Abstract
Cognitive impairment and post-concussive symptoms (PCS) represent hallmark sequelae of pediatric mild traumatic brain injury (pmTBI). Few studies have directly compared cognition as a function of PCS status longitudinally. Cognitive outcomes were therefore compared for asymptomatic, symptomatic pmTBI, and healthy controls (HC) during sub-acute (SA; 1-11 days) and early chronic (EC; approximately 4-months) post-injury phases. We predicted worse cognitive performance for both pmTBI groups relative to HC at the SA visit. At the EC visit we predicted continued impairment from the symptomatic group, but no difference between asymptomatic pmTBI and HCs. A battery of clinical (semi-structured interviews and self-report questionnaires) and neuropsychological measures were administered to 203 pmTBI and 139 HC participants, with greater than 80% retention at the EC visit. A standardized change method classified pmTBI into binary categories of asymptomatic or symptomatic based on PCS scores. Symptomatic pmTBI performed significantly worse than HCs on processing speed, attention and verbal memory at SA visit, whereas lower performance was only present for verbal memory for asymptomatic pmTBI. Lower performance in verbal memory persisted for both pmTBI groups at the EC visit. Surprisingly, a minority (16%) of pmTBI switched from asymptomatic to symptomatic status at the EC visit. Current findings suggest that PCS and cognition are more closely coupled during the first week of injury but become decoupled several months post-injury. Evidence of lower performance in verbal memory for both asymptomatic and symptomatic pmTBI suggests that cognitive recovery may be a process separate from the resolution of subjective symptomology.
Keywords: Neuropsychological testing, Adolescent, Recovery, Head injury
Introduction
There is a growing body of literature on pediatric mild traumatic brain injuries (pmTBI; used synonymously with concussion in the current manuscript), with approximately 2.5 million TBI-related emergency department visits and 282,000 TBI-related hospitalizations per year in the U.S. alone (Taylor et al., 2017). Children have some of the highest rates for emergency department visits, particularly the 0-4 year old and 15-24 year old age groups having 1,591.5 per 100,000 and 1,080.7 per 100,000 respectively (Taylor et al., 2017). Clinical recovery is typically determined by symptom resolution, normalization of physical examination findings, and tolerance for usual daily activities (Silverberg et al., 2020). The Center for Disease Control and several expert consensus panels (e.g., Concussion in Sport Group, Four Corners Youth Consortium) suggest using symptom scales to diagnose mTBI, and provide instructions on return to activity based on the patient’s self-reported post-concussive symptoms (PCS; Lumba-Brown et al., 2018; McCrory et al., 2017; Rivara et al., 2020). Cognition can be considered a domain in symptom presentation (e.g., difficulty concentrating, mental fogginess) on some symptom inventories, but it can also be measured objectively through neuropsychological testing. While many studies have explored cognition (Babikian et al., 2011; Babikian et al., 2013; Konrad et al., 2011) and PCS (Barlow et al., 2015; de Koning et al., 2018; Ewing-Cobbs et al., 2018; Ledoux et al., 2018; Taylor et al., 2010) separately in pmTBI, few studies have directly compared cognition as a function of symptom status longitudinally.
The typical course of recovery from mTBI is symptomatic to asymptomatic, with a varying timeline of recovery for each individual. Rapid recovery can occur within a few days to a few weeks (Lau et al., 2011; Lau et al., 2012; Mayer et al., 2017), while children are posited to have a slightly longer recovery period (~4 weeks) relative to adults (McCrory et al., 2017; Zemek et al., 2016). The nature of persistent post-concussion symptoms (PPCS) has long been a topic of debate among mTBI researchers (Ewing-Cobbs et al., 2018; Novak et al., 2016; Zemek et al., 2016), in part due to the poor psychometric properties associated with PCS reporting (Iverson et al., 2015; Mayer et al., 2020). Specifically, test-retest reliability of symptom reporting is typically lower than recommended levels over prolonged periods of time (Hergert et al., 2021; Mayer et al., 2020), and has been shown to be subject to a number of different biases (Chrisman et al., 2013; Mittenberg et al., 1992). Specificity/sensitivity is strongly affected by premorbid factors when defining PPCS (Iverson et al., 2015), and the “rate” of PPCS has been shown to vary by over 49% in the same sample depending on the way that it is calculated (Mayer et al., 2020). Finally, other studies have reported that anywhere from 10% (Barlow et al., 2015) to 50% (de Koning et al., 2018) of “asymptomatic” pmTBI patients as measured at the sub-acute injury stage may transition to “symptomatic” status at longer follow-up periods.
Similarly, cognitive deficits and PCS burden are expected to be more severe during the acute and semi-acute stages of injury, and to decrease as a function of time post-injury and natural recovery (Babikian et al., 2011; Ewing-Cobbs et al., 2018; McCrory et al., 2017). Mixed evidence has been reported in terms of the relationship between PCS and cognitive performance (Babikian et al., 2013; Beauchamp et al., 2018; Hanna-Pladdy et al., 2001; Sicard et al., 2020). Cognition was found to be predictive of PCS load when used acutely in the emergency department (Brooks et al., 2016), whereas other studies have reported that measures of cognition are not predictive of PCS load in the sub-acute phase (Takagi et al., 2020; Teh et al., 2020). When using a group of asymptomatic concussed athletes for comparison, one study noted that the asymptomatic group performed significantly better on memory, visual memory, reaction time, and processing speed during computerized testing relative to a symptomatic group, but had worse performance than controls across all composite scores (Fazio et al., 2007). Another study that examined adult athletes before the start of the season and again within 11 days of their injury found that the symptomatic group had impairments with simple motor and attentional domains of cognition after concussion, while the asymptomatic group had impairments on a test of divided attention (Collie et al., 2006). Importantly, a number of these studies were cross-sectional (Hanna-Pladdy et al., 2001; Kunker et al., 2020; Yumul et al., 2020) or focused on sport-related concussion in older groups (Collie et al., 2006; Fazio et al., 2007), such that findings may not generalize to pediatric samples.
The current study compared cognitive performance in asymptomatic and symptomatic pmTBI participants over a 4-month period relative to a large sample of matched healthy controls (HC). PCS burden was separately determined at both the sub-acute (SA: within 11 days of injury) and early chronic (EC: approximately 4 months post-injury) visits. We predicted symptomatic pmTBI participants would exhibit worse cognitive performance relative to asymptomatic pmTBI participants and HC, with asymptomatic patients exhibiting impairments relative to HC only at the SA visit.
