Abstract
The objective was investigating the effect of age on speech-in-noise perception (SINP) using word perception score in white noise (WPS in WN). This cross-sectional study was conducted on 76 participants, including 30 elderly (older than 61 years) and 46 young adults (between 14 and 35 years) with normal levels of stress, night sleep and mini-mental states. Audiological evaluations included acoustic immittance testing, pure tone audiometry, determination of speech reception threshold and WPS in WN. Data analysis were performed using Mann–Whitney and Tukey HSD tests. Based on the results of the tests, the participants were divided into three groups: (1) young adults with normal hearing (n = 30), (2) elderly adults with normal hearing (n = 16), (3) elderly adults with mild to moderate high frequency hearing loss (n = 14). In both groups of old adults, the means WPS in WN differences were significant only in the left ears (Pv = 0.008, 0.033, 0.025 for SNR = 0, +5, +10 dB). In the three groups and in the right ears, there were the significant differences between the means of WPS in WN (Pv = 0.002, 0.000, 0.001 for SNR = 0, +5, +10 dB), and also the left ears (Pv = 0.000, 0.002, 0.002 for SNR = 0,+5, +10 dB).There is a relationship between increasing age and decreasing WPS in WN. The deleterious effects of aging on SINP decline are greater than that of hearing loss.
Keywords: Aging, Speech, Noise, Perception
Introduction
Presbycusis, which is predominantly associated with progressive bilateral high-frequency hearing loss [1] has become a pervasive public health issue and is often associated with social isolation [2], communication, language, and speech difficulties [1, 3].
Clinical features of presbycusis include slow central processing of auditory information [4], SINP problems [3], temporal synchrony disorders, widening of auditory filters [5], and mechanical changes in the middle ear due to which there is a possibility of conductive hearing loss [6].
The type and level of background noise, regional vocabulary, the ability to understand vowel sounds, the type of pronunciation and loudness of the speaker's voice, the speed of speech, bilingualism, the level of hearing loss and the listener's linguistic knowledge are effective on SINP scores. However, the specific neurobiological mechanisms underlying these difficulties remain unclear [7].
Age-related decline in SINP is associated with decreased brain activation in the right superior temporal cortex and arising stimulation to noise in the left anterior temporal lobe, which is related to structural changing in auditory brain [8]. Significant structural destruction in multiple brain areas complicated in perceiving and producing speech show that gray matter density declines with age. However, SINP are affected both changing in brain structure and functioning [9]. Therefore, this research was designed with the objective of investigating the effect of age on SINP using WPS in WN.
Material and Methods
This research was a cross-sectional study. Its practical work was done in the fall season of 2022 in Hamedan city, Iran. The study population involve 186 participants, which was included 93 elderly (over 61 years old) living in nursing homes and 93 young adults (between 14 and 35 years old). All 186 participants were educated in elementary and high school in Hamedan city.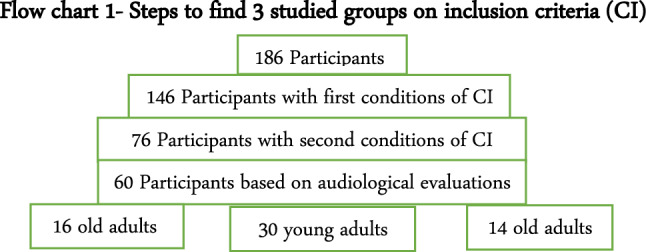
The inclusion criteria were monolingualism (native speakers of Persian language with Iranian dialect), normal stress level, normal night sleep, normal mental state, no self-reported speech, voice, language, and swallowing disorders, no history of underlying diseases, no acute or chronic ear infections, no rheumatological diseases, vasculitis, acoustic neuroma, known neurological disorders and ear surgery, no smoking (even one cigarette per day) and drugs, as well as immunosuppressive drugs including interferons before the study, corten, anticonvulsants and sedatives.
The exclusion criteria contained unwillingness to continue cooperation in our project, suffering from ear diseases and mentioned conditions and diseases during the research.
At the beginning of the work, the study procedures were explained to 186 participants, of which 146 participants (71 elderly and 75 young people) met the basic conditions for entering the study and signed the consent forms, the rest were excluded from the research.
Then the stress level (based on the GHQ28 questionnaire), night sleep position (based on the Petersburg questionnaire) and mini–mental state (based on the mini–mental state examination) were determined. Out of 146 participants, 76 of them had normal results and met the second condition of inclusion criteria (n = 30 old adults + 46 young adults), the rest were excluded from the study.
