Abstract
Inflammatory myofibroblastic tumours are rare lesions that could occur in airways. We report a 10 years old male who complains about dyspnea after physical exercise. Making CT and RMI images and a biopsy, we make an accurate diagnosis of an inflammatory myofibroblastic tumour ALK+. After treatment with a subtotal resection and crizotinib as adjuvant, we achieve a complete remission.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12070-023-03775-5.
Keywords: Dyspnea, neoplasms, operative procedures, chemotherapy
Introduction
An inflammatory myofibroblastic tumour (IMT) is a rare neoplasm with an intermediate malignant potential [1–4] due to the high rate of local relapses and the possibility of metastasizing [2, 4]. It mainly occurs in children and young patients and usually appears in the lower respiratory tract, abdomen and pelvis, but it’s possible the development in many other areas [1, 2].
A chromosomal rearrangement involving the anaplastic lymphoma kinase receptor (ALK) gene exists in 50% of IMTs [2, 3, 5–7]. This fact implies the production of ALK fusion proteins which have potential oncogenic functions [4].
Clinical manifestations depend on the place of appearance [1, 4] being stridor, dyspnea and cough the most common symptoms in case of affectation of the trachea.
[1, 7]. A non-specific syndrome with fever and weight loss with raised inflammatory markers has been reported too [1, 4], and occurs in 15–30% of cases [4].
The optimal treatment is the complete surgical resection [2, 4, 6, 7]. Nevertheless, there are some cases where this is not possible, and chemotherapy and radiotherapy could be employed as exclusive or adjuvant treatment [2, 6].
Case Report
A 10 years old male is attended by his paediatrician due to respiratory distress and cough after intense physical exercise. Initially he starts treatment with antibiotics and anti-inflammatory therapy without achieving a complete remission of his symptoms.
A few weeks later, he goes to the emergency because of the increase of the intensity of the dyspnea. Now he has problems for breathing after making physical activity and resting. After rejecting bronchopulmonary pathology, the patient is examined by ENT.
The nasofibroscopic examination shows a subglottic stenosis over 50% of tracheal lumen (Cotton Meyer grade II) with normal activity of both cords.
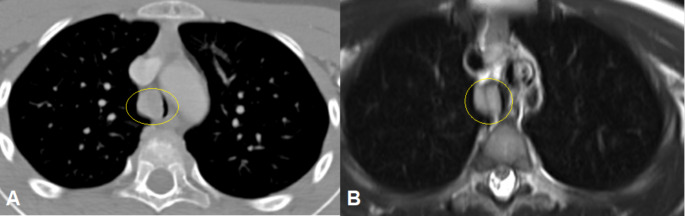
A cervical CT is requested. The images show a rounded and homogeneous lesion with soft tissue density in the right wall of the trachea 9 mm over the carina. Its size is 18 × 15 × 12 mm and it causes a significant stenosis of the tracheal lumen (Fig. 1A).
Fig. 1.
CT (A) and T2 RMI (B) imagesof the lesion before starting the treatment. They show a mass with soft tissuedensity occluding more than 50% of tracheal lumen (Circle).
A cervical MRI is also requested for completing the study. The images show a hyperintense mass in T2 with enhancement with gadolinium contrast (Fig. 1B).
A biopsy is made. The lesion is composed of neoplastic myofibroblasts in a myxoid stroma with other inflammatory cells. These myofibroblasts are immunoreactive for vimentin, smooth muscle actin and muscle specific actin. There is cytoplasmic immunoreactivity with anaplastic lymphoma kinase too.
We make the diagnosis of inflammatory myofibroblastic tumour ALK+.
Under general anaesthesia, a partial resection of the mass is made by microscopic translaryngeal approach, employing CO2 laser to eliminate approximately the 90% of the mass, respecting the tracheal mucosa and the pathological tissue closest to the tracheal wall. Afterwards, he stars treatment with oral crizotinib 200 mg twice a day for 6 months.
The follow up is made with a quarterly MRI during the first year, and biannual during the second one. After two years, we offer an annual MRI if there are not new symptoms.
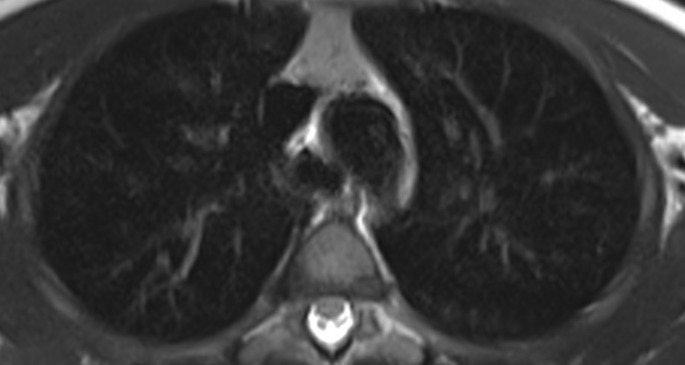
Nowadays, 2 years after the treatment we achieve a complete remission. There is a complete removal of the lesion in the last RMI images (Fig. 2).
