Abstract
Background
Objective markers of ultraprocessed foods (UPF) may improve the assessment of UPF intake and provide insight into how UPF influences health.
Objectives
To identify metabolites that differed between dietary patterns (DPs) high in or void of UPF according to Nova classification.
Methods
In a randomized, crossover, controlled-feeding trial (clinicaltrials.govNCT03407053), 20 domiciled healthy participants (mean ± standard deviation: age 31 ± 7 y, body mass index [kg/m2] 22 ± 11.6) consumed ad libitum a UPF-DP (80% UPF) and an unprocessed DP (UN-DP; 0% UPF) for 2 wk each. Metabolites were measured using liquid chromatography with tandem mass spectrometry in ethylenediaminetetraacetic acid plasma, collected at week 2 and 24-h, and spot urine, collected at weeks 1 and 2, of each DP. Linear mixed models, adjusted for energy intake, were used to identify metabolites that differed between DPs.
Results
After multiple comparisons correction, 257 out of 993 plasma and 606 out of 1279 24-h urine metabolites differed between UPF-DP and UN-DP. Overall, 21 known and 9 unknown metabolites differed between DPs across all time points and biospecimen types. Six metabolites were higher (4-hydroxy-L-glutamic acid, N-acetylaminooctanoic acid, 2-methoxyhydroquinone sulfate, 4-ethylphenylsulfate, 4-vinylphenol sulfate, and acesulfame) and 14 were lower following the UPF-DP; pimelic acid, was lower in plasma but higher in urine following the UPF-DP.
Conclusions
Consuming a DP high in, compared with 1 void of, UPF has a measurable impact on the short-term human metabolome. Observed differential metabolites could serve as candidate biomarkers of UPF intake or metabolic response in larger samples with varying UPF-DPs.
This trial was registered at clinicaltrials.gov as NCT03407053 and NCT03878108.
Keywords: processed foods, food processing, United States adults, dietary assessment, dietary biomarkers, dietary patterns, metabolomics, urine metabolites, plasma metabolites
Introduction
Processing foods is a central component of the United States food system for safety, security, and to help meet nutrient needs [1]. The Nova is 1 of several classification systems used to classify foods based on the degree of food and beverage processing that has gained traction [2,3]. As defined in Nova [[2], [3], [4]], foods classified as ultraprocessed (ie, ultraprocessed foods or UPF) contain ingredients or additives rarely used in household kitchens (eg, hydrogenated oils, modified starches, and artificial sweeteners [5]) to improve palatability, sensory properties, stability, or convenience. In the United States, adults [6] and children aged 2–18 y [7] consume >60% of total EI from UPF, as defined by the Nova. Additionally, UPF contributes ∼45% of the energy consumed by infants and toddlers aged 6–23 mo in the United States, outside of human milk and formula intake [8]. Observational research using self-reported dietary intake data suggests that high intake of UPF is associated with increased risk of obesity [[9], [10], [11]], cancer [12,13], CVD [[14], [15], [16]], and mortality [[17], [18], [19]]. The tension between the food system benefits and public health concerns of highly processed food intake has become a pressing issue in the fields of nutrition science and epidemiology [20,21].
Concerns have been raised about the reproducibility of Nova classification, which is compounded by limitations of dietary assessment tools and underlying food and nutrient databases [[22], [23], [24]]. Nova classification requires ingredient-amount data on each food and beverage item [4,25]. FFQs are widely used to assess usual intake in large prospective studies. FFQs query about a predefined list of commonly consumed food and beverage items but do not typically collect information on food processing or source to provide adequate detail for Nova classification. Shorter-term dietary assessment methods, such as multiple 24-h recalls or weighted food records, include more detailed probes about each food and beverage consumed within a 24-h period [26]. However, the underlying food and nutrient databases often lack ingredient-amount details and label information needed for Nova classification. Therefore, there may be varying approaches used by researchers to classify self-reported dietary intake based on the degree of food processing [25,27]. Identification of objective biomarkers of UPF intake and metabolic response to intake has the potential to: 1) improve the validity and reproducibility of estimated intake, 2) strengthen estimates of associations with disease [26], and 3) provide insight into biological mechanisms linking UPF intake to health. Domiciled controlled-feeding studies provide an opportunity to facilitate the discovery of objective markers of foods classified as UPF because researchers can design menus, document ingredients from food packages and recipes, control available foods, and objectively monitor intake [28]. The objective of this exploratory analysis was to identify metabolites, of both exogenous and endogenous origin, that differed between dietary patterns (DPs) that are either high in or void of foods classified as ultraprocessed according to Nova using data from a domiciled, randomized, crossover, controlled-feeding trial (RCfT) [29].
Methods
Experimental design
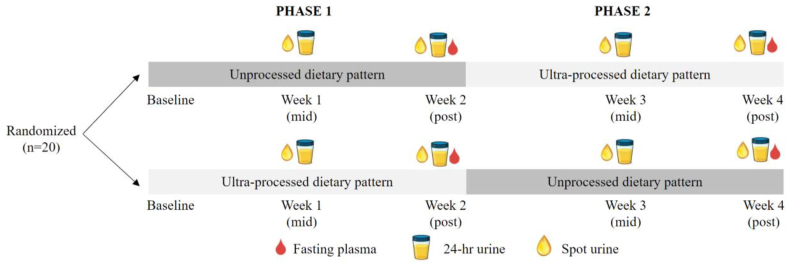
This was a secondary analysis using biospecimens from a domiciled, crossover RCfT; the study design and main results have been described previously [29]. The study’s primary aim was to investigate differences in ad libitum EI and weight gain between the consumption of a UPF-DP and a minimally processed or unprocessed (UN)-DP. In brief, 20 participants were given a UPF-DP and an UN-DP to consume ad libitum for 2 wk each, in a randomly assigned order with no washout period. Both DPs had 7-d rotating menus with breakfast, lunch, and dinner, along with snacks and bottled water available continuously. Fasting blood samples, 24-h urine, and spot urine were collected throughout the study (Figure 1). Participants consumed 508 ± 106 kcal/d more during the UPF-DP than UN-DP and gained 0.9 ± 0.3 kg on the UPF-DP and lost 0.9 ± 0.3 kg on the UN-DP [29]. For this post hoc analysis, we analyzed EDTA plasma, 24-h urine, and spot urine samples, which had been stored at –57 °C since collection and not previously thawed, to identify metabolites that differed between the UPF-DP and UN-DP.
FIGURE 1.
A randomized, controlled, crossover, domiciled feeding study comparing metabolomic responses following consumption of an ultraprocessed dietary pattern and an unprocessed dietary pattern. Metabolites were measured in EDTA plasma, 24-h urine, and spot urine at the time points indicated above for (n = 20) participants. Within-individual differences in relative levels of metabolites were compared between dietary intervention phases (ultraprocessed dietary pattern compared with unprocessed dietary pattern) at week 2 for plasma and at week 1 and week 2 for 24-h and spot urine. Relative levels of metabolites were also compared over time (changes from week 1 to week 2 within an individual) for 24-h and spot urine during the ultraprocessed dietary pattern and during the unprocessed dietary pattern. EDTA, ethylenediaminetetraacetic acid.
Participants
Participants were eligible to enroll if they were aged 18–50 y, had a BMI of >18.5 kg/m2, and were self-reported as weight stable (<5 kg change in the prior 6 mo). Participants were excluded if they had anemia, diabetes, cancer, thyroid disease, eating disorders, diagnosed psychiatric disorders (eg, depression and bipolar disorder), food allergies, or if they adhered to a specialized DP (eg, kosher and vegan).
Ethics
The study protocol was approved by the institutional review board of the National Institute of Diabetes & Digestive & Kidney Diseases and was registered at clinicaltrials.gov (Identifier NCT03407053). All participants provided written informed consent prior to participation and the clinical trial conducted in the Metabolic Clinical Research Unit at the NIH Clinical Center.
