycopene from 11 tissues). Lycopene concentration was enriched in the hepatic mitochondria (where BCO2 resides) independently of the expression of BCO2. Additionally, the similar lycopene isomer profile of the whole liver and isolated mitochondria suggests that there is no preferential cleavage of cis forms of lycopene compared to all-trans lycopene. Finally, SRB1 appears to play a role in facilitating the uptake of lycopene in the intestines, and lycopene’s metabolites may act on RAR receptors to influence the ISX/SRB1 axis.
Funding
This research was partially funded by NIH grant 1R01HL147252, the USDA NIFA program (W5002) to JA.
MJB was supported by a Jonathan Baldwin Turner Fellowship, University of Illinois at Urbana Champaign; JRA was supported by a Kraft Heinz Company Human Nutrition Fellowship; the rest of the authors have nothing to disclose.
Author disclosures
The authors report no conflicts of interest.
Acknowledgments
All authors have read and approved this manuscript for submission to the Journal of Nutrition.
The author’s responsibilities were as follows—MJB, JE, JA, and JA designed the research; MJB conducted the research; MB provided technical inputs and advice in conducting the research; MJB analyzed the data and performed statistical analysis; MJB, JE, JA, and MB; prepared the manuscript; all authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.05.025.
Contributor Information
Jaume Amengual, Email: Jaume6@illinois.edu.
John W. Erdman, Jr., Email: jwerdman@illinois.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Arballo J., Amengual J., Erdman J.W., Jr. Lycopene: a critical review of digestion, absorption, metabolism, and excretion. Antioxidants. 2021;10:342. doi: 10.3390/antiox10030342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cichon M.J., Moran N.E., Riedl K.M., Schwartz S.J., Clinton S.K. Identification of an epoxide metabolite of lycopene in human plasma using 13C-labeling and QTOF-MS. Metabolites. 2018;8:24. doi: 10.3390/metabo8010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdman J.W., Jr., Ford N.A., Lindshield B.L. Are the health attributes of lycopene related to its antioxidant function? Arch. Biochem. Biophys. 2009;483:229–335. doi: 10.1016/j.abb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrison E. Carotenoids, β-apocarotenoids, and retinoids: the long and the short of it. Nutrients. 2022;14:1411. doi: 10.3390/nu14071411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopec R., Riedl K., Harrison E., Curley R., Jr., Hruszkewycz D.P., Clinton S., et al. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J. Agric. Food Chem. 2010;58:3290–3296. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindshield B., Canene-Adams K., Erdman J.W., Jr. Lycopenoids: are lycopene metabolites bioactive? Arch. Biochem. Biophys. 2007;458:136–140. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Wang X.D. Lycopene metabolism and its biological significance. Am. J. Clin. Nutr. 2012;96:1214S–1222S. doi: 10.3945/ajcn.111.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran N., Mohn E., Hason N., Erdman J.W., Jr., Johnson E. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv. Nutr. 2018;9:465–492. doi: 10.1093/advances/nmy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Våge D.I., Boman I.A. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries) BMC Genet. 2010;11:10. doi: 10.1186/1471-2156-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Lintig J., Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J. Biol. Chem. 2000;275:11915–11920. doi: 10.1074/jbc.275.16.11915. [DOI] [PubMed] [Google Scholar]
- 11.Amengual J., Lobo G.P., Golczak M., Li H.N., Klimova T., Hoppel C.L., et al. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011;25:948–959. doi: 10.1096/fj.10-173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiefer C., Hessel S., Lampert J., Vogt K., Lederer M., Breithaupt D., et al. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 13.dela Seña C., Narayanasamy S., Riedl K.M., Curley R.W., Jr., Schwartz S.J., Harrison E.H. Substrate specificity of purified recombinant human β-carotene 15,15'-oxygenase (BCO1) J. Biol. Chem. 2013;288:37094–37103. doi: 10.1074/jbc.M113.507160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.dela Seña C., Sun J., Narayanasamy S., Riedl K., Yuan Y., Curley R., Jr., et al. Substrate specificity of purified recombinant chicken β-carotene-9′, 10′-oxygenase (BCO2) Metabolism. 2016;29:14609–14619. doi: 10.1074/jbc.M116.723684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford N., Elsen A., Erdman J.W., Jr. Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice. Nutr. Res. 2013;33:733–742. doi: 10.1016/j.nutres.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coronel J., Yu J., Pilli N., Kane M., Amengual J. The conversion of β-carotene to vitamin A in adipocytes drives the anti-obesogenic effects of β-carotene in mice. Mol. Metab. 2022;66:101640. doi: 10.1016/j.molmet.2022.101640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lobo G.P., Hessel S., Eichinger A., Noy N., Moise A.R., Wyss A., et al. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta, beta-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amengual J., Coronel J., Marques C., Aradillas-Garcia C., Vargas Morales J.M., Andrade F., et al. β-carotene oxygenase 1 activity modulates circulating cholesterol concentrations in mice and humans. J. Nutr. 2020;150:2023–2030. doi: 10.1093/jn/nxaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran N., Clinton S., Erdman J.W., Jr. Differential bioavailability, clearance, and tissue distribution of the acyclic tomato carotenoids lycopene and phytoene in Mongolian Gerbils. J. Nutr. 2013;143:1920–1926. doi: 10.3945/jn.113.181461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuniga K.E., Erdman J.W., Jr. Combined consumption of soy germ and tomato powders results in altered isoflavone and carotenoid bioavailability in rats. J. Agric. Food Chem. 2011;59:5335–5341. doi: 10.1021/jf2004157. [DOI] [PubMed] [Google Scholar]