Fig. 2.
T2 RMI image of the tracheal lumen two years after starting treatment and one year after finishing it
Discussion
An inflammatory myofibroblastic tumour is a mesenchymal neoplasm with neoplastic myofibroblasts in a myxoid to collagenous stroma admixed with inflammatory cells [1–6, 8–11] with 150–200 new cases reported annually in the USA [9]. It mainly affects young people [2,4–7,9-,11] and the main places of appearance is lower airway, abdomen and pelvis [3–7, 10, 11]. Local recurrences may occur after primary surgery, with a low risk of distant metastases [8]. This is the reason because of it’s considered a tumour with intermediate biologic potential [1–11].
Clinical manifestations depend on the place of appearance [1, 2, 4, 10]. A non-specific syndrome with fever and weight loss with raised inflammatory markers occurs in 15–30% of cases [4, 10].
Rearrangements involving the ALK locus on chromosome 2p23 happen in 50% of IMTs [2–11]. There are many fusion partners thar serve to activate ALK [8, 9]. This fact implies the production of ALK fusion proteins which have potential oncogenic functions [4]. To differentiate ALK positive and ALK negative IMTs, cytoplasmic immunoreactivity with anaplastic lymphoma kinase must be studied, and it occurs in approximately half of cases [11].
In the past, ALK positivity was recognized as bad prognosis factor in comparation with negativity due to a higher tendency for recurrence, large size or abdominopelvic place [2, 3]. Current studies show that this is not real, because ALK negative IMTs are related with a higher rate of metastases and deaths by the tumour [10, 11]. The metastases from IMTs have a predilection for lung, brain, liver and bones [10].
The differential diagnosis is extensive and depends on the anatomic site. It includes the inflammatory pseudotumour, denditric cell neoplasm, low grade sarcomas as myxoid fibrosarcoma, sarcomatoid variant of anaplastic large cell lymphoma and nodular fasciitis [11].
For making an accurate diagnosis, the immunohistochemical studies are essential. The myofibroblastic cells are frequently immunoreactive for vimentin, smooth muscle actin, muscle specific actin, and less commonly for desmin, cytokeratin and CD68 [11]. They are no immunoreactive for S-100, CD117, CD23 and c-kit [2]. Cytoplasmic immunoreactivity with anaplastic lymphoma kinase occurs in 50% of cases [11].
The main treatment is surgical. When possible, a total resection of the lesion is curative [2, 4, 6, 7, 9]. Nevertheless, the upper airway adds an extra level of complexity because the healing process may result in stenosis as result of inflammation and scar formation [7].
There are some reported surgical techniques as the electrocautery or the KTP laser with very good results [7].
In cases where surgical treatment is not possible (cases with locally advanced neoplasm), there is multifocal or metastatic disease, or it’s not possible achieving a complete resection of the lesion, radiotherapy and chemotherapy are useful tools [2–4, 9]. There are many combinations reported employing chemoterapics, but there is not a standard treatment. [9]. Crizotinib is an ALK inhibitor useful in ALK positive cases [2, 3, 6, 9]. Due to its efficacy, the U.S National Comprehensive Cancer-Network recommends the use of crizotinib as the standard of care for locally advanced or metastatic ALK positive IMT [9]. In this case, we achieve a nearly total resection, so we employ crizotinib as adjuvant treatment. Nowadays, two years after the end of treatment, this patient has no relapse.
Conclussion
Inflammatory myofibroblastic tumours are rare in upper airways. Its treatment and prognosis are different in relation with a rearrangement involving the ALK locus on chromosome 2p23. The optimal treatment is the total resection. In other cases where there is not possibility of achieving a complete resection, crizotinib could be employed as adjuvant tool in ALK + neoplasms.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to this excellent journal for employing some time reading and assessing our paper. It is an uncommon case and we hope you find it interesting as we have considered it. We wish to publish this and other future papers in this journal.
Author Contribution
The entire author contributed equally to this work. Each one made a part of the paper and then we supervised the final article together.
Funding
There are no funding for preparing this paper.
Declarations
The authors are in accordance with the Code of Ethics of the World Medical Association (Helsinki Declaration).
