Abstract
Argonaute (Ago) proteins are conserved programmable nucleases present in eukaryotes and prokaryotes and provide defense against mobile genetic elements. Almost all characterized pAgos prefer to cleave DNA targets. Here, we describe a novel pAgo from Verrucomicrobia bacterium (VbAgo) that can specifically cleave RNA targets rather than DNA targets at 37°C and function as a multiple-turnover enzyme showing prominent catalytic capacity. VbAgo utilizes DNA guides (gDNAs) to cleave RNA targets at the canonical cleavage site. Meanwhile, the cleavage activity is remarkably strengthened at low concentrations of NaCl. In addition, VbAgo presents a weak tolerance for mismatches between gDNAs and RNA targets, and single-nucleotide mismatches at positions 11‒12 and dinucleotide mismatches at positions 3‒15 dramatically reduce target cleavage. Moreover, VbAgo can efficiently cleave highly structured RNA targets at 37°C. These properties of VbAgo broaden our understanding of Ago proteins and expand the pAgo-based RNA manipulation toolbox.
Keywords: RNA target specificity, Verrucomicrobia bacterium , prokaryotic argonaute, programmable nucleases
Introduction
Eukaryotic Argonautes (eAgos) are key players in the RNA-induced silencing complex (RISC), which uses small RNA as a guide to recognize and cleave complementary nucleic acid targets [1]. eAgos participate in gene regulation, protect their host from invading RNA viruses and preserve genome integrity [2]. Although recent studies have reported that some prokaryotic Agos (pAgos) function in DNA replication and protect cells from foreign genetic elements in vivo[ 3– 7] , the cellular functions of pAgos remain poorly understood.
Genomic studies have shown that the diversity of pAgos is much greater than that of eAgos [8] and the family of pAgos can be classified into long pAgos, short pAgos and PIWI-RE proteins [9]. The conserved six-domain architecture of most pAgos is similar to that of eAgos, both of which contain N-terminal (N), linker 1 (L1), middle (MID), linker 2 (L2), P-element induced wimpy testis (PIWI), and PIWI-Ago-Zwille (PAZ) domains [5]. Agos with a complete catalytic tetrad DEDX (X is D, H, or N) in the PIWI domain can exert endonuclease activity [ 10, 11] . Bound with small nucleic acid guides, active Agos can cleave complementary targets at a site between the 10 th and 11 th nucleotides starting from the 5′-end of the guide [ 3, 5, 12– 15] .
Although eAgos exclusively cleave RNA targets, pAgos show more diversity in binding guides and cleaving targets. pAgos can utilize either DNA guides or RNA guides to cleave DNA targets or RNA targets, such as DNA-guided DNA target cleavage (gD-tD), DNA-guided RNA target cleavage (gD-tR), RNA-guided DNA target cleavage (gR-tD) and RNA-guided RNA target cleavage (gR-tR). Most characterized pAgos show a preference for DNA target cleavage when loaded with DNA or RNA guide. Early studies of pAgos from thermophilic prokaryotes were DNA- or RNA-guided nucleases that target DNA at elevated temperatures, such as TtAgo ( Thermus thermophilus) [16], PfAgo ( Pyrococcus furiosus) [17] and MjAgo ( Methanocaldococcus jannaschii) [ 18, 19] . Subsequent studies focused on pAgos from mesophilic prokaryotes, including KmAgo ( Kurthia massiliensis) [ 13, 20] , CbAgo ( Clostridium butyricum) [ 21, 22] , LrAgo ( Limnothrix rosea) [21], RsuAgo ( Rummeliibacillus suwonensis) [14], CpAgo ( Clostridium perfringens) and IbAgo ( Intestinibacter bartlettii) [23]. Some of these (such as KmAgo, CbAgo, RsuAgo and CpAgo) can cleave both DNA targets and RNA targets, but they all prefer DNA targets. More recently, pAgos that prefer RNA targets were reported, including MbpAgo [15] ( Mucilaginibacter paludis), PliAgo ( Pseudooceanicola lipolyticus), HpeAgo ( Hydrobacter penzbergensis), PnyAgo ( Pedobacter nyackensis) and RslAgo ( Runella slithyformis) [24]. Compared to characterized pAgos, these RNA-targeting pAgos not only have different targets but also show some other differences. For example, in the MID domain of MbpAgo, HpeAgo and PnyAgo, the conserved amino acid used to bind the guide terminal nucleotide is the HK-motif, while the conserved amino acid of DNA-targeting pAgos is mainly the YK-motif. In addition, the MID pocket contains an unconventional RHSY motif and KHTY motif in PliAgo and RslAgo, respectively, and they cleave RNA targets at a site between the 9 th and 10 th nucleotides starting from the 5′-end of the guide. The mechanisms and functions of RNA-targeting pAgos are only beginning to be explored, and excavating novel RNA-targeting pAgos will improve our understanding of pAgos and may lead to the development of a series of pAgos-based RNA manipulation tools.
To search for novel RNA-targeting pAgos, we selected 50 kinds of candidate pAgos genes from mesophilic bacteria by the web interface of the BLASTp program with the PIWI domain sequence of KmAgo [13] (WP 010289662.1) as a query and tested their cleavage activity at 37°C. VbAgo (MBK8002123.1; Verrucomicrobia bacterium) identified in metagenomic sequences from activated sludge [25] was the only pAgo that uses gDNAs to specifically cleave RNA targets. We regarded VbAgo as one of the gD-tR pAgos and performed its biochemical analysis. The biochemical characterization of VbAgo broadens our understanding of pAgos and expands the toolkit for RNA manipulations.
