Abstract
Aims
The present study examines the role of demographic and pathological features of primary tumours in predicting neck metastasis in early oral cavity cancers, which has been a matter of debate.
Methods
A single-centre, retrospective, institution review was conducted of all the patients presented to our centre from January 2014 to December 2021. Patient characteristics were compared between the two lymph node groups (lymph node positive and lymph node negative) and significant prognostic factors were determined.
Results
A total of 462 oral squamous cell carcinoma (OSCC) patients were included, 407 male and 55 female. Tobacco chewing (59.2%) was a major habit with buccal mucosa (49.5%) and tongue (44.8%) as primary sites. The majority of the patient’s histology was of SCC (96.8%) with grade II (moderately differentiated, 74.5%). Univariate logistic regression analysis to predict lymph node metastasis showed pT size (< 0.001), LVI (< 0.001), and PNI (< 0.001) as significant tumor characteristics. On multivariate, pT size (OR-1.58, P – 0.0001) and LVI (OR-19.70, P – 0.0001) were reported to be statistically significant to predict lymph node metastasis.
Conclusion
Reporting and studying the clinico-pathological features of primary tumors can give vital information in predicting the neck node metastasis in OSCC patients.
Keywords: Depth of invasion, Lymphovascular invasion, Perineural invasion, Neck node metastasis, Oral cavity cancers
Introduction
Oral cancer of the head and neck is the most common and aggressive form of malignancy with a poor prognosis [1]. The oral squamous cell carcinoma (OSCC) has an especially high propensity for metastasis, so it is often controlled through traditional surgical resection (elective neck dissection - END) or irradiation of the primary tumor, with treatment of the clinically positive neck [2].
The American Joint Committee on Cancer (AJCC)/ International Union Against Cancer (UICC) Tumor-Node‐Metastasis (TNM) staging system is the most widely used staging scheme in patients with cancer, defining prognosis, and guiding the most appropriate treatment plans [3]. Recently, AJCC in its 8th edition has proposed to incorporate the depth of invasion (DOI) as a modifier for the T category in the TNM staging [2, 4, 5]. Substantially, histopathologic evidence such as DOI of the primary tumor (thin, ≤ 5 mm,T1; intermediate, > 5 mm and ≤ 10 mm, T2; and thick, > 10 mm, T3/T4) has also identified as a possible predictor of cancer growth beneath the epithelial surface and regional metastasis in OSCC [2, 3]. Apart from DOI, the role of other histological adverse pathological features such as lymphovascular invasion (LVI), perineural invasion (PNI), tumour thickness (TT), and poor differentiation were also incorporated for initial risk group stratification, treatment decision, and to predict the need for END based on the tumour behaviour and the risk of recurrence/ occult lymph node metastasis (LNM) [6–10]. Studies have also established these pathological features as poor prognostic factors in many human malignancies [10–12].
LNM tumors occur in about 40% of patients with oral cancer [13]. Among all oral cancers, OSCC and oral tongue have high potential for local invasion and cervical lymph node metastasis. At the time of initial diagnosis, identifying the presence of such node metastasis can play a crucial in patient’s treatment planning, prognosis, and survival [14]. LNM is also a well-known and clinically important prognostic factor in head neck cancer patients [15–17].
This retrospective study was aimed to investigate the role of various demographical (age) and pathological features (Grade, LVI, PNI, DOI) of primary tumours of the patients in predicting the neck metastasis in early oral cavity cancers.
Methods
A single-centre, retrospective, institution review was conducted of all the patients presented to our centre from January 2014 to December 2021.
Study Population
Inclusion criteria:
-
A)
Clinically early stage oral cavity cancer.
-
B)
Pathological T (pT) size ≤ 4 cm with SCC histology.
-
C)
With complete histopathological information available.
Exclusion criteria:
-
A)
Presence of a synchronous head and neck SCC.
-
B)
History of head and neck cancer/ or previous resection of an oral cancer.
-
C)
Previous treatment with chemotherapy and/or radiation specifically for head and neck cancer.
-
D)
Distant metastasis at presentation.
