Abstract
Background
Previous studies have demonstrated that there are sex-related differences in atherosclerosis. However, in terms of the nature of in-stent restenosis (ISR) neointima, the effect of gender on the results is unclear.
Methods
Patients with ISR of drug-eluting stents (DES) who underwent OCT examination in Senior Department of Cardiology of the Chinese People's Liberation Army General Hospital from March 2010 to March 2022 were included. The patients were divided into male and female groups.
Results
In this study, a total of 230 DES-ISR patients and 249 DES-ISR target lesions were analyzed. OCT data showed that compared to females, males have a higher incidence of thin-cap fibrous atherosclerosis (TCFA) (37.4% [n = 77] vs. 9.3% [n = 4], p < 0.001) and in-stent neoatherosclerosis (ISNA) (82.0% [n = 169] vs. 62.8% [n = 27]). p = 0.005). In addition, a multivariate logistic regression analysis demonstrated that male was independently associated with a higher incidence of ISNA (OR: 2.659, 95% CI: 1.239–5.707; p = 0.012) and TCFA (OR: 4.216, 95% CI: 1.370–12.976; p = 0.012).
Conclusion
For DES-ISR patients, the incidence of ISNA in female was significantly lower than that in male and vulnerability of neointimal pattern is lower in female than in male.
Keywords: Gender, In-stent restenosis, Drug-eluting stent, Optical coherence tomography, Percutaneous coronary intervention
1. Introduction
It is well known that there are inherent biological differences between male and female that contribute to sex differences in cardiovascular disease. Previous studies showed that females develop cardiovascular disease an average of 10 years later than males [1]. ADAPT-DES studies showed that plaque erosion plays an important role in the pathogenesis of acute coronary syndrome (ACS) in females, while acute plaque rupture is more typical in males [2], and females have a higher risk of adverse outcomes after percutaneous coronary intervention (PCI) [3]. This also applies to PCI for in-stent restenosis (ISR) patients. In the era of drug-eluting stents (DES), DES surface drugs have a strong inhibitory effect on neointima, which may have a certain impact on the relationship between sex and ISR incidence [4]. It has been confirmed that male patients are at greater risk of ISR than female patients, but heterogeneity exists among different studies [5,6]. With the development of intracoronary imaging, optical coherence tomography (OCT) can clearly elucidate the mechanism of ISR and the characteristics of neointima, but few studies reported the gender differences in the characteristics of DES-ISR neointima. The main purpose of this study was to investigate the differences in the characteristics of neointima between different genders of DES-ISR patients by OCT, so as to provide certain reference for future clinical treatment.
2. Methods
2.1. Study population
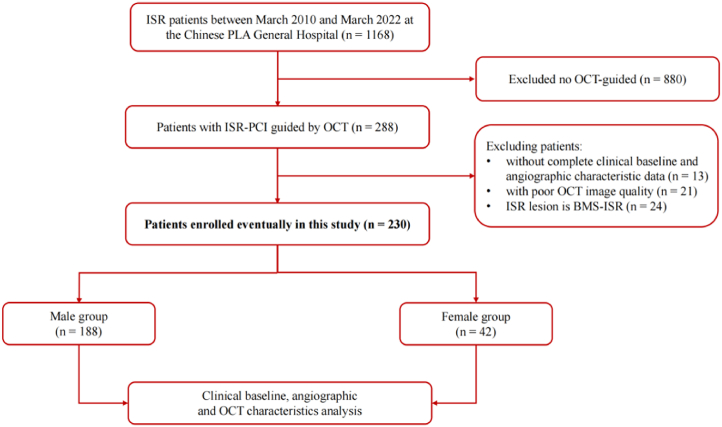
This study was a single-center, retrospective observational study. This study retrospectively analyzed 1168 DES-ISR patients who underwent PCI at the Chinese People's Liberation Army (PLA) General Hospital between March 2010 and March 2022, and all of these patients underwent coronary angiography before PCI. ISR was defined as a reduction ≥50% of the luminal diameter within the vessel segment of the implanted stent or 5 mm the proximal or distal edge to the stent, as assessed by coronary angiography [7]. This study included patients who underwent OCT at ISR lesions during PCI. Exclusion criteria were as follows: 1. DES-ISR patients who did not undergo OCT examination; 2. Bare metal stent (BMS) ISR patients; 3. Patients with incomplete clinical and angiographic data; 4. Patients with poor quality OCT or angiographic images. We excluded 880 patients with ISR who did not undergo OCT; 13 patients with ISR who lacked complete clinical baseline or angiographic characteristic data; 21 with poor image quality; and 24 ISR that were BMS-ISR (Fig. 1).
Fig. 1.
Study flow. According to gender, the patients were divided into 2 groups (male, n = 188; or female, n = 42).
The patient cohort was segregated into two distinct groups based on gender, and a comprehensive comparison of all analysis results in this paper was conducted between the male and female. The analysis encompassed the patients' clinical baseline data, angiographic and procedural data, and the relevant OCT data. The study complied with the Declaration of Helsinki. The Chinese PLA General Hospital committee approved the research protocol (No. S2018-049-01). We obtained written informed consent from all patients enrolled in this study before the procedure.
2.2. Clinical baseline and angiographic characteristic
In this study, the inpatient records of all patients were obtained from the medical record management system of PLA General Hospital. And the baseline data for all patients were compiled and subjected to analysis by the first author of this paper. Baseline data included age, sex, history of hypertension, diabetes, hyperlipidemia, smoking, etc. ST-segment elevation myocardial infarction (STEMI) was defined as the presence of persistent chest discomfort lasting more than 30 min, accompanied by ST-segment elevation of greater than 0.1 mV in at least two contiguous leads.
