Abstract
Background
A rapid 4-compartment (4C) model integrates dual-energy x-ray absorptiometry (DXA) and multi-frequency bioimpedance analysis (MFBIA), which may be useful for clinical and research settings seeking to employ a multi-compartment model.
Objectives
This study aimed to determine the added benefit of a rapid 4C model over stand-alone DXA and MFBIA when estimating body composition.
Methods
One hundred and thirty participants (n = 60 male; n = 70 female) of Hispanic descent were included in the present analysis. A criterion 4C model that employed air displacement plethysmography (body volume), deuterium oxide (total body water), and DXA (bone mineral) was used to measure fat mass (FM), fat-free mass (FFM), and body fat percent (%BF). A rapid 4C model (DXA-derived body volume and bone mineral; MFBIA-derived total body water) and stand-alone DXA (GE Lunar Prodigy) and MFBIA (InBody 570) assessments were compared against the criterion 4C model.
Results
Lin’s concordance correlation coefficient values were >0.90 for all comparisons. The standard error of the estimates ranged from 1.3 to 2.0 kg, 1.6 to 2.2 kg, and 2.1 to 2.7% for FM, FFM, and %BF, respectively. The 95% limits of agreement ranged from ±3.0 to 4.2 kg, ±3.1 to 4.2 kg, and ±4.9 to 5.2% for FM, FFM, and %BF, respectively.
Conclusions
Results revealed that all 3 methods provided acceptable body composition results. The MFBIA device used in the current study may be a more economically friendly option than DXA or when there is a need to minimize radiation exposure. Nonetheless, clinics and laboratories that already have a DXA device in place or that value having the lowest individual error when conducting a test may consider continuing to use the machine. Lastly, a rapid 4C model may be useful for assessing body composition measures observed in the current study and those provided by a multi-compartment model (e.g., protein).
Keywords: validation, adiposity, lean mass, bod pod, dilution, hydration
Introduction
The molecular 2-compartment (2C) model is commonly used in clinical and research settings to assess body composition [1,2]. Methods based upon a 2C model employ assumptions regarding FM and FFM [3,4]. For instance, DFFM (density of FFM) is assumed at 1.100 g/cm3. Moreover, the DFFM consists of water, bone mineral (MO), and residual, representing 73.8%, 5.6%, and 20.6% of FFM, respectively [4]. Hydration assessment is often considered the most important for accurate body composition analysis since it comprises the largest component of FFM [2,5,6]. This is problematic since FFM hydration can vary substantially within and across racial/ethnicities regardless of health status. For instance, previous work has shown the hydration of FFM to vary from 63.76–79.55% in Hispanic males and females. Accordingly, body composition methods such as DXA and BIA, which assume FFM hydration, may be impacted when used as a stand-alone method in Hispanic populations.
A conventional 4-compartment (4C) model can account for variations in FFM characteristics (i.e., hydration, BMC, and residual). The ability to partition FFM into different constituents is why a 4C model is often more accurate than simpler 2C models [[7], [8], [9]]. Though effective, a conventional 4C model is impractical for use in clinical settings because of the need for measures of body volume (BV), which traditionally required the use of hydrostatic weighing or air displacement plethysmography (ADP). These limitations have led to the development of BV assessments using clinical methods such as DXA [[10], [11], [12], [13]]. In addition, a conventional 4C model also requires dilution methods that are expensive and time-consuming. Consequently, BIA is often used as an alternative to dilution methods for total body water (TBW) assessments.
The use of DXA-derived BV and BIA-derived TBW has led to the emergence of a rapid 4C model [14,15]. A rapid 4C model is advantageous compared to a conventional 4C model because it only takes 10–15 min to administer. As a result, the time required for assessing body composition via a rapid 4C model is ideal in clinical testing centers. In addition, a rapid 4C model could be beneficial for clinicians working with Hispanic populations, which are known to have variations in FFM from assumed constants, as previously discussed. Using DXA and BIA as stand-alone methods may be problematic when assessing Hispanics’ body composition. Nonetheless, the increased precision of a rapid 4C model over stand-alone 2C models needs further research. Therefore, this study aimed to determine the added benefit of a rapid 4C model over stand-alone DXA and multi-frequency bioimpedance analysis (MFBIA) when estimating body composition. We hypothesize that the rapid 4C model will provide better accuracy than stand-alone DXA and MFBIA because of the ability to account for variations in FFM hydration.
Methods
Participants
One hundred and thirty participants (n = 60 male; n = 70 female) of Hispanic descent were included in the present analysis (Table 1). Body composition was measured with a criterion and rapid 4C model and a stand-alone DXA and MFBIA. The inclusion criteria consisted of Hispanic females and males that were: 1) 18–65 y of age and apparently healthy (i.e., free from orthopedic disorders and who had no known signs or symptoms of CVD, pulmonary, or metabolic diseases); 2) <159 kg because of DXA table restrictions; and 3) did not have conditions or take medications that may affect body composition. Participants were asked to complete an overnight fasting protocol, which consisted of not eating or drinking 8 h prior to participation and to also avoid exercise 24 h before testing. Prior to testing, participants provided written informed consent and completed a self-reported medical history questionnaire to ensure inclusion criteria were met. Institutional review board approval for subject participation was approved by the host university (IRB # 2016-10-16).