Materials and Methods
Participants
Two-hundred and twelve adolescents diagnosed with pmTBI (12–18 years) were consecutively recruited from local emergency department and urgent care settings between July 2016 and July 2020 for this prospective cohort study (see Figure 1). Inclusion criteria were based on the American Congress of Rehabilitation Medicine (Kay et al., 1993) and Zurich Concussion in Sport Group guidelines (McCrory et al., 2013) guidelines. Specifically, all enrolled pmTBI participants experienced head trauma resulting in a Glasgow Coma Score ≥13, an alteration in mental status or at least two new post-concussive symptoms, a loss of consciousness (LOC) limited to 30 minutes (if present), or post-traumatic amnesia limited to 24 hours (if present). In addition, 149 age-matched HC participants were recruited from the local community and evaluated at equivalent time-points.
Figure 1.
Flow chart for participant inclusion/exclusion after enrollment.
Exclusion criteria for both pmTBI and HC participants were as follows: 1) a history of neurological diagnosis, previous moderate or severe TBI with >30 minutes LOC, autism spectrum disorder or intellectual disability, any psychiatric disorders other than adjustment disorder, or substance abuse/dependence, or 2) a non-English speaking child or guardian. HC participants were also excluded if they received a diagnosis of attention-deficit/hyperactivity disorder (ADHD), or a learning disability (LD), or if they were wearing orthodontic braces at time of their visit, as the current sample is a part of a parent study that includes neuroimaging. Urine-based drug screens were conducted for all participants at both visits, and participants with positive results (N=6) were excluded from the current study. The study was approved by the Institutional Review Board of the University of New Mexico Health Sciences Center. All participants provided informed consent or assent according to institutional guidelines at the University of New Mexico School of Medicine.
The SA sample consisted of 203 pmTBI (94 females; age 14.88±1.95 years; 7.34±2.27 days post-injury) and 139 HC (60 females; age 14.83±2.06 years) after accounting for incomplete data collection and exclusions. Study attrition and additional exclusions (change in medical status, drug use) resulted in a sample of 166 pmTBI (80 females; age 14.73±1.94 years; 132.05±19.68 days post-injury) and 126 HC (55 females; age 14.81±2.0 years) at the EC visit (see Figure 1).
Procedures
A Common Data Elements battery of tests were administered at SA/EC visits. The Post-Concussion Symptom Inventory (PCSI) was selected as primary clinical outcome measure. Symptomatic versus asymptomatic PCS status was calculated (see below) based on HC data (Mayer et al., 2020). A modified version of the 5P clinical risk score was also calculated (see Supplemental Materials) in order to stratify the risk of PPCS. A battery of neuropsychological tests was also administered to determine potential cognitive deficits in primary (attention and processing speed) and secondary (working memory, executive functioning, and verbal memory) cognitive domains, as well as estimates of effort (TOMMe10; Denning, 2012; Donders and Gardner, 2020; Loughan et al., 2016) and reading ability (WRAT-4; Wilkinson and Robertson, 2006). DKEFS color-word interference conditions 1-3 comprised the attention domain, whereas WAIS-IV/WISC-V digit symbol coding and symbol search comprised processing speed. Similarly, WISC-V/WAIS-IV digit span backwards trial comprised working memory, DKEFS trail making test condition 4, verbal fluency, color-word interference condition 4 comprised executive function, and HVLT Delay (forms 1 & 4) was used for verbal memory recall.
A standardized model was used to classify pmTBI participants as asymptomatic or symptomatic at each visit (Mayer et al., 2020), using the following method:
Briefly, this method determines symptom status by taking the sum of self-reported PCSI ratings, calculating the common logarithm (Log10) to reduce skew, standardizing (z-scores) based on HC ratings (classification cutoff z≈1.64) and adjusting z-scores for distributional bias.
Statistical Analyses
Demographic information from asymptomatic pmTBI, symptomatic pmTBI, and HC participants was compared through a series of one-way ANOVAs, Kruskal-Wallis, or chi-squared tests, as appropriate. Differences in returning versus non-returning pmTBI (i.e., study attrition) were also examined for the SA visit. One-way ANCOVAs were performed for each cognitive composite (processing speed, attention, executive functioning, and working memory) and verbal memory scores at both visits using the SA WRAT-4-Word Reading subtest t-score as a covariate. Where available, normative data were used to transform cognitive measures. However, the HVLT-R has not been normed for the full range of included ages, and therefore was not normalized. Two-way random effect, absolute agreement, single measure model intraclass correlation coefficients were calculated on the WRAT-4 scores to establish reliability between SA and EC visits (see Supplemental Materials for more details).
Except for demographic comparisons, Bonferroni corrections (0.05/5 cognitive domains=0.01) were used to control for family-wise error separately at each visit. Partial eta-squared (partial-η2) are reported as measures of effect size using the following guidelines: η2<0.06=small; η2=0.06-0.14=medium, η2>0.14=large (Cohen, 1988). All statistical analyses were performed using IBM SPSS Statistics for Windows, version 20 (IBM Corp., Armonk, NY, USA).
Results
SA Visit
One-hundred and twenty-two pmTBI patients were classified as asymptomatic and 81 as symptomatic at the SA visit (Mayer et al., 2020). Omnibus tests indicated no significant differences in age or handedness across groups (all p’s≥0.169), and no differences in type or mechanism of injury between asymptomatic and symptomatic pmTBI participants (p’s≥0.109; see Table 1). Although HC were matched on sex relative to the combined pmTBI group, a greater number of female participants comprised the symptomatic group at the SA visit (χ2=5.96, p=0.015). HC had significantly higher parental education relative to both pmTBI groups (Wald-χ2’s = 23.13-24.08 p’s<0.001), with no difference observed between symptomatic and asymptomatic pmTBI groups (Wald-χ2=0.52, p=0.820). HC also had higher WRAT-4 scores relative to both pmTBI groups (all p’s<0.001), with no significant differences between asymptomatic and symptomatic groups (see Supplemental Materials for results on WRAT-4 reliability). Removal of two symptomatic and two asymptomatic patients scoring abnormally low on a measure of effort (<8 on TOMMe10) during the SA visit did not influence primary results. The groups significantly differed (all p’s<0.001) with respect to their 5P risk score in the expected direction (symptomatic pmTBI > asymptomatic pmTBI > HC). Retrospective PCSI scores also differed significantly, as expected based on the literature (Ewing-Cobbs et al., 2018; Ledoux et al., 2022; Mayer et al., 2020).
Table 1.
