Abstract
Background
Multiple sclerosis (MS) is traditionally managed using disease-modifying pharmaceutical therapies as a first line approach for treatment, yet there is increasing interest in lifestyle factors, particularly diet, for managing disease outcomes. Lutein has neuroprotective properties in healthy adults, but no previous research has examined the effects of lutein supplementation in persons with MS.
Objectives
This study aimed to investigate the efficacy of 4-mo lutein supplementation on carotenoid status and cognition in persons with relapse-remitting MS (RRMS).
Methods
A randomized controlled, single-blind research design was used among adults with RRMS (N = 21). Participants were randomized into placebo (n = 9) or treatment (20-mg/d lutein, n = 12) groups with outcomes measured before and after 4 mo. Macular pigment optical density (MPOD) was assessed using heterochromatic flicker photometry. Skin carotenoids were assessed using reflection spectroscopy. Serum lutein was measured using high-performance liquid chromatography. Cognition was assessed via the Eriksen flanker with event-related potentials, spatial reconstruction, and the symbol digit modalities tests.
Results
There was a significant group by time interaction for MPOD (F = 6.74, P = 0.02), skin carotenoids (F = 17.30, P < 0.01), and serum lutein (F = 24.10, P < 0.01), whereby the treatment group improved in all carotenoid outcomes. There were no significant group by time interactions for cognitive and neuroelectric outcomes. However, increase in MPOD was positively associated with accuracy during the flanker incongruent trials (r = 0.55, P = 0.03) and the spatial memory task (r = 0.58, P = 0.02) among treatment participants.
Conclusions
Lutein supplementation increases carotenoid status among persons with RRMS. There is no significant effect on cognitive function but change in macular carotenoids is selectively associated with improved attention and memory. This study provides preliminary support for a fully powered study targeting retinal and neural carotenoids for cognitive benefits in persons with MS.
This trial was registered at clinicaltrials.gov as NCT04843813.
Keywords: multiple sclerosis, lutein, carotenoids, macular pigment, cognition
Introduction
Multiple sclerosis (MS) is an immune-mediated and neurodegenerative disease resulting in demyelination and transection of axons and subsequent loss of neurons in the CNS [1]. The extent and location of CNS tissue damage manifests in outcomes such as cognitive dysfunction (eg, slowed processing speed) among many others. The first-line approach for managing MS and disease pathophysiology involves application of disease-modifying therapies (DMTs) that target cytokines and lymphocytes, but these agents have little benefit in restoring cognitive dysfunction. To that end, there has been increasing interest in lifestyle factors as an adjuvant for DMTs, as lifestyle might provide complementary benefits necessary for restoration of cognitive function decline in MS [2]. The focus on lifestyle has largely included physical activity, smoking, and diet/nutrition [[3], [4], [5]]. Interestingly, diet/nutrition was the number one topic of interest for the management of outcomes among persons with MS [6,7]. However, there is still limited knowledge on the efficacy of specific nutrients for restoring function in MS [8], and we believe that carotenoids represent a safe, tolerable, and feasible direction for improving cognition in MS.
Carotenoids are anti-inflammatory plant pigments that cannot be synthesized de novo and accumulate across various tissues in the human body including adipose, skin, retina, and brain [9]. Several carotenoids have been detected in the skin, and these carotenoids provide protection against free radicals and support innate resistance against damaging UV light [10,11]. In the retina, the macula is composed of 3 xanthophyll carotenoids: zeaxanthin, lutein, and mesozeaxanthin [12], collectively referred to as macular pigment. Macular pigment filters out damaging short wavelength blue light and protects photoreceptor cells and retinal pigment epithelium from oxidative stress [[13], [14], [15], [16]]. Additionally, among the handful of carotenoids detected in human brain tissue, lutein accumulates in disproportionately greater quantities (up to 5-fold) relative to other carotenoids that are more commonly consumed in the diet, eg, β-carotene [17]. Although the exact role of lutein in brain tissue is unclear, postmortem metabolomic analyses of human brain tissue have demonstrated that brain lutein concentrations are correlated with lipid pathway metabolites, energy pathway metabolites, brain osmolytes, amino acid neurotransmitters, and other antioxidants [18]. Additionally, lutein may support brain structure and function through protective effects against oxidation of DHA, the major polyunsaturated fatty acid in the brain [19]. Nonenzymatic oxidation of DHA has been documented in conditions of oxidative stress such as aging and neurodegenerative disease [20,21]. Therefore, consumption of antioxidants known to colocalize with DHA in brain tissue—such as lutein—may confer neuroprotective effects in MS. Anti-inflammatory and antioxidant properties of carotenoids have been hypothesized to have the potential of downregulating inflammatory molecules in MS [2]. Lutein supplementation has improved measures of cognition [[22], [23], [24], [25]] and vision [26,27] in adults from the general population, and we have reported that people with MS have lower MPOD (macular pigment optical density) and serum lutein than age-matched healthy controls [28]. Additionally, higher MPOD is associated with better processing speed in persons with MS [29]. Finally, MPOD has been associated with neuroelectric measures of cognitive processing as assessed by event-related potentials acquired through electroencephalography (EEG) recording. Specifically, previous work has shown that fluctuations in the P3 component, an index of stimulus evaluation and context updating, are explained by MPOD in persons with MS [29].
We conducted a randomized, single-blind, placebo-controlled trial of daily lutein supplementation over a 4-mo period on carotenoid and cognitive outcomes in persons with MS. The primary aim of this study was to examine the effects of lutein supplementation on MPOD. The secondary aim was to examine the effects of lutein supplementation on other carotenoid measures as well as behavioral and neuroelectric measures of cognition. We hypothesized that lutein supplementation would improve biological markers of lutein status in the retina, skin, and serum and benefit cognitive performance compared with placebo. We further examined the relationship between carotenoid changes in the different biomarkers and cognitive performance changes across the 4-mo period in the intervention group. As an exploratory aim, we investigated the relationship between changes in biological markers of lutein status with changes in cognition within the treatment group. If macular lutein is reflective of neural lutein status, we anticipated that improvement in MPOD would have a stronger association with changes in neurocognitive function than skin and serum concentrations.
Methods
Research design
The Lutein and Multiple Sclerosis Experimental Study was a lutein compared with placebo-controlled, single-blind, randomized trial conducted in central Illinois (https://clinicaltrials.gov, NCT04843813) from 2019 to 2022. Due to the COVID-19 pandemic, we were unable to recruit the proposed number of participants; however, the study remained sufficiently powered to address the primary aim. Participants were randomized through a simple randomization [30], wherein participants were randomized using a random number generator into either the treatment or control group after participants were determined eligible but before participants visited the laboratory for their baseline appointment. The trial was a 2-group single (ie, participant) blind parallel design. The supplement was provided in the form of a daily soft gel. The single-blind approach was utilized because we had a small research team due to limited funds, and it was necessary for the experimenters to be involved in the receival and packaging of the supplements—which were received with an unblinded label—as well as the data collection. The treatment comprised 20 mg/d of lutein (FloraGLO) and safflower oil whereas the placebo contained safflower oil without added lutein. Participants were instructed to consume the supplement daily with a meal for 4 mo. The study duration was based on previous research that showed significant improvement in MPOD following a similar supplementation dose in 4 mo [22,23]. Compliance and participant experience were assessed throughout the study whereas treatment effects were assessed at baseline and at 4-mo follow-up via laboratory visits. This study was approved by the University of Illinois Urbana-Champaign Institutional Review Board (20012).
Participants
All participants provided written informed consent prior to study participation. Persons with MS between the ages of 18 and 64 y from the East-Central Illinois region were recruited for this study using university e-mails, flyers posted in community buildings, clinics, hospitals, and verbal announcements at regional events/gatherings of persons with MS. All participants completed a phone screening to determine eligibility for the study. Inclusion criteria included self-reported relapsing-remitting MS (RRMS), 20/20 corrected vision, no presence of color blindness, no history of age-related macular degeneration or epileptic seizures, and a score of ≤6 on the Patient-Determined Disease Steps (PDDS) scale [31]. The PDDS scale was utilized as a screener of disease progression to recruit a sample with mild to moderate impairment to limit fatigue to the participant during the testing procedures. Exclusion criteria included being <18 or >64 y of age, an MS diagnosis other than RRMS, uncorrected vision, presence of color blindness, PDDS score >7, pregnancy, prior diagnosis of age-related macular degeneration, or epileptic seizures. None of the participants were current smokers.