Materials and Methods
Protein expression and purification
The VbAgo gene (MBK8002123.1; Verrucomicrobia bacterium) and VbAgo double mutant (VbAgo_DM) (D550A, D617A) gene were codon-optimized for expression in Escherichia coli. The codon-optimized genes were synthesized by Wuhan GeneCreate Biological Engineering (Wuhan, China). and cloned into pET23a expression vectors in the frame with a C-terminal 6× His tag, as shown in Supplementary Figure S1. VbAgo and VbAgo_DM proteins were expressed in E. coli BL21(DE3) (Novagen, Beijing, China). Cultures were grown at 37°C in Luria-Bertani (LB) medium containing 100 μg/mL ampicillin until the OD 600 reached 0.8. VbAgo expression was induced by the addition of isopropyl-β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. Cells were incubated at 18°C for 16 h with continuous shaking for expression. Centrifugally collected cells were stored at –80°C for further protein purification.
The cell pellet was resuspended in Buffer A (20 mM Tris-HCl, pH 7.5, 500 mM NaCl, 10 mM imidazole) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) and disrupted by sonication with SCIENTZ-IID (SCIENTZ, Ningbo, China) at 400 W with 2 s on/ 4 s off for 15 min. The lysate was clarified by centrifugation, and the supernatant was loaded onto Ni-NTA agarose resin for 60 min with rotation. The beads were washed with Buffer A, then with the same buffer containing 50 mM imidazole and eluted with Buffer A containing 100 mM imidazole. The eluted protein was concentrated against Buffer B (20 mM HEPES, pH 7.5, 0.5 M NaCl) by ultrafiltration using an Amicon 50K filter unit (Millipore, Boston, USA). Next, the protein was diluted in 20 mM HEPES (pH 7.5) to lower the final salt concentration to 125 mM NaCl. The diluted protein was applied to a Heparin column (HiTrap Heparin HP; GE Healthcare, Boston, USA) equilibrated with Buffer C (20 mM HEPES, pH 7.5, 125 mM NaCl), washed with at least 10 column volumes of the same buffer and eluted with a linear NaCl gradient (0.125–2 M). Fractions containing pAgos were concentrated by ultrafiltration using the Amicon 50K filter unit. Purified VbAgo was diluted in Buffer D (20 mM HEPES, pH 7.5, 100 mM NaCl), aliquoted and flash-frozen in liquid nitrogen.
Single-stranded nucleic acid cleavage assay
Single-stranded nucleic acid cleavage assays were performed at 37°C with a molar ratio of pAgo:guide:target of 4:2:1 unless otherwise indicated. 5′-FAM-labelled targets and 5′-P guides were synthesized for most experiments. pAgos (800 nM) and guides (400 nM) were mixed in reaction buffer containing 10 mM HEPES-NaOH, pH 7.5, 100 mM NaCl, 5 mM MnCl 2, and 5% glycerol and then incubated at 37°C for 10 min for guide loading, and 200 nM targets were added. The reaction was stopped after the indicated time intervals by mixing the samples with equal volumes of 2× RNA loading dye (95% formamide, 18 mM EDTA, 0.025% SDS, and 0.025% bromophenol blue) and heating for 5 min at 95°C. The cleavage products were separated by 20% denaturing polyacrylamide gel electrophoresis (PAGE) and stained with SYBR Gold (Invitrogen, Carlsbad, USA). To analyse the effects of the concentration of NaCl, the concentration of NaCl varied from 50 to 1250 mM. To test the effects of pH, the pH of the reactions varied from 7.0 to 8.0. To test the temperature dependence of RNA cleavage, VbAgo was mixed with gDNA at 37°C for 10 min, and then RNA targets were added to a final concentration of 200 nM. The samples were incubated at the indicated temperature in T100 PCR thermocycler (Bio-Rad, Hercules, USA) for 1 h. Follow-up assays were carried out at 50°C for 15 min if not indicated.
Kinetic analyses of RNA cleavage were carried out under single- or multiple-turnover conditions using methods described in literature [ 15, 21, 22] . In single-turnover reactions, the VbAgo/guide ratio was 1:1, and the [VbAgo–gDNA]/RNA target ratio was ≥ 1. The data were fitted to the single-exponential equation: C= C max×[1 – exp(– k obs× t)], where C is the cleavage efficiency at a given time point, C max is the maximum cleavage, and k obs is the observed rate constant. In multiple-turnover reactions, the VbAgo/guide ratio was 1:1 and the [VbAgo–gDNA]/RNA target ratio was <1, and the linear parts of the reaction kinetics were used to calculate the steady-state velocities of RNA cleavage. All the nucleic acids used in cleavage assays are listed in Supplementary Table S1.
Preparation of HIV-1 ΔDIS 5′UTR transcript and its cleavage assays
Dayeh et al. [26] demonstrated that the HIV-1 ΔDIS 5′UTR is a highly structured RNA. The HIV-1 ΔDIS 5′UTR was transcribed in vitro using T7 RNA polymerase and synthetic DNA templates carrying a T7 promoter sequence. The transcripts used for assays were treated with DNase I and precipitated with ethanol. The guide DNAs (gDNAs) targeting the HIV-1 ΔDIS 5′UTR sequence were 5′-phosphorylated using T4 PNK (New England Biolabs, Ipswitch, USA). Then, 400 nM gDNA and 800 nM VbAgo were mixed and incubated at 37°C for 10 min. Then, 200 ng RNA targets were added to the samples and incubated at 37°C and 50°C for 15 min, respectively. The reactions were stopped by mixing the samples with equal volumes of 2× RNA loading dye and heating at 95°C for 5 min. The cleavage products were resolved by 8% denaturing PAGE and stained with SYBR Gold.
