Abstract
Although it was described previously for estrogen (E2) regulation of intestinal epithelial Cl− and HCO3− secretion in sex difference, almost nothing is known about the roles of estrogen receptor (ER) subtypes in regulating E2-modulated epithelial ion transports and epithelial restitution. Here, we aimed to investigate ERα and ERβ subtypes in the regulation of E2-modulated colonic epithelial HCO3− and Cl− secretion and epithelial restitution. Through physiological and biochemical studies, in combination of genetic knockdown, we showed that ERα attenuated female colonic Cl− secretion but promoted Ca2+-dependent HCO3− secretion via store-operated calcium entry (SOCE) mechanism in mice. However, ERβ attenuated HCO3− secretion by inhibiting Ca2+via the SOCE and inhibiting cAMP via protein kinases. Moreover, ERα but not ERβ promoted epithelial cell restitution via SOCE/Ca2+ signaling. ERα also enhanced cyclin D1, proliferating cell nuclear antigen, and β-catenin expression in normal human colonic epithelial cells. All ERα-mediated biological effects could be attenuated by its selective antagonist and genetic knockdown. Finally, both ERα and ERβ were expressed in human colonic epithelial cells and mouse colonic tissues. We therefore conclude that E2 modulates complex colonic epithelial HCO3− and Cl− secretion via ER subtype-dependent mechanisms and that ERα is specifically responsible for colonic epithelial regeneration. This study provides novel insights into the molecular mechanisms of how ERα and ERβ subtypes orchestrate functional homeostasis of normal colonic epithelial cells.
Keywords: ERα subtype, ERβ subtype, intestinal ion transport, colonic epithelial restitution
Epithelial ion transports are pivotal physiological process in several human organs, such as gastroenterological (GI) tract, respiratory tract, reproductive tract, and skin. Intestinal epithelium either absorbs electrolytes or secretes anions (such as HCO3− and Cl−), providing the driving force for fluid transport to maintain fluid homeostasis in human body (1, 2). Intestinal epithelial anion secretion is under control of endogenous neurohumoral factors, such as acetylcholine, prostaglandin E2, 5-HT, and nitric oxide. These neurohumoral factors trigger intracellular Ca2+, cAMP, and cGMP signaling to mediate intestinal epithelial anion secretion via plasma membrane ion channels and transporters, such as cystic fibrosis transmembrane conductance regulator and Cl−/HCO3− exchanger (i.e., DRA) (3, 4), etc.
The duodenal mucosa not only senses luminal nutrients but also regulates ion transports (particularly HCO3− and Cl− secretion), which in turn is important for nutrient absorption and mucosal protection (5, 6). It was reported that patients with duodenal ulcer had significantly diminished proximal duodenal HCO3− secretion compared with healthy volunteers (7, 8), suggesting not only that normal duodenal HCO3− secretion is pivotal to mucosal protection but that diminished duodenal HCO3− secretion contributes to duodenal ulcer. We previously revealed a sex difference in duodenal HCO3− secretion in mice and found the expression and function of estrogen receptors (ER) in murine duodenal epithelium (9). We further demonstrated that estrogen (E2) may protect human duodenum against the acid-induced injury by mediating duodenal HCO3− secretion likely via ER activation, which explains the lower incidence of duodenal ulcer in women than age-matched men (10). However, it is largely unknown about the involvement of different ER subtypes (ERα and ERβ) in the process of intestinal epithelial HCO3− secretion, let alone the underlying mechanisms of ERα- and ERβ-mediated HCO3− secretion.
Colonic epithelial anion secretion is a well-established physiological process closely linked to overall fluid and electrolyte movement in the colon (11, 12, 13). It is vital to maintain normal colonic HCO3− secretion, which loss (such as in diarrhea) may cause not only imbalance of pH values and electrolytes in whole body but also local disruption of colonic environments, such as epithelial barrier and microbiome (11, 12, 13). Therefore, the studies on colonic HCO3− secretion may offer an opportunity for improving human GI health. Unfortunately, it has not been explored if E2 regulates colonic HCO3− secretion via ER activation so far; and if so, what ER subtypes and mechanisms are involved.
While E2 promotes duodenal HCO3− secretion (9, 10), it inhibits colonic Cl− secretion in sex difference (14, 15, 16). However, the underlying mechanisms are unclear, and it is even unknown if E2 inhibition of Cl− secretion is via ER or not. Moreover, integrity and homeostasis of intestinal mucosa are crucial for GI function, which depends upon the balance between mucosal injury and healing (17). Although epithelial cell restitution plays an important role in healing process (17), little is known about the involvement of ER subtypes in colonic mucosal healing. Therefore, in the present study, we hypothesized that ER subtypes may play different roles in the regulation of E2-modulated colonic HCO3− and Cl− secretion, and we examined the underlying mechanisms of ER subtypes in E2-mediated colonic epithelial HCO3− secretion and epithelial repair.
Results
ERα stimulation of HCO3− secretion from the male duodenum and distal colon
Since the lack of information on ER subtypes in intestinal HCO3− secretion, we performed Ussing chamber experiments with pH-stat to examine the roles of ER subtypes in HCO3− secretion from the duodenum and distal colon in male mice. First, we found that both estradiol-17β (E2, 100 nM) and ERα selective activator propyl pyrazole triol (PPT, 10 nM) stimulated rapid duodenal HCO3− secretion; however, ERβ selective activator diarylpropionitrile (DPN, 10 nM) did not (Fig. 1A). Second, we tested if there is any regional heterogeneity for the ER subtype-mediated HCO3− secretion in between the duodenum and distal colon. Like in the duodenum, both E2 and PPT stimulated colonic HCO3− secretion but DPN alone did not (Fig. 1B). Moreover, compared to PPT alone, PPT plus DPN did not further stimulate additional colonic HCO3− secretion (Fig. 1B). Thus, ERα activation stimulated HCO3− secretion from the duodenum and distal colon, which has no regional heterogeneity in GI tract. In the subsequent experiments, distal colonic HCO3− secretion was focused because duodenal HCO3− secretion has been extensively studied (10, 18, 19).
Figure 1.
Activation of ERα but not ERβ stimulates epithelial HCO3−secretion in both duodenum and distal colon.A, effects of estradiol-17β (E2, 100 nM, n = 6), propyl pyrazole triol (PPT, 10 nM, n = 6), and diarylpropionitrile (DPN, 10 nM, n = 5) on duodenal net peak HCO3− secretion. B, effects of E2 (100 nM, n = 7), PPT (10 nM, n = 5), DPN (10 nM, n = 5) and PPT plus DPN (n = 5) on distal colonic net peak HCO3− secretion. E2, PPT, and DPN were added to both sides, but CCh and forskolin were add to serosal side. ∗∗p < 0.01. ns, no significant differences. Data are presented as mean ± SD. The statistical significance of differences in the means of experimental groups was determined using Student's t test or one-way ANOVA followed by post hoc test for multiple pairwise comparisons. CCh, carbachol; ER, estrogen receptor.
