Abstract
Our study aims to evaluate the functions of the middle and inner ear and the efferent auditory system in psoriasis. Hearing thresholds, resonance frequency, otoacoustic emission amplitudes with and without contralateral acoustic stimulation, contralateral suppression levels were evaluated in 35 psoriasis patients and 40 controls. The air-conduction hearing thresholds of the patients were significantly higher than the controls at 125, 250, 500, 1000, 2000, 4000, and 12,000 Hz frequencies in the left ear, and 125, 250, 500, 1000, 2000, and 4000 Hz frequencies in the right ear, and the bone-conduction hearing thresholds of the patients were found to be significantly higher than the controls at all frequencies in the left ear, and 250, 500, 1000 and 2000 Hz in the right ear (p < 0.05). The resonance frequencies of patients were found to be significantly lower than the controls (p < 0.001). The emission amplitudes obtained with contralateral acoustic stimulation were significantly lower than without contralateral acoustic stimulation at 1 kHz frequency in both groups (p < 0.01). There was no significant difference in contralateral suppression levels of the two groups (p > 0.05). Our findings indicate that middle ear mechanics can be affected by psoriasis. There was no significant difference between psoriasis patients and healthy controls in terms of efferent auditory functions. There was a significant difference at limited frequency in hearing thresholds and emission amplitudes between the groups.
Keywords: Psoriasis, Middle ear, Inner ear, Hearing, Hearing loss
Introduction
Psoriasis is an Immune-Mediated Inflammatory Disease (IMID) of the skin characterized by erythematous, scaly, indurated, pruritic plaques which tend to be located particularly on the scalp and extensor surfaces [1]. IMIDs, characterized by dysregulation including psoriasis, ankylosing spondylitis (AS), rheumatoid arthritis (RA), and inflammatory bowel disease, are at greater risk for the development of systemic comorbidities that extend beyond the primary target organ. IMIDs are thought to affect these comorbid conditions through the complex interactions between environmental and genetic factors [2].
The role of autoimmune causes in the etiology of sensorineural hearing loss is emphasized. The inner ear is an organ sensitive to autoimmune attacks. Sensorineural hearing loss may occur as a complication of non-organ-specific autoimmune diseases [3]. In 1979, McCabe first described immune-mediated sensorineural hearing loss which is characterized by the damage to the inner ear structures due to an autoimmune response [4]. In many diseases such as inflammatory bowel disease and AS, hearing loss induced by autoimmune and inflammatory diseases has been reported [4–7]. However, few studies have been performed on functions of the auditory system in patients with psoriasis [3, 8–12]. Sensorineural hearing loss has been investigated in patients with psoriasis in the studies. The studies show that sensorineural hearing loss can be seen in psoriasis patients [9, 10]. The presence of sensorineural hearing loss in these patients shows degenerative changes in the structures such as outer hair cells, stria vascularis, and spiral ganglion. Otoacoustic emissions (OAE) reflect outer hair cell activity and the functional integrity of outer hair cells is essential for the generation of OAEs [13]. The number of studies evaluating OAE responses in psoriasis patients is limited [3, 8]. Outer hair cells have a unique motility ability. The vertical movement caused by the length variation of the outer hair cells, known as the cochlear amplifier, increases the basilar membrane movement and leads to higher hearing sensitivity and frequency selectivity. The outer hair cells receive a rich efferent innervation from the central nervous system. The efferent auditory system descends from the cortex via the brain stem and reaches the outer hair cells and modifies the movements of the outer hair cells to control cochlear amplification [13]. Cochlear amplification is controlled by the hyperpolarization of outer hair cells through the medial olivocochlear efferent system. In this way, the auditory system is protected from acoustic trauma, and speech discrimination is increased in a noisy environment. Studies show that medial olivocochlear efferent function may be affected in immune-mediated diseases such as AS [14]. No study evaluating the efferent hearing system in psoriasis patients has been found in the literature. In addition, no study evaluating middle ear resonance frequency (RF) in psoriasis patients has been found. A limited number of studies in autoimmune diseases show that the RF value may be affected in patients [15]. Our research aims to evaluate the efferent auditory system, middle ear RF, and inner ear functions in psoriasis patients.
Materials and Methods
This study was approved by the Institutional Review Board (Project no: KA15/357). All procedures were performed by the ethical standards of the Institutional Review Board, and Helsinki Declaration in this study. Written informed consent was obtained from all participants.
