Abstract
Introduction
Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplasia that often affects children, presenting a broad clinical spectrum.
Methods
Here, we report a 13-year-old male Salvadorian patient who was referred presenting a nodular swelling at the mandibular angle region, mildly symptomatic, few weeks ago, which relevantly was associated with limited mouth opening. Intraoral examination was unremarkable. Imaginological exams revealed an osteolytic lesion affecting the vestibular cortex at the right mandibular angle. The blood test results were normal, except for eosinophilia (21%; absolute eosinophil count 4 × 109/L). After an incisional biopsy, microscopical and immunohistochemical analyses were consistent with LCH diagnosis, which corresponded to a single system-single site category. After a few weeks, the mandibular movements were re-established, and complete resolution of blood eosinophilia was observed.
Conclusion
LCH with blood eosinophilia is rarely reported. To our knowledge, 3 cases have been previously published.
Keywords: Langerhans cell histiocytosis, Blood eosinophilia, Restriction mouth opening, Mandible, Immunohistochemistry
Introduction
Langerhans cell histiocytosis (LCH) is an abnormal clonal proliferation of Langerhans cells (LCs). LCH cells, in combination with eosinophils, lymphocytes, and histiocytes, form typical LCH lesions, which can be found in almost any organ or tissue [1–3]. LCH more frequently affects children, with most cases harboring BRAFV600E mutations [1, 3]. Overall, with similar histopathological and immunohistochemical features, the clinical characteristics of LCH can vary notably, from trivial single bone lesions to life-threatening diseases with disseminated lesions [1, 2].
In the oral cavity, LCH presents as a single or multiple lesions, with oral mucosa and/or alveolar bone involvement, clinically showing ulcerations, periodontal lesions, bleeding, dental mobility, and premature loss of teeth [2, 4]. When mandibular angle/ramus or temporomandibular joint (TMJ) involvement by LCH is observed, clinical manifestations can include pain, swelling, and limited mouth opening [5], in addition to TMJ noises [6]. Moreover, exclusive skeletal muscle tissue involvement by LCH is rare, affecting non-masticatory muscles [7, 8]. By microscopy, eosinophilic infiltration in LCH is a common finding [1, 9]; however, data about blood eosinophilia in these patients are scarce, as shown in a recent study review of LCH in the oral and maxillofacial region [4].
In fact, LCH with eosinophilia is rarely reported. To our knowledge, 3 cases have been previously published [10–12]. Here, we report a mandibular LCH case affecting a pediatric patient who presented limited mouth opening and blood eosinophilia, which resolved after a few weeks of the biopsy procedure.
Case description and results
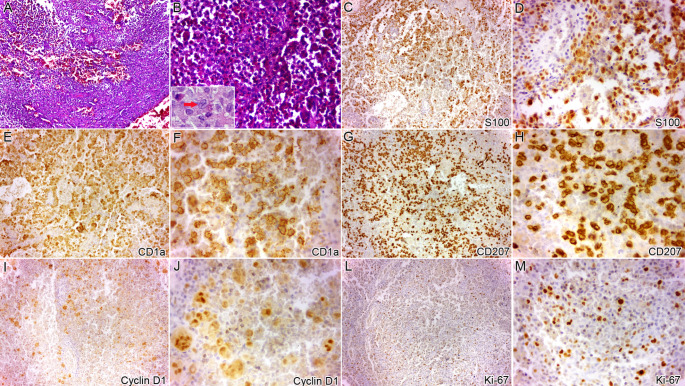
A 13-year-old male Salvadorian patient was referred, presenting a mildly symptomatic nodular swelling at the mandibular angle region a few weeks ago. Relevantly, he reported progressive restriction of mouth opening. The mouth opening was 28 mm, while lateral excursion and protrusion were 2 mm each. The patient was otherwise healthy, without personal and familiar medical history remarkable. The intraoral examination did not show lesion or abnormality. Panoramic radiograph, computerized tomography (CT) scans, and 3D reconstruction revealed an osteolytic lesion affecting the vestibular cortex at the right mandibular angle (Fig. 1). The blood test showed eosinophilia (21%; absolute eosinophil count [AEC] 4 × 109/L), while all other values were normal. After biopsy with curettage of the lesion, which also included the extraction of tooth #48, the microscopical analysis exhibited an infiltration of large cells presenting abundant, pale eosinophilic cytoplasm with prominent nuclear grooves and folds, occasional multinucleated giant cells, which were permeated by numerous eosinophils. The immunohistochemical analysis showed positivity for S100, CD1a, CD207, and cyclin D1. The Ki-67 labeling index was 10% (Fig. 2). These findings were consistent with LCH diagnosis. Tooth #48 showed normal-appearing microscopical features. Systemic examination, including CT scan images of the whole body, revealed no alterations or abnormalities. These findings corresponded to the single system-single site category of LCH [1]. After a few weeks, the mandibular movements were re-established, and, noteworthy, all blood test results were normal. After 2-year of follow-up, the patient is well, without recurrence or alteration at the lesional site.
