Abstract
Study Design
Retrospective cohort.
Objectives
To compare outcomes of minimally invasive surgery (MIS) vs open surgery (OPEN) for lumbar spinal stenosis (LSS) in patients with diabetes.
Methods
Patients with diabetes who underwent spinal decompression alone or with fusion for LSS within the Canadian Spine Outcomes and Research Network (CSORN) database were included. MIS vs OPEN outcomes were compared for 2 cohorts: (1) patients with diabetes who underwent decompression alone (N = 116; MIS n = 58 and OPEN n = 58), (2) patients with diabetes who underwent decompression with fusion (N = 108; MIS n = 54 and OPEN n = 54). Modified Oswestry Disability Index (mODI) and back and leg pain were compared at baseline, 6–18 weeks, and 1-year post-operation. The number of patients meeting minimum clinically important difference (MCID) or minimum pain/disability at 1-year was compared.
Results
MIS approaches had less blood loss (decompression alone difference 100 mL, P = .002; with fusion difference 244 mL, P < .001) and shorter length of stay (LOS) (decompression alone difference 1.2 days, P = .008; with fusion difference 1.2 days, P = .026). MIS compared to OPEN decompression with fusion had less patients experiencing adverse events (AEs) (difference 13 patients, P = .007). The MIS decompression with fusion group had lower 1-year mODI (difference 14.5, 95% CI [7.5, 21.0], P < .001) and back pain (difference 1.6, 95% CI [.6, 2.7], P = .002) compared to OPEN. More patients in the MIS decompression with fusion group exceeded MCID at 1-year for mODI (MIS 75.9% vs OPEN 53.7%, P = .028) and back pain (MIS 85.2% vs OPEN 70.4%, P = .017).
Conclusions
MIS approaches were associated with more favorable outcomes for patients with diabetes undergoing decompression with fusion for LSS.
Keywords: spinal stenosis, spinal fusion, diabetes, patient-reported outcome measures, decompression, low back pain
Introduction
Lumbar spinal stenosis (LSS) is narrowing of the spinal canal which may result in compression of neural structures. 1 LSS can cause neurogenic claudication with or without radiculopathy resulting in low back and leg pain, cramping, weakness, and numbness.1,2 Neurogenic claudication and/or radiculopathy has a negative impact on health-related quality of life.1-3 Surgical treatment of symptomatic LSS is typically spinal decompression to alleviate pressure on neural structures; spinal fusion may also be performed to stabilize the vertebral segments.1,4
Diabetes has been shown to be a risk factor for development of LSS and is correlated with more adverse events (AEs) and worse outcomes following spine surgery.5-12 It is postulated that because of comorbidities, micro-/macro-vascular degeneration, and impaired tissue healing, patients with diabetes have worse outcomes following spinal surgery.9-12 This makes the outcomes of patients with diabetes undergoing surgery for LSS of particular importance.
Minimally invasive surgical approaches for the lumbar spine have gained popularity as they are associated with less soft tissue damage and more favorable short-term outcomes, compared to conventional open operative approaches (OPEN).4,13-15 Theoretically, this makes minimally invasive surgery (MIS) an attractive alternative procedure for patients with specific comorbities, such as diabetes, because of their increased risk of perioperative and acute post-operative complications.8-11
Two recent studies assessed the impact of diabetes specifically in MIS lumbar surgery.16,17 Regev et al., found MIS decompression is equally effective in patients with and without diabetes regarding post-operative complications, hospital length of stay (LOS), and functional outcome scores. 16 Narain et al., showed no significant difference in post-operative complications, LOS, or hospital costs following MIS lumbar interbody fusion comparing patients with and without diabetes. 17 These recent articles suggest MIS approaches may improve the outcomes of patients with diabetes making them comparable to patients without diabetes. Despite this, to our knowledge, the outcomes of MIS compared to OPEN surgery solely in patients with diabetes have not yet been studied. Therefore, to assess the potential benefits of MIS surgery in this patient population, we compared the outcomes of patients with diabetes undergoing MIS compared to OPEN surgery for LSS.
Materials and Methods
Study Design
We conducted a multicentre, retrospective review of prospectively collected data on spine surgery patients enrolled in the Canadian Spine Outcomes and Research Network (CSORN) national registry as of June 2020.
This study received approval from the Horizon Health Network Research Ethics Board (REB) and the CSORN national steering committee. Each participating site received institutional REB approval prior to collecting data. Written informed consent for use of patient data was obtained from all participating patients prior to enrollment in the CSORN registry.
CSORN is a group of over 50 neurosurgical and orthopedic spine surgeons from 20 tertiary care academic and non-academic hospitals across Canada that prospectively collect data on patients with spinal conditions. This database serves as a national registry created to answer research questions and to facilitate the implementation of best practices.
A national database research coordinator audits data quality and performance and sends reports to each contributing hospital site coordinator on a quarterly basis. Reports track data completion and follow-up rates to facilitate internal data validation at each site. A national privacy and security framework was created for CSORN that includes a governance structure, standard operating procedures, training processes, physical and technical security, and privacy impact assessments. This model ensures privacy and security of personal health information. Written informed consent is obtained from all participating patients. Patient identification is anonymized to ensure that patients cannot be individually identified. All participating sites obtained REB approval prior to any data collection. Decisions regarding data collection, storage, and analysis are independent of any particular company or commercial interest.