Participants
The study was conducted at the Audiology Unit in the Department of Otorhinolaryngology from May 2015 until May 2016 on 35 patients who were diagnosed with psoriasis by the Department of Dermatology and 40 healthy controls.
Inclusion criteria for all subjects were as follows: aged between 18 and 65 years (The mean ages of 35 psoriasis patients were 42.31 ± 14.36 years and of 40 control subjects were 41.48 ± 12.77 years.), having normal otoscopy, and type A tympanogram, and prevailing ipsilateral and contralateral acoustic reflexes. Subjects with an external or middle ear disease, a history of acoustic or head trauma, a history of ear disease which affects hearing thresholds such as Meniere, recurrent/chronic otitis media, otosclerosis, a history of ear surgery or ototoxic drug exposure were excluded from the study. The patients using Tumor necrosis factor-α (TNF-α) antagonists were also excluded from the research since the TNF-α antagonists affect the hearing functions [16].
Methods
After otoscopic examination, pure-tone audiometry, high-frequency audiometry (8000–16,000 Hz), speech audiometry, immittance, transient evoked otoacoustic emissions (TEOAE), and contralateral suppression test, multifrequency tympanometry (MFT) were administered to the participants. Pure-tone audiometry, TEOAE, and contralateral suppression tests were conducted in soundproof rooms.
An interacoustics-clinical audiometer (AC40) was used for pure-tone audiograms. Air-conduction (AC) hearing thresholds were obtained through TDH-39P Telephonic HB-7 earphones between 125 and 8000 Hz octave frequencies and Sennheiser HDA 200 circumaural earphones in 10,000, 12,000, 14,000, and 16,000 Hz octave frequencies. Bone-conduction (BC) hearing thresholds were evaluated using a Radioear B71 bone vibrator between 250 and 4000 Hz octave frequencies. Based on the hearing loss classification in the American speech-language-hearing association audiology information series (2015), pure tone average (PTA) of 0.5, 1, and 2 kHz frequencies at least 16 dB hearing level (HL) was defined as the hearing loss [17]. The speech reception threshold (SRT), and speech recognition scores (SRS) of the participants were obtained in speech audiometry. A word list consisting of three syllable words was used in SRT and the test was started at 30–40 dB sensation level (SL) according to the PTA. The SRT word list used in this study was developed by Department of Audiology at Hacettepe University [18]. By using the ascending method, the threshold level at which 2/3 or 50% of the words are repeated correctly was determined. The difference between SSO and SRT is expected to be no more than ± 10 dB. A word list consisting of monosyllabic phonetically balanced (PB-300) words was used in SRS. The PB-300 word lists developed by the Department of Audiology at Hacettepe University in 1972 were standardized by Kilincarslan in 1986 [18, 19]. The test was started at Most Comfortable Loudness Level (MCL) (approximately 40 dB above SRT − 40 dB SL). Words were presented to the participants using a carrier phrase, and the number of words they repeated correctly was determined. The Speech Recognition Test Score was determined as a percentage according to the number of words that the participants repeated correctly.
GSI Tympstar (Version 2) (Grason Stadler Inc., MN, USA) middle ear analyzer was used for immittance tests, middle ear pressure, acoustic reflex measurements, and middle ear RF. Middle ear RF was evaluated between 250 and 2000 Hz frequency with MFT. The test device performed MFT in two steps; the first step was to search for standard tympanometry data such as static admittance, tympanometric peak pressure and gradient value by changing the pressure between + 200 and − 400 daPa by giving a fixed frequency probe tone and obtaining a tympanogram. The second step was to determine the middle ear RF value by keeping the pressure constant and giving stimulus to the ear of the individual in the frequency range of 250–2000 Hz consecutively at 50 Hz intervals.
