Abstract
Study Design:
Retrospective Cohort Study.
Objective:
Octogenarians living with spinal metastases are a challenging population to treat. Our objective was to identify the rate, types, management, and predictors of complications and survival in octogenarians following surgery for spinal metastases.
Methods:
A retrospective review of a prospectively collected cohort of patients aged 80 years or older who underwent surgery for metastatic spinal tumor treatment between 2008 and 2019 were included. Demographic, intraoperative, complications, and postoperative follow-up data was collected. Cox proportional hazards regression and logistic regression were used to associate variables with overall survival and postoperative complications, respectively.
Results:
78 patients (mean 83.6 years) met inclusion criteria. Average operative time and blood loss were 157 minutes and 615 mL, respectively. The median length of stay was 7 days. The overall complication rate was 31% (N = 24), with 21% considered major and 7% considered life-threatening or fatal. Blood loss was significantly associated with postoperative complications (OR = 1.002; P = 0.02) and mortality (HR = 1.0007; P = 0.04). Significant associations of increased risk of death were also noted with surgeries with decompression, and cervical/cervicothoracic index level of disease. For deceased patients, median time to death was 4.5 months. For living patients, median follow-up was 14.5 months. The Kaplan-Meier based median overall survival for the cohort was 11.6 months (95% CI: 6.2-19.1).
Conclusions:
In octogenarians undergoing surgery with instrumentation for spinal metastases, the median overall survival is 11.6 months. There is an increased complication rate, but only 7% are life-threatening or fatal. Patients are at increased risk for complications and mortality particularly when performing decompression with stabilization, with increasing intraoperative blood loss, and with cervical/cervicothoracic tumors.
Keywords: octogenarian, elderly, separation surgery, spinal metastasis, spine tumor, percutaneous stabilization
Introduction
The spine is the most common musculoskeletal organ affected by metastatic disease, occurring in up to 10% of cancer patients. 1 Indications for surgery in these patients include high grade epidural neural compression or stabilization of mechanically unstable spines. A major component of the decision-making paradigm is which patients can optimally proceed to spinal surgery. This is determined by a thorough understanding of the risk-benefit analysis of surgical complication rates, survival, and postoperative quality of life. Advances in healthcare over the past decade have resulted in extended life expectancies for patients living with a number of different malignant cancers. 2 Behind these advances are a better understating of molecular drivers of tumorigenesis concomitant with the development of targeted immunotherapies, improvement in stereotactic radiation delivery technologies, and innovations in surgical techniques and instrumentation.3,4
In this regard, cancer in octogenarians has historically been difficult to treat, given a high incidence of medical comorbidities and lack of quality, long-term survival data following complex operations.5-8 However, a number of recent studies have evaluated the safety and effectiveness of surgery for other primary malignancies in this age group, which have shown acceptable outcomes for patients diagnosed with colorectal,9,10 breast,11,12 lung,13-15 endometrial, 11 renal,11,16,17 and bladder17,18 cancers. Patients requiring spinal surgery with instrumentation for stabilization and or fusion on the other hand, remain a difficult population to manage in the setting of traumatic, degenerative, and neoplastic pathologies. The major issue these individuals experience following surgery is underscored by pain and limitations with ambulation, which may then propagate a cascade of other local or systemic complications including wound infections, pneumonias and deep venous thromboses, for which advanced stage cancer patients are already at increased risk.6,19-21
In the present study, our goals were 3-fold. First, to identify the rate of postoperative complications in octogenarians following surgery for metastatic spinal disease with posterolateral instrumentation. Second, to determine the median survival of octogenarian patients following surgery for spinal metastases. Third, to identify which risk factors correlate with adverse outcomes in order to better define clinical characteristics that should be strongly considered in the preoperative evaluation.
Materials and Methods
A retrospective study of a prospectively collected cohort of patients aged 80 or greater years old undergoing surgery for metastatic spine disease at a single tertiary institution between 2008 and 2019 was performed. Institutional review board (IRB) approval for the study was obtained (IRB #16-1263). Informed consent was obtained from all patients, including detailed discussions regarding risks, benefits, and alternatives for surgery and for inclusion in the study protocol. Patients with intradural tumors, primary spine tumors, and previous operations for degenerative disease or trauma were excluded. Age at time of surgery, gender, primary tumor histology, index level of disease, length of stay, discharge destination, postoperative complication, indication for return to OR for complication, mortality status, and survival time after surgery were collected. Operative data including stabilization technique (open versus minimally invasive surgery (MIS)), levels decompressed, levels instrumented, use of cement augmentation, kyphoplasty levels, operative time, blood loss was collected. Index level of disease was classified as cervical, cervicothoracic, thoracic, thoracolumbar, or lumbar. Complications were classified as wound-related (dehiscence or infection), hematoma, deep venous thrombosis, pulmonary (pneumonia, effusion), cardiac (arrest, myocardial infarction), spinal cord infarct, seroma, sepsis, hardware failure, kyphoplasty/cement migration, and C5 palsy. Complications were also divided into 1 of 5 classes based on the proposed system by Dindo et al. 22 Discharge destination was classified as either home, rehabilitation center, hospice, or death in hospital. Survival time was classified as months between surgery and death for deceased patients, or as months from surgery to last known follow-up for patients still alive.