The features of coronary angiography in all patients were collected and analyzed by two independent technicians (Xiaohang Yuan and Mengting Jiang) at the Imaging Core Laboratory of the PLA General Hospital. Among them, quantitative coronary angiography (QCA) analysis was performed by a blinded trained technician using QAngio XA software (Medis Medical Imaging Systems, Leiden, Netherlands). The calculation of diameter stenosis involved utilizing the minimum lumen diameter and interpolated reference vessel diameter, whereby formula [1-minimum lumen diameter/reference vessel diameter] × 100 was applied. To determine lesion length, measurements were taken from the proximal to the distal shoulder in the projection that exhibited the least foreshortening. The angiographic classification of ISR was according to Mehran's classification [8].
2.3. OCT image acquisition and assessment
OCT examination was performed using either frequency-domain (C7-XR™ or ILUMIEN™ OPTIS™ OCT Intravascular Imaging System; St Jude Medical) OCT system. During PCI, an OCT catheter (Dragonfly™ or Dragonfly™ Duo™; St. Jude Medical, St. Paul, MN, USA) was used to pass the ISR lesion and contrast agent was injected at a rate of 3–4 mL/s while pullback was performed [9]. All OCT images were subjected to analysis by two experienced physicians (Mengting Jiang and Yan Han) who were unaware of the patients' clinical and angiographic information. An offline proprietary software (St. Jude Medical) was employed for this image analysis process. When the views of the two observers diverged, a third independent expert assessed the decision. In addition, to evaluate the intra-observer changes in diagnosing OCT features (neointimal pattern properties, backscatter features, ISNA and TCFA), one of the two observers (Yan Han) conducted a re-evaluation of all OCT images four months after concluding the initial OCT image evaluation process.
2.4. Definitions
For the quantitative and qualitative analysis of OCT images, we examined OCT images at 1-mm intervals between the proximal and distal reference sections. The proximal and distal reference lumen regions were identified as the sections with the largest lumen area within 5 mm of the proximal and distal edges of the stent, respectively. In terms of quantitative analysis, we calculated the percentage of maximum neointimal hyperplasia (NIH) as ([stent-lumen area]/stent area) × 100%. Underexpansion was determined when the minimal stent area (MSA) divided by the mean of the distal and proximal area was less than 80% [10]. Malapposition was defined as a measured distance between the stent surface and the adjacent vessel surface that exceeded the thickness of the stent [11].
For the qualitative assessment, the presence of any ISR neointima feature on the cross-section within 5 mm at both ends of the stent is regarded as an indication of the presence of that specific feature. Neoatherosclerosis was defined as a lipid or calcified neointima [12]. ISNA found by OCT is defined as OCT-ISNA. A calcified neointima was well defined, with low signal reflection and sharp boundaries (Fig. 2D). A lipid neointima was defined as low reflection signal, diffuse boundary unclear (Fig. 2F). In the measurement of lipid patch correlation, the maximum lipid arc was measured on the cross-sectional image with the largest lipid content, and the minimum fiber cap thickness was measured at its thinnest part. Among lipid neointima, those with a maximum lipid arc of >180° were defined as lipid-rich plaques (LRP). TCFA in neointima was fiber cap thickness ≤65 μm [13]. TCFA found by OCT is defined as OCT-TCFA. A thrombus was defined as an irregular mass with a minimum diameter of at least 250 mm attached to the vessel wall or floating in the lumen [14]. Neointimal macrophages were defined as a linear, strong OCT image of the neointima surface with high attenuation [15] (Fig. 2E). Microvessel was defined as tubular structures <200 μm in diameter [16].
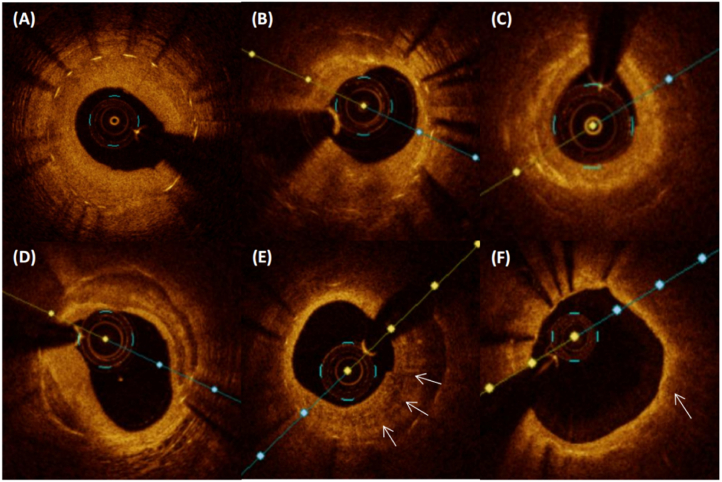
Fig. 2.
Representative optical coherence tomography. Various classical images of OCT. A) Homogenous hyperplasia, high-intensity. B)Heterogeneous, low-intensity. C)Layered, low-intensity. D)Neointimal calcium. E)Macrophage (arrows). F) Lipidic neointimal hyperplasia, thin-cap fibroatheroma (arrows).
Neointimal rupture was defined as a break in the fibrous cap connecting the lumen with the underlying lipidic NIH. Three types of restenosis tissue structure characteristics are defined as follows:1) homogeneous, with uniform optical properties, and does not show focus changes in the backscatter mode (Fig. 2A); 2) Heterogeneous, with optical properties that vary in focus and exhibit various backscattering patterns (Fig. 2B); 3) Layered type, with different optical properties, and the appearance of concentric layers, consisting of a bottom cavity high scattering layer and a bottom cavity low scattering layer (Fig. 2C). Regarding the type of scattering, high backscatter appears as high backscatter of most tissues and appears bright. Low backscatter shows that most tissues have low backscatter and appear dark or black. According to the distribution characteristics, ISR could be divided into: 1) focal (<10 mm in length); 2) multifocal (<10 mm in length and >5 mm apart); 3) diffuse (≥10 mm in length). The representative OCT images of restenosis tissue structure and vulnerability involved in this study are shown in Fig. 2.