Differing effects of ERα and ERβ receptors on Ca2+-mediated and cAMP-mediated colonic HCO3− secretion
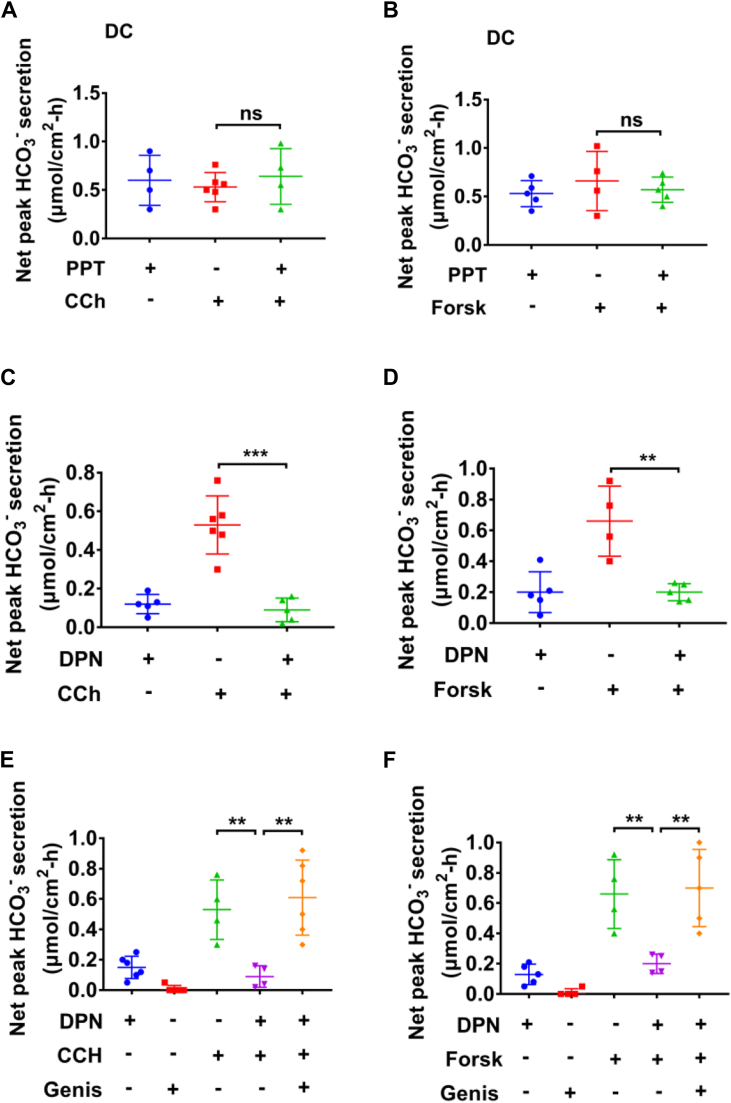
It is well-known that Ca2+ and cAMP signaling pathways play crucial roles in regulating intestinal anion secretion (11, 12, 13, 18, 19, 20, 21). Since carbachol (CCh, a cholinergic agonist) and forskolin (an adenylcyclase activator) stimulate colonic anion secretion by triggering intracellular Ca2+ and cAMP signaling respectively (15), we examined if ER signaling may impact CCh (Ca2+ signaling)- or forskolin (cAMP signaling)-stimulated HCO3− secretion. As shown in Fig. 2, A and B, the selective ERα activator PPT (10 nM) pretreatment did not affect CCh (100 μM)- or forskolin (20 μM)-induced colonic HCO3− secretion, indicating that ERα-stimulated HCO3− secretion did not utilize the same signaling pathways as CCh or forskolin. In contrast, although the selective ERβ activator DPN (10 nM) did not alter basal colonic HCO3− secretion, it almost abolished both CCh (100 μM)- and forskolin (20 μM)-induced colonic HCO3− secretion (Fig. 2, C and D), suggesting that ERβ activation may alter Ca2+- and cAMP-mediated colonic HCO3- secretion.
Figure 2.
Activation of ERβ but not ERα inhibits carbachol- and forskolin-induced male colonic HCO3−secretion via inhibition of CFTR.A and B, effect of PPT (10 nM) on carbachol (CCh, 100 μM)- and forskolin (Forsk, 20 μM)-induced colonic HCO3− secretion. C and D, effect of DPN (10 nM) on CCh- and Forsk-induced colonic HCO3− secretion. E and F, genistein (Genis, 20 μM) reversed DPN (10 nM)-inhibited CCh- and Forsk-induced colonic HCO3− secretion. PPT, DPN, and genistein were add to both sides, but CCh and forskolin were added to serosal side. ∗∗p < 0.01 and ∗∗∗p < 0.001. n = 5 to 6 tissues for each group. ns, no significant differences. Data are presented as mean ± SD. The statistical significance of differences in the means of experimental groups was determined using Student's t test. CFTR, cystic fibrosis transmembrane conductance regulator; DPN, diarylpropionitrile; ER, estrogen receptor; PPT, propyl pyrazole triol.
ERβ inhibits male colonic HCO3− secretion via protein kinase pathways
After demonstrating ERβ inhibition of CCh- and forskolin-induced colonic HCO3− secretion, we further elucidated the underlying mechanisms. Since protein kinase, such as tyrosine kinase (TK), protein kinase C (PKC), and phosphoinositide 3-kinase (PI3K) pathways play critical roles in intestinal HCO3− secretion (6, 22, 23, 24, 25), we next studied the intracellular signaling mechanisms behind ERβ inhibition of Ca2+- and cAMP-stimulated HCO3− secretion. First, as shown in Figure 2, E and F, genistein (20 μM), a commonly used TK inhibitor, inhibited basal HCO3− secretion, suggesting its role in basal HCO3− secretion, consistently with the report by Osamu Furukawa et al (25). Furthermore, genistein reversed DPN inhibition of CCh- and forskolin-induced HCO3- secretion (Fig. 2, E and F), suggesting that ERβ activation inhibits Ca2+- and cAMP-mediated colonic HCO3− secretion likely via TK pathway. Although genistein may also activate ER (26), at 20 μM, it inhibited rather than stimulated basal HCO3- secretion, excluding the possibility that genistein at this concentration stimulates HCO3− secretion via ER activation.
Second, rottlerin (10 μM), a selective PKC inhibitor (27), attenuated basal HCO3− secretion (Fig. 3, A and C), suggesting its role in regulating basal HCO3− secretion, which is consistent with the report by Odes HS et al (26). However, a selective PI3K inhibitor wortmannin (100 nM) (24) did not alter basal HCO3− secretion (Fig. 3, B and D). Neither rottlerin nor wortmannin affected DPN inhibition of CCh-induced male colonic HCO3− secretion (Fig. 3, A and B). In contrast to CCh-induced colonic HCO3− secretion, both rottlerin and wortmannin reversed DPN inhibition of forskolin-induced colonic HCO3− secretion (Fig. 3, C and D). These data suggest that ERβ activation inhibits cAMP-mediated colonic HCO3− secretion via PKC and PI3K pathways, which are not involved in ERβ inhibition of Ca2+-mediated HCO3− secretion.
Figure 3.
ERβ activation inhibits carbachol- and forskolin-stimulated male colonic HCO3−secretion via different signaling pathways.A, rottlerin (Rott, 10 μM) did not affect DPN (10 nM)-inhibited CCh (100 μM)-stimulated HCO3− secretion. DPN (n = 6), rottlerin (n = 6), CCh (n = 4), DPN + CCh (n = 5), and rottlerin + DPN + CCh (n = 6). B, Wortmannin (Wort, 100 nM) did not affect DPN (10 nM)-inhibited CCh (100 μM)-stimulated HCO3− secretion. DPN (n = 5), wortmannin (n = 5), CCh (n = 4), DPN + CCh (n = 5), and wortmannin + DPN + CCh (n = 5). C, rottlerin (10 μM) reversed DPN (10 nM)-inhibited forskolin (Forsk, 20 μM)-stimulated HCO3− secretion. DPN (n = 6), rottlerin (n = 6), forskolin (n = 4), DPN + forskolin (n = 5), and rottlerin + DPN + forskolin (n = 6). D, wortmannin (100 nM) reversed DPN (10 nM)-inhibited forskolin (20 μM)-stimulated HCO3− secretion. DPN (n = 6), wortmannin (n = 7), forskolin (n = 4), DPN + forskolin (n = 4), and wortmannin + DPN + forskolin (n = 6). PPT, DPN, rottlerin, and wortmannin were added to both sides, but CCh and forskolin were added to serosal side. ∗p < 0.05 and ∗∗p < 0.01. ns, no significant differences. Data are presented as mean ± SD. The statistical significance of differences in the means of experimental groups was determined using Student's t test or one-way ANOVA followed by post hoc test for multiple pairwise comparisons. CCh, carbachol; DPN, diarylpropionitrile; ER, estrogen receptor; PPT, propyl pyrazole triol.
ERα specifically inhibits CCh- and forskolin-induced female colonic short-circuit current
After demonstrating the roles and the mechanisms of ER subtypes in the regulation of male colonic HCO3− secretion, we examined their roles in colonic Cl− secretion that is poorly understood although it is known about E2 inhibition of Cl− secretion in rat distal colonic epithelium with a gender-specific mechanism (14, 15, 28). None of E2 (1 μM), PPT (500 nM), and DPN (500 nM) at high concentrations affected basal colonic short-circuit current (Isc) in both sexes (Fig. 4, A and B). Moreover, none of them affected CCh- and forskolin-induced male colonic Isc (Fig. 4, C and D), consistently with the previous reports that E2 did not alter colonic epithelial Cl− secretion in male rats (15, 28).
Figure 4.