General procedures
All procedures were administered in accordance with the Declaration of Helsinki. Persons who were eligible after screening were invited to the laboratory to complete testing; consisting of a baseline visit and a follow-up visit. Supplements, a compliance log, and surveys were deployed at the end of the baseline visit, and participants were asked to consume either the placebo or treatment daily for 4 mo before returning for follow-up testing. Compliance during the 4 mo was self-reported via a daily compliance log indicating whether they consumed the supplement or not. Participants e-mailed records of compliance biweekly and returned the paper record at their follow-up appointment. The baseline and follow-up appointments consisted of all the procedures outlined below. Visits to the laboratory consisted of 3 h of testing; surveys were e-mailed to limit participant fatigue. Participants were compensated via Amazon gift card or check for a total of $200 ($50 after baseline and $150 after follow-up) with an additional compensation of $30 for travel to the laboratory of distances ≥60 miles.
Anthropometrics and demographic information
Height and weight measures were conducted in triplicate and averaged at each visit with participants wearing lightweight clothing and no shoes. A stadiometer (model 240; SECA) and a digital scale (WB-300 Plus) were used to measure height and weight, respectively. Participants completed a survey to report demographic and health history information. Surveys were completed electronically to limit participant fatigue and appointment burden.
Expanded Disability Status Scale (EDSS)
The EDSS [32] was administered to assess disability status. The scale ranges from 0 (normal) to 10 (death due to MS), with a higher score indicating greater impairment in function system and gait. The EDSS is a validated tool that is commonly used clinically to measure and evaluate MS patients’ level of functioning.
Habitual dietary intake
Dietary intake was assessed using the Diet History Questionnaire Version III [33] (DHQIII; version 3.0, National Cancer Institute) FFQ distributed via e-mail and completed through the DHQII website. To assess habitual dietary intake at baseline, the DHQIII past year with portion sizes was administered during the first week of the intervention. Dietary intake for follow-up testing was assessed by asking participants to complete the DHQIII with questions about the past month, completed in the week after completion of their follow-up testing appointment. DHQIII is a validated FFQ that outputs a multitude of dietary data on food and nutrient intake for each participant. For the present work, unadjusted dietary lutein and zeaxanthin intake was the main dietary outcome of interest.
MPOD
MPOD was assessed using a heterochromatic flicker photometry (HFP) technique administered via a macular densitometer (Macular Metrics Corporation). The principles and validation of this task are described in more detail in Wooten et al. [34]. Utilizing a similar procedure to Cerna et al. [28,29], participants completed a 2-step process administered by a trained member of the research staff. The first step involved focusing on a flickering stimulus in their central line of vision, where macular pigment is most dense. The stimulus was presented between 2 wavelengths, 460 nm and 570 nm, at a rate that was optimized for the subject’s null zone. The operator adjusted the radiance until the participant could no longer observe a flicker, indicating a null-flicker zone. The same procedure was then repeated at a 7° parafoveal angle where macular pigment is least dense. The MPOD value was calculated as the differential between the foveal and parafoveal log sensitivity measurements after normalization at 570 nm. The same eye was used at both the baseline and follow-up appointment.
Skin carotenoid assessment
Skin carotenoid status was measured noninvasively using the Veggie Meter device (Longevity Link Corporation). This device uses reflection spectroscopy at the fingertip to provide a quantitative estimation of skin carotenoids. Before each assessment, the device was calibrated using a light and dark reference. Participants placed their nondominant hand index finger on top of a convex lens through which a light source was projected, and a dark clamp was placed on top to apply slight pressure and hold the finger in place on the lens. This position temporarily pushes the blood away from the measured area to minimize the confounding effect of hemoglobin absorptions. The Veggie Meter scores are logarithmic scale values based on reflectivity and range from 0 to 800, with a higher score indicating greater skin carotenoid concentrations [35]. Three scans were taken at baseline and follow-up testing; an average of the 3 scans was used for analyses. This technique is validated and described in more detail in Ermakov et al. [36].
Serum lutein assessment
A venous blood draw was completed following an overnight fast to quantify serum concentration of lutein. Serum lutein was assessed utilizing a high-performance liquid chromatography procedure previously described in Jeon et al. [37] and Edwards et al. [38]. In summary, 250 μL of serum was mixed with an equal volume of ethanol containing 0.1% of butylated hydroxytoluene and vortexed. Serum lutein was extracted using 3 consecutive 1-mL hexane extraction processes. Hexane layers were combined, dried under nitrogen, taken up into 90% methyl tert-butyl ether, 8% methanol, and 2% ammonium acetate in water solution (1.5% solution) and then analyzed, in duplicate, for carotenoid concentrations using the Alliance high-performance liquid chromatography system (e2695 Separation Module) equipped with 2998 photodiode array detector (Waters) and a reverse-phase C30 column (4.6 × 150 nm, 3 μm, YMC). Lutein standard was obtained from Carotenature. For quantification, a standard lutein curve was run, and serum lutein concentrations were quantified using a 2550 ethanol extinction coefficient in a 1% solution.
Attentional inhibition
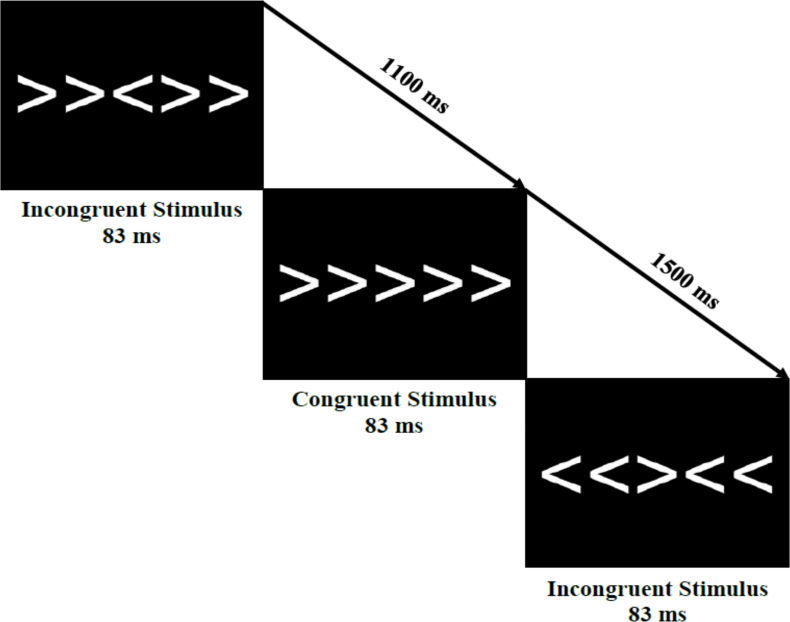
Attentional inhibition was assessed using a modified Eriksen flanker task [39]. The stimuli for the task were presented as 5 white arrows on a black background (Figure 1), with participants instructed to use a response pad to identify the directionality of the middle arrow, referred to as the target. The task comprised 2 types of trials: congruent and incongruent. During the congruent trials, the flanking arrows were oriented in the same direction as the target (eg, “>>>>>”), whereas in incongruent trials, the target arrow pointed in the opposite direction to the 4 flanking arrows (eg, “>><>>”). Participants completed 40 practice trials before completing 400 experimental trials (2 blocks of 200 trials), with each trial having a stimulus duration of 83 ms. The blocks included an equal and random distribution of congruent and incongruent trials as well as left and right trials. Furthermore, the experimental trials were separated by jittered intertrial intervals of 1100, 1300, or 1500 ms. Behavioral performance was assessed in terms of accuracy and reaction time for each trial type.
FIGURE 1.
Schematic of the modified Eriksen flanker task used to measure attentional inhibition. Congruent or incongruent stimuli were presented for 83 ms, with the participant responding to the direction of the middle arrow. Stimuli were presented in a jittered interstimulus interval of 1100, 1300, or 1500 ms.