All these 76 participants were evaluated by general audiological evaluations, which included acoustic immittance testing, pure tone audiometry and speech reception threshold test. Based on the results, the participants were divided into three groups:
Young adults with normal hearing that included 15 women (50%) and 15 men (50%).
-
Old adults, which included 10 women (62.5%) and 6 men (37.5%).
The hearing thresholds of groups 1 and 2 were within the normal level, which were less than 25 dBHL in the frequency range of 250 to 8000 HZ [10].
Old adults, which included 6 women (86.42%) and 8 men (14.57%). The hearing thresholds of these participants were normal in the frequency range of 250 to 2000 HZ, but in 4000 to 8000 HZ (high frequency) had 40–55 dBHL (mild to moderate) hearing loss [10].
Considering that until the date of conducting this research, there was no norm for WPS in WN of Persian language with Iranian dialect. It was necessary to determine its norm in a population consisting of students and school teachers. Therefore, we considered the minimum age for the norm group to be 14 years. Because, the right ear matures at the age of 10 and becomes similar to adult performance, while the maturity of the left ear is around 13 and 14 years old [11]. We considered the maximum age of the norm group to be 35 years; the ministry of sports and youth of Iran has also declared the maximum age of youth to be 35 years [12].
Our last test was WPS in WN, by which we evaluated 76 participants. The WPS in WN was measured by phonetically balanced monosyllabic words. In each trial, one word was presented, at an individually adjusted intensity based on participants’ comfort level. The words were presented 200 ms apart to minimize working memory demand. Participants were asked to determine if the words were identical or different. The presentation of the second words was followed by a question mark cueing participant to respond. They were asked to answer as quickly. The inter-trial interval was 1000 ms. If no response was made, the next trial automatically begun 2000 ms after the last stimulus was played. All stimuli were presented using presentation software through high-quality headphones, by a woman voice with the accompanying carrier phrase (Say the word…), while participants were comfortably seated in a soundproof room. The pairing of the responses and button on the response box was counterbalanced across participants. The SNR was at three levels: 0, + 5, + 10 dB. All the results are recorded in the main questionnaire of the project. For this reason, we had to evaluate each participant three times, and in order not to distort the results due to their fatigue, we evaluated each participant on three separate days.
Designing Word Perception Score in White Noise Test (WPS in WN)
To make phonetically balanced monosyllabic words, we selected 150 words based on 6 Persian vowels, which are: /Á/, /O/, /Æ/, /Â/, /OU/, /EÌ/.
The words were prepared in the order /consonant/vowel/consonant/ and in each list of 25 words had only one similar vowel (Table 1). Examining their frequency spectrum were done based on Fourier analysis. All of them were meaningful and familiar. To determine the validity of them, the lists were presented to 20 professionals of linguistics, speech therapy and persian literature.
Table 1.
The phonetically balanced monosyllabic words lists of the Persian language for word perception score in white noise test (WPS in WN)
| N | Consonant vowel of Æ consonant | Consonant | Consonant | Consonant vowel of  consonant | Consonant | Consonant |
|---|---|---|---|---|---|---|
| Vowel of O consonant | Vowel of EÌ consonant | Vowel of Á consonant | Vowel of OU consonant | |||
| 1 | Shæn | Shol | Seìb | Kâr | Sár | Goush |
| 2 | Sæn | Pol | Sheìb | Bâr | Dár | Moush |
| 3 | Jæn | Gol | Jeìb | Mâr | Kár | Doush |
| 4 | Gæl | Kol | Seìr | Ghâr | Shár | Noush |
| 5 | Dæl | Khol | Peìr | Zâr | Pár | Housh |
| 6 | Hæl | Ghom | Deìr | Yâr | Zár | Joush |
| 7 | Sæl | Gom | Sheìr | Hâr | Khár | Shoush |
| 8 | Tæl | Khom | Gheìr | Dâr | Nár | Mour |
| 9 | Væl | Dom | Zeir | Nâr | Tár | Shour |
| 10 | Bæh | Som | Jeìr | Sâr | Kár | Sour |
| 11 | Mæh | Ton | Meìr | Khâl | Sám | Dour |
| 12 | Dæh | Bon | Teìr | Sâl | Nám | Zour |
| 13 | Zæh | Nok | Meìsh | Mâl | Khám | Kour |
| 14 | Sæh | Shok | Neìsh | Bâl | Jám | Gour |
| 15 | Mæsh | Kor | Peìsh | Zâl | Dám | Jour |
| 16 | Kæsh | Sor | Keìsh | Kâl | Bám | Nour |
| 17 | Shæsh | Shor | Feìl | Shâl | Shám | Bour |
| 18 | Fær | Lor | Peìl | Vâl | Ghám | Tour |
| 19 | Shær | Ghor | Meìl | Nâm | Rám | Doud |
| 20 | Ghær | Boz | Beìl | Shâm | Sháb | Soud |
| 21 | Zær | Hoz | Meìm | Dâm | Táb | Ooud |
| 22 | Væz | Poz | Neìm | Jâm | Láb | Koud |
| 23 | Tæz | Moz | Beìm | Kâm | Shák | Boud |
| 24 | Mæs | Khosh | Teìm | Vâm | Ták | Zoud |
| 25 | Hæs | Shosh | Seìm | Râm | Ság | Roud |
The reason for our work was that brainstem is responsive to pitch and rhythm. The cerebral cortex is sensitive to meanings and concepts [13]. In other words, the brainstem participates in the detection of the main frequency of the human voice (F0) or pitch of the speech, which is transmitted by the vowels [13, 14].