ERα activation specifically inhibited carbachol- or forskolin-induced female colonic Isc. A, E2 (1 μM), PPT (500 nM), and DPN (500 nM) did not affect basal colonic short-circuit current (Isc) in male mice. Baseline (n = 6), E2 (n = 5), PPT (n = 4), and DPN (n = 4). B, E2 (1 μM), PPT (500 nM), and DPN (500 nM) did not affect basal colonic short-circuit current (Isc) in female mice. Baseline (n = 5), E2 (n = 5), PPT (n = 5), and DPN (n = 4). C, E2 (1 μM), PPT (500 nM), and DPN (500 nM) did not affect carbachol (CCh, 50 μM)-induced male colonic Isc. Control (Ctrl, n = 10), E2 (n = 5), PPT (n = 10), and DPN (n = 5). D, E2, PPT, and DPN did not affect forskolin (Forsk, 5 μM)-induced male colonic Isc, Control (Ctrl, n = 9), E2 (n = 5), PPT (n = 8), and DPN (n = 6). E, PPT inhibited CCh (50 μM)-induced Isc of female mouse colon, Ctrl (n = 5), PPT (10 nM, n = 6), PPT (100 nM, n = 6), and PPT (500 nM, n = 6). F, PPT inhibited forskolin (Forsk, 5 μM)-induced Isc of female mouse colon, Ctrl (n = 5), PPT (10 nM, n = 6), PPT (100 nM, n = 5), and PPT (500 nM, n = 5). G, DPN (500 nM) did not affect CCh (50 μM)-induced Isc of female mouse colon. Ctrl (n = 5) and DPN (n = 7). H, DPN (500 nM) did not affect forskolin (Forsk, 5 μM)-induced Isc of female mouse colon. Ctrl (n = 5) and DPN (n = 5). Ctrl represents as the control with CCh or forskolin treatment only. PPT, DPN, and E2 were added to both sides. CCh and forskolin were added to serosal side. ∗p < 0.05 and ∗∗p < 0.01. ns, no significant differences. Data are presented as mean ± SD. The statistical significance of differences in the means of experimental groups was determined using Student's t test or one-way ANOVA followed by post hoc test for multiple pairwise comparisons. CCh, carbachol; DPN, diarylpropionitrile; ER, estrogen receptor; PPT, propyl pyrazole triol.
We further examined the role of ER subtypes in female colonic Cl− secretion. As shown in Figure 4, E and F, ERα selective activator PPT (100 nM) significantly inhibited CCh- and forskolin-induced Isc of female mouse colon, which is consistent with the previous reports that E2 inhibited colonic epithelial Cl− secretion through KCNQ1 channels in female rats (14, 15, 28). However, ERβ selective activator DPN at high concentration 500 nM did not affect CCh- and forskolin-induced Isc of female mouse colon (Fig. 4, G and H). Taken together, ERα subtype is specifically responsible for the inhibition of female colonic Isc.
ERα induces Ca2+ signaling via the store-operated Ca2+ entry, but ERβ inhibits the store-operated Ca2+ entry in human colonic epithelial cell line
It is well documented that Ca2+ signaling triggers intestinal HCO3− secretion (9, 18, 20, 29), and E2 may induce HCO3− secretion via Ca2+ signaling (9); however, little is known about ER-mediated colonic epithelial Ca2+ signaling. In other cell types, store-operated Ca2+ entry (SOCE) plays a pivotal role in controlling Ca2+ signaling. Initially, we applied single cell Ca2+ imaging to characterize the SOCE in human colonic epithelial cell line (HCoEpiC). Cyclopiazonic acid (CPA, 5 μM), an inhibitor of the sarcoendoplasmic reticulum Ca2+ ATPase and a commonly used SOCE activator, significantly induced free cytoplasmic Ca2+ [Ca2+]cyt in Ca2+-free solutions and then a sustained [Ca2+]cyt increase in Ca2+-containing solutions, suggesting that intracellular Ca2+ release causes extracellular Ca2+ influx in HCoEpiC (i.e, SOCE) (Fig. 5A). Pretreatment with three different SOCE blockers, GSK7975A (10 μM) (30), ML-9 (100 μM) (31), and SKF96365 (50 μM) (32), markedly eliminated the CPA-induced SOCE (Fig. 5, B–J), verifying SOCE function in HCoEpiC.
Figure 5.
Characterization of store-operated Ca2+entry (SOCE) function in HCoEpiC. A, Summary tracings of [Ca2+]cyt time course in response to cyclopiazonic acid (CPA) (5 μM, n = 27) in Ca2+-free solutions (0 Ca2+) and in Ca2+-containing solutions (2 Ca2+). B, E and H, summary tracings of [Ca2+]cyt time course showing the inhibitory effect of GSK7975A (10 μM, n = 10), ML-9 (100 μM, n = 10), and SKF96365 (50 μM, n = 11) on CPA-induced Ca2+ release and Ca2+ influx. C and D, summary data showing the peaks of CPA-increased [Ca2+]cyt signaling as described in (A and B). F and G, summary data showing the peaks of CPA-increased [Ca2+]cyt signaling as described in (A and E). I and J, summary data showing the peaks of CPA-increased [Ca2+]cyt signaling as described in (A and H). K, summary tracings of [Ca2+]cyt time course showing the inhibitory effect of SKF96365 (50 μM, n = 19) on CPA-induced Ca2+ influx. L and M, summary data showing the peaks of CPA-increased [Ca2+]cyt signaling as described in (A and K). ∗∗∗p < 0.001. ns, no significant differences. Data are presented as mean ± SD. The statistical significance of differences in the means of experimental groups was determined using Student's t test. [Ca2+]cyt, free cytoplasmic Ca2+; HCoEpiC, human colonic epithelial cell line.
Although the initial Ca2+ transient produced by CPA in Ca2+-free solutions is presumably Ca2+ release from stores, it was reduced by pretreatment with SOCE blockers (Fig. 5, C, F, and I). We assume it is because the SOCE is important to refill the intracellular Ca2+ store, and Ca2+ release from stores would be reduced by pretreatment with SOCE blockers. To test this possibility, we added SOCE blockers after the store depletion but before extracellular Ca2+ influx. Indeed, SKF96365 (50 μM) markedly reduced CPA-induced Ca2+ influx (Fig. 5, K–M). These data verify not only SOCE function per se but also its important role in refilling intracellular Ca2+ store in HCoEpiC.
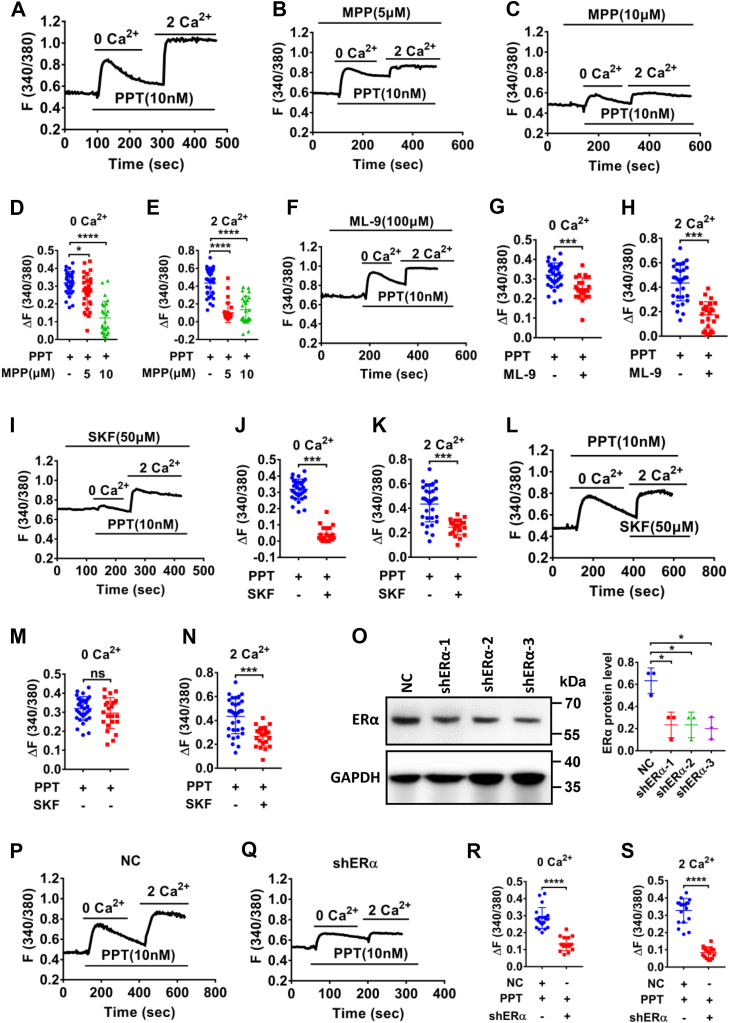
After characterizing the SOCE in HCoEpiC, we next examined whether ER stimulates colonic epithelial Ca2+ signaling. ERα selective activator PPT (10 nM) significantly stimulated [Ca2+]cyt in Ca2+-containing solutions (Fig. 6, A and C), but ERβ selective activator DPN (10–500 nM) did not alter basal [Ca2+]cyt (Fig. 6, B and C). However, DPN (10 nM) significantly inhibited CPA (5 μM)-induced SOCE (Fig. 6, D–G). Together, these data suggest that ERα triggers Ca2+ signaling, but ERβ inhibits Ca2+ signaling likely via the SOCE.
