Abstract
The animal gut harbors diverse microbes that play an essential role in the well-being of their host. Specific diets, such as those rich in dietary fiber, are vital in disease prevention and treatment because they affect intestinal flora and have a positive impact on the metabolism, immunity, and intestinal function of the host. Dietary fiber can provide energy to colonic epithelial cells, regulate the structure and metabolism of intestinal flora, promote the production of intestinal mucosa, stimulate intestinal motility, improve glycemic and lipid responses, and regulate the digestion and absorption of nutrients, which is mainly attributed to short-chain fatty acids (SCFA), which is the metabolite of dietary fiber. By binding with G protein-coupled receptors (including GPR41, GPR43 and GPR109A) and inhibiting the activity of histone deacetylases, SCFA regulate appetite and glucolipid metabolism, promote the function of the intestinal barrier, alleviate oxidative stress, suppress inflammation, and maintain immune system homeostasis. This paper reviews the physicochemical properties of dietary fiber, the interaction between dietary fiber and intestinal microorganisms, the role of dietary fiber in maintaining intestinal health, and the function of SCFA, the metabolite of dietary fiber, in inhibiting inflammation. Furthermore, we consider the effects of dietary fiber on the intestinal health of pigs, the reproduction and lactation performance of sows, and the growth performance and meat quality of pigs.
Keywords: Dietary fiber, Short-chain fatty acid, Intestinal microorganism, Inflammation, Pig, Lactation performance
1. Introduction
The animal intestine contains trillions of microorganisms, that are significant to body health and perform metabolic, immune and protective functions for the host (Holscher, 2017). Gut flora is affected by such factors as hereditary, the age and disease of the host, and the environment (Goodrich et al., 2014; Wang et al., 2011; Yatsunenko et al., 2012). In recent years, it has been increasingly recognized that diet is a key environmental factor in regulating the structure and metabolic function of the intestinal flora (Bai et al., 2022; Sonnenburg and Bäckhed, 2016). Diet can interact with microorganisms to directly influence their growth, alter the intestinal microenvironment, and indirectly affect the metabolism and immune system of the host. The consumption of specific dietary components, such as prebiotics and fibers, has emerged as a method to prevent and treat diseases through the regulation of gut microbiota.
Dietary fiber, a complex carbohydrate from plants, is decomposed by special enzymes produced by intestinal bacteria and is the main energy source of colon bacteria (Sonnenburg and Sonnenburg, 2014). Dietary fiber can promote intestinal health by regulating the composition and metabolism of bacterial communities, providing energy to colonic epithelial cells, promoting the production of intestinal mucosa, stimulating intestinal motility, and maintaining intestinal integrity (Ye et al., 2022). Meanwhile, dietary fiber can also regulate appetite, improve glycemic and lipid responses, regulate plasma cholesterol by limiting bile salt absorption, enhance digestive function, and regulate the digestion and absorption of nutrients. When it is decomposed by intestinal microbiota, dietary fiber produces short-chain fatty acids (SCFA) including acetate, propionate and butyrate, and gases including methane and carbon dioxide. Once released from the enteric cavity, SCFA are absorbed by the colon, muscle, liver and other tissues, providing basic energy for the body in the form of nutrients, while acetate can also promote the generation of ATP through the citric acid cycle (Shah et al., 2020). In addition to supplying energy to the colon, SCFA can also reduce pH and inhibit the excessive growth of harmful bacteria (Greenberg et al., 2006). Furthermore, SCFA can regulate the inflammatory process by inhibiting the activity of histone deacetylase (HDAC) and activating G protein-coupled receptors (GPCR) such as GPR41 and GPR43. At the same time, the production of certain cytokines, such as interleukin-12 (IL-12), IL-6 and IL-10, tumor necrosis factor-α (TNF-α), is reduced under the action of SCFA (den Besten et al., 2013).
Numerous studies have shown that dietary fiber plays a vital role in pig nutrition. Adding dietary fiber to sow feed can increase the satiety, lactation performance, and reproductive performance of sows, as well as litter size, weaning litter weight, and butterfat content in colostrum (Che et al., 2011; Loisel et al., 2013). In addition, the SCFA produced by dietary fiber play a significant role in regulating the cell proliferation, immunity, and metabolism of sows and affect the expression of inflammatory factors, pro-inflammatory factors and antioxidant enzymes in sows and piglets (Guo et al., 2020; Li et al., 2019; Xu et al., 2020b). Therefore, this paper reviews the physicochemical properties of dietary fiber, the interaction between dietary fiber and microorganisms, and the protective effect of dietary fiber on intestinal health. Furthermore, we consider the role of dietary fiber and SCFA in pig nutrition, including intestinal health, reproductive performance, lactation performance and growth performance.
2. Dietary fiber
2.1. Definition and classification of dietary fiber
Fiber, a group of heterogeneous materials, is composed of plant-derived carbohydrates and is not digested by typical human amylase in the absence of the cellulase required for decomposition (O'Grady et al., 2019). Microorganisms are needed to digest fiber through anaerobic fermentation in human, which results in the production of SCFA. Terms such as microbiota-accessible carbohydrate are used to describe fiber. In 1953, Hipsley first proposed the term “dietary fiber” as a shorthand for indigestible components in food (Hipsley, 1953). Over time, the physiological effects of dietary fiber have been gradually explored, and the definition has changed considerably. In 2009, the definition of dietary fiber was provided by the Codex Alimentarius Commission and was unanimously recognized (Makki et al., 2018; Stephen et al., 2017). Dietary fiber is defined as a carbohydrate polymer with 10 or more monomer units, that is neither digested nor absorbed in the human small intestine. Dietary fiber can be categorized as follows: (1) edible carbohydrate polymers naturally present in food when consumed; (2) edible carbohydrate polymers obtained from food raw materials by physical, enzymatic or chemical methods with beneficial physiological effects proven by recognized scientific evidence; and (3) edible synthetic carbohydrate polymers with recognized scientific evidence of beneficial physiological effects (Waddell and Orfila, 2022). However, evidence shows that lignin and indigestible oligosaccharides also exhibit beneficial effects similar to those of fiber, potentially promoting the health and stability of intestinal microbiota, the generation of SCFA in the colon, and the absorption of calcium. Therefore, in 2010, the expert group of the European Food Safety Agency further defined dietary fiber indigestible carbohydrates plus lignin in 2010 (Ye et al., 2022).
There are many ways of classifying dietary fiber. Generally, it is divided into soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) according to its solubility in water. Soluble dietary fiber include several types of polysaccharides, such as hemicellulose (e.g., arabinoxylan and β-glucan) and pectin (Evans, 2020), which are abundant in beans, fruits, vegetables, oats, wheat and rice (Nakashima et al., 2018). In addition, SDF includes seaweed polysaccharides (e.g., carrageenan, sodium alginate, and guar gum) and soluble oligosaccharides (e.g., inulin and raffinose-like oligosaccharides). Insoluble dietary fiber is mainly composed of cell wall components, including lignin, cellulose and some hemicellulose (Chuang et al., 2012). Insoluble dietary fiber is the most abundant fiber type in most foods, and can be found in wheat, oats, barley, rye and other whole grains (Waddell and Orfila, 2022). Dietary fiber can be divided into plant dietary fiber and animal dietary fiber according to its source, while plant dietary fiber can be further divided into cereal dietary fiber, fruit dietary fiber and vegetable dietary fiber. The main dietary fiber consumed by the human body is cereal dietary fiber, followed by vegetable dietary fiber, which account for about 50% and 30% respectively, and with approximately 16% coming from fruits (Dhingra et al., 2012). In addition, dietary fiber can be classified according to several other parameters, including chemical structure, viscosity and fermentability (Deehan et al., 2017). Currently, dietary fiber is divided into the following 4 categories: non-starch polysaccharides (including cellulose, hemicellulose, pectin and β-dextran), resistant oligosaccharides (including fructose oligosaccharides and galacto-oligosaccharides [GOS]), resistant starch (RS), and lignin (Table 1) (Armstrong et al., 2020; Ye et al., 2022).
Table 1.
Classification, sources and physicochemical characteristics of common dietary fibers, and intestinal microorganisms that can degrade them.
| Dietary fiber | Classification | Sources | Physicochemical characteristics |
Intestinal microorganisms | ||
|---|---|---|---|---|---|---|
| Solubility | Viscosity | Fermentability | ||||
| Arabinoxylan | Non-starch polysaccharides | Oats, wheat, rice | Medium | Medium | High | Roseburia, Bacteroides, Prevotella, Porphyromonas |
| Beta-glucan | Non-starch polysaccharides | Oat, barley, fungi, mushrooms | Medium | High | High | Lactobacillus, Enterococcus, Bifidobacterium |
| Beta-fructans | Non-starch polysaccharides | Chicory root, agave, artichoke | High | Low | High | Bifidobacterium, Lactobacillus, Streptococcus, Flavobacterium |
| Pectin | Non-starch polysaccharides | Fruits, vegetables, nuts | High | High | High | Dictyostelium, Bacillus, Pseudomonas |
| Inulin | Non-starch polysaccharides | Cereals, fruits, vegetables | Low | High | High | Bacteroides, Bifidobacterium |
| Galacto-oligosaccharide | Resistant oligosaccharides | Beans, peas, lentils | High | Low | High | Faecalibacterium prausnitzii |
| Resistant starch | Resistant starch | Raw fruits, vegetables | Low | Non-viscous | High | Bifidobacterium breve, Bifidobacterium adolescentis |
| Lignin | Lignin | Grains, vegetables | Insoluble | High | Low | Bjerkandera, Fomitopsis, Schizophyllum |
2.2. Physicochemical properties of dietary fiber
Fermentability, viscosity, solubility, water holding capacity, adsorption, and particle size constitute the physicochemical properties of fiber, which vary due to differences in the structure and molecular weight of dietary fiber, and affect the fermentation and therapeutic effects (Table 1) (Armstrong et al., 2020; Gill et al., 2021; Holscher, 2017; Ye et al., 2022). Solubility refers to the degree to which dietary fiber dissolves in water. Dietary fibers that are highly crystallized are usually insoluble in water, while those with irregular structures are more soluble. In addition, the solubility of dietary fiber and the granularity and integrity of the plant cell wall are affected by the charge content of the group (Elleuch et al., 2011; Grundy et al., 2017). Soluble dietary fiber can form a sticky gel in the intestine and produce substances such as SCFA after intestinal microbial fermentation, while IDF is not easily fermentable, but increases the intestinal transport rate and water holding capacity of intestinal contents, thus reducing the fermentation time of nondigestible food in the colon, increasing stool volume, and slowing down gastric emptying (Bliss et al., 2013). Viscosity refers to the degree of flow resistance. The viscosity of dietary fiber is affected by its molecular weight, chemical composition, water-holding capacity, particle size, solution temperature, processing time and pH value. Higher dietary fiber viscosity increases the viscosity of intestinal contents, thus preventing intestinal epithelial cells from absorbing nutrients (Dikeman and Fahey, 2006; Theuwissen and Mensink, 2008). Some intestinal microorganisms have enzymes capable of hydrolyzing chemical bonds in certain dietary fibers in vegetable foods, and these fermentable fibers include inulin-type fructans, resistant starch, and GOS (El Kaoutari et al., 2013). In fact, except for synthetic fibers, such as methyl cellulose, that are completely non fermentable, all natural plant fibers have a measure of fermentability, even including cellulose and lignin with low fermentability (Stephen et al., 2017).
Highly fermentable fibers with high solubility and viscosity include β-glucan and pectin, which are naturally found in whole grains (e.g., oats and barley) and apples, respectively (Holscher, 2017). The psyllid cannot be fermented, but its solubility and viscosity are very high and help control blood sugar and reduce blood cholesterol levels (McRorie, 2015). Non-sticky soluble fibers easily fermented by intestinal microorganisms include RS, resistant maltodextrin, inulin, soluble corn fiber, and polydextrose (Holscher et al., 2015a, 2015b). Inulin can be found in bananas, garlic, artichokes, onions, chicory roots, leeks, and wheat and, in studies with rodents, has been proven to lower the concentration of cholesterol and glucose in the blood and body weight (Márquez-Aguirre et al., 2013; Rendón-Huerta et al., 2012).
