Abstract
Rhabdomyolysis is a syndrome resulting from striated muscular breakdown, which may occur due to drug therapy with agents such as selective serotonin reuptake inhibitors (SSRIs). Although studies have shown that fluvoxamine can rarely cause myalgia, there are no reported cases of rhabdomyolysis due to fluvoxamine monotherapy. Here we describe a case of rhabdomyolysis due to fluvoxamine monotherapy for obsessive-compulsive disorder. The young adolescent developed pain in the extremities, and an increase in serum creatine kinase (CK) and myoglobin during fluvoxamine treatment. These adverse reactions were reversed immediately after the medicine was changed to another SSRI—sertraline. This is the first reported case of fluvoxamine-associated rhabdomyolysis. It is advisable to determine serum CK levels before starting fluvoxamine treatment, and then at regular intervals, to avoid the occurrence of severe acute kidney injury with possible life-threatening complications.
Keywords: Case Reports, DRUG-RELATED SIDE EFFECTS AND ADVERSE REACTIONS, MENTAL HEALTH, PSYCHIATRY, CLINICAL MEDICINE
Background
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI), widely used in the treatment of anxiety, obsessive-compulsive disorder, and depressive disorder. Increasing clinical data suggest that fluvoxamine use in early-stage subjects with COVID-19 might be associated with a reduced risk of intubation or death.1 Fluvoxamine is relatively safe compared with other psychiatric medications. It can affect cardiac conduction, causes hypotension, prolongs QTc interval, and increases the propensity for seizures.2 3 However, there is no report that fluvoxamine could induce rhabdomyolysis.
Rhabdomyolysis is a condition that arises from striated muscle injury causing the release of massive amounts of uric acid, the heme-containing protein myoglobin, phosphate ions, potassium ions, and creatine kinase into the circulation. Serious rhabdomyolysis could lead to acute renal failure and life-threatening arrhythmia. Causes of rhabdomyolysis are varied and include excessive muscular activities, trauma, infection, drugs, electrolyte imbalance, and toxins. Antipsychotics and statins are the most common causes of drug-related rhabdomyolysis.4 There are also some reports regarding the occurrence of rhabdomyolysis following the use of SSRIs such as sertraline, escitalopram and paroxetine. Here we present a case of rhabdomyolysis in a male adolescent who received fluvoxamine monotherapy for obsessive-compulsive disorder. Notably, the reaction regressed following the switch to sertraline. To the best of our knowledge, this is the first reported case of fluvoxamine-associated rhabdomyolysis.
Case presentation
This male early adolescent was admitted due to exercise-induced extremity pain for 2 weeks (day 0). He had 1 day of dark urine and loss of appetite, but no other symptoms, such as headache, dizziness, nausea or vomiting. A blood investigation showed his serum creatine kinase (CK) (28 855.0 U/L) and creatine kinase-MB (CK-MB) (245.4 U/L) were notably elevated. A test for urine myoglobulin was positive, thus the patient was diagnosed with rhabdomyolysis and given intravenous hydration, and alkalisation of urine for symptomatic treatment. Seven days after treatment, laboratory tests showed decreases in CK (1356.8 U/L), CK-MB (21.2 U/L) and myoglobin (313.9 ng/mL), demonstrating recovery from rhabdomyolysis.
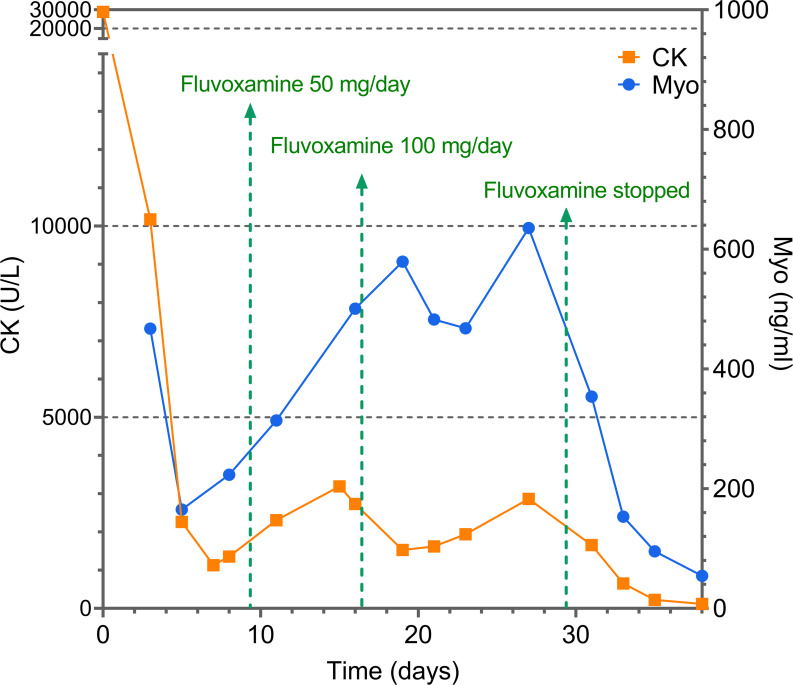
The patient had a history of obsessive-compulsive disorder and social phobia for 2 years, characterised by checking excessively recurrent worries about doing things incorrectly and incompletely, and avoiding being around others. However, he had not received any medication for obsessive-compulsive disorder in the past year. On day 7, his psychotic symptoms of checking and doubting worsened and treatment with fluvoxamine 50 mg/day was started. On day 15, because of exacerbation of the patient’s obsessive-compulsive disorder, the psychiatrist adjusted the fluvoxamine dose to 100 mg/day. During fluvoxamine treatment from day 7 to day 27, a series of laboratory tests showed the patient’s CK and myoglobin levels continued to increase (figure 1). Under the suspicion that the increased CK level was triggered by fluvoxamine, the psychiatrist stopped fluvoxamine and switched to sertraline. After fluvoxamine was discontinued, the CK level continued to decrease and returned to normal.