Figure 6.
Differential effects of ERα and ERβ activation on SOCE-mediated [Ca2+]cytin HCoEpiC.A, summary tracings of [Ca2+]cyt time course in response to PPT (10 nM, n = 17) in Ca2+-containing solutions. B, summary tracings of [Ca2+]cyt time course in response to DPN (10–500 nM, n = 15) and 5 mM Ca2+ in Ca2+-containing solutions. C, summary data showing the peaks of PPT-, DPN- and 5 mM Ca2+-induced [Ca2+]cyt signaling as described in (A and B). D and E, CPA (5 μM, n = 25)-induced Ca2+ release and Ca2+ influx, which was attenuated by DPN (10 nM, n = 28). F and G, summary data showing the peaks of CPA-increased [Ca2+]cyt signaling with or without DPN as described in (D and E). ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001. ns, no significant differences. Data are presented as mean ± SD. The statistical significance of differences in the means of experimental groups was determined using Student's t test. [Ca2+]cyt, free cytoplasmic Ca2+; CPA, cyclopiazonic acid; DPN, diarylpropionitrile; ER, estrogen receptor; HCoEpiC, human colonic epithelial cell line; PPT, propyl pyrazole triol; SOCE, store-operated Ca2+ entry.
We further elucidated the underlying mechanisms of ERα-induced Ca2+ signaling in HCoEpiC. Like SOCE inducer CPA, PPT (10 nM) significantly stimulated [Ca2+]cyt in Ca2+-free solutions and then a sustained [Ca2+]cyt increase in Ca2+-containing solutions (Fig. 7A). Further studies showed that either MPP dihydrochloride (MPP) at 5 to 10 μM (Fig. 7, B–E), a ERα selective inhibitor, or ML-9 (100 μM) (Fig. 7, F–H) and SKF96365 (50 μM) (Fig. 7, I–K), two different inhibitors of the SOCE, markedly eliminated PPT-induced Ca2+ release and Ca2+ influx, suggesting that ERα activation triggers [Ca2+]cyt via the SOCE. Moreover, SKF96365 (50 μM) markedly reduced PPT-induced Ca2+ entry when it was added after the store depletion but before extracellular Ca2+ entry (Fig. 7, L–N). Finally, after shERα was applied to successfully knock down the protein expression of ERα in HCoEpiC (Fig. 7O), PPT-induced [Ca2+]cyt was significantly attenuated by shERα-3 as well (Fig. 7, P–S), verifying ERα activation of the SOCE in colonic epithelial cells.
Figure 7.
ERα activation raised [Ca2+]cytvia the SOCE in HCoEpiC. A, summary tracings of [Ca2+]cyt time course in response to PPT (10 nM, n = 33) in Ca2+-free solutions and in Ca2+-containing solutions. B, C, F, and I, summary tracings of [Ca2+]cyt time course showing the inhibitory effect of MPP (5–10 μM, n = 30), ML-9 (100 μM, n = 25) and SKF96365 (50 μM, n = 21) on PPT-induced Ca2+ release and Ca2+ influx. D and E, summary data showing the peaks of PPT-induced [Ca2+]cyt signaling with or without MPP as described in (A–C). G and H, summary data showing the peaks of PPT-induced [Ca2+]cyt signaling with or without ML-9 as described in (A and F). J and K, summary data showing the peaks of PPT-induced [Ca2+]cyt signaling with or without SKF96365 as described in (A and I). L, summary tracings of [Ca2+]cyt time course showing the inhibitory effect of SKF96365 (50 μM, n = 23) on PPT-induced Ca2+ influx. M and N, summary data showing the peaks of PPT-induced [Ca2+]cyt signaling as described in (A and L). O, Western blotting analysis of ERα proteins expression in NC and shERα (left) and the summary data of ERα protein expression (right) (n = 3). P and Q, summary tracings of [Ca2+]cyt time course in response to PPT (10 nM) of NC (n = 20) and shERα-1 (n = 16) in Ca2+-free solutions and in Ca2+-containing solutions. R and S, summary data showing the peaks of PPT-increased [Ca2+]cyt signaling with NC or shERα as described in (P and Q). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001. ns, no significant differences. Data are presented as mean ± SD. The statistical significance of differences in the means of experimental groups was determined using Student's t test or one-way ANOVA followed by post hoc test for multiple pairwise comparisons. [Ca2+]cyt, free cytoplasmic Ca2+; ER, estrogen receptor; HCoEpiC, human colonic epithelial cell line; MPP, MPP dihydrochloride; PPT, propyl pyrazole triol; SOCE, store-operated Ca2+ entry.
ERα promoted Ca2+-dependent proliferation and migration of HCoEpiC
Since proliferation and migration of epithelial cells are critical for the restitution of injured intestinal epithelium (33, 34), we examined the roles of ER subtypes in epithelial regeneration. PPT at 5 to 50 nM promoted proliferation of HCoEpiC (Fig. 8A), which was attenuated by ERα selective inhibitor MPP (1 μM, Fig. 8E) and shERα (Fig. 8F), but not by ERβ selective inhibitor PHTPP (5 μM) (Fig. 8E). However, DPN at 1 to 50 nM did not affect proliferation of HCoEpiC (Fig. 8B). Finally, [Ca2+]cyt chelator BAPTA-AM (1 μM) also inhibited PPT-induced cell proliferation (Fig. 8H). MPP (1 μM), PHTPP (5 μM), and BAPTA-AM (1 μM) were chosen at these concentrations because they per se did not directly alter HCoEpiC proliferation (Fig. 8, C, D, and G).
Figure 8.
ERα activation promotes Ca2+-dependent proliferation of HcoEpiC. A, PPT (5–50 nM) dose-dependently enhanced cell proliferation. B, DPN (1–50 nM) did not affect cell proliferation. C, MPP (1–20 μM) dose-dependently inhibited cell proliferation. D, PHTPP did not affect cell proliferation. E, the inhibitory effect of MPP (1 μM) on PPT (10 nM)-induced cell proliferation. F, the inhibitory effect of shERα on PPT (10 nM)-induced cell proliferation. G, BAPTA-AM (1–10 μM) dose-dependently inhibited cell proliferation. H, the inhibitory effect of BAPTA-AM (1 μM) on PPT (10 nM)-induced cell proliferation. I, PPT (10 nM) at 48 h-enhanced cyclin D1, PCNA, and β-catenin protein expression. J, DPN (10 nM) did not affect protein expression of PCNA, cyclin D1, and β-catenin. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001, ns, no significant differences. Data are presented as mean ± SD. The statistical significance of differences in the means of experimental groups was determined using Student's t test or one-way ANOVA followed by post hoc test for multiple pairwise comparisons. DPN, diarylpropionitrile; ER, estrogen receptor; HCoEpiC, human colonic epithelial cell line; MPP, MPP dihydrochloride; PCNA, proliferating cell nuclear antigen; PPT, propyl pyrazole triol.
Since cyclin D1, proliferating cell nuclear antigen (PCNA), and β-catenin play crucial roles in enterocyte proliferation (35, 36), we examined their protein expression following ER subtype stimulation. As shown in Fig. 8I, the pretreatment with PPT (10 nM) for 48 h enhanced the expression of cyclin D1, PCNA, and β-catenin. However, DPN (10 nM) did not affect their expression (Fig. 8J). Therefore, ERα promotes Ca2+-dependent proliferation of HCoEpiC.
Next, we performed cell scratch assays to examine the roles of ER subtypes in HCoEpiC migration. As shown in Fig. 9, A and B, PPT at 10 to 50 nM promoted cell migration, but DPN (10–50 nM) did not affect it. Moreover, ERα selective inhibitor MPP (1 μM) and BAPTA-AM (1 μM) and shERα abolished PPT-induced cell migration (Fig. 9, C–E). Therefore, ERα also promotes Ca2+-dependent migration of HCoEpiC.
Figure 9.
ERα activation promotes HCoEpiC migration following injury. A, PPT (10–50 nM) dose-dependently enhanced cell migration. B, DPN (10–50 nM) did not affect cell migration. C–E, the inhibitory effect of MPP (1 μM), BAPTA-AM (1 μM), and shERα on PPT (10 nM)-induced cell migration. The scale bar represents 200 μm for each image. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. ns, no significant differences. Data are presented as mean ± SD. The statistical significance of differences in the means of experimental groups was determined using Student's t test or one-way ANOVA followed by post hoc test for multiple pairwise comparisons. DPN, diarylpropionitrile; ER, estrogen receptor; HCoEpiC, human colonic epithelial cell line; MPP, MPP dihydrochloride; PPT, propyl pyrazole triol.