TABLE 1.
Participant characteristics
| All participants (n = 130) |
Males (n = 60) |
Females (n = 70) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| Age (y) | 29.2 | 11.3 | 18.0 | 63.0 | 28.2 | 11.5 | 18 | 63 | 30.0 | 11.2 | 18 | 58 |
| Height (cm) | 166.6 | 8.8 | 150.0 | 186.0 | 173.4 | 6.2 | 161.0 | 186.0 | 160.7 | 5.8 | 150 | 174 |
| Weight (kg) | 78.2 | 17.8 | 46.3 | 131.4 | 85.4 | 16.7 | 57.0 | 131.4 | 72.1 | 16.5 | 46.3 | 118.6 |
| BMI (kg/m2) | 28.1 | 5.8 | 19.0 | 44.4 | 28.3 | 5.1 | 19.7 | 42.1 | 27.9 | 6.3 | 19.0 | 44.4 |
| 4C body fat (%) | 31.9 | 10.1 | 11.2 | 54.7 | 26.4 | 8.8 | 11.2 | 48.4 | 36.7 | 8.6 | 14.5 | 54.7 |
| 4C FM (kg) | 25.8 | 12.3 | 7.0 | 60.3 | 23.6 | 11.9 | 7.0 | 58.7 | 27.6 | 12.4 | 7.3 | 60.3 |
| 4C FFM (kg) | 52.5 | 10.8 | 29.1 | 76.4 | 61.7 | 7.5 | 44.9 | 76.4 | 44.5 | 5.6 | 29.1 | 58.3 |
| ADP body volume (L) | 76.2 | 18.3 | 44.0 | 129.8 | 82.2 | 17.4 | 53.6 | 129.8 | 71.0 | 17.6 | 44.0 | 120.5 |
| D2O TBW (L) | 38.2 | 7.9 | 20.9 | 58.7 | 44.7 | 5.7 | 31.5 | 58.7 | 32.6 | 4.6 | 20.9 | 45.5 |
| DXA BMC (kg) | 2.6 | 0.5 | 1.6 | 4.2 | 3.0 | 0.4 | 2.2 | 4.2 | 2.3 | 0.3 | 1.6 | 3.1 |
| DXA body fat (%) | 34.9 | 9.0 | 14.7 | 54.4 | 29.5 | 7.8 | 14.7 | 47.7 | 39.5 | 7.3 | 20.8 | 54.4 |
| DXA body volume (L) | 75.9 | 18 | 43.9 | 128.7 | 82.1 | 16.9 | 54.3 | 128.7 | 70.7 | 17.3 | 43.9 | 121.3 |
| DXA FM (kg) | 27.0 | 11.5 | 8.7 | 62.3 | 25.1 | 10.9 | 8.7 | 56.2 | 28.6 | 11.8 | 10.1 | 62.3 |
| DXA FFM (kg) | 51.3 | 11 | 27.6 | 78.9 | 60.3 | 8.0 | 41.9 | 78.9 | 43.5 | 6.3 | 27.6 | 59.3 |
| MFBIA body fat (%) | 32.4 | 10.1 | 8.9 | 53.1 | 27.0 | 9.0 | 8.9 | 49.5 | 37.1 | 8.5 | 19.2 | 53.1 |
| MFBIA FM (kg) | 26.2 | 12.4 | 5.7 | 61.9 | 24.2 | 12 | 5.7 | 60.0 | 27.9 | 12.6 | 10.2 | 61.9 |
| MFBIA FFM (kg) | 52.0 | 10.7 | 30.5 | 78.2 | 61.2 | 7.4 | 45.5 | 78.2 | 44.2 | 5.6 | 30.5 | 56.7 |
| MFBIA TBW (L) | 38.1 | 7.8 | 22.3 | 56.7 | 44.8 | 5.4 | 33.4 | 56.7 | 32.4 | 4.1 | 22.3 | 41.9 |
| Rapid 4C body fat (%) | 31.2 | 9.1 | 12.5 | 54.6 | 26.2 | 7.5 | 15.2 | 43.1 | 35.6 | 8.0 | 17.8 | 54.6 |
| Rapid 4C FM (kg) | 25.2 | 11.5 | 8.1 | 64.7 | 23.3 | 10.5 | 8.1 | 56.1 | 26.8 | 12.2 | 9.0 | 64.7 |
| Rapid 4C FFM (kg) | 53.1 | 10.9 | 30.5 | 81.1 | 62.1 | 8.1 | 44.9 | 81.1 | 45.4 | 5.8 | 30.5 | 57.1 |
| D2O TBW/4C FFM (%) | 72.8 | 2.3 | 68.0 | 83.0 | 72.4 | 2.3 | 68.3 | 83.0 | 73.1 | 2.1 | 68.0 | 78.0 |
ADP, air displacement plethysmography; D2O, deuterium oxide; MFBIA, multi-frequency bioimpedance analysis; TBW, total body water; 4C, 4-compartment.
Procedures
Upon completion of the informed consent and medical history questionnaire, participants’ hydration status was assessed from a urine sample using a handheld refractometer. Urine-specific gravity values <1.029 were required for inclusion in this analysis. After assessing hydration, height was measured (to the nearest 0.1 cm) with a stadiometer with a maximum capacity of 205 cm (SECA 213; Seca Ltd.).