2.5. Statistical analysis
For measurement data with normal distribution, the mean ± standard deviation was used to describe, and t-test was used to compare the two groups. For data exhibiting skewed distribution, we utilized the median as a descriptive measure, and comparisons between two groups were conducted using the rank-sum test. Count data were presented as rates (%) and compared between the two groups using the χ2 test. Univariate and multivariate logistic regression analyses were carried out to identify independent factors associated with OCT-ISNA and OCT-TCFA. Additionally, these regression analyses were used to explore the relationship between factors, including gender, and the presence of ISNA and TCFA. A multivariable logistic regression model was constructed with the objective of discerning the factors that exhibit associations with ISNA and TCFA. Variables of clinical significance identified in prior studies [17], along with variables that demonstrated statistical significance in the univariate analysis (p-value <0.05). Age, sex, hypertension, current smoking, diabetes, Hyperlipidaemia, LDL cholesterol, statin use at the time of ISR, NIH >50% and minimum lumen area were chosen as variables according to a previous report. Results of the model are presented as the odds ratio (OR) and 95% confidence interval (CI). A p-value below 0.05 was adopted as the threshold for statistical significance. All statistical analyses were executed employing SPSS software, Version 20.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Clinical characteristics
This was a retrospective study which conducted on 1168 ISR patients and after excluding patients who did not undergo OCT, we identified 288 patients who were evaluated for ISR culprit lesions using OCT. According to the inclusion and exclusion criteria, 230 ISR patients with 249 ISR target lesions were finally included in this study. The patients were divided into two groups according to gender [male group, 188 patients (81.7%) with 206 ISR target lesions; or female group, 42 patients (18.3%) with 43 ISR target lesions]. In the baseline clinical data, the prevalence of smoking in male ISR patients was significantly higher than that in female patients (56.4% [n = 106] vs. 7.1% [n = 3], p < 0.001), but the prevalence of hypertension in male patients was significantly lower than that in female patients (58.0% [n = 109] vs. 76.2% [n = 32], p = 0.028). In addition, the time since implantation in male patients was significantly longer than that in female patients (6.2 ± 4.3 years vs. 4.4 ± 3.9 years, p = 0.015). Other baseline clinical and angiographic characteristics were not significantly different (Table 1, Table 2).
Table 1.
Clinical characteristics.
| Overall population (n = 230) | Sex |
p value | ||
|---|---|---|---|---|
| Male (n = 188) | Female (n = 42) | |||
| Age, years | 63.1 ± 10.4 | 62.5 ± 10.4 | 65.7 ± 10.2 | 0.070 |
| Current smoker | 109 (47.4) | 106 (56.4) | 3 (7.1) | <0.001 |
| Hypertension | 141 (61.3) | 109 (58.0) | 32 (76.2) | 0.028 |
| Diabetes mellitus | 108 (47.0) | 88 (46.8) | 20 (47.6) | 0.924 |
| Hyperlipidaemia | 68 (29.6) | 54 (28.7) | 13 (31.0) | 0.774 |
| Chronic renal insufficiency | 7 (3.0) | 5 (2.7) | 2 (4.8) | 0.826 |
| Chronic cardiac insufficiency | 23 (10.0) | 18 (9.6) | 5 (11.9) | 0.864 |
| Prior coronary artery bypass grafting | 0 (0) | 0 (0) | 0 (0) | – |
| ST-segment elevation myocardial infarction | 12 (5.2) | 10 (5.3) | 2 (4.8) | 1.000 |
| Medication at the time of in-stent restenosis | ||||
| Aspirin | 219 (95.2) | 178 (94.7) | 41 (97.6) | 0.684 |
| Clopidogrel | 157 (68.3) | 129 (68.6) | 28 (66.7) | 0.806 |
| Ticagrelor | 56 (24.3) | 43 (22.9) | 13 (31.0) | 0.270 |
| Statin | 202 (87.8) | 165 (87.8) | 37 (88.1) | 0.953 |
| Fasting blood-glucose, mmol/L | 5.8 (5.0, 8.3) | 5.7 (5.0, 8.3) | 6.2 (5.1, 8.3) | 0.649 |
| LDL cholesterol, mg/dL | 1.8 (1.4, 2.3) | 1.8 (1.4, 2.3) | 1.9 (1.5, 2.4) | 0.359 |
Values are n (%) or mean and standard deviation or median (1st quartile, 3rd quartile). LDL: low-density lipoprotein.
Table 2.
Lesion characteristics and quantitative coronary angiography findings.
| Overall population (n = 249) | Sex |
p value | ||
|---|---|---|---|---|
| Male (n = 206) | Female (n = 43) | |||
| Time since implantation, years | 5.9 ± 4.3 | 6.2 ± 4.3 | 4.4 ± 3.9 | 0.015 |
| Culprit vessel | 0.827 | |||
| Left anterior descending | 162 (65.1) | 132 (64.1) | 30 (69.8) | |
| Circumflex | 34 (13.6) | 30 (14.6) | 4 (9.3) | |
| Right | 51 (20.5) | 42 (20.4) | 9 (20.9) | |
| Other | 2 (0.8) | 2 (0.9) | 0 (0) | |
| Type of previous stent | 0.152 | |||
| Drug-eluting stent | ||||
| First-generation | 56 (22.5) | 49 (23.8) | 7 (16.3) | |
| New-generation | 168 (67.5) | 134 (65.0) | 34 (79.1) | |
| Unknown | 25 (10.0) | 23 (11.2) | 2 (4.7) | |
| Lesion characteristics | ||||
| Bifurcation (>1.5 mm) | 89 (35.7) | 73 (35.4) | 16 (37.2) | 0.825 |
| Ostial location | 16 (6.4) | 14 (6.8) | 2 (4.7) | 0.857 |
| In-stent restenosis pattern* | 0.925 | |||
| Type Ⅰ | 88 (35.3) | 74 (35.9) | 14 (32.6) | |
| Type Ⅱ | 72 (28.9) | 59 (28.6) | 13 (30.2) | |
| Type Ⅲ | 73 (29.3) | 59 (28.6) | 14 (32.5) | |
| Type Ⅳ | 16 (6.4) | 14 (6.8) | 2 (4.7) | |
| QCA analysis of lesion segment | ||||
| Restenosis lesion length, mm | 11.3 (7.9, 15.4) | 11.2 (7.7, 15.0) | 13.3 (8.2, 18.8) | 0.189 |
| Reference vessel diameter, mm | 2.7 (2.3, 3.1) | 2.7 (2.3, 3.1) | 2.7 (2.1, 3.0) | 0.447 |
| Minimum lumen diameter, mm | 1.0 (0.7, 1.3) | 1.0 (0.7, 1.4) | 1.0 (0.4, 1.3) | 0.769 |
| Diameter stenosis,% | 62.0 (50.7, 74.1) | 61.9 (50.5, 74.0) | 62.6 (51.0, 74.5) | 0.869 |
Values are n (%) or mean or median with first and third quartiles. *In-stent restenosis pattern was defined as per Mehran's classification.