ER subtype expression in HCoEpiC and native mouse colonic epithelia
Since there is no information available in literature on ER subtype expression in HCoEpiC, we examined ER subtype expression in HCoEpiC and human umbilical vein endothelial cells (HUVECs) as a positive control (37, 38). As shown in Figure 10, A and B, both ERα and ERβ mRNA expression were detected in HCoEpiC. Western blots analysis further confirmed ERα and ERβ protein expression in HCoEpiC like in HUVEC (Fig. 10, C and D). Moreover, we also performed immunofluorescence to examine the expression and localization of ER subtypes in HCoEpiC. As shown in Figure 10E, ERα proteins were predominately expressed in the cytoplasm and nucleus, but ERβ were mostly expressed in the cytoplasm compared to the nucleus. However, the immunofluorescence staining was not observed without the primary antibodies against ERα and ERβ in the negative control, indicating specific staining on these proteins in HCoEpiC.
Figure 10.
The expression of ER subtypes in HCoEpiC and mouse colon. A and B, RT-PCR showing mRNA expression of ERα and ERβ in HCoEpiC and HUVEC cells (n = 3). C and D, Western blots showing protein expression of ERα and ERβ in HCoEpiC and HUVEC cells and the summary data of ERα and ERβ protein expression (n = 3). E, the expression and localization of ERα and ERβ detected by immunofluorescence staining. Upper panel: ERα proteins staining (in red) and merge with nuclei stained with DAPI (in blue). Middle panel: ERβ proteins staining (in red) and merge with nuclei stained with DAPI. Lower panel: nuclei of the cells stained with DAPI without primary antibodies against ERα and ERβ as a negative control. The scale bar represents 20 μm for each image. F and G, representative Western blots and quantification of ERα and ERβ subtype protein expression in male and female mouse colon (n = 3). ER, estrogen receptor; HCoEpiC, human colonic epithelial cell line; HuVEC, human umbilical vein endothelial cell.
ERα and ERβ mRNA expression was previously detected in human and mouse colonic epithelia (38, 39, 40); however, the protein expression of these receptors in colonic epithelia is unknown. So, we performed Western blots analysis to examine protein expression of ER subtypes in mouse colonic epithelia. As shown in Figure 10, F and G, both proteins were expressed in native colonic epithelia in male and female mice, further supporting both ERα and ERβ expression in mice (38, 39).
Discussion
Although sex-based differences in E2 regulation of intestinal Cl− and HCO3− secretion have previously been described (9, 10, 14, 15, 16), it has been unknown what specific ER subtypes may be responsible for intestinal ion transport and epithelial restitution. In the present study, for the first time, we demonstrate ER subtypes in E2-mediated colonic ion transports and epithelial restitution in distinct ways (please see Fig. 11). Here we show that (1) both ERα and ERβ subtypes are expressed in HCoEpiC and mouse colonic epithelia, (2) ERα attenuates E2-mediated colonic Cl− secretion but promotes E2-mediated HCO3− secretion via SOCE/Ca2+ signaling, (3) ERβ attenuates E2-mediated HCO3− secretion by inhibiting SOCE/Ca2+ signaling and by inhibiting cAMP signaling via activation of TK, PKC, and PI3K, (4) Erα but not Erβ promotes E2-mediated epithelial cell restitution via the SOCE, and (5) ERα enhances the expression of cyclin D1, PCNA, and β-catenin in HCoEpiC.
Figure 11.
Schematic depicting the proposed mechanisms of ER subtype–mediated colonic ion transport and epithelial restitution. The left: ERα activation inhibits colonic Cl− secretion but promotes colonic HCO3− secretion and cell restitution to produce epithelial protection via the SOCE/Ca2+ signaling. The right: ERβ activation attenuates colonic HCO3− secretion by blocking the SOCE/Ca2+ signaling to inhibit adenylyl cyclase (AC) and by activating protein kinases to inhibit cAMP signaling. ERα and ERβ subtypes orchestrate the homeostasis of colonic epithelial ion transports. ER, estrogen receptor; EC, epithelial cell; SOCE, store-operated Ca2+ entry; PPT, propyl pyrazole triol; DPN, diarylpropionitrile; KCNQ1, a potassium channel.
We previously reported that E2 stimulated murine duodenal HCO3− secretion (9) and revealed gender differences in human duodenal HCO3− secretion (10), strongly supporting a protective role of E2 against mucosal acid exposure in the duodenum of females. Although our studies have provided a potential explanation for the lower prevalence of duodenal ulcer in women (9, 10), so far it is unclear whether E2 plays these roles via different ER subtypes (ERα and ERβ) to exert various biological actions through genomic and nongenomic pathways (41, 42). Genomic pathway involves nucleus/transcription factors to modulate specific gene expression, but nongenomic pathway induces immediate responses through multiple signaling pathways, such as Ca2+, cAMP, TK, PKC, and PI3K pathways (42). Although genomic action of E2 has been well studied, its nongenomic action on GI function is less understood. In the present study, we found divergent roles for ERα and ERβ subtypes in regulating colonic ion transport and HCO3− secretion. ERα activation promoted immediate E2-mediated HCO3− secretion but did not alter CCh- and forskolin-stimulated HCO3− secretion, suggesting a direct activation of ERα via nongenomic action. However, although ERβ activation alone did not affect colonic HCO3− secretion, it attenuated CCh- and forskolin-stimulated colonic HCO3− secretion likely via protein kinases (22, 25).
Most well-known secretagogues, such as forskolin and CCh usually stimulate both epithelial HCO3− and Cl− secretion in parallel (43, 44), but in the case of E2, it appears that Cl- and HCO3− secretion may be regulated in distinct ways. Although E2 inhibited colonic Cl− secretion through KCNQ1 channels (14, 15, 28), we showed here E2 rapid stimulation of HCO3− secretion via nongenomic action through distinct effects of ERα and ERβ subtypes on intracellular signaling responsible for colonic Cl- and/or HCO3- secretion. Since E2 could promote duodenal HCO3− secretion via Ca2+ signaling (9), it is reasonable to infer that ER may promote Ca2+-dependent HCO3− secretion. Ca2+ enters nonexcitable epithelial cells mainly through the SOCE, and its critical role has been descried in intestinal epithelial cells (45, 46, 47, 48). In the present study, we found that ERα promotes but ERβ inhibits SOCE/Ca2+ signaling, suggesting divergent roles of ER subtypes in regulating SOCE/Ca2+ signaling.
Interestingly, ERβ activation did not affect basal colonic HCO3− secretion but markedly attenuated CCh- and forskolin-stimulated HCO3− secretion. Since it is well-known that CCh and forskolin stimulated HCO3− secretion via Ca2+ and cAMP signaling, respectively, we further elucidated the underlying mechanisms of ERβ-inhibited colonic HCO3− secretion via Ca2+ and cAMP pathways. We not only found that ERβ inhibited SOCE mechanism (49) but also revealed that inhibition of protein kinases reverses ERβ-inhibited colonic HCO3− secretion via cAMP pathway, suggesting that ERβ-activated protein kinases are involved in this process. In contrast, inhibition of protein kinases did not reverse ERα-inhibited HCO3− secretion via Ca2+-pathway, further supporting the notion that ERβ inhibits colonic HCO3− secretion by suppressing Ca2+ signaling via SOCE and cAMP signaling via protein kinases.
We previously showed that activation of the Ca2+-sensing receptor in the duodenum resulted in [Ca2+]cyt increase but cAMP decrease (19); the end result being increased duodenal HCO3− secretion without simultaneously altering Isc. Therefore, the data from Ca2+-sensing receptor and ER further support our notion that epithelial HCO3− and Cl− secretion could be triggered differentially by Ca2+ and cAMP signaling (9, 19). Determining how to specifically modulate Cl− versus HCO3− secretion is important as one develops drugs to improve acid–base balance and epithelial repair in GI diseases (e.g., duodenal ulcer disease, cystic fibrosis, ulcerative colitis [UC]) without triggering excessive Cl− secretion that might induce the unwanted diarrheal side effects.
While epithelial restitution plays a critical role in the healing process of intestinal mucosa (17), little is known about the role of ER in colonic mucosal healing. Diseases like UC, a global intestinal autoimmune disease with no available cure, require an ongoing process of healing from injury. The foundational target of UC therapies is to suppress the immune system, thereby leading to less autoreactivity. However, large amounts and long-term immunosuppressive therapy increases the risk for infections and cancers (50). Therapies that may promote mucosal healing without immunosuppression are enticing as stand-alone or adjunctive therapies. In addition to modulating epithelial ion transport, ERα, but not ERβ, activation promotes Ca2+-dependent cell proliferation and migration, leading to colonic epithelial restitution; a process that may be beneficial to colonic disease like UC. Moreover, we found that activation of ERα but not ERβ increases protein levels of cyclin D1, PCNA, and β-catenin, indicating increased proliferation and migration.