Deuterium oxide
TBW for the criterion 4C model was conducted using deuterium oxide (D2O) (99.8% 2H; Cambridge Isotope Laboratories, Inc.). Prior to D2O ingestion, urine samples were collected from all participants. Each participant was instructed to void their bladders as much as possible. Urine samples obtained during this time point were used for baseline analysis. After completely voiding the bladder, participants ingested ≈11 g of D2O along with a 100 ml rinse of deionized water. The exact amount of D2O ingested by each participant was recorded. Subjects were then asked to void their bladder at 3.5 h to clear the bladder of any urine that had not been completely diluted. Next, subjects waited for another 30-min and were instructed to provide a post-urine sample at 4-h. Participants were asked to remain in the Body Composition Laboratory during the 4-h equilibration period to ensure that eating, physical exertion, and other factors that can impact results did not occur prior to the post-urine sample collection. Urine-diluted D2O was analyzed in triplicate using an isotope-ratio mass spectrometer at an independent laboratory (Metabolic Solutions, Inc.). Isotope abundances in the urine were calculated following the method of Wong et al. [16]. TBW was calculated from the dilution of isotopic water and corrected for the exchange of D2O with nonaqueous tissue [17].
Air displacement plethysmography
BV for the criterion 4C model was obtained using ADP via the BOD POD (COSMED USA Inc.). Prior to each testing day, the BOD POD was calibrated according to manufacturer specifications. Participants were required to wear minimal lycra compression clothing, and lycra swim-caps were provided and required for testing. To assess BV, participants were instructed to sit in the BOD POD chamber for 2 trials of roughly 50 s for each trial. A third trial was necessary if the first 2 trials did not agree within 150 mL of each other. Thoracic gas volume was estimated for all assessments.
Dual-energy x-ray absorptiometry
BMC for the criterion and rapid 4C model was measured using DXA (GE Lunar Prodigy, software version 14.10.022; GE Lunar Corporation). Prior to each use, the DXA was calibrated according to manufacturer guidelines using a standardized QA block phantom. Participants were positioned supine on the DXA platform with arms resting along the sides of the body and feet secured with Velcro straps around the ankles to reduce movement for the duration of the scan. The BMC values obtained from DXA were converted to total body MO for use in the criterion and rapid 4C model [18]. The equation from Nickerson et al. [10], previously developed in Hispanics, was used to compute BV in the rapid 4C model: Volume (L) = (FM/0.91) + (LM/1.06) + (BMC/16.95) + 0.268. The $%Fat of each participant was also utilized for analysis for each DXA scan.
Multi-frequency bioimpedance analysis
Participants had their TBW measured with MFBIA for the rapid 4C model. The $%Fat of each participant was also computed from each MFBIA test. All tests were conducted based on the manufacturer’s instructions using the proprietary built-in algorithm of the MFBIA device (InBody 570; InBodyUSA). The MFBIA device employed in the current study utilized a tetrapolar 8-point tactile electrode system, which sends 3 separate frequencies (i.e., 5, 50, and 500 kHz) of alternating currents through the body. For testing, participants’ feet were centered on the electrodes, and the hand electrodes were grasped with arms being held wide enough so there was no contact between the arms and torso. The position was held for the test duration (∼45 s). Once the assessment was completed, participants were prompted to return the hand electrodes and step off the device.
4C calculation model
The criterion 4C model was computed using ADP (BV), DXA (MO), and D2O (TBW). The rapid 4C model was calculated using DXA (BV and MO) and MFBIA (TBW). The 4C model is based on the equation described by Wang et al. [19]:
| FM (kg) = 2.748 (BV) – 0.699 (TBW) + 1.129 (MO) – 2.051 (BM) |
| FFM (kg) = (BM-FM) |
| %BF = (FM/BM) × 100 |
Statistical analysis
For BV analysis, ADP was considered the criterion method, with the alternate estimate provided by DXA. For TBW analysis, D2O was considered the criterion method, with the alternate estimate provided by MFBIA. For body composition analyses, alternate estimates provided by the rapid 4C model, DXA, and MFBIA were compared against the criterion 4C model. Analyses were performed for FM, FFM, and body fat percent (%BF). The primary results presented in the text reflect performance in the entire sample (n = 130), with results for each sex individually presented in the Supplemental Materials. Equivalence testing was performed with 90% confidence limits for two one-sided t-tests (TOST) to assess whether each method demonstrated equivalence with the criterion 4C model, using a ±1.5 kg equivalence region for FM and FFM and a ±2% equivalence region for %BF, consistent with previous research [20,21]. Equivalence between ADP and DXA BV and between D2O and MFBIA TBW were examined using ±1 L equivalence regions.
Bland-Altman analysis was performed [22], including calculation of the 95% limits of agreement and linear regression to allow for examination of proportional bias (i.e., a slope differing from 0). Ordinary least squares regression was performed to compare the slope and intercept of the linear relationship observed between methods to the line of identity (i.e., a perfect linear relationship, with an intercept of 0 and slope of 1). The constant error was calculated as the mean of the individual differences in each variable between the criterion 4C model and each other technique (i.e., alternate method value minus 4C value), and total error (TE) was calculated as the RMSE. SEE was defined as the residual SE value from ordinary least squares regression. Other values of interest included Lin’s concordance correlation coefficient (CCC) and R2. All data analysis was performed using R version 4.2.1 and the software packages TOSTER version 0.4.2 and DescTools version 0.99.46 [[23], [24], [25]]. Statistical significance was accepted at P < 0.05. Eligible subjects, body composition measures, and statistical analysis can also be observed in the online supporting material.