3.2. OCT analysis of ISR lesions
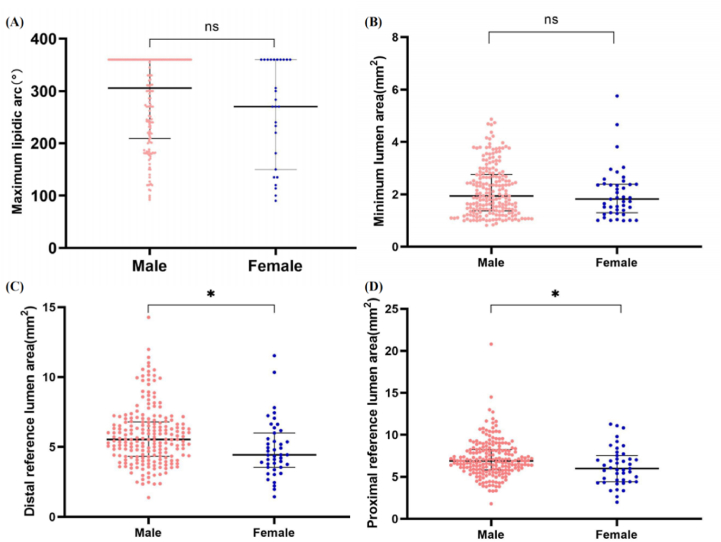
First, in the target lesion of ISR, distal reference lumen area [4.4 (3.6, 6.0) vs. 5.5 (4.3, 6.8), p = 0.003] and proximal reference cross section area [6.0 (4.4, 7.6) vs. 6.9 (5.8, 8.2), p = 0.010] of female were smaller than those of male, and the difference was statistically significant (Fig. 3C and D). Nevertheless, there was no significant difference in maximum lipidic arc and minimum lumen area between male and female (Fig. 3A and B).
Fig. 3.
Comparison of maximum lipid arc, minimum lumen area, distal reference lumen area and proximal reference lumen area between males and females. Females have smaller distal reference lumen [4.4 (3.6, 6.0)vs. 5.5 (4.3, 6.8), p = 0.003] area and smaller proximal reference lumen area [6.0 (4.4, 7.6)vs. 6.9 (5.8, 8.2), p = 0.010]. There were no significant differences in the maximum lipid Arc (p = 0.322) and minimum lumen area (p = 0.315) between male and female ISR patients.
In terms of ISR neointima structure, there was a significant difference in the distribution pattern of ISR stenosis between the two groups (p = 0.047): the proportion of homogeneous neointima in women was significantly higher than that in men (37.2% vs. 19.9%). In contrast, the proportion of heterogeneous neointima (44.2% vs. 59.7%) and layered neointima (18.6% vs. 20.4%) in female ISR was lower than that in male. In addition, there were also fewer neointima with high backscatter in target lesions in female patients with ISR than that in male patients (48.8% [n = 21] vs. 32.5% [n = 67], p = 0.042).
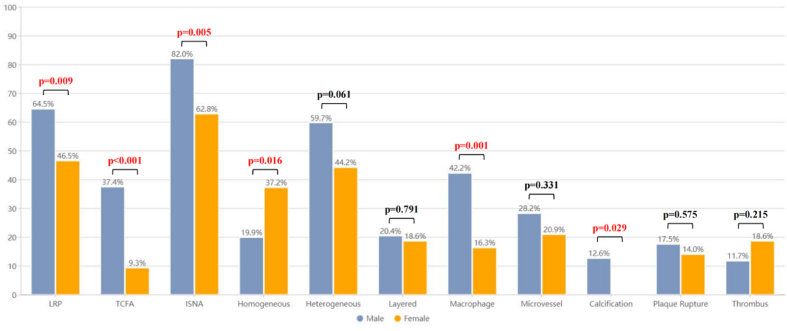
OCT-ISNA (62.8% [n = 27] vs. 82.0% [n = 169], p = 0.005) and OCT-TCFA (9.3% [n = 4] vs.37.4% [n = 77], p < 0.001) in the female group was significantly lower than that in the male group (Fig. 4). In addition, there was a significant difference in the characteristics of ISR lesions between male and female in this study: female patients with ISR had a lower incidence of lipid neointima than patients with male ISR (62.8% [n = 27] vs. 77.7% [n = 160], p = 0.040). There was no significant difference in the maximum lipidic arc (p = 0.315), but it tended to be smaller in the female group than in the male group. Neointimal macrophages (16.3% [n = 7] vs. 42.2% [n = 87], p = 0.001) and calcified neointima (0.0% [n = 0] vs. 12.6% (n = 26), p = 0.029) was less frequent in female patients with ISR than in male patients (Table 3).
Fig. 4.
Prevalence of OCT-Defined plaque characteristics in ISR lesions. LRP, TCFA, ISNA, macrophage, calcification were detected in male ISR patients compared with female ISR patients. ISNA = in-stent neoatherosclerosis; LRP = lipid-rich plaque; OCT = optical coherence tomography; TCFA = thin-cap fibroatheroma.
Table 3.