Patient Sample
All patients ≥18 years old with diabetes who underwent spinal decompression alone or decompression with fusion for LSS and were enrolled in the CSORN registry with available 1-year follow-up as of June 2020 were included. Data from 15 Canadian sites contributed to this study. MIS procedures were completed by surgeons with a minimum of 2 years’ experience in MIS spine techniques. Patients with American Society of Anesthesiologists (ASA) scores >3, 18 levels operated on >3, or with missing patient-reported outcome measures (PROMs) of interest at follow-up time points were excluded.
The patients with diabetes were separated into 2 cohorts: (1) patients with diabetes who underwent decompression alone and (2) patients with diabetes who underwent decompression with fusion. Only patients with LSS without spondylolisthesis were included in the decompression alone cohort; patients with primary LSS or LSS secondary to spondylolisthesis were included in the decompression with fusion cohort. Within each cohort there were 2 groups based on surgical technique, MIS and OPEN. MIS procedures were defined by CSORN as any muscle sparring or muscle-splitting approach which minimizes trauma to the paraspinal soft tissue. Surgical technique was reported by each individual surgeon. For both cohorts, the MIS group had a smaller number of patients available. To avoid any possible increase in type 1 error and unequal variances that can occur due to unequal groups, patients from the OPEN group were selected using random sampling to create an equal number of patients in both arms of each cohort.
Study Measures
Baseline variables of interest were biological sex, body mass index, smoking status, exercise history, working status, number of comorbidities, ASA scores, and number of levels operated on.
The treating surgeons recorded operative and post-operative variables including type of procedure, operating time, blood loss, and AEs utilizing the Spinal Adverse Events Severity System, version 2, a validated and reliable tool for spine AEs. 19 The research coordinators tabulated the LOS.
Research coordinators, unaware of the study hypothesis, collected PROMs and AEs at baseline, 6–18 weeks, and 1-year post-operatively. Collection was in-person, over the phone, via post, or employing an online patient portal. PROMs were the modified Oswestry Disability Index (mODI), 20 and an 11-point (0–10, higher scores indicate worse pain) numerical rating scale (NRS) for back and leg pain. 21
Adverse events were those captured during the initial operation, during hospital admission prior to discharge, and at follow-up.
Data Analysis
The data were analyzed using IBM Statistical Package for the Social Sciences version 25.0 and graphs were created using Prism 8. The data were analyzed separately for the 2 cohorts of patients with diabetes: (1) decompression alone and (2) decompression with fusion. A priori significance level of P < .05 was set for all statistical comparisons.
The continuous variables were described as means ± standard deviations and compared between the MIS and OPEN groups for each cohort using independent t-tests. The categorical variables were described as percentages and compared using X2 tests.
The PROMs were analyzed at baseline, 6–18 weeks post-operation, and 1-year post-operation using a 2 (MIS vs OPEN) × 3 (baseline; 6–18 weeks; and 1-year) mixed measures analysis of covariance (ANCOVA) with ASA scores controlled as a covariate. ASA scores were controlled for as they were the only variable which significantly differed between the groups within cohorts at baseline (Table 1), and due to a recent publication finding that patients with ASA scores >2 undergoing operation for LSS were more likely to have a poor outcome. 22 Bonferroni post hoc t-tests were conducted for significant ANCOVA findings.
Table 1.
Baseline Demographics of Both Cohorts.
| Variables | Decompression alone | Decompression with fusion | ||||
|---|---|---|---|---|---|---|
| MIS | OPEN | P-value | MIS | OPEN | P-value | |
| N = | 58 | 58 | 54 | 54 | ||
| Female | 26 (44.8%) | 20 (34.5%) | .255 | 29 (53.7%) | 25 (46.3%) | .441 |
| Male | 32 (55.2%) | 38 (65.5%) | 25 (46.3%) | 29 (53.7%) | ||
| Age | 69.7 ± 8.1 | 67.8 ± 9.2 | .245 | 64.9 ± 7.6 | 63.1 ± 8.3 | .262 |
| Missing | 1 (1.9%) | |||||
| BMI | 30.4 ± 6.6 | 30.5 ± 4.5 | .887 | 30.5 ± 5.6 | 32.5 ± 8.3 | .162 |
| Missing | 5 (8.6%) | 1 (1.7%) | 2 (3.7%) | 2 (3.7%) | ||
| Nicotine user | 7 (12.1%) | 5 (8.6%) | .542 | 11 (20.4%) | 8 (14.8%) | .448 |
| Exercise once or less/week | 12 (20.7%) | 10 (17.2%) | .731 | 13 (24.1%) | 14 (25.9%) | .561 |
| Twice or more/week | 22 (37.9%) | 24 (41.4%) | 12 (22.2%) | 12 (22.2%) | ||
| Never due to physical limitations | 24 (41.4%) | 23 (39.7%) | 27 (50.0%) | 28 (51.9%) | ||
| Missing | 1 (1.7%) | 2 (3.7%) | ||||
| Number of comorbidities incl. diabetes | 4.2 ± 2.0 | 4.3 ± 2.0 | .744 | 4.1 ± 2.0 | 4.9 ± 2.2 | .052 |
| Number of levels operated on | 1.3 ± 0.5 | 1.4 ± 0.5 | .198 | 1.2 ± 0.5 | 1.4 ± 0.6 | .119 |
| ASA score 1 2 3 | 1 (1.7%) 35 (60.3%) 22 (37.9%) | 3 (5.2%) 22 (37.9%) 33 (56.9%) | .046* | 0 33 (61.1%) 21 (38.9%) | 0 22 (40.1%) 32 (59.3%) | .034* |
Abbreviations: MIS, minimally invasive surgery; OPEN, open surgery; BMI, body mass index; ASA, American Society of Anesthesiologists. Continuous and categorical variables were analyzed using independent two-tailed t-tests and X2 tests, respectively. Continuous variables are reported as mean ± SD, categorical variables as n (%). *P < .05.