EEG recording and event-related potential analyses
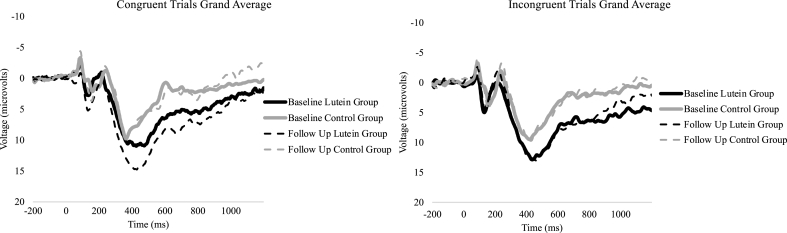
EEG data were collected using a 64-channel Neuroscan Quik-cap (Compumedics). During the recording, an electrode between Cz and CPz served as the reference, while the AFz electrode was used as a ground. To record electro-oculographic activity, electrodes were placed above and below the orbit and on the outer canthus of the eyes. Impedances were kept <10 kΩ. Data were digitized at a sampling rate of 500 Hz and amplified 500 times with a direct current to a 70-Hz filter and a 60-Hz notch filter using a Neuroscan Syn-amps2 amplifier. EEGlab and ERPlab toolboxes were used for data processing [40,41]. During offline processing, the average of 2 mastoid electrodes (M1 and M2) were used for rereferencing and subjected to a 0.1-Hz high-pass filter to account for slow drifts before utilizing an independent component analysis to identify eye blink artifacts. Components with correlation of ≥0.35 with the vertical electro-oculogram electrode were considered eye blinks and removed. Continuous data were then epoched for each trial from −200 prior to and 1200 ms following stimulus onset, with −200 to 0 ms used as a baseline correction. All data were filtered using a 30-Hz zero-phase shift low-pass filter. Trials containing a peak-to-peak amplitude exceeding 100 μV in a 100-ms width and 50-ms moving window were rejected as artifacts. Correct trials from individuals with ≥50% artifact-free trials were included for analyses. The P3 was determined using the collapsed localizer method [42,43]; a lateralized region of interest identical to that in the study by Cerna et al. [29] was created using 8 central-parietal electrodes, FZ, F2, F4, FC2, FCZ, FC4, C2, and C4. Mean amplitude and peak latency were extracted between 300 and 700 ms in relation to stimulus onset. Waveforms separated by trial type are shown in Figure 2.
FIGURE 2.
Grand average event-related potential waveforms for the P3 region of interest for both trial types of the Eriksen flanker task. The P3 mean amplitude and latency were extracted from 300 to 700 ms after stimulus onset.
Hippocampal-dependent relational memory
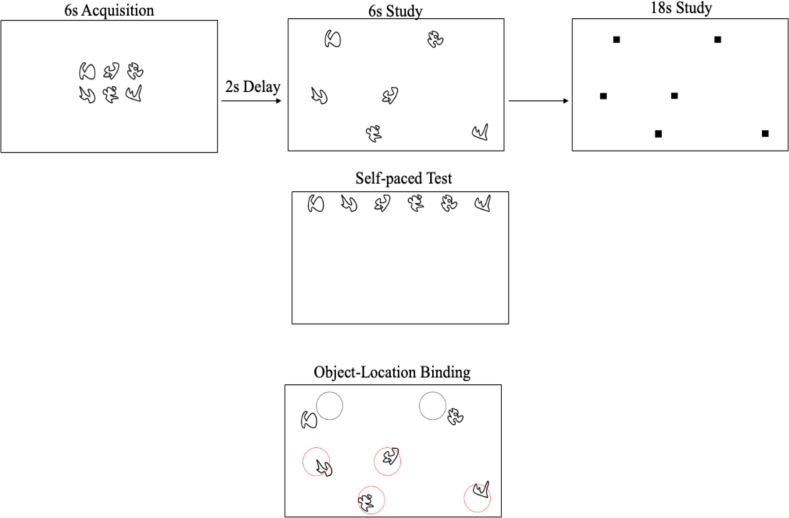
To assess hippocampal-dependent relational memory, a computerized spatial reconstruction task was administered using Presentation Software (Neurobehavioral Systems), as shown in Figure 3. The task consisted of an acquisition phase, a study phase, and a test phase. In the acquisition phase, 6 abstract shapes were presented in the center of the screen for 6 s to familiarize participants with the stimuli. The stimuli then disappeared and reappeared in a randomized array on the screen for 6 s. Subsequently, small squares replaced the shapes, and participants were given 18 s to click each box to individually learn the location of each shape. After the boxes disappeared and a 2-s fixation, the shapes reappeared at the top of the screen, and participants were tasked with reconstructing the array they had previously studied at their own pace. This same task was repeated in 4 blocks of 5 trials for a total of 20 trials.
FIGURE 3.
Schematic of the phases of the spatial reconstruction task and the scoring metric. Participants viewed 6 ambiguous creatures in a standard array during acquisition, the same 6 objects were viewed in a randomized array and studied one at a time, and lastly, participants completed a self-paced reconstruction of the randomized array. Object-location binding was utilized for scoring, with a point given to every object placed correctly in the predefined radius.
Object-location binding was used to assess performance. Object-location binding was defined as the number of times a participant correctly placed an item within a predefined radius around its studied location. For each trial, a score of 0 to 6 was assigned for this metric, with a higher score indicating better performance. Performance was averaged across trials. A more detailed explanation of this error metric can be found in Horecka et al. [44].
Processing speed
The assessment of cognitive processing speed was conducted using an oral administration of the symbol digit modalities test (SDMT), which is a widely used clinical measure [45]. The SDMT involves presenting several lines of symbols, each corresponding to a number from 1 to 9, and a corresponding key at the top of the page for reference. Participants were instructed to decode the symbols and report the corresponding numbers verbally, with the total number of correctly reported numbers within a 90-s time frame being the scoring metric.
Statistical analysis
Statistical analyses were performed using IBM SPSS, version 24. An a priori power calculation was conducted to determine an adequate sample size for the primary aim. Assuming an effect size of 0.3 (f), α of 0.05, and power of 80%, it was estimated that a minimum of 20 participants (10 per group) would be necessary to conduct the MPOD analyses. Independent samples t tests were conducted to confirm that the groups did not differ statistically in demographics and carotenoid status at baseline. Pearson correlations were conducted to determine covariates for subsequent analyses. We then proceeded with group (lutein compared with placebo) by time (baseline compared with follow-up) repeated measures analysis of variance controlling for covariates, as necessary, on the outcomes of MPOD, skin carotenoid status, and serum lutein. The same analytic model was applied for examining intervention effects on flanker, spatial reconstruction, and SDMT performance. To assess the relationship between changes in carotenoid status variables and cognitive measures in the intervention group, we conducted Pearson correlations on the change scores for cognitive and carotenoid variables. The change scores were calculated by subtracting the baseline value from the follow-up value. Normality for the data was assessed using the Shapiro-Wilk test, Q-Q plots, histograms, and boxplot analysis. Outliers, values exceeding 3 standard deviations from the mean, were winsorized (baseline MPOD, n = 1; follow-up serum lutein, n = 1). The Mauchly test of sphericity was performed to assess equal sphericity between groups. Analyses were performed using a pairwise deletion approach. We applied study-wise α of 0.05 for statistical significance.
Results
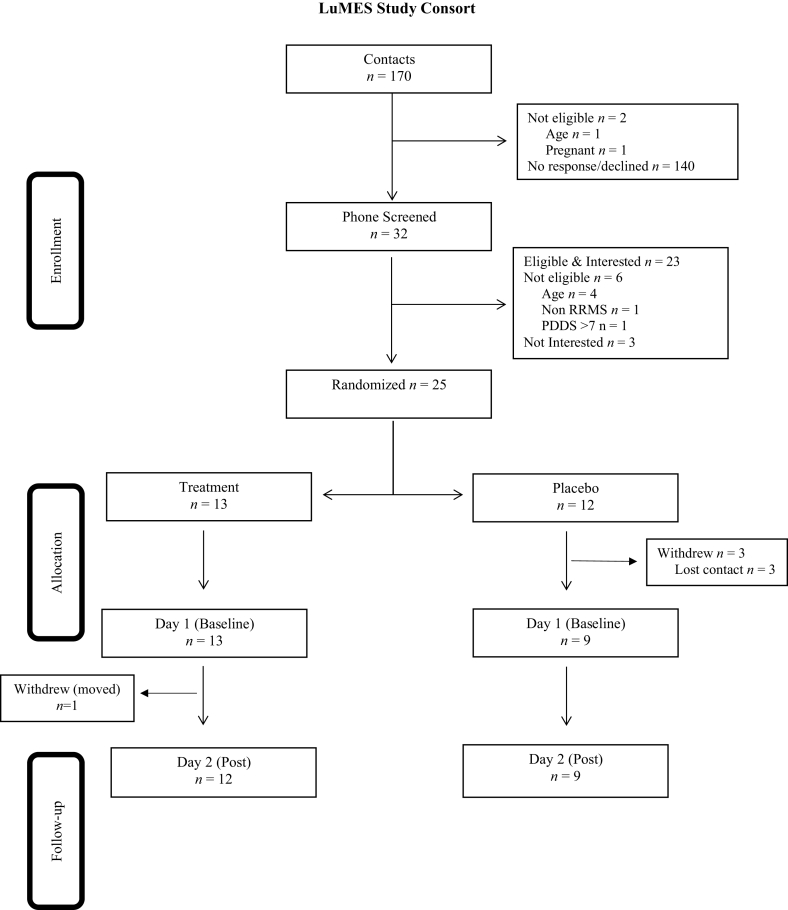
The study CONSORT diagram is presented in Figure 4. Thirty-two individuals were phone screened for the study, 6 were deemed ineligible based on criteria, and 3 were not interested in participating. While 25 individuals were randomized into groups after determining eligibility, 3 participants declined participation before completing the baseline testing. Twenty-two participants completed a baseline laboratory visit and were randomized into the lutein (n = 13) or placebo (n = 9) groups, and there was one dropout in the treatment group between baseline and follow-up due to participant relocation. While a total of 21 participants completed the study, 1 participant did not complete the blood draw at baseline, resulting in a sample size of 20 (placebo: n = 8; lutein: n = 12) for the main analyses of carotenoid status; intervention analyses on cognition had a sample size of 21.