Demographics and injury characteristics.
| Variable | SA | EC | ||||||
|---|---|---|---|---|---|---|---|---|
| HC | Asym | Sym | p | HC | Asym | Sym | p | |
| n=139 | n=122 | n=81 | n=126 | n=100 | n=66 | |||
| Age, Mean (SD) | 14.83 (2.1) | 14.68 (2.0) | 15.17 (1.8) | 0.223 | 14.81 (2.0) | 14.56 (2.0) | 15.00 (1.8) | 0.351 |
| Ethnicity, n (%) | 0.133 | 0.191 | ||||||
| Hispanic/Latino | 72 (51.8) | 79 (64.8) | 47 (58.0) | 65 (51.6) | 64 (64.0) | 39 (59.1) | ||
| Non-Hispanic/Latino | 67 (48.2) | 42 (34.4) | 33 (40.7) | 61 (48.4) | 35 (35.0) | 27 (40.9) | ||
| Unknown | 0 (0.0) | 1 (0.8) | 1 (1.2) | 0 (0.0) | 1 (1.0) | 0 (0.0) | ||
| Race, n (%) | 0.000 | 0.000 | ||||||
| Black/African-American | 2 (1.4) | 1 (0.8) | 7 (8.6) | 2 (1.6) | 1 (1.0) | 7 (10.6) | ||
| White | 126 (90.6) | 95 (77.9) | 54 (66.7) | 115 (91.2) | 76 (76.0) | 45 (68.2) | ||
| Asian | 1 (0.7) | 0 (0.0) | 3 (3.7) | 1 (0.8) | 0 (0.0) | 1 (1.5) | ||
| Native Hawaiian/other Pacific Islander | 1 (0.7) | 0 (0.0) | 3 (3.7) | 1 (0.8) | 0 (0.0) | 2 (3.0) | ||
| American Indian or Alaska Native | 3 (2.2) | 16 (13.1) | 10 (12.3) | 3 (2.4) | 14 (14.0) | 7 (10.6) | ||
| Multiple | 6 (4.3) | 10 (8.2) | 4 (4.9) | 4 (3.2) | 9 (9.0) | 4 (6.1) | ||
| Female sex, n (%) | 60 (43.2) | 48 (39.3) | 46 (57.8) | 0.043 | 55 (43.7) | 44 (44.0) | 36 (54.5) | 0.305 |
| WRAT-4 T-scores | 56.0 (11.0) | 50.18 (10.3) | 48.80 (9.8) | 0.000 | 55.83 (10.9) | 51.15 (10.2) | 48.88 (10.5) | 0.000 |
| PCSI Total, Mean (SD) | 19.0 (20.7) | 22.9 (20.6) | 48.0 (15.4) | 0.000 | 19.7 (18.6) | 21.2 (19.3) | 24.8 (19.4) | 0.217 |
| Parental Ed, Mean (SD) | 4.08 (1.3) | 3.39 (1.3) | 3.40 (1.2) | 0.000 | 4.07 (1.3) | 3.38 (1.4) | 3.42 (1.2) | 0.000 |
| ADHD, n (%) | - | 13 (10.7) | 6 (7.4) | 0.437 | - | 7 (7.0) | 5 (7.6) | 0.889 |
| LD, n (%) | - | 22 (18.0) | 10 (12.3) | 0.276 | - | 19 (19.0) | 9 (13.6) | 0.366 |
| LOC, n (%) | - | 63 (51.6) | 42 (51.9) | 0.976 | - | 49 (49.0) | 32 (48.5) | 0.948 |
| PTA, n (%) | - | 41 (33.6) | 36 (44.4) | 0.119 | - | 34 (34.0) | 30 (45.5) | 0.138 |
| Injury Cause, n (%) | 0.664 | 0.912 | ||||||
| Struck by object | - | 19 (15.6) | 12 (14.8) | 15 (15.0) | 11 (16.7) | |||
| Fall | - | 28 (23.0) | 21 (25.9) | - | 26 (26.0) | 19 (28.8) | ||
| Struck by person | - | 25 (20.5) | 16 (19.8) | - | 21 (21.0) | 13 (19.7) | ||
| MVC | - | 34 (28.0) | 23 (28.4) | - | 26 (26.0) | 18 (27.3) | ||
| Assault | - | 6 (4.9) | 7 (8.6) | - | 4 (4.0) | 3 (4.5) | ||
| Bicycle related | - | 9 (7.4) | 2 (2.5) | - | 7 (7.0) | 2 (3.0) | ||
| Other | - | 1 (0.8) | 0 (0.0) | - | 1 (1.0) | 0 (0.0) | ||
| Sport-related, n (%) | - | 76 (62.3) | 41 (50.6) | 0.099 | - | 64 (64.0) | 36 (54.5) | 0.223 |
Notes: HC-healthy control; Asym-asymptomatic; Sym-symptomatic; PCSI – post concussion symptom inventory; ADHD – attention-deficit/hyperactivity disorder; LD – learning disability; LOC- loss of consciousness; PTA- post traumatic amnesia; MVC- motor vehicle crash; SA- sub acute; EC- early chronic; ISCED Cat = International Standard Classification of Education category. Parental education categories are based on the ISCED levels. Asym and Sym classifications at both visits are based on symptom report at SA visit.
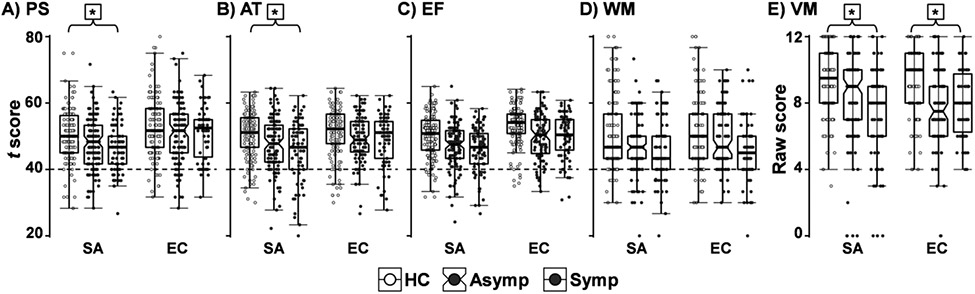
All primary cognitive measures using WRAT-4 scores as a covariate except working memory and executive functioning (p’s≥0.012) showed significant group effects (processing speed: F2, 337=5.14, p=0.006, partial-η2=0.03; attention: F2, 336=5.07, p=0.007, partial-η2=0.03; verbal memory: F2, 338=10.35, p<0.001, partial-η2=0.06) at the SA visit following multiple comparison corrections. Supplemental results indicated that the verbal memory findings were not changed when age was included as a covariate in the analyses (see Supplemental Material). As expected, WRAT-4 was significantly related to each of these domains (p’s≤0.002, partial-η2’s≥0.03). HC showed higher performance on verbal memory scores than symptomatic (p<0.001) and asymptomatic (p=0.001) participants. Further, HC had significantly higher processing speed and attention scores than symptomatic participants (all p’s≤0.003), while HC and asymptomatic participants did not differ on any of these measures following Bonferroni correction (all p’s≥0.019). Notably, the two pmTBI groups did not differ from each other for any of the cognitive measures (all p’s≥0.143; see Figure 2 and Table 2). Supplementary results indicated no changes to the reported findings when sex and parental education were used as additional covariates. Similarly, the removal of participants with ADHD/LD or positive CT or MRI imaging findings did not change conventional significance criteria or significantly alter effect sizes (see Supplemental Material).