OCT analysis of culprit lesion.
| Overall population (n = 249) | Sex |
p value | ||
|---|---|---|---|---|
| Male (n = 206) | Female (n = 43) | |||
| Quantitative assessment | ||||
| Distal reference lumen area, mm2 | 5.4 (4.1, 6.7) | 5.5 (4.3, 6.8) | 4.4 (3.6, 6.0) | 0.003 |
| Proximal reference lumen area, mm2 | 6.8 (5.7, 8.2) | 6.9 (5.8, 8.2) | 6.0 (4.4, 7.6) | 0.010 |
| Minimum lumen area, mm2 | 1.9 (1.4, 2.7) | 1.9 (1.4, 2.8) | 1.8 (1.3, 2.4) | 0.322 |
| Minimum lumen diameter, mm | 1.5 (1.3, 1.8) | 1.6 (1.3, 1.9) | 1.5 (1.3, 1.7) | 0.316 |
| Minimum stent area, mm2 | 6.6 (5.4, 7.8) | 6.6 (5.6, 7.9) | 6.1 (4.7, 7.5) | 0.073 |
| Maximal NIH, % | 0.7 (0.6, 0.8) | 0.7 (0.6, 0.8) | 0.7 (0.5, 0.8) | 0.861 |
| Mean intimal thickness | 0.7 (0.5, 0.8) | 0.7 (0.5, 0.8) | 0.6 (0.4, 0.8) | 0.285 |
| Qualitative assessment, n (%) | ||||
| Neointima characteristics at lesion | ||||
| Neoatherosclerosis | 196 (78.7) | 169 (82.0) | 27 (62.8) | 0.005 |
| Lipid neointima | 187 (75.1) | 160 (77.7) | 27 (62.8) | 0.040 |
| Lipid-rich plaque | 159 (63.9) | 139 (67.5) | 20 (46.5) | 0.009 |
| Maximum lipidic arc, | 300.0 (205.8, 360.0) | 305.5 (209.4, 360.0) | 270.0 (150.0, 360.0) | 0.315 |
| Calcified neointima | 26 (10.4) | 26 (12.6) | 0 (0) | 0.029 |
| Spotty calcification | 15 (6.0) | 15 (7.3) | 0 (0) | 0.141 |
| Re-stenotic tissue structure | 0.047 | |||
| Homogeneous | 57 (22.9) | 41 (19.9) | 16 (37.2) | |
| Heterogeneous | 142 (57.0) | 123 (59.7) | 19 (44.2) | |
| Layered | 50 (20.1) | 42 (20.4) | 8 (18.6) | |
| Distribution of ISR | 0.401 | |||
| Focal | 57 (22.9) | 45 (21.8) | 12 (27.9) | |
| Multifocal | 95 (38.2) | 77 (37.4) | 18 (41.9) | |
| Diffuse | 97 (38.9) | 84 (40.8) | 13 (30.2) | |
| Restenotic tissue backscatter | 0.042 | |||
| High | 88 (35.3) | 67 (32.5) | 21 (48.8) | |
| Low | 161 (64.7) | 139 (67.5) | 22 (51.2) | |
| TCFA | 81 (32.5) | 77 (37.4) | 4 (9.3) | <0.001 |
| Neointimal rupture | 42 (16.9) | 36 (17.5) | 6 (14.0) | 0.575 |
| Thrombus | 32 (12.9) | 24 (11.7) | 8 (18.6) | 0.215 |
| Neointimal macrophages | 94 (37.8) | 87 (42.2) | 7 (16.3) | 0.001 |
| Microvessels | 67 (26.9) | 58 (28.2) | 9 (20.9) | 0.331 |
| Underexpansion | 7 (2.8) | 5 (2.4) | 2 (4.7) | 0.768 |
Values are n (%) or median with first and third quartiles. ISR: in-stent restenosis; MLA: minimum lumen area; NIH: neointimal hyperplasia; TCFA:thin-cap fibrous atherosclerosis.
3.3. Prediction of ISNA and TCFA
Data of 249 lesions were used for logistic regression. As shown in Table 4, Table 5, univariate and multivariate analyses indicated that male had a higher risk of ISNA (OR: 2.659, 95% CI: 1.239–5.707; p = 0.012) and TCFA (OR: 4.216, 95% CI: 1.370–12.976; p = 0.012) than femal. Besides, multivariable analysis revealed that NIH >50% was also independently associated with ISNA (OR: 3.215, 95% CI: 1.408–7.341; p = 0.006) and TCFA (OR: 3.360, 95% CI: 1.330–8.487; p = 0.010).
Table 4.
Multivariate logistic regression analysis for independent predictors affecting the presence of OCT-ISNA.
| Univariable |
p value | Multivariate analysis |
p value | |
|---|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | |||
| Age >65 years | 0.823 (0.447–1.513) | 0.530 | ||
| Male | 2.827 (1.380–5.792) | 0.005 | 2.659 (1.239–5.707) | 0.012 |
| Current smoker | 1.278 (0.694–2.355) | 0.431 | ||
| Hypertension | 0.830 (0.439–1.569) | 0.566 | ||
| Diabetes mellitus | 0.661 (0.359–1.216) | 0.183 | ||
| Hyperlipidaemia | 0.644 (0.340–1.217) | 0.175 | ||
| LDL cholesterol, mg/dL | 1.249 (0.802–1.944) | 0.326 | ||
| Statin use at the time of ISR | 1.091 (0.442–2.689) | 0.851 | ||
| NIH >50% | 4.005 (1.978–8.108) | <0.001 | 3.215 (1.408–7.341) | 0.006 |
| Time since implantation, years | 1.092 (1.009–1.182) | 0.028 | 1.076 (0.990–1.170) | 0.085 |
| Minimum lumen area, mm2 | 0.701 (0.519–0.948) | 0.021 | 0.836 (0.582–1.201) | 0.333 |
ISNA: in-stent neoatherosclerosis; ISR: in-stent restenosis; LDL: low-density lipoprotein; NIH: neointimal hyperplasia.
