Summary
Prolonged withdrawal from opioids leads to negative emotions. Kappa opioid receptor (KOR) plays an important role in opioid addiction and affective disorders. However, the underlying mechanism of KOR in withdrawal-related depression is still lacking. We found that escitalopram treatment had a limited effect in improving depression symptoms in heroin-dependent patients. In mice, we demonstrated prolonged (4 weeks) but not acute (24 h) withdrawal from morphine induced depressive-like behaviors. The number of c-Fos positive cells and the expression of KOR in the nucleus accumbens (NAc), were significantly increased in the prolonged morphine withdrawal mice. Conditional KOR knockdown in NAc significantly improved depressive-like behaviors. Repeated but not acute treatment with the KOR antagonist norBNI improved depressive-like behaviors and reversed PSD95, synaptophysin, p-ERK, p-CREB, and BDNF in NAc. This study demonstrated the important role of striatal KOR in morphine withdrawal-related depressive-like behaviors and offered therapeutic potential for the treatment of withdrawal-related depression.
Subject areas: Addiction medicine, Behavioral neuroscience, Molecular neuroscience, Neuroscience
Graphical abstract
Highlights
-
•
Prolonged withdrawal from opioids induced depression in both humans and mice
-
•
NAc KOR upregulation correlates with withdrawal-related depressive behavior in mice
-
•
Conditional KOR knockdown in NAc improved depressive-like behavior in mice
-
•
Repeated norBNI treatment reversed the ERK-CREB-BDNF pathway in NAc
Addiction medicine; Behavioral neuroscience; Molecular neuroscience; Neuroscience
Introduction
Drug addiction is characterized by compulsive use of drugs, withdrawal, and relapse regardless of the negative consequences.1 A neuroadaptive view of drug addiction focuses on long-term plasticity that leads to a persistent negative affective state and altered function of key motivational systems as the proximal cause of relapse.2,3 Studies on opioid addiction find that most heroin abuse patients developed depressive symptoms after withdrawal.4,5 Importantly, depressive symptoms could be an important factor in relapse of opioid addiction. Depressed heroin addicts are more likely to relapse compared to those without depression.6 However, withdrawal-related depression still lacks a complete understanding of opioid addiction. Classical antidepressants, mainly selective serotonin reuptake inhibitors (SSRI), have been reported to have limited effects on the treatment of withdrawal-related depression.7,8 The study found that escitalopram did not produce faster or greater reductions in depressive symptoms after withdrawal from opioids.9 Based on this evidence, we speculate that there may be another mechanism of withdrawal-related depression that is worth studying in more detail.
The opioid receptors include three subtypes of the G protein-coupled receptor: mu, delta, and kappa opioid receptor (KOR).10 Although they share the common analgesic effect on brain circuits, the different subtypes of opioid receptors have unique effects and a specific distribution in brain regions.11,12 The mu opioid receptor (MOR), highly expressed in the periaqueductal gray, cerebral cortex, and thalamus, stimulates respiratory depression, physical dependence, and euphoria by binding endorphins. In different regions of the brain, such as the vomeronasal nucleus and the basolateral amygdala, MOR triggers the motivational properties of euphoric and rewarding stimuli.13,14 Delta opioid receptor (DOR), located mainly in the basal ganglia, induces anxiolytic effects by binding enkephalins.15 KOR, highly expressed in the hypothalamus, periaqueductal gray, and nucleus accumbens, binds to dynorphins and triggers sedation and dysphoric effects.15 Recent evidence implicated the role of KOR in mediating behaviors relevant to depression and anxiety.16 KOR agonists have been reported to elicit negative emotions such as anhedonia, aversion, and depression.17,18,19 A selective KOR antagonist JNJ-67953964 has been shown to reverse stress-induced decrease in sucrose pref.20 However, other studies found opposing results. The KOR agonist salvinorin A induced an antidepressant effect in rodents, including reduced immobility in the forced swim test and increased sucrose pref.21 The KOR agonist U50,488 attenuated the escape failure induced by pre-exposure to shock in the learned helplessness model of depression in mice.22 Dynorphin has been found to modulate emotional control, and mice lacking pro-dynorphin exhibit marked anxiety.23 Therefore, it is urgent to investigate the role of KOR in opioid withdrawal-related depression.
The nucleus accumbens (NAc) has been recognized as a key brain region that mediates reward valence and drug-seeking behaviors. NAc is one of the target brain regions for the clinical treatment of drug abuse.24 The increasing evidence indicates that NAc plays an important role in emotional changes.25,26,27 This study first asked whether withdrawal from heroin causes significant emotional changes in the addiction patient and whether depression symptoms could be improved by antidepressant treatment. To further study the mechanism of withdrawal-related depression in patients, we established the mouse model of withdrawal-related depression from chronic morphine addiction. We then investigated the role of KOR in NAc in depressive-like behaviors related to morphine withdrawal. Furthermore, we examined synaptic markers and the classical KOR downstream signaling pathway ERK-CREB-BDNF in NAc to further explore the possible molecular mechanisms underlying opioid withdrawal-related depression.
Results
Escitalopram had limited effects for ameliorating depression symptoms in heroin-dependent patients
Among 356 heroin-dependent participants, 73 (20.51%) were diagnosed with major depression. Sixty-one of these participants were randomly assigned to the escitalopram treatment group (n = 32) and the placebo group (n = 29) (Tables S1–S4). The two-way ANOVA analysis results of Tables 1, 2, and 3 showed the HAMD scores of the participants at baseline and at weeks 2, 4, 6 and 8 after the escitalopram intervention. At the end of 8 weeks of treatment, the escitalopram group included 21 participants with moderate depression and 11 participants with severe depression, of which only 9 and 4 participants responded to the escitalopram treatment. The placebo group included 17 participants with moderate depression and 12 participants with severe depression, of which only 4 and 2 participants responded to escitalopram treatment. As shown in Table 4, 13 of 32 subjects in the intervention group (40.63%) and 6 of 29 placebo subjects (20.69%) were considered responders in total. The comparison of intervention therapy between groups by chi-square test did not show significant differences (Table 4, p = 0.093). These results showed that the treatment of escitalopram had limited effects for ameliorating depression symptoms in heroin-dependent patients.
Table 1.
Change in HAMD scores from baseline: within group (merge) comparison
| Change in HAMD scores from baseline (merge) | Placebo | Escitalopram |
|---|---|---|
| Baseline (mean ± SEM) | 22.724 ± 0.520 | 22.844 ± 0.598 |
| 2w post-test (mean ± SEM) | 20.310 ± 0.699 | 19.813 ± 0.733 |
| Change between baseline and 2w post-test (95% CI) | −2.414(-0.6179,5.445) | −3.031(0.1452,5.917) |
| 4w post-test (mean ± SEM) | 18.793 ± 0.846 | 18.563 ± 0.943 |
| Change between baseline and 4w post-test (95% CI) | −3.931(0.8994,6.963) | −4.281(1.395,7.167) |
| 6w post-test (mean ± SEM) | 17.655 ± 0.899 | 16.625 ± 0.946 |
| Change between baseline and 6w post-test (95% CI) | −5.069(2.037,8.101) | −6.219(3.333,9.105) |
| 8w post-test (mean ± SEM) | 17.759 ± 1.012 | 14.500 ± 0.962 |
| Change between baseline and 6w post-test (95% CI) | −4.966(1.934,7.997) | −8.344(5.458,11.23) |
Table 2.
Change in HAMD scores from baseline: within group (moderate) comparison
| Change in HAMD scores from baseline (moderate) | Placebo | Escitalopram |
|---|---|---|
| Baseline (mean ± SEM) | 21.118 ± 0.528 | 20.857 ± 0.449 |
| 2w post-test (mean ± SEM) | 18.765 ± 0.872 | 18.095 ± 0.765 |
| Change between baseline and 2w post-test (95% CI) | −2.353 (−1.416, 6.122) | −2.762 (−0.6292,6.153) |
| 4w post-test (mean ± SEM) | 17.294 ± 1.091 | 16.190 ± 1.064 |
| Change between baseline and 4w post-test (95% CI) | −3.824 (0.05451, 7.593) | −4.667 (1.276, 8.058) |
| 6w post-test (mean ± SEM) | 16.353 ± 1.254 | 14.667 ± 1.124 |
| Change between baseline and 6w post-test (95% CI) | −4.765 (0.9957, 8.534) | −6.190 (2.799, 9.582) |
| 8w post-test (mean ± SEM) | 16.412 ± 1.383 | 13.143 ± 1.137 |
| Change between baseline and 6w post-test (95% CI) | −4.706 (0.9369, 8.475) | −7.714 (4.323, 11.11) |
Table 3.
Change in HAMD scores from baseline: within group (severe) comparison
| Change in HAMD scores from baseline (severe) | Placebo | Escitalopram |
|---|---|---|
| Baseline (mean ± SEM) | 25.000 ± 0.537 | 26.636 ± 0.509 |
| 2w post-test (mean ± SEM) | 22.500 ± 0.830 | 23.091 ± 0.986 |
| Change between baseline and 2w post-test (95% CI) | −2.500 (−1.103, 6.103) | −3.545 (−0.2179, 7.309) |
| 4w post-test (mean ± SEM) | 20.917 ± 1.118 | 23.091 ± 0.756 |
| Change between baseline and 4w post-test (95% CI) | −4.083 (0.4802, 7.686) | −3.545 (−0.2179, 7.309) |
| 6w post-test (mean ± SEM) | 19.500 ± 1.098 | 20.364 ± 1.055 |
| Change between baseline and 6w post-test (95% CI) | −5.500 (1.897, 9.103) | −6.273 (2.509, 10.04) |
| 8w post-test (mean ± SEM) | 19.667 ± 1.339 | 17.091 ± 1.546 |
| Change between baseline and 6w post-test (95% CI) | −5.333 (1.730, 8.936) | −9.545 (5.782, 13.31) |
Table 4.