Figure 2.
t-scores for the cognitive domains processing speed (PS), attention (AT), executive function (EF), and working memory (WM) for healthy controls (HC; no shading), asymptomatic pmTBI (Asymp; light grey), and symptomatic pmTBI (Symp; dark grey). Raw scores are used for verbal memory (VM). Results from ANCOVA models, using WRAT-4 ratings as a covariate, are denoted for significant Group effects after Bonferroni correction (*) at each time point.
Table 2.
Performance on major cognitive domains across all three groups.
| HC | HC vs. Asym p(eff) |
Asym | Asym vs. Sym p(eff) |
Sym | HC vs. Sym p(eff) |
|
|---|---|---|---|---|---|---|
| Cognitive Measures at SA | ||||||
| AT | 49.71±7.78 | 0.051(0.016) | 47.82±7.63 | 0.158(0.008) | 46.27±7.67 | 0.002(0.046)* |
| PS | 50.14±7.83 | 0.019(0.020) | 47.85±7.69 | 0.354(0.004) | 46.83±7.73 | 0.003(0.044)* |
| EF | 49.23±6.53 | 0.047(0.015) | 47.61±6.41 | 0.257(0.006) | 46.58±6.44 | 0.004(0.038)* |
| WM | 49.02±8.69 | 0.135(0.008) | 47.39±8.53 | 0.940(0.000) | 47.49±8.58 | 0.212(0.004) |
| VM | 9.10±2.38 | 0.001(0.038)* | 8.14±2.34 | 0.143(0.009) | 7.65±2.35 | <0.001(0.092)* |
| Cognitive Measures at EC | ||||||
| AT | 50.24±7.09 | 0.325(0.006) | 49.29±6.98 | 0.820(0.000) | 49.55±7.07 | 0.538(0.001) |
| PS | 52.39±8.80 | 0.284(0.006) | 51.12±8.67 | 0.719(0.002) | 51.62±8.76 | 0.569(0.003) |
| EF | 52.34±6.01 | 0.010(0.033)* | 50.26±5.91 | 0.531(0.003) | 50.84±5.97 | 0.107(0.016) |
| WM | 49.94±9.10 | 0.807(0.000) | 49.64±8.96 | 0.067(0.026) | 47.03±9.06 | 0.039(0.017) |
| VM | 9.11±2.07 | <0.001(0.094)* | 7.74±2.04 | 0.449(0.002) | 7.98±2.06 | <0.001(0.068)* |
Notes: HC-healthy control; Asym-asymptomatic; Sym-symptomatic; SA-sub-acute; EC-early chronic; AT-attention; PS-processing speed; EF-executive functioning; WM-working memory; VM-verbal memory; eff-partial eta. All values are covariate-adjusted for WRAT-4 word reading.
Indicates significance. Bonferroni corrected level of 0.01. All cognitive measures are norm-referenced T-scores except for VM which uses raw scores.
EC Visit
There were no differences between returning and non-returning patients on any of the demographic variables or injury characteristics tested, (age, sex, WRAT-4 scores, parental education, LOC, PTA, or mechanism of injury; see [S-Table 1). Removal of one HC, three asymptomatic, and one symptomatic participant with low effort did not change primary outcomes.
A total of 41 of the 66 returning pmTBI symptomatic at SA showed evidence of recovery at the EC visit based on the PCSI score (i.e., changed to asymptomatic status). Conversely, 19 out of 100 asymptomatic pmTBI at SA were symptomatic at their EC follow-up. As exhibited in Figure 3, this was the result of both smaller (less than 1 standard deviation: N = 10) as well as larger (greater than 1 standard deviation: N = 9) changes in normalized PCSI scores for pmTBI who were reclassified based to symptomatic at the EC visit. For the EC visit, the WRAT-4 covariate remained significantly related to all cognitive domains (p’s≤0.009, partial-η2’s≥0.02).
Figure 3.
Change in Post-Concussion Symptom Inventory (PCSI) Z scores for 19 asymptomatic pmTBI at the sub-acute (SA) visit that changed status to symptomatic at the early chronic (EC) visit.
A main effect of group was again observed for verbal memory scores (F2, 287=13.57, p<0.001, partial-η2=0.09). Supplemental analyses further confirmed that verbal memory findings were not changed when age was included as a covariate in the analyses (see Supplemental Material). HC scored higher relative to both asymptomatic and symptomatic groups (p’s<0.001), with no differences between the two pmTBI groups (p=0.449; see Figure 2 and Table 2). No significant group omnibus effects were observed for the processing speed, attention, executive function, or working memory domains following Bonferroni correction (all p’s≥0.067). Supplementary results indicated no changes to the reported findings when accounting for individuals with positive imaging findings, ADHD/LD, sex, or parental education (see Supplemental Material).
Discussion
The purpose of the current study was to compare cognitive performance in symptomatic and asymptomatic pmTBI participants (defined by PCS symptom load) during the first week of injury and at approximately 4 months post-injury. Results indicated that ~40% of pmTBI were classified as symptomatic at the SA visit, whereas only ~27% were classified as symptomatic at the EC visit. As expected, symptomatic pmTBI scored higher on the 5P risk score relative to asymptomatic patients and HC. Lower performance on measures of executive function, attention, processing speed, and verbal memory were present for symptomatic patients relative to HC at the SA visit, but, with the exception of verbal memory, were absent for asymptomatic patients relative to the control group. The effect sizes associated with lower scores in the asymptomatic group were in the medium range, suggesting that non-significant findings were not the result of low power. Finally, verbal memory was the only domain that was significantly different between asymptomatic participants and HC, and did not show evidence of normalization even at 4 months post-injury.