FIGURE 4.
CONSORT diagram. Thirty-two individuals were phone screened for the study; 6 were deemed ineligible based on criteria, and 3 were not interested in participating. Twenty-two participants completed their baseline laboratory visit and were randomized into the lutein treatment group (n = 13) or control group (n = 9), there was 1 dropout in the lutein supplementation group between baseline and follow-up. LuMES, Lutein and Multiple Sclerosis Experimental.
Adherence, safety, and tolerability
Of the participants who completed the study (n = 21), self-reported compliance for both groups was 98% with no adverse side effect reported. Analyses were conducted only for individuals with >80% compliance. All participants met this threshold (n = 21).
Baseline between-group differences
Table 1 provides demographic information for both groups, and Table 2 describes the baseline and follow-up values for all carotenoid outcomes. At baseline, there were no significant differences in age (t = 0.30, P = 0.38), BMI (t = 0.63, P = 0.54), years with MS (t = 1.07, P = 0.30), and EDSS (t = −0.66, P = 0.52) between the treatment and control groups. Additionally, there were no significant differences between MPOD (t = 1.19, P = 0.25), skin carotenoids (t = 1.75, P = 0.10), dietary lutein and zeaxanthin (t = −0.003, P = 0.99), or serum lutein (t = 1.58, P = 0.07) between the 2 groups at baseline.
TABLE 1.
| Variable | Treatment group | Control group |
|---|---|---|
| n | 12 | 9 |
| Age, y | 51.6 ± 10.0 | 52.8 ± 8.1 |
| Sex, n female, n male | 10, 2 | 9, 0 |
| Race/ethnicity, % | ||
| Caucasian | 100 | 77.8 |
| African American | 0 | 11.1 |
| Latino/a | 0 | 11.1 |
| Highest level of education, % | ||
| High school | 8.3 | 0 |
| Some college | 50 | 11.1 |
| College graduate | 33.3 | 44.4 |
| After college | 8.3 | 44.4 |
| Years with diagnosed MS | 10.3 ± 6.2 | 14 ± 9.04 |
| BMI, kg/m2 | 29.9 ± 7.1 | 32.30 ± 9.00 |
| Expanded Disabilities Scale | 3.5 ± 2.4 | 2.78 ± 2.44 |
| Dietary L + Z intake, mg3 | 2.47 ± 2.22 | 2.46 ± 2.11 |
| Macular pigment optical density | 0.32 ± 0.26 | 0.46 ± 0.27 |
| Skin carotenoids, VM units | 240 ± 57 | 292 ± 84 |
| Serum lutein, μmol/L4 | 0.13 ± 0.06 | 0.19 ± 0.11 |
L + Z, lutein and zeaxanthin; MS, multiple sclerosis; VM, Veggie Meter.
Continuous data expressed as mean ± SD.
Baseline group equivalence was assessed at baseline using independent t tests. No significant differences were observed between the 2 groups at baseline.
Treatment group n = 5, control group n = 7.
Control group n = 8.
TABLE 2.
Bivariate correlations between baseline and change in carotenoid status measures across groups1
| Timepoint | Carotenoid variable | Change (follow-up − baseline) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group (n = 12) |
Control group (n = 9) |
||||||||
| Serum L, μmol/L | MPOD | Skin carotenoids | Dietary LZ, mg2 | Serum L, μmol/L3 | MPOD | Skin carotenoids | Dietary LZ, mg2 | ||
| Baseline | Dietary LZ2 | −0.91 (0.09) | 0.45 (0.44) | −0.21 (0.73) | −0.99 (0.08) | −0.64 (0.17) | −0.13 (0.78) | −0.20 (0.61) | −0.63 (0.26) |
| Serum L | −0.29 (0.38) | 0.87 (<0.01)4 | −0.15 (0.64) | −0.65 (0.55) | −0.50 (0.20) | 0.07 (0.87) | 0.30 (0.47) | −0.53 (0.35) | |
| Skin carotenoids | −0.12 (0.73) | 0.75 (<0.01)4 | −0.22 (0.49) | −0.97 (0.15) | −0.40 (0.33) | 0.38 (0.32) | −0.20 (0.61) | −0.86 (0.06) | |
| MPOD | 0.30 (0.37) | −0.19 (0.57) | 0.34 (0.29) | −0.83 (0.37) | 0.12 (0.77) | −0.40 (0.29) | −0.27 (0.48) | −0.68 (0.21) | |
L, lutein; LZ, lutein and zeaxanthin; MPOD, macular pigment optical density.
Data presented as Pearson r (P).
Treatment group n = 5, control group n = 7.
Control group n = 8.
Denotes significance at P < 0.05 (2 tailed).
Relationship between carotenoid measures and demographic variables
TABLE 3, TABLE 4 summarize the bivariate correlations that were conducted to determine covariates for the ANCOVA models. Baseline serum lutein was significantly correlated with change in MPOD (r = 0.87, P < 0.01) and baseline skin carotenoids (r = 0.74, P < 0.01) for the treatment group. Therefore, the effects of lutein supplementation on lutein accumulation in the skin and eye may be confounded by baseline serum lutein concentrations and the subsequent models adjusted for this relationship. No demographic variables were related to markers of carotenoid status.
TABLE 3.
Bivariate correlations between baseline carotenoid status measures and demographic variables in the separate groups1
| Timepoint | Variables | Baseline |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group (n = 12) |
Control group (n = 9) |
||||||||
| Serum L, μmol/L | MPOD | Skin carotenoids | Dietary LZ, mg2 | Serum L, μmol/L3 | MPOD | Skin carotenoids | Dietary LZ, mg2 | ||
| Baseline | Age | 0.24 (0.42) | 0.19 (0.56) | −0.04 (0.90) | 0.15 (0.81) | 0.24 (0.42) | 0.19 (0.56) | −0.50 (0.17) | 0.46 (0.30) |
| EDSS | 0.07 (0.82) | 0.13 (0.69) | −0.07 (0.82) | 0.33 (0.59) | 0.07 (0.82) | 0.13 (0.69) | −0.85 (<0.01)4 | 0.06 (0.90) | |
| Years with MS | −0.04 (0.89) | 0.24 (0.49) | 0.01 (0.99) | 0.01 (0.98) | −0.50 (0.21) | −0.61 (0.08) | 0.02 (0.96) | −0.69 (0.09) | |
EDSS, Expanded Disability Status Scale; L, lutein; LZ, lutein and zeaxanthin; MPOD, macular pigment optical density; MS, multiple sclerosis.
Data presented as Pearson r (P).
Treatment group n = 5, control group n = 7.
Control group n = 8.
Denotes significance at P < 0.05 (2 tailed).
TABLE 4.
Baseline and follow-up carotenoid means based on group and time1
| Variable | Treatment group (n = 12) |
Control group (n = 9) |
||
|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |
| Carotenoid status | ||||
| Macular pigment optical density | 0.32 ± 0.26 | 0.52± 0.41 | 0.48 ± 0.282 | 0.46 ± 0.352 |
| Skin carotenoids, VM units | 240 ± 57 | 380 ± 89 | 280 ± 822 | 276 ± 982 |
| Serum lutein, μmol/L | 0.13 ± 0.06 | 0.63 ± 0.46 | 0.19 ± 0.112 | 0.18 ± 0.092 |
| Cognitive performance | ||||
| Eriksen flanker task | ||||
| Congruent accuracy, % | 97.92 ± 1.44 | 95.38 ± 8.25 | 97.76 ± 2.08 | 98.39 ± 1.95 |
| Congruent RT, ms | 433 ± 57 | 426 ± 66 | 410 ± 68 | 415 ± 52 |
| Incongruent accuracy, % | 95.04 ± 3.94 | 93.71 ± 7.33 | 93.38 ± 4.99 | 95.00 ± 3.06 |
| Incongruent RT, ms | 487 ± 56 | 478 ± 64 | 453 ± 60 | 457 ± 44 |
| P3 congruent mean amplitude, μV | 8.02 ± 5.10 | 9.58 ± 8.30 | 5.59 ± 4.25 | 5.52 ± 5.67 |
| P3 congruent peak latency, ms | 422 ± 59 | 447 ± 89 | 407 ± 56 | 447 ± 81 |
| P3 incongruent mean amplitude μV | 8.99 ± 5.11 | 9.57 ± 5.71 | 6.46 ± 2.92 | 6.15 ± 6.54 |
| P3 incongruent peak latency, ms | 449 ± 70 | 505 ± 75 | 440 ± 49 | 448 ± 69 |
| Spatial reconstruction task | ||||
| Object-location binding | 2.2 ± 0.85 | 2.3 ± 0.63 | 2.4 ± 0.80 | 2.2 ± 0.83 |
| SDMT | ||||
| Score | 57 ± 14 | 57 ± 12 | 62 ± 9 | 62 ± 13 |
RT, reaction time; SDMT, symbol digits modality test.