Copurification of nucleic acids
The copurified nucleic acids were isolated with minor modifications according to Hegge et al. [22]. Briefly, 3 mg of purified VbAgo and VbAgo_DM in Buffer D were mixed with 5 mM CaCl 2 and 1 mg/mL proteinase K (Zomanbio, Beijing, China). The mixture was incubated at 50°C for 4 h, and then Roti-phenol/chloroform/isoamyl alcohol (pH 7.5–8.0) at a 25:24:1 ratio was added. The top layer was separated, and nucleic acids were precipitated by adding 99% ethanol at a 1:2 ratio. The sample was incubated at –20°C for 20 h and centrifuged at 10,000 g for 15 min. Finally, the sample was washed with 500 μL of 70% ethanol twice and dissolved in 50 μL nuclease-free water. The purified nucleic acids were treated with either 100 μg/mL RNase A (Thermo Fisher Scientific, Waltham, USA), 2 units DNase I (New England Biolabs), or both for 1 h at 37°C, resolved on 20% denaturing PAGE and stained with SYBR Gold.
Results
VbAgo can specifically cleave RNA targets at physiological temperatures
Phylogenetic tree analysis revealed that VbAgo is correlated with the gD-tR pAgos family ( Figure 1A). The multiple sequence alignment shows the low sequence identity between VbAgo and other characterized pAgos (sequence identity <30%), and among the gD-tR pAgos, VbAgo is closer to MbpAgo (sequence identity: 22.58%) than to other gD-tR pAgos ( Supplementary Figure S2A). The PIWI domain of VbAgo contains a typical catalytic tetrad (D550, E584, D617 and D756; Supplementary Figure S2A), and the MID domain contains a group of conserved amino acid residues (Y484, K488, Q497, T503, N521 and A529; Supplementary Figure S2B) for anchoring the 5′-end of the guide. To study the cleavage activity of VbAgo, the gene expressing VbAgo was chemically synthesized and cloned into the pET23a plasmid. The purified VbAgo and VbAgo_DM were consistent with the predicted molecular weights ( Supplementary Figure S2C). To confirm the nucleic acid specificity of VbAgo, cleavage assays were performed by using synthetic fluorescently labelled oligonucleotide targets ( Figure 1B). VbAgo or VbAgo_DM was incubated with 16 nt gDNA or gRNA containing 5′P or 5′OH at 37°C for 10 min in buffer containing 5 mM Mn 2+, and then the complementary 5′-FAM-labelled 45 nt-long ssDNA or RNA targets were added to a final concentration of 200 nM. The sample was incubated at 37°C for 1 h; subsequently, the cleavage products were resolved by 20% denaturing gel electrophoresis ( Figure 1C,D). Unlike the majority of studied pAgos, which notably prefer to cleave DNA targets [ 13, 14, 21, 23] , VbAgo can exclusively cleave RNA targets.
Figure 1 .
VbAgo exhibits DNA-guided RNA cleavage activity at 37°C
(A) Maximum likelihood phylogenetic tree analysis of VbAgo based on amino acid sequences. The numbers at the nodes indicate the bootstrap values for maximum likelihood analysis of 1000 resampled data sets. (B) The sequence of the synthetic guide and target. gDNAs and RNA targets (T-RNA) were used in most experiments. The black triangle indicates the cleavage site. (C) VbAgo exhibits DNA-guided RNA cleavage activity. (D) VbAgo exhibits no DNA cleavage activity. Positions of T-RNA and products (P) are indicated on the left of the gels. VbAgo, guide and target were mixed at a 4:2:1 molar ratio (800 nM VbAgo preloaded with 400 nM guide, plus 200 nM target) and incubated at 37°C for 60 min. Catalytic mutant VbAgo_DM was used as a control. Lanes M1 and M2 contain chemically synthesized 5′-end, FAM-labelled, 34 nt RNA and DNA corresponding to the cleavage products of target RNA and target DNA (T-DNA), respectively.
To demonstrate the cleavage site of VbAgo, we synthesized FAM-labelled 33 nt,34 nt and 35 nt ssRNA as markers. The expected lengths of the products were 34 nt, and the RNA targets were cleaved at a single site between the 10 th and 11 th nucleotides starting from the guide 5′-end ( Supplementary Figure S3A), as expected. The cleavage activity of pAgo is dependent on the complete catalytic DEDX tetrad in the PIWI domain, and VbAgo_DM eliminated the cleavage activity as expected. In conclusion, VbAgo shows RNA target cleavage specificity at 37°C and can be a potential novel genome editing tool. In addition, the copurified nucleic acids were analysed ( Supplementary Figure S3B). Small DNAs between 15 nt and 18 nt and RNAs of unknown length were observed to be associated with VbAgo and VbAgo_DM. Therefore, we need to analyse the results of copurification nucleic acid sequencing in the future to prove whether the binding of VbAgo to RNA targets is dependent on small gDNAs in E. coli.
Effects of NaCl, pH, temperature and different divalent metal ions on RNA cleavage by VbAgo
Considering that the concentration of NaCl may affect cleavage activity [23], we measured the efficiency of ssRNA cleavage under different reaction conditions. The assays were performed with 5′P-gDNA and complementary RNA targets at 37°C for 1 h, where the final concentration of NaCl varied from 50 mM to 1250 mM. The cleavage activity was remarkably strengthened at low concentrations of NaCl ( Figure 2A). In addition, VbAgo showed cleavage activity at all tested pH values (7.0–8.0) ( Figure 2B). Moreover, the results of the temperature-dependent RNA cleavage activity revealed that VbAgo obtained stronger cleavage activities between 50°C and 60°C when loaded with 5′P-gDNA or 5′OH-gDNA, and the absence of activity with 5′-OH guide DNA at 65°C may indicate that 5′-P guides are better bound under these conditions ( Figure 2C). In conclusion, although VbAgo originates from a mesophilic bacterium, it is not only active at moderate temperatures but also stable enough to cleave RNA at elevated temperatures.