Prescribed dietary interventions
All foods and beverages were prepared and provided to participants to consume ab libitum by the metabolic kitchen at the NIH Clinical Center. The presented UPF-DP and UN-DP were matched for total calories, macronutrient composition (∼47% carbohydrate, 36% fat, and 17% protein), total sugars, fiber, and sodium and are described in detail previously [29]. The foods and beverages were classified according to the Nova system [2]. Nova classifies foods and beverages into 4 groups according to the degree of processing [4]. Group 1 includes “unprocessed or minimally processed foods,” such as fresh, dry, or frozen fruits or vegetables, grains, legumes, meat, fish, and milk, that have undergone minimal processing techniques, such as grinding, cooking, or pasteurization. Group 2 includes “processed culinary ingredients,” such as table sugar, oils, fats, salt, and other substances that have been extracted, pressed, or centrifuged from foods used for culinary preparation. Group 3 includes “processed foods,” which include group 1 foods that have culinary ingredients from group 2 added, such as canned fruits, artisanal bread, cheese, or smoked meat. Group 4 includes “ultraprocessed foods,” which are foods with group 2 ingredients as well as additives not used in culinary preparations, such as flavors, colors, nonnutritive sweeteners, emulsifiers, and other substances, used to increase palatability and sensorial properties of the food or beverage.
The UPF-DP was composed of 5% of total EI (%en) from minimally processed foods (Nova group 1), <1 %en culinary ingredients (Nova group 2), 14 %en processed foods (Nova group 3), and 81 %en UPF (Nova group 4). The UN-DP was composed of 88%en minimally processed foods (Nova group 1), 12%en culinary ingredients (Nova group 2), 0%en processed foods (Nova group 3), and 0%en UPF (Nova group 4). Presented meals were identical in composition and amount for each participant [29] and were consumed ad libitum. Pictures and descriptions, including brand names, of all meals and snacks provided to participants can be found here in the Supplementary Materials of the original article [29]. All recipes are available upon reasonable request.
Meals were delivered to participants’ rooms 3 times/d, and participants were given 60 min to consume each meal. Participants were instructed to eat as little or as much food as desired. A variety of snacks and bottled water were provided each morning that could be consumed throughout the day ad libitum. The total food provided to participants each day substantially exceeded their estimated energy requirements (∼200% of energy requirements as determined by 1.6× resting EE measured at screening) and was anticipated to be more than the participants could eat in 1 sitting [29]. At each mealtime, remaining food and beverages were reweighed by study staff, and nutrient and metabolizable EIs were calculated via ProNutra software version 3.4 (Viocare, Inc.) using the USDA National Nutrient Database for Standard Reference, Release 26 and the USDA Food and Nutrient Database for Dietary Studies, 4.0.
Metabolomics analysis
Discovery metabolomics analyses were conducted on stored (–57FC since collection) plasma and urine samples identified in Figure 1 by Metabolon Inc. Samples were analyzed using ultra-HPLC with tandem MS/MS for a broad range of metabolites (<1 kDa), representing multiple metabolic pathways, including endogenously derived amino acids, carbohydrates, lipids, cofactors and vitamins, intermediates of energy metabolism, as well as xenobiotics derived from exogenous sources, such as food or drugs. In brief, serum samples were prepared using the automated MicroLab STAR system (Hamilton Company). Recovery standards were added, and the protein fraction was extracted with methanol, followed by vigorous shaking and centrifugation. Sample extracts were dried and reconstituted using recovery solvents containing fixed concentrations of standards. These extracts were analyzed using reverse phase/ultra-HPLC-MS/MS in positive ion mode electrospray ionization and negative ion mode electrospray ionization. Raw data were extracted, peak-identified, and processed by Metabolon Inc. using proprietary software and a biochemical reference library of >4500 known metabolites based on authentic standards. The reliability of Metabolon’s platform for related research has been demonstrated previously [[30], [31], [32], [33]].
Blinded plasma or urine replicate pooled quality control (QC) samples (n = 30) were randomly distributed and used to calculate within and (for urine) between batch variability by calculating CV. All study plasma samples (n = 70) and plasma QC samples (n = 8) were analyzed as a single batch. Spot urine and 24-h urine study samples (n = 200) were analyzed in 2 batches, each with 100 study samples and 11 QCs. The median CV for metabolites in plasma was 9.5% (25th–75th percentile: 6.4%–15.1%), and metabolites in urine were 12.8% (25th–75th percentile: 8.6%–19.7%).
A total of 1091 metabolites were identified in plasma, and 1416 were identified in 24-h and spot urine. Before imputation, metabolites with >80% of values below the detection limit were excluded (n = 38 for plasma, n = 37 for 24-h urine, and n = 46 for spot urine); these consisted mainly of compounds related to prescription or over-the-counter drugs or tobacco. Metabolites with a CV >30.0% were also excluded (n = 60 for plasma and n = 97 for 24-h and spot urine). Three additional 24-h urine metabolites were excluded because the models failed to converge (xanthine, 3-methoxytyramine-sulfate, and unknown metabolite X-12729). In total, we considered 993 plasma, 1279 24-h urine, and 1273 spot urine metabolites for this analysis, including unidentified (or “unknown”) and partially characterized metabolites. Values below the detection limit were assigned to the minimum detectable value for a given metabolite. All values were scaled to a median of 1, and log2 was transformed to improve symmetry and approximate normality.
Power calculations
With the 20 participants who completed the original study, we estimated a priori that we would have 80% power to detect an association if metabolites were ∼35% higher after 2-wk on the UPF-DP compared with UN-DP. Power calculations assumed: 1) the use of a paired t test comparing normalized log-metabolite levels between 2 groups with a difference equal to log(1+Δ), where Δ is the proportional increase in metabolites with UPF-DP, 2) between-subject variability accounts for 90% of sample variability, and 3) α-level = 0.05 before correction for multiple comparisons.
Statistical analysis
Metabolites that differ between UPF-DP and UN-DP
We used a linear mixed model (PROC MIXED) in SAS version 9.4 (SAS Institute Inc.) to investigate which plasma and urine metabolites differed between the UPF-DP and UN-DP intervention within-individual at the relevant time points shown in Figure 1 [34]. Each univariate model (ie, 1 model per metabolite per sample type) incorporated subject-specific random intercepts and was adjusted for the study phase (phase 1 compared with phase 2) and diet sequence (UN-DP in phase 1, then UPF-DP in phase 2 compared with UPF-DP in phase 1 then UN-DP in phase 2) to account for potential correlations among repeated measurements in the crossover design. The phase covariate allowed us to determine if there was a chronologic effect of time across the duration of the study, and the sequence covariate allowed us to determine if there was a carry-over or residual effect between study phases [35]. An additional covariate for time point (mid and post) and an interaction term (diet∗time point) were included in urine models to test for changes in 24-h and spot urine metabolites from week 1 to week 2 within each DP as well as differences between the DPs at week 1. Spot urines were used to explore if metabolite differences observed in 24-h urine samples extended to a shorter-term collection method. The urine models are considered doubly repeated, as both phase (phase 1 and phase 2) and timepoint (mid and post) are repeated in the crossover design [[36], [37], [38]] (Figure 1). For plasma and urine metabolites that differed between DPs, we characterized the proportion in each broad biochemical class (ie, Metabolon assigned “super pathway”) and metabolic pathway (ie, Metabolon assigned “sub pathway”). We also adjusted for differences in calculated EI using the participants’ mean EI from the week prior to each specimen collection to account for random daily fluctuations during the relevant time period because participants consumed 508 ± 106 kcal/d more during the UPF-DP than UN-DP and gained 0.9 ± 0.3 kg after the UPF-DP and lost 0.9 ± 0.3 kg after the UN-DP [29]. Thus, our main results can be interpreted as individual differences in measured metabolites between the 2 DPs, independent of differential EI. EI was calculated by weighing and measuring foods before and after the presentation to participants and entering values into ProNutra software version 3.4 (Viocare, Inc.). We also conducted a sensitivity analysis adjusting for crude changes in body weight from baseline to week 2 (Supplementary Appendix).
To control the family-wise false positive rate, we applied a Benjamini-Hochberg (BH) correction within each of the sets of comparisons [39,40]. This correction was applied separately for plasma at week 2, 24-h urine at week 1, 24-h urine at week 2, spot urine at week 1, and spot urine at week 2, and within the phase and sequence, P values for each sample type and time point.
Consistent metabolites across time points and sample types
For metabolites that differed between the 2 DPs across all time points and sample types, we created a bubble plot illustrating the magnitude and direction of relative metabolite changes from plasma at week 2, 24-h urine at weeks 1 and 2, and spot urine at weeks 1 and 2. Figures were generated using RStudio version 4.2.2 (RStudio Team (2022)).
Pathway analysis
In secondary analyses, we used Fisher’s method for combining metabolite P values, estimated from linear mixed models, across 8 super-pathways for plasma, 24-h, and spot urine as well as 103 and 85 subpathways for plasma and both urine types, respectively; we applied a BH correction within a given biospecimen type. Our choice was predicated on the efficacy of Fisher’s method when dealing with smaller sample sizes. To account for the dependency among tests from which the P values were extracted, we employed an empirically derived null distribution grounded on pseudo replicates that simulate the properties of a rigorous permutation test [41]. Disregarding this interdependence when pooling the P values could skew the results, resulting in either overly conservative or overly liberal type 1 error rates.
