Abstract
Pythiosis is a fatal disease which has high incidence in tropical regions. In contrast with vascular pythiosis, cutaneous and subcutaneous pythiosis are both uncommon. Here, we report a case of subcutaneous pythiosis in a pregnant farmer manifested with a progressively larger and more painful mass at right deltoid. The tissue culture and molecular test were negative for fungi. The diagnosis was supported by the positivity of serum immunochromatographic test (ICT) for pythiosis. Patients responded well to the combination therapy of itraconazole, terbinafine and azithromycin.
Keywords: Cutaneous pythiosis, Subcutaneous pythiosis, Pythiosis
Introduction
Pythiosis, caused by Pythium insidiosum [1], is an emerging infectious disease in both human and animals which had a high prevalence in tropical and subtropical areas, with over 770 cases reported in the literatures up to December 2022 [2]. In Thailand, the first human pythiosis case was reported in 1985 [3]. It can now be found throughout Thailand, most notably in the central region (46 %), followed by the northeastern (27 %), the northern (16 %), the southern (8 %), and the eastern (3 %) regions [4]. Clinical features of pythiosis included vascular pythiosis, ocular pythiosis, cutaneous/subcutaneous pythiosis and disseminated infection [2], [5]. Vascular pythiosis, the most common presentation in Thailand [4], can manifest as chronic ulcer or acute limb ischemia. Most patients with vascular pythiosis had underlying disease of thalassemia and had water exposure as part of their jobs or other activities [4]. In contrast to vascular pythiosis, cutaneous and subcutaneous forms are less common and can manifest in non-thalassemic patients [4]. Here, we present a pregnant woman whose chief complaint was a progressively growing and more painful mass at right deltoid.
Case presentation
A 26-year-old previously healthy pregnant woman in her second trimester presented with right deltoid mass developing over 6 months when she was in Myanmar. She reported to be afebrile throughout the duration. The lesion initially appeared as a small, painless erythematous patch at right deltoid area and progressed into a large painful mass around her right shoulder within a month, when she started to seek medical care. She had a history of intramuscular injection for vitamin supplement after giving birth at right deltoid 3 years ago at non-healthcare facility, but never had symptoms of skin or soft tissue infections since then. She was a farmer and had a history of frequently swimming in the river. She visited a private clinic several times and received painkillers, oral antimicrobials including doxycycline and cloxacillin, and a week of intravenous amphotericin B without clinical improvement.
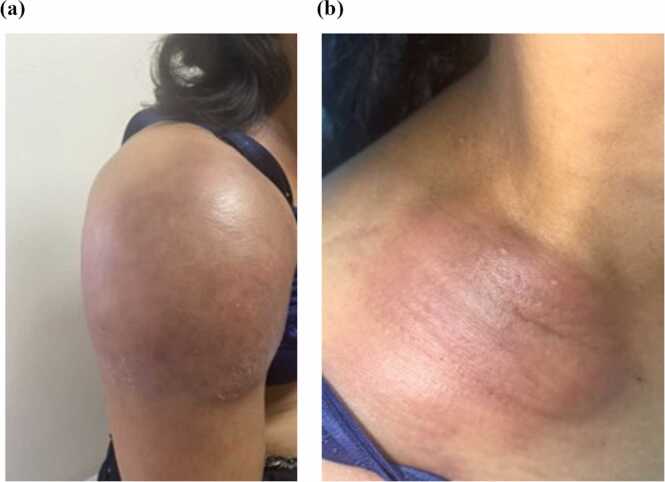
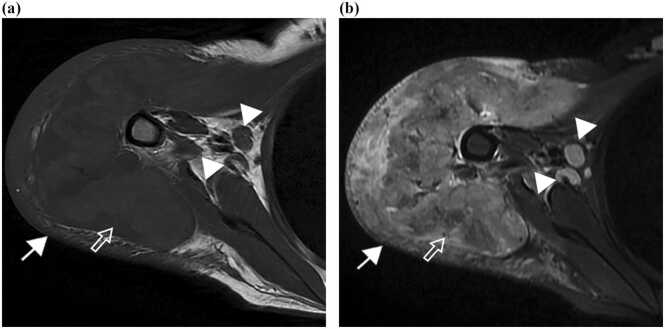
Physical examinations revealed body temperature of 39 °C, pulse rate 124 beats per minute, blood pressure 96/64 mmHg, and respiratory rate 20 times per minute. A large painful erythematous mass approximately 16 × 18 centimeters in size at right deltoid with swelling of the right arm was noted, resulting in limited range of motion (Fig. 1a). A hard well-defined erythematous plaque at the right clavicle 5 × 6 centimeters in size was also observed (Fig. 1b). The magnetic resonance imaging (MRI) showed infiltrative lesion in the subcutaneous tissue and muscles, as well as right axillary lymphadenopathy (Fig. 2). Magnetic resonance angiography (MRA) revealed no vascular involvement.