The criteria to pursue surgical intervention was determined using the NOMS paradigm, which takes into account neurologic status, degree of epidural spinal cord or cauda equina compression, predicted response to oncologic treatment (i.e. radiosensitivity of primary tumor histology), presence of mechanical instability based on the Spinal Neoplastic Instability Score,23-25 and burden of systemic disease. 26 Surgeries were performed via an open or percutaneous (MIS) posterolateral approach with screw and bilateral rod fixation. Decompressions in open surgeries were performed using a posterior only laminectomy and transpedicular approach for circumferential tumor decompression. This “separation surgery,” as previously described by our group, 27 did not include en bloc resections, or anterior expandable cage, titanium mesh, or strut graft reconstruction. The only type of anterior reconstruction that was performed in select cases was a transpedicular approach for vertebroplasty with polymethyl methacrylate (PMMA) cement. None of the surgeries performed required staging or separate anterior/lateral approaches. The type of surgery (open versus MIS) was individually determined for each patient by the operating surgeon based on anatomic, radiographic, and clinical factors. All surgeries were performed by 1 of 3 surgeons. Mortality status, date of death, and last known follow-up were determined by a combination of outpatient visits, follow-up phone calls, and electronic medical record review.
Statistical Analysis
Descriptive statistics such as medians, ranges, and proportions were used to characterize the cohort under study. The Kaplan Meier method was used to determine and graphically display overall survival which was defined from date of surgery until death for those with an event or until last follow up for those who were censored. Overall median survival using Kaplan-Meier methodology was defined at the time that 50% of our cohort was still alive. Univariable and multivariable Cox proportional hazards regression was used to associate variables of interest with overall survival in time-to-event analyses. Univariable associations of variables of interest with postoperative complications occurring during postoperative inpatient stay were explored using the Student’s t-test for continuous variables and Fisher’s test for categorical variables. Multivariable logistic regression was used to associate variables of interest in an adjusted fashion with postoperative complications occurring during postoperative inpatient stay. All statistical tests were 2-sided. P-values less than 0.05 were considered statistically significant. All analyses were performed in SAS v9.4 (SAS Institute Inc., Cary, NC) and R v3.6.0.
Results
A total of 78 patients met inclusion criteria (65.4% male). Median and mean age at time of surgery was 82.7 and 83.6 years old, respectively (range 80-91 years old). The most common primary tumor histologies were non-small cell lung (N = 18; 23%), prostate (N = 17; 21.8%), renal cell carcinoma (N = 8; 10.2%) and breast (N = 7; 8.9%) (Table 1). 63 and 15 patients underwent open and percutaneous instrumented stabilization, respectively, where 85.9% of all surgeries involved the thoracic and/or lumbar spine segments as the index level of pathology. 65 patients (83.3%) had decompression performed in addition to stabilization. Cement augmentation of pedicle screws was used in 21 cases (26.9%) and kyphoplasty was performed in 14 cases (17.9%). Average operative time and blood loss were 2 hours 37 minutes and 615 mL, respectively (Table 1).
Table 1.
Patient and Operative Characteristics.
| Characteristic | N (% or range) |
|---|---|
| Median age at surgery (years) | 82.8 (80.1-91.1) |
| Sex | |
| Male | 51 (65) |
| Female | 27 (35) |
| Metastatic tumor diagnosis | |
| Lung (NSCLC) | 18 (23) |
| Prostate | 17 (22) |
| Renal | 8 (10) |
| Breast | 7 (9) |
| Sarcoma | 5 (6) |
| Urothelial/Bladder | 5 (6) |
| Other | 18 (23) |
| Type of Surgery | |
| Open | 63 (81) |
| MIS | 15 (19) |
| Treatment level, No. (%) | |
| Cervical | 8 (10) |
| Cervicothoracic | 3 (4) |
| Thoracic | 42 (54) |
| Thoracolumbar | 2 (3) |
| Lumbar | 23 (29) |
| Length of Construct | |
| 3 levels | 17 (22) |
| 4 levels | 13 (17) |
| 5 levels | 18 (23) |
| 6 levels | 18 (23) |
| ≥ 7 levels | 12 (15) |
| Decompression performed | |
| Yes | 65 (83) |
| No | 13 (17) |
| Median estimated blood loss (mL) | 500 (10-3250) |
| Median length of surgery (minutes) | 146 (68-290) |
Abbreviations: NSCLC, non-small cell lung cancer; MIS, Minimally invasive surgery.
24 patients (31%) developed postoperative complications. The most common complications were wound dehiscence or infection (N = 6; 8%), pulmonary (pneumonia or effusion) (N = 4; 5.1%), and hematoma (N = 3; 3.8%). 9 patients (11.5%) had to return to the operating room for postoperative complications; 6 for wound washout and revision and 3 for hematoma evacuation. 3 patients (3.8%) required interventional procedures for postoperative complications; 1 for pleural effusion tap and 2 for drainage of seromas. 4 patients (5.1%) died the same admission as a result of complications (2 from cardiac arrest, 1 from sepsis, 1 from respiratory failure). When stratifying based on the severity of complications described by Dindo et al. 16 10% of patients had complications that were considered minor, requiring no or only pharmacological treatment (Grades I and II). 21% of all patients had major complications. 14% (N = 11) were Grade III requiring an operative or interventional procedure. 7% (N = 5) of all patients were considered severe (Grades IV and V), based on life-threatening complications requiring intensive care monitoring or resulting in death (Table 2). No patients in our cohort had complications related to acute renal failure, hepatic failure, or gastrointestinal bleeding.
Table 2.
Postoperative Complications.
| Complication | N (% of total cohort) | Return to OR or interventional procedure* N (% of total cohort) | |
|---|---|---|---|
| Total | 24 (31) | 12 (16) | |
| Wound | 6 (8) | 6 (8) | |
| Pulmonary (PNA, effusion) | 4 (5) | 1 (1)* | |
| Hematoma | 3 (4) | 3 (4) | |
| DVT | 2 (3) | — | |
| Seroma | 2 (3) | 2 (3)* | |
| Cardiac arrest | 2 (3) | — | |
| Spinal cord stroke | 1 (1) | — | |
| Sepsis | 1 (1) | ||
| C5 palsy | 1 (1) | — | |
| Kyphoplasty/cement complication | 1 (1) | — | |
| Hardware failure | 1 (1) | — | |
|
Grade† |
N |
% of total complications |
% of total cohort |
| Grade I | 3 | 13 | 4 |
| Grade II | 5 | 21 | 6 |
| Grade III | 11** | 46 | 14 |
| Grade IV | 1 | 4 | 1 |
| Grade V | 4 | 16 | 6 |
Abbreviations: PNA, pneumonia; DVT, deep venous thrombosis; OR, operating room.