Results
All 20 participants who successfully screened for the study also completed the study (Supplementary Figure 1), which is described in Table 1. The study was racially and ethnically diverse. Overall, 55% self-identified as Black; 15% as White; 15% as Asian; and 15% as multiracial or undeclared. Additionally, 25% self-identified as Hispanic or Latino. Calculated energy, macronutrient, fiber, and sodium intake for each DP are described in Table 2.
TABLE 1.
Participant baseline characteristics
| Characteristic | Mean ± SD or n |
|---|---|
| Age (y) | 31 ± 7 |
| Weight (kg) | 78.2 ± 21.20 |
| BMI (kg/m2) | 22.2 ± 11.57 |
| Males | 10 |
| Females | 10 |
| Asian1 | 3 |
| Black/African American1 | 11 |
| Multiracial1 | 2 |
| White1 | 3 |
| Undeclared race1 | 1 |
| Hispanic or Latino1 | 5 |
BMI, body mass index; SD, standard deviation.
Participants (n = 20) were asked during screening to identify with 1 of these categories.
TABLE 2.
Dietary intake during the ultraprocessed and unprocessed dietary pattern intervention phases
| Dietary component | UPF-DP | UN-DP |
|---|---|---|
| Total energy (kcal) | 2978 ± 944 | 2471 ± 728 |
| Energy from protein (kcal) | 490 ± 153 | 492 ± 139 |
| Energy from fat (kcal) | 1387 ± 470 | 1106 ± 366 |
| Energy from CHO (kcal) | 1102 ± 336 | 872 ± 267 |
| %en from protein | 16% | 20% |
| %en from fat | 37% | 35% |
| %en from carbohydrates | 47% | 45% |
| Monounsaturated fat (g) | 43 ± 13 | 49 ± 14 |
| Polyunsaturated fat (g) | 30 ± 9 | 25 ± 11 |
| Saturated fat (g) | 39 ± 12 | 19 ± 5 |
| Fiber (g) | 49 ± 20 | 46 ± 15 |
| Sodium (mg) | 5817 ± 1843 | 4607 ± 1485 |
Data are presented as mean ± SD for n = 20 participants. Statistical comparisons of intake are presented in the original manuscript [29]. Energy and nutrient intakes were estimated by weighing and measuring foods before and after presentation to participants and entering values into ProNutra software version 3.4 (Viocare, Inc). Pictures and descriptions, including brand names, of all meals and snacks provided to participants can be found here from the Supplementary Materials of the original article [29]. All recipes are available upon reasonable request.
Metabolites that differed between UPF-DP and UN-DP
Plasma
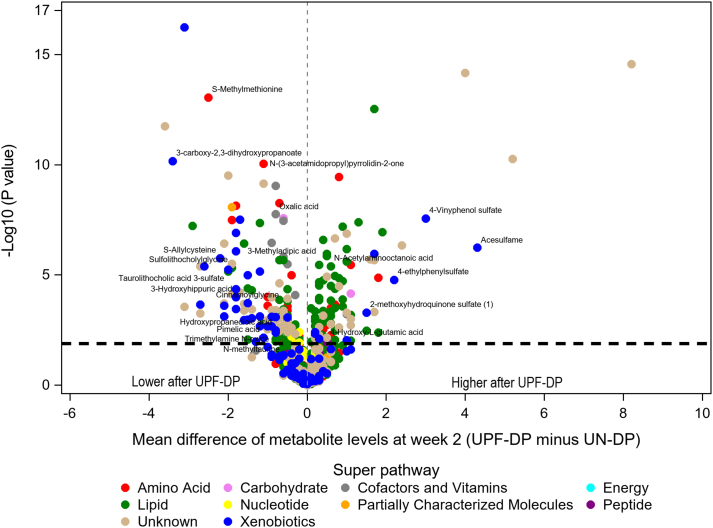
After adjustment for calculated EI and correction for multiple comparisons, 257 metabolites differed between the UPF-DP and UN-DP within-individual (Supplementary Table 1 and Supplementary Figure 2). The study phase and diet sequence covariates were NS for any of these metabolites. Most of these 257 plasma metabolites were lipids (42%; n = 108), followed by unknowns (21%; n = 54), xenobiotics (16%; n = 40), and amino acids (14%; n = 36) (Supplementary Table 2 and Figure 2). Over half of the statistically significant lipid (59%; n = 64) and amino acid (53%; n = 19) metabolites, but only 23% of xenobiotic (n = 9) metabolites were higher after consuming the UPF-DP compared with UN-DP (Supplementary Table 2).
FIGURE 2.
Volcano plot of mean difference at week 2 in plasma metabolites after consumption of a UPF-DP compared with UN-DP. Mean differences in metabolites (n = 993) were estimated for 20 participants via a linear mixed model adjusted for diet, phase, sequence and calculated EI during the week prior to sample collection with subject-specific random intercepts. The metabolites labeled in the figure consistently differed between the UPF-DP and UN-DP across all sample types and time points, as shown in Table 3. The metabolites above the horizontal black dashed line were statistically significantly different at week 2 after the Benjamini-Hochberg correction for multiple comparisons (n = 257). Super pathway (ie, general biochemical class) was assigned by Metabolon, Inc. for sorting compounds by broad biochemical classes. EI, energy intake; UN-DP, unprocessed dietary pattern; UPF-DP, ultraprocessed dietary pattern.
Urine
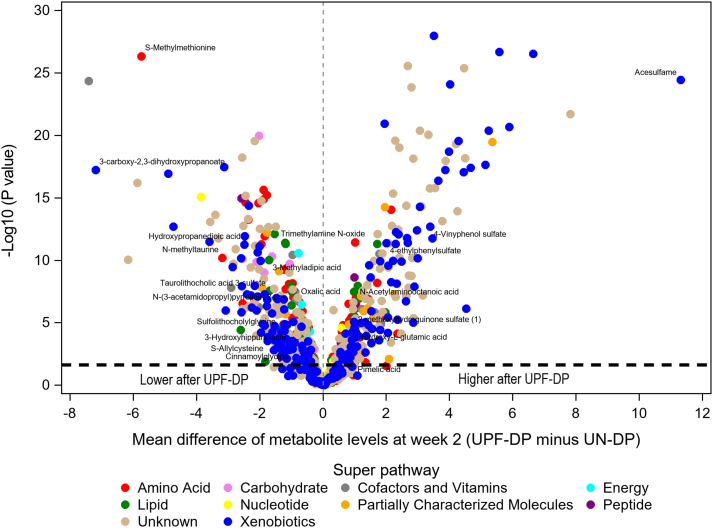
After adjustment for EI and correction for multiple comparisons, 606 24-h urine metabolites differed with-individual between the UPF-DP and UN-DP at week 2 (Supplementary Table 3 and Supplementary Figure 3). The study phase and diet sequence covariates were NS for any of these metabolites, except for unknown metabolite X-11847, which had a significant phase P value. There were no changes in relative levels of chemically identified urine metabolites within a 2-wk study phase (ie, urine collected at week 1 compared with week 2 within UPF-DP or within UN-DP). Most metabolites (78%, n = 475) statistically differed within-individual between the UPF-DP and UN-DP at week 1 and week 2 (Supplementary Table 3), with a consistent direction of change within phase for all but 4 metabolites. Thus, most 24-h urine metabolites were relatively stable during a given dietary intervention phase, with most differences large enough to detect between the UPF-DP compared with UN-DP after both 1 and 2 wk of consumption.
A majority of the 475 24-h urine metabolites that differed within-individual between UPF-DP and UN-DP at week 1 and week 2 were from unknown (34%; n = 161) or xenobiotic (28%; n = 133) pathways, of which 77 metabolites were food or plant components, and 24 were related to benzoate metabolism (Supplementary Table 4 and Figure 3). Similar to plasma, 16% (n = 77) of differential metabolites in 24-h urine were from amino acid pathways, whereas only 7% (n = 32) of metabolites were lipids (compared with 42% of plasma metabolites). Unlike plasma, only 34% (n = 11) of statistically significant lipids and 36% of amino acid (n = 28) metabolites, but 49% of xenobiotic (n = 65) metabolites were higher after consuming the UPF-DP compared with UN-DP (Supplementary Table 4).
FIGURE 3.