-
E)
Histology’s other than SCC.
-
F)
Missing histopathological data.
Statistical Analysis
A total of 478 patients were included in this retrospective study. Sixteen of 478 patients were excluded for not meeting the study criterion. All demographic and clinical characteristics were recorded from the case record forms of patients maintained in an electronic database. Data of DOI, tumor characteristics, clinical N-classification, LVI, and PNI were reviewed [7, 8, 10, 18].
Patient characteristics were compared between the two LN groups (lymph node positive and lymph node negative) using Chi-square test for categorical data. A Receiver-Operator-Curve (ROC) analysis was performed to determine the optimal cut-off values of DOI with LN metastases. Univariate logistic regression analysis was used to assess relations between predictor variables (i.e. demographic and all histopathological factors) and lymph node status. A multiple logistic regression model was constructed using forward selection. For all tests, a p-value of < 0.05 was considered statistically significant. All statistical data analyses were performed using SPSS (IBM SPSS Statistics version 22.0, IBM Corp., USA).
Results
Over a period of 8 years (January 2014- December 2021), a total of 462 OSCC patients have met all our study criteria and were analysed retrospectively. All demographic and clinical characteristics are summarized in Table 1. Of patients, a distinct male (88.1%) predominance was observed with average age at presentation being 46.5 years (IQR: 21–89 years). Almost 62% of patients were of age < 50 years with no co-morbidities (83.1%) on presentation. Tobacco chewing (59.2%) was a major habit with buccal mucosa (49.5%) and tongue (44.8%) as primary sites. Majority of the patient’s histology was of SCC (96.8%) with grade II (moderately differentiated, 74.5%). In 462 patients, 410 patients have undergone surgery/treatment at our centre and the remaining 52 patients were referred to our centre from outside after their surgery for adjuvant treatment (either adjuvant RT or adjuvant CTRT).
Table 1.
Baseline patients and tumour characteristics of the study cohort (n = 462)
| Patients characteristics | Number (%) | |
|---|---|---|
| Age | Median (Range) | 46.5 (21–89) |
| Gender |
Male Female |
407 (88.1%) 55 ( 11.9%) |
| Age group |
< 30 31–40 41–50 51–60 61–70 > 70 |
15 (3.2%) 110 (23.8%) 160 (34.6%) 111 (24%) 53 (11.5%) 13 (2.8%) |
| Comorbidities |
Hypertension DM IHD Others No |
31 (6.7%) 31 (6.7%) 4 (0.1%) 12 (2.6%) 384 (83.1%) |
| Habits |
Alcohol + tobacco Tobacco Bidi/Cigarette smoking Others No |
42 (9.2%) 273 (59.2%) 13 (2.8%) 16 (3.6%) 116 (25.2%) |
| Primary sites |
Lip Hard palate Alveolus Buccal mucosa Tongue Floor of mouth |
9 (1.9%) 4 (0.9%) 10 (2.1%) 228 (49.1%) 207 (44.8%) 3 (4.9%) |
| Laterality |
Right Left Midline |
212 (46%) 240 (52%) 10 (2%) |
| Tumour characteristics | ||
| Histology |
SCC Verrucous carcinoma Sarcomaoid SCC |
447 (96.8%) 10 (2.2%) 5 (1.0%) |
| Grade |
Grade I (WD) Grade II (MD) Grade III (WD) |
76 (16.5%) 344 (74.5%) 42 (9%) |
| Lympho-vascular invasion |
Positive Negative |
97 (21%) 365 (79%) |
| Perineural invasion |
Positive Negative |
137(29.7%) 325 (70.3%) |
| Margin status |
Positive Close margin Negative |
9 (1.9%) 111 (24%) 342 (74.1%) |
| LN status |
Positive Negative |
153 (33.1%) 309 (66.9%) |
| DOI |
< 0.4 > 0.4 |
109 (23.6%) 353 (76.4%) |
DM Diabetes mellitus, IHD Ischemic heart disease, SCC Squamous cell carcinoma, WD Well differentiated, MD Moderately differentiated, PD Poorly Differentiated, LN Lymph Node, DOI Depth of Invasion
More than half of the patient’s pathological characteristics such as LVI (21%), PNI (29.7%), LN (33.1%), and margin status (1.9%) were reported to be positive.