* Interventional procedure defined as percutaneous tap or drain placement.
† Based on complication classification by Dindo et al Grades I-II are “minor.” Grades III and greater and “major.”
** One patient underwent an interventional procedure then subsequently died, therefore classified as Grade V and not Grade III.
No variables of interest were associated with postoperative complications (Table 3) in the univariate setting. In the multivariable logistic and Cox regression models, intraoperative blood loss was significantly associated with postoperative complications (OR = 1.002; P = 0.02) and mortality (HR = 1.0007; P = 0.04), respectively. Age, operative time, construct length, index level, and type of surgery (open vs. MIS) were not significantly associated with complications in the multivariable logistic regression model (Table 4). No variables of interest were associated with survival (Table 5) in the univariate setting. Multivariable Cox regression analysis demonstrated significant associations of increased risk of death with increased blood loss, shorter operative time, surgeries with decompression, and cervical/cervicothoracic index level of disease (Table 5). However, given the low number of events per category of analysis, we were unable to demonstrate the same kind of stability in multivariable model estimates as we were in the estimates from univariable models.
Table 3.
Patient Characteristics and Unadjusted Associations With Postoperative Complications.
| Postoperative complications | No postoperative complications | ||||
|---|---|---|---|---|---|
| Variable | N* | % | N* | % | P-value |
| Age in years, median (range) | 82.9 | 80.1-89.8 | 82.7 | 80.4-91.1 | 0.87 |
| Length of Construct in number of levels, median (range) | 5 | 3-10 | 5 | 3-9 | 0.64 |
| Surgery time in minutes, median (range) | 152 | 102-289 | 137.5 | 68-290 | 0.13 |
| Blood loss in mL, median (range) | 575 | 25-3250 | 450 | 10-2000 | 0.08 |
| Decompression | |||||
| No | 3 | 13 | 10 | 19 | 0.74 |
| Yes | 21 | 88 | 44 | 81 | |
| Index Level | |||||
| Thoracic | 12 | 50 | 30 | 56 | 0.52 |
| Cervical/Cervicothoracic | 5 | 21 | 6 | 11 | |
| Lumbar/Thoracolumbar | 7 | 29 | 18 | 33 | |
| MIS | |||||
| No | 19 | 79 | 44 | 81 | 1.00 |
| Yes | 5 | 21 | 10 | 19 | |
* Results presented are number and percentage unless otherwise indicated.
Table 4.
Patient Characteristics and Adjusted Associations With Postoperative Complications.
| Adjusted | |||
|---|---|---|---|
| Variable | OR | 95% CI | P-value |
| Age (years) | 1.05 | 0.86-1.27 | 0.66 |
| Length of Construct (# of levels) | 0.98 | 0.62-1.56 | 0.94 |
| Surgery time (minutes) | 0.999 | 0.987-1.012 | 0.89 |
| Blood loss (mL) | 1.002 | 1.000-1.003 | 0.02 |
| Decompression | |||
| No | ref | — | — |
| Yes | 6.16 | 0.31-121.32 | 0.23 |
| Index Level | |||
| Thoracic | ref | — | — |
| Cervical/Cervicothoracic | 4.67 | 0.87-25.08 | 0.07 |
| Lumbar/Thoracolumbar | 0.91 | 0.24-3.52 | 0.90 |
| MIS | |||
| No | ref | — | — |
| Yes | 18.31 | 0.85-394.55 | 0.06 |
Abbreviations: CI, confidence interval; Ml, milliliters; MIS, minimally invasive surgery; OR, odds ratio; ref, reference.
Table 5.
Patient Characteristics and Unadjusted and Adjusted Associations With Overall Survival.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age (years) | 0.94 | 0.83-1.06 | 0.31 | 0.98 | 0.85-1.13 | 0.80 |
| Length of Construct (# of levels) | 1.11 | 0.93-1.33 | 0.24 | 1.09 | 0.82-1.45 | 0.57 |
| Surgery time (minutes) | 1.00 | 0.99-1.00 | 0.37 | 0.99 | 0.98-0.999 | 0.02 |
| Blood loss (mL) | 1.0005 | 0.99 997-1.00 096 | 0.07 | 1.0007 | 1.00 003-1.001 | 0.04 |
| Decompression | ||||||
| No | ref | — | — | ref | — | — |
| Yes | 2.12 | 0.90-5.03 | 0.09 | 7.46 | 1.28-43.46 | 0.03 |
| Index Level | ||||||
| Thoracic | ref | — | — | ref | — | — |
| Cervical/Cervicothoracic | 1.93 | 0.89-4.22 | 0.10 | 2.52 | 1.05-6.05 | 0.04 |
| Lumbar/Thoracolumbar | 0.96 | 0.50-1.83 | 0.90 | 1.01 | 0.51-1.99 | 0.98 |
| MIS | ||||||
| No | ref | — | — | ref | — | — |
| Yes | 0.56 | 0.25-1.26 | 0.16 | 5.57 | 0.91-34.08 | 0.06 |
Abbreviations: CI, confidence interval; mL, milliliters; MIS, minimally invasive surgery; HR, hazard ratio; ref, reference.
Boldface values indicates statistically significant.