Figure 1.
Creatine kinase (CK) and myoglobin (Myo) levels from onset to outcome.
Investigations
This case yielded a score of 7 according to the Naranjo Adverse Drug Reaction Probability Scale.5 The adverse event appeared after the suspected drug was administered and improved when the drug was discontinued. During the treatment, there were no other alternative causes that could on their own have caused the reaction. The reaction became more severe when the fluvoxamine dose was increased. The adverse event was confirmed by clinical laboratory tests of CK and myoglobin levels. All the evidence indicated that fluvoxamine was the highly probable cause of the rhabdomyolysis.
Treatment
Under the suspicion that the increased CK level was triggered by fluvoxamine, the psychiatrist stopped fluvoxamine and switched to sertraline.
Outcome and follow-up
After fluvoxamine was discontinued, the CK level continued to decrease and returned to normal value, and the unusual signs and symptoms gradually disappeared.
Discussion
The use of SSRIs in child and adolescent populations has been rising, and typically include fluoxetine, sertraline, citalopram, escitalopram, paroxetine and fluvoxamine. Reported cases throughout the world regarding the occurrence of rhabdomyolysis with SSRIs have concerned sertraline, escitalopram and paroxetine.6–8 A young adult in their 20s took a dose of 100 mg of sertraline monotherapy and developed signs consistent with rhabdomyolysis.9 Notably, the symptoms of our patient regressed as the treatment was switched to sertraline. Pharmacokinetic data suggest that CYP2C19 is the major metabolic pathway of sertraline, while fluvoxamine is extensively metabolised by CYP2D6 to compounds with little pharmacological activity towards serotonin reuptake inhibition.10 11 However, we did not investigate the related genetic background of the patient, thus variations in polymorphic cytochrome P450 enzymes may result in lower or greater exposure to these drugs.
Although the mechanism by which SSRIs cause rhabdomyolysis is unsure, there are some hypotheses. SSRIs inhibit the presynaptic reuptake of serotonin at the serotonin transporter, subsequently increasing serotonin levels at the postsynaptic membrane in the serotonergic synapse. A study showed that an increment of serotonin (5-HT) in animals may accumulate in skeletal muscle by passive diffusion and become toxic to skeletal muscle, thereby leading to necrosis and massive elevation of CK.12 High levels of serotonin in the synaptic cleft may cause an overactivation of the central and peripheral serotonin receptors, leading to serotonin syndrome. A subset of patients with serotonin syndrome developed recurrent episodes of rhabdomyolysis.13
An alternative central mechanism for dopamine has also been proposed. The dopaminergic block in the hypothalamus or striatum always causes generalised muscular contraction, rigidity and potentially rhabdomyolysis.14 15 This theory explained the fewer elevations in the CK level with antipsychotics that have less dopamine type 2 receptor blockade, such as aripiprazole, clozapine and quetiapine, compared with olanzapine.16 A study showed that acute administration of fluvoxamine inhibits the basal firing rate of dopaminergic neurons in the ventral tegmental area of rats.17 However, it was interesting to find that long-term treatment with fluvoxamine may downregulate dopamine transporters and preserve dopaminergic innervation in the striatum.18 Therefore, the dopaminergic mechanism of fluvoxamine-associated rhabdomyolysis remains unclear.