The number of patients with PROM changes that met meaningful change using the minimum clinically important difference (MCID) or who reached minimal disability/pain scores were compared. The MCID levels for this study were set using common thresholds: 12.8 change for mODI, 1.2 change for the NRS back pain, and 1.6 change for the NRS leg pain.23,24 Minimal disability on the mODI is described as a score of ≤20.24,25 Minimal pain is described as 0–3 on the 11-point NRS for both back and leg pain.21,25
The proportion of each patient group meeting the MCID change or categorized as minimal disability or pain at 1-year post-operation for the 3 PROMs (mODI, NRS back and leg pain) was compared using a X2 test between the MIS and OPEN groups within each cohort (decompression alone and decompression with fusion).
Results
Baseline Demographics/Variables
Of patients eligible for this study, 9.8% were not included due to loss to follow-up. 116 patients with diabetes were included in the decompression alone cohort (MIS n = 58; OPEN n = 58); 108 patients with diabetes were included in the decompression with fusion cohort (MIS n = 54; OPEN n = 54). Patients in the OPEN group had significantly higher ASA scores (Table 1). The OPEN and MIS groups of both cohorts did not significantly differ in any other baseline demographics/variables (Table 1).
Intra/Perioperative Outcomes
Table 2 represents the operative outcomes. Estimated blood loss and LOS were significantly lower in the MIS group for both cohorts.
Table 2.
Perioperative Outcomes of Interest for Both Cohorts.
| Variable | Decompression alone | Decompression with fusion | ||||
|---|---|---|---|---|---|---|
| MIS | OPEN | P-value | MIS | OPEN | P-value | |
| N = | 58 | 58 | 54 | 54 | ||
| Operative time: Incision to closure (minutes) | 93.2 ± 33.4 | 99.8 ± 37.5 | .325 | 201.5 ± 65.1 | 186.5 ± 71.6 | .270 |
| Missing | 4 (6.9%) | 1 (1.7%) | 3 (5.6%) | 3 (5.6%) | ||
| Estimated blood loss (mL) | 82 ± 121 | 182 ± 208 | .002* | 230 ± 176 | 474 ± 369 | <.001* |
| Missing | 3 (5.2%) | 4 (6.9%) | 1 (1.9%) | 2 (3.7%) | N/A | |
| Length of stay in hospital (days) | 1.3 ± 1.8 | 2.5 ± 2.8 | .008* | 4.2 ± 3.1 | 5.4 ± 3.3 | .026* |
| Missing | 8 (13.8%) | 3 (5.2%) | 4 (7.4%) | |||
Abbreviations: MIS, minimally invasive surgery; OPEN, open surgery; mL, milliliters. Continuous variables were analyzed using independent two-tailed t-tests. Continuous variables are reported as mean ± SD. *P < .05.
Adverse Events
Table 3 represents the AEs that were experienced by the patients in each group. Adverse events were captured intra-operatively, in-hospital, and at follow-up. There was no significant difference in the number of MIS vs OPEN patients who experienced AEs in the decompression alone cohort (X2(1) = .6, P = .429). Significantly more patients who underwent OPEN decompression with fusion experienced an AE compared to the patients who underwent MIS decompression with fusion (X2(1) = 7.4, P = .007).
Table 3.
Adverse Events for Both Cohorts.