Figure 2 .
Effects of the reaction system on VbAgo cleavage activity
(A) Effects of NaCl concentration on RNA target cleavage activity. (B) Effects of pH value on RNA target cleavage activity. (C) Effects of temperature on RNA target cleavage. (D) Effects of different divalent metal ions on target cleavage. Data are presented as the mean±standard deviation (SD) from three independent experiments. In all experiments, VbAgo, guide and target were mixed at a 4:2:1 molar ratio (800 nM VbAgo preloaded with 400 nM guide, plus 200 nM target). Experiments in (A) and (C) were incubated at 37°C for 60 min, experiments in (B) were incubated at 37°C for 15 min and experiments in (D) were incubated at 50°C for 15 min.
To optimize RNA cleavage activity, subsequent assays were performed in Buffer E containing 10 mM NaCl and 10 mM HEPES-NaOH, pH 7.5 at 50°C. The RNA cleavage activity was dramatically promoted in Buffer E at 50°C, as expected ( Supplementary Figure S4A). In addition, only extremely weak DNA target cleavage was present after 6 h above 45°C ( Supplementary Figure S4B). Although the reaction time was extended to 24 h, DNA target cleavage was not observed at 37°C, which suggested that VbAgo specifically cleaves RNA targets at 37°C and possesses weak DNA targets cleavage activity at elevated temperatures. Thus, we regard it as gD-tR pAgos.
To test the effects of divalent metal ions on VbAgo cleavage activity, assays were performed with different divalent metal ions at 50°C for 15 min ( Figure 2D). The RNA targets were only cleaved in the presence of Mn 2+ or Mg 2+. In addition, titration of Mn 2+ and Mg 2+ ions showed that VbAgo incubated with 5′P-gDNA or 5′OH-gDNA was active at Mn 2+ concentration ≥ 0.05 mM and showed stronger cleavage activity when the Mn 2+ concentration was between 0.5 and 1 mM ( Supplementary Figure S5A). VbAgo was active at Mg 2+ concentration ≥ 0.5 mM. Meanwhile, the most efficient cleavage occurred in the presence of Mg 2+ concentration ≥ 10 mM, which was superior to that of Mn 2+ ( Supplementary Figure S5B). Thus, most experiments were performed with a Mg 2+ concentration of 10 mM.
Effects of guide length on VbAgo activity
It has been reported that the guide length influences cleavage efficiency and results in cleavage site shifting comparable to the canonical cleavage site [20]. Thus, we tested a series of gDNAs from 8 to 44 nt on RNA target cleavage by VbAgo. We found that VbAgo was most active at 15–19 nt for both 5′P-gDNA and 5′OH-gDNA ( Figure 3A,B). With 13 or 14 nt 5′P-gDNA or 5′OH-gDNA, only very weak RNA target cleavage was observed, while RNA target cleavage was not observed with shorter (8–12 nt) 5′P-gDNA or 5′OH-gDNA. Different from MbpAgo [15], the cleavage site was shifted with 5′P-gDNAs longer than 30 nt or 5′OH-gDNAs longer than 25 nt. However, cleavage occurred between the 10 th and 11 th guide positions with 13 to 25 nt 5′P-gDNA, and the same phenomenon was observed with 13 to 21 nt 5′OH-gDNA.
Figure 3 .
Effects of the guide length on RNA target cleavage
(A) Cleavage assays with 5′P-gDNA (upper panel) and 5′OH-gDNA (lower panel) of varying lengths. The positions of RNA targets (T) and products (P) are indicated on the left of the gels. (B) Quantification of cleavage efficiencies by VbAgo (the percentage of target cleavage). The results from three independent experiments were quantified. (C–E) Kinetics analyses of RNA cleavage by VbAgo with 15, 16 and 18 nt gDNAs. The kobs values were analysed from the single-exponential fits of the data. Data are presented as the mean±standard deviation (SD) from three independent experiments. In all experiments, VbAgo, guide and target were mixed at a 4:2:1 molar ratio (800 nM VbAgo preloaded with 400 nM guide, plus 200 nM target) and incubated at 50°C for 15 min.
Due to the effects of gDNA length on RNA target cleavage, we analysed the kinetics of VbAgo-mediated RNA target cleavage with 15 and 18 nt gDNA, which showed a different cleavage efficiency compared to 16 nt gDNA. Under single-turnover conditions, we found that the observed rates ( k obs) of RNA cleavage with 15 nt gDNA were slightly higher than those with 16 nt or 18 nt gDNA ( Figure 3C–E). For the 15 nt gDNA, the k obs value of 5′P-gDNA (0.450± 0.074 min –1) was almost identical to that of 5′OH-gDNA (0.469± 0.028 min –1). For the 16 nt gDNA, the k obs value of 5′P-gDNA (0.371± 0.050 min –1) was nearly equal to that of 5′OH-gDNA (0.323± 0.048 min –1). However, the k obs value of 5′OH-gDNA (0.219±0.038 min –1) was almost two times higher than that of 5′P-gDNA (0.128± 0.028 min –1) for the 18 nt gDNA. Multiple sequence alignment showed that the MID domain of VbAgo contains the YK motif ( Supplementary Figure S2B), which is also found in CbAgo, RsAgo and hAgo2, and directly interacts with the 5′P of the bound guide molecules. In summary, the more appropriate length of the guides is 15 or 16 nt for VbAgo compared to 18 nt. With no obvious distinction, 16 nt gDNAs were adopted in most experiments.