TEOAE test was performed at two stages for each group respectively: without contralateral acoustic stimulation (CAS) and with contralateral narrowband (NB) noise. TEOAEs were recorded using Titan (Interacoustics A/S Assens, Denmark) with 80 ± 3 dB Sound Pressure Level (SPL) nonlinear click stimuli. Signal to noise ratio (SNR), response level, and reproducibility was determined at 1, 1.4, 2, 2.8, and 4 kHz frequencies. Reproducibility ≥ 70%, stimulus stability ≥ 70%, and SNR ≥ 3 dB SPL were accepted for the analysis. Transient impulses averaged 260 times and 20 ms window. TEOAE test parameters were also used for the contralateral suppression test. The measurement was repeated without removing the probe from the ear while giving NB noise into the contralateral ear through insert earphones using Interacoustics-Clinical Audiometer AC33. The intensity of the NB was fixed at 70 dB SPL to evoke the contralateral medial olivocochlear (MOC) reflex without evoking the contralateral stapedius muscle reflex. The results were recorded for the evaluation of the contralateral suppression level. The difference between the amplitude in dB SPL of the total response without CAS and with contralateral narrow band noise was evaluated as the level of suppression of TEOAE.
Statistical Analyses
Statistical Package for the Social Sciences (SPSS), v25.0 for Windows program (SPSS Inc.; Chicago, IL, USA) was used for the statistical evaluation. The Pillai's Trace test statistic in one-way multivariate analysis of variance (one-way MANOVA) was used to determine whether there were any differences between independent groups on more than one continuous dependent variable. In the case of differences in more than one continuous dependent variable between independent groups, The ANOVA test statistic was used to determine whether there was a statistically significant difference between the two categorical groups. The differences between the AC and BC hearing thresholds of the right and left ears in both groups were compared with the Student t-test when they indicate normal distribution; or else the Mann–Whitney U test was conducted. TEOAE amplitudes recorded with and without CAS within groups were compared with the Paired samples t-test when they indicate normal distribution; or else the Wilcoxon test was used. The p-value of less than 0.05 was considered statistically significant.
Results
Audiological Findings
There was a significant difference between psoriasis and control groups in terms of dependent variables (p = 0.021). AC hearing thresholds between 125 and 16,000 Hz and BC hearing thresholds between 250 and 4000 Hz of patients with psoriasis and controls were obtained and compared with each other. The AC hearing thresholds of the psoriasis patients were significantly higher than the control group at frequencies of 125, 250, 500, 1000, 2000, and 4000 Hz in the right ear, and 125, 250, 500, 1000, 2000, 4000, and 12,000 Hz in the left ear, and the BC hearing thresholds of the psoriasis patients were found to be significantly higher than the controls at all frequencies in the left ear, and 250, 500, 1000 and 2000 Hz frequencies in the right ear (p < 0.05). The mean AC hearing thresholds of both groups are given for the right ear in Fig. 1 and for the left ear in Fig. 2. The mean BC hearing thresholds of both groups are given for the right ear in Fig. 3 and for the left ear in Fig. 4. The standard deviation (SD) values for the mean AC and BC hearing thresholds of the patient and control groups at each frequency are shown with error bars in Figs. 1, 2, 3 and 4. The mean PTA of AC hearing thresholds of psoriasis patients was statistically higher than the controls for the left ear (p = 0.000) and right ear (p = 0.001). The mean PTA of AC hearing thresholds of psoriasis group was 10 ± 5.20 dB HL for the right ear, and 9.76 ± 5.97 dB HL for the left ear, and of control group was 6.33 ± 3.76 dB HL for the right ear, and 5.33 ± 3.62 dB HL for the left ear. Based on the hearing loss classification in the American Speech-Language-Hearing Association Audiology information series (2015), bilateral slight sensorineural hearing loss was observed in 4 patients with psoriasis, and unilateral slight sensorineural hearing loss was observed in a psoriasis patient. When the AC and BC hearing thresholds of the right and left ears were compared, no significant difference was found between the ears in both groups (p > 0.05). The SRTs of all participants were consistent with their PTAs. The SRSs of all participants were above 88%.
Fig. 1.
Air conduction hearing thresholds of psoriasis and control groups between 125 and 16,000 Hz in the right ear
Fig. 2.
Air conduction hearing thresholds of psoriasis and control groups between 125 and 16,000 Hz in the left ear
Fig. 3.

Bone conduction hearing thresholds of psoriasis and control groups between 250 and 4000 Hz in the right ear
Fig. 4.