3. Interaction between dietary fiber and microorganisms
The intestine is a chamber for the survival and reproduction of various microorganisms that can influence the digestive process of the host. These microorganisms are influenced by several factors, including individual genetic background, physiological status, the environment and drugs (Shah et al., 2020). Among those factors, diet has been widely studied as a key factor. Dietary fiber is a high-quality source of carbohydrates for the microbiota, and can be used as an energy source by gut microbes and as a carbon source by the host (Pistollato et al., 2016; Sonnenburg and Sonnenburg, 2014). Soluble fibers are easily obtained by intestinal microorganisms in the enteric cavity and are mainly metabolized in the proximal colon and ileum (Koropatkin et al., 2012). Insoluble fibers are difficult to obtain for microorganisms and are mainly degraded in the distal colon. Dietary fiber has important effects on intestinal microorganisms, and the degradation of different types of fiber requires different microorganisms (Table 1) (Armstrong et al., 2020; Ye et al., 2022). Therefore, there is an interaction between dietary fiber and intestinal microbiota.
Dietary fiber cannot be directly degraded by human digestive enzymes. When dietary fiber enters the intestine, intestinal microorganisms can degrade it through different systems and absorb the energy generated (Singh, 2019). Many microorganisms are needed for the host to obtain energy from these reactions. Intestinal microorganisms have the ability to encode a series of carbohydrate active enzymes (CAZymes), including glycoside hydrolase, glycosyltransferase, polysaccharide lyase and carbohydrate esterase, which can hydrolyze a variety of fibers (Hamaker and Tuncil, 2014; Lombard et al., 2014). Therefore, compared to a refined diet, diet containing various dietary fibers and resistant starches can support multiple gastrointestinal microbial communities (El Kaoutari et al., 2013). Microbe diversity in the intestine is lower in individuals with a low-fiber diet, and the gut of these individuals is often dominated by microorganisms dependent on amino acids and lipids, as the decrease of dietary fiber in a diet is often accompanied by the increase of animal protein and fat (Makki et al., 2018). Dietary fiber can increase probiotic level and promote intestinal homeostasis, thus functioning as an effective prebiotic (Roberfroid et al., 2010). Prebiotic sources include fiber-rich whole grains and indigestible oligosaccharides, such as fructans, polydextrose, inulin, fructo-oligosaccharides (FOS), GOS, and araboxylan. Different types of dietary fiber have been found to have different effects on the microbiota of the host and many intestinal microorganisms use dietary fiber through different strategies. For instance, Bacteroides is a typical Gram-negative bacterium with an efficient polysaccharide degradation system and is the most frequently mentioned microorganism in the study of the use and transportation of polysaccharides in the gut microbiota (Singh, 2019). However, glycoside hydrolases fixed on the cell surface are required by Gram-positive bacteria for the degradation of complex polysaccharides due to the lack of periplasmic space (Guan et al., 2021). Complex polysaccharides are first degraded into oligosaccharides outside the cell, and then transported to the cytoplasm for further degradation, or used as nutrition for other microorganisms in the intestine (Koropatkin et al., 2012). The types of microorganisms and the chain length of the fibers affect the ability of bacteria to degrade fibers. Short-chain FOS can be fermented by many microorganisms including Bacteroides, Faecalibacterium, Bifidobacterium, and Lactobacillus, while long chain fructosan can only be degraded by few microorganisms (De Vuyst and Leroy, 2011; Ramirez-Farias et al., 2009).
Arabinoxylan (AX), a hemicellulose molecule, that consists of β-(1,4)-linked xylose backbone containing arabinose side chains, is the main dietary fiber in cereals and other plant and animal dietary sources (Armstrong et al., 2020; Mendis et al., 2016). It was shown to be fermented in the gut by Porphyromonas, Roseburia, Prevotella and Bacteroides to produce SCFA (Grootaert et al., 2009). AX upregulates the bile acid metabolic pathway in the intestines of mice with a high-fat diet by modulating the intestinal microbiota, thereby reducing obesity and liver injury (Cheng et al., 2020). β-Glucan is a glucose polysaccharide that is widely found in the cell walls of bacteria, fungi, and plants such as oats and barley (El Khoury et al., 2012). Microorganisms such as Bifidobacterium, Lactobacillus, and Enterococcus have been shown to degrade dietary β-glucan to produce varying amounts of SCFA depending on the source of the fiber and the species of the microorganism (Lam et al., 2018). A study in rats showed that β-glucan promoted the reproduction of Lactobacillus and Bifidobacterium and inhibited that of Enterobacteriaceae in a dose-dependent manner (Shen et al., 2012). Yeast derived β-glucan can also alleviate the metabolic syndrome induced by a high-fat diet by regulating the intestinal microbiota of mice and reducing the levels of proinflammatory cytokines such as IL-6 and IL-1β in plasma (Chen et al., 2021). Beta-fructans, including FOS and inulin, are β-(2 → 1) linked fructose oligosaccharides and polysaccharides, and are mainly derived from chicory roots, agave, and artichokes (Armstrong et al., 2020). Microorganisms such as Bifidobacterium, Lactobacillus, Streptococcus, Flavobacterium, and various yeasts have been shown to ferment β-fructans (Fraberger et al., 2018). FOS can be fermented by Collinsella aerofaciens and Bifidobacterium (Cook and Russell, 1994). It is also effective in increasing the abundance of Bifidobacterium and Lactobacillus, as well as the diversity of intestinal microorganisms (Tandon et al., 2019). Inulin has been shown to promote the health of body by modulating the gut microbiota, and diets containing inulin reduce the accumulation of fat and enhance the glucose tolerance of the body (Song et al., 2019), as well as improve the diversity and activity of the intestinal microflora, increase the abundance of Bifidobacterium and decrease that of Desulfovibrio (Holscher et al., 2015a). In addition, in mice, inulin supplementation can repair the intestinal epithelial barrier by regulating intestinal microorganisms, with hyperuricemia also being alleviated (Guo et al., 2021). GOS is composed of different galactose residues and β-glycosidic bonds linked terminal glucose molecules, and can be fermented by Faecalibacterium prausnitzii (Davis et al., 2011). In rats, GOS intake helped alleviate neuroinflammation and cognitive dysfunction, significantly increased the β diversity of the intestinal microbiota and promoted the proliferation of Bifidobacterium and other microorganisms with potential anti-inflammatory functions (Yang et al., 2018). Pectin is a complex polysaccharide composed of α-1,4-linked D-galacturonic acid residues and is widely present in the cell walls of fruits and vegetables (Dongowski et al., 2000). Various microorganisms such as Dictyostelium, Bacillus, Pseudomonas, and yeast have been shown to be capable of almost complete fermentation of pectin in the colon through an enzymatic process or oxidative pathway to produce a variety of SCFA and several gases (Dittoe et al., 2020; Kaur and Gupta, 2017; Larsen et al., 2019; Luis et al., 2018). Pectin extracted from citrus significantly promoted the production of Bifidobacterium and Lactobacillus and reduced the pH of the intestine (Mao et al., 2019). Another experiment showed that the application of pectin can increase the diversity of intestinal microorganisms in mice, thereby alleviating colitis caused by Bacteroids (Beukema et al., 2021). Moreover, pectin oligosaccharides can reduce cholesterol by regulating specific bacteria and their metabolites (Hu et al., 2019).
Cellulose, a linear chain of β (1 → 4) linked glucose monomers, is a major structural component of plant cell walls (Lattimer and Haub, 2010). Microorganisms such as Bacteroidetes, Clostridium, and Fibrobacter have been shown to ferment cellulose to produce acetate (Flint et al., 2012). In mice, a high cellulose diet can maintain intestinal homeostasis and alleviate intestinal inflammation and prevent colitis by altering intestinal microbiota and metabolites (Kim et al., 2020). In addition, cellulose promoted intestinal barrier function and secretion of mucin in cupped cells, which may due to the increase of Akkermansia (Kim et al., 2020). Lignin is a complex polymer that exists in many plants, and the degradation of it is complex and difficult. However, certain fungi such as Bjerkandera, Schizophyllum and Fomitopsis are able to ferment lignin to produce ethanol using peroxidases, lignases, and laccase (Horisawa et al., 2015; Janusz et al., 2017). Besides, Pseudomonas, Rhodococcus, and Sphingobacterium have been shown to use the aromatic components of lignin during the fermentation of lignin to produce biofuels (Anthony et al., 2019; Lee et al., 2019; Ponnusamy et al., 2019). Both RS type 2 and type 4 consist of glucose monosaccharides linked by α-1,6 glycosidic bonds (Martínez et al., 2010). Enzymes encoded by Bifidobacterium adolescentis and Bifidobacterium breve have been showen to degrade RS (Ryan et al., 2006), while RS with different structures can promote the growth of different species of intestinal microorganisms. The abundance of Actinomycetes and Bacteroid were significantly increased with the administration of RS4, and a diet with RS2 increased the proportion of Ruminococcus bromii and Eubacterium rectale (Martínez et al., 2010).
4. Regulation of dietary fiber on intestinal health
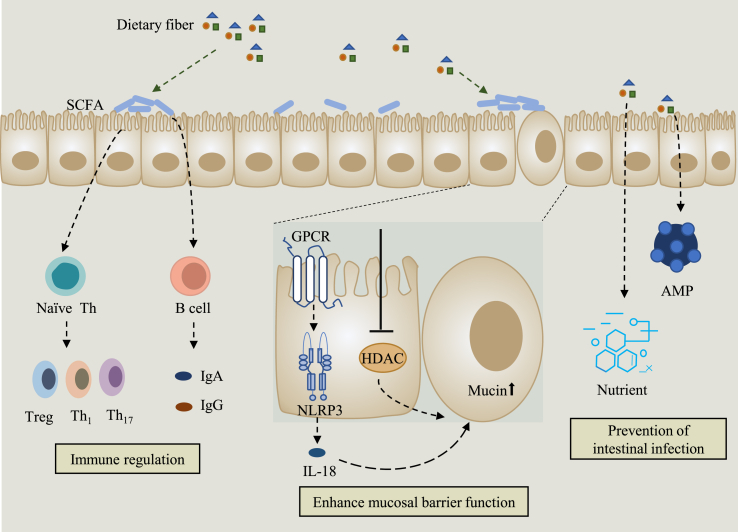
The effects of dietary fiber on the intestine are manifold and include immune regulation, promotion of mucosal barrier function and prevention of intestinal infection (Fig. 1). The intake of dietary fiber changes the length and size of the small intestine, cecum, colon and other digestive organs, which may be affected by the morphology of the intestinal epithelium and may ultimately influence intestinal digestion and hydrolysis function ultimately (Costello et al., 2017). As the main component of the digestive system, the intestine not only bears a massive bioburden in the lumen, but also prevents harmful substances from being transmitted to other parts of the body through the lymphatic and blood systems (Cui et al., 2019). The integrity of the epithelial layer is essential in ensuring that the contents of the intestinal cavity are separated from the lymphatic and blood systems. There are many causes that may destroy the homeostasis of the intestinal environment, such as epithelial damage due to diet, changes in intestinal microflora and mucous layer, which may lead to increased intestinal permeability, and the contents of the lumen will be transferred to the underlying mucosa (Mu et al., 2017). The function of the gut barrier is closely related to the integrity of the above structures, and many gastrointestinal diseases occur due to misadjusted of these components, such as IBD, colitis, IBS, intestinal overgrowth and allergic food intolerance (Camilleri et al., 2012). Dietary fiber is fermented by microorganisms in the colon under anaerobic conditions to produce SAFC such as acetic acid, propionic acid, butyric acid, valeric acid and isovaleric acid (Dongowski et al., 2002). The SAFC produced by the fermentation of different types of dietary fiber vary. For example, pectin and arabinoxylan are usually fermented to produce acetate, arabinogalactan is fermented to produce acetate and propionate, and butyrate is fermented from starch (Macfarlane et al., 2006). Short-chain fatty acids are involved in the regulation of environmental stability in the colon, the activation of epithelial cell proliferation and differentiation, the maintenance of mucosal integrity, and the alleviation of inflammation (Klampfer et al., 2003; Tsukahara et al., 2003).
Fig. 1.