Effect of escitalopram treatment in heroin patients with depression
| Treatment Results |
Placebo (n %) | Escitalopram (n %) | χ2 | p |
|---|---|---|---|---|
| Effect | 6 (20.69) | 13 (40.63) | 2.819 | 0.093 |
| No effect | 23 (79.31) | 19 (59.37) |
Depressive-like behaviors and levels of NAc opioid receptors in mice with acute withdrawal from morphine
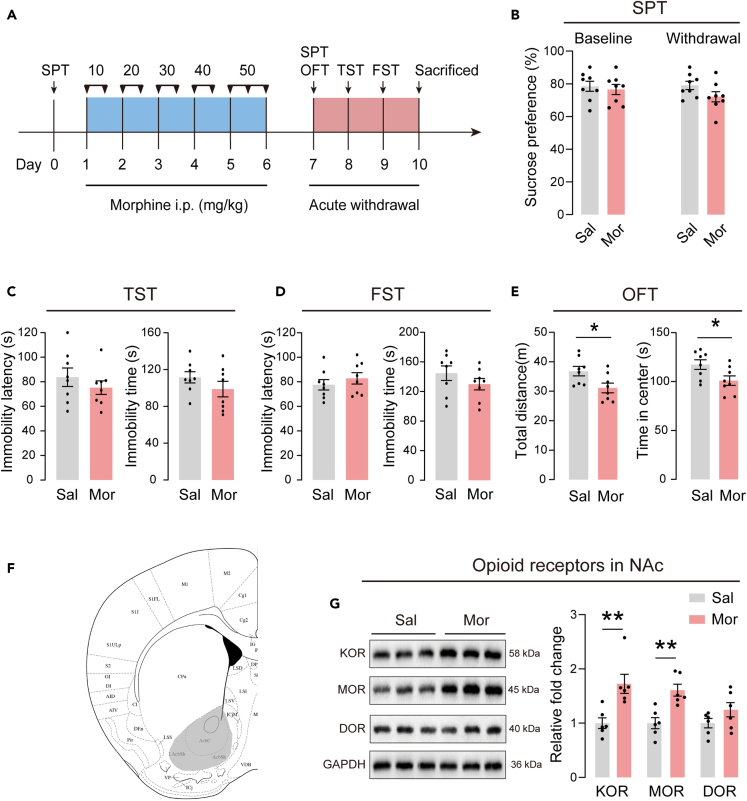
To model the affective changes that are observed in humans during protracted opioid withdrawal, mice were administered escalating doses of morphine and tested for depressive-like behaviors 24 h after discontinuation of morphine. The timeline of the experiment is illustrated in Figure 1A. Depressive-like behaviors were assessed using SPT, TST, and FST. After injection of the escalating dose of morphine, no differences were found in sucrose preference in SPT (Figure 1B), immobility time and latency in TST and FST (Figures 1C and 1D), indicating that the acute withdrawal from morphine did not induce depressive-like behaviors in mice. In OFT, acute withdrawal mice showed a significantly decrease in distance and center time compared to saline control mice (Figure 1E), suggesting impairment of locomotor activity in these mice. Next, we evaluated the expression levels of three opioid receptors in NAc. Representative immunoblots of the KOR, MOR and DOR are shown in Figure 1G. Expression levels of KOR and MOR increased significantly in mice with acute withdrawal from morphine, while the expression level of DOR was similar to those of saline control mice.
Figure 1.
Depressive-like behaviors and NAc opioid receptors levels in acute withdrawal mice
(A) Timeline of the experiment.
(B) The sucrose preference at baseline and after acute withdrawal.
(C) The immobility latency and immobility time in the TST.
(D) The immobility latency and immobility time in the FST.
(E) The locomotor activity in the OFT. n = 8/group. ∗p < 0.05. The behavioral data were analyzed with unpaired t-test. Sal mice were compared with Mor mice.
(F) Schematic diagram of brain regions. The gray shaded areas represent the portion of NAc for the analysis of protein expression.
(G) Left: Representative western blot images. Right: Effects on the protein expression of opioid receptors after morphine acute withdrawal, including KOR, MOR, and DOR. The protein levels of the saline treated group are set as 1. n = 6/group for analysis. ∗∗p < 0.01. Protein levels were analyzed with unpaired t-test. Sal group was compared with Mor group. The values represent the mean ± SEM.
Prolonged withdrawal from morphine-induced depressive-like behaviors in mice and distinct KOR upregulation in NAc
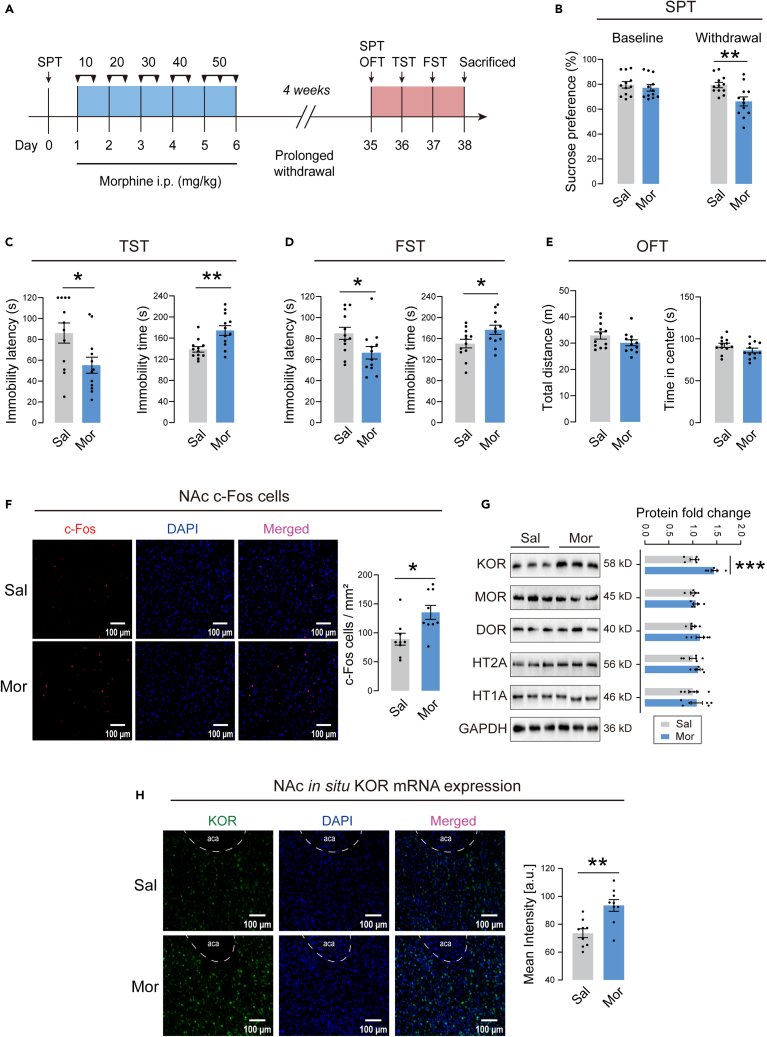
Next, we explored whether prolonged withdrawal from morphine could induce depressive-like behaviors in mice. The timeline of the experiment is illustrated in Figure 2A. The sucrose preference was significantly decreased in morphine withdrawal mice compared to saline control mice (Figure 2B). The morphine withdrawal mice exhibited decreased immobility latency and increased immobility time in both TST and FST (Figures 2C and 2D). There were no significant differences in total distance and center time in OFT between the two groups (Figure 2E), suggesting unchanged locomotor activity. These results clearly demonstrated that 4 weeks of withdrawal from morphine induced depressive-like behaviors in mice.
Figure 2.
Depressive-like behaviors and NAc molecular alterations in mice with 4 weeks withdrawal from morphine
(A) Timeline of the experiment.
(B) The sucrose preference at baseline and after 4 weeks withdrawal.
(C) The immobility latency and immobility time in the TST.
(D) The immobility latency and immobility time in the FST.
(E) The locomotor activity in the OFT. n = 12/group. ∗p < 0.05, ∗∗p < 0.01. The behavioral data were analyzed with unpaired t-test. Sal mice were compared with Mor mice.
(F) Left: Distribution of c-Fos cells in NAc in saline or morphine treated mice. Scale bar, 100 μm. Right: Number of c-Fos cells per mm2 imaging area. n = 9 slices from 3 animals/group. ∗p < 0.05 by unpaired t-test. Sal group was compared with Mor group.
(G) Left: Representative western blot images. Right: Effects of prolonged morphine withdrawal on protein expression, including KOR, MOR, DOR, HT2A, and HT1A. The protein levels of the Sal group are set as 1. n = 6/group for analysis. ∗∗∗p < 0.001. Protein levels were analyzed with unpaired t-test. Sal group was compared with Mor group.
(H) Left: Representative FISH images of KOR mRNA. Scale bar, 100 μm. Right: The mean intensity of KOR mRNA in NAc of saline or morphine treated mice. n = 9 slices from 3 animals/group. ∗∗p < 0.01 by unpaired t-test. Sal group was compared with Mor group. The values represent the mean ± SEM.
To further determine whether NAc was involved in morphine withdrawal-related depressive-like behaviors, we detected c-Fos (an immediate-early gene marker of neural activity) expression in NAc from morphine withdrawal mice after behavioral testing. Representative photos of c-Fos immunofluorescent staining in NAc are shown in Figure 2F. Quantitative analysis revealed a significantly increased number of c-Fos positive cells in morphine withdrawal mice, indicating that NAc was activated after prolonged withdrawal of morphine. We then assessed the protein expression levels of opioid receptors in NAc. Since evidence suggested a critical role for 5-HT receptors in major depressive disorder,28,29 we also evaluated protein expression levels of the 5-HT1A and 5-HT2A receptors. Representative immunoblots for opioid receptors and 5-HT receptors are shown in Figure 2G. Interestingly, KOR was significantly upregulated in NAc in morphine withdrawal mice. There were no significant differences in MOR and DOR expression between the morphine and saline groups. Furthermore, the 5-HT1A and 5-HT2A receptors did not change between morphine and saline mice. We performed fluorescence in situ hybridization (FISH) to examine the level of KOR mRNA expression in NAc. Representative FISH photos are shown in Figure 2H. Consistent with the result of immunoblotting, the level of KOR mRNA in NAc increased in morphine withdrawal mice (Figure 2H). These results indicated that KOR was markedly upregulated in NAc after prolonged withdrawal from morphine.