Fig. 1.
(a) Mass at right deltoid and (b) well-defined erythematous plaque at right clavicle.
Fig. 2.
Magnetic resonance imaging of the right shoulder on axial (a) T1-and (b) short tau inversion recovery (STIR) images show infiltrative lesion in the subcutaneous tissue (arrow) and deltoid muscle (open arrow), and enlarged axillary lymph nodes (arrowheads).
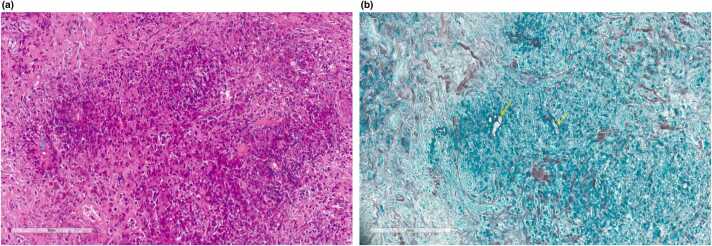
Skin biopsy showed granulomatous inflammation with many eosinophils, granular eosinophilic island, and fungal hyphae with Splendore-Hoeppli phenomenon within the eosinophilic island without vascular invasion. The Grocott methenamine silver (GMS) stain showed fungal hyphae (Fig. 3). Neither bacteria nor fungus was detected from skin cultures. Polymerase chain reaction (PCR) for mycobacteria, 16s ribosomal RNA and 18s ribosomal RNA gene sequencing was negative for bacteria and fungi, respectively. However, the result of serum immunochromatography test (ICT) for pythiosis was positive, leading to the final diagnosis of subcutaneous pythiosis.
Fig. 3.
Pathological findings show granular eosinophilic island and fungal hyphae. with Splendore-Hoeppli phenomenon (a) Hematoxylin and Eosin stain (200 ×) and. (b) Grocott’s methenamine silver stain shows sparsely septate hyphae (400 ×). Arrow indicates fungal hyphae.
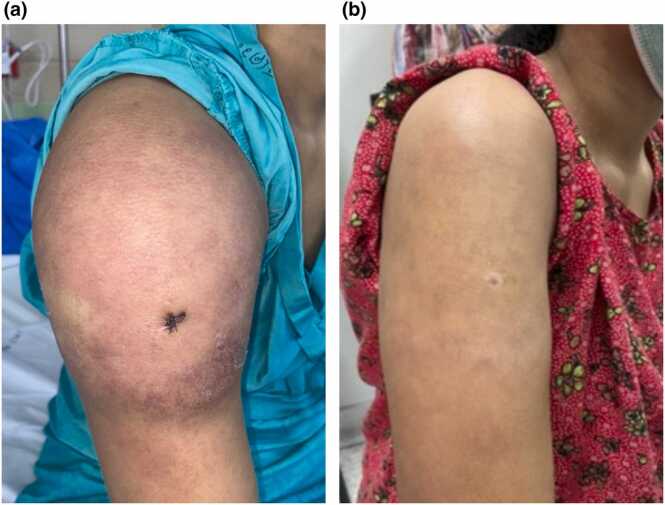
Itraconazole 600 mg/day for the first 3 days followed by 400 mg/day, azithromycin 500 mg/day and terbinafine 500 mg/day were prescribed orally. The lesion gradually regressed, and follow-up images were taken at one week as well as three months after treatment (Figs. 4a and 4b, respectively). She later delivered a healthy baby.
Fig. 4.
Lesion after treatment (a) 1 week, (b) 3 months.
Discussion
Pythiosis is a rare and elusive emerging disease in tropical countries, notorious for its difficulty to be correctly diagnosed. The main pathogen is Pythiosis insidosum, an aquatic fungus-like organism, which can be found in soil and water throughout Thailand [6]. The life cycle of P. insidosum starts from the development of undifferentiated sporangia which are then cleaved, and several biflagellate zoospores are released. The zoospores then swim and preferentially attach to plant and animal tissues. They subsequently become encysted zoospore which then elongate their germ tubes to penetrate and assist their hyphae’s invasion into the host tissues [7].