Table 5.
Multivariate logistic regression analysis for independent predictors affecting the presence of OCT-TCFA.
| Univariable |
p value | Multivariate analysis |
p value | |
|---|---|---|---|---|
| Odds ratio (95% CI) | Odds ratio (95% CI) | |||
| Age >65 years | 0.919 (0.528–1.571) | 0.757 | ||
| Male | 5.627 (1.934–16.376) | 0.002 | 4.216 (1.370–12.976) | 0.012 |
| Current smoker | 1.939 (1.132–3.322) | 0.016 | 1.508 (0.833–2.728) | 0.175 |
| Hypertension | 0.943 (0.546–1.630) | 0.835 | ||
| Diabetes mellitus | 0.947 (0.557–1.613) | 0.842 | ||
| Hyperlipidaemia | 0.809 (0.449–1.456) | 0.480 | ||
| LDL cholesterol, mg/dL | 1.056 (0.734–1.520) | 0.768 | ||
| Statin use at the time of ISR | 1.205 (0.528–2.752) | 0.657 | ||
| NIH >50% | 3.531 (1.424–8.754) | 0.006 | 3.360 (1.330–8.487) | 0.010 |
| Time since implantation, years | 1.064 (1.000–1.131) | 0.048 | 1.055 (0.988–1.126) | 0.109 |
| Minimum lumen area, mm2 | 0.787 (0.588–1.053) | 0.106 |
ISR: in-stent restenosis; LDL: low-density lipoprotein; NIH: neointimal hyperplasia; TCFA:thin-cap fibrous atherosclerosis.
3.4. Reproducibility of analyzed OCT results
In the qualitative OCT assessment, the inter-researcher Kappa coefficients of LRP, TCFA and ISNA were 0.89, 0.91 and 0.93, respectively. The intra-investigator Kappa coefficients of LRP, TCFA and ISNA were 0.94, 0.91 and 0.93, respectively.
4. Discussion
In this large single-center retrospective study, we analyzed the neointimal characteristics of DES-ISR patients by OCT, and compared the differences in the features of DES-ISR neointima between different genders for the first time. According to the results of this study, the main findings were as follows:1) The incidence of OCT-ISNA was significantly higher in males than in females. 2)In ISR patients, neointimal vulnerability (OCT-TCFA, neointimal macrophage, LRP and calcified neointima) in male patients was higher than that in female patients. 3) Multivariate logistic regression analysis demonstrated male and higher NIH burden were independent predictors for OCT-ISNA and OCT-TCFA.
So far, no studies have shown the relationship between gender and neointima characteristics of DES-ISR, and there was no consensus on the mechanism of gender's influence on vulnerability of neointima. In this study, the incidence of ISNA and the neointimal vulnerability in male DES-ISR patients were higher than female. This may account for the different rates of adverse cardiovascular events between male and female. Therefore, the reasons for this difference in neointimal characteristics were worth further investigation. Modern epidemiological data consistently showed that young women had a lower risk of cardiovascular disease and a lower incidence of myocardial infarction than men, but the incidence of these disease in female catch up with men at age 60–79 and surpass men at age 80, possibly related to the gradual loss of the protective effect of estrogen on blood vessels as women age [18]. The results of another study showed that benefitting from female estrogen, the lipid level of females at the same age was lower than that of males [19], which may be one of the reasons why the incidence of LRP and ISNA in males was significantly higher than that in females. Women also respond better to statin therapy than men. SATURN Study (Study of Coronary Atheroma by inTravascular Ultrasound: The Effect of Rosuvastatin Versus Atorvastatin) demonstrated that women with LDL levels ≤70 mg/dL were independently associated with a greater reduction in atherosclerosis after statin treatment, but the exact mechanism of occurrence has not been clarified [20]. This means that blood lipid levels in men are more difficult to control than in women, which provides the basis for the formation of necrotic lipid cores. In addition to lipid levels, inflammation is also one of the important factors affecting the incidence of ISNA [21], and there are differences in the inflammatory response between different genders. Previous studies on human carotid artery AS pathological sections showed that males were significantly associated with increased inflammatory cell infiltration in AS plaque sites [19,22]. In this study, it was also found through OCT that the degree of macrophage infiltration in the intima of male DES-ISR patients was significantly higher than that of female patients, but the specific mechanism of this difference remains to be explored.
In addition to ISNA, vulnerability of neointima was also associated with the occurrence of TCFA, LRP, calcification, macrophages, and microvessels. Previous studies showed that LRP, TCFA, obvious inflammatory cell infiltration and microvessels were the basic characteristics of neointimal rupture and the risk of potentially fatal coronary events [23,24]. In the occurrence and development of atherosclerosis, the occurrence of TCFA, LRP, calcification, macrophages and microvessels were mostly related to the level of inflammation, renal function, diabetes and lipid spectrum disorders [25]. Similarly, the formation of vulnerability of neointima depended on inflammatory cells and lipids, leading to the thinning of fibrous cap and the formation of TCFA accelerated by microvessels and necrotic core [21,24]. In addition, recent investigations have provided compelling evidence regarding the significant involvement of neutrophil extracellular traps (NETs) in diverse pathophysiological processes associated with atherosclerosis, encompassing thrombosis, inflammation, and activation of the fibrinolytic system [26]. Locally, NETs can inflict substantial tissue damage within blood vessels and are independently correlated with inflammatory response and the severity of atherosclerosis [[26], [27], [28]]. Nevertheless, the association between NETs and intimal proliferation in patients with ISR necessitates further comprehensive investigation for conclusive validation. As the present study did not encompass the assessment of NETs in patients with thrombosis, we are unable to ascertain the potential impact of NETs on the outcomes of this investigation.
However, previous studies did not mention whether gender was an influencing factor for the vulnerability of DES-ISR neointima. According to our findings, there were significant differences in the incidence of TCFA, LRP, calcification and macrophage between males and females with DES-ISR. In addition, multivariate analysis suggested that male and NIH >50% were risk factors for the occurrence of TCFA in DES-ISR patients, indicating that we may need to take more active treatment for male patients.