The median length of stay was 7 days. 39 patients (50%) were discharged home and 32 (41%) were discharged to rehab. 3 patients (3.8%) went to hospice and 4 (5.1%) died in the hospital. The overall median follow-up time was 6 months (range 0.3-76.1). For deceased patients, median time to death was 4.5 months. For patients still alive, median follow-up period was 14.5 months (Table 6). The Kaplan-Meier based median overall survival, defined at the time 50% of the cohort was still alive, was 11.6 months (95% CI: 6.2-19.1) (Figure 1).
Table 6.
Postoperative Clinical Outcomes.
| Variable | N (% or range) |
|---|---|
| Median length of stay (days) | 7 (2-46) |
| Discharge Destination | |
| Home | 39 (50) |
| Rehab | 32 (41) |
| Hospice | 3 (4) |
| Death in hospital | 4 (5) |
| Status at last follow-up | |
| Alive | 28 (36) |
| Deceased | 50 (64) |
| Median follow-up time based on status (months) | |
| Total | 6.0 (0.3-76.1) |
| Alive | 14.5 (0.8-46.4) |
| Deceased | 4.5 (0.3-76.1) |
| Kaplan-Meier Median Overall Survival (months) | 11.6 (95%CI 6.2-19.1) |
Abbreviation: CI, confidence interval.
Figure 1.
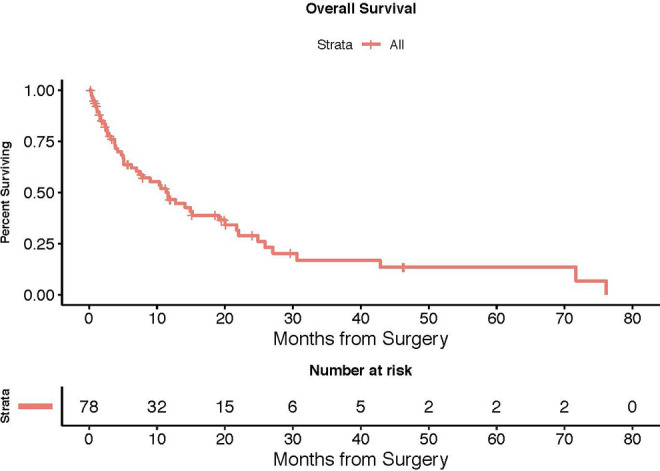
Kaplan-Meier curve. The Kaplan-Meier based median overall survival, defined at the time 50% of the cohort was still alive, was 11.6 months (95% CI: 6.2-19.1).
Discussion
The data presented in this manuscript represents the largest analysis of surgical outcomes for metastatic spine disease in the octogenarian and older population. Our cohort generally reflects the most frequently seen malignancies among the general population, including lung, prostate, renal, and breast. The vast majority of patients (91%) had outcomes that resulted in discharge home or to rehabilitation centers after a median of 7 days in the hospital. There was a 5.1% same-admission mortality rate with an overall 31% postoperative complication rate. When stratifying by severity, 10% of all patients had minor complications and 21% had major complications. The most common surgical complications were related to wound healing, hematoma, and seromas, all of which required re-operations or interventional drainage. The most common non-spinal complication was for pulmonary etiologies, including pneumonia and effusions, one of which required an interventional tap. Median survival was 11.6 months, with follow-up time for patients who died during the course of the study being 4.5 months. Patients who were still alive at last follow-up were a median of 14.5 months out from surgery.
Overall mortality rates following elective degenerative spinal fusion surgery in octogenarians have been quoted to be as high as 8.4% with wide variation in complication rates ranging from 10-71% reported in the literature.28-31 Surgeries with instrumentation, number of levels instrumented, operative time, and lumbar surgeries have been shown to correlate with complications in these studies.28,32 Specific to the cancer population, overall complication rates for patients undergoing spine surgery are 14.4% with a 3.3% 30-day mortality rate. 33 Surgical site infections, deep venous thromboses, and systemic infections are the most common complications. The most comprehensive analysis regarding age-related morbidity from spine tumor surgery was reported by Amelot et al as part of a multi-center study. 6 This study reported outcomes for elderly patients (not specifically octogenarians) undergoing surgery for spinal metastases and found an overall complication rate of 33.3%. Compared with individuals aged 70-79 years, patients aged > = 80 years of age (N = 51) had lower survival and lower likelihood of neurologic recovery with pre-existing deficits. Despite the higher complication rate, these patients still experienced significant improvements in quality of life based on patient reported outcome measures. 6
Interestingly, increasing age within this population and length of instrumentation construct did not demonstrate significant associations with complications nor overall survival in our study. Associations between tumor histology and outcomes could not be assessed due to the heterogeneity and relatively small sample size for each group. Intraoperative blood loss was statistically significantly associated with complications and mortality, but by a minimal degree (OR = 1.002; P = 0.02 and HR = 1.0007; P = 0.04, respectively). Surgeries with decompression in addition to instrumented stabilization and those with cervical/cervicothoracic index level of disease were significantly associated with 7.5- and 2.5-times increased risk of death, respectively.