Ethical Approval
It is not necessary an ethical approval due the absence of investigation with patients.
Conflicts of Interest
There are no conflicts of interest or supports for writing and preparing this paper.
Informed Consent
Parents ‘patient have given their consent for preparing and publishing this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar N, Saravanamuthu T, Srinivasan A, Raman T, Scott JX Pediatric Inflammatory Myofibroblastic Tumors of the Airway: two case reports with varying clinical presentation.Iran J Otorhinolaryngol. 2018 May; 30(98):171–176. PMID: 29876333; PMCID: PMC5985620. [PMC free article] [PubMed]
- 2.Lisi R, Abate G, D’Urso P, Martinetti MT, Siniscalchi B, Marampon F, Bulzonetti N, Tombolini V (2019 Dec) Successful role of adjuvant radiotherapy in a rare case of tracheal inflammatory myofibroblastic tumor: a case report. Tumori 105(6):NP1–NP3. 10.1177/0300891619838333Epub 2019 Mar 22. PMID: 30900517 [DOI] [PubMed]
- 3.Pecoraro Y, Diso D, Anile M, Russo E, Patella M, Venuta F (2014 Dec) Primary inflammatory myofibroblastic tumor of the trachea. Respirol Case Rep 2(4):147–149. 10.1002/rcr2.81Epub 2014 Oct 15. PMID: 25530866; PMCID: PMC4263498 [DOI] [PMC free article] [PubMed]
- 4.Liu W, Duan Q, Gong L, Yang Y, Huang Z, Guo H, Niu X (2021) Feb;39(1):278–282 A novel LRRFIP1-ALK fusion in inflammatory myofibroblastic tumor of hip and response to crizotinib. Invest New Drugs. doi: 10.1007/s10637-020-00984-5. Epub 2020 Sep 11. PMID: 32915420 [DOI] [PubMed]
- 5.Elktaibi A, Benzerdjeb N, Ameur F, Daveau C, Tantot J, Costes Martineau VA, Novel (2020 Jun) ALK-THBS1 Fusion in a laryngeal inflammatory myofibroblastic tumour: a Case Report and Literature Review. Head Neck Pathol 14(2):454–458. 10.1007/s12105-019-01061-xEpub 2019 Jul 31. PMID: 31368077; PMCID: PMC7235104 [DOI] [PMC free article] [PubMed]
- 6.Theilen TM, Soerensen J, Bochennek K, Becker M, Schwabe D, Rolle U, Klingebiel T, Lehrnbecher T Crizotinib in ALK+ inflammatory myofibroblastic tumors-current experience and future perspectives.Pediatr Blood Cancer. 2018Apr; 65(4). doi: 10.1002/pbc.26920. Epub 2017 Dec 29. PMID: 29286567. [DOI] [PubMed]
- 7.Kieu MQ, Thottam PJ, DaCosta V, Gonzalez-Krellwitz L, Poulik JM, Madgy DN (2014) Nov;28(6):841.e1-4 Treatment of inflammatory myofibroblastic tumor of the subglottis with KTP laser: a case report. J Voice. doi: 10.1016/j.jvoice.2014.03.016. Epub 2014 Jun 24. PMID: 24972538 [DOI] [PubMed]
- 8.Butrynski JE, D’Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ, Ramaiya N, Kwak EL, Clark JW, Wilner KD, Christensen JG, Jänne PA, Maki RG, Demetri GD, Shapiro GI (2010) Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. Oct 28;363(18):1727-33. doi: 10.1056/NEJMoa1007056. PMID: 20979472; PMCID: PMC3014292 [DOI] [PMC free article] [PubMed]
- 9.Casanova M, Brennan B, Alaggio R, Kelsey A, Orbach D, van Noesel MM, Corradini N, Minard-Colin V, Zanetti I, Bisogno G, Gallego S, Merks JHM, De Salvo GL, Ferrari A (2020 Mar) Inflammatory myofibroblastic tumor: the experience of the european pediatric soft tissue Sarcoma Study Group (EpSSG). Eur J Cancer 127:123–129 Epub 2020 Jan 30. PMID: 32007712 [DOI] [PubMed]
- 10.Coffin CM, Hornick JL, Fletcher CD Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007 Apr;31(4):509 – 20. doi: 10.1097/01.pas.0000213393.57322.c7. PMID: 17414097 [DOI] [PubMed]
- 11.Gilani SM, Kowalski PJ (2014 Mar) Inflammatory myofibroblastic tumour: a rare entity with wide differential diagnosis. Pathologica 106(1):1–6 PMID: 24897773 [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.