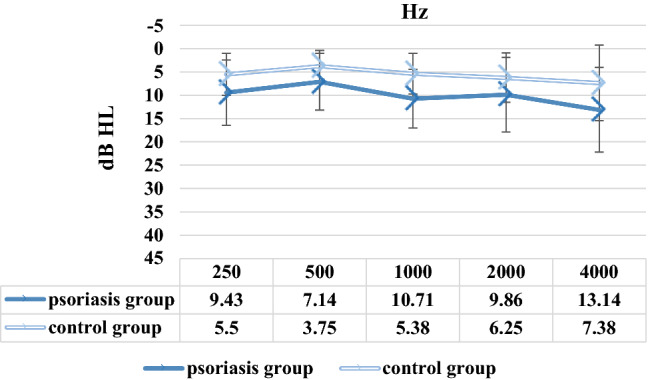
Bone conduction hearing thresholds of psoriasis and control groups between 250 and 4000 Hz in the left ear
Multifrequency Tympanometry Findings
Type A tympanograms were obtained in all participants of both ears. Ipsilateral and contralateral acoustic reflexes were present in all participants of both ears. When the mean RF values of psoriasis patients were compared with the controls, the mean RF of psoriasis group was statistically higher than the control group (p = 0.000). The mean RF values of psoriasis patients were 744.29 ± 118.68 and of 40 control subjects were 845.00 ± 105.79.
TEOAE and Contralateral Suppression Findings
TEOAE amplitudes with and without CAS of psoriasis and control groups at 1, 1.4, 2, 2.8, and 4 kHz frequencies were given in Table 1. When the mean TEOAE amplitudes of the psoriasis and control groups were compared, the average amplitudes of psoriasis patients at 2.8 kHz (p = 0.024) frequency were significantly lower than the controls. There was no significant difference between the two groups in the other frequencies evaluated (p > 0.05). The mean emission amplitude values obtained with CAS were significantly lower values than without CAS at 1 kHz frequency in psoriasis (p = 0.004) and control (p = 0.000) groups. There was no significant difference between TEOAE amplitudes with and without CAS in the other frequencies evaluated in both groups (p > 0.05). Comparison of contralateral suppression levels (dB) at 1, 1.4, 2, 2.8, and 4 kHz frequencies between two groups were given in Table 2. There was not a statistically significant difference in contralateral suppression levels of the two groups (p > 0.05).
Table 1.
TEOAE amplitudes with and without CAS of psoriasis and control groups at 1, 1.4, 2, 2.8, and 4 kHz frequencies
| Frequency | TEOAE without CAS Mean ± SD | TEOAE with CAS Mean ± SD | p |
|---|---|---|---|
| Psoriasis group | |||
| 1 kHz | 18.47 ± 5.46 | 14.72 ± 4.92 | 0.004 |
| 1.4 kHz | 19.69 ± 4.74 | 18.14 ± 4.25 | 0.156 |
| 2 kHz | 15.50 ± 4.19 | 14.49 ± 4.74 | 0.347 |
| 2.8 kHz | 11.82 ± 3.21 | 11.24 ± 3.66 | 0.486 |
| 4 kHz | 12.64 ± 3.24 | 12.26 ± 3.50 | 0.638 |
| Control group | |||
| 1 kHz | 20.60 ± 4.11 | 16.26 ± 3.79 | 0.000 |
| 1.4 kHz | 20.67 ± 4.11 | 18.79 ± 4.17 | 0.060 |
| 2 kHz | 16.95 ± 2.83 | 16.18 ± 3.62 | 0.293 |
| 2.8 kHz | 13.61 ± 3.51 | 12.63 ± 3.44 | 0.209 |
| 4 kHz | 13.40 ± 3.85 | 13.26 ± 3.21 | 0.577 |
TEOAE transient evoked otoacoustic emission; CAS contralateral acoustic stimulation; kHz kilohertz; SD standard deviation
Table 2.
Comparison of contralateral suppression levels (dB) at 1, 1.4, 2, 2.8, and 4 kHz frequencies between two groups
| Frequency | Contralateral suppression levels (dB) | p | |
|---|---|---|---|
| Psoriasis group Mean ± SD | Control group Mean ± SD | ||
| 1 kHz | 3.75 ± 4.04 | 4.34 ± 2.56 | 0.828 |
| 1.4 kHz | 1.55 ± 3.14 | 1.88 ± 2.21 | 0.393 |
| 2 kHz | 1.01 ± 3.20 | 0.77 ± 2.59 | 0.716 |
| 2.8 kHz | 0.58 ± 2.45 | 0.99 ± 2.25 | 0.454 |
| 4 kHz | 0.38 ± 2.11 | 0.14 ± 2.79 | 0.629 |
dB decibel; kHz kilohertz; SD standard deviation
Discussion
Psoriasis is currently considered a multi-systemic chronic inflammatory disease. The idea that the disease affects not only the skin and is associated with several comorbidities came into prominence. This has paved the way for investigations on psoriasis in terms of morbidities that may accompany it [20].