Protective effects of dietary fiber on intestinal health. First, the activity of GPCR is promoted by SCFA, the fermentation products of dietary fiber; then, NLRP3 is activated, which increases the production of the epithelial healing cytokine IL-18. Moreover, SCFA can enhance the barrier function of the mucosa by inhibiting HDAC activity. Second, SCFA promote the development of Treg, Th1 and Th17 cells, and indirectly increase the production of IgA and IgG through the activation of B cells. Finally, dietary fiber can bind different nutrients and promote the production of AMP, which contributes to the prevention of intestinal infections. GPCR = G protein-coupled receptor; SCFA = short-chain fatty acids; NOD = nucleotide-binding oligomerization domain; IL-18 = interleukin-18; HDAC = histone deacetylase; Treg = regulatory T cells; AMP = antimicrobial peptides; NLRP3 = NOD-like receptor thermal protein domain associated protein 3.
The mucus layer, the first line of defense against harmful bacteria, is composed of glycoproteins produced by goblet epithelial cells (Bergstrom et al., 2008). Mucosa is one of the main defense mechanisms of the intestine and acts as a barrier to protect the intestinal epithelium against chemical and biological hazards. Dietary fiber increases the release of mucins from intestinal mucosa into the enteric cavity and provides more substrates for the growth and multiplication of commensal bacteria. It has been shown that the proportion of mucin species changes after the intake of dietary fiber (Shan et al., 2013), and the SCFA produced by fermentation of fermentable dietary fiber also positively affect the integrity of the epithelial mucosa (Cui et al., 2019). Insufficient intake of dietary fiber reduces the abundance of probiotics and cause the metabolism of intestinal microorganisms to produce metabolites that are harmful to the host such as amines, ammonia, N-nitroso compounds and branched-chain fatty acids, thus leading to intestinal mucosal damage (Windey et al., 2012). Therefore, a high fat and high sugar diet but low fiber may lead to the occurrence of chronic inflammatory diseases such as allergies, obesity, cardiovascular disease, colorectal cancer and inflammatory bowel disease (Makki et al., 2018; Schroeder et al., 2018; Zou et al., 2018). Adequate consumption of dietary fiber helps avoid excessive degradation of mucopolysaccharides, maintains a safe range of mucus-consuming flora, and thus prevents inflammatory responses (Leatham et al., 2009).
In addition to the mucosa, the intestinal epithelium is another barrier outside that not only physically separates the host and intestinal microorganisms, but also supports the communication between them (Peterson and Artis, 2014). The integrity of epithelium is the backbone of the health of gut and body. Stem cells at the bottom of the crypt often differentiate into a large number of transport cells that continuously move closer to the epithelium of intestinal cavity to better differentiate themselves into different types of cells, such as absorptive cells, intestinal endocrine cells, goblet cells, and M cells at specific locations, all of which are crucial to the barrier function of the colonic epithelium (Barker, 2014). Mucin is an important component of the mucus layer. Short-chain fatty acids can ensure the integrity of epithelium by increasing tolerance to the drug and promoting the secretion of mucus (Fukuda et al., 2011; Wrzosek et al., 2013), as well as increasing the secretion of prostaglandin, which promotes the expression of epithelial mucin (Willemsen et al., 2003). Butyrate is a significant substrate for nourishing colonic epithelial cells and protecting the gut epithelium. It can not only upregulate the expression of mucin by inhibiting the activity of HDAC (Gaudier et al., 2004), but also protect the gut barrier function through the reduction of the concentration of oxygen inducible factor in gut epithelium (Kelly et al., 2015).
Antimicrobial peptides (AMP) are first-line defense effectors that have the function of inhibiting the growth and reproduction of pathogenic bacteria (Parada Venegas et al., 2019), which is one of the important mechanisms of epithelial barrier function. In GPR43 knockout mice, the expression of AMP, RegIIIγ and β-defensins, was significantly inhibited, while butyrate could induce the secretion of AMPs (Zhao et al., 2018b). Dietary fiber from different sources has been shown to be effective in reducing the adhesion of pathogenic Escherichia coli to intestinal epithelial cells. For example, β galactomannan from yeast reduced the adhesion of ETEC to Caco-2 cells (Roussel et al., 2018).
Besides, dietary fiber can combine with different nutrients including calcium, zinc and copper, and transport them to the distal intestine, where they are released when fiber is metabolized by microorganisms in the colon (Baye et al., 2017). Some of these ions have antimicrobial effects under specific conditions and help prevent intestinal infections. For example, zinc can promote the metabolic activity of the intestinal microbiota in weaned piglets (Højberg et al., 2005), thus improving metabolic health parameters. Meanwhile, studies in chickens have shown that zinc-deficient diets lead to reduced intestinal microbial diversity, which is associated with reduced SCFA production (Reed et al., 2015). Several studies in humans have shown that SCFA produced by specific fibers, such as glycans and GOS, resulted in a decrease in pH and an increase in the solubility of calcium, thus increasing the passive transport of calcium in the intestine (Abrams et al., 2007; Whisner et al., 2013). The intake of dietary fiber can also increase the absorption of substances such as vitamin A, vitamin B1, vitamin C, and vitamin E by the human body (Chan et al., 2019), prevent the conversion of bile into secondary bile, and accelerate the consumption of cholesterol (Cronin et al., 2021). In addition to producing SCFA, microorganisms can also ferment dietary fiber to produce other substrates that benefit health. Ferulic acid, a phenolic compound that exists in the plant cell wall, can not only regulate intestinal physiology, but also be transported into the blood and thus affecting the health of the body (Makki et al., 2018). Lactobacillus fermentus can metabolize dietary fiber to produce ferulic acid, regulate intestinal physiology, and exert antioxidant and anti-inflammatory properties, with potential therapeutic benefits for many chronic diseases such as neurodegeneration, obesity, diabetes and cancer (Tomaro-Duchesneau et al., 2012).
5. SCFA are the main fermentation products of dietary fiber
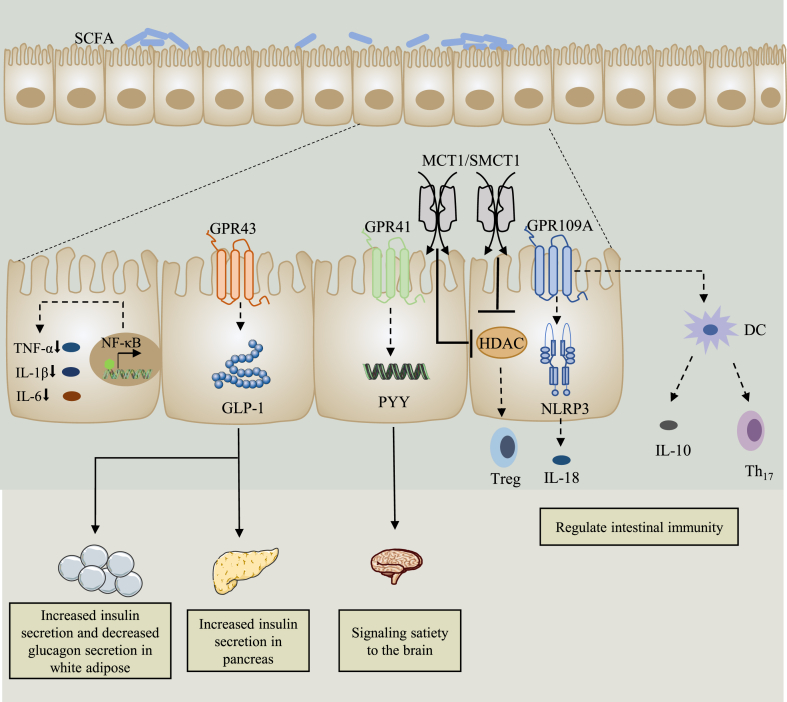
Through the fermentation of host microorganisms, dietary fiber provides many health benefits, including intestinal immunity regulation, insulin secretion promotion, and satiety transmission signal to the brain (Fig. 2). Dietary fiber is fermented by intestinal bacteria to produce monosaccharides, SCFA and gases such as methane and carbon dioxide (Rinninella et al., 2019). Short-chain fatty acids are the main product of dietary fiber fermentation, in which acetate, propionate and butyrate account for 60%, 25% and 15% respectively. They can regulate the physiological functions of the intestine, including the provision of energy to colon cells, the maintenance of the fluidity of the colon, and the regulation of the transport of electrolytes and nutrients in the intestinal cavity (Akhtar et al., 2022; Tazoe et al., 2008). Acetate is a precursor of cholesterol synthesis and fat generation, which can be produced from pyruvate through acetyl coenzyme A or Wood-Ljungdahl pathway (Koh et al., 2016; Ragsdale and Pierce, 2008). Propionate is a gluconeogenic substrate (Levy et al., 2016) that can be synthesized from acrylate via the acrylate pathway and propylene glycol pathway, with lactic acid as precursor (Koh et al., 2016; Scott et al., 2006). In addition, acetate and propionate can also act as substrates for the metabolism of cholesterol, glucose, and lipids when they are absorbed by the liver through the portal vein. There are two ways to synthesize butyric acid. Carbohydrates produce pyruvate through sugar metabolism, and the pyruvate is then converted into acetyl coenzyme A under the action of pyruvate dehydrogenase. A part of acetyl coenzyme A is converted into acetic acid and the rest is converted to butyryl coenzyme A under the action of a series of enzymes and generates butyric acid under the action of butyric acid kinase (Louis et al., 2004). At the same time, butyryl coenzyme A can also be directly converted into butyric acid under the action of coenzyme A transferase (Duncan et al., 2002). Butyrate plays a vital role in the maintenance of tissue barrier function, regulation of gene expression and immunity, in addition to being the main source of energy for colon cells and the preferred metabolic substrate (Hong et al., 2015; Rinninella et al., 2019). Through the action of microorganisms, these SCFA molecules can be converted to each other, acetic acid can be transformed into butyric acid for example (Boets et al., 2017). The type and quantity of SCFA produced by dietary fiber are related to the type of dietary fiber and the species of gut microbes (Kovatcheva-Datchary et al., 2015; Yang and Rose, 2014). Generally speaking, the concentration of SCFA circulating in the body is relatively low, many important metabolic processes in the host are regulated by these effective signal molecules (Makki et al., 2018).
Fig. 2.
SCFA action mechanisms. On the one hand, SCFA act as HDAC inhibitors to promote the production of Treg and alleviate intestinal inflammation. On the other hand, SCFA act as GPCR (GPR43, GPR41, GPR109A) ligands to promote the secretion of GLP-1 and PYY, increase the secretion of insulin in white adipose tissue and pancreatic tissue, and transmit satiety signals to the brain. In addition, SCFA can effectively inhibit the NF-κB signaling pathway and reduce the expression of inflammatory cytokines. SCFA = short-chain fatty acids; Treg = regulatory T cells; HDAC = histone deacetylase; GLP-1 = glucagon-like peptide 1; PYY = peptide YY; NF-κB = nuclear factor κB; TNF-α = tumor necrosis factor-α; IL = interleukin; DC = dendritic cell.
5.1. SCFA can act as an HDAC inhibitor
Histone acetylation occurs in the ε-amino group of lysine residues, and histone acetyltransferases add acetyl groups to the tail of histones to cause the acetylation of histones, thus promoting gene transcription, while HDAC can inhibit this process. Histone deacetylase inhibitors have been reported to have therapeutic effects in relation to cancer, in addition to exhibiting anti-inflammatory and immunosuppressive properties. Studies have shown that SCFA can exert anti-inflammatory effects by inhibiting HDAC. Most SCFA transported across the mucosa involve active transport mediated by two receptors, monocarboxylate transporter 1 and sodium coupled monocarboxylate transporter 1, which can regulate gene expression by directly inhibiting HDAC to affect and regulate the physiological function and biological response of the host (Kumar et al., 2020). Butyrate can inhibit HDAC activity and induce the histone acetylation, which affects the processes of cell proliferation, differentiation, apoptosis, and gene expression (Fung et al., 2012). Researches demonstrated that SCFA downregulated pro-inflammatory cytokines, including IL-12 and IL-6, in macrophages of the colon and distinguished dendritic cells from bone marrow stem cells (Chang et al., 2014; Singh et al., 2010). In addition, SCFA regulate the production of regulatory T cells (Tregs) by suppressing HDAC (Furusawa et al., 2013). It has been shown that propionate and butyrate can induce the differentiation of Tregs, thus inducing the expression of Foxp3, a transcription factor that plays a vital role in the control of intestinal inflammation in mice, by increasing the acetylation of Foxp3 gene sites (Arpaia et al., 2013). Besides, butyrate and propionate can activate the AP-1 signaling pathway, thus controlling the proliferation and inducing apoptosis in colon cancer cells (Nepelska et al., 2012).