Volcano plot of mean difference at week 2 in urine metabolites after consumption of a UPF-DP compared with UN-DP. Mean differences in metabolites (n = 1279) were estimated for 20 participants via a linear mixed model adjusted for diet, phase, sequence, and time point and calculated EI during the week prior to sample collection with subject-specific random intercepts. The metabolites labeled in the figure consistently differed between the UPF-DP and UN-DP across all sample types and time points, as shown in Table 3. The metabolites above the horizontal black dashed line were statistically significantly different at week 2 after the Benjamini-Hochberg correction for multiple comparisons (n = 606). Super pathway (ie, general biochemical class) was assigned by Metabolon, Inc. for sorting compounds by broad biochemical classes. EI, energy intake; UN-DP, unprocessed dietary pattern; UPF-DP, ultraprocessed dietary pattern.
Findings for spot urine were generally reflective of 24-h urine, though fewer in number. After adjustment for EI and correction for multiple comparisons, 176 spot urine metabolites differed within-individual between the UPF-DP and UN-DP, and 44% (n = 78) of differential spot urine metabolites changed during the UPF-DP from weeks 1 to 2 as well as 34% (n = 60) during UN-DP, indicating higher temporal variability for spot urine than 24-h urine metabolites. Of the 176 differential spot urine metabolites, 91 metabolites overlapped with 24-h urine results (Supplementary Table 5 and Supplementary Figure 4). Of these 91 metabolites, the phase and sequence covariates were NS for any, and all metabolites differed within-individual between UPF-DP and UN-DP at both week 1 and week 2.
Consistent metabolites across time points and sample types
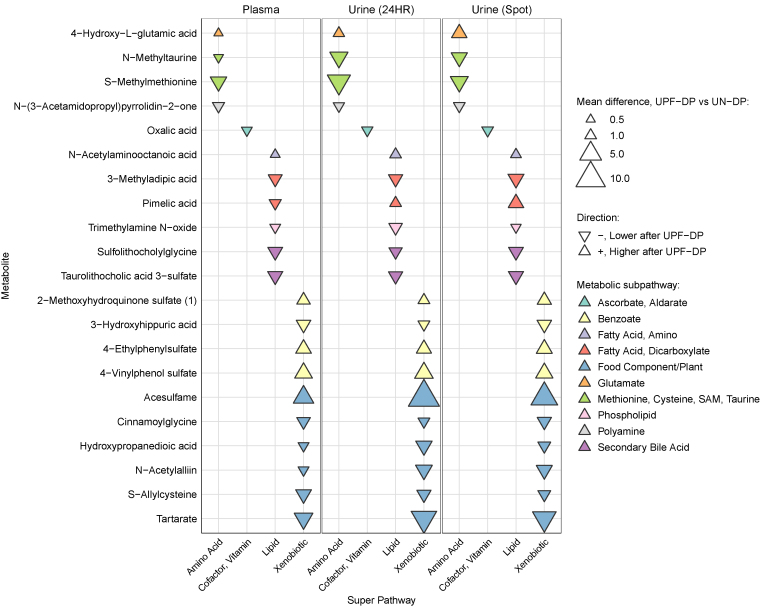
Seventy-nine metabolites differed within-individual between the UPF-DP and UN-DP in plasma at week 2 and in 24-h urine at weeks 1 and 2 (Table 3). Twenty-one of these metabolites, with known identities, differed within-individual between the UPF-DP and UN-DP for spot urine at weeks 1 and 2 (Figure 4), and an additional 9 unidentified metabolites also differed across all biospecimen types (Supplementary Figure 5). The direction and magnitude of change in relative levels of these metabolites after the UPF-DP was remarkably consistent across the plasma, 24-h, and spot urine. The largest differences between DPs were observed for the food component, acesulfame, which was higher after the UPF-DP than the UN-DP. Notably, 3 metabolites (2-methoxyhydroquinone sulfate, 4-ethylphenyl sulfate, and 4-vinylphenol sulfate) related to benzoate metabolism were higher after the UPF-DP than the UN-DP consistently in all 3 sample types.
TABLE 3.
Metabolites that consistently differed within-individual between an ultraprocessed and unprocessed dietary pattern in both plasma and 24-h urine across all time points
| Super pathway | Sub pathway | Metabolite | MSI metabolite identification level3 |
|---|---|---|---|
| Amino acid | |||
| Glutamate metabolism | 4-Hydroxy-L-glutamic acid1 | 1 | |
| Histidine metabolism | 3-Methylhistidine | 1 | |
| Histidine metabolism | Imidazoleacetic acid riboside | 2 | |
| Leucine, isoleucine and valine metabolism | 2,3-Dihydroxy-2-methylbutyrate | 1 | |
| Methionine, cysteine, SAM, and taurine metabolism | Cysteine-S-sulfate | 1 | |
| Methionine, cysteine, SAM, and taurine metabolism | N-Methyltaurine1 | 1 | |
| Methionine, cysteine, SAM, and taurine metabolism | S-Methylmethionine1 | 1 | |
| Polyamine metabolism | N-(3-Acetamidopropyl)pyrrolidin-2-one1 | 1 | |
| Polyamine metabolism | N1-Acetylspermidine | 1 | |
| Polyamine metabolism | N-Acetyl-isoputreanine | 1 | |
| Tryptophan metabolism | Indoxyl sulfate | 1 | |
| Tyrosine metabolism | Gentisic acid | 1 | |
| Urea cycle; arginine and proline metabolism | Homocitrulline | 1 | |
| CHO | |||
| Advanced glycation end-product | N6-carboxymethyllysine | 1 | |
| Glycolysis, gluconeogenesis, and pyruvate metabolism | Glyceric acid | 1 | |
| Cofactors and vitamins | |||
| Ascorbate and aldarate metabolism | Ascorbic acid-3-sulfate | 2 | |
| Ascorbate and aldarate metabolism | Ascorbic acid-2-sulfate | 1 | |
| Ascorbate and aldarate metabolism | L-threonic Acid | 1 | |
| Ascorbate and aldarate metabolism | Oxalic acid1 | 1 | |
| Tocopherol metabolism | Gamma-CEHC | 1 | |
| Vitamin B6 metabolism | 4-Pyridoxic acid | 1 | |
| Lipid | |||
| FA metabolism (acyl glutamine) | Hexanoylglutamine | 1 | |
| FA, amino | DL-2-Aminooctanoic acid | 1 | |
| FA, amino | N-Acetylaminooctanoic acid1 | 2 | |
| FA, dicarboxylate | 3-Methyladipic acid1 | 1 | |
| FA, dicarboxylate | Pimelic acid1 | 1 | |
| Inositol metabolism | Myo-inositol | 1 | |
| Phospholipid metabolism | Trimethylamine N-oxide (TMAO)1 | 1 | |
| Secondary bile acid metabolism | Sulfolithocholylglycine1 | 2 | |
| Secondary bile acid metabolism | Taurolithocholic acid 3-sulfate1 | 1 | |
| Partially characterized molecules | |||
| Partially characterized molecules | Pentoic acid | 3 | |
| Peptide | |||
| Acetylated peptides | 4-Hydroxyphenylacetylglutamine | 1 | |
| Xenobiotics | |||
| Benzoate metabolism | 2-Methoxyhydroquinone sulfate (1)1, 2 | 1 | |
| Benzoate metabolism | 3-Hydroxyhippuric acid1 | 1 | |
| Benzoate metabolism | 4-Allylcatechol sulfate | 1 | |
| Benzoate metabolism | 4-Ethylphenylsulfate1 | 1 | |
| Benzoate metabolism | 4-Vinylphenol sulfate1 | 1 | |
| Benzoate metabolism | Pyrocatechol sulfate | 1 | |
| Chemical | 2-Methoxyresorcinol sulfate | 1 | |
| Chemical | Pyrogallol-1-O-sulfate | 1 | |
| Drug - topical agents | 2,6-Dihydroxybenzoic acid | 1 | |
| Food component/plant | 4-Vinylguaiacol sulfate | 1 | |
| Food component/plant | 2-Acetamidophenol sulfate | 1 | |
| Food component/plant | 2-Aminophenol sulfate | 1 | |
| Food component/plant | 3-Carboxy-2,3-dihydroxypropanoate (tartarate)1 | 1 | |
| Food component/plant | 4-Allylphenol sulfate | 1 | |
| Food component/plant | Acesulfame1 | 1 | |
| Food component/plant | Cinnamoylglycine1 | 1 | |
| Food component/plant | Ethyl beta-D-glucopyranoside | 1 | |
| Food component/plant | Hydroxypropanedioic acid1 | 1 | |
| Food component/plant | Maltol sulfate | 1 | |
| Food component/plant | Methyl vanillate sulfate | 1 | |
| Food component/plant | N-Acetylalliin1 | 1 | |
| Food component/plant | Piperine | 1 | |
| Food component/plant | S-Allylcysteine1 | 1 | |
| Food component/plant | Sulfate of piperine metabolite C16H19NO3 (2)2 | 3 | |
| Food component/plant | Sulfate of piperine metabolite C16H19NO3 (3)2 | 3 | |
| Food component/plant | Sulfate of piperine metabolite C18H21NO3 (1)2 | 3 | |
| Food component/plant | Sulfate of piperine metabolite C18H21NO3 (3)2 | 3 | |
The 79 metabolites listed here differed within-individual (n = 20 participants) between UPF-DP and UN-DP at week 2 for plasma as well as week 1 and week 2 for 24-h urine. Mean differences in metabolites were estimated via a linear mixed model adjusted for diet, phase, sequence, time point for urine only, and calculated EI during the week prior to sample collection with subject-specific random intercepts. P values were Benjamini-Hochberg corrected for multiple comparisons. Super pathway (ie, general biochemical class) and sub pathway names were assigned via the Metabolon, Inc. discovery platform.