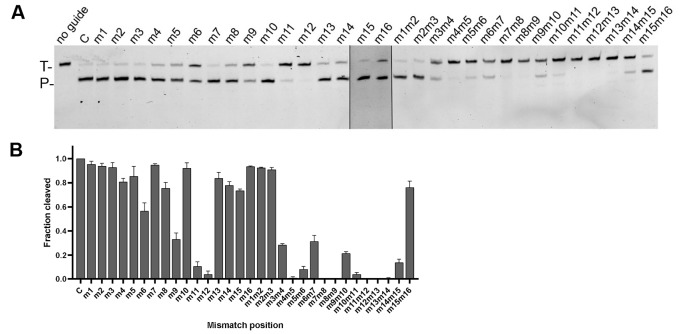
Effects of guide-target mismatches on VbAgo cleavage activity
Previous studies have demonstrated that mismatches between guide and target could affect target cleavage [ 26– 28] . To analyse the mismatch tolerance of VbAgo, we synthesized a series of gDNAs containing single-nucleotide or dinucleotide mismatches at a certain position. For single-nucleotide mismatches, mismatches at positions 6, 9, 11 and 12 caused a sharp drop in cleavage efficiency, especially at position 12, and no dramatic decrease in cleavage efficiency was observed at other mismatch positions ( Figure 4A,B). However, dinucleotide mismatches at positions 3–15 markedly reduced target cleavage, especially at positions 4–5, 7–9 and 11–14, eliminating the cleavage activity. Therefore, VbAgo presents a weak tolerance for mismatches between gDNAs and RNA targets.
Figure 4 .
Effects of the guide-target mismatches on target cleavage
(A) Cleavage assays with single-nucleotide or dinucleotide mismatches at a certain position. The positions of RNA targets (T) and products (P) are indicated on the left of the gels. (B) Quantification of cleavage efficiencies by VbAgo (the percentage of target cleavage). Data are presented as the mean±standard deviation (SD) from three independent experiments. In all experiments, VbAgo, guide and target were mixed at a 4:2:1 molar ratio (800 nM VbAgo preloaded with 400 nM guide, plus 200 nM target) and incubated at 50°C for 15 min.
Effects of the 5′-nucleotide of the guide on target cleavage and VbAgo as a multiple-turnover enzyme
Previous studies showed that the nucleotide residue at the first position of the guide was bound in the MID pocket of Ago [ 19, 29, 30] , and many reported eAgos and pAgos showed a bias for a specific guide 5′-nucleotide [ 13, 14, 31] . To determine whether VbAgo has a preference for the first nucleotide of the guide, four guide variants with different 5′-terminal nucleotides but otherwise identical sequences were synthesized for assays. The k obs value of VbAgo loaded with gDNA containing a 5′-T (0.623± 0.096 min –1), 5′-A (0.523± 0.135 min –1) or 5′-C (0.532± 0.115 min –1) was slightly higher than that containing a 5′-G (0.389± 0.132 min –1) ( Figure 5A). This suggested that VbAgo showed no apparent preference to guide the 5′-nucleotide ( Figure 5B). Therefore, VbAgo can potentially be programmed for specific recognition and cleavage of any RNA target sequence.
Figure 5 .
Effects of the 5′-nucleotide of the guide on target cleavage and VbAgo is a multiple-turnover enzyme
(A) Effects of the 5′-nucleotide of the guide on target cleavage. The assays were performed at 50°C for 15 min. The kobs values were analysed from the single-exponential fits of the data. Data are presented as the mean±standard deviation (SD) from three independent experiments. (B) P values for all comparisons of kobs values from (A). nsP>0.05, compared to the 5′-T guide using Student’s t-test. (C) VbAgo was loaded with 16 nt 5′P-gDNA at 37°C for 60 min. In the turnover experiments, 200 nM RNA target was mixed with increasing concentrations of VbAgo–gDNA (50-800 nM). (D) VbAgo was loaded with 16 nt 5′P-gDNA at 50°C for 60 min. In the turnover experiments, 200 nM RNA target was mixed with increasing concentrations of VbAgo–gDNA (50-800 nM). The numbers in panels C and D are the ratios of VbAgo to RNA targets. Data were fitted to a single-exponential equation if the [VbAgo–gDNA]/RNA target ratio was ≥ 1. The linear parts of the reaction kinetics were used for the calculation of the steady-state velocities of RNA cleavage if the [VbAgo–gDNA]/RNA target ratio was <1. Data are presented as the mean±standard deviation (SD) from three independent experiments.
To further investigate the catalytic properties of VbAgo, the kinetics of RNA cleavage were tested at 37°C and 50°C ( Figure 5C,D).In single-turnover conditions (when the [VbAgo–gDNA]/RNA target ratio was 4), the k obs at 50°C (0.229± 0.019 min ‒1) was higher than that at 37°C (0.093± 0.017 min ‒1). Moreover, in multiple-turnover conditions, no burst phase was observed, but a linear steady state was detected, which is similar to LrAgo [21] and TtAgo [16]. There was no obvious distinction for the steady-state velocity at both 37°C (0.014± 0.003 min ‒1) and 50°C (0.016± 0.004 min ‒1) when the [VbAgo–gDNA]/RNA target ratio was 0.25. The absence of burst might reflect a slower rate of ternary complex formation and/or catalysis by VbAgo compared to MbpAgo [15] and CbAgo [21], suggesting that the product of dissociation was no longer rate-limiting in the steady-state reaction for VbAgo.