Values presented as unadjusted mean ± SD.
Control group, n = 8.
Intervention effects on carotenoid outcomes
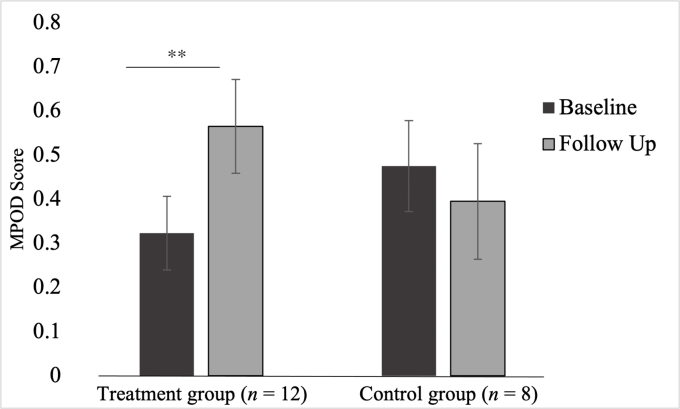
There was a statistically significant interaction between group and time for MPOD (F [1,18] = 6.74, P = 0.02, partial η2 = 0.28) while controlling for baseline serum lutein whereby there was an increase in MPOD only in the treatment group (Δẋ = 0.24, P < 0.01) and not in the control group (Δẋ = 0.08, P = 0.42). There was no significant main effect of time (F [1,18] = 1.62, P = 0.22, partial η2 = 0.09) or group for the outcome of MPOD (F [1,18] < 0.01, P = 0.97, partial η2 < 0.01). The covariate, baseline serum lutein, was significantly related to effects on MPOD (F [1,18] = 4.89, P = 0.04, partial η2 = 0.22).
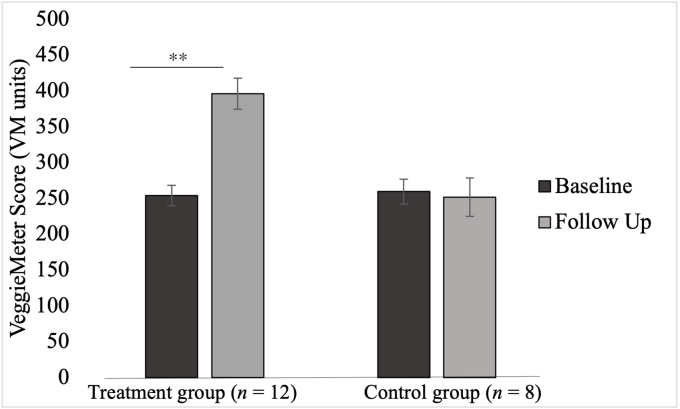
There was a significant interaction between group and time for skin carotenoids (F [1,18] = 17.30, P < 0.01, partial η2 = 0.50) whereby significant improvement in skin carotenoids was only observed in the lutein supplementation group (Δẋ = 142.0, P < 0.001) but not in the control group (Δẋ = 7.8, P = 0.76). There was a significant main effect of group (F [1,18] = 8.14, P = 0.01, partial η2 = 0.32), whereby skin carotenoids were higher in the treatment group (ẋDifference = 69.40, P = 0.01) at follow-up. There was no significant main effect of time for skin carotenoids (F [1,18] = 3.33, P = 0.09, partial η2 = 0.16). Baseline serum lutein (F [1, 18] < 0.01, P = 0.98, partial η2 < 0.01) was not significantly related to effects on skin carotenoids.
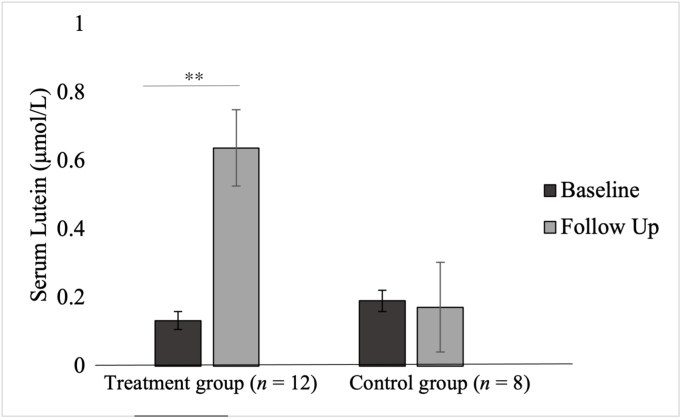
There was a significant interaction between group and time for serum lutein (F [1,18] = 24.10, P < 0.01, partial η2 = 0.59) whereby an improvement in serum lutein was only observed in the lutein supplementation group (Δẋ = 0.39, P < 0.01) but not in the control group (Δẋ = −0.01, P = 0.88). There was a significant main effect of time (F [1,18] = 21.92, P < 0.01, partial η2 = 0.56) and group (F [1,18] = 4.95, P = 0.041, partial η2 = 0.24) for serum lutein whereby serum lutein at follow-up was higher than at baseline (Δẋ = 0.24, P = 0.01). There was a significant main effect for group (F [1,18] = 9.43, P < 0.01, partial η2 = 0.36). The difference in adjusted means across the intervention groups and time periods are illustrated inFIGURE 5, FIGURE 6, FIGURE 7.
FIGURE 5.
Intervention effects on macular pigment optical density. Results of the post hoc t tests conducted to determine the nature of the interaction effects of the repeated measures ANCOVA for macular pigment optical density (MPOD). The figure presents the adjusted means after adjusting for age and baseline serum lutein. A significant difference in MPOD over time was observed only in the treatment group. ∗∗Denotes significant difference at P < 0.01.
FIGURE 6.
Intervention effects on skin carotenoids. Results of the post hoc t tests conducted to determine the nature of the interaction effects of the repeated measures ANCOVA for skin carotenoids. The figure presents the adjusted means after adjusting for age and baseline serum lutein. A significant difference in skin carotenoids over time was observed only in the treatment group. ∗∗Denotes significant difference at P < 0.01. VM, Veggie Meter.
FIGURE 7.
Intervention effects on serum lutein. Results of the post hoc t tests conducted to determine the nature of the interaction effects of the repeated measures ANCOVA for serum lutein. The figure presents the adjusted means after adjusting for age. A significant difference in serum lutein over time was observed only in the treatment group. ∗∗Denotes significant difference at P < 0.01.
Cognitive function intervention effects
Table 2 provides the mean values at baseline and follow-up for the cognitive outcomes. There was no significant group by time interactions for performance on the Eriksen flanker task, flanker P3 amplitude and latency, spatial reconstruction task, and SDMT. Table 5 summarizes the repeated measures ANOVA values for flanker accuracy and reaction time, flanker P3 amplitude and latency, spatial reconstruction task performance, and SDMT score.
TABLE 5.
