Cervical cancer is the most common malignancy of the reproductive system and is a serious threat to a woman’s health. In China, cervical cancer ranks after breast cancer, colon cancer, and lung cancer in terms of incidence and has become the fourth leading cause of cancer-related deaths among women [1]. Although most patients can benefit from surgery and radiotherapy in the early stage of cervical cancer, the prognosis of patients with advanced or recurrent disease is poor [ 2, 3] . Furthermore, the incidence rate of cervical cancer increases annually, and there is a lack of targeted drugs [4]. Therefore, it is of utmost importance to elucidate the molecular mechanisms underlying the pathogenesis of cervical cancer for the development of novel targeted and adjuvant drugs.
Curcumin is a polyphenol extracted from the rhizome of Curcumaceae plants and has significant antitumour, anti-inflammatory, and immunomodulatory effects [5]. However, due to its low water solubility, poor absorption in vivo and low bioavailability, the clinical application of curcumin is greatly limited [ 5, 6] . Dimethoxycurcumin (DMC) is a natural derivative of curcumin that lacks the methoxy group combined with benzene and shows greater water solubility and bioavailability [7]. In this study, the effect of DMC on cervical cancer progression was evaluated, and its potential molecular mechanism was explored.
Peroxisome proliferator activated receptor γ (PPARγ) is a ligand-activated transcription factor that regulates genes involved in metabolism, inflammation, and tumorigenesis [8]. In a previous study, DMC was shown to upregulate PPARγ and induce autophagy and subsequently inhibit lipopolysaccharide (LPS)-induced activation of the NF-κB pathway and NLRP3 inflammasome [9]. Given the role of PPARγ in tumorigenesis, we hypothesized that DMC may abrogate the progression of cervical cancer by inducing PPARγ. To this end, 6-week-old female BALB/c nude mice (Shanghai Experimental Animal Center, Shanghai, China) were injected subcutaneously with 5×10 6 HeLa cells (Cell Resource Center, IBMS, CAMS&PUMC, Beijing, China) and treated (i.p.) with 50 mg/kg dimethoxycurcumin (DMC; Sigma, St Louis, USA). The expression of PPARγ in HeLa xenografts resected from untreated and DMC-treated nude mice was first determined by immunohistochemical staining. As shown in Figure 1A,B, DMC significantly increased the expression of PPARγ protein in tumor tissues. To further evaluate the role of PPARγ in the antitumour effects of DMC, tumor-bearing mice were treated with the PPARγ antagonist T0070907. As shown in Figure 1C,D, tumors in the DMC group were smaller than those in the control group, and the difference in size increased until the endpoint of 30 days. However, the therapeutic effects of DMC were neutralized by treatment (i.p.) with T0070907 (1 mg/kg; MedChem Express, Monmouth Junction, USA), which indicated that the antitumour effects of DMC were mediated, at least in part, by PPARγ.
Figure 1 .
Role of PPARγ in dimethoxycurcumin-regulated growth of cervical cancer xenografts
Six-week-old female nude mice (BALB/c) were injected subcutaneously with 5×10 6 HeLa cells and treated with dimethoxycurcumin (DMC; 50 mg/kg, i.p.) or the vehicle (50 μL PBS, i.p.) with or without T0070907 (1 mg/kg, i.p.) every 2 days for 30 days. (A) Representative immunohistochemistry (IHC) images for PPARγ-staining. Scale bar: 20 μm. (B) Percentage of PPARγ-positive cells. (C) Images of tumors dissected on day 30. (D) Change in tumor volume in the indicated groups. Data are presented as the mean±SD ( n=6 mice/group). * P<0.05 vs the control group; #P<0.05 vs the DMC group. i.p., intraperitoneally.
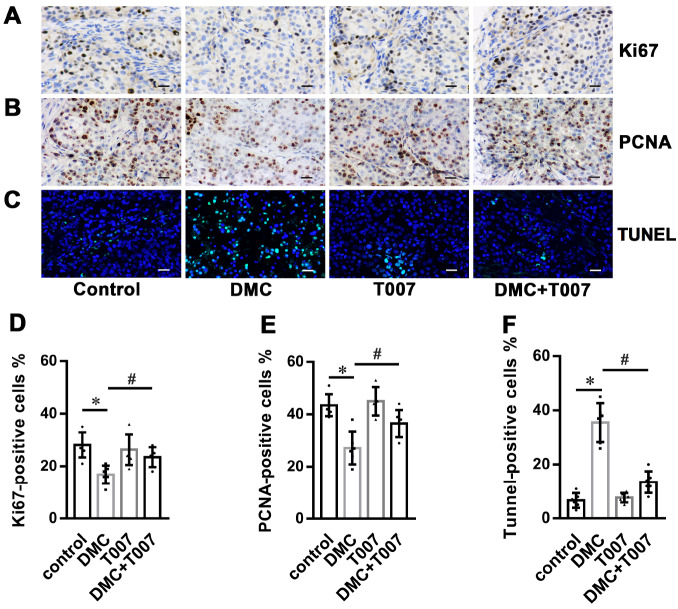
PPARγ and its ligands inhibit the proliferation of tumor cells and promote apoptosis [10]. Therefore, the effects of DMC on the proliferation and apoptosis rates of transplanted cervical cancer cells were further investigated by immunostaining and TUNEL assays, respectively. The percentages of proliferating cells expressing Ki67 and PCNA were significantly reduced in the DMC group compared to that in the control group, and this difference was partly offset by treatment with T0070907 ( Figure 2A,B,D,E). Furthermore, DMC treatment increased the proportion of TUNEL-positive apoptotic cells in the xenografts, while treatment with T0070907 partly rescued tumor cells from apoptosis ( Figure 2C,F). Taken together, DMC inhibited the proliferation of HeLa cells in vivo and promoted cell apoptosis by upregulating PPARγ.