In conclusion, we demonstrate for the first time that E2 modulates colonic epithelial ion transports and epithelial restitution via ER subtype-dependent mechanisms. Since ERα and ERβ subtypes usually play opposite roles in regulating colonic epithelial function, E2 may coordinate different ER subtypes to orchestrate functional homeostasis of colonic epithelial cells. Disruption of ERα- and ERβ-coordinated gut homeostasis may have underpinnings behind sex-based differences in GI disease, especially those involving epithelial injury and repair. While this requires further study, we have provided new insights into the cellular mechanisms of E2-mediated colonic epithelial ion transports and epithelial repair via different ER subtypes.
Experimental procedures
Cell culture
Human colonic epithelial cells (HCoEpiC) (Cat #2950) and HUVEC (Cat #8000) were purchased from ScienCell Research Laboratory. Cells were cultured in DMEM-HIGH GLUCOSE or RPMI-1640 (HyClone) supplemented with 10% fetal bovine serum (HyClone) in a 37 °C humidified atmosphere containing 5% CO2.
Animal studies and ethics
All animal studies were approved by the Ethics Committee of the Qingdao University Medical College. All animal care and experimental procedures complied with the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health. Animal studies are reported in compliance with the ARRIVE guidelines (51). The C57BL/6 mice (6–8 weeks old; 18–22 g) were purchased from HFK Bioscience Co., Ltd. Animals were assigned randomly to different experimental groups. Randomization and single-blinding were used for the measurement.
Measurement of intestinal Isc and HCO3− secretion in Ussing chamber experiments
Ussing chamber experiments were performed as previously described (52, 53). C57BL/6J mice were anesthetized by halothane, and the abdomen was opened with a midline incision. Duodenal and colonic tissues were removed, stripped of seromuscular layers, divided, and mounted in Ussing chambers (aperture area, 0.1 cm2). The experiments were performed under continuous short-circuited conditions (Voltage-Current Clamp, VCC MC6; Physiologic Instruments), and luminal pH was maintained at 7.0 by the continuous infusion of 5 mm HCl under the automatic control of a pH-stat system (ETS 822; Radiometer America). The volume of the titrant infused per unit time was used to quantitate HCO3− secretion. Measurements were recorded at 5-min intervals, and mean values for consecutive 5- or 10-min periods were averaged. The rate of luminal HCO3− secretion is expressed as micromoles per square centimeter per hour. The transepithelial Iscs were measured via an automatic voltage clamp, in which μA was used for the original recordings, but μA·cm-2 was used for summary data. After a 30 min basal period, inhibitor or control vehicle was added for another 30 min, followed by addition of stimulus to both sides of the tissue. Electrophysiological parameters and HCO3− secretion were then recorded for 60 min. The mucosal solution contained the following (mM): 115 NaCl, 25 sodium-D-gluconate, 5.2 potassium-D-gluconate, 1.2 CaCl2, 1.2 MgCl2, and 10 mannitol. The serosal solution contained the following (mM): 115 NaCl, 25 NaHCO3, 2.2 K2HPO4, 1.2 CaCl2, 1.2 MgCl2, 0.8 KH2PO4, 10 glucose, and 0.01 indomethacin. The osmolalities for both solutions were ∼300 mosmol·kg-1 of H2O.
The maximal volumes of concentrated stock solutions of all compounds added to 3 ml Ussing chamber solutions were less than 30 μl. All added compounds did not alter pH values in chamber solutions, which is consistent with other report (25). The concentrations of all pharmacological inhibitors used in the present study were based on their IC50 and the data from others’ reports.
Measurement of [Ca2+]cyt by single-cell imaging
[Ca2+]cyt imaging experiments were performed as previously described (52). Briefly, cells were grown on glass coverslips for 24 h and incubated with 5 μM fura-2/AM (Invitrogen) for 1 h in physiological salt solution (PSS) at 37 °C humidified atmosphere containing 5% CO2 in the dark and then washed with PSS for 20 min. Then, cells on coverslips were mounted in a standard perfusion chamber on the stage of an inverted fluorescence microscope (Leica). Fluorescence signals were imaged using an intensified CCD camera (ICCD200) attached to an inverted fluorescence microscope (Leica) and recorded with MetaFluor software (Universal Imaging Corporation). Images were acquired every 3 s. The dual wavelength excitation method for the measurement of fura-2 fluorescence was used. The excitation wavelengths were 340 and 380 nm, and the emitted fluorescence was collected at 510 nm. [Ca2+]cyt was presented as fluorescence ratios (F340/F380) after background subtraction. The PSS contained the following: 140 mM Na+, 5 mM K+, 2 mM Ca2+, 147 mM Cl−, 10 mM Hepes and 10 mM glucose (pH 7.4). The 0 Ca2+ solution (0 Ca2+) contained the following: 140 mM Na+, 5 mM K+, 145 mM Cl−, 0.5 mM EGTA, 10 mM Hepes, and 10 mM glucose (pH 7.4). The osmolality for the solution was ∼300 mosmol.kg−1 of H2O.
Western blotting
Western blotting was performed as previously described (52, 54). Briefly, the cells and tissues were harvested and lysed using RIPA lysis buffer. The protein sample were separated using 4 to 20% SDS-PAGE and transferred to polyvinylidene difluoride membrane. Following blocking with 5% nonfat milk for 2 h at room temperature, the membranes were incubated with the following primary antibodies at 4 °C overnight: anti-ERα (1:1000) (Cat. No. ab32063, Abcam), anti-ERβ (1:1000) (Cat. No. PA1-311, ThermoFisher) antibody, and anti-GAPDH (1:10,000) (Cat. No. 60004-1-Ig, Proteintech), anti-β-catenin (1:1000) (Cat. No. 8480, Cell Signaling Technology), anti-Cyclin D1 (1:1000) (Cat. No. 2978, Cell Signaling Technology), anti-PCNA (1:1000) (Cat. No. ab29, Abcam). Then, the membranes were washed with Tris-buffered saline with 0.1% Tween 20 detergent three times and incubated with corresponding secondary antibodies for 2 h at room temperature. The signals were visualized using enhanced chemiluminescence (Millipore) in an ImageQuant LAS 400 digital biomolecular imaging system. Each experiment was repeated three times. The gray value of the bands was measured by ImageJ software for statistics.
Quantitative real-time PCR
Quantitative real-time PCR was performed as previously described (54, 55). Briefly, Total RNA was extracted by RNAiso Plus reagent (Cat. No. 9109, Takara). cDNA was synthesized using PrimeScript RT-polymerase (Cat. No. R050A, Takara). Next, qPCR was performed using a SteponePlus device (Art. No. 272008342, Life Technologies) with a SYBR Premix Ex TaqTM II kit (Cat. No. RR820A, Takara). All samples were run in triplicate, and GAPDH was used as an internal control. Primers were as follows:
ERα: 5′- GGGAAGTATGGCTATGGAATCTG-3′ (Forward),
ERα: 5′- TGGCTGGACACATATAGTCGTT-3′ (Reverse).
ERβ: 5′-AGCACGGCTCCATATACATACC-3′ (Forward),
ERβ: 5′- TGGACCACTAAAGGAGAAAGGT-3′ (Reverse).
GAPDH: 5′- ACAACTTTGGTATCGTGGAAGG-3′ (Forward),
GAPDH: 5′- GCCATCACGCCACAGTTTC-3′ (Reverse).
Immunofluorescence staining
After fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, and blocked with bovine serum albumin, the HCoEpiC were incubated with anti-ERα (1:100) (Cat. No. ab32063, Abcam) or anti-ERβ (1:200) (Cat. No. PA1-311, ThermoFisher) antibody overnight at 4 °C. Then, the cells were incubated with Cy3 labeled anti-rabbit secondary antibody (Cat. No. A0516, Beyotime Biotechnology). Finally, nuclei were stained with DAPI for 5 min, and images were captured using confocal microscope.
Cell proliferation and scratch assays
Cell proliferation assay was performed as previously described (56). Briefly, cells were plated in 96-well plates. After 24 h, medium was replaced with medium containing different drugs. CCK-8 reagent (Cat. No. C0038, Beyotime Biotechnology) was added to each well at 0.5 to 2 h before the endpoint of incubation. A microplate reader (Thermo Fisher Scientific) was used to quantify viable cells by measuring the absorbance at 450 nm. Cell scratch assay was performed as previously described (57). After scratching, cell monolayers were gently washed to remove detached cells and replenished with serum free medium (with or without drugs) to inhibit cell proliferation. Images were obtained at 0 and 6 h postscratch. Experiments were repeated at least three times.