A hurdle in the accurate reporting of complications for patients following spine surgery is in the inconsistency in defining what should and should not be considered a complication. 34 While there are generally accepted complications universal to all surgeries, 22 different indications (i.e. deformity, neoplastic, degenerative, trauma) may justify a different set of complications that are ultimately reported that are relevant to that particular patient population. For example, large multi-center studies have reported complications divided into intraoperative or postoperative, surgical or medical, major or minor, neurologic or radiographic, and early or late classes.35-41 Lee et al described perioperative complications in 200 patients surgically treated for spinal metastases and described an overall complication rate of 16.5%. 20 These included wound dehiscence (4.5%), pneumonia (3.5%), epidural hematoma (2.5%) acute renal failure (2.5%), sepsis (2.5%), hepatic failure (1.0%) and cardiac arrest (1.0%). When stratifying complications as a function of surgical aggressiveness based on 3 groups (en bloc resections, debulking/curettage surgeries, and palliative surgeries), they found that the rates of complications were 12.9%, 20.7%, and 14.3%, respectively. Cumulatively these complication rates were lower than those found in our paper, likely due to the fact that adults with a mean age of 51.7 years were analyzed. An extensive literature review by Luksanapruksa et al found that complication rates can range from 5.3-76.2% depending on the study with an average rate of 26.87%. 21 The most common surgical-related complications were wound dehiscence/infections (10.2%), hematomas (2.29%), and hardware problems (1.52%). The most common medical complications were delirium (11.2%), pneumonia (3.98%), and DVT (2.36%). Igoumenou et al similarly performed a literature review of complications in spine surgery for metastases and found DVT and PE rates ranging from 2-5.6%, hematomas ranging from 5.9-12%, dural tears ranging from 2-16%, wound dehiscence/infections ranging from 1.5-30%, and instrumentation failure ranging 0.3-5%. 19 Other less commonly reported complications they note are prolonged postoperative intubation, hypercalcemic crisis, and retroperitoneal hemorrhages. Some complications described in these studies are difficult to definitively and objectively diagnose based on our available data (e.g., delirium, ileus). After reviewing the literature on complications in spinal metastases surgery, we ultimately elected to include only those that required some form of medical, procedural, or surgical intervention, or those that resulted in permanent disability (e.g., spinal cord stroke) or death (e.g., cardiac arrest). Other complications that have been reported in the spine literature but beyond the scope of this analysis include urinary tract infections, side effects of pain medication, electrolyte imbalances, and requirement of blood transfusions. Given the retrospective nature of this analysis and based on the data we have available, we did not feel that we can reliably describe all minor/outpatient related complications without significant bias leading to under or over reporting of systemic complications. Future and more granular studies should consider including these variables based on the endpoint of interest.
Minimally invasive strategies for spinal decompression and stabilization are becoming more prevalent with advances in technology and surgical techniques. The advantages of less blood loss, earlier ambulation, shorter hospital stays, reduction in narcotic requirement, and infection reduction have been extensively reported in the spine literature for degenerative conditions and deformity.42-45 The use of tubular retractors, endoscopic approaches, percutaneous pedicle screw stabilization, and fenestrated pedicle screws with cement augmentation for shorter constructs have shown significant benefits over traditional open techniques in the general spine tumor population.46-48 However, most cases in our study were performed open (81%), and there was no statistically significant advantage to lowering complications or increasing survival with MIS surgery. Hansen-Algenstaedt et al 49 performed a prospective propensity score-matched study of 30 patients each undergoing MIS or open surgery for spinal metastases. They did not find a statistically significant difference in Eastern Cooperative Oncology Group (ECOG), Karnofsky scores, visual analog scale (VAS) for pain, and neurological status postoperatively between the 2 groups. However, the MIS group had less blood loss, blood transfusions, and shorter hospital stays. Collectively, these findings suggest that larger studies are required to more reliably detect a difference in outcomes between traditional and MIS approaches in elderly patients with spinal metastases.
An emerging concept that originated in geriatric studies and subsequently validated in the surgical literature is that of “frailty.” In general, frailty is a score that works to more objectively define physiologic reserves, functional status, nutritional status, and comorbidities in older and sicker patients.50-56 Various frailty indices have been described in the spine surgery population,57-60 but few have focused on patients undergoing surgery for spinal metastases. De la Garza Ramos et al developed the Metastatic Spine Tumor Frailty Index (MSTFI) utilizing a large Nationwide Inpatient Sample database. 61 The authors used multiple logistic regression modeling to identify 9 parameters that were then used to build the MSTFI. They found an overall perioperative complication rate of 19.3% and patients designated as “severely” frail demonstrated 5.7- and 7-fold increased risk of inpatient mortality and major in-hospital complication, respectively.
These studies, among others, highlights another important strategy in the management of elderly patients with metastatic disease. The inclusion of geriatric-specialists as part of the multi-disciplinary team involving medical oncologists, radiation oncologists, and surgeons may ultimately improve the quality of care and these patients receive, which in turn can improve health-related quality of life and survival outcomes. A study by Festen et al assessed 197 patients aged 70 or older with solid malignancies and found that 27% had different treatment recommendations based on a geriatric multi-disciplinary team as compared with their traditional tumor board. 62 The differences in recommendations were predominantly toward less intensive curative or palliative treatments. Shahrokni and Alexander highlighted that geriatrician involvement in the surgical management of the elderly with cancer should place emphasis on prehabilitation, geriatric co-management in the postoperative period, and enrollment in transitional care models. 63
There are several limitations with the present study that must be acknowledged. First, the relatively small patient population meeting inclusion criteria for the study may have been underpowered to detect significant associations between demographic and operative variables with complications and survival. Second, it was not possible to collect a clinically-matched cohort of patients less than 80 years old (including histology, neurologic presentation, level and extent of disease) to evaluate head-to-head outcomes and comparisons. Third, preoperative baseline characteristics of the patients included in our study were not collected. There is likely bias in that patients who ultimately were offered surgery were healthier patients with lower burden of systemic disease. Fourth, post-operative adjuvants including radiation or chemotherapy/immunotherapy, which likely influence patient survival, were beyond the scope of this analysis. Notably, at our institution all octogenarians considered for surgery undergo a formal geriatric and medical oncology evaluation and patients’ ability to tolerate the planned procedure and further oncologic therapies are thoroughly evaluated. Fifth, discharge destination is not only dependent upon postoperative functional status, but often times due to social situations that are not reflected by our data (e.g., patient lives alone with no additional care).