Results
Body volume
DXA-derived BV demonstrated statistical equivalence with ADP BV (90% TOST interval: –0.35, –0.15 L; P < 0.001; Figure 1A) and an essentially perfect correlation (R2 and CCC values of 1.00). Narrow limits of agreement (±1.4 L) were also observed (Figure 1B). Statistically significant but small-magnitude proportional bias was observed (95% CI for slope: –0.02, –0.01).
FIGURE 1.
Correlation and Bland-Altman analyses for DXA- and ADP-derived BV. (A) Scatter plot illustrating the relationship between DXA- and ADP-derived BV. X-axis, ADP-derived BV; y-axis, DXA-derived BV. (B) Bland-Altman plot showing the agreement between DXA- and ADP-derived BV. Dashed line, LOA, solid line, mean difference; bold solid line, regression line. ADP, air displacement plethysmography; BV, body volume; CE, constant error; LOA, limits of agreement; TE, total error.
Body water
MFBIA TBW demonstrated statistical equivalence with D2O TBW (90% TOST interval: –0.19, 0.38 L; P < 0.001; Figure 2A) and a strong correlation (R2 of 0.94 and CCC 0.97). Moderate limits of agreement (±3.8 L) were also observed (Figure 2B). No proportional bias was observed (95% CI for slope: –0.03, 0.06).
FIGURE 2.
Correlation and Bland-Altman analyses for MFBIA- and D2O-derived TBW. (A) Scatter plot illustrating the relationship between MFBIA- and D2O-derived TBW. X-axis, D2O-derived TBW; y-axis, MFBIA-derived TBW. (B) Bland-Altman plot showing the agreement between MFBIA- and D2O-derived TBW. Dashed line, LOA, solid line, mean difference; bold solid line, regression line. CE, constant error; D2O, deuterium oxide; LOA, limits of agreement; MFBIA, multi-frequency bioimpedance analysis; TBW, total body water; TE, total error.
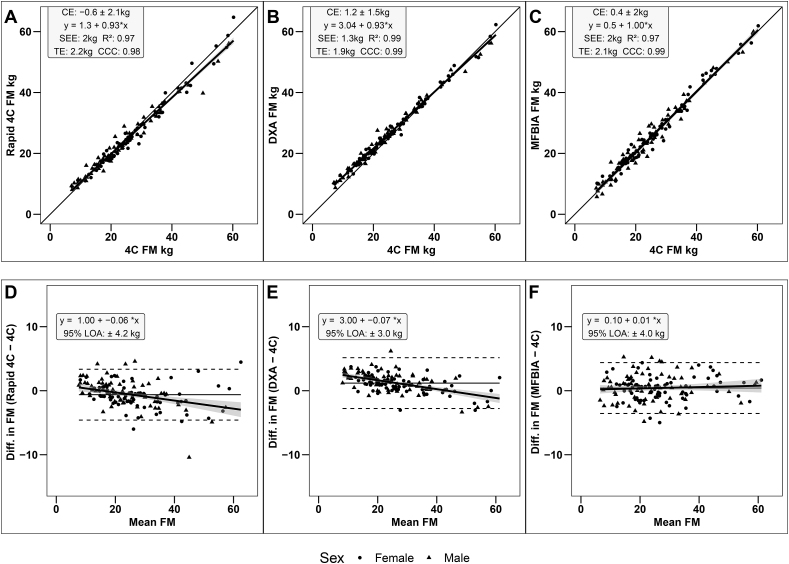
Fat mass
Strong, statistically significant correlations were observed between the criterion 4C model and all other methods (R2: 0.97–0.99; CCC: 0.98–0.99; P < 0.001). Statistical equivalence was observed between the criterion 4C model and DXA (90% TOST interval: 0.96, 1.41 kg; P = 0.01), rapid 4C model (90% TOST interval: –0.93, –0.31 kg; P < 0.001), and MFBIA (90% TOST interval: 0.12, 0.71 kg; P < 0.001). SEE values were 1.3 kg for DXA and 2.0 kg for the rapid 4C model and MFBIA (Figure 3A–C), with TE values ranging from 1.9 to 2.2 kg. No proportional bias was observed for MFBIA (95% CI for slope: –0.02, 0.04), but slight negative proportional bias was observed for the rapid 4C model (95% CI for slope: –0.09, –0.03) and DXA (95% CI for slope: –0.09,–0.05) (Figure 3D–F).
FIGURE 3.
Correlation and Bland-Altman analyses for FM estimates. (A) Scatterplot illustrating relationship between rapid 4C model and criterion 4C model; (B) Scatterplot illustrating relationship between DXA and criterion 4C model; (C) Scatterplot illustrating relationship between MFBIA and criterion 4C model; (D) Bland-Altman plot showing the agreement between rapid 4C model and criterion 4C model; (E) Bland-Altman plot showing the agreement between DXA and criterion 4C model; (F) Bland-Altman plot showing the agreement between MFBIA and criterion 4C model. Dashed line, LOA, solid line, mean difference; bold solid line, regression line. CE, constant error; LOA, limits of agreement; MFBIA, multi-frequency bioimpedance analysis; TE, total error; 4C, 4-compartment.