Baseline and follow-up cognitive outcomes repeated measures ANOVA based on group and time
| Cognitive variable | Interaction effect |
Main effect: time |
Main effect: group |
||||||
|---|---|---|---|---|---|---|---|---|---|
| F (1,19) | Partial η2 | P | F (1,19) | Partial η2 | P | F (1,19) | Partial η2 | P | |
| Eriksen flanker task | |||||||||
| Congruent accuracy | 1.19 | 0.059 | 0.29 | 0.43 | 0.022 | 0.52 | 0.94 | 0.047 | 0.35 |
| Congruent RT | 0.60 | 0.031 | 0.45 | 0.022 | 0.001 | 0.88 | 0.45 | 0.023 | 0.51 |
| Incongruent accuracy | 1.07 | 0.003 | 0.31 | 0.010 | 0.00 | 0.92 | 0.008 | 0.049 | 0.93 |
| Incongruent RT | 0.95 | 0.047 | 0.34 | 0.19 | 0.010 | 0.67 | 1.31 | 0.065 | 0.27 |
| P3 congruent mean amplitude | 0.41 | 0.03 | 0.53 | 0.34 | 0.02 | 0.57 | 2.03 | 0.11 | 0.17 |
| P3 congruent peak latency | 0.13 | 0.01 | 0.72 | 2.17 | 0.12 | 0.16 | 0.08 | 0.01 | 0.78 |
| P3 incongruent mean amplitude | 0.10 | 0.01 | 0.75 | 0.00 | 0.00 | 0.96 | 2.18 | 0.11 | 0.16 |
| P3 incongruent peak latency | 1.79 | 0.10 | 0.20 | 3.15 | 0.16 | 0.10 | 1.49 | 0.09 | 0.24 |
| Spatial reconstruction task | |||||||||
| Object-location binding | 0.001 | 0.00 | 0.97 | 0.29 | 0.016 | 0.60 | 0.50 | 0.03 | 0.49 |
| SDMT | |||||||||
| Score | 0.052 | 0.003 | 0.82 | 0.00 | 0.00 | 0.99 | 0.97 | 0.049 | 0.34 |
RT, reaction time; SDMT, symbol digits modality test.
Relationship between change in cognitive function and change in carotenoid status
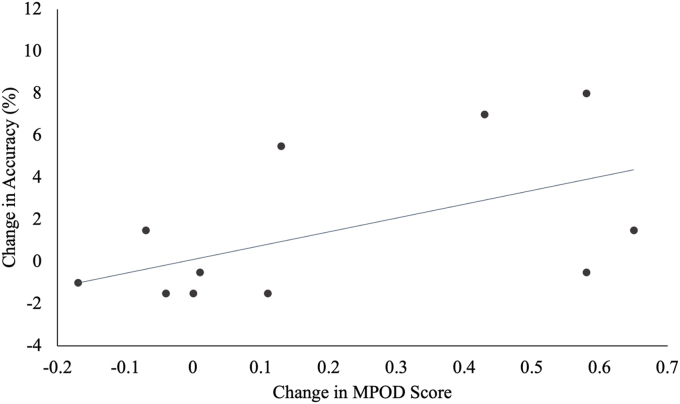
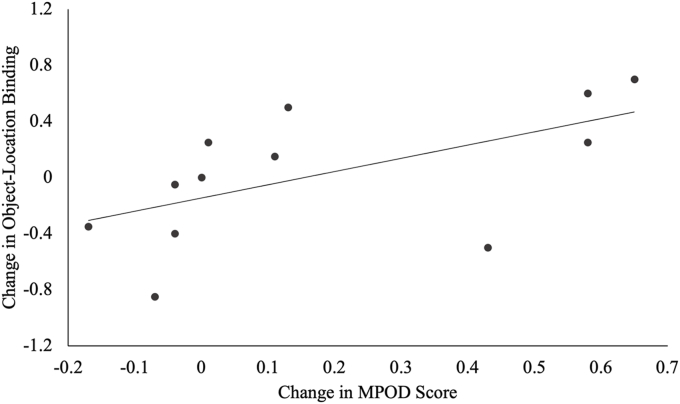
Table 6 summarizes the correlation coefficients and P values for the correlations between change in carotenoid status and change in cognitive function for participants in the intervention group. There was a significant positive association between change in MPOD with change in flanker incongruent accuracy (r = 0.55, P = 0.03) and change in object-location binding (r = 0.58, P = 0.02) in the spatial reconstruction task. FIGURE 8, FIGURE 9 display the significant relationships between the change in MPOD and cognitive performance across the intervention.
TABLE 6.
Change score correlations between carotenoid status and cognitive variables in the treatment group subsample (n = 12)1
| Change (follow-up − baseline) |
||||
|---|---|---|---|---|
| MPOD | Skin carotenoids | Serum lutein | ||
| Change (follow-up − baseline) | Eriksen flanker task | |||
| Congruent accuracy | 0.25 (0.22) | 0.10 (0.38) | 0.45 (0.10) | |
| Congruent RT | 0.14 (0.33) | 0.07 (0.41) | 0.00 (0.50) | |
| Incongruent accuracy | 0.55 (0.03)2 | 0.09 (0.39) | −0.23 (0.24) | |
| Incongruent RT | 0.01 (0.48) | −0.02 (0.47) | −0.03 (0.47) | |
| P3 congruent mean amplitude | −0.22 (0.26) | 0.06 (0.43) | 0.16 (0.32) | |
| P3 congruent peak latency | 0.34 (0.15) | 0.49 (0.07) | 0.43 (0.09) | |
| P3 incongruent mean amplitude | 0.001 (0.50) | 0.22 (0.26) | 0.34 (0.15) | |
| P3 incongruent peak latency | 0.49 (0.06) | 0.40 (0.11) | 0.37 (0.13) | |
| Spatial reconstruction task | ||||
| Object-location binding | 0.58 (0.02)2 | −0.10 (0.67) | −0.06 (0.80) | |
| SDMT | ||||
| Score | −0.38 (0.22) | −0.21 (0.51) | −0.45 (0.17) | |
RT, reaction time; SDMT, symbol digits modality test.
Data presented as Pearson r (P).
Denotes significance at P < 0.05 (1 tailed).
FIGURE 8.
Relationship between macular pigment ocular density (MPOD) and incongruent accuracy across the intervention. The relationships between the change in MPOD and change in incongruent flanker accuracy in the lutien subsample are shown. There was a significant positive association between change in MPOD and change in flanker incongruent accuracy.
FIGURE 9.
Relationship between macular pigment optical density (MPOD) and object-location binding across the intervention. The relationships between change in MPOD and change in object-location binding in the lutein subsample are shown. There was a significant positive association between change in MPOD and change in object-location binding.
Discussion
Despite nutrition being recognized as the lifestyle factor of highest interest among persons with MS [6,7], there have been very few randomized, controlled clinical trials investigating the effects of nutrient intake in this population [46,47]. To our knowledge, this is the first study to assess the effects of lutein supplementation on multiple biological markers of carotenoids using a placebo-controlled, single-blind, randomized trial among persons with MS. Herein we investigated the effects of a 4-mo lutein randomized controlled trial among persons with MS on biological markers of carotenoids in the macula, blood, and skin. Consistent with the a priori hypotheses, the group receiving daily lutein had a significant improvement in carotenoid status across the 3 measures of carotenoids whereas no such change was observed in the placebo control group. To our knowledge, these findings are the first to describe changes in carotenoid status among persons with MS following lutein supplementation. Additionally, there were no significant effects of supplementation on cognitive and neuroelectric outcomes of attentional control, memory function, and information processing speed. However, participants in the lutein treatment group with the greatest increase in MPOD exhibited greater improvements during the attention inhibition and spatial memory tasks. We did not screen for cognitive impairment; therefore, the lack of improvements of lutein supplementation on cognition could be explained by ceiling effects. The necessary next step is a lutein intervention trial in persons with MS with confirmed cognitive impairment.
Dietary antioxidant nutrients have been hypothesized to reduce oxidative stress and modulate immune responses of inflammatory cells [48]. Although carotenoids have not been widely studied in MS, other anti-inflammatory nutrients have been explored in the context of MS disease regulation [49]. Animal models of MS have provided evidence that curcumin, vitamin A, PUFAs, polyphenols, and vitamin D have beneficial properties, eg, protection against oxidative stress, demyelination, axonal injury, and decreased inflammation and improved remyelination [50]. Clinical studies of MS have focused primarily on supplementation with polyunsaturated fatty acids, vitamin D, and vitamin B-12 [46]. Preliminary evidence has shown varying results on the effectiveness of these supplements. Although there is limited knowledge on the nutrients to target for MS, there is support for anti-inflammatory dietary supplements to complement DMTs [2]. Therefore, this research provides novel evidence of the possible role of the carotenoid, lutein, to be studied as a complementary nutrient that could support DMTs in persons with MS.