Antidepressant treatment had only limited effects on depressive-like behaviors in morphine withdrawal mice
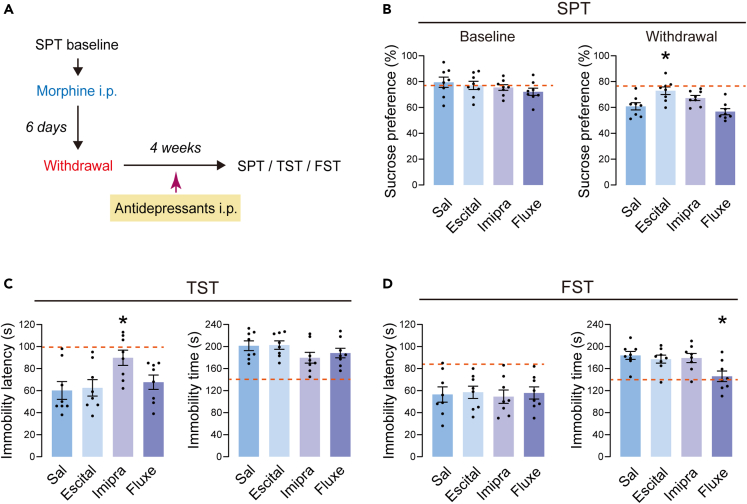
We evaluated the effect of classic antidepressants on depressive-like behaviors in morphine-treated mice with prolonged withdrawal. Studies show that escitalopram,30 imipramine31 and fluoxetine32 significantly reversed depressed emotion. In this experiment, mice received escitalopram (15 mg/kg, i.p.), imipramine (20 mg/kg, i.p.), and fluoxetine (10 mg/kg, i.p.) every day during the 4-week withdrawal period (Figure 3A). Depressive-like behaviors were evaluated using SPT, TST, and FST. In SPT, escitalopram, but not imipramine or fluoxetine, reversed the withdrawal-induced decrease in sucrose preference (Figure 3B). In TST, immobility latency increased in the imipramine group (Figure 3C). In FST, the immobility time decreased in the fluoxetine group (Figure 3D). Over all, these results suggested that the three classic antidepressants had only limited effects on depressive-like behaviors induced by morphine withdrawal.
Figure 3.
Depressive-like behaviors after the treatment of antidepressants
(A) Timeline of the experiment.
(B) Left: Baseline of the sucrose preference before the injection of morphine. Right: The sucrose preference after the 4 weeks withdrawal.
(C) The immobility latency and immobility time in the TST.
(D) The immobility latency and immobility time in the FST. n = 8/group. The orange dashed line represents the level of mice in the Sal-Sal group. ∗p < 0.05. The behavioral data were analyzed with one-way ANOVA, followed by Tukey’s post hoc test. Mor+Escital mice, Mor+Imipra mice and Mor+Fluxe mice were compared with Mor+Sal mice. The values represent the mean ± SEM.
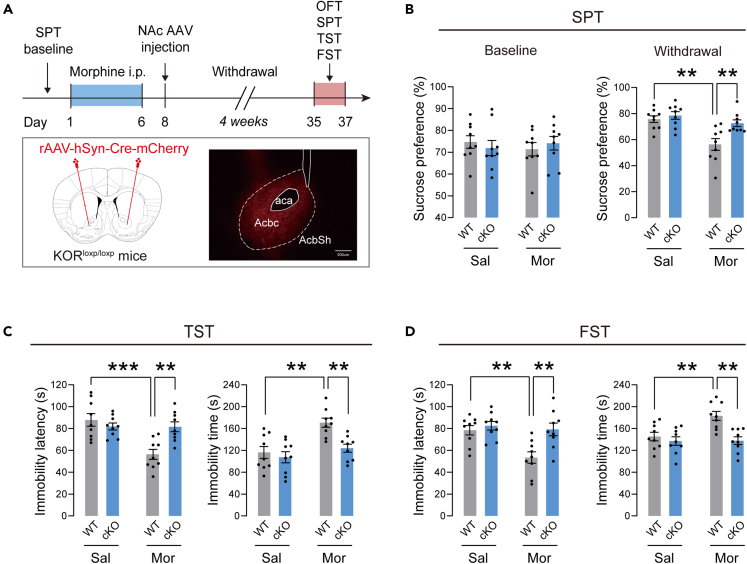
Conditional knockdown of KOR in NAc prevented depressive-like behaviors in mice after withdrawal from morphine
We used KORloxp/loxP mice to knockdown KOR in NAc. After the last injection of morphine, the viruses (AAV-hSyn-Cre-mCherry or AAV-hSyn-mCherry) were microinjected bilaterally in NAc in mice. The KOR protein expression levels were dramatically decreased in the KORloxp/loxP mice expressing Cre-recombinase, implicating that KOR transgene mice were an effective tool to manipulate the expression of KOR (Figures S1B and S1C). The mice were then subjected to 4 weeks of spontaneous withdrawal and tested for depressive-like behaviors. The timeline of this experiment is illustrated in Figure 4A. Schematic pictures show the injection sites and the representative photo of virus-expressed fluorescence in NAc. At baseline, no differences were found in sucrose preference between the different groups of mice (Figure 4B). However, after 4 weeks of morphine withdrawal, mice in the NAc KOR conditional knockout (cKO) group showed significantly higher sucrose preference than KOR wild-type (WT) controls. Furthermore, cKO mice exhibited a higher immobility latency, as well as a shorter immobility time in TST (Figure 4C) and FST (Figure 4D) compared to WT mice. In OFT, there were no significant differences in total distance and center time between these groups (Figure S2), suggesting unchanged locomotor activity in these mice. These results indicated that the KOR in NAc is required for depressive-like behaviors after prolonged morphine withdrawal.
Figure 4.
The effect of KOR conditional knockdown for the depressive-like behaviors
(A) Above: Timeline of the experiment. Below: Diagram of virus injection and representative coronal images of the injection site. The virus AAV-hSyn-Cre-mCherry was microinjected bilaterally in the NAc of KORloxp/loxP mice to knockdown the KOR.
(B) Left: The baseline of the sucrose preference before the injection of morphine. Right: The sucrose preference after the 4 weeks withdrawal.
(C) The immobility latency and immobility time in the TST.
(D) The immobility latency and immobility time in the FST. n = 9 per group. ∗∗p < 0.01, ∗∗∗p < 0.001. The behavioral data were analyzed with two-way ANOVA, followed by Sidak’s post hoc test. Sal-treated WT mice were compared with Mor-treated WT mice. Mor-treated WT mice were compared with Mor-treated cKO mice. The values represent the mean ± SEM.
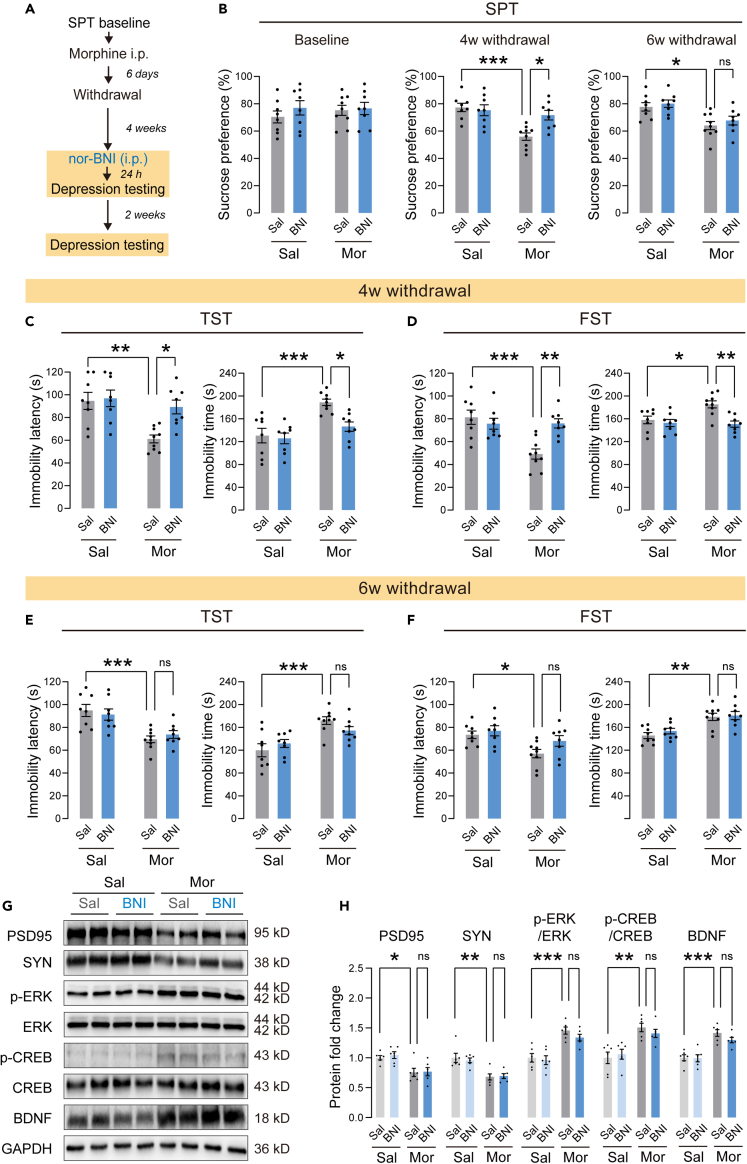
A single norBNI application induced a short antidepressant effect in morphine withdrawal mice
To validate the effect of KOR on morphine withdrawal-related depression, we applicated a single dose of the KOR antagonist norBNI (10 mg/kg, i.p.) 24 h before behavioral testing.33 Two weeks later, the depressive-like behaviors were assessed again to assess the potency and duration of norBNI (Figure 5A). We found that a single norBNI injection reversed sucrose preference in morphine withdrawal mice (Figure 5B). Similar results were observed in the FST and the TST. In the TST, a single nor-BNI injection reversed the decreased immobility latency and the increased immobility time (Figure 5C). In the FST, a single norBNI injection also reversed the decreased immobility latency and the increased immobility time (Figure 5D). However, the antidepressant effect of norBNI disappeared two weeks after injection. Compared to saline-treated morphine withdrawal mice, norBNI-treated morphine withdrawal mice exhibited a similar decreased sucrose preference (Figure 5B), decreased immobility latency, and increased immobility time in TST and FST (Figures 5E and 5F). In addition, a correlation analysis was conducted on the data of immobility behavior 4 weeks and 6 weeks after morphine withdrawal. The results did not show a correlation, which can be inferred that the loss of effectiveness of norBNI over the course of two weeks could not be the result of learned immobility (Figure S3). These results indicated that a single norBNI application induced a short antidepressant effect in mice with morphine withdrawal.