Previous studies with pediatric and adult mTBI have reported differences in attention (Belanger and Vanderploeg, 2005; Collie et al., 2006; Sicard et al., 2022), processing speed (Brooks et al., 2016; Fazio et al., 2007; Hannah et al., 2021), executive functioning (Belanger and Vanderploeg, 2005; Broadway et al., 2019), and memory (Belanger and Vanderploeg, 2005; Fazio et al., 2007) during the acute and sub-acute injury phases. However, most previous studies did not stratify group comparisons based on the presence/absence of PCS. This approach may be more sensitive and specific for detecting children who may require cognitive or additional rehabilitation services (Kirkwood et al., 2008; Marshall et al., 2015; Teh et al., 2020). Current findings suggest that the majority of pmTBI patients (~60%) are symptomatically recovered at approximately one week post-injury, with mixed evidence for cognitive function. Other large studies (Zemek et al., 2016) have reported approximately ~68% recovered at 1-month post-injury. Although different methods were used in the current versus previous study to define persistent symptoms (Mayer et al., 2020), collectively these results suggest a more rapid symptom recovery for the majority of patients followed by a slower trajectory in neurocognitive functioning (Ledoux et al., 2018; Sady et al., 2014).
Specifically, the current study observed lower verbal memory performance in both symptomatic and asymptomatic patients that persisted up to 4 months post-injury. Previous studies in more severely injured TBI patients have observed evidence of encoding and consolidation deficits that were significantly associated with verbal memory performance, with consolidation identified as the most chronically affected function (Vanderploeg et al., 2014; Wright and Schmitter-Edgecombe, 2011). Persisting verbal memory deficits have also been associated with reduced usage of efficient internally driven strategies (semantic clustering) following mTBI (Broadway et al., 2019; Geary et al., 2011). No other cognitive domains were affected at the EC visit with relatively small effect sizes, suggesting that current null findings are more reflective of non-meaningful clinical differences in cognitive functioning rather than due to low statistical power. However, previous pediatric and adults studies have indicated continued alterations of executive function (Howell et al., 2013; Konrad et al., 2011; Kunker et al., 2020; Mani et al., 2017), working memory (Dean and Sterr, 2013; Konrad et al., 2011; Kumar et al., 2013), attention (Catale et al., 2009; Jeter et al., 2013; Konrad et al., 2011; Mani et al., 2017; Pontifex et al., 2012), and processing speed (Cicerone, 1996; Dean and Sterr, 2013; Jeter et al., 2013) in some participants at more chronic time-points. Thus, additional studies are required to clarify these findings of mixed cognitive recovery profiles following mTBI in both adults and children.
When considered collectively, the current study adds to the evidence of PCS tracking cognition within the first few weeks of injury (Collie et al., 2006; Fazio et al., 2007; Sicard et al., 2020), with a decoupling of the two at longer assessment times (Babikian et al., 2013; Yeates et al., 2012). This decoupling of PCS and cognition at 4 months post-injury is similar to other studies that reported prolonged issues after PCS “recovery” (de Koning et al., 2018; Hammond et al., 2004; Hanna-Pladdy et al., 2001; Novak et al., 2016). Previous studies indicate that only a minority of sport-related concussion studies use multiple measures or have consistent criteria to define recovery, with symptom report typically used in isolation, or in conjunction with varying criteria (Haider et al., 2018). A number of studies highlight the importance of a consistent definition of recovery, suggesting that clinical assessments should involve both subjective and objective measures of cognition, in addition to other measures (physical, somatic, etc.) for a multifaceted view of recovery (Anderson, 2021; Collie et al., 2006; Gosselin et al., 2006; Haider et al., 2018; Kunker et al., 2020; Stenberg et al., 2020). Follow-up visits and/or thorough cognitive assessment may therefore also be warranted for asymptomatic patients, representing an important consideration when determining treatment plans, academic accommodations, or return-to-play protocols (Gioia et al., 2008; Kim and Priefer, 2020; Marshall et al., 2015; Sady et al., 2014).
Several other findings were observed in the current study. First, female sex was over-represented in the symptomatic group in our study. Evidence for sex differences in mTBI is mixed (Mollayeva et al., 2019; Yeates et al., 2022). However, previous studies indicate that female sex represents a risk factor for PPCS (Ewing-Cobbs et al., 2018; Ledoux et al., 2018; Lin et al., 2018; Zemek et al., 2016), is associated with a higher risk for concussion in similar sports (Zuckerman et al., 2015), and that females experience more objective and subjective adverse effects than males after concussion (Broshek et al., 2005; Hannah et al., 2021). In addition to reduced neck strength, females have smaller diameter axons and cerebral vasculature, both of which increase the likelihood of diffuse axonal and vascular injuries (Dolle et al., 2018; Sutton et al., 2019).
Approximately 62% of the symptomatic pmTBI converted to asymptomatic status at EC visit, as would be expected from a typical course of recovery. In addition, 19% of the asymptomatic pmTBI converted to symptomatic status at 4 months post-injury, a finding that was not expected from a neurological perspective. The most parsimonious explanation of these findings is the non-specific (secondary to other clinical disorders such as anxiety, depression, etc.) and the unreliable nature of PCS self-reporting in relationship to mTBI (Hergert et al., 2021; Iverson et al., 2015; Mayer et al., 2020). However, other studies have also provided evidence of asymptomatic participants developing injury-related subjective complaints over time (de Koning et al., 2018), and symptoms frequently emerge during more strenuous physical activity during the acute injury setting (Kozlowski et al., 2013). Symptom exacerbation upon full integration into regular life activities may provide a basis through which a minority of “recovered” patients experience increased symptoms months post-injury.
The current study had a number of strengths. In addition to a sample large enough to sufficiently determine differences at the observed effect sizes, there was 80% retention among our sample. Further, our findings in regard to verbal memory were supported by medium effect sizes. There are several limitations to the study. As previously discussed, PCS reporting has been critiqued both in terms of psychometric properties and the non-specific nature of most symptoms (Iverson et al., 2015; Mayer et al., 2020). Other studies have used orthopedically injured controls in addition to uninjured HC. Orthopedic injuries may better control for nonspecific injury related symptoms or premorbid risk factors due to sampling bias (Wilde et al., 2019). Studies that used orthopedically injured controls found similar rates of persistent symptoms longitudinally compared with pmTBI (Babikian et al., 2011; Babikian et al., 2013; Ewing-Cobbs et al., 2018). As expected, the retrospective PCSI scores differed between groups (Ewing-Cobbs et al., 2018; Ledoux et al., 2022), and pre-injury symptoms were unattainable through baseline assessments prior to injury. Children and adolescents may perceive pain and other symptoms differently from adults, and some children may not understand how to relate their symptoms to a Likert scale (Jeter et al., 2013; Sady et al., 2014). Further, other studies (Babikian et al., 2013; Beauchamp et al., 2015; Beauchamp et al., 2018) have adopted alternative methods to t-scores such as the summing of individual test results (e.g., 1.5 SD or more below mean), which may be more sensitive to the heterogeneity of pmTBI.