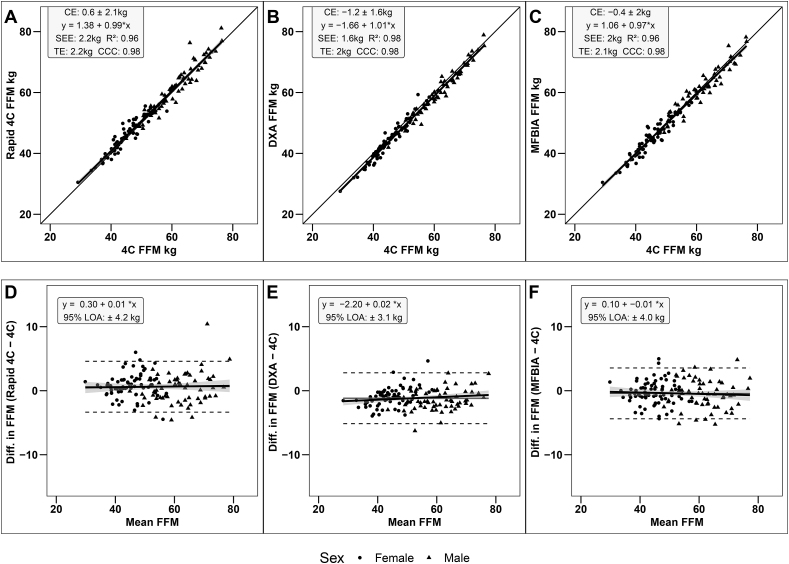
Fat-free mass
Strong, statistically significant correlations were observed between the criterion 4C model and all other methods (R2: 0.96–0.98; CCC: 0.98; P < 0.001). Statistical equivalence was observed between criterion 4C model and DXA (90% TOST interval: –1.41, –0.95 kg; P = 0.01), rapid 4C model (90% TOST interval: 0.31, 0.93 kg; P < 0.001), and MFBIA (90% TOST interval: –0.71, –0.12 kg; P < 00.01). SEE values ranged from 1.6 to 2.2 kg, with TE values ranging from 2.0 to 2.2 kg (Figure 4A–C). No proportional bias was observed for the rapid 4C model (95% CI for slope: –0.03, 0.04), DXA (95% CI for slope: –0.01, 0.04), or MFBIA (95% CI for slope: –0.04, 0.02) (Figure 4D–F).
FIGURE 4.
Correlation and Bland-Altman analyses for FFM estimates. (A) Scatterplot illustrating relationship between rapid 4C model and criterion 4C model; (B) Scatterplot illustrating relationship between DXA and criterion 4C model; (C) Scatterplot illustrating relationship between MFBIA and criterion 4C model; (D) Bland-Altman plot showing the agreement between rapid 4C model and criterion 4C model; (E) Bland-Altman plot showing the agreement between DXA and criterion 4C model; (F) Bland-Altman plot showing the agreement between MFBIA and criterion 4C model. Dashed line, LOA, solid line, mean difference; bold solid line, regression line. CE, constant error; LOA, limits of agreement; MFBIA, multi-frequency bioimpedance analysis; TE, total error; 4C, 4-compartment.
Body fat percent
Strong, statistically significant correlations were observed between the criterion 4C model and all other methods (R2: 0.93–0.94; CCC: 0.92–0.96; P < 0.001). Statistical equivalence was observed for the rapid 4C model (90% TOST interval: –1.1, –0.3%; P < 0.001) and MFBIA (90% TOST interval: 0.13, 0.91%; P < 0.001). In contrast, statistical equivalence was not observed between the criterion 4C model and DXA (90% TOST interval: 2.6, 3.3%; P = 1.0). SEE values ranged from 2.1 to 2.7%, with TE values ranging from 2.6 to 3.9% (Figure 5A–C). Negative proportional bias was observed for the rapid 4C model (95% CI for slope: –0.15, –0.06) and DXA (95% CI for slope: –0.15, 0.07), but not MFBIA (95% CI for slope: –0.05, 0.05) (Figure 5D–F).
FIGURE 5.
Correlation and Bland-Altman analyses for body fat percent estimates. (A) Scatterplot illustrating relationship between rapid 4C model and criterion 4C model; (B) Scatterplot illustrating relationship between DXA and criterion 4C model; (C) Scatterplot illustrating relationship between MFBIA and criterion 4C model; (D) Bland-Altman plot showing the agreement between rapid 4C model and criterion 4C model; (E) Bland-Altman plot showing the agreement between DXA and criterion 4C model; (F) Bland-Altman plot showing the agreement between MFBIA and criterion 4C model. Dashed line, LOA, solid line, mean difference; bold solid line, regression line. %BF, body fat percent; CE, constant error; LOA, limits of agreement; MFBIA, multi-frequency; bioimpedance analysis; TE, total error; 4C, 4-compartment.