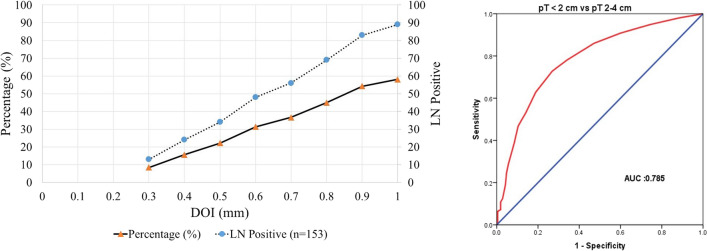
A total 153 of 462 patients were LN positive. Most of them were under the age of ≤ 50 years (70%) with grade I/II tumors (86%), size ranging from 2 to 4 cm (72%). On univariate analysis (Table 2), the most significant tumor influencing characteristics between LN negative and LN positive patients were reported to be pT size (< 0.001), LVI (< 0.001), PNI (< 0.001) followed by DOI (0.002), age (0.018), and tumor grade (0.036), respectively. DOI (> 0.5 cm, 78%) was observed to be the most significant factor compared to other pathological parameters and patients with DOI ≤ 0.5 cm (22%). On multivariate analysis of LN positive patients (Table 3), pT size (OR-1.58, P – 0.0001) and LVI (OR-19.70, P–0.0001) were reported to be the most statistically significant factors. A proportional increase in LN positivity was reported with increase in DOI (Fig. 1) in LN positive patients. From ROC, the area under ROC (AUROC) was observed to be 0.78 (close to 1, Fig. 1) confirming its accuracy.
Table 2.
Univariate analysis of tumor characteristics in lymph node negative and lymph node positive patients
| Tumour characteristics | Lymph node Negative |
Lymph node Positive |
P-value | |||
|---|---|---|---|---|---|---|
| Number (n = 309) | (%) | Number (n = 153) | (%) | |||
| Age (years) |
≤ 50 > 50 |
179 130 |
58% 42% |
106 47 |
70% 30% |
0.018 |
| History of addiction |
Yes No |
222 87 |
72% 28% |
122 31 |
80% 20% |
0.067 |
| pT Size |
0–2 cm 2–4 cm |
169 140 |
54% 46% |
43 110 |
28% 72% |
< 0.001 |
| Subsite* |
L/B/A Tongue / FOM |
171 138 |
56% 44% |
80 73 |
53% 47% |
0.535 |
| Grades |
Grade I / II Grade III |
287 22 |
92% 8% |
133 20 |
86% 14% |
0.036 |
| LVI |
Positive Negative |
15 294 |
5% 95% |
82 71 |
53% 47% |
< 0.001 |
| PNI |
Positive Negative |
67 242 |
21% 79% |
70 83 |
45% 55% |
< 0.001 |
| DOI (cm) |
≤ 0.5 > 0.5 |
113 196 |
36% 64% |
34 119 |
22% 78% |
0.002 |
pT Tumor size, DOI Depth of invasion, LVI Lympho vascular invasion, PNI Perineural invasion, LBA Lip/ BM/ Alveolus
*Data is dichotomised in Tongue versus Lip/BM/Alveolus
# Data is dichotomised in Positive versus Close and Negative margin
$ Data is dichotomised in Grade III versus Grade I/II
Table 3.
Multivariate analysis of prognostic factors in lymph node positive patients
| Prognostic factors | Lymph node Positive |
||||
|---|---|---|---|---|---|
| Odds ratio (OR) | 95% CI | p - value | |||
| Age (years) |
< 50 (Ref) > 50 |
1.58 | 0.95 | 2.62 | 0.076 |
| pT (cm) |
2–4 (Ref) ≤ 2 |
2.75 | 1.59 | 4.75 | < 0.0001 |
| Grade |
Grade III (Ref) Grade I / II |
1.38 | 0.60 | 3.14 | 0.44 |
| DOI (cm) |
> 0.5 ≤ 0.5 |
0.98 | 0.54 | 1.78 | 0.95 |
| LVI |
Present Absent |
19.70 | 10.31 | 37.63 | < 0.0001 |
| PNI |
Present Absent |
1.23 | 0.70 | 2.16 | 0.46 |
pT Tumor size, DOI Depth of Invasion, LVE Lympho vascular invasion, PNI Perineural Invasion
Fig. 1.