CEHC, gamma-carboxyethyl hydroxychroman; CHO, carbohydrate; EI, energy intake; FA, fatty acid; MSI, metabolomics standards initiative; SAM, S-adenosyl methionine; UN-DP, unprocessed dietary pattern; UPF-DP, ultraprocessed dietary pattern.
21 metabolites also differed within-indiviudal between UPF-DP and UN-DP at week 1 and week 2 for spot urine.
A (#) is assigned by Metabolon and indicates a compound that is a structural isomer of another compound in the Metabolon spectral library. For example, a steroid that may be sulfated at one of several positions that are indistinguishable by the mass spectrometry data or a diacylglycerol for which more than one stereospecific molecule exists.
MSI level 1 are identified compounds, level 2 are putatively annotated compounds, level 3 are putatively characterized compound classes, and level 4 are unknown [42].
FIGURE 4.
Magnitude and direction of mean differences at week 2 in metabolites after consumption of a UPF-DP compared with UN-DP. Metabolites listed (n = 21) here differed within-individual (n = 20 participants) between UPF-DP and UN-DP at week 2 for plasma as well as week 1 and week 2 for 24-h urine. Mean differences in metabolites were estimated via a linear mixed model adjusted for diet, phase, sequence, and time point for urine only and calculated EI during the week prior to sample collection with subject-specific random intercepts. P values were Benjamini-Hochberg corrected for multiple comparisons. Super pathway (ie, general biochemical class) and subpathway were assigned by Metabolon, Inc. EI, energy intake; UN-DP, unprocessed dietary pattern; UPF-DP, ultraprocessed dietary pattern.
Pathway analysis
Overall, Fisher’s combined P values for 6 (ie, xenobiotics, cofactors/vitamins, amino acid, lipid, carbohydrate, and nucleotide) and 2 (ie, xenobiotics and cofactors/vitamins), out of 8, super-pathways were statistically significant at a BH-corrected α of 0.05 in plasma as well as 24-h and spot urine, respectively (Supplementary Table 6). Additionally, 40 out of 103 and 12 out of 85 subpathways were statistically significant in plasma and both urine types, respectively (Supplementary Tables 7 and 8). Of these, 9 subpathways overlapped between the biospecimen types; these include food component/plant; benzoate metabolism; secondary bile acid (BA) metabolism; tyrosine metabolism; tocopherol metabolism; ascorbate and aldarate metabolism; chemical; methionine, cysteine, S-adenosyl methionine (SAM) and taurine metabolism; and FA, dicarboxylate.
Discussion
Metabolomics analyses of RCfT provide a powerful tool for identifying metabolites that change with the intake of DPs [[43], [44], [45], [46]]. In the current study, we found that consuming a DP high in foods classified as ultraprocessed, according to Nova, for 2 wk had a measurable impact on the plasma and urine metabolomes in a diverse group of generally healthy adults. Hundreds of plasma and urine metabolites associated with a UPF-DP included both endogenous and exogenous compounds and represented a wide range of biochemical pathways. Similar within-individual metabolite changes were observed across plasma and urine. Specifically, metabolites related to benzoate preservatives and industrial ingredients (artificial sweeteners and flavorings) were identified as being higher after the UPF-DP than the UN-DP. These results suggest that ingredients common to UPF [5] affect the human metabolome and justify further research as dietary biomarkers of a UPF-DP. In addition, numerous perturbations in metabolites of human and microbial origin suggest that UPF intake broadly impacts human metabolism in the short term.
Sodium and potassium benzoate are widely used preservatives in the United States food supply [47,48]. During the UPF-DP, ginger ale, Fig Newtons, and margarine contained benzoate preservatives, and 1 or more of these items were provided each day. No benzoate preservatives were listed as ingredients for foods or beverages consumed during UN-DP, which may explain higher concentrations of 2-methoxyhydroquinone sulfate [1], 4-ethylphenyl sulfate, and 4-vinylphenol sulfate during the UPF-DP than UN-DP. Prior studies have reported that 4-ethylphenyl sulfate, which is produced by gut bacteria, is associated with soy intake [49,50] and sodium restriction [51]. Changes in benzoate metabolites can also occur with the consumption of high-protein/low-carbohydrate DPs [52,53]. Protein intake was similar across UPF-DP and UN-DP, but participants consumed ∼230 kcal more from carbohydrates during UPF-DP than UN-DP. Thus, macronutrient differences could have influenced benzoate metabolism, independent of processing. However, our results suggest that benzoate metabolites may indicate intake of foods classified as ultraprocessed by Nova because these foods commonly contain sodium and potassium benzoate preservatives.
Several compounds that are hallmark properties of foods classified as UPF differed between the DPs. For example, the following metabolites were higher after UPF-DP than UN-DP: acesulfame, an artificial sweetener; methyl vanillate sulfate, a flavoring agent; and chemically modified hydroxylated lecithin, a common emulsifier, all of which are additives indicative of UPF according to Nova. In contrast, several metabolites that were lower after UPF-DP compared with UN-DP are associated with fruit and vegetable intake (cinnamoylglycine [plant foods [54]], tartarate [grapes [55]]; 4-allylphenol sulfate [bananas and apples [56]]; allantoic acid [peaches, asparagus, and tomatoes [57]] hydroxypropanedioic acid [potatoes [58]]; S-allylcysteine [onions and garlic [59]]) or with DPs high in fruits and vegetables (2-aminophenol sulfate, threonate derivatives, and glyceric acid [45]). The UN-DP meals were markedly higher in minimally processed fruits and vegetables than the UPF-DP meals. According to Nova, fruits and vegetables can be classified as minimally processed (group 1), processed (group 3), or ultraprocessed (group 4), depending on formulations and added ingredients. Therefore, these xenobiotics are likely not specific to minimally processed DPs but may be useful to distinguish ultraprocessed from minimally processed DPs in community-dwelling populations.
Plasma better reflects endogenous metabolism, whereas urine better captures the metabolism of recent dietary intake [45]. Nevertheless, some lipid compounds consistently differed in both sample types, including metabolites related to BAs and trimethylamine N-oxide (TMAO). Most BAs (as identified by Metabolon) from plasma (n = 10) and urine (n = 2) in our study were lower after UPF-DP, despite ∼280 kcal more energy from total fat and ∼20 g more saturated fat consumed during UPF-DP than UN-DP. In line with our findings, another RCfT found that a DP characterized by whole grains, legumes, and fruits and vegetables compared with a DP high in refined grains and added sugars led to modest increases in taurocholic, glycocholic, and taurolithocholic acid [60]. However, plasma cholic acid (a major primary BA) was higher after the UPF-DP than UN-DP, which complements results from prior RCfTs assessing fecal BA changes following Western animal-based DPs compared with rural African plant-based DPs [61,62]. Although fecal BAs may increase with higher-fat intake [63], associations between dietary fat intake and circulating BA concentrations are inconsistent [64]. For example, serum BA concentrations were positively associated with trans-fat and polyunsaturated fat intake but inversely associated with monounsaturated fat intake among fasting men but not among nonfasting men and women [65]. Future studies, including blood and stool collections, must clarify the relationship between dietary fat intake and gut microbial metabolites, such as secondary BAs. TMAO was also consistently lower after UPF-DP than UN-DP in both plasma and urine. TMAO has been associated with fish and whole-grain intake [66]. Seafood (cod, shrimp, and salmon) was presented to participants 3 times/wk but not consumed during UPF-DP. Whole grains (farro, oatmeal, bulgur, and quinoa) were presented 9 times/wk during UN-DP, and foods containing whole-grain flour were presented 3 times/wk during UPF-DP. Thus, it is likely that TMAO concentrations reflect specific foods consumed rather than the Nova classification.