Figure 5.
Effect of a single norBNI application for depressive-like behaviors
(A) Timeline of the experiment.
(B) Left: The baseline of the sucrose preference before the injection of morphine. Middle: The sucrose preference after the 4 weeks withdrawal. Right: The sucrose preference after the 6 weeks withdrawal.
(C and D) The immobility latency and immobility time in the TST (C) and FST (D) after morphine withdrawal of 4 weeks.
(E and F) The immobility latency and immobility time in the TST (E) and FST (F) after morphine withdrawal of 6 weeks n = 8–9/group. Ns: non-significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. The behavioral data were analyzed with two-way ANOVA, followed by Sidak’s post hoc test. Sal+Sal mice were compared with Mor+Sal mice. Mor+Sal mice were compared with Mor+BNI mice.
(G) Representative western blot images.
(H) The protein expression levels of PSD95, synaptophysin, p-ERK, p-CREB and BDNF following the single norBNI treatment. The protein levels of the Sal+Sal group are set as 1. n = 6/group for analysis. Ns: non-significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Protein levels were analyzed with two-way ANOVA, followed by Sidak’s post hoc test. Sal+Sal group was compared with Mor+Sal group. Mor+Sal group was compared with Mor+BNI group. The values represent the mean ± SEM.
Chronic administration of morphine induces extensive molecular and biochemical adaptive changes34 and activates the KOR downstream signaling pathway.35 After the final behavioral tests, we evaluated changes in the synaptic marker PSD95, synaptophysin, and the ERK-CREB-BDNF pathway, which is a known downstream pathway of KOR, in NAc by Western blotting. Representative immunoblots are presented in Figure 5G. As shown in Figure 5H, compared to saline control mice, PSD95 and synaptophysin expression levels decreased significantly in mice with morphine withdrawal. Furthermore, ERK-CREB-BDNF signaling was significantly activated in NAc after withdrawal from morphine. Surprisingly, the single norBNI treatment failed to reverse the changes of these molecules in morphine withdrawal mice.
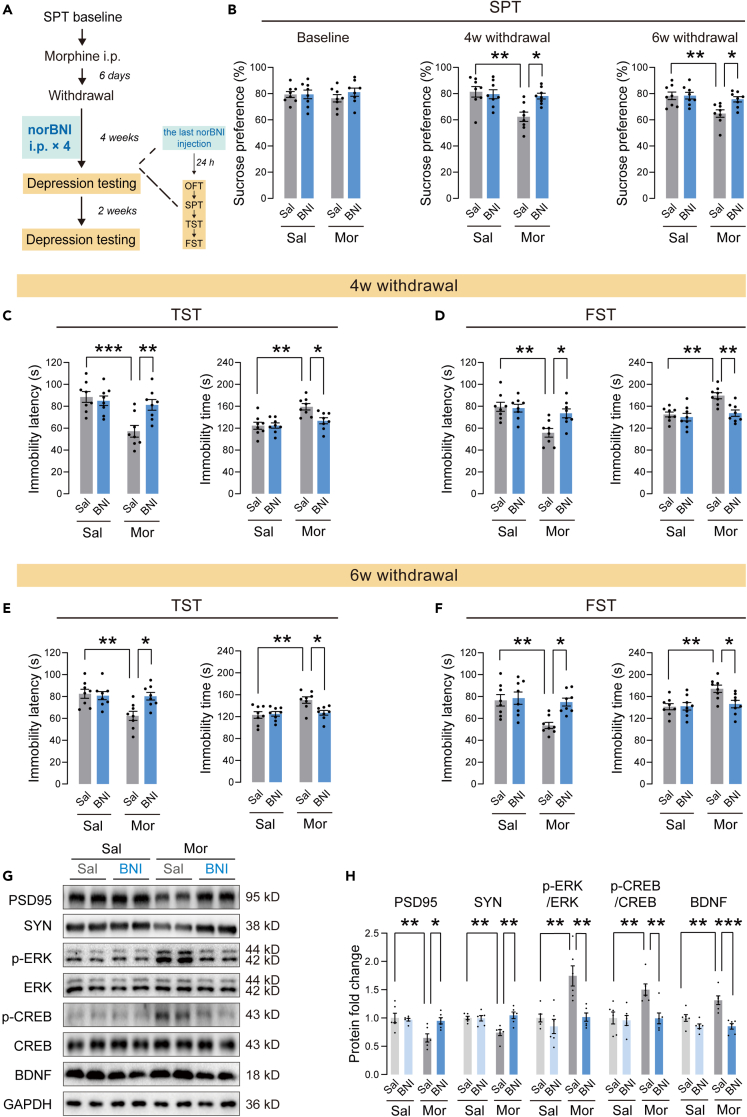
Repeated norBNI application improved depressive-like behaviors in morphine withdrawal mice at long-term
To explore whether KOR antagonism in NAc could improve long-term depressive-like behaviors, morphine withdrawal mice were injected with norBNI (10 mg/kg, i.p.) once a week during 4-week withdrawal periods (Figure 6A). After 4 weeks of withdrawal, the mice showed a significantly lower sucrose preference than the saline control mice (Figure 6B). norBNI treatment for 4 weeks significantly improved the reduction of sucrose preference in morphine withdrawal mice. In the TST, repeated injection of norBNI for 4 weeks reversed the decrease in immobility latency and the increased immobility time (Figure 6C). In the FST, repeated injection of norBNI also reversed the decreased immobility latency and the increased immobility time (Figure 6D). In OFT, there were no significant differences in total distance and center time between these groups (Figure S4), suggesting unchanged locomotor activity in these mice. Furthermore, compared to saline-treated morphine withdrawal mice, norBNI-treated mice showed a normal preference for sucrose 6 weeks after withdrawal (Figure 6B), indicating a long-term improvement in anhedonia. NorBNI-treated morphine withdrawal mice also exhibited an increase in immobility latency and a decrease in immobility time in both TST and FST 6 weeks after withdrawal (Figures 6E and 6F). These results indicated that the antidepressant effect of repeated norBNI treatment lasted at least two weeks. Similarly, after the final behavioral tests, we assessed changes of the synaptic marker PSD95, synaptophysin and the ERK-CREB-BDNF pathway by Western blotting. Representative immunoblots are presented in Figure 6G. As shown in Figure 6H, repeated norBNI treatment reversed the decrease in PSD95 and synaptophysin and the increase in p-ERK, p-CREB and BDNF in morphine withdrawal mice.
Figure 6.
Effect of repeated norBNI application for depressive-like behaviors
(A) Timeline of the experiment.
(B) Left: The baseline of the sucrose preference before the injection of morphine. Middle: The sucrose preference after the 4 weeks withdrawal. Right: The sucrose preference after the 6 weeks withdrawal.
(C and D) The immobility latency and immobility time in the TST (C) and FST (D) after morphine withdrawal of 4 weeks.
(E and F) The immobility latency and immobility time in the TST (E) and FST (F) after morphine withdrawal of 6 weeks n = 8/group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. The behavioral data were analyzed with two-way ANOVA, followed by Sidak’s post hoc test. Sal+Sal mice were compared with Mor+Sal mice. Mor+Sal mice were compared with Mor+BNI mice.
(G) Representative western blot images.
(H) The protein expression levels of PSD95, synaptophysin, p-ERK, p-CREB, and BDNF following the repeated norBNI treatment. The protein levels of the Sal+Sal group are set as 1. n = 6/group for analysis. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Protein levels were analyzed with two-way ANOVA, followed by Sidak’s post hoc test. Sal+Sal group was compared with Mor+Sal group. Mor+Sal group was compared with Mor+BNI group. The values represent the mean ± SEM.
The statistics of results presented in this study are summarized in supplementary data file (Data S1: Statistics related to data presented in this study. Related to STAR methods.).
Discussion
In our study, we established a mouse model of morphine withdrawal to investigate the role of striatal KOR in depressive-like behaviors after prolonged withdrawal from opioids. First, we found that heroin-dependent participants developed depressive symptoms after withdrawal and antidepressant escitalopram had limited effects in ameliorating depression symptoms. In mice, we established a mouse model of morphine addiction and found that prolonged but not acute withdrawal from morphine produced significant depressive-like behaviors. Importantly, treatment with classic antidepressants, including escitalopram, imipramine, and fluoxetine, had only limited effects on depressive-like behaviors induced by prolonged withdrawal. The Petrakis study showed that fluoxetine is not an effective agent in treating cocaine-related depression.7 Carpenter presented that sertraline was no different than placebo in reducing depressive symptoms or the use of illicit substances in the sample of opiate-dependent patients with syndromic depressive disorders.8 Therefore, we assumed that there may be another mechanism in withdrawal-related depression. Although currently no animal model can mimic all the characteristics of human depression, our results suggested that our mouse model could be a useful animal model that recapitulates the major clinical symptoms observed in human subjects with withdrawal-related depression. Furthermore, it is possible that there is a comparable mechanism of depression after withdrawal in both humans and mice. Therefore, the use of this mouse model of morphine withdrawal presents an opportunity to investigate the mechanism of depression after withdrawal in the human. NAc is a key region of the brain involved in the reward and affective process. Dopamine (DA) terminal in the medial shell of NAc is excited by aversive outcome, whereas the lateral shell of NAc is excited by rewards.36 Thus, NAc dysfunction is believed to be related to addiction and depressive-like behaviors.37,38 In this study, we found that morphine withdrawal-related depressive-like behaviors strongly evoked the expression of c-Fos in NAc, suggesting that NAc neurons were activated in opioid withdrawal mice with depressive-like behaviors.