Additionally, electrolyte imbalance may contribute to rhabdomyolysis. Hypokalaemia is the most common cause related to an electrolyte imbalance that causes rhabdomyolysis. A report indicated that fluvoxamine mediated potassium channel inhibition and promoted the potential risk of arrhythmia.19 The patient had no electrolyte imbalance in our case. So here the possibility of hypokalaemia associated with fluvoxamine can be excluded.
Fluvoxamine is also a potent sigma-1 receptor agonist.1 Fluvoxamine, sertraline, fluoxetine, and citalopram have a high to moderate affinity for sigma-1 receptors in the rat brain, and sertraline may be an antagonist at the sigma-1 receptor chaperone.20 Mutations in the sigma-1 gene are associated with distal neuropathies with skeletal muscle dysfunction, such as muscle wasting, but there are no reports on the mechanism of the sigma-1 receptor and rhabdomyolysis. New research showed the presence of abnormal mitochondria and derangements in dystrophin localisation in skeletal muscles from sigma-1 knockout mice.21 It suggests that sigma-1 activation plays an important role in maintaining the structural and functional aspects of healthy skeletal muscle. This may explain the rarity of fluvoxamine-induced rhabdomyolysis compared with other SSRIs such as sertraline and paroxetine.
SSRIs target the serotonin transporter, thereby influencing the serotonergic tone. Nearly 95% of the serotonergic system is localised in the gastrointestinal tract. The gut microbiota synthesises tryptophan and promotes serotonin biosynthesis in the host organism.22 Correspondingly, a range of common medications has a direct influence on the growth of human gut microbiota.23 Serotonin affects the growth of microbes such as Escherichia coli and Rhodospirillum rubrum, and exacerbates in vivo infection in Pseudomonas aeruginosa.24 Fluoxetine administration results in the selective depletion of lactobacilli and increases the abundance phylotypes related to dysbiosis.25 It has been reported that Lactobacillus and Bifidobacterium may influence tight junction proteins of the intestine and regulate muscle size.26 One theory suggested the release of bacterial endotoxin into the bloodstream, causing a vasoconstrictive effect and local ischaemic damage to myocytes.27 This suggests that SSRIs are capable of altering the gut microbiota; in turn, gut microbiota can also modulate drug efficacy and cause negative clinical side effects. However, there are no studies on SSRI-induced alterations in the composition of the intestinal bacterial community in patients with rhabdomyolysis. High-quality studies are needed to elucidate the SSRI-associated rhabdomyolysis with gut microbiota abnormalities.
In conclusion, rhabdomyolysis associated with fluvoxamine use not been previously reported. In such cases, it is not always possible to predict how a particular adverse drug reaction occurs. Nevertheless, rhabdomyolysis can be asymptomatic. Thus, it is advisable to determine serum CK levels before treatment and then at regular intervals to avoid the occurrence of severe acute kidney injury with possible life-threatening complications.
Learning points.
This is the first reported case of fluvoxamine monotherapy associated with rhabdomyolysis.
Fluvoxamine causes rhabdomyolysis by a mechanism different from that of sertraline.
Regular monitoring of serum creatine kinase is recommended for patients being treated with fluvoxamine.
Footnotes
Contributors: FZ and YL reviewed the literature and wrote the report. WG and ZC contributed to writing the case presentation. YL and JL followed up the patient during treatment. All authors read and approved the final manuscript. FZ is responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
Not applicable.