Immunological diseases of the inner ear have been a common research topic for a long time. In 1958, Lenhardt published a case of bilateral sensorineural hearing loss caused by anticochlear antibodies [21]. Kikuchi published a case of 'sympathetic otitis' in 1959. After surgery on a patient's ear, hearing loss also occurred in the patient’s healthy ear. Bleichert published histopathology of autoimmune reactions in the cochlea in 1961 [22]. However, all these studies drew attention in 1979 after McCabe published his work on ‘Autoimmune Sensorineural Hearing Loss’. In his study, McCabe described a series of eighteen patients with asymmetric and bilateral progressive sensorineural hearing loss and sometimes with vestibular symptoms. McCabe suggested the possibility of autoimmune pathogenesis in the etiology of sensorineural hearing loss upon noticing improvement following treatment with immunosuppressive medications [4].
Sensorineural hearing loss can take place as a result of autoimmune diseases since autoimmune attacks affect the inner ear [5]. The pathogenesis of sensorineural hearing loss accompanying immunologically mediated diseases has not been fully elucidated. The emphasized causal mechanisms are vasculitis, immune complex deposition, and T lymphocyte-mediated cytotoxicity. Although the mechanism of T-lymphocyte-mediated cytotoxicity is not known exactly, it is thought that the cochlear damage occurs first, then the circulating T cells are sensitized with the subsequently released antigens, and later the target organ is damaged by the type IV hypersensitivity. Another proposed mechanism is the type III reaction. This mechanism is the accumulation of circulating immune complexes in the target organ, followed by the damage to chemotactic factors, vasoactive amines, and complex fixation. In the vasculitic mechanism, it is suggested that hearing loss due to ischemia and damage to the organ of Corti may occur.
Kumar et al. reported that prolonged high serum levels of proinflammatory cytokines and TNF-α may cause cochlear injury [23]. As in ulcerative colitis and Crohn’s disease, TNF-α plays a crucial role in the pathogenesis of psoriasis [24]. TNF-α, which is a proinflammatory cytokine, was found in high levels in psoriatic skin [25]. Guvenc et al. [11] found that hearing thresholds, particularly at high frequencies were significantly higher in the psoriasis group than in the controls. They reported that it might be connected with higher serum levels of TNF-α which is the severe stage of psoriasis and cochlear damage might occur as a result of this.
Recently, limited studies have been conducted reportedly associated auditory functions with psoriasis and/or psoriatic arthritis [3, 8–12, 26–30]. When Amor-Dorado et al. [26] compared PTAs and speech reception thresholds in the psoriatic arthritis patients and controls, they found a significant difference between groups. They detected symmetrical hearing loss in 22 of the patients with psoriatic arthritis and asymmetrical bilateral hearing loss in 6 of them. Giani et al. [27] presented the case of autoimmune bilateral and asymmetric sensorineural hearing loss in a young patient with juvenile psoriatic arthritis. In our research, bilateral slight hearing loss was observed in 5 patients with psoriasis, and unilateral slight hearing loss was observed in a psoriasis patient. When the AC and BC hearing thresholds of the right and left ears were compared, no significant difference was found between the ears in both groups (p > 0.05).