5.2. SCFA are important ligands for GPCR
Short-chain fatty acids can not only act as HDAC inhibitors, but also play an important role as ligands for GPCR. Three GPCR are involved in immune regulation, GPR41, GPR43 and GPR109A. They have been shown to have specific responses to fatty acids and hydroxycarboxylic acids, and are thus named free fatty acid receptor 2 (FFAR2), FFAR3 and HCAR2, respectively (Brown et al., 2003).
The function of GPR43/FFAR2, a Gi/o- and Gq-double-coupled GPCR, is mainly mediated by Gi/o. However, in the gut, GPR43 is Gq-coupled and can promote the secretion of glucagon-like peptide 1 (GLP-1) in L cells (Tolhurst et al., 2012). Acetate and propionate can effectively activate GPR43, while their concentration range in the colon lumen and EC50 is 10–100 μM and 250–500 μM, respectively (Le Poul et al., 2003). Therefore, GPR43 should be continuously saturated with ligands when expressed in colonic epithelial cells, and signaling should not be affected by subtle changes in SCFA concentration. However, the thick mucus layer and continuous mucus flow in the colon may result in an SCFA concentration gradient (Donohoe et al., 2012), so that their concentration in the colon may reach a range capable of activating the biological activity of GPR43 in the epithelium. GPR43 affects the differentiation and activation of the monocytes and neutrophils that mediate inflammation, as well as intracellular signaling pathways, while mitogen-activated protein kinase, protein kinase C, and phospholipase C trigger leukocyte recycling in the inflammation location (Vinolo et al., 2011). In contrast to GPR43, GPR41/FFAR3 can only be coupled with Gi and can be activated by propionate, butyrate and acetate, of which propionate is the most effective activator, with an EC50 of approximately 12–274 μM (Le Poul et al., 2003). GPR109A/HCAR2 is only activated by butyrate (Blad et al., 2012), which is produced at a high concentration (10–20 μM) in the colon cavity and acts as an endogenous agonist of GPR109A (Thangaraju et al., 2009).
In the intestine and white adipose tissue of mice, the stimulation of GPCR43 by SCFA triggers the production of the intestinal GLP-1 (Kimura et al., 2013), which can directly promote the secretion of insulin and inhibit that of glucagon by interacting with pancreatic β-cells (Wei and Mojsov, 1995). Butyric acids play an important role in this process. First, butyrate enhances the secretion of GLP-1 by upregulating the genes responsible for the synthesis and secretion of GLP-1, and enhances the activity of GLP-1 and the sensitivity of insulin. Second, the secretion of GLP-1 and glucose-dependent insulinotropic polypeptide is induced by the stimulation of pancreatic β-cells, and butyrate can maximally transmit the signal to induce GLP-1 production through FFAR3 (Lin et al., 2012). Short-chain fatty acids the proliferation and protection of islet cells mediated by protein kinase B and pancreatic and duodenal homeobox factor 1 (PDX1) by activating the receptor of GLP-1 (Li et al., 2005; Lin et al., 2012; Perfetti and Hui, 2004). There is also evidence that SCFA can prevent the dedifferentiation of pancreatic β cells caused by forkhead box O1 (FOXO1) through GLP-1-mediated inhibition of intracellular FOXO1 and upregulation of PDX1 (Kitamura, 2013; Talchai et al., 2012). In addition, sodium butyrate may be a pancreatic development factor, inducing nidogen enhanced green fluorescent protein progenitor cells to transform into insulin secreting cells to promote insulin secretion in the presence of GLP-1 (Li et al., 2008). In addition, by activating GPR41, SCFA induce intestinal endocrine cells located in the colon to secrete peptide YY and then release it into systemic circulation (Samuel et al., 2008; Tolhurst et al., 2012). Once it enters circulation, it can send signals to the central nervous system to delay intestinal movement and gastric emptying, thus prolonging the ability to absorb nutrients (Cronin et al., 2021).
In addition, SCFA are important in regulating intestinal immunity. Researchers found that butyric acid can induce the transcription of IL-18 by stimulating the signals of GPR109A and GPR43, with the NLRP3 inflammasome also be activated (Macia et al., 2015; Singh et al., 2014). By releasing IL-18, the inflammatory response of the mucosal immune system caused by commensal microorganisms in the intestine is alleviated, and the integrity of the intestinal barrier is promoted, thus helping prevent bacterial invasion and infection (Kau et al., 2011; Koh et al., 2016; Macia et al., 2015). Butyrate prevents leukocyte migration by downregulating the expression of human vascular endothelial cell adhesion molecule 1, which is mediated by TNF-α and induces IL-10-mediated suppression of inflammation by Tregs (Meijer et al., 2010). Then, GPR109A is activated, which promotes the maturation of macrophages and dendritic cells in the colon, stimulates the production of transforming growth factors, and induces T cells that produce Tregs and IL-10 (Singh et al., 2014). Dendritic cells produce IL-10 and retinoic acid, stimulate the conversion of naive T cells into Tregs and suppress the production of helper T cells 17 that promote inflammation and colon cancer (Kalala et al., 2018). In addition, propionate and butyrate effectively inhibit the activation of the nuclear factor κB (NF-κB) signaling pathway (Singh et al., 2014), the expression of inflammatory cytokine genes, and exert anti-inflammatory effects by downregulating pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 (Mattace Raso et al., 2013).
6. The role of dietary fiber in the health and development of pigs
6.1. The microecology and health of the pig intestine
Generally, the microbial flora in the animal intestine is in a relatively stable state. When the body encounters stress, the imbalance of intestinal flora leads to intestinal inflammation and diarrhea, which have a negative impact on the growth and development of animals (Duarte and Kim, 2022; Ma et al., 2022). The addition of dietary fiber to the feed has a beneficial effect on the intestinal health of both sows and piglets. When sows consume feeds supplemented with fermented fiber, beneficial bacteria can rapidly colonize in the intestine of sows and form a biological barrier, promoting the growth of beneficial intestinal flora while inhibiting that of pathogenic bacteria, which may be related to the production of a variety of metabolites, such as SCFA, by the fermentation of dietary fiber, thus reducing the intestinal pH and competitively inhibiting the growth of harmful bacteria (Montagne et al., 2003). Study has shown that the supplement of dietary fiber to sow diet can decrease the abundance of E. coli and increase that of acetic acid in the intestine (Wang et al., 2003). Furthermore, a high-fiber diet increased the abundance of Lactobacillus, as well as the content of butyric acid, acetic acid and propionic acid in the colon of Taoyuan pigs and Duroc pigs (Liu et al., 2022). In addition, in sows, a high level of dietary fiber diet can promote intestinal peristalsis, improve the speed of chyme passing through the gastrointestinal tract, and prevent constipation (Oliviero et al., 2009). According to studies, increasing the proportion of dietary fiber in the diet of sows increased the speed of chyme passing through the intestine by several times (Jørgensen et al., 1996), and greatly improved the constipation of sows, while the postpartum feed intake, water intake and piglet birth weight were significantly higher than those of the control group (Oliviero et al., 2009). In addition, the addition of appropriate amounts of dietary fiber to piglet diets can block the adhesion of harmful bacteria to the gastrointestinal mucosa, regulate the intestinal microecological balance and maintain a normal microbiota, thus preventing digestive disorders and intestinal diseases (Chen et al., 2019). Similar to the role of dietary fiber in sows, suitable proportions of soluble and insoluble fiber can reduce the residence time of chyme in the intestines of piglets and reduce the fermentation of proteins in the hindgut, allowing pathogenic bacteria to be rapidly excreted with feces and reducing the proliferation of pathogenic bacteria in the small intestine (Gerritsen et al., 2012).
6.2. Reproductive performance of sows
Dietary fiber fermented feed can enable sows to reach their full reproductive potential. In recent years, scholars have conducted numerous studies on the effects of dietary fiber on sow fertility, founding that sows can obtain more energy from fermented dietary fiber and improve their reproductive performance. There is evidence that the diet of sows prior to mating can have a significant effect on pre-partum survival and litter size in subsequent pregnancies, possibly due to the fact that the developing oocytes within the follicles are highly sensitive to changes in maternal nutritional intake, which in turn affects hormone and metabolite levels, thereby altering ovarian function (Ashworth et al., 2009). Sows undergo changes in hormone levels and experience labor stress during labor, while dietary fiber and its metabolites can help regulate stress and hormone level changes during labor (Pastuszewska et al., 2000). Dietary fiber has been reported to reduce digestive tract disorders, regulate the blood insulin, estrogen, progesterone, and prolactin levels in sows prior to parturition, and promote feeding during lactation as well as growth and development of offspring, which may be achieved by modulating multiple activities such as intestinal immunity, inflammatory response, and insulin sensitivity through pathways such as intestine-liver or intestine-brain (de Leeuw et al., 2004; Quesnel et al., 2009). According to Ferguson et al., follicular development and oocyte maturation were promoted in sows that were fed diets rich in dietary fiber prior to mating, possibly due to changes in estradiol hormone levels (Ferguson et al., 2007). This reproductive benefit was also observed in sows that were fed with lupin before fertilization, and the embryo survival rate of sows increased 27–29 days after mating (Ferguson et al., 2007; Weaver et al., 2013). For sows that were fed diets supplemented with fiber, the live litter size increased significantly (Wu et al., 2020). The same results were observed for sows fed with wheat straw, with significant increases in total litter size, the number of piglets weaned per litter, and number of litters weaned (Veum et al., 2009). In addition, short-term supplementation of dietary fiber in the late gestation period reduced the total mortality of lactating piglets and percentage of stillborn piglets (Feyera et al., 2017). In summary, feeds with fermented dietary fiber contains many beneficial active ingredients that can improve sows' intestinal health, immune function, and digestion, as well as the absorption and utilization of nutrients. Furthermore, these beneficial effects improve the intestinal health of offspring through the maternal effect, which improves sows' reproductive performance and promotes offspring growth and development. In addition, feeds with fermented dietary fiber have good appetite and palatability, which promotes greater feed intake by sows and ultimately improves sow performance. Finally, sows' improved reproductive performance may be related to the promotion of their reproductive organ development by dietary fiber and the growth of intestinal microflora associated with SCFA (Abdelsattar et al., 2022).
The interaction between dietary fiber and intestinal microorganisms can produce beneficial effects that can be passed on to sow offspring. Adding inulin and guar gum to the diet of sow altered the gut flora of piglets, with the abundance of probiotics in the gut was increased, and the diarrhea rate of piglets was reduced (Cheng et al., 2018; Paβlack et al., 2015). Increasing the proportion of dietary fiber in the diet of pregnant sows also improved antioxidant capacity and inhibited colonic inflammation in piglets (Li et al., 2019). Feeding a diet rich in fiber to pregnant sows also affected the growth rate of piglets. When sows were fed dietary fiber in late pregnancy, the daily gains of their offspring piglets were higher until the fifth day of lactation (Oliviero et al., 2009). A similar situation was observed when sows were fed dietary fiber from the 25th day of gestation to delivery, with the offspring piglets of the fiber fed sows grew faster than those of the control sows (Quesnel et al., 2009). Similarly, when high-fiber diets were fed to primiparous sows from 5 weeks of gestation to parturition, the growth rate in the first week of postpartum and the weight at weaning of piglets were increased (Guillemet et al., 2007).