Conclusion
Surgery for spinal metastatic disease with posterior pedicle screw stabilization in patients who are 80 years of age or older carries a 31% postoperative complication rate, but only 7% develop life-threatening or fatal complications. Blood loss is significantly associated with postoperative complications. There is increased mortality in patients with increasing blood loss, those undergoing concomitant decompression with instrumented stabilization, and those with cervical/cervicothoracic index level of disease. For deceased patients, median time to death was 4.5 months. For patients still alive, median follow-up period was 14.5 months. The Kaplan-Meier based median overall survival for the cohort was 11.6 months (95% CI: 6.2-19.1). Given the expected increased rate of complications, patients may benefit from geriatric preoperative risk assessment and post-operative co-management.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Ilya Laufer receives consulting fees from DePuy/Synthes, Globus, Spinewave, and Medtronic. Mark H. Bilsky receives consulting fees for Varian, Stryker and royalties from Globus and DePuy/Synthes.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was funded by an NIH/NCI Cancer Center support grant no. P30 CA008748.
ORCID iDs: Ibrahim Hussain, MD  https://orcid.org/0000-0002-6343-2228
https://orcid.org/0000-0002-6343-2228
Anne S. Reiner, MPH  https://orcid.org/0000-0002-1258-6112
https://orcid.org/0000-0002-1258-6112
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Dal Maso L, Panato C, Guzzinati S; et al. AIRTUM Working group. Prognosis and cure of long-term cancer survivors: a population-based estimation. Cancer Med. 2019;8(9):4497–4507. doi:10.1002/cam4.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothrock RJ, Barzilai O, Reiner AS, et al. Survival trends after surgery for spinal metastatic tumors: 20-year cancer center experience. Neurosurgery. 2021;88(2):402–412. doi:10.1093/neuros/nyaa380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothrock R, Pennington Z, Ehresman J, et al. Hybrid therapy for spinal metastases. Neurosurg Clin N Am. 2020;31(2):191–200. doi:10.1016/j.nec.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 5.Aebi M. Spinal metastasis in the elderly. Eur Spine J. 2003;12(suppl 2):S202–S213. doi:10.1007/s00586-003-0609-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amelot A, Balabaud L, Choi D, et al. Surgery for metastatic spine tumors in the elderly. Advanced age is not a contraindication to surgery! Spine J. 2017;17(6):759–767. doi:10.1016/j.spinee.2015.07.440 [DOI] [PubMed] [Google Scholar]
- 7.Rades D, Evers JN, Bajrovic A, Veninga T, Schild SE. Re-irradiation of spinal cord compression due to metastasis in elderly patients. Anticancer Res. 2014;34(5):2555–2558. [PubMed] [Google Scholar]
- 8.Given B, Given CW. Older adults and cancer treatment. Cancer. 2008;113(12 suppl):3505–3511. doi:10.1002/cncr.23939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weerink LBM, Gant CM, van Leeuwen BL, et al. Long-term survival in octogenarians after surgical treatment for colorectal cancer: prevention of postoperative complications is key. Ann Surg Oncol. 2018;25(13):3874–3882. doi:10.1245/s10434-018-6766-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan Kim Y. Surgical treatment for colorectal cancer in octogenarians and nonagenarians. J BUON. 2017;22(3):578–585. [PubMed] [Google Scholar]
- 11.Damhuis RA, Meurs CJ, Meijer WS. Postoperative mortality after cancer surgery in octogenarians and nonagenarians: results from a series of 5,390 patients. World J Surg Oncol. 2005;3:71. doi:10.1186/1477-7819-3-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser R, Marinopoulos S, Dimitrakakis C. Breast cancer treatment in women over the age of 80: a tailored approach. Maturitas. 2018;110:29–32. doi:10.1016/j.maturitas.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 13.Dominguez-Ventura A, Allen MS, Cassivi SD, Nichols FC III, Deschamps C, Pairolero PC. Lung cancer in octogenarians: factors affecting morbidity and mortality after pulmonary resection. Ann Thorac Surg. 2006;82(4):1175–1179. doi:10.1016/j.athoracsur.2006.04.052 [DOI] [PubMed] [Google Scholar]
- 14.Pagni S, Federico JA, Ponn RB. Pulmonary resection for lung cancer in octogenarians. Ann Thorac Surg. 1997;63(3):785–789. doi:10.1016/s0003-4975(96)01150-2 [DOI] [PubMed] [Google Scholar]
- 15.Tanita T, Hoshikawa Y, Tabata T, et al. Functional evaluations for pulmonary resection for lung cancer in octogenarians. Investigation from postoperative complications. Jpn J Thorac Cardiovasc Surg. 1999;47(6):253–261. [DOI] [PubMed] [Google Scholar]
- 16.Miller C, Raza SJ, Davaro F, May A, Siddiqui S, Hamilton ZA. Trends in the treatment of clinical T1 renal cell carcinoma for octogenarians: analysis of the national cancer database. J Geriatr Oncol. 2019;10(2):285–291. doi:10.1016/j.jgo.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 17.Liguori G, Trombetta C, Pomara G, et al. Major invasive surgery for urologic cancer in octogenarians with comorbid medical conditions. Eur Urol. 2007;51(6):1600–1604; discussion 1605. doi:10.1016/j.eururo.2006.07.046 [DOI] [PubMed] [Google Scholar]
- 18.De Groote R, Gandaglia G, Geurts N, et al. Robot-assisted radical cystectomy for bladder cancer in octogenarians. J Endourol. 2016;30(7):792–798. doi:10.1089/end.2016.0050 [DOI] [PubMed] [Google Scholar]
- 19.Igoumenou VG, Mavrogenis AF, Angelini A, et al. Complications of spine surgery for metastasis. Eur J Orthop Surg Traumatol. 2020;30(1):37–56. doi:10.1007/s00590-019-02541-0 [DOI] [PubMed] [Google Scholar]