VbAgo can cleave highly structured RNA
Considering that VbAgo loaded with 5′P-gDNA or 5′OH-gDNA could cleave RNA targets with high efficiency, we next tested whether the VbAgo-guide complex can cleave target sequences in a highly structured RNA containing a diverse set of conformational features, such as varying lengths of bulges, hairpin loops, and single-stranded regions. Dayeh et al. [26] predicted the secondary structure of the HIV-1 ΔDIS 5′-UTR with SHAPE and designed a set of gDNAs to span the HIV-1 ΔDIS 5′-UTR sequence in 23-nt increments ( Supplementary Figure S6). Since 45 nt unstructured RNA targets can be best cleaved by VbAgo with 15‒16 nt gDNA ( Figure 3A,B), we synthesized 12 gDNAs (16 nt long) ( Figure 6A and Supplementary Table S2) corresponding to target regions of 2 and 4–14 [26] to test whether predicted products could be observed. Cleavage products were detected with most gDNAs except gDNA-1 when VbAgo loaded with 5′P-gDNA was incubated at both 37°C and 50°C for 15 min with 10 mM Mg 2+ ( Figure 6B). Especially when VbAgo was loaded with gDNA-3, 7, 8, 11 and 12 at 50°C, the targets could be almost completely cleaved. VbAgo was most efficient when loaded with gDNA-7 and 11 at 37°C. However, the secondary structure of target RNA can inhibit target cleavage by VbAgo, since no cleavage was observed with gDNA-1 corresponding to a double-stranded region similar to MbpAgo [15], and the cleavage efficiency was also significantly diminished for several other guides targeting partially double-stranded regions. These results implied that it is necessary to excavate potential pAgos cleaving highly structured RNA with high efficiency. Meanwhile, further experiments should be carried out to test whether longer double-strand stems affect the cleavage activity of VbAgo, since HIV-1 ΔDIS 5′-UTR contains only short double-stranded stems. These results implied the possibility of the combined use of VbAgo and these RNA manipulation tool enzymes, suggesting possible application for RNA editing.
Figure 6 .
Cleavage of highly structured HIV-1 ΔDIS 5′-UTR RNA by VbAgo
(A) Schematic overview of the HIV-1 ΔDIS 5′-UTR. Twelve regions (shown in different colors) were selected from the RNA target sequence, with each region targeted by a 16 nt 5′P-gDNA. (B) VbAgo cleaved HIV-1 ΔDIS 5′-UTR RNA with 16 nt 5′P-gDNA at 37°C and 50°C for 15 min, and products (indicated by red triangle) were observed at predicted positions. The positions of the targets (T), gDNAs (G) and products (P) are indicated on the left of the gels. M, RNA marker.
Discussion
In this study, we characterized a novel gD-tR pAgo from the mesophilic bacterium Verrucomicrobia. Compared to most pAgos strongly preferring DNA targets, such as TtAgo, CpAgo, IbAgo and MpAgo [ 3, 13, 23, 27, 32] , VbAgo shows RNA target cleavage specificity at 37°C in vitro. Meanwhile, VbAgo functions as a multiple turnover enzyme, since approximately 40% of RNA targets are cleaved for 60 min when the [VbAgo–gDNA]/RNA target ratio is 0.25. Recent studies have reported some gD-tR pAgos, such as MbpAgo, PliAgo, RslAgo, PnyAgo and HpeAgo [ 15, 24] . There are some similarities and differences between VbAgo and these gD-tR pAgos, as shown in Supplementary Table S3. Their similarities mainly lie in the following points. First, they all prefer RNA targets, and they all utilize both 5 ′-P and 5 ′-OH gDNAs to cleave RNA targets. VbAgo cleaves RNA targets dependent on Mg 2+ or Mn 2+, similar to gD-tR pAgos. In addition, all of these gD-tR pAgos are sensitive to the mismatch at position 13, indicating that this position is critical for target cleavage. Additionally, the cleavage efficiency drops with gDNAs shorter than 14 nt for all these gD-tR pAgos. The cleavage efficiency of VbAgo, MbpAgo, PliAgo and RslAgo is reduced when loaded with gDNAs shorter than 14 nt, and the cleavage efficiency of PnyAgo and HpeAgo is reduced when loaded with gDNAs shorter than 16 nt[ 15, 24] . At the same time, except RslAgo, all of these gD-tR pAgos show no obvious preference to guide 5′-nucleotide [ 15, 24] .
However, they also have obvious differences. Phylogenetic tree analysis reveals that VbAgo is correlated with gD-tR pAgos, but VbAgo has low sequence identity to other characterized gD-tR pAgos (sequence identity<30%). These results indicate that VbAgo is a novel gD-tR pAgo. Furthermore, the MID pocket differs between VbAgo and other pD-tR pAgos. Previous studies have reported that the MID pocket of pAgos contains a conserved six-amino-acid motif [8] for anchoring the 5′-end of the guide. The first two residues are the most conserved and can be classified into several subtypes, such as YK, HK, and MID-OH [8]. The MID domain of VbAgo contains a YK motif identical to CbAgo, KmAgo and NgAgo. The MID pockets of MbpAgo, PnyAgo and HpeAgo contain the HK motif. The MID domains of PliAgo and RslAgo contain unconventional RHSY motifs and KHTY motifs, respectively. VbAgo utilizes both 5′-P and 5′-OH gDNAs to cleave the RNA targets at a site between the 10 th and 11 th nucleotides starting from the 5′-end of the guide, similar to MbpAgo [15]. However, PliAgo and RslAgo loaded with 5 ′-P gDNAs cleave RNA targets at a site between the 9 th and 10 th nucleotides starting from the 5′-end of the guide [24]. PnyAgo and HpeAgo loaded with 5 ′-OH gDNAs cleave RNA targets at a site between the 11 th and 12 th nucleotides starting from the 5′-end of the guide [24]. The weak cleavage of DNA targets can be observed by PnyAgo and MbpAgo [ 15, 24] , while VbAgo specifically cleaves RNA targets at 37°C, which is identical to PliAgo, HpeAgo and RslAgo, and cleavage of DNA targets is not observed even when extending the reaction time up to 24 h. Therefore, we hypothesize that VbAgo may not cause DNA damage when used in RNA targeting. Furthermore, the cleavage efficiency of PliAgo, RslAgo, PnyAgo and HpeAgo drops dramatically at elevated temperatures above 50°C, but the cleavage efficiency of VbAgo and MbpAgo drops dramatically at elevated temperatures above 65°C [ 15, 24] .