Clinical manifestations of subcutaneous pythiosis resemble many fungal diseases such as entomopthoromycosis and mucormycosis [8]. Host factor is one of the important clues of cutaneous and subcutaneous pythiosis. Unlike vascular pythiosis which mostly occurs in thalassemic patients, cutaneous and subcutaneous pythiosis can present in non-thalassemic and non-hematologic patients, predominantly in the agricultural population [4]. In addition, majority of the previously reported cases of cutaneous and subcutaneous pythiosis were strongly associated with water exposure [5]. This patient’s risk of exposure to the pathogen was supported by her occupation as well as history of frequently swimming in the river. Although shoulder mass is more common in entomopthoromycosis (especially basidiobolomycosis), pain is not a typical symptom [8]. Several diagnostic methods have been developed to aid the diagnosis of pythiosis. However, identification of P. insidosum on culture remains the most reliable method. P. insidosum can grow in 24–48 h on standard agars such as Sabouraud agar, potato dextrose agar, corn meal agar, and blood agar with optimal temperature between 28 and 32 °C [9], [10]. Serologic testing may help to support the diagnosis of pythiosis, particularly when culture results are negative. Several techniques exist for detecting P. insidosum-specific antibody, including immunodiffusion, enzyme-linked immunosorbent assay (ELISA), western blot, hemagglutination test, and immunochromatographic test (ICT) [5]. Immunodiffusion has poor sensitivity (61 %) and longer turnaround time but is relatively simple to use [11], whereas ICT and ELISA have higher sensitivity and specificity [11], [12] but may not be widely available in some hospitals. ICT demonstrated 88–96 % sensitivity, 100 % specificity, 100 % positive predictive value, and 98 % negative predictive value [11], [13]. This supports the value of serologic tests in the diagnosis of pythiosis. However, false positive results for ICT have been reported in animal with basidiobolomycosis [14].
All patients with suspected subcutaneous pythiosis should perform computerized tomography angiogram (CTA) of affected part to rule out vascular involvement [5]. MRI also plays a major role in the evaluation of disease extension and MRA can be used in place of CTA if necessary. Treatment of subcutaneous pythiosis include surgery, antimicrobial and immunotherapy [5]. Since P. insidosum has incomplete set of sterol biosynthesis enzyme, antifungal therapy alone typically does not provide effective treatment [15]. Combination of itraconazole and terbinafine shows benefit in synergistic effect in vitro study in Brazil [16]. In Thai strain, meanwhile, the concentration of drug needed to inhibit P. insidosum growth is higher and more difficult to achieve in vivo [15]. However, combination of terbinafine and itraconazole together results in an effective treatment in many cases [17], [18]. Azithromycin has immunomodulatory effect and can inhibit growth of P. insidosum in vivo when used alone or combined with minocycline [19]. This patient responded well to the combination of itraconazole, terbinafine, and azithromycin. In previous study conducted in Thailand [4], 5 patients with cutaneous and subcutaneous pythiosis presented with chronic painful subcutaneous mass or ulcer on the arm or leg. Three of them responded well to saturated solution of potassium iodide, while another patient responded to the combination of itraconazole, terbinafine, and surgical debridement. The last patient was lost to follow up and the outcome could not be determined [4].
In conclusion, pythiosis is a rare yet emerging disease in tropical countries. However, physicians taking care of patients with risk factors, such as underlying hematologic diseases or having a history of environmental exposure should be aware of this disease.
Ethical approval
This study received certificate of exemption (exemption no. 0085/2023) from the research ethics committee panel 5 of the Faculty of Medicine, Chiang Mai University.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Athitaya Luangnara: Conceptualization, Writing – original draft. Mati Chuamanochan: Writing – review & editing. Siri Chiewchanvit: Writing – review & editing. Nuttaya Pattamapaspong: Writing – review & editing. Parichat Salee: Writing – review & editing. Romanee Chaiwarith: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Conflict of Interest Statement
All authors declared no conflict of interests.
Acknowledgement
The authors would like to thank Jetanat Chantrapitak, MD for his role as an English proofreader.
Consent
Written informed consent was obtained from the patient for publication.