- 21.Folch J., Lees M., Stanley G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 22.Amengual J., Petrov P., Bonet M., Ribot J., Palou A. Induction of carnitine palmitoyl transferase 1 and fatty acid oxidation by retinoic acid in HepG2 cell. Int. J. Biochem. Cell Biol. 2012;44:2019–2027. doi: 10.1016/j.biocel.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Solesio M.E., Xie L., McIntyre B., Ellenberger M., Mitaishvili E., Bhadra-Lobo S., et al. Depletion of mitochondrial inorganic polyphosphate (polyP) in mammalian cells causes metabolic shift from oxidative phosphorylation to glycolysis. Biochem. J. 2021;478:1631–1646. doi: 10.1042/BCJ20200975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palczewski G., Amengual J., Hoppel C., von Lintig J. Evidence for compartmentalization of mammalian carotenoid metabolism. FASEB. 2014;28:4457–4469. doi: 10.1096/fj.14-252411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hessel S., Eichinger A., Isken A., Amengual J., Hunzelmann S., Hoeller U., et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J. Biol. Chem. 2007;282:3355–33561. doi: 10.1074/jbc.M706763200. [DOI] [PubMed] [Google Scholar]
- 26.Lim J., Liu C., Hu K., Smith D., Wang X.D. Ablation of the carotenoid cleavage enzymes (BCO1 and BCO2) induced hepatic steatosis by altering the farnesoid X receptor/miR-34a/sirtuin 1 pathway. Arch. Biochem. Biophys. 2018;654:1–9. doi: 10.1016/j.abb.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Wu L., Guo X., Wong S., Lu P., Hartson S., Medeiros D., et al. Deficiency of β-carotene oxygenase 2 induces mitochondrial fragmentation and activates the STING-IRF3 pathway in the mouse hypothalamus. J.N.B. 2021;88:108542. doi: 10.1016/j.jnutbio.2020.108542. [DOI] [PubMed] [Google Scholar]
- 28.Boileau A., Merchen N., Wasson K., Atkinson A., Erdman J.W., Jr. Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. J. Nutr. 1999;129:1176–1181. doi: 10.1093/jn/129.6.1176. [DOI] [PubMed] [Google Scholar]
- 29.Ford N., Clinton S., von Lintig J., Wyss A., Erdman J.W., Jr. Loss of carotene-9',10'-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J. Nutr. 2010;140:2134–2138. doi: 10.3945/jn.110.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boileau T.W.–M., Boileau A.C., Erdman J.W., Jr. Bioavailability of all-trans and cis–Isomers of lycopene. Exp. Biol. Med. (Maywood) 2002;227:914–919. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 31.Lindshield B., King J., Wyss A., Goralczyk R., Lu C.–H., Ford N., et al. Lycopene biodistribution is altered in 15,15'-carotenoid monooxygenase knockout mice. J. Nutr. 2008;138:2367–2371. doi: 10.3945/jn.108.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindqvist A., He Y.G., Andersson S. Cell type-specific expression of β-carotene-9′,10′ monooxygenase in human tissues. J. Histochem. Cytochem. 2005;53:11. doi: 10.1369/jhc.5A6705.2005. [DOI] [PubMed] [Google Scholar]
- 33.Proteinatlas.org [Internet] The Human Protein Atlas; [cited 2023 Jan 30] Available from: https://www.proteinatlas.org/ENSG00000135697-BCO1.
- 34.Uhlen M., Fagerberg L., Hallstrom B., Lindskog C., Oksvold P., Mardinoglu A. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 35.Kiefer C., Sumser E., Wernet M., von Lintig J. A class B scavenger receptor mediates the uptake of carotenoids in Drosophila. PNAS. 2002;99:10581–10586. doi: 10.1073/pnas.162182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shyam R., Vachali P., Gorusupudi A., Nelson K., Bernstein P.S. All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids. Arch. Biochem. Biophys. 2017;634:21–28. doi: 10.1016/j.abb.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohn T., de Lera A., Landrier J.–F., Carlsen H., Merk D., et al. State-of-the-art methodological investigation of carotenoid activity and metabolism-from organic synthesis via metabolism to biological activity-exemplified by a novel retinoid signaling pathway. Food Funct. 2023;14:621–638. doi: 10.1039/d2fo02816f. [DOI] [PubMed] [Google Scholar]
- 38.Aydemir G., Kasiri Y., Birta E., Beke G., Garcia A., Bartok E.M., et al. Lycopene-derived bioactive retinoic acid receptors/retinoid X receptors-activating metabolites may be relevant for lycopene’s anti-cancer potential. Mol. Nutr. Food Res. 2013;57:739–747. doi: 10.1002/mnfr.201200548. [DOI] [PubMed] [Google Scholar]
- 39.Moran N.E., Cichon M.J., Riedl K.M., Grainger E.M., Schwartz S.J., Novotny J.A., et al. Compartmental and noncompartmental modeling of 1³C-lycopene absorption, isomerization, and distribution kinetics in healthy adults. Am. J. Clin. Nutr. 2015;102:1436–1449. doi: 10.3945/ajcn.114.103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu K.Q., Liu C., Ernst H., Krinsky N.I., Russell R.M., Wang X.D. The biochemical characterization of ferret carotene-9',10'-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J. Biol. Chem. 2006;281:19327–19338. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.