Whereas, F0 does not convey the required cues to processing the concept and lexical information, and the auditory cortex is also not sensitive to the main frequency of the human voice, it is only activate by the differentiation between the frequencies of the first and second formant of the vowels [14]. The crucial specific of F0 perception is that listeners native a language are able to recognize a certain voice as uniform feature regardless of the speaker’s F0 [10, 13]. In a situation where the rhythm of words and their tonality change, it will be easier to recognize them with the help of phonetic information [10, 14]. If the vowels in each list were unsimilar, we had checked both the function of the brainstem and auditory cortex. We wanted to have a specific test, which is only to evaluate brain function and concept comprehension.
Data Analysis
All analysis were done by means of the statistics software SPSS17. Descriptive statistics were Kolmogorov–Smirnov and Shapiro–Wilk tests were used for evaluation of normal test distribution. Intera class correlation and Cronbach's Alpha used to study the stability of the WPS in WN. The normatic values expressed as mean ± standard deviation and as percentages. Mann–Whitney and Tukey HSD tests were used for the multiple comparisons. The significance level was determined to be less than 0.05.
Results
To determine the stability of WPS in WN with SNR between 0, + 5 and + 10 dB, the total intraclass correlation coefficient (0.39) and Cronbach's alpha (0.88) were evaluated. There was a high correlation between initial test and retest performance, confirming high test/retest reliability.
The participants were divided into three groups based on the WPS in WN results: youth group (mean age = 24 ± 7.68 years), elderly group 1 (mean age 75 ± 9.32 years), and elderly group 2 (mean age = 72 ± 8.95 years).
In each groups, the means WPS in WN of the right ears (with SNR between 0, + 5, + 10 dB) were greater than the left ears (Tables 2, 3, 4). But, the differences were not significance (Mann–Whitney, Pv > 0.05).
Table 2.
Mean ± standard deviation (S.D) of word perception score in white noise (with SNR between 0, + 5, + 10 dB) of youngsters with normal hearing (Right ears = 30, Left ears = 30)
| Ear | SNR | Mean ± S.D (%) | Max | Min |
|---|---|---|---|---|
| Right | 0 | 54.20 ± 13.780 | 88 | 26 |
| Left | 0 | 52.47 ± 17.624 | 80 | 22 |
| Right | 5+ | 67.47 ± 17.059 | 92 | 32 |
| Left | 5+ | 66.67 ± 15.548 | 92 | 28 |
| Right | 10+ | 88.13 ± 9.126 | 100 | 72 |
| Left | 10+ | 87.73 ± 13.683 | 100 | 48 |
Table 3.
Mean ± standard deviation (S.D) of word perception score in white noise (with SNR between 0, + 5, + 10 dB) of oldsters1 with normal hearing (Right ears = 16, Left ears = 16)
| Ear | SNR | Mean ± S.D (%) | Max | Min |
|---|---|---|---|---|
| Right | 0 | 25.47 ± 15.447 | 52 | 0 |
| Left | 0 | 19.53 ± 14.621 | 48 | 0 |
| Right | +5 | 41.32 ± 19.137 | 72 | 6 |
| Left | +5 | 34.88 ± 24.377 | 84 | 4 |
| Right | 10+ | 65.05 ± 26.794 | 100 | 12 |
| Left | 10+ | 58.35 ± 25.022 | 88 | 8 |
Table 4.