Infection of lentiviruses
Lentiviruses were purchased from Sangon Biotech Co, Ltd. The sequences for ERα shRNA and NC were as follows: shRNA-1 (5′-TTGTGTGCCTCAAATCTATTA-3′), shRNA-2 (5′-AGGCCAAATTCAGATAATCGA-3′), shRNA-3 (5′- CAGGTCCACCTTCTAGAATGT-3′), shNC (5′- TTCTCCGAACGTGTCACGT -3′). HCoEpiC were infected with lentiviruses according to the protocol of the manufacturer.
Drugs
PPT, DPN, MPP, PHTPP, E2, forskolin, genistein, rottlerin, wortmannin, GSK-7975A, ML-9, SKF-96365, CPA, and BAPTA-AM were purchased from MedChemExpress and dissolved in DMSO. CCh and indomethacin were purchased from Sigma-Aldrich. CCh was dissolved in ultrapure water. Indomethacin was dissolved in anhydrous alcohol. All salts were supplied by Sangon Biotech and dissolved in ultrapure water.
Data and statistical analysis
GraphPad Prism 7.0 (RRID: SCR_002798, USA) software was used for analysis and graph generation. All results shown are means ± SD. All experiments were repeated at least three times. The number of biological repeats (n) in the figures is the number of individual tissues or cells obtained from at least three mice or three independent experiments. The statistical significance of differences in the means of experimental groups was determined using Student's t test or one-way ANOVA followed by post hoc test for multiple pairwise comparisons. Significant differences (∗p < 0.05) are expressed in the figures and figure legends.
Data availability
The data supporting the findings of this study are available within the article.
Conflicts of interest
The authors declare no conflicts of interest with the content of this article.
Acknowledgments
Author contributions
H. W. and H. D. methodology; H. W., J. L., and X. C. investigation; H. W., J. L., and X. C. data curation; H. W. validation; H. W. and Z. M. S. formal analysis; H. W. writing–original draft; H. W. and H. D. funding acquisition; H. D. and Z. M. S. writing-review and editing; Z. M. S. and H. D. conceptualization; H. D. supervision.
Funding and additional information
These studies were supported by research grants from the National Natural Science Foundation of China (81972328 to H. W. and No. 82273115 to H. D.).
Reviewed by members of the JBC Editorial Board. Edited by Mike Shipston
Contributor Information
Zachary M. Sellers, Email: zsellers@stanford.edu.
Hui Dong, Email: donghui@qdu.edu.cn.
References
- 1.Bachmann O., Juric M., Seidler U., Manns M.P., Yu H. Basolateral ion transporters involved in colonic epithelial electrolyte absorption, anion secretion and cellular homeostasis. Acta Physiol. (Oxf) 2011;201:33–46. doi: 10.1111/j.1748-1716.2010.02153.x. [DOI] [PubMed] [Google Scholar]
- 2.Seidler U., Song P., Xiao F., Riederer B., Bachmann O., Chen M. Recent advances in the molecular and functional characterization of acid/base and electrolyte transporters in the basolateral membranes of gastric and duodenal epithelial cells. Acta Physiol. (Oxf) 2011;201:3–20. doi: 10.1111/j.1748-1716.2010.02107.x. [DOI] [PubMed] [Google Scholar]
- 3.Flemstrom G., Heylings J.R., Garner A. Gastric and duodenal HCO3- transport in vitro: effects of hormones and local transmitters. Am. J. Physiol. 1982;242:G100–110. doi: 10.1152/ajpgi.1982.242.2.G100. [DOI] [PubMed] [Google Scholar]
- 4.Flemstrom G., Garner A. Gastroduodenal HCO3(-) transport: characteristics and proposed role in acidity regulation and mucosal protection. Am. J. Physiol. 1982;242:G183–193. doi: 10.1152/ajpgi.1982.242.3.G183. [DOI] [PubMed] [Google Scholar]
- 5.Sjoblom M. The duodenal mucosal bicarbonate secretion. Ups J. Med. Sci. 2005;110:115–149. doi: 10.3109/2000-1967-076. [DOI] [PubMed] [Google Scholar]
- 6.Tuo B., Wen G., Song P., Xu J., Liu X., Seidler U., et al. Genistein stimulates duodenal HCO(3)(-) secretion through PI3K pathway in mice. Eur. J. Pharmacol. 2011;651:159–167. doi: 10.1016/j.ejphar.2010.10.070. [DOI] [PubMed] [Google Scholar]
- 7.Isenberg J.I., Selling J.A., Hogan D.L., Koss M.A. Impaired proximal duodenal mucosal bicarbonate secretion in patients with duodenal ulcer. N. Engl. J. Med. 1987;316:374–379. doi: 10.1056/NEJM198702123160704. [DOI] [PubMed] [Google Scholar]
- 8.Bukhave K., Rask-Madsen J., Hogan D.L., Koss M.A., Isenberg J.I. Proximal duodenal prostaglandin E2 release and mucosal bicarbonate secretion are altered in patients with duodenal ulcer. Gastroenterology. 1990;99:951–955. doi: 10.1016/0016-5085(90)90612-5. [DOI] [PubMed] [Google Scholar]
- 9.Smith A., Contreras C., Ko K.H., Chow J., Dong X., Tuo B., et al. Gender-specific protection of estrogen against gastric acid-induced duodenal injury: stimulation of duodenal mucosal bicarbonate secretion. Endocrinology. 2008;149:4554–4566. doi: 10.1210/en.2007-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuo B., Wen G., Wei J., Liu X., Wang X., Zhang Y., et al. Estrogen regulation of duodenal bicarbonate secretion and sex-specific protection of human duodenum. Gastroenterology. 2011;141:854–863. doi: 10.1053/j.gastro.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder H.J., Rajendran V., Sadasivan V., Geibel J.P. Bicarbonate secretion: a neglected aspect of colonic ion transport. J. Clin. Gastroenterol. 2005;39:S53–58. doi: 10.1097/01.mcg.0000155521.81382.3a. [DOI] [PubMed] [Google Scholar]
- 12.Vidyasagar S., Rajendran V.M., Binder H.J. Three distinct mechanisms of HCO3- secretion in rat distal colon. Am. J. Physiol. Cell Physiol. 2004;287:C612–621. doi: 10.1152/ajpcell.00474.2003. [DOI] [PubMed] [Google Scholar]
- 13.Tang L., Peng M., Liu L., Chang W., Binder H.J., Cheng S.X. Calcium-sensing receptor stimulates Cl(-)- and SCFA-dependent but inhibits cAMP-dependent HCO3(-) secretion in colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;308:G874–883. doi: 10.1152/ajpgi.00341.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Mahony F., Alzamora R., Betts V., LaPaix F., Carter D., Irnaten M., et al. Female gender-specific inhibition of KCNQ1 channels and chloride secretion by 17beta-estradiol in rat distal colonic crypts. J. Biol. Chem. 2007;282:24563–24573. doi: 10.1074/jbc.M611682200. [DOI] [PubMed] [Google Scholar]
- 15.Condliffe S.B., Doolan C.M., Harvey B.J. 17beta-oestradiol acutely regulates Cl- secretion in rat distal colonic epithelium. J. Physiol. 2001;530:47–54. doi: 10.1111/j.1469-7793.2001.0047m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Mahony F., Thomas W., Harvey B.J. Novel female sex-dependent actions of oestrogen in the intestine. J. Physiol. 2009;587:5039–5044. doi: 10.1113/jphysiol.2009.177972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oncel S., Basson M.D. Gut homeostasis, injury, and healing: new therapeutic targets. World J. Gastroenterol. 2022;28:1725–1750. doi: 10.3748/wjg.v28.i17.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogan D.L., Crombie D.L., Isenberg J.I., Svendsen P., Schaffalitzky de Muckadell O.B., Ainsworth M.A. CFTR mediates cAMP- and Ca2+-activated duodenal epithelial HCO3- secretion. Am. J. Physiol. 1997;272:G872–878. doi: 10.1152/ajpgi.1997.272.4.G872. [DOI] [PubMed] [Google Scholar]
- 19.Xie R., Dong X., Wong C., Vallon V., Tang B., Sun J., et al. Molecular mechanisms of calcium-sensing receptor-mediated calcium signaling in the modulation of epithelial ion transport and bicarbonate secretion. J. Biol. Chem. 2014;289:34642–34653. doi: 10.1074/jbc.M114.592774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J., Yang X., Guo Y., Zhang F., Wan H., Sun X., et al. Ca(2+) signaling in HCO3(-) secretion and protection of upper GI tract. Oncotarget. 2017;8:102681–102689. doi: 10.18632/oncotarget.21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunzelmann K., Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol. Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- 22.Odes H.S., Reimer R., Muallem R., Schwenk M., Beil W., Sewing K.F. Role of protein kinase C in duodenal mucosal bicarbonate secretion in the Guinea pig. Pharmacology. 1996;53:60–65. doi: 10.1159/000139415. [DOI] [PubMed] [Google Scholar]