Previous research on MPOD and MS has shown that persons with MS have lower MPOD compared to healthy controls [28]. Although this is the first study to examine the effects of lutein supplementation in MS, lutein supplementation has been previously studied in persons without MS. Healthy adults consuming 12 mg/d of lutein had a significant increase in serum lutein after 4 mo; however, at this dose, MPOD effects were not significant until 8 mo [25]. Bovier et al. [22] conducted a trial in healthy adults at the same dose and length as our current study and found significant increase in MPOD, supporting that 20 mg/d of lutein does significantly increase this biomarker in 4 mo. However, the magnitude of change for our treatment group (ẋ = 0.24) was 2-fold greater than the sample of healthy adults (ẋ = 0.10) in the study by Bovier et al. [22]. Given that persons with MS have lower MPOD status, it is possible that supplementation with lutein could allow for greater improvement in MPOD. Lutein supplementation studies in adults without MS have been found to improve visual processing speed [22], contrast and glare sensitivity [26], and visual performance at low luminance [27]. Similarly, lutein supplementation has also been associated with increases in cognitive abilities including verbal fluency, memory, and rates of learning [24] as well as complex attention and cognitive flexibility [25]. Although the current study did not reveal significant main effects of lutein supplementation on cognitive function, changes in MPOD were positively associated with changes in attentional inhibition and spatial memory. Previous research in primates has found that macular pigment is significantly related to lutein levels in the brain, suggesting that macular pigment measures can serve as a proxy for brain lutein status [51]. Therefore, our findings that MPOD had a positive relationship with cognition support this theory and provide preliminary support for targeting neural carotenoids for potential cognitive benefits in persons with MS. Given that lutein supports the visual system and thus improvements in cognition could be potentially confounded by these improvements, future studies should include cognitive tasks that are not dependent on the visual system. Performance on SDMT, frequently utilized clinically in patients with MS, was consistent with previous studies in patients with RRMS [52]. However, previous research has shown that more severe MS types have greater neurological impairment. Thus, future studies should expand beyond RRMS to explore whether persons with progressive MS types have greater cognitive benefits from lutein supplementation. Nevertheless, future research with a larger sample size to test the effects of lutein supplementation on cognitive function is needed to confirm the findings observed in the present study. Given that the observed change values in MPOD for the treatment group were larger than previously observed in adults without MS, future studies should include approaches beyond HFP (eg, autofluorescence) of retinal xanthophyll assessment to confirm the magnitude of effects observed in persons with MS. Additionally, future studies are needed to understand how the reliability of the HFP approach in persons with MS compares with the HFP approach in subjects without MS. Overall, MPOD assessment in populations with neurodegenerative disease are lacking, and additional research is needed to better understand the effect of disease on different approaches of assessing MPOD.
There are several limitations of this study worth considering. First, while adequately powered to address the lutein supplementation effects on carotenoid status, our sample size might not have been large enough to detect group by time differences across the different cognitive tasks. Further, we had a limited degree of racial and ethnic diversity among our sample, which limits the translation of the findings. Additionally, this study only explored a subset of the MS population by only examining how lutein affected people with RRMS. Although RRMS represents the majority of MS cases (approximately 75%), it is important to investigate lutein effects among individuals with other disease subtypes in future trials to better understand the relationship between lutein supplementation and disease progression. Study design and statistical method limitations include the single-blind approach, being underpowered to address the effects of lutein supplementation on cognition, and the method of randomization. The single-blind approach may introduce potential bias because there were subjective outcome methods. Regardless, a double-blind approach will be utilized in future clinical trials. Another limitation includes the method of randomization; simple randomization before the intervention had disadvantages of potentially leading to imbalanced samples. However, the current study reports balanced samples between each group with no demographic differences at baseline. Additional limitations include that immune function and other medical conditions were not monitored throughout the study. Because lutein has been negatively associated with inflammation, these data could inform individual differences in responses to lutein supplementation. Further limitations include that other factors that influence cognitive performance such as psychotropic medications, caffeine intake, and sleep were not considered in this study.
Future directions include a large-scale lutein supplementation trial that confirms the preliminary findings on effects on cognition. Interestingly, our current study had observed that individuals with higher baseline serum lutein had the greatest gains in MPOD following lutein supplementation. This is somewhat surprising given that individuals with lower lutein status may be expected to exhibit the greatest gains following supplementation. One possible explanation for this finding could be genetic and/or immune factors that might confer participants with greater ability to absorb dietary carotenoids—as reflected in serum—exhibited greater ability to improve macular deposition of lutein. Nevertheless, given the robust effect of baseline serum lutein status changes in MPOD, the observed effects warrant further exploration. Given that 4-mo lutein supplementation had the largest effect on serum lutein in comparison to the measures in the skin and retina, a longer supplementation trial could potentially confer larger gains in these biomarkers and have beneficial cognitive effects. Additionally, it would be advantageous to include MRI in future studies to be able to measure structural markers of brain myelination. MRI has been a critical technique for detecting and monitoring cognitive impairment in MS; however, there is still little known about the mechanisms and endogenous factors that protect against cognitive decline [53]. MRI measures would provide novel insight into the possible structural protection of lutein to the brain. Finally, there is a need for animal studies to explore lutein’s effects on MS models to better understand neuronal effects such as remyelination or reduced inflammation mechanisms of lutein.
In conclusion, this study utilized a randomized controlled trial design to investigate the impact of 4-mo lutein supplement consumption on biological markers of carotenoid status among a sample of persons with MS. We observed that lutein intake significantly increased macular pigmentation, skin carotenoids, and serum lutein. There were no significant supplementation effects on cognitive function. However, we found evidence that increase in MPOD was selectively related to attentional inhibition and spatial memory improvement. Further studies are needed to confirm the relationship between these measures and markers of disease progression and symptoms in persons with MS to further understand the functional and structural aspects of these biological markers.
Funding
The study was funded by the University of Illinois Division of Nutritional Sciences, NIH National Rehabilitation Research Resource to Enhance Clinical Trials.
Author contributions
The authors’ responsibilities were as follows – JWE, BA, RWM, NAK: designed research; SGM, JK, CNC, TDM: conducted research; SGM, NAK: analyzed data; SGM: wrote the paper; NAK: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request.
References
- 1.Kurtzke J.F. Epidemiology and etiology of multiple sclerosis. Phys. Med. Rehabil. Clin. N. Am. 2005;16(2):327–349. doi: 10.1016/j.pmr.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Riccio P., Rossano R. Nutrition facts in multiple sclerosis. ASN Neuro. 2015;7(1) doi: 10.1177/1759091414568185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicoletti A., Messina S., Bruno E., Mostile G., Quattrocchi G., Raciti L., et al. Risk factors in multiple sclerosis: a population-based case–control study in Sicily. Background and methods. Neurol. Sci. 2016;37(12):1931–1937. doi: 10.1007/s10072-016-2685-8. [DOI] [PubMed] [Google Scholar]
- 4.Halawani A.T., Zeidan Z.A., Kareem A.M., Alharthi A.A., Almalki H.A. Sociodemographic, environmental and lifestyle risk factors for multiple sclerosis development in the western region of Saudi Arabia: a matched case control study. Saudi Med. J. 2018;39(8):808–814. doi: 10.15537/smj.2018.8.22864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfredsson L., Olsson T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb. Perspect. Med. 2019;9(4):a028944. doi: 10.1101/cshperspect.a028944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn M., Bhargava P., Kalb R. Your patients with multiple sclerosis have set wellness as a high priority—and the national multiple sclerosis society is responding. US. Neurol. 2015;11(2):80–86. doi: 10.17925/USN.2015.11.02.80. [DOI] [Google Scholar]