Significance of depth of invasion on lymph node positivity (on left). Receiver operator characteristic curve (ROC) for differentiating depth of Invasion among the defined groups of oral squamous cell carcinoma based on pT < 2 cm and pT 2–4 cm (on right)
Discussion
OSCC was reclassified in AJCC 8th edition by including DOI in the pT staging and clinical management guidelines [9].
The trend of increase in incidence of OSCC has increased many folds over the decades, especially in young males (< 50 years) [19, 20]. Such male preponderance of OSCC over females was quite evident from our study and same pattern was reported earlier in multiple studies [6, 9, 21, 22]. This trend might be due to the high oral habits of Southern Asian and East Asian population compared to West [1, 23]. The primary site was in line with the habits possessed by the patients, where the buccal mucosa and tongue are the most effected [6, 7, 18, 22]. As reported in studies [6, 7, 18, 22, 24], most of the patients with OSCC were also reported to have moderately differentiated tumor characteristics (grade II), strongly predicting the locoregional recurrence and occult metastasis [25, 26].
Node metastasis is another important prognostic factor, influenced by many other factors other than DOI such as LVI, PNI, and LN status. The AJCC 8th edition recommendation was to keep the DOI cut-off for decision making on ENDs at ≥ 4 mm, but the studies published later have showed some large variances in cut-off values. For instance, Tam et al. showed 7.25 mm, Faisal et al. showed 10 mm, Yassine et al. and Lanschot et al. confirmed at 4 mm, Brockhoff et al. calculated the cut-off values based on the sub-sites (i.e., tongue = 2 mm, floor of mouth = 2–3 mm, retromolar trigone/alveolus/hard palate = 3–4 mm, and all sites = 2–4 mm), and Kozak et al. did not specified any DOI cut-off value itself [5–7, 27–29]. Therefore, the cut-off values based on the sub site should be validated in future studies, based on the tumor and its distance from the lymphatic vessels. Such cut-off values will be playing a vital role in metastasis management and prognosis of the patient. Based on the popular reports published after AJCC 8th edition recommendation, we have considered a DOI cut-off of ≥ 4 mm for all ENDs in our patients.
Other than DOI, other tumor and pathological characteristics like pT size, differentiation grade, margin status, LVI, and PNI were also observed to have significant role in LNM. In the present study among all the pathological characteristics, the pT size (p < 0.001), differentiation grade (p < 0.036), LVI (p < 0.001), and PNI (p < 0.001) were observed to be the most significant factors compared to others in LNM. Findings from a meta-analysis by Shuojin et al. have also reported the importance of LVI as a prognostic predictor for metastasis and prognosis in patients with OSCC [30]. Mascitti et al. have also conducted a review on clinical and prognostic role of LVI in OSCC and confirmed it as a useful marker to better define the therapeutic strategies in OSCC patients [31]. Wei et al. published the importance of investigated features such as margin status, PNI, LVI, and DOI in patients with a pN0 status. The incidence of PNI in wide variety of head-and-neck malignancies ranges from 2.5 to 5.0% [32]. In our study, the incidence of PNI (29.7%) was high compared to existing literature. However, in some exceptional studies the percentage of PNI was observed to be as high as 45.27% [33].
On univariate and multivariate analysis, the strongest prognostic predictor in both the LN negative and LN positive patients leading to their poor prognosis and survival was reported to be pT size and LVI. On multivariate analysis of their retrospective data, Larson et al.[34] have also reported a strong prediction of recurrence and poor survival in the patients with two or more prognostic factors in the tumor such as the DOI > 4 mm and LVI. With increase in DOI, the proportionality for LN positivity and metastasis was also observed to be increasing and it was proven by literature [6, 7, 24, 29].