References
- 1.De Cock A.W., Mendoza L., Padhye A.A., Ajello L., Kaufman L. Pythium insidiosum sp. nov., the etiologic agent of pythiosis. J Clin Microbiol. 1987;25:344–349. doi: 10.1128/jcm.25.2.344-349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yolanda H., Krajaejun T. Global distribution and clinical features of pythiosis in humans and animals. J Fungi. 2022;8 doi: 10.3390/jof8020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imwidthaya P. Human pythiosis in Thailand. Postgrad Med J. 1994;70:558–560. doi: 10.1136/pgmj.70.826.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krajaejun T., Sathapatayavongs B., Pracharktam R., et al. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin Infect Dis. 2006;43:569–576. doi: 10.1086/506353. [DOI] [PubMed] [Google Scholar]
- 5.Chitasombat M.N., Jongkhajornpong P., Lekhanont K., Krajaejun T. Recent update in diagnosis and treatment of human pythiosis. PeerJ. 2020;8 doi: 10.7717/peerj.8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanittanakom N., Szekely J., Khanthawong S., Sawutdeechaikul P., Vanittanakom P., Fisher M.C. Molecular detection of Pythium insidiosum from soil in Thai agricultural areas. Int J Med Microbiol. 2014;304:321–326. doi: 10.1016/j.ijmm.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza L., Hernandez F., Ajello L. Life cycle of the human and animal oomycete pathogen Pythium insidiosum. J Clin Microbiol. 1993;31:2967–2973. doi: 10.1128/jcm.31.11.2967-2973.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontoyiannis D.P., Lewis R.E. In: Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Bennett J.E., Dolin R., Blaser M.J., Mandell, Douglas, editors. Elsevier; Philadelphia: 2000. Agents of mucormycosis and entomopthoramycosis; pp. 3117–3130. [Google Scholar]
- 9.Grooters A.M., Whittington A., Lopez M.K., Boroughs M.N., Roy A.F. Evaluation of microbial culture techniques for the isolation of Pythium insidiosum from equine tissues. J Vet Diagn Invest. 2002;14:288–294. doi: 10.1177/104063870201400403. [DOI] [PubMed] [Google Scholar]
- 10.Krajaejun T., Chongtrakool P., Angkananukul K., Brandhorst T.T. Effect of temperature on growth of the pathogenic oomycete Pythium insidiosum. Southeast Asian J Trop Med Public Health. 2010;41(6):1462. 〈https://www.ncbi.nlm.nih.gov/pubmed/21329324〉 [PubMed] [Google Scholar]
- 11.Krajaejun T., Imkhieo S., Intaramat A., Ratanabanangkoon K. Development of an immunochromatographic test for rapid serodiagnosis of human pythiosis. Clin Vaccine Immunol. 2009;16:506–509. doi: 10.1128/CVI.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chareonsirisuthigul T., Khositnithikul R., Intaramat A., et al. Performance comparison of immunodiffusion, enzyme-linked immunosorbent assay, immunochromatography and hemagglutination for serodiagnosis of human pythiosis. Diagn Microbiol Infect Dis. 2013;76:42–45. doi: 10.1016/j.diagmicrobio.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Intaramat A., Sornprachum T., Chantrathonkul B., et al. Protein A/G-based immunochromatographic test for serodiagnosis of pythiosis in human and animal subjects from Asia and Americas. Med Mycol. 2016;54:641–647. doi: 10.1093/mmy/myw018. [DOI] [PubMed] [Google Scholar]
- 14.Rotchanapreeda T., Sae-Chew P., Lohnoo T., et al. Immunological cross-reactivity of proteins extracted from the oomycete Pythium insidiosum and the fungus basidiobolus ranarum compromises the detection specificity of immunodiagnostic assays for pythiosis. J Fungi. 2021;7 doi: 10.3390/jof7060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerksuthirat T., Sangcakul A., Lohnoo T., Yingyong W., Rujirawat T., Krajaejun T. Evolution of the sterol biosynthetic pathway of Pythium insidiosum and related oomycetes contributes to antifungal drug resistance. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.02352-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argenta J.S., Santurio J.M., Alves S.H., et al. In vitro activities of voriconazole, itraconazole, and terbinafine alone or in combination against Pythium insidiosum isolates from Brazil. Antimicrob Agents Chemother. 2008;52:767–769. doi: 10.1128/AAC.01075-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shenep J.L., English B.K., Kaufman L., et al. Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin Infect Dis. 1998;27:1388–1393. doi: 10.1086/515042. [DOI] [PubMed] [Google Scholar]
- 18.Sermsathanasawadi N., Praditsuktavorn B., Hongku K., et al. Outcomes and factors influencing prognosis in patients with vascular pythiosis. J Vasc Surg. 2016;64:411–417. doi: 10.1016/j.jvs.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Medhasi S., Chindamporn A., Worasilchai N. A review: antimicrobial therapy for human pythiosis. Antibiotics. 2022;11 doi: 10.3390/antibiotics11040450. [DOI] [PMC free article] [PubMed] [Google Scholar]