6.3. Lactation performance of sows
The lactation capacity of sows and the regular nutrient content of their milk are closely related to sows’ reproductive performance, which directly affects the growth and development of offspring during lactation and after weaning. Adding a certain dietary fiber to the diet of sows can improve their lactation performance, probably because the fermentation of dietary fiber produces a variety of organic acids such as acetic acid, propionic acid, butyric acid and lactic acid, which can provide energy for the body. Moreover, these organic acids can enter milk through the blood and improve the levels of lactoprotein and butterfat, further improving the quality of sow milk, which is conducive to the growth and development of offspring, and promoting the survival rate of newborn piglets. According to reports, after increasing the proportion of fiber in the diet of sows, the time to feed colostrum was shortened in newborn piglets, the butterfat content in the breast milk of sows with high-fiber diets was significantly higher than that with low-fiber diets, and the mortality rate of piglets before weaning was reduced (Loisel et al., 2013; Montagne et al., 2014). The use of oat hulls instead of certain conventional feeds also increased the content of butterfat in the milk of postpartum sows, as well as energy in colostrum and standing milk (Mroz et al., 1986). In addition, lactation ADFI can significantly affect the reproductive performance of sows, while various factors such as oxidative stress and inflammation can cause insufficient lactation ADFI. In sows, the imbalance of intestinal flora caused by high metabolism and reduced antioxidant capacity can lead to metabolic syndrome in late gestation and early lactation (Mosnier et al., 2010). Dietary fiber, which promotes beneficial intestinal bacteria, can improve metabolic syndrome by remodeling the flora, thus reducing oxidative stress and inflammation in sows and increasing the ADFI in lactation effectively (Xu et al., 2020a; Zhou et al., 2017). The addition of dietary fiber to the diets of sows during pregnancy also significantly increased their feed intake, thereby increasing the milk yield of sows. Studies found that the feed intake of sows was increased when a certain proportion of wheat bran, sugar beet pulp, soybean hulls, and konjac flour were added to the feed (Quesnel et al., 2009; Renteria-Flores et al., 2008; Tan et al., 2018). Paßlack et al. (2015) showed that for sows, the addition of a certain percentage of dietary fiber during the perinatal period can effectively improve insulin sensitivity. Lower insulin sensitivity reduced the feed intake of sows in late pregnancy, which is conducive to the production of sows and the increase of feed intake during lactation, thus improving the milk yield (Mosnier et al., 2010). Almost all the energy material of piglets before weaning comes from breast milk, and the lactation performance of sows determines the growth performance of piglets before and after weaning, the litter weight of weaned piglets can visually reflect the lactation performance of sows therefore. In a study of 600 sows in three breeding cycles, Veum et al. found that feed with a certain percentage of wheat straw increased the newborn litter weight and weaning litter weight (Veum et al., 2009). Therefore, these studies demonstrate that dietary fiber can regulate the intake of sows by regulating their gut health, which, in turn, improves the milk quality, lactation performance, and reproductive performance of sows and the litter weight of weaned piglets.
6.4. Growth performance and meat quality
The influence of dietary fiber on the growth performance of pigs is related to feed utilization. According to Zhao et al., the addition of corn bran or wheat bran to the diet increased the average weight gain and feed conversion ratio of weaned piglets, which may be related to changes in the intestinal microbiota, increased butyrate production and enhanced intestinal health (Zhao et al., 2018a). First, the various beneficial bacteria and their metabolites contained in dietary fiber fermented feed can promote the balance of gut microbe and reduce the occurrence of diarrhea. Furthermore, the SCFA produced by dietary fiber fermentation can directly or indirectly participate in physiological activities such as energy metabolism and the immune function regulation of the body, that are conducive to the development of the intestinal structure and the improvement of intestinal function, thus facilitating the digestion and utilization of nutrients and promoting piglet growth. Second, fiber fermented feeds can increase appetite, which can improve the feed intake of piglets and eventually improve their growth performance. However, some studies have obtained the opposite results, with high-fiber diets leading to a decrease in the average daily weight gain of weaned piglets and reducing the lean meat of fattening pigs, which are associated with reduced nutrient digestibility and energy deposition caused by dietary fiber (De Jong et al., 2014; Wang et al., 2016).
Muscle fibers in pigs can be divided into fast muscle fibers, including myosin heavy chain (MyHC) IIa, MyHC IIb and MyHC IIx, and slow muscle fibers, which correspond to MyHC I, which are related to energy supply and different modes of glucose utilization, thus affecting the lipid content, function, color and shape of the muscle (Joo et al., 2013). Dietary fiber also has an effect on meat quality, with a high dietary fiber diet reducing glycolysis of fresh pork, which may be related to the improvement of muscle oxidative fiber composition (Li et al., 2015). Furthermore, high fiber diets reduced the mRNA and protein levels of MyHC IIb and MyHC IIx in the longissimus dorsi of Erhualian pigs (Han et al., 2020). Moreover, SCFA produced by dietary fiber fermentation can regulate muscle cell energy metabolism by affecting mitochondrial synthesis and function (Canfora et al., 2015). According to Joven et al. (2014), the replacement of barley with fiber-rich olive cake in the diet can also reduce the drip loss and backfat thickness of fattening pigs.
7. Conclusion and prospect
The impact of dietary fiber as a prebiotic supplement or a natural food on health and disease is related to its effect on the regulation of the intestinal microbiota, which can promote intestinal health and prevent diseases by balancing immune function, promoting intestinal mucus production and membrane integrity, and preventing the growth of pathogenic microorganisms. The main beneficial effects of dietary fiber intake occur through the production of SCFA, which play a significant role in regulating the metabolism, immunity and inflammation of the host through cellular and signaling mechanisms. Here, we discussed the role of dietary fiber and SCFA in maintaining health, preventing inflammation and disease, and improving pig nutrition. However, further work is needed to better understand how dietary fiber interacts with microbiota. The type, dose, solubility, viscosity, and fermentation characteristics of dietary fiber affect its benefits to animals. At the same time, the inhibitory effects of SCFA on inflammation may vary across tissues and cells. Therefore, more studies and reports on these characteristics and mechanisms are needed to determine the clinical therapeutic effects of dietary fiber with different sources, properties and doses on specific diseases. In addition, interventions with single dietary fiber may be insufficient to reverse poor health, and the combined administration of different dietary fibers and the combined intake of dietary fiber with other beneficial substances such as probiotics also need to be explored. Overall, future research should explore the complex interactions between specific dietary fibers and gut microbiota to develop individualized nutritional therapies to reduce the incidence and impact of various diseases.
Author contributions
Xuebing Han: Investigation, Writing – original draft, Writing – review & editing. Yong Ma: Investigation, Writing – review & editing. Sujuan Ding: Investigation, Writing – review & editing. Jun Fang: Supervision. Gang Liu: Supervision.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgment
This study was supported by Hunan Provincial Science and Technology Department (2021JJ30008, 2020NK2004, 2019TP2004) and Double first-class construction project of Hunan Agricultural University (SYL201802003), and Hunan Province Natural Science Foundation (2021JJ30320).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abdelsattar M.M., Vargas-Bello-Pérez E., Zhuang Y., Fu Y., Zhang N. Impact of dietary supplementation of β-hydroxybutyric acid on performance, nutrient digestibility, organ development and serum stress indicators in early-weaned goat kids. Anim Nutr. 2022;9:16–22. doi: 10.1016/j.aninu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams S.A., Griffin I.J., Hawthorne K.M. Young adolescents who respond to an inulin-type fructan substantially increase total absorbed calcium and daily calcium accretion to the skeleton. J Nutr. 2007;137:2524s–2526s. doi: 10.1093/jn/137.11.2524S. [DOI] [PubMed] [Google Scholar]
- Akhtar M., Chen Y., Ma Z., Zhang X., Shi D., Khan J.A., Liu H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim Nutr. 2022;8:350–360. doi: 10.1016/j.aninu.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony W.E., Carr R.R., DeLorenzo D.M., Campbell T.P., Shang Z., Foston M., Moon T.S., Dantas G. Development of rhodococcus opacus as a chassis for lignin valorization and bioproduction of high-value compounds. Biotechnol Biofuels. 2019;12:192. doi: 10.1186/s13068-019-1535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong H., Mander I., Zhang Z., Armstrong D. Wine, E Not all fibers are born equal; variable response to dietary fiber subtypes in ibd. Front Pediatr. 2020;8:620189. doi: 10.3389/fped.2020.620189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., et al. Metabolites produced by commensal bacteria promote peripheral regulatory t-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth C.J., Toma L.M., Hunter M.G. Nutritional effects on oocyte and embryo development in mammals: implications for reproductive efficiency and environmental sustainability. Phil Trans Roy Soc Lond B Biol Sci. 2009;364:3351–3361. doi: 10.1098/rstb.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai K., Jiang L., Li Q., Zhang J., Zhang L., Wang T. Dietary dimethylglycine sodium salt supplementation alleviates redox status imbalance and intestinal dysfunction in weaned piglets with intrauterine growth restriction. Anim Nutr. 2022;10:188–197. doi: 10.1016/j.aninu.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- Baye K., Guyot J.P., Mouquet-Rivier C. The unresolved role of dietary fibers on mineral absorption. Crit Rev Food Sci Nutr. 2017;57:949–957. doi: 10.1080/10408398.2014.953030. [DOI] [PubMed] [Google Scholar]
- Bergstrom K.S., Guttman J.A., Rumi M., Ma C., Bouzari S., Khan M.A., Gibson D.L., Vogl A.W., Vallance B.A. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect Immun. 2008;76:796–811. doi: 10.1128/iai.00093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukema M., Akkerman R., Jermendi É., Koster T., Laskewitz A., Kong C., Schols H.A., Faas M.M., de Vos P. Pectins that structurally differ in the distribution of methyl-esters attenuate citrobacter rodentium-induced colitis. Mol Nutr Food Res. 2021;65 doi: 10.1002/mnfr.202100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blad C.C., Tang C., Offermanns S.G. protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- Bliss D.Z., Weimer P.J., Jung H.J., Savik K. In vitro degradation and fermentation of three dietary fiber sources by human colonic bacteria. J Agric Food Chem. 2013;61:4614–4621. doi: 10.1021/jf3054017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets E., Gomand S.V., Deroover L., Preston T., Vermeulen K., De Preter V., Hamer H.M., Van den Mooter G., De Vuyst L., Courtin C.M., et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595:541–555. doi: 10.1113/jp272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.J., Goldsworthy S.M., Barnes A.A., Eilert M.M., Tcheang L., Daniels D., Muir A.I., Wigglesworth M.J., Kinghorn I., Fraser N.J., et al. The orphan g protein-coupled receptors gpr41 and gpr43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Madsen K., Spiller R., Greenwood-Van Meerveld B., Verne G. N Intestinal barrier function in health and gastrointestinal disease. Neuro Gastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfora E.E., Jocken J.W., Blaak E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- Chan Y.M., Aufreiter S., O'Keefe S.J., O'Connor D.L. Switching to a fibre-rich and low-fat diet increases colonic folate contents among african americans. Appl Physiol Nutr Metabol. 2019;44:127–132. doi: 10.1139/apnm-2018-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che L., Feng D., Wu D., Fang Z., Lin Y., Yan T. Effect of dietary fibre on reproductive performance of sows during the first two parities. Reprod Domestic Anim. 2011;46:1061–1066. doi: 10.1111/j.1439-0531.2011.01787.x. [DOI] [PubMed] [Google Scholar]