Mean ± standard deviation (S.D) of word perception score in white noise (with SNR between 0, + 5, + 10 dB) of oldsters2 with mild-moderate high tone loss (Right ears = 14, Left ears = 14)
| Ear | SNR | Mean ± S.D (%) | Max | Min |
|---|---|---|---|---|
| Right | 0 | 14.91% ± 14.761 | 40 | 0 |
| Left | 0 | 9.23% ± 17.157 | 64 | 0 |
| Right | + 5 | 27.64% ± 31.923 | 92 | 0 |
| Left | + 5 | 23.54% ± 23.808 | 80 | 4 |
| Right | 10 + | 46.55% ± 26.909 | 92 | 8 |
| Left | 10 + | 37.23% ± 24.461 | 84 | 0 |
In all groups and in the right ears, there were the significant differences between the means of WPS in WN (Pv = 0.002, 0.000, 0.001 for SNR = 0, + 5, + 10 dB), the differences in the means of the left ears were also significant (Pv = 0.000, 0.002, 0.002 for SNR = 0, + 5, + 10 dB).
As can be seen in Tables 2, 3 and 4 the standard deviation values are high, which indicate heterogeneity. According to the minimum and maximum values of WPS in WN, the reason is determined. This situation is observed in all three groups, and it is more in two old groups, which may be due to the small number of participants.
The means of WPS in WN of the left and right ears had significantly difference in three groups (Mann–Whitney, Pv = 0.000, 0.002, 0.002 for SNR = 0, + 5, + 10 dB). The differences of the means of WPS in WN in left ears of normal-hearing old adults compared to old adults with mild to moderate high frequency hearing loss were significant (Mann–Whitney, Pv = 0.008, 0.033, 0.025 for SNR = 0, + 5, + 10 dB). While none of the differences in the means of WPS in WN of the right ears were significant in two old groups (Mann–Whitney, Pv = 0.077, Pv = 0.077, Pv = 0.094 for SNR = 0, + 5, + 10 dB).
Discussion
Our objective was investigating the effect of age on speech-in-noise perception (SINP) using word perception score in white noise (WPS in WN). We used of phonetically balanced monosyllabic words lists of the Persian language to eliminate the participation of the brainstem in detection the pitch of the words. The tonality can be effective as a guide key in discovering the meaning and facilitates the recognition of the words [10].
Semantic understanding of speech is essential, everyday conversations and communication take place in background noise [1, 3]. Especially in competitive situations, when several talkers are speaking at the same time [5]. The smallest amount of structural/functional lesions of the auditory cortex can cause great problems in personal relationships, living conditions and cognitive abilities of patients, especially the old adults [4]. Therefore, the use of a test that estimates the vocabulary comprehension capacity can be effective in the diagnosis, treatment and rehabilitation of patients. Our findings showed that SINP decreased with age in both right and left ears, and there was an inverse relationship between means WPS in WN and age. Also, in our participants the amount of SINP was higher for the right ears than the left. The reason for this difference can be the known asymmetry in humans; the dominance of the right ear for listening to speech sounds is thought to confirm the superiority of the left hemisphere of the brain for processing vocal communication [15].
The dissimilarity in SINP between young and normal-hearing old adults suggests dysfunction of central auditory processing, which is associated with impairments in temporal resolution and increased central reaction time in older adults [16]. Sensitivity to temporal envelope, temporal fine structure, and consonant recognition in noise is lower for normal-hearing adults than for young adults, which is due to cognitive and perceptual changes apart from age-related hearing loss. Cognitive abilities guaranteed with aging include short-term memory and sustained attention. Whereas, the elderly are weaker than young people in most cognitive functions [17]. Age-independent intelligibility effects occur in several motor and premotor areas, comprising the left ventral premotor cortex and the right supplementary motor area. Age-dependent intelligibility effects are also happen mostly in sensorimotor cortical areas and in the left dorsal anterior insula [9].
There is a correlation between stimulation of the anterior insula and correcting sentence processing function in which rate are operated to decrease intelligibility. The activation within the anterior insula is poorer in elderly during syllable repetition task in competing situations with worse scores. These failures can explain for the weakening in attention that happens in aging and can have a negative result on SINP [8].