References
- 1. Hashimoto Y, Suzuki T, Hashimoto K. Mechanisms of action of fluvoxamine for COVID-19: a historical review. Mol Psychiatry 2022;27:1898–907. 10.1038/s41380-021-01432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamazaki-Hashimoto Y, Nakamura Y, Ohara H, et al. Fluvoxamine by itself has potential to directly induce long QT syndrome at supra-therapeutic concentrations. J Toxicol Sci 2015;40:33–42. 10.2131/jts.40.33 [DOI] [PubMed] [Google Scholar]
- 3. Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des 2004;10:2463–75. 10.2174/1381612043383872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weisser R, Jalili S, Haen E, et al. [Rhabdomyolysis under treatment with venlafaxine and risperidone]. Nervenarzt 2020;91:153–5. 10.1007/s00115-019-00833-8 [DOI] [PubMed] [Google Scholar]
- 5. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 6. Lee-Kelland R, Zehra S, Mappa P. Fluoxetine overdose in a teenager resulting in serotonin syndrome, seizure and delayed onset rhabdomyolysis. BMJ Case Rep 2018;2018. doi: 10.1136/bcr-2018-225529. [Epub ahead of print: 08 Oct 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Portilla-Botelho M, Ortega-Carnicer J, Gómez-Grande ML, et al. [Paroxetine-induced severe hyponatremic rhabdomyolisis]. Med Intensiva 2008;32:365–6. 10.1016/s0210-5691(08)76217-x [DOI] [PubMed] [Google Scholar]
- 8. Lecardeur L, Lefebvre A, Meunier-Cussac S. Rhabdomyolysis after escitalopram treatment in a young adult with melancholic depression. J Clin Psychopharmacol 2015;35:108–9. 10.1097/JCP.0000000000000234 [DOI] [PubMed] [Google Scholar]
- 9. Snyder M, Kish T. Sertraline-Induced rhabdomyolysis: a case report and literature review. Am J Ther 2016;23:e561–5. 10.1097/MJT.0000000000000196 [DOI] [PubMed] [Google Scholar]
- 10. Kutuk MO, Tufan AE, Topal Z, et al. Cyp450 2D6 and 2C19 genotypes in ADHD: not related with treatment resistance but with over-representation of 2C19 ultra-metabolizers. Drug Metab Pers Ther 2022;37:261–9. 10.1515/dmpt-2021-0163 [DOI] [PubMed] [Google Scholar]
- 11. Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther 2015;98:127–34. 10.1002/cpt.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meltzer HY. Massive serum creatine kinase increases with atypical antipsychotic drugs: what is the mechanism and the message? Psychopharmacology 2000;150:349–50. 10.1007/s002130000465 [DOI] [PubMed] [Google Scholar]
- 13. Kruijt N, van den Bersselaar LR, Wijma J, et al. HyperCKemia and rhabdomyolysis in the neuroleptic malignant and serotonin syndromes: a literature review. Neuromuscul Disord 2020;30:949–58. 10.1016/j.nmd.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 14. Efstratiadis G, Voulgaridou A, Nikiforou D, et al. Rhabdomyolysis updated. Hippokratia 2007;11:129–37. [PMC free article] [PubMed] [Google Scholar]
- 15. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27:501–12. 10.1177/0897190013516509 [DOI] [PubMed] [Google Scholar]
- 16. Tseng C-Y, Chen M-S, Lee K-H, et al. Rhabdomyolysis with clozapine and haloperidol coadministration: a case report. J Clin Psychopharmacol 2020;40:502–4. 10.1097/JCP.0000000000001267 [DOI] [PubMed] [Google Scholar]
- 17. Di Mascio M, Di Giovanni G, Di Matteo V, et al. Selective serotonin reuptake inhibitors reduce the spontaneous activity of dopaminergic neurons in the ventral tegmental area. Brain Res Bull 1998;46:547–54. 10.1016/S0361-9230(98)00054-9 [DOI] [PubMed] [Google Scholar]
- 18. Dallé E, Daniels WMU, Mabandla MV. Long-term treatment with fluvoxamine decreases nonmotor symptoms and dopamine depletion in a postnatal stress rat model of Parkinson's disease. Oxid Med Cell Longev 2020;2020:1941480 10.1155/2020/1941480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morosin M, Dametto E, Bianco FD, et al. An unusual etiology of torsade de pointes-induced syncope. Arch Med Sci 2017;13:686–8. 10.5114/aoms.2017.67287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishima T, Fujita Y, Hashimoto K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur J Pharmacol 2014;727:167–73. 10.1016/j.ejphar.2014.01.064 [DOI] [PubMed] [Google Scholar]
- 21. Aishwarya R, Abdullah CS, Remex NS, et al. Molecular characterization of skeletal muscle dysfunction in sigma 1 receptor (SIGMAR1) knockout mice. Am J Pathol 2022;192:160–77. 10.1016/j.ajpath.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. 10.1016/j.cell.2015.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018;555:623–8. 10.1038/nature25979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knecht LD, O'Connor G, Mittal R, et al. Serotonin activates bacterial quorum sensing and enhances the virulence of Pseudomonas aeruginosa in the host. EBioMedicine 2016;9:161–9. 10.1016/j.ebiom.2016.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lyte M, Daniels KM, Schmitz-Esser S. Fluoxetine-Induced alteration of murine gut microbial community structure: evidence for a microbial endocrinology-based mechanism of action responsible for fluoxetine-induced side effects. PeerJ 2019;7:e6199. 10.7717/peerj.6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grosicki GJ, Fielding RA, Lustgarten MS. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: biological basis for a gut-muscle axis. Calcif Tissue Int 2018;102:433–42. 10.1007/s00223-017-0345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaufman D, Weber K, Gradon JD. Legionella pneumonia: an unusual cause of rhabdomyolysis and acute renal failure. South Med J 2002;95:660. 10.1097/00007611-200206000-00018 [DOI] [PubMed] [Google Scholar]