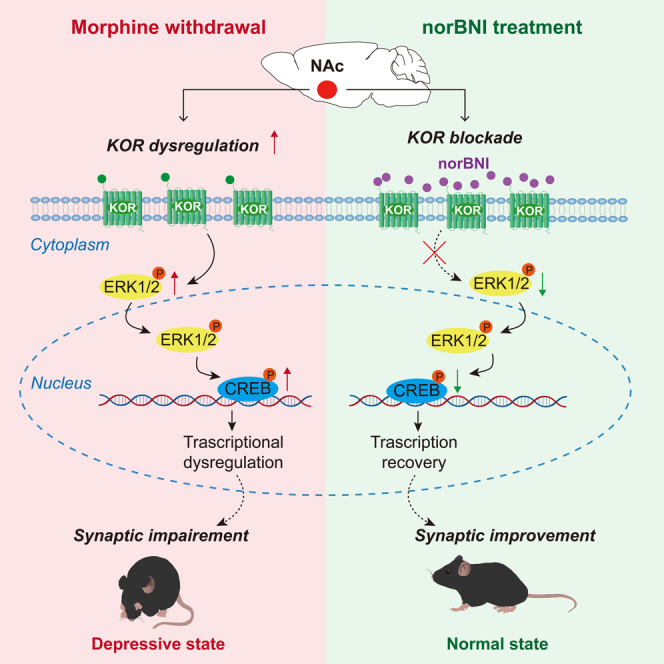
Different subtypes of opioid receptors are distributed differently in the brain and have distinct effects.11,12,13,14,15 MOR, KOR, and DOR modulate a series of physiological processes and behaviors, such as gastrointestinal function, immunity, pain sensation, mood, reward, and aversion.39 Morphine is a potent agonist of opioid receptors, inducing different changes in different subtypes of opioid receptors.40,41,42,43,44 The endogenous opioid system is directly involved in mood regulation and is dysregulated in depression.45 Therefore, we studied the role of NAc opioid receptors in withdrawal-related depressive-like behaviors in mice. We found that the protein expression of KOR and MOR increased 24 h after withdrawal from morphine without depressive-like behaviors. Importantly, high levels of KOR remained 4 weeks after withdrawal and mice exhibited withdrawal-related depressive-like behaviors, while MOR returned to normal levels. MOR is involved in physical dependence and euphoria by binding endorphins.13,14 KOR binds to dynorphins and triggers sedation and dysphoric effects.15 Previous experiments in our lab have shown that mice were in a state of mental euphoria during acute morphine withdrawal and mainly exhibited withdrawal and anxiety-like symptoms within 2 weeks after morphine withdrawal. Also, these symptoms disappeared 4 weeks after morphine withdrawal.46 This study found that during the acute withdrawal period, there were increased levels of MOR and KOR expression in NAc, but the mice did not show depressive-like behaviors. However, 4 weeks later, as MOR levels returned to normal, withdrawal symptoms and anxiety-like behaviors disappeared over time. We speculated that MOR played a dominant role in the regulation of withdrawal symptoms and anxiety-like behaviors during this period, while high levels of KOR played the dominant role in modulating depression-like behaviors in mice. Consistently, Zan proposed that prolonged morphine withdrawal induced depressive-like behaviors in mice, and these depressive-like behaviors were increased along with the duration of abstinence.47 Mounting evidence has indicated that KOR is an important substrate in the comorbidity between depressive disorders and opioid addiction. In animal models of addiction, the activity of KOR is enhanced by multiple stressors and tightly controlled for drug seeking and relapse.48 Furthermore, KOR signaling has been found to mediate despair-like responses to a variety of stressors.10 The systemic application of KOR agonists inhibited dopamine release from NAc.49 Activation of KOR from mPFC glutamatergic projection fibers down-regulates glutamatergic neurotransmission and LTP formation within NAc, causing inhibition of D2-MSN excitatory synaptic transmission function.50 These results suggested that KOR may be involved in the pathophysiological alterations of NAC in aversion. Zan’s study showed that morphine withdrawal-induced depressive-like behaviors were regulated by dynorphin/opioid receptor system, and the application of opioid receptor antagonists norBNI could improve the mood disorders induced by opiate abstinence.47 Studies have suggested that the application of norBNI improved the negative emotions caused by KOR.51,52,53 We hypothesize that the persistent increase in NAc KOR may be related to depressive-like behaviors after prolonged withdrawal from opioids. Using KOR antagonist and virus-mediated KOR knockdown in NAc, we further dissect the role of KOR in depressive-like behaviors. We found that both the application of the KOR antagonist norBNI and the knockdown of KOR in NAc significantly improved depressive-like behaviors. These results showed that the striatal KOR plays a crucial role in opioid withdrawal-related depression.
We also noted the limitation of the striatal KOR antagonism that a single norBNI application only induced a transient antidepressant effect in morphine withdrawal mice. On the contrary, repeated norBNI treatment during opioid withdrawal significantly improved depressive-like behavior for at least 2 weeks. The important parameter that affects the duration of drug action is the distribution of the drug in the body. It has been suggested that prolonged KOR antagonism may be influenced by changes in the pharmacokinetics of norBNI.54 Endoh et al. reported that the time course of antagonistic action of norBNI toward morphine-induced analgesia in the mouse tail pinch test. In this report, the significant antagonistic effect of norBNI against morphine-induced antinociception was maintained for at least 4 days.55,56 According to Patkar’s study, norBNI remained significantly higher than the control in the perfused brain homogenate up to 7 days after a single intraperitoneal pretreatment. In addition, the parallel experiment showed that the prolonged norBNI-induced antagonism of KOR-mediated antinociception persisted for at least 1 week, the amount of norBNI detected fell to levels not statistically different from control over the next 7 days.54 In addition, repeated norBNI injections produced more effective analgesia than the partial analgesic effect produced by a single injection of norBNI.57 We speculated that the limited effect of a single norBNI application against depression in this study was due to the inability of low drug concentrations in the brain to maintain KOR inhibition. On the contrary, repeated norBNI maintains the effective concentration of the drug in the brain. Consistent with the result of pharmacological KOR blockade, virus-mediated KOR knockdown in NAc effectively prevented depressive-like behaviors after morphine withdrawal. Together, we demonstrate that persistent increase in KOR in NAc is involved in the regulation of depressive-like behaviors after prolonged withdrawal from opioids.
KOR is involved in the regulation of neuronal synaptic plasticity. Subchronic administration of U50,488H, a selective KOR agonist, in mice causes profound decreases in several postsynaptic proteins such as postsynaptic density protein 95 (PSD-95) and synaptosomal associated protein 25, while norBNI blocks reduction of these proteins.58 We found that morphine administration significantly decreased the expressions of PSD95 and synaptophysin, while repeated norBNI treatment reversed these changes. This result indicated that the persistent increase in striatal KOR following prolonged withdrawal of morphine may cause dysregulation of synaptic plasticity in NAc neurons and result in altered neural functions, which may be attributed to KOR-induced autophagy according to Karoussiotis’ study.58 Activation of KOR activates the dissociation of G-protein coupled receptor Gα and Gβγ subunits that interact with various intracellular effectors.35 Sustained KOR activation further recruits phosphorylated activation of MAPK signaling cassettes, particularly the ERK-CREB-BDNF signaling pathway.59,60,61 There is disagreement on the ERK-CREB-BDNF signaling in modulating stress responses and affective reward states. Some studies reported that the activation of KOR and ERK signaling was involved in negative emotions in mice and pretreatment with norBNI reversed the increase in p-ERK expression and improved negative emotions.51,60 Others proposed that overexpressed BDNF activated the ERK-CREB-BDNF signaling pathway to alleviate comorbid pain in CRS-induced depression.62 In this study, we found that prolonged morphine withdrawal promoted activation of ERK1/2 and CREB by increasing their phosphorylation levels and increased the expression level of BDNF in NAc. CREB has been reported to transcriptionally regulate the expression of the BDNF gene.63 Striatal BDNF and its receptor TrkB are altered after chronic exposure and withdrawal from cocaine.64 Nestler showed that the increase in BDNF in NAc plays a role in depressive-like behaviors.65 However, interestingly, repeated norBNI injection reversed the activation of the ERK-CREB-BDNF signaling pathway and maintained an improvement in depression 6 weeks after withdrawal. No effects were found in the intervention of single norBNI injection. This study strengthens the notion that long-term drug effects are often attributed to irreversible occupancy of a receptor. Researchers found that norBNI, a selective and competitive KOR antagonist,66 can have its long-term effects blocked by competitive antagonists.33 Additionally, norBNI treatment did not alter the U50488-mediated increases in ERK activity when tested 24 h later.67 Based on the pharmacokinetics of norBNI54 and repeated treatments, the study suggests that norBNI inhibits ERK-CREB-BDNF signaling in a time- and concentration-dependent manner. The specific mechanism needs more detailed exploration. Therefore, our study supports the speculation that prolonged withdrawal from morphine induces a persistent increase in striatal KOR, altering the ERK-CREB-BDNF signaling pathway, which potentially contributes to depressive-like behaviors in mice.
In summary, our study demonstrated that treatment with classic antidepressants had only limited effects on depressive-like behaviors related to opioid withdrawal. Prolonged withdrawal from morphine induced depressive-like behaviors in mice and persistent upregulation of KOR in NAc. Repeated but not a single KOR antagonism or conditional KOR knockdown in NAc improves depressive-like behaviors in morphine withdrawal mice at long-term. The persistent increase in KOR may influence depressive-like behaviors through the regulation of the downstream ERK-CREB-BDNF signaling pathway in NAc. Therefore, striatal KOR may be a promising target for the treatment of depressive-like behaviors following prolonged withdrawal of opioids.