Yen et al. [9] investigated the incidence of sensorineural hearing loss in psoriasis patients and compared them with the controls. They found that the rate of sensorineural hearing loss was higher in the psoriasis patients than in the controls. Borgia et al. [10] found that hearing loss, which is mostly in sensorineural type in patients with psoriasis. The sensorineural hearing loss seen in psoriasis patients can be explained by the triggering of an inflammatory reaction that causes degeneration in the organ of Corti, stria vascularis, and spiral ganglion as a result of the accumulation of leukocytes and local immunoglobulin production. Unlike these findings, there are also studies in which no difference was found between psoriasis patients and healthy controls in terms of inner ear hearing functions evaluated by pure tone audiometry and OAE responses [3, 8]. Karabulut et al. (2010) stated in their study that they did not detect any damage to the outer hair cells in the cochlea in psoriasis patients [3]. In our study, the AC hearing thresholds of the psoriasis group were significantly higher than the control group at frequencies of 125, 250, 500, 1000, 2000, and 4000 Hz in the right ear, and 125, 250, 500, 1000, 2000, 4000, and 12,000 Hz in the left ear, and the BC hearing thresholds of the psoriasis patients were found to be significantly higher than the controls at all frequencies in the left ear, and 250, 500, 1000 and 2000 Hz frequencies in the right ear (p < 0.05). When the mean TEOAE amplitudes of the psoriasis and control groups were compared, the average amplitudes of psoriasis patients at 2.8 kHz (p = 0.024) frequencies were significantly lower than the controls. We think that more studies are required to determine whether there is cochlea involvement in psoriasis because of the differences at limited frequency in AC and BC hearing thresholds and TEOAE amplitudes between the groups in our study.
We carried out MFT and contralateral suppression tests with the pure tone audiometry and TEOAE tests. It was not possible to find any study evaluating the middle ear RF in patients with psoriasis in the literature. MFT is a sensitive measurement method that can detect small changes in mass and solidification within the middle ear system [31]. In one of the studies, RF was evaluated with MFT in patients with RA, an autoimmune disease, and abnormal RF values were obtained in these patients compared to controls. These findings reported that RA might involve the incudostapedial and incudomalleolar joints changing the ossicular mechanics in response to static air pressure modifications [15]. In our study, although the tympanogram results in all individuals were found within standard limits, the RF values of the psoriasis patients were found significantly lower than the controls. The auricle and the external third of the external auditory canal mainly contain elastic cartilage covered with skin and appendages, including hair cells and sebaceous (lipid-producing) and apocrine (ceruminous) glands. Sebumeter shows significantly higher values in non-lesional forearm skin and plaques of psoriatic patients by comparison with the corresponding areas of controls. Cutaneous chronic inflammation and keratinocyte hyperproliferation cause progressive skin thickening, with a tendency to sub-stenosis and cerumen accumulation in the external ear, and stenosis of the tympanic membrane. This pathogenic mechanism is similar to that of keratosis obturans but in a less severe form. The processes mentioned above cause impairment of sound transmission to the middle ear. Additionally in Psoriasis, it is likely that the stiffening of the tympanic-ossicular complex (tympanic membrane alterations and/or arthropathic impairment of the chain of the ossicles) may reduce the elasticity of the system [10]. In our study, the reason as to why RF in psoriasis patients was lower than in the control group could be assumed as being related to the increased stiffness in the middle ear due to soft tissue and inflammation of ligament structures. Yet, further studies are required to evaluate the physiopathology of low RF in psoriasis patients.
Although there are many studies in the literature on the parameters and the disease that may affect MOC reflex [14, 32], we did not encounter any study conducted on patients with psoriasis. In a study evaluating the MOC reflex in AS patients, according to the controls decreased reflex response and suppression were seen in the MOC reflex of the patients [14]. In our research, a significant difference was found in the psoriasis and control groups at 1 kHz frequency while comparing TEOAE amplitudes recorded without and with CAS (p < 0.05). However, the difference was not significant in both ears when contralateral suppression levels in the two groups were compared (p > 0.05). More studies investigating the sensitivity of the peripheral auditory system in psoriasis patients should be carried out.
Our study has some limitations. Disease duration and clinical characteristics such as the severity of the disease were not taken into account. Audiologic evaluation was performed only once, therefore we have no opinion regarding what point of time the middle and inner ear are affected.
Our findings indicate that middle ear mechanics can be affected by psoriasis. There was no significant difference between psoriasis patients and healthy controls in terms of efferent auditory functions. More studies are required to determine whether there is cochlea involvement in psoriasis because of the differences at limited frequency in AC and BC hearing thresholds and TEOAE amplitudes between the groups. Once the psoriasis is diagnosed, it is important to have patients undergo audiological evaluation and to be informed about possible pathological involvement. Future studies should be carried out about middle and inner ear and MOC system functions in psoriasis patients and about interactions that may be connected to the disease characteristics.
Acknowledgements
We would like to thank to Arzu Karataş (M.D.) for the support of this study.
Author Contributions
APP and HSE: idea/concept, design, data collection and/or processing, analysis and/or ınterpretation, literature review, writing the article, critical review, references and fundings, materials. HSE: control/supervision.