Pathological studies demonstrated that in DES-ISR patients, ISNA, closely related to the occurrence of ISR, was independently associated with late thrombotic events after stent implantation [29,30], suggesting that ISNA contributed to the vulnerability of ISR neointima. Therefore, the identification and early intervention of risk factors for ISNA may be more beneficial to patients with ISR. Multiple previous studies indicated that chronic kidney disease, LDL level, duration from stent implantation and DES-ISR were independent predictors for neoatherosclerosis [31,32]. It was also confirmed that neointimal homogenous hyperplasia was more common in BMS-ISR and neoatherosclerosis was more common in DES-ISR [33]. Moreover, OCT studies have demonstrated that ISNA was less common in DES-ISR (early DES-ISR) within 1 year after PCI than in DES-ISR (late DES-ISR) developed 1 year after PCI [30], suggesting that the incidence of ISNA closely depended on the time since implantation and DES. However, it was found that only male and NIH >50% were closely related to ISNA by OCT in this study. This may be subjected to the bias in the patient population which had fewer patients with chronic renal insufficiency. Additionally, this study was a retrospective study, most of the selected ISR patients came to our hospital with obvious symptoms and asymptomatic patients were not included in this study, so the results may be different from previous studies.
5. Limitations
The study is subject to certain limitations. First of all, it is imperative to recognize that this investigation was conducted as a retrospective study in a single center. It is up to the physicians to decide if the patient needs OCT before PCI. Therefore, selection bias may have influenced the results. In addition, many patients with asymptomatic ISR or mild neointimal hyperplasia were not included in this study, so the representation of patients included in this study was limited. Nevertheless, this study is the first to investigate the relationship between neointima of DES-ISR and gender in the largest sample to date. Second, as recently reported by Lutter et al. [34]. OCT qualitative analysis of restenosis tissue may not correspond exactly with pathological findings, creating a risk of misdiagnosis of pathological features. Thirdly, This study is a retrospective study. Clinical data of patients included in this study, such as DES stent type, specific antiplatelet regimens after the index PCI and patients' medication adherence, were not completely collected, and it is impossible to assess whether these factors will affect the results of this study. Although we can confirm that all enrolled patients were implanted with DES and were recommended to receive the optimal medical therapy after the index PCI, this is one of the limitations of this study, which we will address in future prospective studies. Finally, this is a retrospective study without clinical follow-up, so gender differences in the relationship between neointimal vulnerability and future clinical events cannot be known. Future prospective studies will further confirm existing findings.
6. Conclusion
The incidence of ISNA and the neointimal vulnerability in male DES-ISR patients were higher than that in female DES-ISR patients. In addition, male and higher NIH were independent predictors for ISNA and TCFA. These observations support the notion that intervention of risk factors related to ISNA and neointimal vulnerability development may need to be strengthened for male DES-ISR patients.
Author contribution statement
Xiaohang Yuan and Mengting Jiang: Conceived and designed this study; and wrote the paper.
Huanhuan Feng, Yan Han, Xi Zhang: Analyzed and interpreted the data.
Yundai Chen and Lei Gao: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Data availability statement
The data that has been used is confidential.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Lei Gao reports financial support was provided by National Natural Science Foundation of China.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81970443).
Contributor Information
Yundai Chen, Email: cyundai@vip.163.com.
Lei Gao, Email: nkgaolei2010@126.com.
References
- 1.Bigeh A., Shekar C., Gulati M. Sex differences in coronary artery calcium and long-term CV mortality. Curr. Cardiol. Rep. 2020;22(4):21. doi: 10.1007/s11886-020-1267-9. [DOI] [PubMed] [Google Scholar]
- 2.Wang L., Mintz G.S., Witzenbichler B., et al. Differences in underlying culprit lesion morphology between men and women: an IVUS analysis from the ADAPT-DES study. JACC Cardiovasc. Imag. 2016;9(4):498–499. doi: 10.1016/j.jcmg.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Kim H.S., Tonino P.A., De Bruyne B., et al. Fearon WF; FAME Study Investigators, the impact of sex differences on fractional flow reserve-guided percutaneous coronary intervention: a FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) substudy. JACC Cardiovasc. Interv. 2012;5(10):1037–1042. doi: 10.1016/j.jcin.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto A., Sato Y., Kawakami R., et al. Risk prediction of in-stent restenosis among patients with coronary drug-eluting stents: current clinical approaches and challenges. Expert Rev. Cardiovasc Ther. 2021;19(9):801–816. doi: 10.1080/14779072.2021.1856657. [DOI] [PubMed] [Google Scholar]
- 5.Brown R.A., Williams M., Barker C.M., et al. Sex-specific outcomes following revascularization with zotarolimus-eluting stents: comparison of angiographic and late-term clinical results, Catheter. Cardiovasc. Interv. 2010;76(6):804–813. doi: 10.1002/ccd.22624. [DOI] [PubMed] [Google Scholar]
- 6.Rathore S., Terashima M., Katoh O., et al. Predictors of angiographic restenosis after drug eluting stents in the coronary arteries: contemporary practice in real world patients. EuroIntervention. 2009;5(3):349–354. doi: 10.4244/v5i3a55. [DOI] [PubMed] [Google Scholar]
- 7.Fernando A., Coughlan J.J., Daniele G., et al. Management of in-stent restenosis. EuroIntervention. 2022;18(2):e103–e123. doi: 10.4244/EIJ-D-21-01034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehran R., Dangas G., Abizaid A.S., et al. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100(18):1872–1878. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 9.Prati F., Guagliumi G., Mintz G.S., et al. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur. Heart J. 2012;33(20):2513–2520. doi: 10.1093/eurheartj/ehs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Räber L., Mintz G.S., Koskinas K.C., et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018;39(35):3281–3300. doi: 10.1093/eurheartj/ehy285. [DOI] [PubMed] [Google Scholar]