Altogether, timing chosen to evaluate the pathological features were proven to be of greater value. Earlier, all the evaluations were usually done post-surgical leading to re-exploration in exceptional cases based on the final report. To avoid such circumstances, in identifying such occult LNM. At present, performing of pre-operative MRI or intra-operative assessments or sentinel lymph node biopsies (SLNB) were highly encouraged.[29, 35] The intra-operative assessment of resection margins (i.e. frozen section analysis) is also now recommended as a standard of care by the AJCC 8th edition. Brockhoff et al. have also reported the success of such intra-operative assessments and Tim et al. also proven the high sensitivity (95%) of SLNB supporting its role in the diagnostic work-up of OSCC [29, 35]. Like studies conducted by Barroso et al. and Santos et al., recently Lanschot et al. are also investigating the use Raman spectroscopic method pre-operatively to discriminate the OSCC from surrounding healthy tissues.[7, 36,37] The strength of the present study includes its large number of patient population and robust data for analysis. However, the major limitation of this study is its retrospective study design.
Conclusion
Overall results from our study confirms that these clinical and pathological features of primary tumours can give a vital information in predicting the neck node metastasis in OSCC patients.
Acknowledgements
The authors would like to thank Dr. Yasam Venkata Ramesh from HCG Manavata cancer centre, Centre for difficult cancers (CDC), Nashik, India, for his medical writing assistance.
Author Contributions
Concept–PP, RKP, VP; Design–PP, RKP, VP; Supervision–PP, RKP, VP, RN; Materials–PP, RKP, VP, SG, DM, RP, SR, RN; Data Collection and/or Processing–PP, RKP, VP, SG, DM, RP, SR, YVR, VRN, RN; Analysis and/or Interpretation–PP, RKP, VP, SG, DM, RP, SR, YVR, RN; Literature Search—PP, RKP, YVR; Writing Manuscript—YVR; Critical Review—PP, RKP, RN.
Funding
The authors declared that this study has received no financial support.
Data Availability
The data analysed during the current study are not publicly available. They are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for Publication
All the authors have given their consent for publication.
Ethics Approval
The manuscript has got an ethical review exemption from the Ethical review committee of our hospital as retrospective studies are exempted from review according to our ERC’s policy.
Informed Consent
Written informed consent was obtained from all the participants.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ren ZH, Hu CY, He HR, et al. Global and regional burdens of oral cancer from 1990 to 2017: results from the global burden of disease study. Cancer Commun. 2020;40:81–92. doi: 10.1002/CAC2.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuan EC, Clair JMS, Badran KW, St. John MA. How does depth of invasion influence the decision to do a neck dissection in clinically N0 oral cavity cancer? Laryngoscope. 2016;126:547–548. doi: 10.1002/LARY.25707/FULL. [DOI] [PubMed] [Google Scholar]
- 3.Zanoni DK, Patel SG, Shah JP. Changes in the 8th edition of the American joint committee on c(AJCC) staging of head and neck cancer: rationale and implications. Curr Oncol Rep. 2019;21:52. doi: 10.1007/S11912-019-0799-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quintin A, Pontejos Y, Del Mundo DAA. The role of neck dissection in oral cavity carcinoma. Oral Dis. 2020 doi: 10.5772/INTECHOPEN.90925. [DOI] [Google Scholar]