Relationship between brain structure and function in elderly is relative dissociated, suggests that they may be connected to separate risk factors for age-related communication problems [9]. The volume of the left inferior frontal gyrus and the thickness of the left superior frontal gyrus are greater in old adults [18] and there is the microstructural damages in the middle longitudinal fasciculus [19]. Its function is vocabulary selection, sequencing and higher order processing [20], which through the temporal lobe can play a role in primary auditory processing [19].
With aging, the number and even density of neurons and synapses in the nucleus of the cochlea and auditory centers of the brain deteriorate and the size of the cells also decreases [22]. The reduction of cells is not the same in all parts of the brain usually deterioration occurs in hearing areas, which are caused by the decreasing the weight of the brain and its blood flow. The main reason of brain weight loss is the decline of protective tissues and cellular atrophy [9]. Aging affects Heschl’s gyrus/superior temporal gyrus region morphology and can induce SINP problems for old adults already limited by low gray matter volume. The structural differences in this area are happen even after monitoring for hearing loss, suggesting independent effects of aging on the peripheral and central auditory processing [23].
Presbycusis patients with cognitive impairment undergo topological reorganization of the whole-brain functional network and show more obvious changes than patients without cognitive impairment. Structural/functional changes in these patients may compensate for the pathology by mobilizing additional neural resources [21].
The modulation of top-down influences may change the size of the effect of hearing loss in reducing SINP of old adults, which are larger for word scoring than phoneme scoring. Indeed, when word scoring is used, older adults with normal hearing are able to take greater advantage of context cues than their counterparts with hearing loss, possibly relating to the abnormal cognitive functions caused by peripheral hearing impairment [24]. Then hearing aid efficiency is determined, at least in part, by the structural integrity of low-level auditory cortex, and only a minor percentage of old adults who can help from amplification are successful hearing aid users [23].
However, the idea of hidden hearing loss is emerging in the literature, and it is likely that some of the functional difficulties related to these subclinical deficits are present in clinical populations and may best be seen in more complex listening tasks, such as SINP [25]. The interdependence between central auditory function and cognitive processing presents not only challenges to inference but also unique opportunities in identifying targets for prevention. Further studies need to understand why some individuals can set off neural compensations and others seemingly cannot.
Conclusion
Aging can be an important factor in the deterioration of SINP. There is a relationship between decreasing ability to semantic comprehension and increasing age. The destructive effects of old age on the reduction of speech perception are greater than those of hearing loss.
Limitations
Research in the field of old age requires a lot of patience in order to obtain accurate and valid results from the responses of the participants, a case that we faced in this research, as some of our elderly were sensitive and bored, their reactions were very slow and we had to devote a lot of time to gain their trust and cooperation.
Another point is that we wanted to check the relationship between word perception score in white noise and the amount of time they spend reading books during the day, which unfortunately we could not do because of the small number of elderly participants who had normal hearing. Obviously, research at the national level and larger samples can solve this problem.
Acknowledgements
The financial sponsor of this research was Hamadan University of Medical Sciences (Registered No.: 140105183699 and ethics code: IR.UMSHA.REC.1401.419). The authors know to thank and appreciate the esteemed participants who cooperated in this research.
Abbreviations
- SINP
Speech-in-noise perception
- WPS in WN
Word perception score in white noise
- SNR
Signal to noise ratio
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seyede Faranak Emami, Email: faranak_imami@yahoo.com.
Elnaz Shariatpanahi, Email: eshariatpanahi1@gmail.com.
Nasrin Gohari, Email: rasacenter@yahoo.com.
Mobina Mehrabifard, Email: mobinamehrabifard@gmail.com.