Fig. 1.
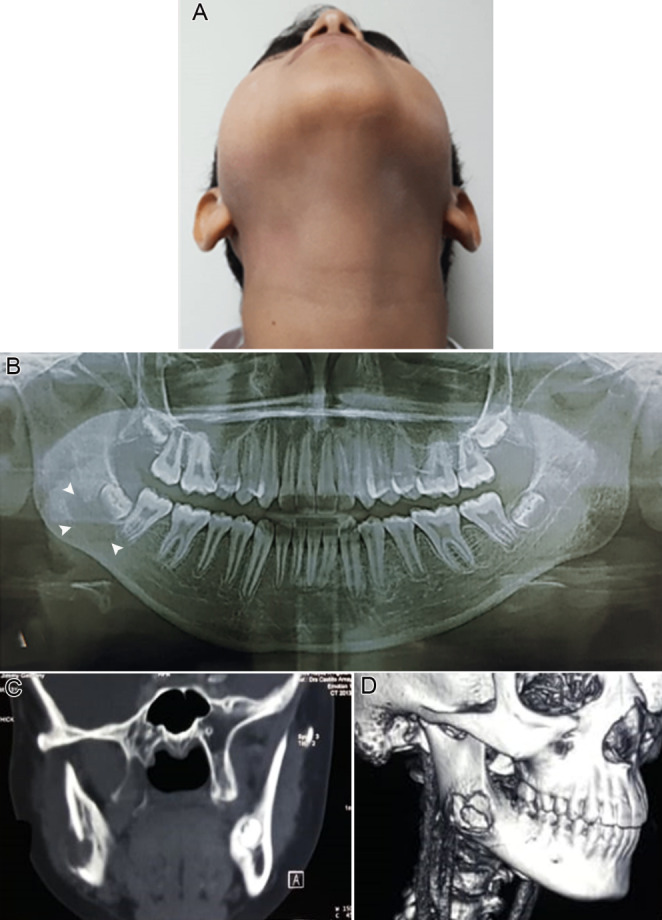
Clinical view showing right facial asymmetry and swelling at mandibular angle region (A and B). Panoramic radiograph exhibiting an ill-defined radiolucent lesion at the periapical region of the tooth # 48 (C). CT scan (D) and 3D reconstruction (E) showing an evident osteolytic lesion affecting the vestibular cortex and adjacent cancellous bone at the right mandibular angle
Fig. 2.
Histopathological analysis (H&E stain) revealed sheets of large eosinophilic cells presenting prominent nuclear grooves and folds, permeated by numerous eosinophils (A, x10; B, x40; inset shows typical LCH cell [arrow]). The large eosinophilic cells were positive for S100 (C, x10; D, x40), CD1a (E, x10; F, x40), and CD207 (G, x10; H, x40). Cyclin D1 also highlighted positivity in several multinucleated giant cells (I, x10; J, x40). The Ki-67 labelling index was 10% (L, x10; M, x40)
Discussion
In the current case, limited mouth opening was observed. Several reports show that when TMJ and mandibular angle/ramus involvement by LCH is observed, clinical manifestations can include pain, swelling, and limited mouth opening [5, 6], with the latter having a varied etiology [13]. Exclusive skeletal muscle tissue involvement by LCH is rare, described as affecting non-masticatory muscles [7, 8].