- 5.Faisal M, Bakar MA, Sarwar A, et al. Depth of invasion (DOI) as a predictor of cervical nodal metastasis and local recurrence in early stage squamous cell carcinoma of oral tongue (ESSCOT) PLos One. 2018;13(8):0202632. doi: 10.1371/journal.pone.0202632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aaboubout Y, van der Toom QM, de Ridder MAJ, et al. Is the depth of invasion a marker for elective neck dissection in early oral squamous cell carcinoma? Front Oncol. 2021;11:434. doi: 10.3389/FONC.2021.628320/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Lanschot CGF, Klazen YP, de Ridder MAJ, et al. Depth of invasion in early stage oral cavity squamous cell carcinoma: the optimal cut-off value for elective neck dissection. Oral Oncol. 2020;111:104940–104940. doi: 10.1016/J.ORALONCOLOGY.2020.104940. [DOI] [PubMed] [Google Scholar]
- 8.Eryılmaz MK, Korkmaz M, Karaağaç M, Artaç M. Perineural invasion is a better prognostic factor than extranodal extension in head and neck cancer. Egypt J Otolaryngol. 2022;381(38):1–8. [Google Scholar]
- 9.Subramaniam N, Balasubramanian D, Murthy S, et al. Predictors of locoregional control in stage I/II oral squamous cell carcinoma classified by AJCC 8th edition. Eur J Surg Oncol. 2019;45:2126–2130. doi: 10.1016/J.EJSO.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Ting KC, Lee TL, Li WY, et al. Perineural invasion/lymphovascular invasion double positive predicts distant metastasis and poor survival in T3–4 oral squamous cell carcinoma. Sci Rep. 2021 doi: 10.1038/S41598-021-99280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones HB, Sykes A, Bayman N, et al. The impact of lymphovascular invasion on survival in oral carcinoma. Oral Oncol. 2009;45:10–15. doi: 10.1016/J.ORALONCOLOGY.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Chatzistefanou I, Lubek J, Markou K, Ord RA. The role of perineural invasion in treatment decisions for oral cancer patients: a review of the literature. J Craniomaxillofac Surg. 2017;45:821–825. doi: 10.1016/J.JCMS.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Metastasis from oral cancer: an overview - PubMed. https://pubmed.ncbi.nlm.nih.gov/22990112/. Accessed 24 May 2022
- 14.Choi KY, Park SC, Kim JH, Lee DJ. The occult nodal metastasis rate of early tongue cancer (T1–T2): a protocol for a systematic review and meta-analysis. Medicine. 2021 doi: 10.1097/MD.0000000000024327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prognostic significance of the distribution of neck node metastasis from oral carcinoma - PubMed. https://pubmed.ncbi.nlm.nih.gov/10748442/. Accessed 24 May 2022 [DOI] [PubMed]
- 16.Gil Z, Carlson DL, Boyle JO, et al. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer. 2009;115:5700–5710. doi: 10.1002/CNCR.24631. [DOI] [PubMed] [Google Scholar]
- 17.Cho JK, Hyun SH, Choi N, et al. Significance of Lymph Node Metastasis in Cancer Dissemination of Head and Neck Cancer. Transl Oncol. 2015;8:119. doi: 10.1016/J.TRANON.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spoerl S, Gerken M, Mamilos A, et al. Lymph node ratio as a predictor for outcome in oral squamous cell carcinoma: a multicenter population-based cohort study. Clin Oral Investig. 2021;25:1705–1713. doi: 10.1007/S00784-020-03471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdulla R, Adyanthaya S, Kini P, et al. Clinicopathological analysis of oral squamous cell carcinoma among the younger age group in coastal Karnataka, India: a retrospective study. J Oral Maxillofac Pathol. 2018;22:180. doi: 10.4103/JOMFP.JOMFP_16_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller S, Pan Y, Li R, Chi AC. Changing Trends in oral squamous cell Carcinoma with Particular Reference to Young Patients: 1971–2006. The Emory University experience. Head Neck Pathol. 2008;2:60. doi: 10.1007/S12105-008-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic Neck dissection in node-negative oral Cancer. N Engl J Med. 2015;373:521–529. doi: 10.1056/NEJMOA1506007/SUPPL_FILE/NEJMOA1506007_DISCLOSURES.PDF. [DOI] [PubMed] [Google Scholar]
- 22.D’Cruz AK, Dhar H, Vaish R, et al. Depth of invasion in early oral cancers- is it an independent prognostic factor? Eur J Surg Oncol. 2021;47:1940–1946. doi: 10.1016/J.EJSO.2021.03.243. [DOI] [PubMed] [Google Scholar]