Considering the high RNA target cleavage specificity and prominent highly structured RNA cleavage by VbAgo, it remains to be established whether VbAgo can be applied in RNA-centric in vivo and in vitro methods, such as RNA targeting, antiviral and nucleic acid detection [ 33, 34] . Although CRISPR/Cas systems have achieved great progress in genome editing, there are obvious limitations. For instance, Type VI CRISPR-Cas systems (CRISPR/Cas13) loaded with crRNA can exclusively cleave RNA targets regulated by protospacer flanking sequences of RNA targets (PFS) and present a high risk of off-target effects, such as cleaving both target RNA and bystander RNAs that lack crRNA base-pairing potential [35]. In contrast, VbAgo can cleave RNA targets independent of special sequences such as PFS, and the cleavage site is exclusive, which makes it convenient to design guides without the limitation of the cleavage site. In addition, it is cheaper to synthesize guide DNA than to synthesize guide RNA, which reduces the cost. Furthermore, the RNA targeting capabilities of VbAgo may be selectively utilized by the combination of VbAgo_DM and other effector components. VbAgo fused to various effector protein domains might expand the functionality of VbAgo beyond RNA cleavage, such as RNase-defective dCas13, which may provide an effective strategy [ 13, 35] .
In conclusion, this study characterizes a novel pAgo specifically cleaving RNA targets at 37°C with a YK motif in the MID pocket, which provides complementary findings to gD-tR pAgos and could expand the pAgo-based RNA manipulation toolbox.
Supplementary Data
Supplementary data is available at Acta Biochimica et Biphysica Sinica online.
Supporting information
COMPETING INTERESTS
Hubei University has applied for a patent (application No. 202210794665.9) for the application of VbAgo with L. Ma, Q. Liu, Y. Liu, F. Wang and W. Chen listed as co-inventors.
Funding Statement
This work was supported by the grants from the China National Key Research and Development (R&D) Program (No. 2021YFC2100100), the Wuhan Science and Technology Bureau Knowledge Innovation Special Dawning Program (No. 2022020801020326), the Postdoctoral Innovation Project of Shandong Province (No. SDCX-ZG-202203010), and the China Postdoctoral Science Foundation (No. 2022M721074).
References
- 1.Swarts DC, Makarova K, Wang Y, Nakanishi K, Ketting RF, Koonin EV, Patel DJ, et al. The evolutionary journey of Argonaute proteins. Nat Struct Mol Biol. . 2014;21:743–753. doi: 10.1038/nsmb.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ketting RF. The Many Faces of RNAi. Dev Cell. . 2011;20:148–161. doi: 10.1016/j.devcel.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Swarts DC, Jore MM, Westra ER, Zhu Y, Janssen JH, Snijders AP, Wang Y, et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature. . 2014;507:258–261. doi: 10.1038/nature12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makarova KS, Wolf YI, van der Oost J, Koonin EV. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol Direct. . 2009;4:29. doi: 10.1186/1745-6150-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisitskaya L, Aravin AA, Kulbachinskiy A. DNA interference and beyond: structure and functions of prokaryotic Argonaute proteins. Nat Commun. . 2018;9:5165. doi: 10.1038/s41467-018-07449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuzmenko A, Oguienko A, Esyunina D, Yudin D, Petrova M, Kudinova A, Maslova O, et al. DNA targeting and interference by a bacterial Argonaute nuclease. Nature. . 2020;587:632–637. doi: 10.1038/s41586-020-2605-1. [DOI] [PubMed] [Google Scholar]
- 7.Jolly SM, Gainetdinov I, Jouravleva K, Zhang H, Strittmatter L, Bailey SM, Hendricks GM, et al. Thermus thermophilus argonaute functions in the completion of DNA replication . Cell. . 2020;182:1545–1559.e18. doi: 10.1016/j.cell.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryazansky S, Kulbachinskiy A, Aravin AA. The expanded universe of prokaryotic argonaute proteins. mBio. 2018, 9: e01935–18 . [DOI] [PMC free article] [PubMed]
- 9.Hegge JW, Swarts DC, van der Oost J. Prokaryotic argonaute proteins: novel genome-editing tools? Nat Rev Microbiol. 2018, 16: 5–11 . [DOI] [PubMed]
- 10.Nakanishi K, Weinberg DE, Bartel DP, Patel DJ. Structure of yeast argonaute with guide RNA. Nature. . 2012;486:368–374. doi: 10.1038/nature11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan YR, Pei Y, Ma JB, Kuryavyi V, Zhadina M, Meister G, Chen HY, et al. Crystal structure of A. aeolicus argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage . Mol Cell. . 2005;19:405–419. doi: 10.1016/j.molcel.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Yang J, Cho WC, Zheng Y. Argonaute proteins: structural features, functions and emerging roles. J Adv Res. . 2020;24:317–324. doi: 10.1016/j.jare.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Li W, Jiang X, Wang Y, Zhang Z, Liu Q, He R, et al. A programmable omnipotent Argonaute nuclease from mesophilic bacteria Kurthia massiliensis . Nucleic Acids Res. . 2021;49:1597–1608. doi: 10.1093/nar/gkaa1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Liu Y, Liu Q, Ma L. Characterization of a programmable argonaute nuclease from the mesophilic bacterium Rummeliibacillus suwonensis . Biomolecules. . 