Limitations of the study
KOR is highly expressed in the NAc medium spiny neurons (MSNs).68 The NAc MSNs are segregated into dopamine D1 receptor-containing MSN (D1 MSN) and D2 receptor-containing MSN (D2 MSN).69 In this study, we demonstrated that KORs in NAc play a vital role in withdrawal-related depressive-like behaviors, but the specific role of KOR in D1 or D2 MSN in this model is still unclear. In addition, the functions of the two subregions of the NAc differ significantly in many behavioral paradigms.70,71 However, the NAc subregion in the analysis of protein expression or the intervention of the viruses was not discriminated in this study. Finally, we did not determine the underlying mechanism of the ERK-CREB-BDNF signaling pathway in depressive-like behaviors following prolonged withdrawal of opioids.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-c-Fos | Abcam | Cat#ab208942; RRID:AB_2747772 |
| Rabbit monoclonal anti-KOR | Abcam | Cat#ab183825; RRID: AB_2936198 |
| Rabbit polyclonal anti-MOR | Abcam | Cat#ab17934; RRID: AB_2283186 |
| Rabbit monoclonal anti-DOR | Abcam | Cat#ab176324; RRID:AB_2936199 |
| Rabbit polyclonal anti-5HT1A | Servicebio | Cat#GB11887; RRID:AB_2936213 |
| Rabbit polyclonal anti-5HT2A | Servicebio | Cat#GB111001; RRID:AB_2936214 |
| Mouse monoclonal anti-ERK1/2 | Novus | Cat#216703; RRID:AB_2936202 |
| Rabbit polyclonal anti- phospho-ERK1/2 | Novus | Cat#AF1018; RRID:AB_2936203 |
| Mouse monoclonal anti-CREB | Abcam | Cat#ab178322; RRID:AB_2827810 |
| Rabbit monoclonal anti- phospho-CREB | Abcam | Cat#ab32096; RRID:AB_731734 |
| Rabbit polyclonal anti-BDNF | Servicebio | Cat# GB11559; RRID:AB_2936215 |
| Rabbit polyclonal anti-PSD95 | Abclonal | Cat# A6194; RRID:AB_2766806 |
| Rabbit polyclonal anti-Synaptophysin | Servicebio | Cat# GB11553; RRID: AB_2937063 |
| Mouse monoclonal anti-GAPDH | Abcam | Cat# ab8245; RRID:AB_2107448 |
| Cy3 Goat Anti-Mouse IgG | Abclonal | Cat#AS008; RRID:AB_2769088 |
| Fluorescein Anti-Avidin D | Sigma | Cat#A2901; RRID:AB_2936205 |
| Peroxidase-conjugated anti-DIG | Roche | Cat#11207733910; RRID:AB_514500 |
| HRP Goat Anti-Rabbit IgG | Abclonal | Cat#AS063; RRID:AB_2864057 |
| HRP Goat Anti-Mouse IgG | Abclonal | Cat# AS064; RRID:AB_2864058 |
| Bacterial and virus strains | ||
| rAAV2/9-hSyn-mCherry | OBio, Shanghai | This paper |
| rAAV2/9-hSyn-Cre-mCherry | OBio, Shanghai | This paper |
| Chemicals, peptides, and recombinant proteins | ||
| Escitalopram oxalate tablets | Lundbeck, Denmark | N/A |
| Morphine hydrochloride | Third Research Institute of the Ministry of Public Security | N/A |
| Escitalopram | Sigma | CAS Number:219861-08-2 |
| Imipramine hydrochloride | Sigma | CAS Number:113-52-0 |
| Fluoxetine | Sigma | CAS Number:56296-78-7 |
| NorBNI | Abcam | CAS Number:105618-26-6 |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J | Vital River laboratory | N/A |
| Mouse: KORloxp/loxP | Jackson Laboratory | RRID: IMSR_JAX:030076 |
| Software and algorithms | ||
| Any-maze | Stoeling | RRID:SCR_014289 |
| ImageJ | NIH | https://ImageJ.net/ |
| Image Lab | Bio-Rad | RRID:SCR_014210 |
| GraphPad Prism version 8.0 | GraphPad Software | RRID:SCR_002798 |
| Photoshop CC | Adobe | RRID:SCR_014199 |
Resource availability
Lead contact
Further information and requests for resources and regents can be directed to the lead contact, Yunpeng Wang (wyp033@xjtu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Participants
A total of 356 male heroin-dependent participants with Han population (mean age = 33.11 ± 7.46 years), were recruited from the Xi’an Lantian detoxification center. Only male participants were recruited because males comprise a much higher proportion of drug abusers (88.3%) than females in China.72 Participants were included if: (i) they are first-time hospitalized, (ii) they used heroin more than one month, and (iii) they never received methadone maintenance treatment, (iiii) they have no history of psychiatric illness. Major depression was diagnosed by a structured clinical interview according to the DSM-V criteria. Depressive symptoms were rated using the 24-item Hamilton depression rating scale (HAMD, MD ≥ 17). An experienced psychiatrist rated the participants before they entered the study. The classification of participants with major depressive disorders was carried out in a clinic using the HAMD scale.73 A score ranging from 17 to 24 is defined as moderate depression. Score >24 is defined as severe depression. We did not attempt to distinguish between heroin-induced depression and primary depressive disorders. Participants had not received any antidepressants, antipsychotic drugs, or mood stabilizers for at least two months prior to the study. A total of 73 heroin-dependent participants were diagnosed with major depression (moderate depression, n = 45; severe depression, n = 28). Twelve patients were lost during the study. This investigation was approved by the First Affiliated Hospital of Xi’an Jiaotong University and was registered in the Chinese Clinical Trial Registry (ChiCTR2000037788). The enrollment date of the first subject is February 27, 2021. Written informed consent was obtained from all participants before experiments.
Animals
In the present study, male C57/Bl6J mice and KORloxp/loxP transgenic mice were used. Animals (8–10 weeks old, 26 ± 2 g) were kept in a room with a 12/12 h light-dark cycle, controlled temperature (22 ± 2°C) and humidity (50 ± 5%). All animals were housed 4 per cage in Plexiglas cages with free access to food and tap water. Experiments were conducted during the light cycle. All procedures were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University.
Method details
Escitalopram treatment
The heroin-dependent participants who were diagnosed with major depression were randomly assigned to the escitalopram treatment group (n = 32) and the placebo group (n = 29) and received escitalopram and placebo treatment for 8 weeks. A tablet of escitalopram (10 mg daily) was administered orally for the first week, after which the dose was increased to 2 tablets of escitalopram per day for the remainder of the trial. Therefore, the prominent dose of escitalopram was 20 mg/day. Participants were not allowed to use any other psychotropic drug or undergo behavioral intervention therapy during the trial course. Patients take the tablets in the presence of the medical staff when administered the medicine. Participants were evaluated using HAMD at baseline and at weeks 2, 4, 6 and 8 post-interventions. The outcome measure was to evaluate the efficacy of escitalopram in improving depressive symptoms, response to treatment (defined as 50% reduction in the HAMD score) and remission (defined as the HAMD score ≤7) rates. Treatment failure was defined as the persistence of clinical signs and symptoms. The criteria of “50% reduction in the HAMD score” was as the criteria for ameliorating depression symptoms in heroin-dependent patients. Adverse events were systematically evaluated at each time point using a checklist. Patients were also asked to immediately inform the research team of any unexpected symptom during the trial course.
Drugs and treatments
Morphine hydrochloride was prepared in 0.9% sodium chloride. Repeated morphine administration paradigm was performed by using a previously established procedure with a minor modification.46,74,75 Briefly, mice were injected intraperitoneally (i.p.) with saline or an escalating dose of morphine (from 10 mg/kg to 50 mg/kg) twice daily for 5 consecutive days. Thus, the total dose of morphine was 20–100 mg/kg daily. On the sixth day, 50 mg/kg of morphine hydrochloride was administered once at 9:00 a.m. The doses of escitalopram, imipramine, fluoxetine and norBNI were selected as previously reported.30,31,32,33 Escitalopram (15 mg/kg), imipramine hydrochloride (20 mg/kg), and fluoxetine (10 mg/kg) were administered i.p. daily within the 4 weeks after withdrawal from morphine, norBNI (10 mg/kg) was administered i.p. 24 h before the behavior test or once a week during the withdrawal period of morphine.
Behavioral tests
Sucrose preference test (SPT)
Prior to SPT, mice were deprived of water for 24 h. In SPT, mice were exposure to two identical bottles for 1.5 h. One bottle was filled with 1% sucrose solution and the other bottle was filled with water. In order to prevent possible side preference in the test, the position of the bottles was exchange after 45 min. At the end of SPT, sucrose and water consumption were measured by weighing. Sucrose preference = sucrose consumption/(sucrose consumption + water consumption).
Open field test (OFT)
The mice were placed into an open field box (45 × 45 × 30 cm) under dim light for 15 min. The arena was divided into a peripheral zone and a center zone (30 × 30 cm). The locomotor activity and the movement trace of mice were recorded by the ANY-maze software (Stoelting Company, Wood Dale, IL, USA). The total distance traveled in the arena and the time spent in the center zone were evaluated.
Tail suspension test (TST)
The mice were suspended by the tail from a horizontal bar (35 cm above the floor) using an adhesive tape applied 1 cm from the tip of the tail. The test session was recorded for 6 min. The immobile latency for the first 2 min and the time spent immobile during the last 4 min were determined by the observer who was blind to allocations of the mouse group.
Forced swim test (FST)
The mice were placed in the cylinder (diameter: 15 cm, height: 25 cm) filled with water (24 ± 1°C) to 15 cm. The test session was recorded for 6 min. The water in the cylinder was changed between each mouse during the test session. The immobile latency for the first 2 min and the time spent immobile during the last 4 min were determined by the observer who was blind to allocations of the mouse group.
Western blot
After the behavioral experiments, the mice were killed and the NAc tissue was quickly removed and placed on dry ice. NAc tissues were sonicated in RIPA buffer containing protease and phosphatase inhibitors. Protein samples were separated by 10% SDS-PAGE, transferred to PVDF membranes, blotted with 5% fat milk for 3 h and incubated with primary antibodies overnight at 4°C. The membranes were then washed and incubated with HRP-conjugated secondary antibody. Signals were developed using the enhanced chemiluminescence kit. Densitometry was performed to quantify signal intensity using Image Lab software.
Immunofluorescent histochemistry and fluorescence in situ hybridization (FISH)
Under terminal anesthesia, mice were transcardially perfused with 0.01M PBS and 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS). The brains were further post-fixed in 4% PFA at 4°C and subsequently stored in 30% sucrose solution in 0.2M PB at 4°C. The entire brain was serially cut into 30-μm-thick transverse sections with a freezing microtome (Leica, Germany). NAc sections were incubated with primary anti-c-fos antibody overnight at 4°C and then incubated with Cy3 conjugated goat anti-mouse secondary antibody at room temperature for 4 h in the dark.