- Chen G., Chen D., Zhou W., Peng Y., Chen C., Shen W., Zeng X., Yuan Q Improvement of metabolic syndrome in high-fat diet-induced mice by yeast β-glucan is linked to inhibited proliferation of lactobacillus and lactococcus in gut microbiota. J Agric Food Chem. 2021;69:7581–7592. doi: 10.1021/acs.jafc.1c00866. [DOI] [PubMed] [Google Scholar]
- Chen T., Chen D., Tian G., Zheng P., Mao X., Yu J., He J., Huang Z., Luo Y., Luo J., et al. Soluble fiber and insoluble fiber regulate colonic microbiota and barrier function in a piglet model. BioMed Res Int. 2019;2019:7809171. doi: 10.1155/2019/7809171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Wei H., Xu C., Xie X., Jiang S., Peng J Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl Environ Microbiol. 2018;84 doi: 10.1128/aem.01047-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Jiang X., Li J., Zhou S., Bai T., Qin W., Li H., Luo Y., Huang Z., Liu Y., et al. Xyloglucan affects gut-liver circulating bile acid metabolism to improve liver damage in mice fed with high-fat diet. J Funct Foods. 2020;64:103651. doi: 10.1016/j.jff.2019.103651. [DOI] [Google Scholar]
- Chuang S.C., Norat T., Murphy N., Olsen A., Tjønneland A., Overvad K., Boutron-Ruault M.C., Perquier F., Dartois L., Kaaks R., et al. Fiber intake and total and cause-specific mortality in the european prospective investigation into cancer and nutrition cohort. Am J Clin Nutr. 2012;96:164–174. doi: 10.3945/ajcn.111.028415. [DOI] [PubMed] [Google Scholar]
- Cook G.M., Russell J.B. Energy-spilling reactions of streptococcus bovis and resistance of its membrane to proton conductance. Appl Environ Microbiol. 1994;60:1942–1948. doi: 10.1128/aem.60.6.1942-1948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S.P., Soo W., Bryant R.V., Jairath V., Hart A.L., Andrews J.M. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Therap. 2017;46:213–224. doi: 10.1111/apt.14173. [DOI] [PubMed] [Google Scholar]
- Cronin P., Joyce S.A., O'Toole P.W., O'Connor E.M. Dietary fibre modulates the gut microbiota. Nutrients. 2021;13 doi: 10.3390/nu13051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Lian Y., Zhao C., Du H., Han Y., Gao W., Xiao H., Zheng J Dietary fibers from fruits and vegetables and their health benefits via modulation of gut microbiota. Compr Rev Food Sci Food Saf. 2019;18:1514–1532. doi: 10.1111/1541-4337.12489. [DOI] [PubMed] [Google Scholar]
- Davis L.M., Martínez I., Walter J., Goin C., Hutkins R.W. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong J.A., DeRouchey J.M., Tokach M.D., Dritz S.S., Goodband R.D. Effects of dietary wheat middlings, corn dried distillers grains with solubles, and net energy formulation on nursery pig performance. J Anim Sci. 2014;92:3471–3481. doi: 10.2527/jas.2013-7350. [DOI] [PubMed] [Google Scholar]
- de Leeuw J.A., Jongbloed A.W., Verstegen M.W. Dietary fiber stabilizes blood glucose and insulin levels and reduces physical activity in sows (sus scrofa) J Nutr. 2004;134:1481–1486. doi: 10.1093/jn/134.6.1481. [DOI] [PubMed] [Google Scholar]
- De Vuyst L., Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol. 2011;149:73–80. doi: 10.1016/j.ijfoodmicro.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Deehan E.C., Duar R.M., Armet A.M., Perez-Muñoz M.E., Jin M., Walter J Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.BAD-0019-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra D., Michael M., Rajput H., Patil R.T. Dietary fibre in foods: a review. J Food Sci Technol. 2012;49:255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikeman C.L., Fahey G.C. Viscosity as related to dietary fiber: a review. Crit Rev Food Sci Nutr. 2006;46:649–663. doi: 10.1080/10408390500511862. [DOI] [PubMed] [Google Scholar]
- Dittoe D.K., Barabote R.D., Rothrock M.J., Ricke S.C. Assessment of a potential role of dickeya dadantii dsm 18020 as a pectinase producer for utilization in poultry diets based on in silico analyses. Front Microbiol. 2020;11:751. doi: 10.3389/fmicb.2020.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongowski G., Huth M., Gebhardt E., Flamme W. Dietary fiber-rich barley products beneficially affect the intestinal tract of rats. J Nutr. 2002;132:3704–3714. doi: 10.1093/jn/132.12.3704. [DOI] [PubMed] [Google Scholar]
- Dongowski G., Lorenz A., Anger H. Degradation of pectins with different degrees of esterification by bacteroides thetaiotaomicron isolated from human gut flora. Appl Environ Microbiol. 2000;66:1321–1327. doi: 10.1128/aem.66.4.1321-1327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D.R., Collins L.B., Wali A., Bigler R., Sun W., Bultman S.J. The warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M.E., Kim S.W. Intestinal microbiota and its interaction to intestinal health in nursery pigs. Anim Nutr. 2022;8:169–184. doi: 10.1016/j.aninu.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S.H., Barcenilla A., Stewart C.S., Pryde S.E., Flint H. J Acetate utilization and butyryl coenzyme a (coa):Acetate-coa transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. 2002;68:5186–5190. doi: 10.1128/aem.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kaoutari A., Armougom F., Gordon J.I., Raoult D., Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- El Khoury D., Cuda C., Luhovyy B.L., Anderson G.H. Beta glucan: health benefits in obesity and metabolic syndrome. J Nutr Metabol. 2012;2012:851362. doi: 10.1155/2012/851362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleuch M., Bedigian D., Roiseux O., Besbes S., Blecker C., Attia H. Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem. 2011;124:411–421. doi: 10.1016/j.foodchem.2010.06.077. [DOI] [Google Scholar]
- Evans C.E.L. Dietary fibre and cardiovascular health: a review of current evidence and policy. Proc Nutr Soc. 2020;79:61–67. doi: 10.1017/s0029665119000673. [DOI] [PubMed] [Google Scholar]
- Ferguson E.M., Slevin J., Hunter M.G., Edwards S.A., Ashworth C. J Beneficial effects of a high fibre diet on oocyte maturity and embryo survival in gilts. Reproduction. 2007;133:433–439. doi: 10.1530/rep-06-0018. [DOI] [PubMed] [Google Scholar]
- Feyera T., Højgaard C.K., Vinther J., Bruun T.S., Theil P.K. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J Anim Sci. 2017;95:5430–5438. doi: 10.2527/jas2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint H.J., Scott K.P., Duncan S.H., Louis P., Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microb. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraberger V., Call L.M., Domig K.J., D'Amico S. Applicability of yeast fermentation to reduce fructans and other fodmaps. Nutrients. 2018;10 doi: 10.3390/nu10091247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- Fung K.Y., Cosgrove L., Lockett T., Head R., Topping D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr. 2012;108:820–831. doi: 10.1017/s0007114512001948. [DOI] [PubMed] [Google Scholar]
- Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gaudier E., Jarry A., Blottière H.M., de Coppet P., Buisine M.P., Aubert J.P., Laboisse C., Cherbut C., Hoebler C. Butyrate specifically modulates muc gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1168–G1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- Gerritsen R., van der Aar P., Molist F. Insoluble nonstarch polysaccharides in diets for weaned piglets. J Anim Sci. 2012;90(Suppl 4):318–320. doi: 10.2527/jas.53770. [DOI] [PubMed] [Google Scholar]
- Gill S.K., Rossi M., Bajka B., Whelan K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. 2021;18:101–116. doi: 10.1038/s41575-020-00375-4. [DOI] [PubMed] [Google Scholar]
- Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J.T., et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg N.A., Gassull M.A., Meier R. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006;21:639–640. doi: 10.1177/0115426506021006639. author reply 40. [DOI] [PubMed] [Google Scholar]
- Grootaert C., Van den Abbeele P., Marzorati M., Broekaert W.F., Courtin C.M., Delcour J.A., Verstraete W., Van de Wiele T. Comparison of prebiotic effects of arabinoxylan oligosaccharides and inulin in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol. 2009;69:231–242. doi: 10.1111/j.1574-6941.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- Grundy M.M.L., Quint J., Rieder A., Ballance S., Dreiss C.A., Cross K.L., Gray R., Bajka B.H., Butterworth P.J., Ellis P.R., et al. The impact of oat structure and β-glucan on in vitro lipid digestion. J Funct Foods. 2017;38:378–388. doi: 10.1016/j.jff.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z.W., Yu E.Z., Feng Q Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules. 2021;26 doi: 10.3390/molecules26226802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemet R., Hamard A., Quesnel H., Père M.C., Etienne M., Dourmad J.Y., Meunier-Salaün M.C. Dietary fibre for gestating sows: effects on parturition progress, behaviour, litter and sow performance. Animal. 2007;1:872–880. doi: 10.1017/s1751731107000110. [DOI] [PubMed] [Google Scholar]
- Guo Q., Li F., Duan Y., Wen C., Wang W., Zhang L., Huang R., Yin Y. Oxidative stress, nutritional antioxidants and beyond. Sci China Life Sci. 2020;63:866–874. doi: 10.1007/s11427-019-9591-5. [DOI] [PubMed] [Google Scholar]
- Guo Y., Yu Y., Li H., Ding X., Li X., Jing X., Chen J., Liu G., Lin Y., Jiang C., et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in uox-knockout mice. Eur J Nutr. 2021;60:2217–2230. doi: 10.1007/s00394-020-02414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker B.R., Tuncil Y.E. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol. 2014;426:3838–3850. doi: 10.1016/j.jmb.2014.07.028. [DOI] [PubMed] [Google Scholar]
- Han P., Li P., Zhou W., Fan L., Wang B., Liu H., Gao C., Du T., Pu G., Wu C., et al. Effects of various levels of dietary fiber on carcass traits, meat quality and myosin heavy chain i, iia, iix and iib expression in muscles in erhualian and large white pigs. Meat Sci. 2020;169:108160. doi: 10.1016/j.meatsci.2020.108160. [DOI] [PubMed] [Google Scholar]
- Hipsley E.H. Dietary “fibre” and pregnancy toxaemia. Br Med J. 1953;2:420–422. doi: 10.1136/bmj.2.4833.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Højberg O., Canibe N., Poulsen H.D., Hedemann M.S., Jensen B.B. Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl Environ Microbiol. 2005;71:2267–2277. doi: 10.1128/aem.71.5.2267-2277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microb. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher H.D., Bauer L.L., Gourineni V., Pelkman C.L., Fahey G.C., Jr., Swanson K.S. Agave inulin supplementation affects the fecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145:2025–2032. doi: 10.3945/jn.115.217331. [DOI] [PubMed] [Google Scholar]
- Holscher H.D., Caporaso J.G., Hooda S., Brulc J.M., Fahey G.C., Jr., Swanson K.S. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: follow-up of a randomized controlled trial. Am J Clin Nutr. 2015;101:55–64. doi: 10.3945/ajcn.114.092064. [DOI] [PubMed] [Google Scholar]
- Hong M.Y., Turner N.D., Murphy M.E., Carroll R.J., Chapkin R.S., Lupton J.R. In vivo regulation of colonic cell proliferation, differentiation, apoptosis, and p27kip1 by dietary fish oil and butyrate in rats. Cancer Prev Res. 2015;8:1076–1083. doi: 10.1158/1940-6207.Capr-15-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisawa S., Ando H., Ariga O., Sakuma Y. Direct ethanol production from cellulosic materials by consolidated biological processing using the wood rot fungus schizophyllum commune. Bioresour Technol. 2015;197:37–41. doi: 10.1016/j.biortech.2015.08.031. [DOI] [PubMed] [Google Scholar]
- Hu H., Zhang S., Liu F., Zhang P., Muhammad Z., Pan S. Role of the gut microbiota and their metabolites in modulating the cholesterol-lowering effects of citrus pectin oligosaccharides in c57bl/6 mice. J Agric Food Chem. 2019;67:11922–11930. doi: 10.1021/acs.jafc.9b03731. [DOI] [PubMed] [Google Scholar]
- Janusz G., Pawlik A., Sulej J., Swiderska-Burek U., Jarosz-Wilkolazka, Paszczynski A. A Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev. 2017;41:941–962. doi: 10.1093/femsre/fux049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S.T., Kim G.D., Hwang Y.H., Ryu Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95:828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Jørgensen H., Zhao X.Q., Eggum B.O. The influence of dietary fibre and environmental temperature on the development of the gastrointestinal tract, digestibility, degree of fermentation in the hind-gut and energy metabolism in pigs. Br J Nutr. 1996;75:365–378. doi: 10.1079/bjn19960140. [DOI] [PubMed] [Google Scholar]