Additionally, while the HVLT-R has not been normed for children under 16, it has been used in previous pediatric studies (Nagle et al., 2006; Smelror et al., 2019; Smith et al., 2022; Stone et al., 2016), though there have been mixed findings in the literature (Guskiewicz et al., 2001). Further, the current study statistically controlled for premorbid reading ability which varied between groups (see Supplemental Material for information on additional analyses). This approach is more conservative due to the removal of shared variance, but has been critiqued due to the specious conclusion that one can disambiguate clinically comorbid conditions with potentially shared etiologies through a statistical process (Miller and Chapman, 2001). Importantly, excellent test-retest reliability (Koo and Li, 2016) was also observed for the WRAT-4 across visits separately in both groups (mTBI ICC=0.837; HC ICC=0.869), suggesting that reading performance was not adversely affected by other non-specific factors (pain, lack of concentration) associated with acute injury. Finally, there was a lack of power due to small subgroup sizes for a number of potential post hoc tests, specifically in regard to the 19 participants who went from asymptomatic at SA to symptomatic at EC, those with intracranial findings, and those who did not return for the follow up visit.
Conclusions
In summary, current results suggest greater agreement between subjectively defined PCS and lower cognitive performance in the first week of injury rather than several months post-injury. Both symptomatic and asymptomatic pmTBI continued to exhibit lower performance in verbal memory up to 4 months post-injury suggesting that cognitive recovery post pmTBI may be an on-going process separate from subjective symptom resolution (Sady et al., 2014). Current results highlight the importance of assessing multiple domains of functioning (cognitive, behavioral, and subjective symptoms) to determine full clinical recovery from pmTBI. Future studies should include the use of orthopedically injured controls, as well as monitoring the trajectory of recovery through longer durations (six months to one year post-injury).
Supplementary Material
Funding
This research was supported by grants from the National Institutes of Health [https://www.nih.gov; grant numbers NIH 01 R01 NS098494-01A1, R01 NS098494-03S1A1, P30 GM122734, and S10 OD025313] to Andrew R. Mayer. The NIH had no role in study review, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial Disclosure
The authors report there are no competing interests to declare.
Reference List
- Anderson JFI (2021). Cognitive complaint and objective cognition during the post-acute period after mild traumatic brain injury in pre-morbidly healthy adults. Brain Inj 35, 103–113. [DOI] [PubMed] [Google Scholar]
- Babikian T, McArthur D, and Asarnow RF (2013). Predictors of 1-month and 1-year neurocognitive functioning from the UCLA longitudinal mild, uncomplicated, pediatric traumatic brain injury study. J Int Neuropsychol Soc 19, 145–154. [DOI] [PubMed] [Google Scholar]
- Babikian T, Satz P, Zaucha K, et al. (2011). The UCLA longitudinal study of neurocognitive outcomes following mild pediatric traumatic brain injury. J Int Neuropsychol Soc 17, 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow KM, Crawford S, Brooks BL, et al. (2015). The Incidence of Postconcussion Syndrome Remains Stable Following Mild Traumatic Brain Injury in Children. Pediatr Neurol 53, 491–497. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Aglipay M, Yeates KO, et al. (2018). Predictors of neuropsychological outcome after pediatric concussion. Neuropsychology 32, 495–508. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Brooks BL, Barrowman N, et al. (2015). Empirical Derivation and Validation of a Clinical Case Definition for Neuropsychological Impairment in Children and Adolescents. J Int Neuropsychol Soc 21, 596–609. [DOI] [PubMed] [Google Scholar]
- Belanger HG, and Vanderploeg RD (2005). The neuropsychological impact of sports-related concussion: A meta-analysis. J Int Neuropsychol Soc 11, 345–357. [DOI] [PubMed] [Google Scholar]
- Broadway JM, Rieger RE, Campbell RA, et al. (2019). Executive function predictors of delayed memory deficits after mild traumatic brain injury. Cortex 120, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BL, Daya H, Khan S, et al. (2016). Cognition in the emergency department as a predictor of recovery after pediatric mild traumatic brain injury. J Int Neuropsychol Soc 22, 379–387. [DOI] [PubMed] [Google Scholar]
- Broshek DK, Kaushik T, Freeman JR, et al. (2005). Sex differences in outcome following sports-related concussion. J Neurosurg 102, 856–863. [DOI] [PubMed] [Google Scholar]
- Catale C, Marique P, Closset A, et al. (2009). Attentional and executive functioning following mild traumatic brain injury in children using the Test for Attentional Performance (TAP) battery. J Clin Exp Neuropsychol 31, 331–338. [DOI] [PubMed] [Google Scholar]
- Chrisman SP, Quitiquit C, and Rivara FP (2013). Qualitative study of barriers to concussive symptom reporting in high school athletics. J Adolesc Health 52, 330–335. [DOI] [PubMed] [Google Scholar]
- Cicerone KD (1996). Attention deficits and dual task demands after mild traumatic brain injury. Brain Inj 10, 79–89. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. Hillsdale,NJ. [Google Scholar]
- Collie A, Makdissi M, Maruff P, et al. (2006). Cognition in the days following concussion: comparison of symptomatic versus asymptomatic athletes. J Neurol Neurosurg Psychiatry 77, 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning ME, Scheenen ME, van der Horn HJ, et al. (2018). From 'miserable minority' to the 'fortunate few': the other end of the mild traumatic brain injury spectrum. Brain Inj 32, 540–543. [DOI] [PubMed] [Google Scholar]
- Dean PJ, and Sterr A (2013). Long-term effects of mild traumatic brain injury on cognitive performance. Front Hum Neurosci 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning JH (2012). The efficiency and accuracy of the Test of Memory Malingering trial 1, errors on the first 10 items of the test of memory malingering, and five embedded measures in predicting invalid test performance. Arch Clin Neuropsychol 27, 417–432. [DOI] [PubMed] [Google Scholar]