| Decompression alone | Decompression with fusion | |||
|---|---|---|---|---|
| MIS | OPEN | MIS | OPEN | |
| Wound infection/drainage | 1 | 0 | 1 | 3 |
| Gastric/urological infection, obstruction, and retention | 7 | 6 | 2 | 5 |
| Fever | 0 | 0 | 1 | 2 |
| Hypokalemia | 0 | 1 | 0 | 2 |
| Cardiovascular | 1 | 1 | 2 | 5 |
| Dural tear/CSF leak | 4 | 6 | 1 | 4 |
| Cognitive decline | 2 | 1 | 1 | 2 |
| Airway | 1 | 1 | 1 | |
| New onset pain/slow mobilization | 2 | 3 | 3 | 5 |
| Rash | 0 | 1 | 0 | 0 |
| Post-op revision | 2 | 0 | 0 | 1 |
| Hypoglycemia | 1 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 1 |
| Diabetic foot amputation (unrelated to spine surgery) | 1 | 0 | 0 | 0 |
| Spinal column stenosis, fibrosis, and segment disease | 1 | 2 | 2 | 2 |
| Implant/instrumentation related | 0 | 0 | 2 | 1 |
| Total number of AEs | 23 | 22 | 16 | 33 |
| Number of patients who had an AE | 21 (36.2%) | 17 (29.3%) | 10 (18.5%) | 23 (42.6%)* |
Abbreviations: MIS, minimally invasive surgery; OPEN, open surgery; CSF, cerebrospinal fluid; AE, adverse event. Continuous and categorical variables were analyzed using independent two-tailed t-tests and X2 tests, respectively. Continuous variables are reported as mean ± SD, categorical variables as n (%). *P < .05.
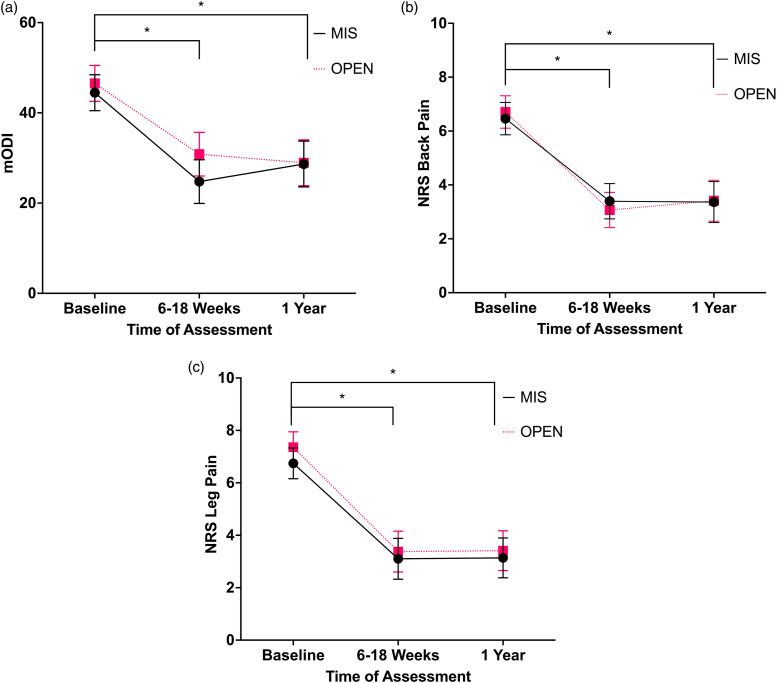
Patient-Reported Outcome Measures
Figure 1 represents the PROMs for the decompression alone cohort. The 2 × 3 mixed measures ANCOVA found a significant main effect of time for mODI F(2,226) = 8.8, P < .001, back pain F(2,226) = 9.4, P < .001, and leg pain F(2,226) = 4.7, P = .010. There was no significant interaction effect for time*MIS/OPEN for mODI F(2,226) = 1.9, P = .147, back pain F(2,226) = .5, P = .592, or leg pain F(2,226) = .2, P = .822. There was also no significant effect of the covariate time*ASA score for mODI F(2,226) = .9, P = .428, back pain F(2,226) = 1.0, P = .385, or leg pain F(2,226) = .1, P = .998.
Figure 1.
(A) Mean mODI adjusted for ASA scores for decompression alone cohort, error bars represent ± standard error of mean × 2. Data analyzed using ANCOVA with ASA scores as covariate. (B) Mean NRS back pain adjusted for ASA scores for decompression alone cohort, error bars represent ± standard error of mean × 2. Data analyzed with ANCOVA with ASA scores as covariate. (C) Mean NRS leg pain adjusted for ASA scores for decompression alone cohort, error bars represent ± standard error of mean × 2. Data analyzed with ANCOVA with ASA scores as covariate. Abbreviations: ASA, American Society of Anesthesiologists; ANCOVA, analysis of covariance; MIS, minimally invasive surgery; OPEN, open surgery; mODI, modified Oswestry Disability Index; NRS, numerical rating scale. *P < .05.
The follow-up post hoc t-tests for the main effect of time showed significant improvement, for mODI and NRS back and leg pain from baseline to 6–18 weeks (mODI mean difference 17.7, 95% CI [13.9, 21.5], P < .001; back pain mean difference 3.4, 95% CI [2.6, 4.1], P < .001; leg pain mean difference 3.8, 95% CI [3.1, 4.6], P < .001) and from baseline to 1-year (mODI, mean difference 16.7, 95% CI [13.0, 20.4], P < .001; back pain mean difference 3.2, 95% CI [2.5, 3.9], P < .001; leg pain mean difference 3.8, 95% CI [3.0, 4.6], P < .001).