- Joven M., Pintos E., Latorre M.A., Suárez-Belloch J., Guada J.A., Fondevila M. Effect of replacing barley by increasing levels of olive cake in the diet of finishing pigs: growth performances, digestibility, carcass, meat and fat quality. Anim Feed Sci Technol. 2014;197:185–193. doi: 10.1016/j.anifeedsci.2014.08.007. [DOI] [Google Scholar]
- Kalala G., Kambashi B., Everaert N., Beckers Y., Richel A., Pachikian B., Neyrinck A.M., Delzenne N.M., Bindelle J Characterization of fructans and dietary fibre profiles in raw and steamed vegetables. Int J Food Sci Nutr. 2018;69:682–689. doi: 10.1080/09637486.2017.1412404. [DOI] [PubMed] [Google Scholar]
- Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S.J., Gupta V.K. Production of pectinolytic enzymes pectinase and pectin lyase by bacillus subtilis sav-21 in solid state fermentation. Ann Microbiol. 2017;67:333–342. doi: 10.1007/s13213-017-1264-4. [DOI] [Google Scholar]
- Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J., Wilson K.E., Glover L.E., Kominsky D.J., Magnuson A., et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial hif augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Hwang S.W., Kim S., Lee Y.S., Kim T.Y., Lee S.H., Kim S.J., Yoo H.J., Kim E.N., Kweon M.N. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microb. 2020;11:944–961. doi: 10.1080/19490976.2020.1730149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I., Ozawa K., Inoue D., Imamura T., Kimura K., Maeda T., Terasawa K., Kashihara D., Hirano K., Tani T., et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor gpr43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T. The role of foxo1 in β-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9:615–623. doi: 10.1038/nrendo.2013.157. [DOI] [PubMed] [Google Scholar]
- Klampfer L., Huang J., Sasazuki T., Shirasawa S., Augenlicht L. Inhibition of interferon gamma signaling by the short chain fatty acid butyrate. Mol Cancer Res : MCR. 2003;1:855–862. [PubMed] [Google Scholar]
- Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Koropatkin N.M., Cameron E.A., Martens E.C. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., De Vadder F., Arora T., Hallen A., Martens E., Björck I., Bäckhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metabol. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Kumar J., Rani K., Datt C. Molecular link between dietary fibre, gut microbiota and health. Mol Biol Rep. 2020;47:6229–6237. doi: 10.1007/s11033-020-05611-3. [DOI] [PubMed] [Google Scholar]
- Lam K.-L., Keung H.-Y., Ko K.-C., Kwan H.-S., Cheung P.C.-K. In vitro fermentation of beta-glucans and other selected carbohydrates by infant fecal inoculum: an evaluation of their potential as prebiotics in infant formula. Bioact Carbohydr Diet Fibre. 2018;14:20–24. doi: 10.1016/j.bcdf.2017.07.009. [DOI] [Google Scholar]
- Larsen N., Bussolo de Souza C., Krych L., Barbosa Cahú T., Wiese M., Kot W., Hansen K.M., Blennow A., Venema K., Jespersen L. Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Front Microbiol. 2019;10:223. doi: 10.3389/fmicb.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattimer J.M., Haub M.D. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2:1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E., Loison C., Struyf S., Springael J.Y., Lannoy V., Decobecq M.E., Brezillon S., Dupriez V., Vassart G., Van Damme J., et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- Leatham M.P., Banerjee S., Autieri S.M., Mercado-Lubo R., Conway T., Cohen P.S. Precolonized human commensal escherichia coli strains serve as a barrier to e. Coli o157:H7 growth in the streptomycin-treated mouse intestine. Infect Immun. 2009;77:2876–2886. doi: 10.1128/iai.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kang M., Bae J.H., Sohn J.H., Sung B.H. Bacterial valorization of lignin: strains, enzymes, conversion pathways, biosensors, and perspectives. Front Bioeng Biotechnol. 2019;7:209. doi: 10.3389/fbioe.2019.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M., Thaiss C.A., Elinav E Metabolites. Messengers between the microbiota and the immune system. Genes Dev. 2016;30:1589–1597. doi: 10.1101/gad.284091.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lili R., Hui Q., Min W., Xue W., Xin S., Jing L., Yan L., Yeqiang L., Fenrong H., et al. Combination of glp-1 and sodium butyrate promote differentiation of pancreatic progenitor cells into insulin-producing cells. Tissue Cell. 2008;40:437–445. doi: 10.1016/j.tice.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Li Y., Cao X., Li L.X., Brubaker P.L., Edlund H., Drucker D. J Beta-cell pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes. 2005;54:482–491. doi: 10.2337/diabetes.54.2.482. [DOI] [PubMed] [Google Scholar]
- Li Y., Li J., Zhang L., Yu C., Lin M., Gao F., Zhou G., Zhang Y., Fan Y., Nuldnali L. Effects of dietary energy sources on post mortem glycolysis, meat quality and muscle fibre type transformation of finishing pigs. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu H., Zhang L., Yang Y., Lin Y., Zhuo Y., Fang Z., Che L., Feng B., Xu S., et al. Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int J Mol Sci. 2019;21 doi: 10.3390/ijms21010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.V., Frassetto A., Kowalik E.J., Jr., Nawrocki A.R., Lu M.M., Kosinski J.R., Hubert J.A., Szeto D., Yao X., Forrest G., et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Luo Y., Kong X., Yu B., Zheng P., Huang Z., Mao X., Yu J., Luo J., Yan H., et al. Effects of dietary fiber on growth performance, nutrient digestibility and intestinal health in different pig breeds. Animals. 2022;12 doi: 10.3390/ani12233298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel F., Farmer C., Ramaekers P., Quesnel H. Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance. J Anim Sci. 2013;91:5269–5279. doi: 10.2527/jas.2013-6526. [DOI] [PubMed] [Google Scholar]
- Lombard V., Golaconda Ramulu H., Drula E., Coutinho P.M., Henrissat B. The carbohydrate-active enzymes database (cazy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P., Duncan S.H., McCrae S.I., Millar J., Jackson M.S., Flint H. J Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186:2099–2106. doi: 10.1128/jb.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis A.S., Briggs J., Zhang X., Farnell B., Ndeh D., Labourel A., Baslé A., Cartmell A., Terrapon N., Stott K., et al. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic bacteroides. Nat Microbiol. 2018;3:210–219. doi: 10.1038/s41564-017-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Duan Y., Li R., Liang X., Li T., Huang X., Yin Y., Yin J. Gut microbial profiles and the role in lipid metabolism in shaziling pigs. Anim Nutr. 2022;9:345–356. doi: 10.1016/j.aninu.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S., Macfarlane G.T., Cummings J.H. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Therap. 2006;24:701–714. doi: 10.1111/j.1365-2036.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- Macia L., Tan J., Vieira A.T., Leach K., Stanley D., Luong S., Maruya M., Ian McKenzie C., Hijikata A., Wong C., et al. Metabolite-sensing receptors gpr43 and gpr109a facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Mao G., Li S., Orfila C., Shen X., Zhou S., Linhardt R.J., Ye X., Chen S. Depolymerized rg-i-enriched pectin from citrus segment membranes modulates gut microbiota, increases scfa production, and promotes the growth of bifidobacterium spp., lactobacillus spp. and faecalibaculum spp. Food Funct. 2019;10:7828–7843. doi: 10.1039/c9fo01534e. [DOI] [PubMed] [Google Scholar]
- Márquez-Aguirre A.L., Camacho-Ruiz R.M., Arriaga-Alba M., Padilla-Camberos E., Kirchmayr M.R., Blasco J.L., González-Avila M. Effects of agave tequilana fructans with different degree of polymerization profiles on the body weight, blood lipids and count of fecal lactobacilli/bifidobacteria in obese mice. Food Funct. 2013;4:1237–1244. doi: 10.1039/c3fo60083a. [DOI] [PubMed] [Google Scholar]
- Martínez I., Kim J., Duffy P.R., Schlegel V.L., Walter J Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace Raso G., Simeoli R., Russo R., Iacono A., Santoro A., Paciello O., Ferrante M.C., Canani R.B., Calignano A. Meli, R Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRorie J.W., Jr. Psyllium is not fermented in the human gut. Neuro Gastroenterol Motil. 2015;27:1681–1682. doi: 10.1111/nmo.12649. [DOI] [PubMed] [Google Scholar]
- Meijer K., de Vos P., Priebe M.G. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care. 2010;13:715–721. doi: 10.1097/MCO.0b013e32833eebe5. [DOI] [PubMed] [Google Scholar]
- Mendis M., Leclerc E., Simsek S. Arabinoxylans, gut microbiota and immunity. Carbohydr Polym. 2016;139:159–166. doi: 10.1016/j.carbpol.2015.11.068. [DOI] [PubMed] [Google Scholar]
- Montagne L., Loisel F., Le Naou T., Gondret F., Gilbert H., Le Gall M Difference in short-term responses to a high-fiber diet in pigs divergently selected for residual feed intake. J Anim Sci. 2014;92:1512–1523. doi: 10.2527/jas.2013-6623. [DOI] [PubMed] [Google Scholar]
- Montagne L., Pluske J.R., Hampson D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003;108:95–117. doi: 10.1016/S0377-8401(03)00163-9. [DOI] [Google Scholar]
- Mosnier E., Etienne M., Ramaekers P., Père M.C. The metabolic status during the peri partum period affects the voluntary feed intake and the metabolism of the lactating multiparous sow. Livest Sci. 2010;127:127–136. doi: 10.1016/j.livsci.2009.06.023. [DOI] [Google Scholar]
- Mroz Z., Partridge I.G., Mitchell G., Keal H.D. The effect of oat hulls, added to the basal ration for pregnant sows, on reproductive performance, apparent digestibility, rate of passage and plasma parameters. J Sci Food Agric. 1986;37:239–247. doi: 10.1002/jsfa.2740370308. [DOI] [Google Scholar]
- Mu Q., Kirby J., Reilly C.M., Luo X.M. Leaky gut as a danger signal for autoimmune diseases. Front Immunol. 2017;8:598. doi: 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A., Yamada K., Iwata O., Sugimoto R., Atsuji K., Ogawa T., Ishibashi-Ohgo N., Suzuki K. Β-glucan in foods and its physiological functions. J Nutr Sci Vitaminol. 2018;64:8–17. doi: 10.3177/jnsv.64.8. [DOI] [PubMed] [Google Scholar]
- Nepelska M., Cultrone A., Béguet-Crespel F., Le Roux K., Doré J., Arulampalam V., Blottière H. M Butyrate produced by commensal bacteria potentiates phorbol esters induced ap-1 response in human intestinal epithelial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady J., O'Connor E.M., Shanahan F. Review article: dietary fibre in the era of microbiome science. Aliment Pharmacol Therap. 2019;49:506–515. doi: 10.1111/apt.15129. [DOI] [PubMed] [Google Scholar]
- Oliviero C., Kokkonen T., Heinonen M., Sankari S., Peltoniemi O. Feeding sows with high fibre diet around farrowing and early lactation: impact on intestinal activity, energy balance related parameters and litter performance. Res Vet Sci. 2009;86:314–319. doi: 10.1016/j.rvsc.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short chain fatty acids (scfas)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paßlack N., Vahjen W., Zentek J. Dietary inulin affects the intestinal microbiota in sows and their suckling piglets. BMC Vet Res. 2015;11:51. doi: 10.1186/s12917-015-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastuszewska B., Kowalczyk J., Ochtabińska A. Dietary carbohydrates affect caecal fermentation and modify nitrogen excretion patterns in rats. I. Studies with protein-free diets. Archiv fur Tierernahrung. 2000;53:207–225. doi: 10.1080/17450390009381948. [DOI] [PubMed] [Google Scholar]
- Perfetti R., Hui H. The role of glp-1 in the life and death of pancreatic beta cells. Harmone Matabol Res. 2004;36:804–810. doi: 10.1055/s-2004-826167. [DOI] [PubMed] [Google Scholar]
- Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- Pistollato F., Sumalla Cano S., Elio I., Masias Vergara M., Giampieri F., Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of alzheimer disease. Nutr Rev. 2016;74:624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- Ponnusamy V.K., Nguyen D.D., Dharmaraja J., Shobana S., Banu J.R., Saratale R.G., Chang S.W., Kumar G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour Technol. 2019;271:462–472. doi: 10.1016/j.biortech.2018.09.070. [DOI] [PubMed] [Google Scholar]
- Quesnel H., Meunier-Salaün M.C., Hamard A., Guillemet R., Etienne M., Farmer C., Dourmad J.Y., Père M.C. Dietary fiber for pregnant sows: influence on sow physiology and performance during lactation. J Anim Sci. 2009;87:532–543. doi: 10.2527/jas.2008-1231. [DOI] [PubMed] [Google Scholar]