Discussion
This study aimed to determine the added benefit of a rapid 4C model over stand-alone DXA and MFBIA when estimating body composition in Hispanic adults. The mean difference in BV was 0.3 L between DXA and ADP. These results demonstrate that DXA-derived BV appears to provide excellent agreement with ADP-derived BV at the group level. As a result, clinical and research settings in need of BV estimates could use DXA in place of ADP in the event the latter method is unavailable. Similarly, the mean difference in TBW was 0.1 L between D2O and MFBIA, also demonstrating strong group-level agreement, although the individual-level errors in TBW were greater than BV, as indicated by the wider 95% limits of agreement. The current study results also revealed that all 3 body composition methods performed well when compared against the criterion 4C model. Consequently, we reject the hypothesis that a rapid 4C model would be more accurate than a stand-alone DXA and MFBIA. For instance, the rapid 4C model and MFBIA produced similar mean differences, CCC values, and 95% limits of agreements. Additionally, the rapid 4C model and MFBIA provided smaller mean differences than DXA. However, DXA produced the lowest 95% limits of agreements of all methods despite the tendency to have a more profound proportional bias, albeit small, than the rapid 4C model and MFBIA. Altogether, these results suggest that all 3 body composition methods can accurately estimate body composition in a young Hispanic adult population. These findings demonstrate that the lower-costing MFBIA device used in the current study may provide similar body composition estimates as a more costly DXA machine that can be used as a stand-alone method or in a rapid 4C model. These findings are very insightful for clinics seeking to decide on the purchase of new body composition equipment. Specifically, these findings suggest that clinics might consider a more economical MFBIA device, such as the 1 used in the current study, over a more expensive DXA machine.
The current study results conflict with previous research by Nickerson and Snarr [26], which observed significant proportional bias in Hispanic females when comparing MFBIA against DXA. In contrast, Nickerson et al. [10] revealed that a rapid 4C model was valid in a cross-validation sample of Hispanic adults when compared to a 4C model, which is consistent with the present study findings. Additionally, Nickerson et al. [27] found DXA to consistently overestimate %BF by 4% in young Hispanic adults, which is also similar to the present study’s findings. It is worth noting that the aforementioned studies all used the same DXA as that of the current study. Nonetheless, prior to this investigation, the rapid 4C model, DXA, and MFBIA were evaluated in isolation within a Hispanic population. Consequently, the added benefit of a rapid 4C model over a stand-alone DXA and MFBIA measurement was unknown. Accordingly, results from this study will be useful for researchers, healthcare professionals, and physicians who need to make an informed decision concerning whether to utilize a rapid 4C model, DXA, or MFBIA for body composition assessments in Hispanic adults.
Although the rapid 4C model did not add a significant improvement in body composition measurements as initially hypothesized, there are multiple factors worth further consideration. One potential reason for the discrepancies is the criterion method used to develop the DXA-derived BV equation. For instance, Nickerson et al. [10] originally used hydrostatic weighing to develop the DXA-derived BV equation employed in a rapid 4C model. The criterion 4C model in the current study utilized ADP for BV assessments instead of hydrostatic weighing. Previous research has found that body density obtained via ADP is significantly lower than hydrostatic weighing [28]. Thus, its plausible results may have been slightly different with the utilization of hydrostatic weighing in the criterion 4C model. However, the excellent performance of the DXA-derived BV equation, when compared to ADP-derived BV, suggests this had a minimal impact. In addition, Nickerson et al. [29] previously found that DXA-derived BV equations have good agreement with hydrostatic weighing in physically active adults. Altogether, this indicates that other aspects may help explain the current study findings.
Another factor that could have led to conflicting results between the current study and previous research relates to the methods employed in the criterion 4C model. For example, the criterion 4C model in the present study used D2O for hydration assessments. In contrast, previous studies have used bioimpedance spectroscopy as a component in the criterion multi-compartment model when validating the rapid 4C model, MFBIA, or DXA [6,10,11,30]. Although bioimpedance spectroscopy has been validated in young adults when compared against D2O [31], it is possible results of previous research would have been different by using a true criterion multi-compartment model. For instance, the present study employed a more accepted criterion 4C model because of the inclusion of a “gold standard” for TBW measurements instead of an indirect bioimpedance device as an alternative. Despite the discrepancies in hydration assessments used in the criterion 4C model, the present study addresses previous gaps in the literature regarding the added benefit of a rapid 4C model over stand-alone DXA and MFBIA measurements.
Although this study has many strengths, it is not without limitations. For instance, the current study focused solely on the default MFBIA equation for analysis. As a result, our team did not use the MFBIA raw data with other published equations. Utilizing the default values helps maximize the application of our findings. Nonetheless, this is an interesting area that future research might consider pursuing. Another factor to consider is that the study sample consisted primarily of young Hispanic adults. Consequently, the results may not be generalizable to other ethnicities or races, indicating that the possible merits of the rapid 4C model should continue to be investigated in diverse populations. Furthermore, it is unknown whether the results apply to Hispanic children. Altogether, further research is warranted.