References
- 1.Gates GA, Mills JH. Presbycusis Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 2.Dubno JR, Lee FS, Matthews LJ, Ahlstrom JB, Horwitz AR, Mills JH. Longitudinal changes in speech recognition in older persons. J Acoust Soc Am. 2008;123:462–475. doi: 10.1121/1.2817362. [DOI] [PubMed] [Google Scholar]
- 3.Panza F, Solfrizzi V, Logroscino G. Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat Rev Neurol. 2015;11:166–175. doi: 10.1038/nrneurol.2015.12. [DOI] [PubMed] [Google Scholar]
- 4.Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Wingfield A. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol. 2012;23(8):635–666. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Frisina RD, Mapes FM, Hickman ED, Frisina DR. Effect of age on binaural speech intelligibility in normal hearing adults. Speech Commun. 2006;48(6):591–597. doi: 10.1016/j.specom.2005.09.004. [DOI] [Google Scholar]
- 6.Dobrev I. Conductive hearing loss with age; a histologic and audiometric evaluation. Clin Med. 2021;10:2341. doi: 10.3390/jcm10112341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emami SF, Shariatpanahi E (2023) Central representation of speech-in-noise perception: a narrative review. Aud Vestib Res. Article in Press. https://avr.tums.ac.ir/index.php/avr/article/view/1102
- 8.Tremblay P, Perron M, Deschamps I, Kennedy-Higgins D, Houde JC, Dick AS, Descoteaux M. The role of the arcuate and middle longitudinal fasciculi in speech perception in noise in adulthood. Hum Brain Mapp. 2018 doi: 10.1002/hbm.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilodeau-Mercure M, Lortie CL, Sato M, Guitton MJ, Tremblay P. The neuro- biology of speech perception decline in aging. Brain Struct Funct. 2015;220(2):979–997. doi: 10.1007/s00429-013-0695-3. [DOI] [PubMed] [Google Scholar]
- 10.Scott SK, Sinex DG. Speech. In: Rees A, Palmer A, editors. The Oxford handbook of auditory science: the auditory brain. 1. New York: Oxford University Press; 2010. pp. 193–214. [Google Scholar]
- 11.Chandni PG, Vipin Ghosh PG, Chetak KB, Aishwarya L. Maturation of speech perception in noise abilities during adolescence. Int J Pediatr Otorhinolaryngol. 2020 doi: 10.1016/j.ijporl.2020.110459. [DOI] [PubMed] [Google Scholar]
- 12.https://www.isna.ir/news/98022312086/%D8%B3%D9%86-%D8%AC%D9%88%D8%A7%D9%86%DB%8C-%D8%AA%D8%BA%DB%8C%DB%8C%D8%B1-%DA%A9%D8%B1%D8%AF
- 13.Emami SF. Is all human hearing cochlear? Scientific World Journal. 2013 doi: 10.1155/2013/147160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrams DA, Kraus N. Auditory pathway representations of speech sounds in humans. In: Katz J, Chasin M, English K, Hood LJ, Tillery KL, editors. Hand book of clinical audiology. 7. Philadelphia: Wolters Kluwer Health; 2015. pp. 527–544. [Google Scholar]
- 15.Lazard DS, Collette JL, Perrot X. Speech processing: from peripheral to hemispheric asymmetry of the auditory system. Laryngoscope. 2012;122:167–173. doi: 10.1002/lary.22370. [DOI] [PubMed] [Google Scholar]
- 16.Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 1997;106(1–2):95–104. doi: 10.1016/S0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- 17.Fullgrabe C, Moore BC, Stone MA. Age-group differences in speech iden- tification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front Aging Neurosci. 2014;6:347. doi: 10.3389/fnagi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuY BBR, Grady CL, Alain C. Increased activity in frontal motor cortex compensates impaired speech perception in older adults. Nat Commun. 2016;7:12241. doi: 10.1038/ncomms12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay P, Perron M, Deschamps I, Kennedy-Higgins D, Houde JC, Dick AS, Descoteaux M. The role of the arcuate and middle longitudinal fasciculi in speech perception in noise in adulthood. Hum Brain Mapp. 2018 doi: 10.1002/hbm.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong PC, Jin JX, Gunasekera GM, Abel R, Lee ER, Dhar S. Aging and cortical mechanisms of speech perception in noise. Neuropsychologia. 2009;47(3):693–703. doi: 10.1016/j.neuropsychologia.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan B, et al. Reorganized brain functional network topology in presbycusis. Front Aging Neurosci. 2022 doi: 10.3389/fnagi.2022.905487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawool VW (2007) The aging auditory system. Part 2. Slower processing and speech recognition. Hearing review. http://www.hearingreview.com/issues/articles/2007-08_04.asp(accessed 4 August 2007)
- 23.Harris KC, Dubno JR, Keren NI, Ahlstrom JB, Eckert MA. Speech recognition in younger and older adults: a dependency on low level auditory cortex. J Neurosci. 2009;29(19):6078–6087. doi: 10.1523/JNEUROSCI.0412-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billings CJ, Penman TM, Ellis EM, Baltzell LS, McMillan GP. Phoneme and word scoring in speech-in-noise audiometry. Am J Audiol. 2016;25:75–83. doi: 10.1044/2016_aja-15-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after temporary noiseinduced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