Funding
This study was supported by Baskent University Research Fund (Project No: KA15/357).
Data Availability
Not available.
Code Availability
Not available.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
This study was approved by Baskent University Institutional Review Board (Project no: KA15/357).
Informed Consent
Written informed consent was obtained from all participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gudjonsson JE, Elder JT. Psoriasis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick’s dermatology in general medicine. New York: McGraw Hill; 2012. pp. 197–232. [Google Scholar]
- 2.Davidovici BB, Sattar N, Jörg PC, Puig L, Emery P, Barker JN, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Investig Dermatol. 2010;130(7):1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 3.Karabulut H, Karadag AS, Dagli M, Acar B, Babademez MA, Sahin Y, Karasen RM. Investigation of hearing and outer hair cell function of cochlea in patients with psoriasis. J Int Adv Otol. 2010;6(2):239–244. [Google Scholar]
- 4.McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1979;88:585–589. doi: 10.1177/000348947908800501. [DOI] [PubMed] [Google Scholar]
- 5.Maiolino L, Cocuzza S, Conti A, et al. Autoimmune ear disease: clinical and diagnostic relevance in Cogan’s sydrome. Audiol Res. 2017;7(1):162. doi: 10.4081/audiores.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erbek SS, Erbek HS, Yilmaz S, Topal O, Yucel E, Ozluoglu LN. Cochleovestibular dysfunction in ankylosing spondylitis. Audiol Neurootol. 2006;11(5):294–300. doi: 10.1159/000094078. [DOI] [PubMed] [Google Scholar]
- 7.Adam M, Erkan AN, Arslan D, Leblebici B, Ozluoglu L, Akman MN. High frequency sensorineural hearing loss in patients with ankylosing spondylitis: is it an extrarticuler feature of disease? Rheumatol Int. 2008;28(5):413–417. doi: 10.1007/s00296-007-0458-7. [DOI] [PubMed] [Google Scholar]
- 8.Aydın E, Dogan B, Karabacak E, Abuaf OK, Erkul E, Saglam E, et al. Evaluation of hearing with pure tone audiometry and transient evoked otoacoustic emission (TEOAE) in patients with mild to moderate psoriasis. Gulhane Med J. 2015;57(2):98–101. doi: 10.5455/gulhane.178662. [DOI] [Google Scholar]
- 9.Yen YC, Lin YS, Weng SF, Lai FJ. Risk of sudden sensorineural hearing loss in patients with psoriasis: a retrospective cohort study. Am J Clin Dermatol. 2015;16(3):213–220. doi: 10.1007/s40257-015-0117-9. [DOI] [PubMed] [Google Scholar]
- 10.Borgia F, Ciodaro F, Guarneri F, et al. Auditory system involvement in psoriasis. Acta Derm Venereol. 2018;98(7-8):655–659. doi: 10.2340/00015555-2937. [DOI] [PubMed] [Google Scholar]
- 11.Guvenc SC, Turan H, Yilmaz S, Yanik ME, Berada A, Aliagaoglu C. Evaluation of hearing loss in patients with psoriasis. Turkderm. 2012;46(1):15–19. [Google Scholar]
- 12.Semenov YR, Hsiang EY, Huang A, Herbosa CM, Hui X, Kwatra SG, et al. Association between psoriasis with arthritis and hearing impairment in US adults: data from the national health and nutrition examination survey. J Rheumatol. 2019;46(6):587–594. doi: 10.3899/jrheum.171228. [DOI] [PubMed] [Google Scholar]
- 13.Dhar S, Hall JW., III . Overview of otoacoustic emissions. In: Dhar S, Hall JW III, editors. Otoacoustic emissions: principles, procedures, and protocols. USA: Plural Publishing; 2011. [Google Scholar]
- 14.Beyazal MS, Ozgur A, Terzi S, Celiker M, Dursun E. Medial olivocochlear reflex in ankylosing spondylitis patients. Z Rheumatol. 2016;75(10):1016–1020. doi: 10.1007/s00393-016-0100-8. [DOI] [PubMed] [Google Scholar]
- 15.Colletti V, Fiorino FG, Bruni L, Biasi D. Middle ear mechanics in subjects with rheumatoid arthritis. Audiology. 1997;36:136–146. doi: 10.3109/00206099709071967. [DOI] [PubMed] [Google Scholar]