- 7.Motl R.W., Mowry E.M., Ehde D.M., LaRocca N.G., Smith K.E., Costello K., et al. Wellness and multiple sclerosis: the National MS Society establishes a wellness research working group and research priorities. Mult. Scler. 2018;24(3):262–267. doi: 10.1177/1352458516687404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav V., Shinto L., Bourdette D.N. In: Multiple Sclerosis Therapeutics. Cohen J., Rudick R., editors. Cambridge University Press; Cambridge: 2011. Complementary and alternative treatments in multiple sclerosis; pp. 562–573. [DOI] [Google Scholar]
- 9.Parker R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996;10(5):542–551. doi: 10.1096/fasebj.10.5.8621054. [DOI] [PubMed] [Google Scholar]
- 10.Stahl W., Sies H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta. 2005;1740(2):101–107. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Darvin M.E., Sterry W., Lademann J., Vergou T. The role of carotenoids in human skin. Molecules. 2011;16(12):10491–10506. doi: 10.3390/molecules161210491. [DOI] [Google Scholar]
- 12.Roberts R.L., Green J., Lewis B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009;27(2):195–201. doi: 10.1016/j.clindermatol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Sujak A., Gabrielska J., Grudziński W., Borc R., Mazurek P., Gruszecki W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch. Biochem. Biophys. 1999;371(2):301–307. doi: 10.1006/abbi.1999.1437. [DOI] [PubMed] [Google Scholar]
- 14.Barker F.M., Snodderly D.M., Johnson E.J., Schalch W., Koepcke W., Gerss J., et al. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light–induced damage. Invest. Ophthalmol. Vis. Sci. 2011;52(7):3934–3942. doi: 10.1167/iovs.10-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong X., Draper C.S., Allison G.S., Marisiddaiah R., Rubin L.P. Effects of the macular carotenoid lutein in human retinal pigment epithelial cells. Antioxidants. 2017;6(4):100. doi: 10.3390/antiox6040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stringham J.M., Hammond B.R. Dietary lutein and zeaxanthin: possible effects on visual function. Nutr. Rev. 2005;63(2):59–64. doi: 10.1111/j.1753-4887.2005.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson E.J., Vishwanathan R., Johnson M.A., Hausman D.B., Davey A., Scott T.M., et al. Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J. Aging Res. 2013;2013 doi: 10.1155/2013/951786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieblein-Boff J.C., Johnson E.J., Kennedy A.D., Lai C.S., Kuchan M.J. Exploratory metabolomic analyses reveal compounds correlated with lutein concentration in frontal cortex, hippocampus, and occipital cortex of human infant brain. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohn E.S., Erdman J.W., Kuchan M.J., Neuringer M., Johnson E.J. Lutein accumulates in subcellular membranes of brain regions in adult rhesus macaques: relationship to DHA oxidation products. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller E., Morel A., Saso L., Saluk J. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2014;2014:572491. doi: 10.1155/2014/572491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montine T.J., Montine K.S., Reich E.E., Terry E.S., Porter N.A., Morrow J.D. Antioxidants significantly affect the formation of different classes of isoprostanes and neuroprostanes in rat cerebral synaptosomes. Biochem. Pharmacol. 2003;65(4):611–617. doi: 10.1016/s0006-2952(02)01607-6. [DOI] [PubMed] [Google Scholar]
- 22.Bovier E.R., Renzi L.M., Hammond B.R. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson L.M., Tharmarajah S., Jia Y., Semba R.D., Schaumberg D.A., Robinson K.A. The effect of lutein/zeaxanthin intake on human macular pigment optical density: a systematic review and meta-analysis. Adv. Nutr. 2021;12(6):2244–2254. doi: 10.1093/advances/nmab071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012;96(5):1161S–1165S. doi: 10.3945/ajcn.112.034611. [DOI] [PubMed] [Google Scholar]
- 25.Hammond B.R., Miller L.S., Bello M.O., Lindbergh C.A., Mewborn C., Renzi-Hammond L.M. Effects of lutein/zeaxanthin supplementation on the cognitive function of community dwelling older adults: a randomized, double-masked, placebo-controlled trial. Front. Aging Neurosci. 2017;9(254) doi: 10.3389/fnagi.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kvansakul J., Rodriguez-Carmona M., Edgar D.F., Barker F.M., Köpcke W., Schalch W., et al. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic Physiol. Opt. 2006;26(4):362–371. doi: 10.1111/j.1475-1313.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 27.Yao Y., Qiu Q.H., Wu X.W., Cai Z.Y., Xu S., Liang X.Q. Lutein supplementation improves visual performance in Chinese drivers: 1-year randomized, double-blind, placebo-controlled study. Nutrition. 2013;29(7–8):958–964. doi: 10.1016/j.nut.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Cerna J., Anaraki N.S.A., Robbs C.M., Adamson B.C., Flemming I.R., Erdman J.W., et al. Macular xanthophylls and markers of the anterior visual pathway among persons with multiple sclerosis. J. Nutr. 2021;151(9):2680–2688. doi: 10.1093/jn/nxab164. [DOI] [PubMed] [Google Scholar]
- 29.Cerna J., Edwards C.G., Martell S., Anaraki N.S., Walk A.D.M., Robbs C.M., et al. Neuroprotective influence of macular xanthophylls and retinal integrity on cognitive function among persons with multiple sclerosis. Int. J. Psychophysiol. 2023;188:24–32. doi: 10.1016/j.ijpsycho.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Friedman L.M., Furberg C.D., DeMets D.L., Reboussin D.M., Granger C.B. Fundamentals ofClinical Trials. Springer International Publishing; 2015. The randomization process; pp. 123–145. [Google Scholar]
- 31.Learmonth Y.C., Motl R.W., Sandroff B.M., Pula J.H., Cadavid D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013;13(1):1–8. doi: 10.1186/1471-2377-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 33.Subar A.F., Thompson F.E., Kipnis V., Midthune D., Hurwitz P., McNutt S., et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am. J. Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 34.Wooten B.R., Hammond B.R., Land R.I., Snodderly D.M. A practical method for measuring macular pigment optical density. Invest. Ophthalmol. Vis. Sci. 1999;40(11):2481–2489. [PubMed] [Google Scholar]
- 35.Ermakov I.V., Ermakova M., Sharifzadeh M., Gorusupudi A., Farnsworth K., Bernstein P.S., et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 2018;646:46–54. doi: 10.1016/j.abb.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ermakov I.V., Gellermann W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J. Biophotonics. 2012;5(7):559–570. doi: 10.1002/jbio.201100122. [DOI] [PubMed] [Google Scholar]
- 37.Jeon S., Neuringer M., Kuchan M.J., Erdman J.W. Relationships of carotenoid-related gene expression and serum cholesterol and lipoprotein levels to retina and brain lutein deposition in infant rhesus macaques following 6 months of breastfeeding or formula feeding. Arch. Biochem. Biophys. 2018;654:97–104. doi: 10.1016/j.abb.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards C.G., Walk A.M., Thompson S.V., Reeser G.E., Erdman J.W., Burd N.A., et al. Effects of 12-week avocado consumption on cognitive function among adults with overweight and obesity. Int. J. Psychophysiol. 2020;148:13–24. doi: 10.1016/j.ijpsycho.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task, Percept. Psychophys. 1974;16(1):143–149. doi: 10.3758/bf03203267. [DOI] [Google Scholar]
- 40.Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Calderon J., Luck S.J. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014;8(213) doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keil A., Debener S., Gratton G., Junghöfer M., Kappenman E.S., Luck S.J., et al. Committee report: publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology. 2014;51(1):1–21. doi: 10.1111/psyp.12147. [DOI] [PubMed] [Google Scholar]
- 43.Luck S.J., Gaspelin N. How to get statistically significant effects in any ERP experiment (and why you shouldn’t) Psychophysiology. 2017;54(1):146–157. doi: 10.1111/psyp.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horecka K.M., Dulas M.R., Schwarb H., Lucas H.D., Duff M., Cohen N.J. Reconstructing relational information. Hippocampus. 2018;28(2):164–177. doi: 10.1002/hipo.22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith A. 1973. Symbol digit modalities test. PsycTESTS Dataset. [Internet] Available from: [DOI] [Google Scholar]
- 46.Schwarz S., Leweling H. Multiple sclerosis and nutrition. Mult. Scler. 2005;11(1):24–32. doi: 10.1191/1352458505ms1119oa. [DOI] [PubMed] [Google Scholar]
- 47.Qu X., Walsh E.I., Cherbuin N., Black L.J. Mapping the literature on diet and multiple sclerosis: a data-driven approach. Nutrients. 2022;14(22):4820. doi: 10.3390/nu14224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Geldern G., Mowry E.M. The influence of nutritional factors on the prognosis of multiple sclerosis. Nat. Rev. Neurol. 2012;8(12):678–689. doi: 10.1038/nrneurol.2012.194. [DOI] [PubMed] [Google Scholar]
- 49.Bagur M.J., Murcia M.A., Jiménez-Monreal A.M., Tur J.A., Bibiloni M.M., Alonso G.L., et al. Influence of diet in multiple sclerosis: a systematic review. Adv. Nutr. 2017;8(3):463–472. doi: 10.3945/an.116.014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoiloudis P., Kesidou E., Bakirtzis C., Sintila S.A., Konstantinidou N., Boziki M., et al. The role of diet and interventions on multiple sclerosis: a review. Nutrients. 2022;14(6):1150. doi: 10.3390/nu14061150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vishwanathan R., Neuringer M., Snodderly D.M., Schalch W., Johnson E.J. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr. Neurosci. 2013;16(1):21–29. doi: 10.1179/1476830512y.0000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huijbregts S.C.J., Kalkers N.F., de Sonneville L.M.J., de Groot V., Reuling I.E.W., Polman C.H. Differences in cognitive impairment of relapsing remitting, secondary, and primary progressive MS. Neurology. 2004;63(2):335–339. doi: 10.1212/01.wnl.0000129828.03714.90. [DOI] [PubMed] [Google Scholar]
- 53.Paul F. Pathology and MRI: exploring cognitive impairment in MS. Acta Neurolog. Scand. 2016;134(S200):24–33. doi: 10.1111/ane.12649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request.