In conclusion, the current study sought to determine the added benefit of a rapid 4C model over stand-alone DXA and MFBIA when estimating body composition in Hispanic adults. Results revealed that all 3 methods provided acceptable body composition results. It appears the combined error of BV and TBW fails to increase the accuracy of a rapid 4C model beyond that of a stand-alone MFBIA device that employs assumptions. This suggests that the MFBIA device used in the current study may be a more economically friendly option when clinics or laboratories need an accurate measure of body composition but don’t have sufficient funds to purchase and maintain a DXA. Further, MFBIA could serve as a great alternative to DXA when needing to minimize radiation exposure. However, it is worth noting that clinics and laboratories that already have a DXA device in place or that value having the lowest individual error when conducting a test may consider continuing to use the machine. Lastly, the excellent agreement between DXA- and ADP-derived BV suggests that clinics or laboratories could use the former method in situations where a BV measurement is needed. For example, the rapid 4C model is capable of measuring FFM characteristics, unlike stand-alone DXA and MFBIA. Therefore, a rapid 4C model may still be considered useful for assessing the body composition measures observed in the current study and those provided by a multi-compartment model (e.g., protein).
Author contributions
The authors’ responsibilities were as follows– BSN and SAC: contributed to conceptualization, methodology, and funding acquisition; BSN and K-SP: contributed to data collection and project administration; GMT conducted all statistical analysis; BSN, BN, and GMT: contributed to writing the original draft preparation, revisions, and editing and all authors: read and approved the final manuscript.
Conflict of Interest
The authors report no conflicts of interest.
Funding
This publication was supported by the NIGMS of the NIH under award number SC1GM135099. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data availability
Data described in the manuscript will be made available upon request, pending approval.
Acknowledgments
We thank Rocio Gallegos for her efforts in the administrative assistance and data collection of the current study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.06.041.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Demerath E.W., Guo S.S., Chumlea W.C., Towne B., Roche A.F., Siervogel R.M. Comparison of percent body fat estimates using air displacement plethysmography and hydrodensitometry in adults and children. Int. J. Obes. Relat. Metab. Disord. 2002;26(3):389–397. doi: 10.1038/sj.ijo.0801898. [DOI] [PubMed] [Google Scholar]
- 2.Nickerson B.S., Narvaez S.V., Juarez M.I., Czerwinski S.A. Effect of total body water estimates via bioimpedance on bod pod-based three-compartment body fat models. Eur. J. Clin. Nutr. 2022;76(4):581–587. doi: 10.1038/s41430-021-00982-7. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z.M., Deurenberg P., Wang W., Pietrobelli A., Baumgartner R.N., Heymsfield S.B. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am. J. Clin. Nutr. 1999;69(5):833–841. doi: 10.1093/ajcn/69.5.833. [DOI] [PubMed] [Google Scholar]
- 4.Brozek J., Grande F., Anderson J.T., Keys A. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann. N. Y. Acad. Sci. 1963;110:113–140. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 5.Tinsley G.M., Smith-Ryan A.E., Kim Y., Blue M.N.M., Nickerson B.S., Stratton M.T., et al. Fat-free mass characteristics vary based on sex, race, and weight status in US adults. Nutr. Res. 2020;81:58–70. doi: 10.1016/j.nutres.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Nickerson B.S., Tinsley G.M. Utilization of BIA-derived bone mineral estimates exerts minimal impact on body fat estimates via multicompartment models in physically active adults. J. Clin. Densitom. 2018;21(4):541–549. doi: 10.1016/j.jocd.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Moon J.R., Eckerson J.M., Tobkin S.E., Smith A.E., Lockwood C.M., Walter A.A., et al. Estimating body fat in NCAA Division I female athletes: a five-compartment model validation of laboratory methods. Eur. J. Appl. Physiol. 2009;105(1):119–130. doi: 10.1007/s00421-008-0881-9. [DOI] [PubMed] [Google Scholar]
- 8.Blue M.N.M., Hirsch K.R., Brewer G.J., Cabre H.E., Gould L.M., Tinsley G.M., et al. The validation of contemporary body composition methods in various races and ethnicities. Br. J. Nutr. 2022;128(12):2387–2397. doi: 10.1017/S0007114522000368. [DOI] [PubMed] [Google Scholar]
- 9.Blue M.N.M., Hirsch K.R., Trexler E.T., Smith-Ryan A.E. Validity of the 4-compartment model using dual energy X-ray absorptiometry–derived body volume in overweight individuals. Appl. Physiol. Nutr. Metab. 2018;43(7):742–746. doi: 10.1139/apnm-2017-0804. [DOI] [PubMed] [Google Scholar]
- 10.Nickerson B.S., Fedewa M.V., McLester C.N., McLester J.R., Esco M.R. Development of a DXA-derived body volume equation in Hispanic adults for administering in a 4-compartment model. Br. J. Nutr. 2020;123(12):1373–1381. doi: 10.1017/S0007114520000598. [DOI] [PubMed] [Google Scholar]