Figure 2 .
Role of PPARγ in dimethoxycurcumin-regulated proliferation and apoptosis of cervical cancer xenografts
Six-week-old female nude mice (BALB/c) were injected subcutaneously with 5×10 6 HeLa cells and treated with dimethoxycurcumin (DMC; 50 mg/kg, i.p.) or the vehicle (50 μL PBS, i.p.) with or without T0070907 (1 mg/kg, i.p.) every 2 days for 30 days. (A,B) Representative immunohistochemistry (IHC) images for Ki67 and PCNA. (C) Representative images of TUNEL staining. Scale bar: 20 μm. (D) Percentage of TUNEL-positive cells. (E) Percentage of Ki67- and PCNA-positive cells. Scale bar: 20 μm. Data are presented as the mean±SD ( n=6 mice/group). * P<0.05 vs the control group; #P<0.05 vs the DMC group. i.p., intraperitoneally.
Consistent with the in vivo results, DMC decreased the viability of HeLa cells within 24 h in a dose-dependent manner, and the half-maximal inhibitory concentration (IC 50) was approximately 32.49 μM ( Supplementary Figure S1A). Moreover, DMC increased the expression of PPARγ in HeLa cells ( Supplementary Figure S1B). To further investigate the role of PPARγ, HeLa cells were transfected with PPARγ siRNA (sc-156077; Santa Cruz, Santa Cruz, USA) to downregulate the expression of PPARγ, and cells transfected with si-control (sc-36869; Santa Cruz) were used as the control. As shown in Supplementary Figure S1C, PPARγ knockdown significantly decreased its protein expression. Furthermore, transfection with PPARγ siRNA significantly increased the viability ( Supplementary Figure S1D) and proliferation rates and inhibited apoptosis of DMC-treated HeLa cells ( Supplementary Figure S1E,F). Taken together, these data further confirm that PPARγ mediates the effects of DMC on cervical cancer cells.
In conclusion, DMC inhibited the growth of cervical cancer cells by upregulating PPARγ. These findings provide a potential novel strategy for adjuvant therapy of cervical cancer.
Supporting information
Supplementary Data
Supplementary data is available at Acta Biochimica et Biophysica Sinica online.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the Hunan Provincial Natural Science Foundation of China (No. 2017JJ3146) and the Scientific Research Project of Hunan Province Chinese Medicine (No. D2022103).
References
- 1.Zhao F, Qiao Y. Cervical cancer prevention in China: a key to cancer control. Lancet. . 2019;393:969–970. doi: 10.1016/S0140-6736(18)32849-6. [DOI] [PubMed] [Google Scholar]
- 2.Marnitz S, Tsunoda AT, Martus P, Vieira M, Affonso Junior RJ, Nunes J, Budach V, et al. Surgical versus clinical staging prior to primary chemoradiation in patients with cervical cancer FIGO stages IIB–IVA: oncologic results of a prospective randomized international multicenter (Uterus-11) intergroup study. Int J Gynecol Cancer. . 2020;30:1855–1861. doi: 10.1136/ijgc-2020-001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Hu Q, Ye S, Xiang L. Inhibition of the PIN1-NRF2/GPX4 axis imparts sensitivity to cisplatin in cervical cancer cells. Acta Biochim Biophys Sin. . 2022;54:1325–1335. doi: 10.3724/abbs.2022109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Guo E, Yang B, Xiao R, Lu F, You L, Chen G. Trends and age-period-cohort effects on mortality of the three major gynecologic cancers in China from 1990 to 2019: cervical, ovarian and uterine cancer. Gynecol Oncol. . 2021;163:358–363. doi: 10.1016/j.ygyno.2021.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem. . 2017;60:1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. . 2019;24:2930. doi: 10.3390/molecules24162930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teymouri M, Barati N, Pirro M, Sahebkar A. Biological and pharmacological evaluation of dimethoxycurcumin: a metabolically stable curcumin analogue with a promising therapeutic potential. J Cell Physiol. . 2018;233:124–140. doi: 10.1002/jcp.25749. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Quiles M, Broekema MF, Kalkhoven E. PPARgamma in metabolism, immunity, and cancer: unified and diverse mechanisms of action. Front Endocrinol. . 2021;12:624112. doi: 10.3389/fendo.2021.624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang J, Tan X, Huang X, Zhang J, Chen L, Li A, Wang D. Dual targeting of autophagy and NF-κB pathway by PPARγ contributes to the inhibitory effect of demethoxycurcumin on NLRP3 inflammasome priming. Curr Mol Pharmacol. . 2021;14:914–921. doi: 10.2174/1874467214666210301121020. [DOI] [PubMed] [Google Scholar]
- 10.Wu K, Yang Y, Liu D, Qi Y, Zhang C, Zhao J, Zhao S. Activation of PPARγ suppresses proliferation and induces apoptosis of esophageal cancer cells by inhibiting TLR4-dependent MAPK pathway. Oncotarget. . 2016;7:44572–44582. doi: 10.18632/oncotarget.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.