KOR mRNA was detected with FISH using digoxigenin (DIG)-labeled RNA probes, we synthesized the digoxigenin (DIG)-labeled antisense single-strand RNA probes of KOR with a DIG RNA labeling mix. Brains were hybridized with DIG-labeled antisense RNA probes, and immunodetection of the DIG-labeled hybrids was performed with a peroxidase-conjugated anti-DIG Fab. Fluorescence signal was achieved with tyramide signal amplification, the sections were subsequently treated with Fluorescein Avidin D for 4 h. All sections were observed under the fluorescence microscope (Zeiss, Germany).
Virus injection
KORloxp/loxP mice were anesthetized with 2% isoflurane and placed in a stereotaxic device (RWD, Shenzhen, China). The skull was exposed under antiseptic conditions and a small craniotomy was performed with a thin drill above the intended site of injection (NAc: AP +1.4 mm, ML ± 2.0 mm, DV 4.6 mm, 10° angle). The volume of 200 nL of the AAV2/9-hSyn-Cre-mCherry or AAV2/9-hSyn-mCherry was injected bilaterally into NAc using a microsyringe within 10 min. The microsyringe remained for 5 min at the end of the infusion to allow virus diffusion. Behavioral tests were carried out after 3 weeks of the virus injection.
Quantification and statistical analysis
Statistical analyzes were performed with the GraphPad Prism 8.0 software (GraphPad Software, Inc., La Jolla, CA). The data of escitalopram treatment for the patients were analyzed using the chi-square test and repeated measures two-way ANOVA, followed by Sidak’s post hoc test. For the animal’s experiments, statistical significance was determined with Unpaired t test where two groups were compared, or one-way or two-way ANOVA followed by Turkey’s or Sidak’s post hoc test where more than two groups were compared. We used Pearson correlation test to measure linear correlation between two variables. Results are presented as the mean ± standard error of the mean (SEM). Statistical significance was ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. The statistics of results presented in this study are summarized in supplementary data file (Data S1: Statistics related to data presented in this study. Related to STAR methods).
Additional resources
This study is part of a clinical trial (register number ChiCTR2000037788, https://www.chictr.org.cn/).
Acknowledgments
This work was supported by the Natural Science Basic Research Program of Shaanxi Province (2021JM-027, 2022JQ-783) and the Natural Science Foundation of China (82171880).
Author contributions
Conceptualization, Y.W.; Data Acquisition and Investigation, J.Z., Y.L., M.J., Y.B., L.S., Z.D., F.Y., and W.T.; Writing and Supervision, J.Z. and Y.W.; Reviewing and Editing, Y.W. and S.W.
Declaration of interests
The authors declare no conflict of interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: August 3, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107536.
Contributor Information
Shuguang Wei, Email: weisg@xjtu.edu.cn.
Yunpeng Wang, Email: wyp033@xjtu.edu.cn.
Supplemental information
Data and code availability
-
•
No new code was used in these studies.
-
•
No large dataset was generated in these studies.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Zou Z., Wang H., d'Oleire Uquillas F., Wang X., Ding J., Chen H. Definition of Substance and Non-substance Addiction. Adv. Exp. Med. Biol. 2017;1010:21–41. doi: 10.1007/978-981-10-5562-1_2. [DOI] [PubMed] [Google Scholar]
- 2.Wolf M.E. Synaptic mechanisms underlying persistent cocaine craving. Nat. Rev. Neurosci. 2016;17:351–365. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kauer J.A., Malenka R.C. Synaptic plasticity and addiction. Nat. Rev. Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 4.Tolomeo S., Steele J.D., Ekhtiari H., Baldacchino A. Chronic heroin use disorder and the brain: Current evidence and future implications. Prog Neuro Psychoph. 2021;111:110148. doi: 10.1016/j.pnpbp.2020.110148. ARTN 110148. [DOI] [PubMed] [Google Scholar]
- 5.Kessler R.C. The epidemiology of dual diagnosis. Biol Psychiat. 2004;56:730–737. doi: 10.1016/j.biopsych.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 6.Hatsukami D., Pickens R.W. Posttreatment depression in an alcohol and drug abuse population. Am. J. Psychiatry. 1982;139:1563–1566. doi: 10.1176/ajp.139.12.1563. [DOI] [PubMed] [Google Scholar]
- 7.Petrakis I., Carroll K.M., Nich C., Gordon L., Kosten T., Rounsaville B. Fluoxetine treatment of depressive disorders in methadone-maintained opioid addicts. Drug Alcohol Depen. 1998;50:221–226. doi: 10.1016/S0376-8716(98)00032-5. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter K.M., Brooks A.C., Vosburg S.K., Nunes E.V. The effect of sertraline and environmental context on treating depression and illicit substance use among methadone maintained opiate dependent patients: a controlled clinical trial. Drug Alcohol Depen. 2004;74:123–134. doi: 10.1016/j.drugalcdep.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Stein M.D., Herman D.S., Kettavong M., Cioe P.A., Friedmann P.D., Tellioglu T., Anderson B.J. Antidepressant treatment does not improve buprenorphine retention among opioid-dependent persons. J. Subst. Abuse Treat. 2010;39:157–166. doi: 10.1016/j.jsat.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalanne L., Ayranci G., Kieffer B.L., Lutz P.E. The kappa opioid receptor: from addiction to depression, and back. Front Psychiatry. 2014;5:170. doi: 10.3389/fpsyt.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansour A., Khachaturian H., Lewis M.E., Akil H., Watson S.J. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- 12.Wood P.L. The significance of multiple CNS opioid receptor types: a review of critical considerations relating to technical details and anatomy in the study of central opioid actions. Peptides. 1988;9:49–55. doi: 10.1016/0196-9781(88)90223-9. [DOI] [PubMed] [Google Scholar]
- 13.Wassum K.M., Cely I.C., Balleine B.W., Maidment N.T. Micro-opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J. Neurosci. 2011;31:1591–1599. doi: 10.1523/JNEUROSCI.3102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wassum K.M., Ostlund S.B., Maidment N.T., Balleine B.W. Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc. Natl. Acad. Sci. USA. 2009;106:12512–12517. doi: 10.1073/pnas.0905874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zöllner C., Stein C. Opioids. Handb. Exp. Pharmacol. 2007;117:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]
- 16.Browne C.A., Lucki I. Targeting opioid dysregulation in depression for the development of novel therapeutics. Pharmacol. Ther. 2019;201:51–76. doi: 10.1016/j.pharmthera.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlezon W.A., Béguin C., DiNieri J.A., Baumann M.H., Richards M.R., Todtenkopf M.S., Rothman R.B., Ma Z., Lee D.Y.W., Cohen B.M. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J. Pharmacol. Exp. Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 18.Chefer V.I., Bäckman C.M., Gigante E.D., Shippenberg T.S. Kappa Opioid Receptors on Dopaminergic Neurons Are Necessary for Kappa-Mediated Place Aversion. Neuropsychopharmacol. 2013;38:2623–2631. doi: 10.1038/npp.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todtenkopf M.S., Marcus J.F., Portoghese P.S., Carlezon W.A. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology. 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson M.L., Wulf H.A., Browne C.A., Lucki I. The kappa opioid receptor antagonist aticaprant reverses behavioral effects from unpredictable chronic mild stress in male mice. Psychopharmacology. 2020;237:3715–3728. doi: 10.1007/s00213-020-05649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braida D., Capurro V., Zani A., Rubino T., Viganò D., Parolaro D., Sala M. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br. J. Pharmacol. 2009;157:844–853. doi: 10.1111/j.1476-5381.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ukai M., Suzuki M., Mamiya T. Effects of U-50,488H, a kappa-opioid receptor agonist, on the learned helplessness model of depression in mice. J. Neural. Transm. 2002;109:1221–1225. doi: 10.1007/s00702-002-0764-x. [DOI] [PubMed] [Google Scholar]
- 23.Wittmann W., Schunk E., Rosskothen I., Gaburro S., Singewald N., Herzog H., Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacol. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salgado S., Kaplitt M.G. The Nucleus Accumbens: A Comprehensive Review. Stereotact. Funct. Neurosurg. 2015;93:75–93. doi: 10.1159/000368279. [DOI] [PubMed] [Google Scholar]
- 25.Heshmati M., Christoffel D.J., LeClair K., Cathomas F., Golden S.A., Aleyasin H., Turecki G., Friedman A.K., Han M.H., Menard C., Russo S.J. Depression and Social Defeat Stress Are Associated with Inhibitory Synaptic Changes in the Nucleus Accumbens. J. Neurosci. 2020;40:6228–6233. doi: 10.1523/JNEUROSCI.2568-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nestler E.J., Carlezon W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Nestler E.J. Role of the Brain's Reward Circuitry in Depression: Transcriptional Mechanisms. Int. Rev. Neurobiol. 2015;124:151–170. doi: 10.1016/bs.irn.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fakhoury M. Revisiting the Serotonin Hypothesis: Implications for Major Depressive Disorders. Mol. Neurobiol. 2016;53:2778–2786. doi: 10.1007/s12035-015-9152-z. [DOI] [PubMed] [Google Scholar]
- 29.Yohn C.N., Gergues M.M., Samuels B.A. The role of 5-HT receptors in depression. Mol. Brain. 2017;10:28. doi: 10.1186/s13041-017-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burstein O., Franko M., Gale E., Handelsman A., Barak S., Motsan S., Shamir A., Toledano R., Simhon O., Hirshler Y., et al. Escitalopram and NHT normalized stress-induced anhedonia and molecular neuroadaptations in a mouse model of depression. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding Q., Li H., Tian X., Shen Z., Wang X., Mo F., Huang J., Shen H. Zinc and imipramine reverse the depression-like behavior in mice induced by chronic restraint stress. J. Affect. Disord. 2016;197:100–106. doi: 10.1016/j.jad.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Xia J., Xue X., Liu W., Qi Z., Liu W. The Role of Fgf9 in the Antidepressant Effects of Exercise and Fluoxetine in Chronic Unpredictable Mild Stress Mice. Psychosom. Med. 2021;83:795–804. doi: 10.1097/PSY.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 33.Bruchas M.R., Yang T., Schreiber S., Defino M., Kwan S.C., Li S., Chavkin C. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J. Biol. Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemby S.E. Morphine-induced alterations in gene expression of calbindin immunopositive neurons in nucleus accumbens shell and core. Neuroscience. 2004;126:689–703. doi: 10.1016/j.neuroscience.2004.01.056. [DOI] [PubMed] [Google Scholar]