- 20.Lee BH, Park JO, Kim HS, Park YC, Lee HM, Moon SH. Perioperative complication and surgical outcome in patients with spine metastases: retrospective 200-case series in a single institute. Clin Neurol Neurosurg. 2014;122:80–86. doi:10.1016/j.clineuro.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 21.Luksanapruksa P, Buchowski JM, Zebala LP, Kepler CK, Singhatanadgige W, Bumpass DB. Perioperative complications of spinal metastases surgery. Clin Spine Surg. 2017;30(1):4–13. doi:10.1097/BSD.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 22.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher CG, Schouten R, Versteeg AL, et al. Reliability of the spinal instability neoplastic score (SINS) among radiation oncologists: an assessment of instability secondary to spinal metastases. Radiat Oncol. 2014;9:69. doi:10.1186/1748-717X-9-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Versteeg AL, Verlaan JJ, Sahgal A, et al. The spinal instability neoplastic score: impact on oncologic decision-making. Spine (Phila Pa 1976). 2016;41(suppl 20):S231–S237. doi:10.1097/BRS.0000000000001822 [DOI] [PubMed] [Google Scholar]
- 25.Hussain I, Barzilai O, Reiner AS, et al. Patient-reported outcomes after surgical stabilization of spinal tumors: symptom-based validation of the spinal instability neoplastic score (SINS) and surgery. Spine J. 2018;18(2):261–267. doi:10.1016/j.spinee.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18(6):744–751. doi:10.1634/theoncologist.2012-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following separation surgery and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18(3):207–214. doi:10.3171/2012.11.SPINE12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajpal S, Lee Nelson E, Villavicencio AT, et al. Medical complications and mortality in octogenarians undergoing elective spinal fusion surgeries. Acta Neurochir (Wien). 2018;160(1):171–179. doi:10.1007/s00701-017-3384-9 [DOI] [PubMed] [Google Scholar]
- 29.Cloyd JM, Acosta FL, Jr, Ames CP. Complications and outcomes of lumbar spine surgery in elderly people: a review of the literature. J Am Geriatr Soc. 2008;56(7):1318–1327. doi:10.1111/j.1532-5415.2008.01771.x [DOI] [PubMed] [Google Scholar]
- 30.Saleh A, Thirukumaran C, Mesfin A, Molinari RW. Complications and readmission after lumbar spine surgery in elderly patients: an analysis of 2,320 patients. Spine J. 2017;17(8):1106–1112. doi:10.1016/j.spinee.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 31.Bernstein DN, Thirukumaran C, Saleh A, et al. Complications and readmission after cervical spine surgery in elderly patients: an analysis of 1786 patients. World Neurosurg. 2017;103:859–868.e8. doi:10.1016/j.wneu.2017.04.109 [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Kanayama M, Takahata M, et al. Perioperative complications of spine surgery in patients 80 years of age or older: a multicenter prospective cohort study. J Neurosurg Spine. 2019:1–9. doi:10.3171/2019.9.SPINE19754 [DOI] [PubMed] [Google Scholar]
- 33.Karhade AV, Vasudeva VS, Dasenbrock HH, et al. Thirty-day readmission and reoperation after surgery for spinal tumors: a national surgical quality improvement program analysis. Neurosurg Focus. 2016;41(2):E5. doi:10.3171/2016.5.FOCUS16168 [DOI] [PubMed] [Google Scholar]
- 34.Dekutoski MB, Norvell DC, Dettori JR, Fehlings MG, Chapman JR. Surgeon perceptions and reported complications in spine surgery. Spine (Phila Pa 1976). 2010;35(9 suppl):S9–S21. doi:10.1097/BRS.0b013e3181d830de [DOI] [PubMed] [Google Scholar]
- 35.Sciubba DM, Yurter A, Smith JS; et al. International Spine Study Group (ISSG). A comprehensive review of complication rates after surgery for adult deformity: a reference for informed consent. Spine Deform. 2015;3(6):575–594. doi:10.1016/j.jspd.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 36.Kim HJ, Iyer S, Zebala LP; et al. International Spine Study Group (ISSG). Perioperative neurologic complications in adult spinal deformity surgery: incidence and risk factors in 564 patients. Spine (Phila Pa 1976). 2017;42(6):420–427. doi:10.1097/BRS.0000000000001774 [DOI] [PubMed] [Google Scholar]
- 37.Lee NJ, Kothari P, Kim JS, et al. Early complications and outcomes in adult spinal deformity surgery: an NSQIP study based on 5803 patients. Global Spine J. 2017;7(5):432–440. doi:10.1177/2192568217699384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith C, Lamba N, Ou Z, et al. The prevalence of complications associated with lumbar and thoracic spinal deformity surgery in the elderly population: a meta-analysis. J Spine Surg. 2019;5(2):223–235. doi:10.21037/jss.2019.03.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith JS, Klineberg E, Lafage V; et al. International Spine Study Group. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J Neurosurg Spine. 2016;25(1):1–14. doi:10.3171/2015.11.SPINE151036 [DOI] [PubMed] [Google Scholar]
- 40.Soroceanu A, Burton DC, Oren JH; et al. International Spine Study Group. Medical complications after adult spinal deformity surgery: incidence, risk factors, and clinical impact. Spine (Phila Pa 1976). 2016;41(22):1718–1723. doi:10.1097/BRS.0000000000001636 [DOI] [PubMed] [Google Scholar]
- 41.Soroceanu A, Diebo BG, Burton D; et al. International Spine Study Group. Radiographical and implant-related complications in adult spinal deformity surgery: incidence, patient risk factors, and impact on health-related quality of life. Spine (Phila Pa 1976). 2015;40(18):1414–1421. doi:10.1097/BRS.0000000000001020 [DOI] [PubMed] [Google Scholar]