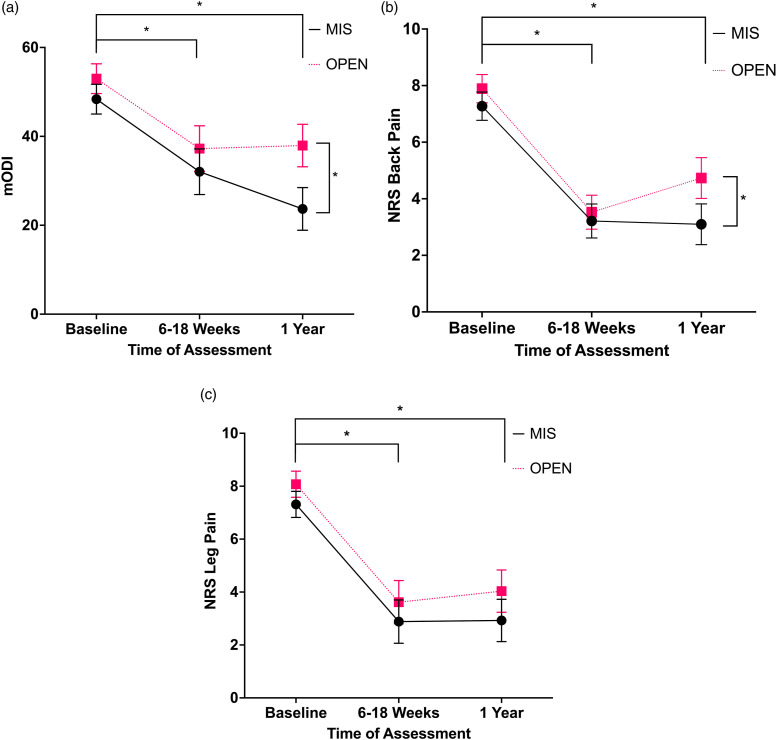
Figure 2 represents the PROMs for the decompression with fusion cohort. The 2 × 3 mixed measures ANCOVA showed a significant main effect of time for mODI F(2,210) = 4.5, P = .013, back pain F(2,210) = 10.2, P < .001, and leg pain F(2,210) = 7.2, P = .001. The ANCOVA also showed a significant interaction effect of time*MIS/OPEN for mODI F(2,210) = 5.0, P = .008 and back pain F(2,210) = 4.2, P = .016. There was not a significant interaction effect for leg pain F(2,210) = .2, P = .803. The covariate did not show a significant main effect for time*ASA score for mODI F(2,210) = .2, P = .825, back pain F(2,210) = .8, P = .753, or leg pain F(2,210) = .3, P = .754.
Figure 2.
(A) Mean mODI adjusted for ASA scores for decompression with fusion cohort, error bars represent ± standard error of mean × 2. Data analyzed with ANCOVA with ASA scores as covariate. (B) Mean NRS back pain adjusted for ASA scores for decompression with fusion cohort, error bars represent ± standard error of mean × 2. Data analyzed with ANCOVA with ASA scores as covariate. (C) Mean NRS leg pain adjusted for ASA scores for decompression with fusion cohort, error bars represent ± standard error of mean × 2. Data analyzed with ANCOVA with ASA scores as covariate. Abbreviations: ASA, American Society of Anesthesiologists; ANCOVA, analysis of covariance; MIS, minimally invasive surgery; OPEN, open surgery; mODI, modified Oswestry Disability Index; NRS, numerical rating scale. *P < .05.
The follow-up post hoc t-tests for the main interaction effect showed significant improvement in mODI and back pain for both surgical techniques from baseline to 6–18 weeks (MIS mODI mean difference 16.3, 95% CI [10.2, 22.5], P < .001; OPEN mODI mean difference 15.7, 95% CI [9.6, 21.9], P < .001; MIS back pain mean difference 4.1, 95% CI [3.3, 4.9], P < .001; OPEN back pain mean difference 4.4, 95% CI [3.6, 5.2], P < .001) and from baseline to 1-year (MIS mODI mean difference 24.6, 95% CI [18.6, 30.8], P < .001; OPEN mODI mean difference 15.0, 95% CI [9.0, 21.1], P < .001; MIS back pain mean difference 4.2, 95% CI [3.3, 5.0] P < .001; OPEN back pain mean difference 3.2, 95% CI [2.3, 4.0], P < .001). Overall, leg pain had significant improvement from baseline to 6–18 weeks (mean difference, 4.4, 95% CI [3.7, 5.2], P < .001) and from baseline to 1-year (mean difference 4.2, 95% CI [3.5, 4.9], P < .001). There were no significant differences between MIS and OPEN patients at baseline (back pain mean difference .6, 95% CI [−.9, 1.3], P = .086; mODI mean difference 4.6, 95% CI [−.1, 9.4], P = .057) or at 6–18 weeks (back pain mean difference .3, 95% CI [−.5, 1.2], P = .086; mODI mean difference 5.2, 95% CI [−2.0, 12.5], P = .161). However, back pain and mODI was significantly higher in OPEN compared to MIS decompression with fusion at 1-year post-operation (back pain mean difference 1.6, 95% CI [.6, 2.7], P = .002; mODI mean difference 14.2, 95% CI [7.5, 21.0], P < .001).