We identified hundreds of metabolites that varied with UPF intake in a tightly controlled domiciled feeding study. In contrast, recently published cross-sectional analyses found that the percentage of energy from UPF intake, estimated using FFQ data, was correlated with a handful of circulating metabolites. More specifically, 12 metabolites (out of 359 measured) were associated with UPF intake in a subset of participants from the ARIC [67]. Of the 12 metabolites, 11 were measured in plasma samples in our study, but only results for stachydrine, a proposed biomarker of citrus fruits and the DASH DP [30,32,49,68], replicated across studies. The second study targeted 2 metabolites that are linked to food processing in a subset of participants from the European Prospective Investigation into Cancer and Nutrition cohort [69]. First, plasma elaidic acid did not differ between DPs in our study. The second, urinary 4-methyl syringol sulfate, was not identified in our study, but concentrations of a related urinary metabolite, syringol sulfate, were higher after the UPF-DP than the UN-DP. Another study used proton nuclear magnetic resonance spectroscopy and identified 231 compounds associated with UPF intake in children but had limited overlap (with the exception of tyrosine metabolism) with our results, likely because of the differences in study design, analytic methods, and population [70]. These studies suggest that candidate biomarkers of UPF intake can be observed in free-living populations, but each of these studies had critical limitations, including their observational designs that cannot establish temporality or be used to infer causality. Future population-based studies with detailed data from multiple 24-h recalls or food records and serially collected blood and urine specimens need to replicate and extend findings from our controlled study.
Designing menus of energy-matched DPs for RCfT is challenging because multiple aspects of intake are changed when 1 food is increased or decreased [71]. The 2 DPs in our study differed in aspects aside from Nova classification, including dietary quality, types and amounts of food groups, and energy density, which may also have contributed to observed metabolite differences. The objective of the current study was to identify metabolomic differences in response to DPs based on the overall percent of EI from foods classified as ultraprocessed, according to Nova. To further elucidate metabolites related to Nova classification independent of variations in food type, future studies are needed to compare similar foods or food groups across Nova categories. For example, comparing metabolomic profiles of grains that are Nova group 1 (eg, minimally processed oatmeal) compared with Nova group 4 (eg, ultraprocessed ready-to-eat granola) or of consuming foods or DPs with and without hallmark UPF ingredients or formulations (eg, emulsifiers, artificial sweeteners). Identifying metabolite markers of UPF intake or metabolic response to UPF intake could facilitate future investigation into UPF intake and health studies and clarify whether food processing classification systems add value beyond current nutrient-based classification systems for dietary advice [20,21].
Strengths of this study include participants that were domiciled in a clinical research center, all foods and beverages were provided, intake was monitored by research staff, and analyses were adjusted for differential EIs between intervention phases. However, the lack of a washout period may be a limitation because of unknown half-lives of many of the metabolites of interest, even in the absence of statistical support for a carry-over effect (which may be because of low power for covariates other than the main effect). Further, the foods provided were classified as ultraprocessed according to Nova using ingredient lists and labels, and the presented food was matched for macronutrients and energy. However, meals were consumed ad libitum inducing differences in energy and macronutrient intake [29]. The main results adjusted for calculated EI were similar to the supplemental results adjusted for measured changes in body weight. However, we cannot rule out that noted metabolite differences were not influenced by differences in intake. Further, there are several classification systems for categorizing processed foods and beverages, and agreement within and across classification systems is limited [[72], [73]]. Our results are based on foods classified as ultraprocessed according to Nova, and participants in our study consumed only 1 UPF-DP. However, there are many ways in which someone could consume 80% of EI from foods classified as ultraprocessed, and diets likely differ by availability and individual preferences. Our study showed that numerous plasma and urine metabolites differed in the short term as a result of the UPF-DP, but, given the small sample size and experimental crossover design, it is not suited for UPF-DP prediction, nor can it address long-term metabolite variability. Thus, future research, preferably in larger cohort studies where metabolomic profiles and diet patterns may each be longitudinal, is required to: extend our findings to other food processing classification systems, varying UPF-DPs, and diverse populations with a range of food preferences; explore prediction models for UPF-DPs; and estimate long-term stability (eg, 1-y intraclass correlation coefficients) for UPF-related metabolites to understand better if they are well suited for long-term risk estimation. Finally, RCfTs, preferably including appropriate washout periods, will need to be conducted to test whether a dose-response relationship between candidate biomarkers of a UPF-DP accurately discriminates between varying amounts (eg, 0%en, 40%en, and 80%en) of UPF intake.
In conclusion, our results suggest that a DP high in, compared with 1 void of, foods classified as ultraprocessed according to Nova had a measurable impact on the plasma and urine metabolomes of generally healthy adults in the short term. Metabolites related to benzoate-containing preservatives, artificial sweeteners, and flavoring agents identified in this exploratory analysis should be considered as candidates in future studies that are designed to validate biomarkers of DPs high in foods classified as ultraprocessed.
Funding
The research leading to these results has received funding from the Intramural Research Program at NCI and NIDDK.
Author disclosures
LEO is principal investigator on a grant administered by the United States Department of Agriculture's National Institute of Food and Agriculture (USDA-NIFA #2022-07671) to coordinate a workshop to establish a research roadmap for the future of food processing, processed food, and human health research. Collaborators on this grant include scientists from Archer Daniels Midland (ADM) with special input from General Mills and Academic Institutions. All other authors report no conflicts of interest. The findings in this article of those of the authors and do not necessarily represent the official position of the National Institutes of Health or USDA. The other authors report no conflicts of interest.
Author contribution
The authors’ responsibilities were as follows – LEO, EL, and KDH: designed research; LEO, EL, and HGH: analyzed data; LEO and EL: wrote the paper with editorial assistance from all coauthors; KDH, STC, MS, and ABC: designed the original trial and collected all samples; PSA and HGH: provided guidance on the statistical analysis and writing of the paper, and all authors: read and approved the final manuscript.