Microscopically, most LCH cases present infiltration by numerous eosinophils, especially in intraosseous location [1, 4, 9], in addition to lymphocytes, plasma cells, macrophages, multinucleated giant cells, mast cells, and dendritic cells [1]. It is suggested that LCH cells could recruit and modulate immune cell infiltration, thus providing reciprocal survival signals [1]. Specifically, eosinophils can be recruited in the inflammatory milieu through chemical mediators, such as CCL-11/eotaxin-1, histamine, monocyte chemotactic protein-5, platelet-activating factor, and interleukin-5 [9, 14]. In view of the above, and considering the systemic effect of these inflammatory mediators, it is reasonable to think that not only local effects could be happening. So, we think this may be applicable in the present case, as the extensive curettage of the lesion must have promoted its resolution and depleted the inflammatory mediators and/or cytokines that would be linked with eosinophilia. After a literature review [1, 4, 9], it is interesting that blood eosinophilia is rarely reported, which could help to better understand the laboratory findings of this condition, with therapeutic and prognostic implications.
In fact, mild blood eosinophilia, defined by AEC between 0.5 and 1.0 × 109/L, is not uncommon, which can be detected in 3–10% of patients, with frequent causes including drug hypersensitivity, atopic disease, asthma, and parasitic infection. Differently, blood hypereosinophilia (HE), defined by AEC ≥ 1.5 × 109/L, is relatively rare, and it should promote an exhaustive assessment to identify a specific cause. HE with end-organ damage attributable to the eosinophilia defines hypereosinophilic syndrome [15]. In the current case, a neoplasm or immunologic disorder subset [15] could be considered in the differential diagnosis of HE.
LCH presenting with HE has been previously reported [10–12]. A 5-year-old male child was incidentally detected to have eosinophilia (72%; AEC 35.8 × 109/L). There were no abnormalities, except for small cervical and inguinal lymph nodes (this latter diagnosed as LCH). PET-CT scans showed no bony abnormality. BCR-ABL fusion and PDGFRA and JAK-2 mutations were negative. Bone marrow aspiration showed an increase in eosinophils and their precursors. After treatment with intravenous vinblastine and prednisolone, the HE resolved [10]. Unlikely, in the current case, HE was resolved after a short-term follow-up, without additional treatment. A 15-year-old male presented with tonsillar enlargement and supraclavicular and mediastinal lymphadenopathy (this latter subsequently diagnosed as LCH), along with marked blood eosinophilia (79%; AEC 24.9 × 109/L) and bone marrow eosinophilia (59% eosinophils with dysplastic changes). RT-PCR for FIP1L1-PDGFRα fusion was negative. This patient was treated similarly to the previous case [10], with a good response [11]. A 25-year-old man who initially presented multiple osteolytic lesions diagnosed as LCH and nondysplastic eosinophilia in peripheral blood received treatment with vinblastine, 6-mercaptopurine, methotrexate, and cladribine. After 1-year, dysplastic eosinophils were detected in the peripheral blood, bone marrow, and pleural effusion, being diagnosed as chronic eosinophilic leukemia with FIP1L1-PDGFRα fusion that evolved from LCH with eosinophilia after chemotherapy [12].
Conclusion
Although these findings are rare, both clinician and pathologist must recognize the characteristics of LCH with eosinophilia for appropriate patient management. In addition, we emphasize that the present case expands the clinicopathological spectrum of LCH due to the limitation of mouth opening and resolution of blood eosinophilia after a short-term follow-up.
Funding
Jorge Esquiche León (2016/11419-0; 2022/07479-9; 2022/12760-9) has received research Grants from State of São Paulo Research Foundation (FAPESP). Jorge Esquiche León (304241/2021-0) has received research Grant from National Council for Scientific and Technological Development (CNPq). Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Data Availability
Data and material used for the manuscript can be made available on request.
Code Availability
Not applicable.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
It was received from the patient directly.