- 11.Song L., Mintz G.S., Yin D., et al. Characteristics of early versus late in-stent restenosis in second-generation drug-eluting stents: an optical coherence tomography study. EuroIntervention. 2017;13(3):294–302. doi: 10.4244/EIJ-D-16-00787. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura D., Attizzani G.F., Toma C., et al. Failure mechanisms and neoatherosclerosis patterns in very late drug-eluting and bare-metal stent thrombosis. Circ. Cardiovasc. Interv. 2016;9(9) doi: 10.1161/CIRCINTERVENTIONS.116.003785. [DOI] [PubMed] [Google Scholar]
- 13.Kubo T., Ino Y., Mintz G.S., et al. Optical coherence tomography detection of vulnerable plaques at high risk of developing acute coronary syndrome. Eur. Heart J. Cardiovasc. Imag. 2021 doi: 10.1093/ehjci/jeab028. jeab028. [DOI] [PubMed] [Google Scholar]
- 14.Tearney G.J., Regar E., Akasaka T., et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012;59(12):1058–1072. doi: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 15.Kato A., Kinoshita D., Nagata T., et al. Lipoprotein (a) levels and vulnerable characteristics in nonculprit plaque in patients with acute coronary syndrome. Int. J. Cardiol. Heart Vasc. 2022;43 doi: 10.1016/j.ijcha.2022.101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jinnouchi H., Kuramitsu S., Shinozaki T., et al. Difference of tissue characteristics between early and late restenosis after second-generation drug-eluting stents implantation - an optical coherence tomography study. Circ. J. : Off. J. Jap. Circ. Soci. 2017;81(4):450–457. doi: 10.1253/circj.CJ-16-1069. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto A., Sato Y., Kawakami R., et al. Risk prediction of in-stent restenosis among patients with coronary drug-eluting stents: current clinical approaches and challenges. Expert Rev. Cardiovasc Ther. 2021;19(9):801–816. doi: 10.1080/14779072.2021.1856657. [DOI] [PubMed] [Google Scholar]
- 18.Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 19.Man J.J., Beckman J.A., Jaffe I.Z. Sex as a biological variable in atherosclerosis. Circ. Res. 2020;126(9):1297–1319. doi: 10.1161/CIRCRESAHA.120.315930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puri R., Nissen S.E., Shao M., et al. Sex-related differences of coronary atherosclerosis regression following maximally intensive statin therapy: insights from SATURN. JACC. Cardiovasc. Imag. 2014;7(10):1013–1022. doi: 10.1016/j.jcmg.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Nusca A., Viscusi M.M., Piccirillo F., et al. In stent neo-atherosclerosis: pathophysiology, clinical implications, prevention, and therapeutic approaches. Life. 2022;12(3) doi: 10.3390/life12030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan X.M., Ward L.J., Forssell C., et al. Carotid atheroma from men has significantly higher levels of inflammation and iron metabolism enabled by macrophages. Stroke. 2018;49(2):419–425. doi: 10.1161/STROKEAHA.117.018724. [DOI] [PubMed] [Google Scholar]
- 23.Räber L., Ueki Y., Otsuka T., et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA. 2022;327(18):1771–1781. doi: 10.1001/jama.2022.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao L., Park S.J., Jang Y., et al. Comparison of neoatherosclerosis and neovascularization between patients with and without diabetes: an optical coherence tomography study. JACC Cardiovasc. Interv. 2015;8(8):1044–1052. doi: 10.1016/j.jcin.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Engelen S.E., Robinson A.J.B., Zurke Y.X., et al. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat. Rev. Cardiol. 2022;19(8):522–542. doi: 10.1038/s41569-021-00668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J., Chen R., Liu C., et al. Associations of NETs with inflammatory risk and atherosclerotic severity in ST-segment elevation myocardial infarction. Thromb. Res. 2021;203:5–11. doi: 10.1016/j.thromres.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Silvestre-Roig C., Braster Q., Wichapong K., et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature. 2019;569(7755):236–240. doi: 10.1038/s41586-019-1167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novotny J., Oberdieck P., Titova A., et al. Thrombus NET content is associated with clinical outcome in stroke and myocardial infarction. Neurology. 2020;94(22):e2346–e2360. doi: 10.1212/WNL.0000000000009532. [DOI] [PubMed] [Google Scholar]
- 29.Brener S.J., Kereiakes D.J., Simonton C.A., et al. Everolimus-eluting stents in patients undergoing percutaneous coronary intervention: final 3-year results of the clinical evaluation of the XIENCE V everolimus eluting coronary stent system in the treatment of subjects with de Novo native coronary artery lesions trial. Am. Heart J. 2013;166(6):1035–1042. doi: 10.1016/j.ahj.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 30.Nakazawa G., Otsuka F., Nakano M., et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J. Am. Coll. Cardiol. 2011;57(11):1314–1322. doi: 10.1016/j.jacc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stettler R., Dijkstra J., Räber L., et al. Neointima and neoatherosclerotic characteristics in bare metal and first- and second-generation drug-eluting stents in patients admitted with cardiovascular events attributed to stent failure: an optical coherence tomography study. EuroIntervention. 2018;13(15):e1831–e1840. doi: 10.4244/EIJ-D-17-00051. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura D., Dohi T., Ishihara T., et al. Predictors and outcomes of neoatherosclerosis in patients with in-stent restenosis. EuroIntervention. 2021;17(6):489–496. doi: 10.4244/EIJ-D-20-00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilickesmez K., Dall'Ara G., Rama-Merchan J.C., et al. Optical coherence tomography characteristics of in-stent restenosis are different between first and second generation drug eluting stents. Int. J. Cardiol. Heart Vessel. 2014;3:68–74. doi: 10.1016/j.ijchv.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutter C., Mori H., Yahagi K., et al. Histopathological differential diagnosis of optical coherence tomographic image interpretation after stenting. JACC Cardiovasc. Interv. 2016;9(24):2511–2523. doi: 10.1016/j.jcin.2016.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.