- 23.Ahluwalia KP. Assessing the oral Cancer risk of South-Asian Immigrants in New York City. Cancer. 2005;104:2959. doi: 10.1002/CNCR.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahoo A, Panda S, Mohanty N, et al. Perinerural, lymphovascular and depths of invasion in extrapolating nodal metastasis in oral cancer. Clin Oral Investig. 2020;24:747–755. doi: 10.1007/S00784-019-02921-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhan KY, Morgan PF, Neskey DM, et al. Preoperative predictors of occult nodal disease in cT1N0 oral cavity squamous cell carcinoma: review of 2623 cases. Head Neck. 2018;40:1967–1976. doi: 10.1002/HED.25178. [DOI] [PubMed] [Google Scholar]
- 26.Almangush A, Bello IO, Coletta RD, et al. For early-stage oral tongue cancer, depth of invasion and worst pattern of invasion are the strongest pathological predictors for locoregional recurrence and mortality. Virchows Arch 2015. 2015;4671 467:39–46. doi: 10.1007/S00428-015-1758-Z. [DOI] [PubMed] [Google Scholar]
- 27.Kozak MM, Shah J, Chen M, et al. Depth of invasion alone as a prognostic factor in low-risk early-stage oral cavity carcinoma. Laryngoscope. 2019;129:2082–2086. doi: 10.1002/LARY.27753. [DOI] [PubMed] [Google Scholar]
- 28.Tam S, Amit M, Zafereo M, et al. Depth of invasion as a predictor of nodal disease and survival in patients with oral tongue squamous cell carcinoma. Head Neck. 2019;41:177–184. doi: 10.1002/HED.25506. [DOI] [PubMed] [Google Scholar]
- 29.Brockhoff HC, Kim RY, Braun TM, et al. Correlating the depth of invasion at specific anatomic locations with the risk for regional metastatic disease to lymph nodes in the neck for oral squamous cell carcinoma. Head Neck. 2017;39:974–979. doi: 10.1002/hed.24724. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, Zhu Y, Cai H, et al. Impact of lymphovascular invasion in oral squamous cell carcinoma: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131:319–328e1. doi: 10.1016/J.OOOO.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Mascitti M, Togni L, Caponio VCA, et al. Lymphovascular invasion as a prognostic tool for oral squamous cell carcinoma: a comprehensive review. Int J Oral Maxillofac Surg. 2022;51:1–9. doi: 10.1016/J.IJOM.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Fowler BZ, Crocker IR, Johnstone PAS. Perineural spread of cutaneous malignancy to the brain: a review of the literature and five patients treated with stereotactic radiotherapy. Cancer. 2005;103:2143–2153. doi: 10.1002/CNCR.21004. [DOI] [PubMed] [Google Scholar]
- 33.Deepthi G, Shyam N, Kumar G, et al. Characterization of perineural invasion in different histological grades and variants of oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2020;24:57. doi: 10.4103/JOMFP.JOMFP_162_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson AR, Kemmer J, Formeister E, et al. Beyond depth of Invasion: adverse pathologic tumor features in early oral tongue squamous cell carcinoma. Laryngoscope. 2020;130:1715–1720. doi: 10.1002/LARY.28241. [DOI] [PubMed] [Google Scholar]
- 35.Govers TM, Hannink G, Merkx MAW. Sentinel node biopsy for squamous cell carcinoma of the oral cavity and oropharynx: A diagnostic meta-analysis. Oral Oncol. 2013;49:726–732. doi: 10.1016/j.oraloncology.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Barroso EM, Smits RW, Bakker STC, et al. Discrimination between oral cancer and healthy tissue based on water content determined by Raman spectroscopy. Anal Chem. 2013;87(4):2419–26. doi: 10.1021/ac504362y. [DOI] [PubMed] [Google Scholar]
- 37.Santos IP, Barroso EM, Bakker STC, et al. Raman spectroscopy for cancer detection and cancer surgery guidance: translation to the clinics. Analyst. 2017;142(17):3025–3047. doi: 10.1039/c7an00957g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analysed during the current study are not publicly available. They are available from the corresponding author on reasonable request.