- Dolle JP, Jaye A, Anderson SA, et al. (2018). Newfound sex differences in axonal structure underlie differential outcomes from in vitro traumatic axonal injury. Exp Neurol 300, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders J, and Gardner V (2020). Utility of abbreviated versions of the test of memory malingering in children with traumatic brain injury. Appl Neuropsychol Child 9, 355–359. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Cox CS Jr., Clark AE, et al. (2018). Persistent postconcussion symptoms after injury. Pediatrics 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio VC, Lovell MR, Pardini JE, et al. (2007). The relation between post concussion symptoms and neurocognitive performance in concussed athletes. NeuroRehabilitation 22, 207–216. [PubMed] [Google Scholar]
- Geary EK, Kraus MF, Rubin LH, et al. (2011). Verbal learning strategy following mild traumatic brain injury. J Int Neuropsychol Soc 17, 709–719. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Collins M, and Isquith PK (2008). Improving identification and diagnosis of mild traumatic brain injury with evidence: psychometric support for the acute concussion evaluation. J Head Trauma Rehabil 23, 230–242. [DOI] [PubMed] [Google Scholar]
- Gosselin N, Theriault M, Leclerc S, et al. (2006). Neurophysiological anomalies in symptomatic and asymptomatic concussed athletes. Neurosurgery 58, 1151–1161. [DOI] [PubMed] [Google Scholar]
- Guskiewicz KM, Ross SE, and Marshall SW (2001). Postural Stability and Neuropsychological Deficits After Concussion in Collegiate Athletes. J Athl Train 36, 263–273. [PMC free article] [PubMed] [Google Scholar]
- Haider MN, Leddy JJ, Pavlesen S, et al. (2018). A systematic review of criteria used to define recovery from sport-related concussion in youth athletes. Br J Sports Med 52, 1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond FM, Hart T, Bushnik T, et al. (2004). Change and predictors of change in communication, cognition, and social function between 1 and 5 years after traumatic brain injury. J Head Trauma Rehabil 19, 314–328. [DOI] [PubMed] [Google Scholar]
- Hanna-Pladdy B, Berry ZM, Bennett T, et al. (2001). Stress as a diagnostic challenge for postconcussive symptoms: sequelae of mild traumatic brain injury or physiological stress response. Clin Neuropsychol 15, 289–304. [DOI] [PubMed] [Google Scholar]
- Hannah TC, Li AY, Spiera Z, et al. (2021). Sex-Related Differences in the Incidence, Severity, and Recovery of Concussion in Adolescent Student-Athletes Between 2009 and 2019. Am J Sports Med 49, 1929–1937. [DOI] [PubMed] [Google Scholar]
- Hergert DC, Sicard V, Stephenson DD, et al. (2021). Test-Retest Reliability of a Semi-Structured Interview to Aid in Pediatric Traumatic Brain Injury Diagnosis. J Int Neuropsychol Soc 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell D, Osternig L, van DP, et al. (2013). Effects of concussion on attention and executive function in adolescents. Med Sci Sports Exerc 45, 1030–1037. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Silverberg ND, Mannix R, et al. (2015). Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr 169, 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter CB, Hergenroeder GW, Hylin MJ, et al. (2013). Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. J Neurotrauma 30, 657–670. [DOI] [PubMed] [Google Scholar]
- Kay T, Harrington DE, Adams R, et al. (1993). Definition of mild traumatic brain injury. Journal of Head Trauma Rehabilitation 8, 86–87. [Google Scholar]
- Kim K, and Priefer R (2020). Evaluation of current post-concussion protocols. Biomed Pharmacother 129, 110406. [DOI] [PubMed] [Google Scholar]
- Kirkwood MW, Yeates KO, Taylor HG, et al. (2008). Management of pediatric mild traumatic brain injury: a neuropsychological review from injury through recovery. Clin Neuropsychol 22, 769–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad C, Geburek AJ, Rist F, et al. (2011). Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol Med 41, 1197–1211. [DOI] [PubMed] [Google Scholar]
- Koo TK, and Li MY (2016). A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 15, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski KF, Graham J, Leddy JJ, et al. (2013). Exercise intolerance in individuals with postconcussion syndrome. J Athl Train 48, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Rao SL, Chandramouli BA, et al. (2013). Reduced contribution of executive functions in impaired working memory performance in mild traumatic brain injury patients. Clin Neurol Neurosurg 115, 1326–1332. [DOI] [PubMed] [Google Scholar]
- Kunker K, Peters DM, and Mohapatra S (2020). Long-term impact of mild traumatic brain injury on postural stability and executive function. Neurol Sci 41, 1899–1907. [DOI] [PubMed] [Google Scholar]
- Lau BC, Collins MW, and Lovell MR (2012). Cutoff scores in neurocognitive testing and symptom clusters that predict protracted recovery from concussions in high school athletes. Neurosurgery 70, 371–379. [DOI] [PubMed] [Google Scholar]
- Lau BC, Kontos AP, Collins MW, et al. (2011). Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med 39, 2311–2318. [DOI] [PubMed] [Google Scholar]
- Ledoux AA, Tang K, Gagnon I, et al. (2022). Association Between Preinjury Symptoms and Postconcussion Symptoms at 4 Weeks in Youth. J Head Trauma Rehabil 37, E90–E101. [DOI] [PubMed] [Google Scholar]
- Ledoux AA, Tang K, Yeates KO, et al. (2018). Natural Progression of Symptom Change and Recovery From Concussion in a Pediatric Population. JAMA Pediatr e183820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Casey E, Herman DC, et al. (2018). Sex Differences in Common Sports Injuries. PM R 10, 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughan AR, Perna R, and Le J (2016). Test of memory malingering with children: The utility of Trial 1 and TOMMe10 as screeners of test validity. Child Neuropsychol 22, 707–717. [DOI] [PubMed] [Google Scholar]
- Lumba-Brown A, Yeates KO, Sarmiento K, et al. (2018). Centers for Disease Control and Prevention Guideline on the Diagnosis and Management of Mild Traumatic Brain Injury Among Children. JAMA Pediatr 172, e182853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani K, Cater B, and Hudlikar A (2017). Cognition and return to work after mild/moderate traumatic brain injury: A systematic review. Work 58, 51–62. [DOI] [PubMed] [Google Scholar]
- Marshall S, Bayley M, McCullagh S, et al. (2015). Updated clinical practice guidelines for concussion/mild traumatic brain injury and persistent symptoms. Brain Inj 29, 688–700. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Quinn DK, and Master CL (2017). The spectrum of mild traumatic brain injury: A review. Neurology 89, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Stephenson DD, Dodd AB, et al. (2020). Comparison of Methods for Classifying Persistent Post-Concussive Symptoms in Children. J Neurotrauma 37, 1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Dvorak J, et al. (2017). Consensus statement on concussion in sport-the 5(th) international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 51, 838–847. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse WH, Aubry M, et al. (2013). Consensus statement on concussion in sport the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 47, 250–258. [DOI] [PubMed] [Google Scholar]