- 16.Gurer MA, Gokalp H. Psoriasis ve obesity. Turkderm. 2012;46(1):3–6. doi: 10.4274/turkderm.98215. [DOI] [Google Scholar]
- 17.American Speech-Language-Hearing Association (2015) Type, degree, and configuration of hearing loss. ASHA, Audiology information series 10802: 2. https://inte.asha.org/siteassets/ais/ais-hearing-loss-types-degree-configuration.pdf. Accessed 20 Jan 2022
- 18.Belgin E. Speech audiometry. In: Erol B, editor. Basic audiology. Ankara: Güneş Medical Bookstore; 2015. pp. 77–82. [Google Scholar]
- 19.Kilincarslan AS. Standardization of phonetically balanced monosyllabic word lists developed for Turkish language (Master’s Thesis) Ankara: Hacettepe University; 1986. [Google Scholar]
- 20.Saleem MD, Feldman SR. Comorbidities in patients with psoriasis: the role of the dermatologist. J Am Acad Dermatol. 2017;77(1):191–192. doi: 10.1016/j.jaad.2017.01.057. [DOI] [PubMed] [Google Scholar]
- 21.Panda N. Understanding autoimmune ear disease a potentially treatable cause of Deafness. Indian J Otolaryngol Head Neck Surg. 2001;53(4):257–260. doi: 10.1007/BF02991541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes GB, Barna BP. Autoimmune inner ear disease: fact or fantasy? Bear Basic Res Clin Otolaryngol. 1991;46:82–91. doi: 10.1159/000419965. [DOI] [PubMed] [Google Scholar]
- 23.Kumar BN, Smith MSH, Walsh RM, Green JRB. Sensorineural hearing loss in ulcerative colitis. Clin Otolaryngol Allied Sci. 2000;2(2):143–145. doi: 10.1046/j.1365-2273.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 24.Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn's disease. J Eur Acad Dermatol Venereol. 2009;23(5):561–565. doi: 10.1111/j.1468-3083.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 25.Baskan EB. Biologic agents in psoriasis/psoriaziste biyolojik ajanlar. Arch Turk Dermatol Veneorol. 2008;42(2):42–51. [Google Scholar]
- 26.Amor-Dorado JC, Barreira-Fernandez MP, Pina T, Vazquez- Rodriguez TR, Llorca J, Gonzalez Gay MA. Investigations into audiovestibular manifestations in patients with psoriatic arthritis. J Rheumatol. 2014;41(10):2018–2026. doi: 10.3899/jrheum.140559. [DOI] [PubMed] [Google Scholar]
- 27.Giani T, Simonini G, Lunardi C, Puccetti A, De Martino M, Falcini F. Juvenile psoriatic arthritis and acquired sensorineural hearing loss in a teenager: is there an association? Clin Exp Rheumatol. 2006;24(3):344–346. [PubMed] [Google Scholar]
- 28.Gunes A, Gundogdu I, Mutlu M, Ozturk EA, Cakci A, Akin I. Functions of the inner ear in psoriatic arthritis. Auris Nasus Larynx. 2016;43(6):626–631. doi: 10.1016/j.anl.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Akdag M, Uçmak D, Özkurt FE, Bozkurt M, Akkurt ZM, Topçu İ. Evaluation of hearing and outer hair cell function of cochlea in patients with psoriatic arthritis. Clin Exp Otorhinolaryngol. 2015;8(3):183–188. doi: 10.3342/ceo.2015.8.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srikumar S, Deepak MK, Basu S, Kumar BN. Sensorineural hearing loss associated with psoriatic arthritis. J Laryngol Otol. 2004;118(11):909–911. doi: 10.1258/0022215042703813. [DOI] [PubMed] [Google Scholar]
- 31.Iacovou E, Vlastarakos PV, Ferekidis E, Nikolopoulos TP. Multi-frequency tympanometry: clinical applications for the assessment of the middle ear status. Indian J Otolaryngol Head Neck Surg. 2013;65(3):283–287. doi: 10.1007/s12070-011-0470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parthasarathy TK. Aging and contralateral suppression effects on transient evoked otoacoustic emissions. J Am Acad Audiol. 2001;12(2):80–85. doi: 10.1055/s-0042-1745583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not available.
Not available.