Consent for Publication
It was obtained from the patient for whom identifying information is uniquely included in this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paredes SEY, Almeida LY, Trevisan GL, Polanco XBJ, Silveira HA, Vilela Silva E, et al. Immunohistochemical characterization of immune cell infiltration in paediatric and adult Langerhans cell histiocytosis. Scand J Immunol. 2020;92:e12950. doi: 10.1111/sji.12950. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Wu X, Wang X, Pan E, Ying L. Molecular and oral manifestations of langerhans cell histiocytosis preceding acute myeloid leukemia. BMC Oral Health. 2022;22:386. doi: 10.1186/s12903-022-02410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J, Gilbert JR, Sutton KS, Goudy SL, Abramowicz S. Head and Neck Langerhans Cell histiocytosis in children. J Oral Maxillofac Surg. 2022;80:545–552. doi: 10.1016/j.joms.2021.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Faustino ISP, Fernandes PM, Pontes HAR, Mosqueda-Taylor A, Santos-Silva AR, Vargas PA, et al. Langerhans cell histiocytosis in the oral and maxillofacial region: an update. J Oral Pathol Med. 2021;50:565–571. doi: 10.1111/jop.13207. [DOI] [PubMed] [Google Scholar]
- 5.Kim JE, Yi WJ, Heo MS, Lee SS, Choi SC, Huh KH. Langerhans cell histiocytosis of the jaw, a mimicker of osteomyelitis on CT and MR images: a retrospective analysis. Med (Baltim) 2019;98:e16331. doi: 10.1097/MD.0000000000016331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JW, Chung JW. Long-term treatment of Langerhans cell histiocytosis of the mandibular condyle with indomethacin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e13–21. doi: 10.1016/j.tripleo.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 7.May DA, Kaushik S, Frable WJ. MR imaging of infiltrative muscle involvement with Langerhans cell histiocytosis. Clin Imaging. 2004;28:301–304. doi: 10.1016/S0899-7071(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 8.Plemel DJA, Benson MD, Tong CM, Mahmood MN, Pollock TJ. Nonosseous Periocular Manifestations of Langerhans Cell histiocytosis: a case report and systematic review. Ophthalmic Plast Reconstr Surg. 2021;37:408–413. doi: 10.1097/IOP.0000000000001906. [DOI] [PubMed] [Google Scholar]
- 9.Cho YA, Yoon HJ, Hong SD, Lee JI, Hong SP. Hypothetical pathogenesis of eosinophilic infiltration in Langerhans cell histiocytosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:734–742. doi: 10.1016/j.oooo.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Rastogi N, Yadav SP. Langerhans Cell Histiocytosis Masquerading as Hypereosinophilia in a child. J Pediatr Hematol Oncol. 2019;41:335–336. doi: 10.1097/MPH.0000000000001445. [DOI] [PubMed] [Google Scholar]
- 11.Parambil AS, Prem S, Jacob PM, Nair RA. Mediastinal Mass with Hyper-eosinophilia in a Young Boy -A Diagnostic Dilemma. J Clin Diagn Res. 2016;10:XD03–04. doi: 10.7860/JCDR/2016/19615.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnishi H, Kandabashi K, Maeda Y, Kawamura M, Watanabe T. Chronic eosinophilic leukaemia with FIP1L1-PDGFRA fusion and T6741 mutation that evolved from Langerhans cell histiocytosis with eosinophilia after chemotherapy. Br J Haematol. 2006;134:547–549. doi: 10.1111/j.1365-2141.2006.06221.x. [DOI] [PubMed] [Google Scholar]
- 13.Augusto-Neto RT, Marinheiro BH, Silveira HA, Polanco XBJ, León JE, Trivellato AE, et al. Complex odontoma restricting mouth opening: an unusual clinical presentation and surgical management. Int J Health Sci (Qassim) 2021;15:60–63. [PMC free article] [PubMed] [Google Scholar]
- 14.Prussin C, Metcalfe DD. 4. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2003;111:S486–S494. doi: 10.1067/mai.2003.120. [DOI] [PubMed] [Google Scholar]
- 15.Klion AD. How I treat hypereosinophilic syndromes. Blood. 2015;126:1069–1077. doi: 10.1182/blood-2014-11-551614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material used for the manuscript can be made available on request.
Not applicable.