- 42.Than KD, Mummaneni PV, Bridges KJ, et al. Complication rates associated with open versus percutaneous pedicle screw instrumentation among patients undergoing minimally invasive interbody fusion for adult spinal deformity. Neurosurg Focus. 2017;43(6):E7. doi:10.3171/2017.8.FOCUS17479 [DOI] [PubMed] [Google Scholar]
- 43.Nerland US, Jakola AS, Solheim O, et al. Minimally invasive decompression versus open laminectomy for central stenosis of the lumbar spine: pragmatic comparative effectiveness study. BMJ. 2015;350:h1603. doi:10.1136/bmj.h1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imada AO, Huynh TR, Drazin D. Minimally invasive versus open laminectomy/discectomy, transforaminal lumbar, and posterior lumbar interbody fusions: a systematic review. Cureus. 2017;9(7):e1488. doi:10.7759/cureus.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine. 2009;11(4):471–476. doi:10.3171/2009.5.SPINE08633 [DOI] [PubMed] [Google Scholar]
- 46.Uei H, Tokuhashi Y, Oshima M, et al. Clinical results of minimally invasive spine stabilization for spinal metastases. Orthopedics. 2017;40(4):e693–e698. doi:10.3928/01477447-20170522-02 [DOI] [PubMed] [Google Scholar]
- 47.Versteeg AL, Verlaan JJ, de Baat P, et al. Complications after percutaneous pedicle screw fixation for the treatment of unstable spinal metastases. Ann Surg Oncol. 2016;23(7):2343–2349. doi:10.1245/s10434-016-5156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barzilai O, McLaughlin L, Amato MK, et al. Minimal access surgery for spinal metastases: prospective evaluation of a treatment algorithm using patient-reported outcomes. World Neurosurg. 2018;120:e889–e901. doi:10.1016/j.wneu.2018.08.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen-Algenstaedt N, Kwan MK, Algenstaedt P, et al. Comparison between minimally invasive surgery and conventional open surgery for patients with spinal metastasis: a prospective propensity score-matched study. Spine (Phila Pa 1976). 2017;42(10):789–797. doi:10.1097/BRS.0000000000001893 [DOI] [PubMed] [Google Scholar]
- 50.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. doi:10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 51.Farhat JS, Velanovich V, Falvo AJ, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526–1530; discussion 1530-1521. doi:10.1097/TA.0b013e3182542fab [DOI] [PubMed] [Google Scholar]
- 52.Al-Khamis A, Warner C, Park J, et al. Modified frailty index predicts early outcomes after colorectal surgery: an ACS-NSQIP study. Colorectal Dis. 2019;21(10):1192–1205. doi:10.1111/codi.14725 [DOI] [PubMed] [Google Scholar]
- 53.Ali TZ, Lehman EB, Aziz F. Modified frailty index can be used to predict adverse outcomes and mortality after lower extremity bypass surgery. Ann Vasc Surg. 2018;46:168–177. doi:10.1016/j.avsg.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 54.Traven SA, McGurk KM, Reeves RA, Walton ZJ, Woolf SK, Slone HS. Modified frailty index predicts medical complications, length of stay, readmission, and mortality following total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(10):1854–1860. doi:10.1016/j.jse.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 55.Traven SA, Reeves RA, Althoff AD, Slone HS, Walton ZJ. New five-factor modified frailty index predicts morbidity and mortality in geriatric hip fractures. J Orthop Trauma. 2019;33(7):319–323. doi:10.1097/BOT.0000000000001455 [DOI] [PubMed] [Google Scholar]
- 56.Vermillion SA, Hsu FC, Dorrell RD, Shen P, Clark CJ. Modified frailty index predicts postoperative outcomes in older gastrointestinal cancer patients. J Surg Oncol. 2017;115(8):997–1003. doi:10.1002/jso.24617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali R, Schwalb JM, Nerenz DR, Antoine HJ, Rubinfeld I. Use of the modified frailty index to predict 30-day morbidity and mortality from spine surgery. J Neurosurg Spine. 2016;25(4):537–541. doi:10.3171/2015.10.SPINE14582 [DOI] [PubMed] [Google Scholar]
- 58.Yagi M, Michikawa T, Hosogane N, et al. Treatment for frailty does not improve complication rates in corrective surgery for adult spinal deformity. Spine (Phila Pa 1976). 2019;44(10):723–731. doi:10.1097/BRS.0000000000002929 [DOI] [PubMed] [Google Scholar]
- 59.Miller EK, Ailon T, Neuman BJ; et al. International Spine Study Group. Assessment of a novel adult cervical deformity frailty index as a component of preoperative risk stratification. World Neurosurg. 2018;109:e800–e806. doi:10.1016/j.wneu.2017.10.092 [DOI] [PubMed] [Google Scholar]
- 60.Miller EK, Neuman BJ, Jain A; et al. International Spine Study Group. An assessment of frailty as a tool for risk stratification in adult spinal deformity surgery. Neurosurg Focus. 2017;43(6):E3. doi:10.3171/2017.10.FOCUS17472 [DOI] [PubMed] [Google Scholar]
- 61.De la Garza Ramos R, Goodwin CR, Jain A, et al. Development of a metastatic spinal tumor frailty index (MSTFI) using a nationwide database and its association with inpatient morbidity, mortality, and length of stay after spine surgery. World Neurosurg. 2016;95:548–555 e4. doi:10.1016/j.wneu.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 62.Festen S, Kok M, Hopstaken JS, et al. How to incorporate geriatric assessment in clinical decision-making for older patients with cancer. An implementation study. J Geriatr Oncol 2019;10(6):951–959. doi:10.1016/j.jgo.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 63.Shahrokni A, Alexander K. The age of talking about age alone is over. Ann Surg Oncol. 2019;26(1):12–14. doi:10.1245/s10434-018-6983-7 [DOI] [PubMed] [Google Scholar]