2022;12:355. doi: 10.3390/biom12030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Liu Y, He R, Wang L, Wang Y, Zeng W, Zhang Z, et al. A programmable pAgo nuclease with RNA target preference from the psychrotolerant bacterium Mucilaginibacter paludis . Nucleic Acids Res. . 2022;50:5226–5238. doi: 10.1093/nar/gkac315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Juranek S, Li H, Sheng G, Wardle GS, Tuschl T, Patel DJ. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature. . 2009;461:754–761. doi: 10.1038/nature08434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swarts DC, Hegge JW, Hinojo I, Shiimori M, Ellis MA, Dumrongkulraksa J, Terns RM, et al. Argonaute of the archaeon Pyrococcus furiosus is a DNA-guided nuclease that targets cognate DNA . Nucleic Acids Res. . 2015;43:5120–5129. doi: 10.1093/nar/gkv415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zander A, Willkomm S, Ofer S, van Wolferen M, Egert L, Buchmeier S, Stöckl S, et al. Guide-independent DNA cleavage by archaeal Argonaute from Methanocaldococcus jannaschii . Nat Microbiol. . 2017;2:17034. doi: 10.1038/nmicrobiol.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willkomm S, Oellig CA, Zander A, Restle T, Keegan R, Grohmann D, Schneider S. Structural and mechanistic insights into an archaeal DNA-guided Argonaute protein. Nat Microbiol. . 2017;2:17035. doi: 10.1038/nmicrobiol.2017.35. [DOI] [PubMed] [Google Scholar]
- 20.Kropocheva E, Kuzmenko A, Aravin AA, Esyunina D, Kulbachinskiy A. A programmable pAgo nuclease with universal guide and target specificity from the mesophilic bacterium Kurthia massiliensis . Nucleic Acids Res. . 2021;49:4054–4065. doi: 10.1093/nar/gkab182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuzmenko A, Yudin D, Ryazansky S, Kulbachinskiy A, Aravin AA. Programmable DNA cleavage by ago nucleases from mesophilic bacteria Clostridium butyricum and Limnothrix rosea . Nucleic Acids Res. . 2019;47:5822–5836. doi: 10.1093/nar/gkz379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegge JW, Swarts DC, Chandradoss SD, Cui TJ, Kneppers J, Jinek M, Joo C, et al. DNA-guided DNA cleavage at moderate temperatures by Clostridium butyricum argonaute . Nucleic Acids Res. . 2019;47:5809–5821. doi: 10.1093/nar/gkz306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y, Sun W, Wang J, Sheng G, Xiang G, Zhang T, Shi W, et al. Argonaute proteins from human gastrointestinal bacteria catalyze DNA-guided cleavage of single- and double-stranded DNA at 37°C. Cell Discov. . 2019;5:38. doi: 10.1038/s41421-019-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisitskaya L, Shin Y, Agapov A, Olina A, Kropocheva E, Ryazansky S, Aravin AA, et al. Programmable RNA targeting by bacterial argonaute nucleases with unconventional guide binding and cleavage specificity. Nat Commun. . 2022;13:4624. doi: 10.1038/s41467-022-32079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singleton CM, Petriglieri F, Kristensen JM, Kirkegaard RH, Michaelsen TY, Andersen MH, Kondrotaite Z, et al. Connecting structure to function with the recovery of over 1000 high-quality metagenome-assembled genomes from activated sludge using long-read sequencing. Nat Commun. . 2021;12:2009. doi: 10.1038/s41467-021-22203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayeh DM, Cantara WA, Kitzrow JP, Musier-Forsyth K, Nakanishi K. Argonaute-based programmable RNase as a tool for cleavage of highly-structured RNA. Nucleic Acids Res. . 2018;46:e98. doi: 10.1093/nar/gky496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Juranek S, Li H, Sheng G, Tuschl T, Patel DJ. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature. . 2008;456:921–926. doi: 10.1038/nature07666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartel David P. MicroRNAs: target recognition and regulatory functions. Cell. . 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elkayam E, Kuhn CD, Tocilj A, Haase AD, Greene EM, Hannon GJ, Joshua-Tor L. The structure of human Argonaute-2 in complex with miR-20a. Cell. . 2012;150:100–110. doi: 10.1016/j.cell.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boland A, Huntzinger E, Schmidt S, Izaurralde E, Weichenrieder O. Crystal structure of the MID-PIWI lobe of a eukaryotic Argonaute protein. Proc Natl Acad Sci USA. . 2011;108:10466–10471. doi: 10.1073/pnas.1103946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalia M, Willkomm S, Claussen J, Restle T, Bonvin A. Novel insights into guide RNA 5′-nucleoside/tide binding by human argonaute 2. Int J Mol Sci. . 2015;17:22. doi: 10.3390/ijms17010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaya E, Doxzen KW, Knoll KR, Wilson RC, Strutt SC, Kranzusch PJ, Doudna JA. A bacterial Argonaute with noncanonical guide RNA specificity. Proc Natl Acad Sci USA. . 2016;113:4057–4062. doi: 10.1073/pnas.1524385113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, Belanto JJ, Verdine V, et al. RNA targeting with CRISPR-Cas13. Nature. . 2017;550:280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbott TR, Dhamdhere G, Liu Y, Lin X, Goudy L, Zeng L, Chemparathy A, et al. Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell. . 2020;181:865–876.e12. doi: 10.1016/j.cell.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terns MP. CRISPR-based technologies: impact of RNA-targeting systems. Mol Cell. . 2018;72:404–412. doi: 10.1016/j.molcel.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.