As seen in Table 4, the X2 tests assessing the number of patients who met MCID change or were at minimal pain/disability at 1-year follow-up for the PROMs found no significant differences in the number of patients who reached meaningful change in the decompression alone cohort. However, in the decompression with fusion cohort, a significantly greater number of patients reached meaningful change at 1-year follow-up for mODI (MIS 75.9% vs OPEN 53.7%, P = .028) and back pain (MIS 85.2% vs OPEN 70.4%, P = .017). Table 4 outlines the full results regarding MCID achievement.
Table 4.
Number and Percentage of Patients Who Met MCID Change in PROM or Met Criteria for Minimal Pain/Disability at 1-Year Post-Operation for mODI, NRS Back Pain, and NRS Leg Pain.
| Variable | Decompression alone | Decompression with fusion | ||||
|---|---|---|---|---|---|---|
| MIS | OPEN | P-value | MIS | OPEN | P-value | |
| N = | 58 | 58 | 54 | 54 | ||
| mODI | 36 (62.1%) | 32 (55.2%) | .451 | 40 (75.9%) | 29 (53.7%) | .028* |
| NRS back pain | 41 (70.7%) | 47 (81.0%) | .193 | 46 (85.2%) | 38 (70.4%) | .017* |
| NRS leg pain | 44 (75.9%) | 49 (84.5%) | .244 | 44 (81.5%) | 42 (77.8%) | .633 |
Abbreviations: MCID, minimum clinically important difference; PROM, patient-reported outcome measures; MIS, minimally invasive surgery; OPEN, open surgery; mODI, modified Oswestry Disability Index; NRS, numerical rating scale. Continuous and categorical variables were analyzed using independent two-tailed t-tests and X2 tests respectively. Continuous variables are reported as mean ± SD, categorical variables as n (%). *P < .05.
Discussion
Diabetes is associated with worse outcomes following spine surgery.8-12 Moreover, diabetes has been shown to be a risk factor for the development of LSS.5-7 As a result, maximizing the surgical benefits for patients with diabetes is of particular importance. In the general population, MIS approaches have been shown to provide perioperative benefits; however, the literature tends to find equivalent PROMs at 1- and 2-year follow-up, regardless of surgical technique (MIS or OPEN).13-15 In this, the first study to the authors knowledge to directly compare the outcomes of MIS vs OPEN surgery in patients with diabetes, we have demonstrated less AEs, better PROMs (mODI and NRS back pain), and a greater proportion of patients meeting MCID for mODI and back pain scores 1-year post-surgery in patients with diabetes who underwent MIS decompression with fusion for LSS compared to those who underwent the OPEN procedure. The benefits of MIS in this analysis are unique to our cohort comprised solely of patients with diabetes and the findings exceeded benchmarks for clinically relevant differences. Additionally, an expected difference in perioperative parameters (EBL and LOS) was shown in favor of MIS for both decompression alone and decompression with fusion.
The significantly lower blood loss seen with the MIS technique is consistent with previous studies on lumbar decompression and lumbar fusion.13-15 The shorter LOS observed in the MIS groups could suggest an earlier return to normal functioning for these patients.
This study showed MIS (compared to OPEN) decompression with fusion yields better PROMs at 1-year post-operation for patients with diabetes. This is contrary to the majority of the literature on the general population which tends to find no benefits in terms of PROMs for MIS compared to OPEN procedures.13,14 McGirt et al. did not find improved 12-month outcomes in a large sample of patients who underwent 1- or 2-level interbody lumbar fusion. 13 Similarly, comparing MIS and OPEN posterior lumbar fusion, Goldstein et al. found no difference in mODI or pain scores 12- and 36-month post-operation. 14 In studies comparing patients with or without diabetes undergoing lumbar surgery, patients with diabetes are found to have worse outcomes and a higher rate of AEs.8-12 However, when comparing patients with and without diabetes using solely MIS lumbar surgeries, recent literature shows patients with diabetes have equivalent AEs, LOS, and functional outcomes.16,17 This along with the results of the present study suggests that MIS techniques for lumbar fusion provides a unique benefit specific to patients with diabetes. Further, our results showed significantly more patients achieving MCID standards for mODI and back pain, suggesting clinical relevance of our results.
These findings may be a result of the smaller muscle-splitting surgical incisions associated with the MIS techniques.4,13,14 Greater injury to back muscles has been associated with worse PROMs 1-year post-operation for lumbar fusion.15,26 Greater injury and incisions may impact patients with diabetes more profoundly as they tend to have impaired wound healing and may be more prone to tissue ischemia, inflammation, and decreased perfusion from retraction and longer operating times.11,12 Research in diabetic rats shows higher rates of local inflammatory cytokines and reduced rate and quality of spinal fusion. 27 The MIS approaches which cause less tissue damage may have reduced inflammation and resulted in local environments more conducive to tissue healing in our study of patients with diabetes.