- 35.Bruchas M.R., Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology. 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Jong J.W., Afjei S.A., Pollak Dorocic I., Peck J.R., Liu C., Kim C.K., Tian L., Deisseroth K., Lammel S. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron. 2019;101:133–151.e7. doi: 10.1016/j.neuron.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drevets W.C., Videen T.O., Price J.L., Preskorn S.H., Carmichael S.T., Raichle M.E. A Functional Anatomical Study of Unipolar Depression. J. Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz P.E., Kieffer B.L. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solecki W., Ziolkowska B., Krowka T., Gieryk A., Filip M., Przewlocki R. Alterations of prodynorphin gene expression in the rat mesocorticolimbic system during heroin self-administration. Brain Res. 2009;1255:113–121. doi: 10.1016/j.brainres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Li X.Y., Sun L., He J., Chen Z.L., Zhou F., Liu X.Y., Liu R.S. The kappa-opioid receptor is upregulated in the spinal cord and locus ceruleus but downregulated in the dorsal root ganglia of morphine tolerant rats. Brain Res. 2010;1326:30–39. doi: 10.1016/j.brainres.2010.02.070. [DOI] [PubMed] [Google Scholar]
- 42.Meuser T., Giesecke T., Gabriel A., Horsch M., Sabatowski R., Hescheler J., Grond S., Palmer P.P. Mu-opioid receptor mRNA regulation during morphine tolerance in the rat peripheral nervous system. Anesth. Analg. 2003;97:1458–1463. doi: 10.1213/01.Ane.0000081721.75663.87. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez V.A., Arttamangkul S., Dang V., Salem A., Whistler J.L., von Zastrow M., Grandy D.K., Williams J.T. mu-Opioid receptors: ligand-dependent activation of potassium conductance, desensitization, and internalization. J. Neurosci. 2002;22:5769–5776. doi: 10.1523/JNEUROSCI.22-13-05769.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erbs E., Faget L., Ceredig R.A., Matifas A., Vonesch J.L., Kieffer B.L., Massotte D. Impact of Chronic Morphine on Delta Opioid Receptor-Expressing Neurons in the Mouse Hippocampus. Neuroscience. 2016;313:46–56. doi: 10.1016/j.neuroscience.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berrocoso E., Sánchez-Blázquez P., Garzón J., Mico J.A. Opiates as Antidepressants. Curr. Pharm. Des. 2009;15:1612–1622. doi: 10.2174/138161209788168100. [DOI] [PubMed] [Google Scholar]
- 46.Deji C., Yan P., Ji Y., Yan X., Feng Y., Liu J., Liu Y., Wei S., Zhu Y., Lai J. The Basolateral Amygdala to Ventral Hippocampus Circuit Controls Anxiety-Like Behaviors Induced by Morphine Withdrawal. Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.894886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zan G.Y., Wang Q., Wang Y.J., Liu Y., Hang A., Shu X.H., Liu J.G. Antagonism of kappa opioid receptor in the nucleus accumbens prevents the depressive-like behaviors following prolonged morphine abstinence. Behav. Brain Res. 2015;291:334–341. doi: 10.1016/j.bbr.2015.05.053. [DOI] [PubMed] [Google Scholar]
- 48.Smith J.S., Schindler A.G., Martinelli E., Gustin R.M., Bruchas M.R., Chavkin C. Stress-induced activation of the dynorphin/kappa-opioid receptor system in the amygdala potentiates nicotine conditioned place preference. J. Neurosci. 2012;32:1488–1495. doi: 10.1523/JNEUROSCI.2980-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chefer V.I., Bäckman C.M., Gigante E.D., Shippenberg T.S. Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharmacol. 2013;38:2623–2631. doi: 10.1038/npp.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks J.M., O'Donnell P. Kappa Opioid Receptors Mediate Heterosynaptic Suppression of Hippocampal Inputs in the Rat Ventral Striatum. J. Neurosci. 2017;37:7140–7148. doi: 10.1523/Jneurosci.0876-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zan G.Y., Sun X., Wang Y.J., Liu R., Wang C.Y., Du W.J., Guo L.B., Chai J.R., Li Q.L., Liu Z.Q., Liu J.G. Amygdala dynorphin/kappa opioid receptor system modulates depressive-like behavior in mice following chronic social defeat stress. Acta Pharmacol. Sin. 2022;43:577–587. doi: 10.1038/s41401-021-00677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laman-Maharg A., Williams A.V., Zufelt M.D., Minie V.A., Ramos-Maciel S., Hao R., Ordoñes Sanchez E., Copeland T., Silverman J.L., Leigh A., et al. Sex Differences in the Effects of a Kappa Opioid Receptor Antagonist in the Forced Swim Test. Front. Pharmacol. 2018;9:93. doi: 10.3389/fphar.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shirayama Y., Ishida H., Iwata M., Hazama G.I., Kawahara R., Duman R.S. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J. Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 54.Patkar K.A., Wu J., Ganno M.L., Singh H.D., Ross N.C., Rasakham K., Toll L., McLaughlin J.P. Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on kappa-opioid receptors. J. Pharmacol. Exp. Ther. 2013;346:545–554. doi: 10.1124/jpet.113.206086. [DOI] [PubMed] [Google Scholar]
- 55.Endoh T., Matsuura H., Tanaka C., Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch. Int. Pharmacodyn. Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- 56.Horan P., Taylor J., Yamamura H.I., Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J. Pharmacol. Exp. Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- 57.Xie J.Y., De Felice M., Kopruszinski C.M., Eyde N., LaVigne J., Remeniuk B., Hernandez P., Yue X., Goshima N., Ossipov M., et al. Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention. Cephalalgia. 2017;37:780–794. doi: 10.1177/0333102417702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karoussiotis C., Sotiriou A., Polissidis A., Symeonof A., Papavranoussi-Daponte D., Nikoletopoulou V., Georgoussi Z. The kappa-opioid receptor-induced autophagy is implicated in stress-driven synaptic alterations. Front. Mol. Neurosci. 2022;15 doi: 10.3389/fnmol.2022.1039135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lefkowitz R.J., Shenoy S.K. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 60.Bruchas M.R., Xu M., Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belcheva M.M., Clark A.L., Haas P.D., Serna J.S., Hahn J.W., Kiss A., Coscia C.J. mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J. Biol. Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X., Hou Z., Han M., Chen K., Wang Y., Qing J., Yang F. Salvianolic acid B alleviates comorbid pain in depression induced by chronic restraint stress through inhibiting GABAergic neuron excitation via an ERK-CREB-BDNF axis-dependent mechanism. J. Psychiatr. Res. 2022;151:205–216. doi: 10.1016/j.jpsychires.2022.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Hall J., Thomas K.L., Everitt B.J. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat. Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 64.Toda S., McGinty J.F., Kalivas P.W. Repeated cocaine administration alters the expression of genes in corticolimbic circuitry after a 3-week withdrawal: a DNA macroarray study. J. Neurochem. 2002;82:1290–1299. doi: 10.1046/j.1471-4159.2002.01083.x. [DOI] [PubMed] [Google Scholar]
- 65.Eisch A.J., Bolaños C.A., de Wit J., Simonak R.D., Pudiak C.M., Barrot M., Verhaagen J., Nestler E.J. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: A role in depression. Biol Psychiat. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Portoghese P.S., Lipkowski A.W., Takemori A.E. Binaltorphimine and nor-binaltorphimine, potent and selective kappa-opioid receptor antagonists. Life Sci. 1987;40:1287–1292. doi: 10.1016/0024-3205(87)90585-6. [DOI] [PubMed] [Google Scholar]
- 67.Jamshidi R.J., Sullivan L.C., Jacobs B.A., Chavera T.A., Berg K.A., Clarke W.P. Long-Term Reduction of Kappa Opioid Receptor Function by the Biased Ligand, Norbinaltorphimine, Requires c-Jun N-Terminal Kinase Activity and New Protein Synthesis in Peripheral Sensory Neurons. J. Pharmacol. Exp. Ther. 2016;359:319–328. doi: 10.1124/jpet.116.235184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meshul C.K., McGinty J.F. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience. 2000;96:91–99. doi: 10.1016/S0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- 69.Kreitzer A.C. Physiology and Pharmacology of Striatal Neurons. Annu. Rev. Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- 70.Di Ciano P., Cardinal R.N., Cowell R.A., Little S.J., Everitt B.J. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J. Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Floresco S.B., Ghods-Sharifi S., Vexelman C., Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J. Neurosci. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun H.Q., Bao Y.P., Zhou S.J., Meng S.Q., Lu L. The new pattern of drug abuse in China. Curr. Opin. Psychiatry. 2014;27:251–255. doi: 10.1097/YCO.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 73.Hamilton M. A Rating Scale for Depression. J Neurol Neurosur Ps. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rauf K., Subhan F., Abbas M., Ali S.M., Ali G., Ashfaq M., Abbas G. Inhibitory effect of bacopasides on spontaneous morphine withdrawal induced depression in mice. Phytother Res. 2014;28:937–939. doi: 10.1002/ptr.5081. [DOI] [PubMed] [Google Scholar]
- 75.Martins C.C., Rosa S.G., Recchi A.M.S., Nogueira C.W., Zeni G. m-Trifluoromethyl-diphenyl diselenide (m-CF3-PhSe)(2) modulates the hippocampal neurotoxic adaptations and abolishes a depressive-like phenotype in a short-term morphine withdrawal in mice. Prog Neuro-Psychoph. 2020;98:109803. doi: 10.1016/j.pnpbp.2019.109803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
No new code was used in these studies.
-
•
No large dataset was generated in these studies.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.