Data availability
Data described in the manuscript will be made available upon request to EL (erikka.loftfield@nih.gov) pending proposal approval and completion of a Data Transfer Agreement via the NCI. Recipes for provided foods are available upon reasonable request to KDH (kevinh@niddk.nih.gov).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.06.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Weaver C.M., Dwyer J., Fulgoni V.L., 3rd, King J.C., Leveille G.A., MacDonald R.S., et al. Processed foods: contributions to nutrition. Am. J. Clin. Nutr. 2014;99(6):1525–1542. doi: 10.3945/ajcn.114.089284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteiro C.A., Canno G., Moubarac J.C., Levy R.B., Louzada M.L.C., Jaime P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. doi: 10.1017/S1368980017000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . 2021. Report of the technical consultation on measuring healthy diet: concepts, methods, and metrics. May 18–20. [Google Scholar]
- 4.Monteiro C.A., Cannon G., Levy R.B., Moubarac J.C., Louzada M.L., Rauber F., et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–941. doi: 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srour B., Kordahi M.C., Bonazzi E., Deschasaux-Tanguy M., Touvier M., Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol. Hepatol. 2022;7(12):1128–1140. doi: 10.1016/S2468-1253(22)00169-8. [DOI] [PubMed] [Google Scholar]
- 6.Juul F., Parekh N., Martinez-Steele E., Monteiro C.A., Chang V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022;115(1):211–221. doi: 10.1093/ajcn/nqab305. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Martínez Steele E., Du M., Pomeranz J.L., O’Connor L.E., Herrick K.A., et al. Trends in consumption of ultraprocessed foods among US youths aged 2-19 years, 1999-2018. JAMA. 2021;326(6):519–530. doi: 10.1001/jama.2021.10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor L., Martinez-Steele E., Wang L., Zhang F.F., Herrick K. Contribution of ultra-processed foods to complimentary foods and beverage intake among US infants and toddlers according to NOVA. Curr. Dev. Nutr. 2022;6(Suppl 1):936. [Google Scholar]
- 9.Neri D., Steele E.M., Khandpur N., Cediel G., Zapata M.E., Rauber F., et al. Ultraprocessed food consumption and dietary nutrient profiles associated with obesity: A multicountry study of children and adolescents. Obes. Rev. 2022;23(Suppl 1) doi: 10.1111/obr.13387. [DOI] [PubMed] [Google Scholar]
- 10.Juul F., Martinez-Steele E., Parekh N., Monteiro C.A., Chang V.W. Ultra-processed food consumption and excess weight among US adults. Br. J. Nutr. 2018;120(1):90–100. doi: 10.1017/S0007114518001046. [DOI] [PubMed] [Google Scholar]
- 11.Mendonça R.D., Pimenta A.M., Gea A., de la Fuente-Arrillaga C., Martinez-Gonzalez M.A., Lopes A.C., et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-UP (SUN) cohort study. Am. J. Clin. Nutr. 2016;104(5):1433–1440. doi: 10.3945/ajcn.116.135004. [DOI] [PubMed] [Google Scholar]
- 12.Fiolet T., Srour B., Sellem L., Kesse-Guyot E., Allès B., Méjean C., et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Sante prospective cohort. BMJ. 2018;360:k322. doi: 10.1136/bmj.k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romaguera D., Fernández-Barrés S., Gracia-Lavedán E., Vendrell E., Azpiri M., Ruiz-Moreno E., et al. Consumption of ultra-processed foods and drinks and colorectal, breast, and prostate cancer. Clin. Nutr. 2021;40(4):1537–1545. doi: 10.1016/j.clnu.2021.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Srour B., Fezeu L.K., Kesse-Guyot E., Allès B., Méjean C., Andrianasolo R.M., et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Sante) BMJ. 2019;365:l1451. doi: 10.1136/bmj.l1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du S., Kim H., Rebholz C.M. Higher ultra-processed food consumption is associated with increased risk of incident coronary artery disease in the atherosclerosis risk in communities study. J. Nutr. 2021;151(12):3746–3754. doi: 10.1093/jn/nxab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z., Jackson S.L., Martinez E., Gillespie C., Yang Q. Association between ultraprocessed food intake and cardiovascular health in US adults: a cross-sectional analysis of the NHANES 2011-2016. Am. J. Clin. Nutr. 2021;113(2):428–436. doi: 10.1093/ajcn/nqaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H., Hu E.A., Rebholz C.M. Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994) Public Health Nutr. 2019;22(10):1777–1785. doi: 10.1017/S1368980018003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnabel L., Kesse-Guyot E., Allès B., Touvier M., Srour B., Hercberg S., et al. Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Intern. Med. 2019;179(4):490–498. doi: 10.1001/jamainternmed.2018.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rico-Campà A., Martínez-González M.A., Alvarez-Alvarez I., Mendonça R.D., de la Fuente-Arrillaga C., Gómez-Donoso C., et al. Association between consumption of ultra-processed foods and all cause mortality: SUN prospective cohort study. BMJ. 2019;365:l1949. doi: 10.1136/bmj.l1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteiro C.A., Astrup A. Does the concept of “ultra-processed foods” help inform dietary guidelines, beyond conventional classification systems? YES. Am. J. Clin. Nutr. 2022;116(6):1476–1481. doi: 10.1093/ajcn/nqac122. [DOI] [PubMed] [Google Scholar]
- 21.Astrup A., Monteiro C.A. Does the concept of “ultra-processed foods” help inform dietary guidelines, beyond conventional classification systems? NO. Am. J. Clin. Nutr. 2022;116(6):1482–1488. doi: 10.1093/ajcn/nqac123. [DOI] [PubMed] [Google Scholar]
- 22.de Araújo T.P., de Moraes M.M., Afonso C., Santos C., Rodrigues S.S.P. Food processing: comparison of different food classification systems. Nutrients. 2022;14(4):729. doi: 10.3390/nu14040729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braesco V., Souchon I., Sauvant P., Haurogné T., Maillot M., Féart C., et al. Ultra-processed foods: how functional is the NOVA system? Eur. J. Clin. Nutr. 2022;76(9):1245–1253. doi: 10.1038/s41430-022-01099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drewnowski A., Detzel P., Klassen-Wigger P. Perspective: achieving sustainable healthy diets through formulation and processing of foods. Curr. Dev. Nutr. 2022;6(6) doi: 10.1093/cdn/nzac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steele E.M., O’Connor L.E., Juul F., Khandpur N., Galastri Baraldi L., Montiero C.A., et al. Identifying and estimating ultraprocessed food intake in the US NHANES according to the NOVA classification system of food processing. J. Nutr. 2023;153(1):225–241. doi: 10.1016/j.tjnut.2022.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Diet assessment primer. Available from: https://dietassessmentprimer.cancer.gov/roadmap.html.
- 27.Khandpur N., Rossato S., Drouin-Chartier J.P., Du M., Steele E.M., Sampson L., et al. Categorising ultra-processed foods in large-scale cohort studies: evidence from the Nurses’ Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study. J. Nutr. Sci. 2021;10:e77. doi: 10.1017/jns.2021.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall K.D. Challenges of human nutrition research. Science. 2020;367(6484):1298–1300. doi: 10.1126/science.aba3807. [DOI] [PubMed] [Google Scholar]
- 29.Hall K.D., Ayuketah A., Brychta R., Cai H., Cassimatis T., Chen K.Y., et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67–77 e3. doi: 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Playdon M.C., Ziegler R.G., Sampson J.N., Stolzenberg-Solomon R., Thompson H.J., Irwin M.L., et al. Nutritional metabolomics and breast cancer risk in a prospective study. Am. J. Clin. Nutr. 2017;106(2):637–649. doi: 10.3945/ajcn.116.150912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampson J.N., Boca S.M., Shu X.O., Stolzenberg-Solomon R.Z., Matthews C.E., Hsing A.W., et al. Metabolomics in epidemiology: sources of variability in metabolite measurements and implications. Cancer Epidemiol. Biomarkers Prev. 2013;22(4):631–640. doi: 10.1158/1055-9965.EPI-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rebholz C.M., Lichtenstein A.H., Zheng Z., Appel L.J., Coresh J. Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am. J. Clin. Nutr. 2018;108(2):243–255. doi: 10.1093/ajcn/nqy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H., Lichtenstein A.H., Wong K.E., Appel L.J., Coresh J., Rebholz C.M. Urine metabolites associated with the Dietary Approaches to Stop Hypertension (DASH) diet: results from the DASH-sodium trial. Mol. Nutr. Food Res. 2021;65(3) doi: 10.1002/mnfr.202000695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X., Niu L., Clerici C., Russo R., Byrd M., Setchell K.D.R. Data analysis of MS-based clinical lipidomics studies with crossover design: A tutorial mini-review of statistical methods. Clin. Mass. Spectrom. 2019;13:5–17. doi: 10.1016/j.clinms.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D. Shen, Z. Lu, Estimate carryover effect in clinical trial crossover designs. Available from: https://www.lexjansen.com/pharmasug/2006/Posters/PO16.pdf.
- 36.O’Connor L.E., Paddon-Jones D., Wright A.J., Campbell W.W. A Mediterranean-style eating pattern with lean, unprocessed red meat has cardiometabolic benefits for adults who are overweight or obese in a randomized, crossover, controlled feeding trial. Am. J. Clin. Nutr. 2018;108(1):33–40. doi: 10.1093/ajcn/nqy075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connor L.E., Li J., Sayer R.D., Hennessy J.E., Campbell W.W. Short-term effects of healthy eating pattern cycling on cardiovascular disease risk factors: pooled results from two randomized controlled trials. Nutrients. 2018;10(11):729. doi: 10.3390/nu10111725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Connor L.E., Campbell W.W. A novel fiber composite ingredient incorporated into a beverage and bar blunts postprandial serum glucose and insulin responses: a randomized controlled trial. Nutr. Res. 2016;36(3):253–261. doi: 10.1016/j.nutres.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57(1):289–300. [Google Scholar]
- 40.Glickman M.E., Rao S.R., Schultz M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 2014;67(8):850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Cinar O., Viechtbauer W. The poolr Package for Combining Independent and Dependent p values. J. Stat. Softw. 2022;101(1):1–42. [Google Scholar]
- 42.Sumner L.W., Amberg A., Barrett D., Beale M.H., Beger R., Daykin C.A., et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI) Metabolomics. 2007;3(3):211–221. doi: 10.1007/s11306-007-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennan L., Hu F.B., Sun Q. Metabolomics meets nutritional epidemiology: harnessing the potential in metabolomics data. Metabolites. 2021;11(10):709. doi: 10.3390/metabo11100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reisdorph N.A., Hendricks A.E., Tang M., Doenges K.A., Reisdorph R.M., Tooker B.C., et al. Nutrimetabolomics reveals food-specific compounds in urine of adults consuming a DASH-style diet. Sci. Rep. 2020;10(1):1157. doi: 10.1038/s41598-020-57979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H., Rebholz C.M. Metabolomic biomarkers of healthy dietary patterns and cardiovascular outcomes. Curr. Atheroscler. Rep. 2021;23(6):26. doi: 10.1007/s11883-021-00921-8. [DOI] [PubMed] [Google Scholar]
- 46.Liang S., Nasir R.F., Bell-Anderson K.S., Toniutti C.A., O’Leary F.M., Skilton M.R. Biomarkers of dietary patterns: a systematic review of randomized controlled trials. Nutr. Rev. 2022;80(8):1856–1895. doi: 10.1093/nutrit/nuac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Safety evaluation of certain food additives and contaminants. WHO FOOD ADDITIVES SERIES: 71World Health Organization. Available at: http://apps.who.int/iris/bitstream/10665/198360/1/9789240694897_eng.pdf?ua=1.