Previous literature has shown that diabetes, regardless of insulin dependence, significantly increases the risk of perioperative AEs following lumbar fusion. 28 Metabolic syndrome, which is common in patients with diabetes also increases the risk of perioperative AEs following posterior lumbar interbody fusion. 29 Further, major perioperative AEs have been shown to result in poorer functional outcomes. 30 MIS approaches are found to decrease medical AEs following lumbar fusion. 14 This study found a significantly greater number of patients with AEs in the OPEN decompression with fusion group compared to the MIS group which may have impacted the PROM differences we observed at 1-year. These factors would have a more profound effect on the recovery of patients with diabetes since diabetes is already associated with poor wound healing, peripheral neuropathy, and angiopathy.9-12 These differences were likely not seen in the decompression alone cohort as it is a much less invasive procedure, regardless of MIS or OPEN approach.
This study has limitations. Although the data were collected prospectively, the analysis was retrospective, and patients were not randomly assigned to different surgical techniques. Therefore, there may have been an element of surgical selection bias inherent to studies of this nature. Of patients eligible for this study, 9.8% were lost to follow-up. The type of OPEN or MIS operative approach was not standardized. While the study was appropriately powered, the cohort size may have been too small to identify noteworthy differences regarding specific AEs, such as infections, which are of particular importance in patients with diabetes. Finally, we recognize that diabetes is a complex condition, and this database did not allow for assessment of all aspects of diabetes and its management such as glycemic control and type 1 vs 2 diabetes. However, as this is the first study comparing MIS vs OPEN surgery for LSS in patients with diabetes and the results show unique benefits regarding MIS techniques in this population, it serves as compelling evidence to promote further research in this area to improve the outcomes of patients with diabetes following spine surgery.
Conclusion
Spinal decompression alone or with fusion, regardless of surgical technique (MIS or OPEN), is effective for treating symptomatic LSS in patients with diabetes. MIS approaches offered short-term advantages in both the decompression alone and decompression with fusion cohorts. MIS approaches also showed improved PROMs 1-year post-operation for patients with diabetes undergoing lumbar decompression with fusion. This is a unique finding to our sample of solely patients with diabetes. Therefore, MIS techniques may be advantageous in this patient population. This study provides strong basis for future prospective controlled trials to assess the impact of MIS techniques on patients with diabetes.
Acknowledgments
We sincerely thank Dr Andrew Flewelling from the Office of Research Services at Horizon Health Network for his support and guidance with statistics and methodology. We would also like to thank everyone involved in the CSORN national registry.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was generously funded by the Dalhousie Medicine New Brunswick Research in Medicine Summer Studentship. Authors CGF, CSB, EA, NM, and YRR receive consulting fees, royalties, or grants from Medtronic. Author CGF receives consulting fees from Nuvasive and grants from the Orthopedic Research and Education Foundation. Author MGJ receives Stryker research support.
Ethical Approval: Ethics approval for a retrospective review of the prospectively collected data contained within the CSORN database was obtained from the Horizon Health Network Research Ethics Board, IRB File #: 100830. Each individual site contributing data to the CSORN national registry received institutional research ethics board approval prior to collecting data. Written informed consent was received from all patients prior to enrollment in the CSORN registry.
ORCID iDs
Kalpesh Hathi https://orcid.org/0000-0003-0362-5623
Greg McIntosh https://orcid.org/0000-0002-0268-6523
Dana El-Mughayyar https://orcid.org/0000-0003-0074-1194
Adrienne Kelly https://orcid.org/0000-0002-7536-8884
References
- 1.Deasy J. Acquired lumbar spinal stenosis. JAAPA. 2015;28(4):19-23. doi: 10.1097/01.JAA.0000462052.47882.fd. [DOI] [PubMed] [Google Scholar]
- 2.Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: The Framingham study. Spine J. 2009;9(7):545-550. doi: 10.1016/j.spinee.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otani K, Kikuchi S, Yabuki S, et al. Lumbar spinal stenosis has a negative impact on quality of life compared with other comorbidities: An epidemiological cross-sectional study of 1862 community-dwelling individuals. Sci World J. 2013;2013:590652. doi: 10.1155/2013/590652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costandi S, Chopko B, Mekhail M, Dews T, Mekhail N. Lumbar spinal stenosis: Therapeutic options review. Pain Pract. 2015;15(1):68-81. doi: 10.1111/papr.12188. [DOI] [PubMed] [Google Scholar]
- 5.Asadian L, Haddadi K, Aarabi M, Zare A. Diabetes mellitus, a new risk factor for lumbar spinal stenosis: A case-control study. Clin Med Insights Endocrinol Diabetes. 2016;9:1-5. doi: 10.4137/CMED.S39035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anekstein Y, Smorgick Y, Lotan R, et al. Diabetes mellitus as a risk factor for the development of lumbar spinal stenosis. Isr Med Assoc J. 2010;12(1):16-20. [PubMed] [Google Scholar]