- 11.Smith-Ryan A.E., Mock M.G., Ryan E.D., Gerstner G.R., Trexler E.T., Hirsch K.R. Validity and reliability of a 4-compartment body composition model using dual energy x-ray absorptiometry-derived body volume. Clin. Nutr. 2017;36(3):825–830. doi: 10.1016/j.clnu.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson J.P., Strauss B.J., Fan B., Duewer F.W., Shepherd J.A. Improved 4-compartment body-composition model for a clinically accessible measure of total body protein. Am. J. Clin. Nutr. 2013;97(3):497–504. doi: 10.3945/ajcn.112.048074. [DOI] [PubMed] [Google Scholar]
- 13.Wilson J.P., Fan B., Shepherd J.A. Total and regional body volumes derived from dual-energy X-ray absorptiometry output. J. Clin. Densitom. 2013;16(3):368–373. doi: 10.1016/j.jocd.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Tinsley G.M. Reliability and agreement between DXA-derived body volumes and their usage in 4-compartment body composition models produced from DXA and BIA values. J. Sports Sci. 2018;36(11):1235–1240. doi: 10.1080/02640414.2017.1369556. [DOI] [PubMed] [Google Scholar]
- 15.Ng B.K., Liu Y.E., Wang W., Kelly T.L., Wilson K.E., Schoeller D.A., et al. Validation of rapid 4-component body composition assessment with the use of dual-energy X-ray absorptiometry and bioelectrical impedance analysis. Am. J. Clin. Nutr. 2018;108(4):708–715. doi: 10.1093/ajcn/nqy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong W.W., Lee L.S., Klein P.D. Deuterium and oxygen-18 measurements on microliter samples of urine, plasma, saliva, and human milk. Am. J. Clin. Nutr. 1987;45(5):905–913. doi: 10.1093/ajcn/45.5.905. [DOI] [PubMed] [Google Scholar]
- 17.Schoeller D.A., van Santen E., Peterson D.W., Dietz W., Jaspan J., Klein P.D. Total body water measurement in humans with 18O and 2H labeled water. Am. J. Clin. Nutr. 1980;33(12):2686–2693. doi: 10.1093/ajcn/33.12.2686. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z.M., Deurenberg P., Guo S.S., Pietrobelli A., Wang J., Pierson R.N., Jr., et al. Six-compartment body composition model: inter-method comparisons of total body fat measurement. Int. J. Obes. Relat. Metab. Disord. 1998;22(4):329–337. doi: 10.1038/sj.ijo.0800590. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z., Shen W., Withers R.T. Human body Composition. 2nd edition. Human Kinetics Publishers; Champaign, IL: 2005. Multicomponent molecular-level models of body composition analysis; pp. 163–176. [Google Scholar]
- 20.Tinsley G.M. Five-component model validation of reference, laboratory and field methods of body composition assessment. Br. J. Nutr. 2021;125(11):1246–1259. doi: 10.1017/S0007114520003578. [DOI] [PubMed] [Google Scholar]
- 21.Tinsley G.M., Harty P.S., Stratton M.T., Smith R.W., Rodriguez C., Siedler M.R. Tracking changes in body composition: comparison of methods and influence of pre-assessment standardization. Br. J. Nutr. 2022;127(11):1656–1674. doi: 10.1017/S0007114521002579. [DOI] [PubMed] [Google Scholar]
- 22.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. 1986. [PubMed] [Google Scholar]
- 23.Lakens D. Equivalence tests: A practical primer for t-tests, correlations, and meta-analyses. Soc. Psychol. Pers. Sci. 2017;1:1–8. doi: 10.1177/1948550617697177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Signorell A. 2022. DescTools: Tools for Descriptive Statistics2022. [Google Scholar]
- 25.R Core Team. R . R Foundation for Statistical Computing; Vienna, Austria: 2022. A Language and Environment for Statistical Computing. [Google Scholar]
- 26.Nickerson B.S., Snarr R.L. Proportional bias of multifrequency bioimpedance analysis is larger in Hispanic females than males. Nutr. Res. 2022;103:40–46. doi: 10.1016/j.nutres.2022.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nickerson B.S., Cicone Z.S., Park K.S., Snarr R.L., Fedewa M.V., Esco M.R. Dual energy X-ray absorptiometry produces larger measurement error in non-Hispanic Caucasians than Hispanics. Clin. Nutr. ESPEN. 2023;53:120–125. doi: 10.1016/j.clnesp.2022.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner D.R., Heyward V.H., Gibson A.L. Validation of air displacement plethysmography for assessing body composition. Med. Sci. Sports Exerc. 2000;32(7):1339–1344. doi: 10.1097/00005768-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Nickerson B.S., Esco M.R., Bishop P.A., Kliszczewicz B.M., Park K.S., Williford H.N. Validity of four-compartment model body fat in physically active men and women when using DXA for body volume. Int. J. Sport Nutr. Exerc. Metab. 2017;27(6):520–527. doi: 10.1123/ijsnem.2017-0076. [DOI] [PubMed] [Google Scholar]
- 30.Graybeal A.J., Moore M.L., Cruz M.R., Tinsley G.M. Body composition assessment in male and female bodybuilders: A 4-compartment model comparison of dual-energy X-ray absorptiometry and impedance-based devices. J. Strength Cond. Res. 2020;34(6):1676–1689. doi: 10.1519/JSC.0000000000002831. [DOI] [PubMed] [Google Scholar]
- 31.Moon J.R., Tobkin S.E., Roberts M.D., Dalbo V.J., Kerksick C.M., Bemben M.G., et al. Total body water estimations in healthy men and women using bioimpedance spectroscopy: a deuterium oxide comparison. Nutr. Metab. (Lond). 2008;5(7):7. doi: 10.1186/1743-7075-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request, pending approval.