- 48.Darch M., Martyn D., Ngo K., Jack M.M. An updated estimate of benzoate intakes from non-alcoholic beverages in Canada and the United States. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2021;38(5):701–717. doi: 10.1080/19440049.2020.1859624. [DOI] [PubMed] [Google Scholar]
- 49.Guertin K.A., Moore S.C., Sampson J.N., Huang W.Y., Xiao Q., Stolzenberg-Solomon R.Z., et al. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am. J. Clin. Nutr. 2014;100(1):208–217. doi: 10.3945/ajcn.113.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pallister T., Jennings A., Mohney R.P., Yarand D., Mangino M., Cassidy A., et al. Characterizing blood metabolomics profiles associated with self-reported food intakes in female twins. PLOS ONE. 2016;11(6) doi: 10.1371/journal.pone.0158568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derkach A., Sampson J., Joseph J., Playdon M.C., Stolzenberg-Solomon R.Z. Effects of dietary sodium on metabolites: the Dietary Approaches to Stop Hypertension (DASH)-Sodium Feeding Study. Am. J. Clin. Nutr. 2017;106(4):1131–1141. doi: 10.3945/ajcn.116.150136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rebholz C.M., Zheng Z., Grams M.E., Appel L.J., Sarnak M.J., Inker L.A., et al. Serum metabolites associated with dietary protein intake: results from the Modification of Diet in Renal Disease (MDRD) randomized clinical trial. Am. J. Clin. Nutr. 2019;109(3):517–525. doi: 10.1093/ajcn/nqy202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H., Lichtenstein A.H., White K., Wong K.E., Miller E.R., 3rd, Coresh J., et al. Plasma metabolites associated with a protein-rich dietary pattern: results from the OmniHeart trial. Mol. Nutr. Food Res. 2022;66(6) doi: 10.1002/mnfr.202100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Metabocard for cinnamoylglycine (HMDB0011621). Human Metabolome Database. Available from: https://hmdb.ca/metabolites/HMDB0011621.
- 55.Regueiro J., Vallverdú-Queralt A., Simal-Gándara J., Estruch R., Lamuela-Raventós R.M. Urinary tartaric acid as a potential biomarker for the dietary assessment of moderate wine consumption: a randomised controlled trial. Br. J. Nutr. 2014;111(9):1680–1685. doi: 10.1017/S0007114513004108. [DOI] [PubMed] [Google Scholar]
- 56.Nieman D.C., Gillitt N.D., Sha W., Meaney M.P., John C., Pappan K.L., et al. Metabolomics-based analysis of banana and pear ingestion on exercise performance and recovery. J. Proteome. Res. 2015;14(12):5367–5377. doi: 10.1021/acs.jproteome.5b00909. [DOI] [PubMed] [Google Scholar]
- 57.Metabocard for allantoic acid (HMDB0001209). Human Metabolome Database. Available from: https://hmdb.ca/metabolites/HMDB0001209.
- 58.Metabocard for Hydroxypropanedioic acid (HMDB0035227). The human metabolome database. Available from: https://hmdb.ca/metabolites/HMDB0035227.
- 59.Metabocard for S-Allylcysteine (HMDB0034323). The human metabolome database. Available from: https://hmdb.ca/metabolites/HMDB0034323.
- 60.Ginos B.N.R., Navarro S.L., Schwarz Y., Gu H., Wang D., Randolph T.W., et al. Circulating bile acids in healthy adults respond differently to a dietary pattern characterized by whole grains, legumes and fruits and vegetables compared to a diet high in refined grains and added sugars: a randomized, controlled, crossover feeding study. Metabolism. 2018;83:197–204. doi: 10.1016/j.metabol.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Keefe S.J., Li J.V., Lahti L., Ou J., Carbonero F., Mohammed K., et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wan Y., Yuan J., Li J., Li H., Zhang J., Tang J., et al. Unconjugated and secondary bile acid profiles in response to higher-fat, lower-carbohydrate diet and associated with related gut microbiota: A 6-month randomized controlled-feeding trial. Clin. Nutr. 2020;39(2):395–404. doi: 10.1016/j.clnu.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 64.Farhat Z., Sampson J.N., Hildesheim A., Safaeian M., Porras C., Cortés B., et al. Reproducibility, temporal variability, and concordance of serum and fecal bile acids and short chain fatty acids in a population-based study. Cancer Epidemiol. Biomarkers Prev. 2021;30(10):1875–1883. doi: 10.1158/1055-9965.EPI-21-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Byrd D.A., Sinha R., Weinstein S.J., Albanes D., Freedman N.D., Sampson J., et al. An investigation of cross-sectional associations of a priori-selected dietary components with circulating bile acids. Am. J. Clin. Nutr. 2021;114(5):1802–1813. doi: 10.1093/ajcn/nqab232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costabile G., Vetrani C., Bozzetto L., Giacco R., Bresciani L., Del Rio D., et al. Plasma TMAO increase after healthy diets: results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am. J. Clin. Nutr. 2021;114(4):1342–1350. doi: 10.1093/ajcn/nqab188. [DOI] [PubMed] [Google Scholar]
- 67.Su D., Chen J., Du S., Kim H., Yu B., Wong K.E., et al. Metabolomic markers of ultra-processed food and incident CKD. Clin. J. Am. Soc. Nephrol. 2023;18(3):327–336. doi: 10.2215/CJN.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazzilli K.M., McClain K.M., Lipworth L., Playdon M.C., Sampson J.N., Clish C.B., et al. Identification of 102 correlations between serum metabolites and habitual diet in a metabolomics study of the prostate, lung, colorectal, and ovarian cancer trial. J. Nutr. 2020;150(4):694–703. doi: 10.1093/jn/nxz300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huybrechts I., Rauber F., Nicolas G., Casagrande C., Kliemann N., Wedekind R., et al. Characterization of the degree of food processing in the European Prospective Investigation into Cancer and Nutrition: application of the Nova classification and validation using selected biomarkers of food processing. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.1035580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Handakas E., Chang K., Khandpur N., Vamos E.P., Millett C., Sassi F., et al. Metabolic profiles of ultra-processed food consumption and their role in obesity risk in British children. Clin. Nutr. 2022;41(11):2537–2548. doi: 10.1016/j.clnu.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y., Hill E.R., Campbell W.W., O’Connor L.E. Plant- and animal-based protein-rich foods and cardiovascular health. Curr. Atheroscler. Rep. 2022;24(4):197–213. doi: 10.1007/s11883-022-01003-z. [DOI] [PubMed] [Google Scholar]
- 72.Bleiweiss-Sande R., Chui K., Evans E.W., Goldberg J., Amin S., Sacheck J. Robustness of food processing classification systems. Nutrients. 2019;11(6):1344. doi: 10.3390/nu11061344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez-Perez C., San-Cristóbal R., Guallar-Castillon P., Martinez-Gonzalez M.A., Salas-Salvado J., Corella D., et al. Use of different food classification systems to assess the association between ultra-processed food consumption and cardiometabolic health in an elderly population with metabolic syndrome (PREDIMED-Plus Cohort) Nutrients. 2021;13(7):2471. doi: 10.3390/nu13072471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript will be made available upon request to EL (erikka.loftfield@nih.gov) pending proposal approval and completion of a Data Transfer Agreement via the NCI. Recipes for provided foods are available upon reasonable request to KDH (kevinh@niddk.nih.gov).