- Ragsdale S.W., Pierce E. Acetogenesis and the wood-ljungdahl pathway of co(2) fixation. Biochim Biophys Acta. 2008;1784:1873–1898. doi: 10.1016/j.bbapap.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Farias C., Slezak K., Fuller Z., Duncan A., Holtrop G., Louis P. Effect of inulin on the human gut microbiota: stimulation of bifidobacterium adolescentis and faecalibacterium prausnitzii. Br J Nutr. 2009;101:541–550. doi: 10.1017/s0007114508019880. [DOI] [PubMed] [Google Scholar]
- Reed S., Neuman H., Moscovich S., Glahn R.P., Koren O., Tako E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients. 2015;7:9768–9784. doi: 10.3390/nu7125497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendón-Huerta J.A., Juárez-Flores B., Pinos-Rodríguez J.M., Aguirre-Rivera J.R., Delgado-Portales R.E. Effects of different sources of fructans on body weight, blood metabolites and fecal bacteria in normal and obese non-diabetic and diabetic rats. Plant Foods Hum Nutr. 2012;67:64–70. doi: 10.1007/s11130-011-0266-9. [DOI] [PubMed] [Google Scholar]
- Renteria-Flores J.A., Johnston L.J., Shurson G.C., Moser R.L., Webel S.K. Effect of soluble and insoluble dietary fiber on embryo survival and sow performance. J Anim Sci. 2008;86:2576–2584. doi: 10.2527/jas.2007-0376. [DOI] [PubMed] [Google Scholar]
- Rinninella E., Cintoni M., Raoul P., Lopetuso L.R., Scaldaferri F., Pulcini G., Miggiano G.A.D., Gasbarrini A., Mele M.C. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11 doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid M., Gibson G.R., Hoyles L., McCartney A.L., Rastall R., Rowland I., Wolvers D., Watzl B., Szajewska H., Stahl B., et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1–S63. doi: 10.1017/s0007114510003363. [DOI] [PubMed] [Google Scholar]
- Roussel C., Sivignon A., de Vallée A., Garrait G., Denis S., Tsilia V., Ballet N., Vandekerckove P., Van de Wiele T., Barnich N., et al. Anti-infectious properties of the probiotic saccharomyces cerevisiae cncm i-3856 on enterotoxigenic e. Coli (etec) strain h10407. Appl Microbiol Biotechnol. 2018;102:6175–6189. doi: 10.1007/s00253-018-9053-y. [DOI] [PubMed] [Google Scholar]
- Ryan S.M., Fitzgerald G.F., van Sinderen D. Screening for and identification of starch-, amylopectin-, and pullulan-degrading activities in bifidobacterial strains. Appl Environ Microbiol. 2006;72:5289–5296. doi: 10.1128/aem.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K., Hammer R.E., Williams S.C., Crowley J., Yanagisawa M., et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding g protein-coupled receptor, gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder B.O., Birchenough G.M.H., Ståhlman M., Arike L., Johansson M.E.V., Hansson G.C., Bäckhed F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe. 2018;23:27–40.e7. doi: 10.1016/j.chom.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K.P., Martin J.C., Campbell G., Mayer C.D., Flint H.J. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “roseburia inulinivorans”. J Bacteriol. 2006;188:4340–4349. doi: 10.1128/jb.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah B.R., Li B., Al Sabbah H., Xu W., Mráz J. Effects of prebiotic dietary fibers and probiotics on human health: with special focus on recent advancement in their encapsulated formulations. Trends Food Sci Technol. 2020;102:178–192. doi: 10.1016/j.tifs.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M., Gentile M., Yeiser J.R., Walland A.C., Bornstein V.U., Chen K., He B., Cassis L., Bigas A., Cols M., et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013;342:447–453. doi: 10.1126/science.1237910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R.L., Dang X.Y., Dong J.L., Hu X.Z. Effects of oat β-glucan and barley β-glucan on fecal characteristics, intestinal microflora, and intestinal bacterial metabolites in rats. J Agric Food Chem. 2012;60:11301–11308. doi: 10.1021/jf302824h. [DOI] [PubMed] [Google Scholar]
- Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H., et al. Activation of gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Thangaraju M., Prasad P.D., Martin P.M., Lambert N.A., Boettger T., Offermanns S., Ganapathy V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285:27601–27608. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.P. Glycan utilisation system in bacteroides and bifidobacteria and their roles in gut stability and health. Appl Microbiol Biotechnol. 2019;103:7287–7315. doi: 10.1007/s00253-019-10012-z. [DOI] [PubMed] [Google Scholar]
- Song X., Zhong L., Lyu N., Liu F., Li B., Hao Y., Xue Y., Li J., Feng Y., Ma Y., et al. Inulin can alleviate metabolism disorders in ob/ob mice by partially restoring leptin-related pathways mediated by gut microbiota. Dev Reprod Biol. 2019;17:64–75. doi: 10.1016/j.gpb.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg E.D., Sonnenburg J.L. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metabol. 2014;20:779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg J.L., Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen A.M., Champ M.M., Cloran S.J., Fleith M., van Lieshout L., Mejborn H., Burley V. J Dietary fibre in europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev. 2017;30:149–190. doi: 10.1017/s095442241700004x. [DOI] [PubMed] [Google Scholar]
- Talchai C., Xuan S., Lin H.V., Sussel L., Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.Q., Sun H.Q., Wei H.K., Tan J.J., Long G., Jiang S.W., Peng J Effects of soluble fiber inclusion in gestation diets with varying fermentation characteristics on lactational feed intake of sows over two successive parities. Animal. 2018;12:1388–1395. doi: 10.1017/s1751731117003019. [DOI] [PubMed] [Google Scholar]
- Tandon D., Haque M.M., Gote M., Jain M., Bhaduri A., Dubey A.K., Mande S.S. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (fos) on human gut microflora. Sci Rep. 2019;9:5473. doi: 10.1038/s41598-019-41837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe H., Otomo Y., Kaji I., Tanaka R., Karaki S.I., Kuwahara A. Roles of short-chain fatty acids receptors, gpr41 and gpr43 on colonic functions. J Physiol Pharmacol. 2008;59(Suppl 2):251–262. [PubMed] [Google Scholar]
- Thangaraju M., Cresci G.A., Liu K., Ananth S., Gnanaprakasam J.P., Browning D.D., Mellinger J.D., Smith S.B., Digby G.J., Lambert N.A., et al. Gpr109a is a g-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.Can-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuwissen E., Mensink R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol Behav. 2008;94:285–292. doi: 10.1016/j.physbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the g-protein-coupled receptor ffar2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaro-Duchesneau C., Saha S., Malhotra M., Coussa-Charley M., Kahouli I., Jones M.L., Labbé A., Prakash S. Probiotic ferulic acid esterase active lactobacillus fermentum ncimb 5221 apa microcapsules for oral delivery: preparation and in vitro characterization. Pharmaceuticals. 2012;5:236–248. doi: 10.3390/ph5020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T., Iwasaki Y., Nakayama K., Ushida K. Stimulation of butyrate production in the large intestine of weaning piglets by dietary fructooligosaccharides and its influence on the histological variables of the large intestinal mucosa. J Nutr Sci Vitaminol. 2003;49:414–421. doi: 10.3177/jnsv.49.414. [DOI] [PubMed] [Google Scholar]
- Veum T.L., Crenshaw J.D., Crenshaw T.D., Cromwell G.L., Easter R.A., Ewan R.C., Nelssen J.L., Miller E.R., Pettigrew J.E., Ellersieck M.R. The addition of ground wheat straw as a fiber source in the gestation diet of sows and the effect on sow and litter performance for three successive parities. J Anim Sci. 2009;87:1003–1012. doi: 10.2527/jas.2008-1119. [DOI] [PubMed] [Google Scholar]
- Vinolo M.A., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell I.S., Orfila C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: from epidemiological evidence to potential molecular mechanisms. Crit Rev Food Sci Nutr. 2022:1–16. doi: 10.1080/10408398.2022.2061909. [DOI] [PubMed] [Google Scholar]
- Wang J.F., Li D.F., Jensen B.B., Jakobsen K., Xing J.J., Gong L.M., Zhu Y.H. Effect of type and level of fibre on gastric microbial activity and short-chain fatty acid concentrations in gestating sows. Anim Feed Sci Technol. 2003;104:95–110. doi: 10.1016/S0377-8401(02)00333-4. [DOI] [Google Scholar]
- Wang L.F., Beltranena E., Zijlstra R.T. Diet nutrient digestibility and growth performance of weaned pigs fed sugar beet pulp. Anim Feed Sci Technol. 2016;211:145–152. doi: 10.1016/j.anifeedsci.2015.11.005. [DOI] [Google Scholar]
- Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver A.C., Kelly J.M., Kind K.L., Gatford K.L., Kennaway D.J., Herde P.J., van Wettere W.H. Oocyte maturation and embryo survival in nulliparous female pigs (gilts) is improved by feeding a lupin-based high-fibre diet. Reprod Fertil Dev. 2013;25:1216–1223. doi: 10.1071/rd12329. [DOI] [PubMed] [Google Scholar]
- Wei Y., Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-i: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- Whisner C.M., Martin B.R., Schoterman M.H., Nakatsu C.H., McCabe L.D., McCabe G.P., Wastney M.E., van den Heuvel E.G., Weaver C.M. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Br J Nutr. 2013;110:1292–1303. doi: 10.1017/s000711451300055x. [DOI] [PubMed] [Google Scholar]
- Willemsen L.E., Koetsier M.A., van Deventer S.J., van Tol E.A. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin e(1) and e(2) production by intestinal myofibroblasts. Gut. 2003;52:1442–1447. doi: 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windey K., De Preter V., Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012;56:184–196. doi: 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- Wrzosek L., Miquel S., Noordine M.L., Bouet S., Joncquel Chevalier-Curt M., Robert V., Philippe C., Bridonneau C., Cherbuy C., Robbe-Masselot C., et al. Bacteroides thetaiotaomicron and faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013;11:61. doi: 10.1186/1741-7007-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Xiong Y., Zhong M., Li Y., Wan H., Wu D., Liu Q Effects of purified fibre-mixture supplementation of gestation diet on gut microbiota, immunity and reproductive performance of sows. J Anim Physiol Anim Nutr. 2020;104:1144–1154. doi: 10.1111/jpn.13287. [DOI] [PubMed] [Google Scholar]
- Xu C., Cheng C., Zhang X., Peng J Inclusion of soluble fiber in the gestation diet changes the gut microbiota, affects plasma propionate and odd-chain fatty acids levels, and improves insulin sensitivity in sows. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21020635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Bai M., Liu H., Duan Y., Zhou X., Wu X., Liao P., Li T., Yin Y. Gut microbiota and blood metabolomics in weaning multiparous sows: associations with oestrous. J Anim Physiol Anim Nutr. 2020;104:1155–1168. doi: 10.1111/jpn.13296. [DOI] [PubMed] [Google Scholar]
- Yang J., Rose D. J Long-term dietary pattern of fecal donor correlates with butyrate production and markers of protein fermentation during in vitro fecal fermentation. Nutr Res (NY) 2014;34:749–759. doi: 10.1016/j.nutres.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Yang X.D., Wang L.K., Wu H.Y., Jiao L. Effects of prebiotic galacto-oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut-brain axis. BMC Anesthesiol. 2018;18:177. doi: 10.1186/s12871-018-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Shah B.R., Li J., Liang H., Zhan F., Geng F., Li B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci Technol. 2022;124:237–249. doi: 10.1016/j.tifs.2022.04.010. [DOI] [Google Scholar]
- Zhao J., Liu P., Wu Y., Guo P., Liu L., Ma N., Levesque C., Chen Y., Zhao J., Zhang J., et al. Dietary fiber increases butyrate-producing bacteria and improves the growth performance of weaned piglets. J Agric Food Chem. 2018;66:7995–8004. doi: 10.1021/acs.jafc.8b02545. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen F., Wu W., Sun M., Bilotta A.J., Yao S., Xiao Y., Huang X., Eaves-Pyles T.D., Golovko G., et al. Gpr43 mediates microbiota metabolite scfa regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mtor and stat3. Mucosal Immunol. 2018;11:752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Zhao Y., Zhang P., Li Y., Gui T., Wang J., Jin C., Che L., Li J., Lin Y., et al. Microbial mechanistic insight into the role of inulin in improving maternal health in a pregnant sow model. Front Microbiol. 2017;8:2242. doi: 10.3389/fmicb.2017.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Chassaing B., Singh V., Pellizzon M., Ricci M., Fythe M.D., Kumar M.V., Gewirtz A.T. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring il-22-mediated colonic health. Cell Host Microbe. 2018;23:41–53.e4. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]