- Miller GA, and Chapman JP (2001). Misunderstanding analysis of covariance. J Abnorm Psychol 110, 40–48. [DOI] [PubMed] [Google Scholar]
- Mittenberg W, DiGiulio DV, Perrin S, et al. (1992). Symptoms following mild head injury: expectation as aetiology. J Neurol Neurosurg Psychiatry 55, 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollayeva T, Mollayeva S, Pacheco N, et al. (2019). The course and prognostic factors of cognitive outcomes after traumatic brain injury: A systematic review and meta-analysis. Neurosci Biobehav Rev 99, 198–250. [DOI] [PubMed] [Google Scholar]
- Nagle AM, Everhart DE, Durham TW, et al. (2006). Deception strategies in children: examination of forced choice recognition and verbal learning and memory techniques. Arch Clin Neuropsychol 21, 777–785. [DOI] [PubMed] [Google Scholar]
- Novak Z, Aglipay M, Barrowman N, et al. (2016). Association of Persistent Postconcussion Symptoms With Pediatric Quality of Life. JAMA Pediatrics 170, e162900. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Broglio SP, Drollette ES, et al. (2012). The relation of mild traumatic brain injury to chronic lapses of attention. Res Q Exerc Sport 83, 553–559. [DOI] [PubMed] [Google Scholar]
- Rivara FP, Tennyson R, Mills B, et al. (2020). Consensus Statement on Sports-Related Concussions in Youth Sports Using a Modified Delphi Approach. JAMA Pediatr 174, 79–85. [DOI] [PubMed] [Google Scholar]
- Sady MD, Vaughan CG, and Gioia GA (2014). Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Arch Clin Neuropsychol 29, 348–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard V, Hergert DC, Pabbathi Reddy S., et al. (2020). Severity of ongoing post-concussive symptoms as a predictor of cognitive performance following a pediatric mild traumatic brain injury. J Int Neuropsychol Soc 27, 686–696. [DOI] [PubMed] [Google Scholar]
- Sicard V, Stephenson DD, Hergert DC, et al. (2022). Investigating the diagnostic accuracy of a paper-and-pencil and a computerized cognitive test battery for pediatric mild traumatic brain injury. Neuropsychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg ND, Iaccarino MA, Panenka WJ, et al. (2020). Management of Concussion and Mild Traumatic Brain Injury: A Synthesis of Practice Guidelines. Arch Phys Med Rehabil 101, 382–393. [DOI] [PubMed] [Google Scholar]
- Smelror RE, Jorgensen KN, Lonning V, et al. (2019). Healthy Adolescent Performance With Standardized Scoring Tables for the MATRICS Consensus Cognitive Battery: A Multisite Study. Schizophr Bull 45, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Thomas J, Friedhoff C, et al. (2022). The Utility of the Test of Memory Malingering Trial 1 in Differentiating Neurocognitive, Emotional, and Behavioral Functioning in a Pediatric Concussion Population. Arch Clin Neuropsychol 37, 322–337. [DOI] [PubMed] [Google Scholar]
- Stenberg J, Karr JE, Terry DP, et al. (2020). Change in self-reported cognitive symptoms after mild traumatic brain injury is associated with changes in emotional and somatic symptoms and not changes in cognitive performance. Neuropsychology 34, 560–568. [DOI] [PubMed] [Google Scholar]
- Stone WS, Mesholam-Gately RI, Giuliano AJ, et al. (2016). Healthy adolescent performance on the MATRICS Consensus Cognitive Battery (MCCB): Developmental data from two samples of volunteers. Schizophr Res 172, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M, Chan V, Escobar M, et al. (2019). Neck Injury Comorbidity in Concussion-Related Emergency Department Visits: A Population-Based Study of Sex Differences Across the Life Span. J Womens Health (Larchmt ) 28, 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M, Hearps SJC, Babl FE, et al. (2020). Does a computerized neuropsychological test predict prolonged recovery in concussed children presenting to the ED? Child Neuropsychol 26, 54–68. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Bell JM, Breiding MJ, et al. (2017). Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ 66, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Dietrich A, Nuss K, et al. (2010). Post-concussive symptoms in children with mild traumatic brain injury. Neuropsychology 24, 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh Z, Takagi M, Hearps SJC, et al. (2020). Acute cognitive postconcussive symptoms follow longer recovery trajectories than somatic postconcussive symptoms in young children. Brain Inj 34, 350–356. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Donnell AJ, Belanger HG, et al. (2014). Consolidation deficits in traumatic brain injury: the core and residual verbal memory defect. J Clin Exp Neuropsychol 36, 58–73. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Ware AL, Li X, et al. (2019). Orthopedic Injured versus Uninjured Comparison Groups for Neuroimaging Research in Mild Traumatic Brain Injury. J Neurotrauma 36, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS and Robertson GJ (2006). WRAT 4: Wide range achievement test; professional manual. Psychological Assessment Resources, Incorporated. [Google Scholar]
- Wright MJ, and Schmitter-Edgecombe M (2011). The impact of verbal memory encoding and consolidation deficits during recovery from moderate-to-severe traumatic brain injury. J Head Trauma Rehabil 26, 182–191. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Kaizar E, Rusin J, et al. (2012). Reliable change in postconcussive symptoms and its functional consequences among children with mild traumatic brain injury. Arch Pediatr Adolesc Med 166, 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates TM, Taylor HG, Bigler ED, et al. (2022). Sex Differences in the Outcomes of Mild Traumatic Brain Injury in Children Presenting to the Emergency Department. J Neurotrauma 39, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumul JN, McKinlay A, Than M, et al. (2020). Concussive Symptoms Following Pediatric Mild Traumatic Brain Injury. J Head Trauma Rehabil 35, 279–287. [DOI] [PubMed] [Google Scholar]
- Zemek R, Barrowman N, Freedman SB, et al. (2016). Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 315, 1014–1025. [DOI] [PubMed] [Google Scholar]
- Zuckerman SL, Kerr ZY, Yengo-Kahn A, et al. (2015). Epidemiology of Sports-Related Concussion in NCAA Athletes From 2009-2010 to 2013-2014: Incidence, Recurrence, and Mechanisms. Am J Sports Med 43, 2654–2662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.