- 7.Lee CK, Choi SK, Shin DA, et al. Influence of diabetes mellitus on patients with lumbar spinal stenosis: A nationwide population-based study. PLoS One. 2019;14(3):e0213858. doi: 10.1371/journal.pone.0213858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrighi-Allisan AE, Neifert SN, Gal JS, et al. Diabetes is predictive of postoperative outcomes and readmission following posterior lumbar fusion. Global Spine J. 2020. In press. doi: 10.1177/2192568220948480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson JM, Silveri CP, Balderston RA, Simeone FA, An HS. The results of operations on the lumbar spine in patients who have diabetes mellitus. J Bone Joint Surg Am. 1993;75(12):1823-1829. doi: 10.2106/00004623-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH, Chung CK, Shin S, et al. The relationship between diabetes and the reoperation rate after lumbar spinal surgery: A nationwide cohort study. Spine J. 2015;15(5):866-874. doi: 10.1016/j.spinee.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Armaghani SJ, Archer KR, Rolfe R, Demaio DN, Devin CJ. Diabetes is related to worse patient-reported outcomes at two years following spine surgery. J Bone Joint Surg Am. 2016;98(1):15-22. doi: 10.2106/JBJS.O.00297. [DOI] [PubMed] [Google Scholar]
- 12.Wukich DK. Diabetes and its negative impact on outcomes in orthopaedic surgery. World J Orthop. 2015;6(3):331-339. doi: 10.5312/wjo.v6.i3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGirt MJ, Parker SL, Mummaneni P, et al. Is the use of minimally invasive fusion technologies associated with improved outcomes after elective interbody lumbar fusion? Analysis of a nationwide prospective patient-reported outcomes registry. Spine J. 2017;17(7):922-932. doi: 10.1016/j.spinee.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: Meta-analysis and systematic review. J Neurosurg Spine. 2016;24(3):416-427. doi: 10.3171/2015.2.SPINE14973. [DOI] [PubMed] [Google Scholar]
- 15.Park Y, Seok SO, Lee SB, Ha JW. Minimally invasive lumbar spinal fusion is more effective than open fusion: A meta-analysis. Yonsei Med J. 2018;59(4):524-538. doi: 10.3349/ymj.2018.59.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regev GJ, Lador R, Salame K, Mangel L, Cohen A, Lidar Z. Minimally invasive spinal decompression surgery in diabetic patients: Perioperative risks, complications and clinical outcomes compared with non-diabetic patients’ cohort. Eur Spine J. 2019;28(1):55-60. doi: 10.1007/s00586-018-5716-8. [DOI] [PubMed] [Google Scholar]
- 17.Narain AS, Haws BE, Jenkins NW, et al. Diabetes does not increase complications, length of stay, or hospital costs after minimally invasive transforaminal lumbar interbody fusion. Clin Spine Surg. 2020;33(7):E307-E311. doi: 10.1097/BSD.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 18.Doyle DJ, Goyal A, Bansal P, Garmon EH. American society of anesthesiologists classification. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 19.Rampersaud YR, Anderson PA, Dimar JR, 2nd, Fisher CG. Spinal adverse events severity system, version 2 (SAVES-V2): Inter- and intraobserver reliability assessment. J Neurosurg Spine. 2016;25(2):256-63. doi: 10.3171/2016.1.SPINE14808. [DOI] [PubMed] [Google Scholar]
- 20.Fritz JM, Irrgang JJ. A comparison of a modified oswestry low back pain disability questionnaire and the quebec back pain disability scale. Phys Ther. 2001;81(2):776-788. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- 21.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S240-S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 22.Hébert JJ, Abraham E, Wedderkopp N, et al. Preoperative factors predict postoperative trajectories of pain and disability following surgery for degenerative lumbar spinal stenosis. Spine. 2020;45(21):E1421-E1430. doi: 10.1097/BRS.0000000000003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: A choice of methods using the Oswestry disability index, medical outcomes study questionnaire short form 36, and pain scales. Spine J. 2008;8(6):968-974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273. [PubMed] [Google Scholar]
- 25.Hanley MA, Masedo A, Jensen MP, Cardenas D, Turner JA. Pain interference in persons with spinal cord injury: Classification of mild, moderate, and severe pain. J Pain. 2006;7(2):129-133. doi: 10.1016/j.jpain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Sihvonen T, Herno A, Paljärvi L, Airaksinen O, Partanen J, Tapaninaho A. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18(5):575-581. doi: 10.1097/00007632-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 27.NaPier Z, Kanim LEA, Nelson TJ, et al. The effect of insulin dependent diabetes on bone metabolism and growth after spinal fusion. Spine J. 2020;20(5):800-808. doi: 10.1016/j.spinee.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glassman SD, Alegre G, Carreon L, Dimar JR, Johnson JR. Perioperative complications of lumbar instrumentation and fusion in patients with diabetes mellitus. Spine J. 2003;3(6):496-501. doi: 10.1016/s1529-9430(03)00426-1. [DOI] [PubMed] [Google Scholar]
- 29.He X, Fei Q, Sun T. Metabolic syndrome increases risk for perioperative outcomes following posterior lumbar interbody fusion. Medicine. 2020;99(38):e21786. doi: 10.1097/MD.0000000000021786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayling OGS, Ailon T, Street JT, et al. The effect of perioperative adverse events on long-term patient-reported outcomes after lumbar spine surgery. Neurosurg. 2021;88(2):420-427. doi: 10.1093/neuros/nyaa427. [DOI] [PubMed] [Google Scholar]