- 23.May O., Yu H., Riederer B., Manns M.P., Seidler U., Bachmann O. Short-term regulation of murine colonic NBCe1-B (electrogenic Na+/HCO3(-) cotransporter) membrane expression and activity by protein kinase C. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuo B.G., Wen G.R., Seidler U. Phosphatidylinositol 3-kinase is involved in prostaglandin E2-mediated murine duodenal bicarbonate secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G279–287. doi: 10.1152/ajpgi.00488.2006. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa O., Hirokawa M., Guth P.H., Engel E., Kaunitz J.D. Role of protein kinases on acid-induced duodenal bicarbonate secretion in rats. Pharmacology. 2003;67:99–105. doi: 10.1159/000067740. [DOI] [PubMed] [Google Scholar]
- 26.Prossnitz E.R., Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gschwendt M., Muller H.J., Kielbassa K., Zang R., Kittstein W., Rincke G., et al. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 28.Alzamora R., O'Mahony F., Harvey B.J. Estrogen inhibits chloride secretion caused by cholera and Escherichia coli enterotoxins in female rat distal colon. Steroids. 2011;76:867–876. doi: 10.1016/j.steroids.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Cooper C.A., Whittamore J.M., Wilson R.W. Ca2+-driven intestinal HCO(3)(-) secretion and CaCO3 precipitation in the European flounder in vivo: influences on acid-base regulation and blood gas transport. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R870–876. doi: 10.1152/ajpregu.00513.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen L., Voronina S., Javed M.A., Awais M., Szatmary P., Latawiec D., et al. Inhibitors of ORAI1 prevent cytosolic calcium-associated injury of human pancreatic acinar cells and acute pancreatitis in 3 mouse models. Gastroenterology. 2015;149:481–492.e487. doi: 10.1053/j.gastro.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smyth J.T., Dehaven W.I., Bird G.S., Putney J.W., Jr. Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J. Cell Sci. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tolstykh G.P., Cantu J.C., Tarango M., Ibey B.L. Receptor- and store-operated mechanisms of calcium entry during the nanosecond electric pulse-induced cellular response. Biochim. Biophys. Acta Biomembr. 2019;1861:685–696. doi: 10.1016/j.bbamem.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Naydenov N.G., Lechuga S., Zalavadia A., Mukherjee P.K., Gordon I.O., Skvasik D., et al. P-cadherin regulates intestinal epithelial cell migration and mucosal repair, but is dispensable for colitis associated colon cancer. Cells. 2022;11:1467. doi: 10.3390/cells11091467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dignass A.U., Tsunekawa S., Podolsky D.K. Fibroblast growth factors modulate intestinal epithelial cell growth and migration. Gastroenterology. 1994;106:1254–1262. doi: 10.1016/0016-5085(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 35.Peng K., Kou L., Yu L., Bai C., Li M., Mo P., et al. Histone demethylase JMJD2D interacts with beta-catenin to induce transcription and activate colorectal cancer cell proliferation and tumor growth in mice. Gastroenterology. 2019;156:1112–1126. doi: 10.1053/j.gastro.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 36.Sharma C., Pradeep A., Pestell R.G., Rana B. Peroxisome proliferator-activated receptor gamma activation modulates cyclin D1 transcription via beta-catenin-independent and cAMP-response element-binding protein-dependent pathways in mouse hepatocytes. J. Biol. Chem. 2004;279:16927–16938. doi: 10.1074/jbc.M309045200. [DOI] [PubMed] [Google Scholar]
- 37.Meng Q., Li Y., Ji T., Chao Y., Li J., Fu Y., et al. Estrogen prevent atherosclerosis by attenuating endothelial cell pyroptosis via activation of estrogen receptor alpha-mediated autophagy. J. Adv. Res. 2021;28:149–164. doi: 10.1016/j.jare.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas M.L., Xu X., Norfleet A.M., Watson C.S. The presence of functional estrogen receptors in intestinal epithelial cells. Endocrinology. 1993;132:426–430. doi: 10.1210/endo.132.1.8419141. [DOI] [PubMed] [Google Scholar]
- 39.Saint-Criq V., Rapetti-Mauss R., Yusef Y.R., Harvey B.J. Estrogen regulation of epithelial ion transport: implications in health and disease. Steroids. 2012;77:918–923. doi: 10.1016/j.steroids.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Campbell-Thompson M., Lynch I.J., Bhardwaj B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001;61:632–640. [PubMed] [Google Scholar]
- 41.Faltas C.L., LeBron K.A., Holz M.K. Unconventional estrogen signaling in health and disease. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuentes N., Silveyra P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019;116:135–170. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazrak A., Thome U., Myles C., Ware J., Chen L., Venglarik C.J., et al. cAMP regulation of Cl(-) and HCO(-)(3) secretion across rat fetal distal lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L650–658. doi: 10.1152/ajplung.00370.2001. [DOI] [PubMed] [Google Scholar]
- 44.Yang X., Wen G., Tuo B., Zhang F., Wan H., He J., et al. Molecular mechanisms of calcium signaling in the modulation of small intestinal ion transports and bicarbonate secretion. Oncotarget. 2018;9:3727–3740. doi: 10.18632/oncotarget.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung H.K., Rathor N., Wang S.R., Wang J.Y., Rao J.N. RhoA enhances store-operated Ca2+ entry and intestinal epithelial restitution by interacting with TRPC1 after wounding. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309:G759–767. doi: 10.1152/ajpgi.00185.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Onodera K., Pouokam E., Diener M. STIM1-regulated Ca2+ influx across the apical and the basolateral membrane in colonic epithelium. J. Membr. Biol. 2013;246:271–285. doi: 10.1007/s00232-013-9528-9. [DOI] [PubMed] [Google Scholar]
- 47.Roy J., Lefkimmiatis K., Moyer M.P., Curci S., Hofer A.M. The {omega}-3 fatty acid eicosapentaenoic acid elicits cAMP generation in colonic epithelial cells via a "store-operated" mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G715–722. doi: 10.1152/ajpgi.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F., Wan H., Chu F., Lu C., Chen J., Dong H. Small intestinal glucose and sodium absorption through calcium-induced calcium release and store-operated Ca(2+) entry mechanisms. Br. J. Pharmacol. 2021;178:346–362. doi: 10.1111/bph.15287. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L.Y., Chen X.Y., Dong H., Xu F. Cyclopiazonic acid-induced Ca(2+) store depletion initiates endothelium-dependent hyperpolarization-mediated vasorelaxation of mesenteric arteries in healthy and colitis mice. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.639857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villablanca E.J., Selin K., Hedin C.R.H. Mechanisms of mucosal healing: treating inflammatory bowel disease without immunosuppression? Nat. Rev. Gastroenterol. Hepatol. 2022;19:493–507. doi: 10.1038/s41575-022-00604-y. [DOI] [PubMed] [Google Scholar]
- 51.Percie du Sert N., Hurst V., Ahluwalia A., Alam S., Avey M.T., Baker M., et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br. J. Pharmacol. 2020;177:3617–3624. doi: 10.1111/bph.15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan H., Chen X.Y., Zhang F., Chen J., Chu F., Sellers Z.M., et al. Capsaicin inhibits intestinal Cl(-) secretion and promotes Na(+) absorption by blocking TRPV4 channels in healthy and colitic mice. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.101847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clarke L.L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1151–1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao N., Yang F., Chen S., Wan H., Zhao X., Dong H. The role of TRPV1 ion channels in the suppression of gastric cancer development. J. Exp. Clin. Cancer Res. 2020;39:206. doi: 10.1186/s13046-020-01707-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang B., Wu J., Zhu M.X., Sun X., Liu J., Xie R., et al. VPAC1 couples with TRPV4 channel to promote calcium-dependent gastric cancer progression via a novel autocrine mechanism. Oncogene. 2019;38:3946–3961. doi: 10.1038/s41388-019-0709-6. [DOI] [PubMed] [Google Scholar]
- 56.Xie R., Xu J., Xiao Y., Wu J., Wan H., Tang B., et al. Calcium promotes human gastric cancer via a novel coupling of calcium-sensing receptor and TRPV4 channel. Cancer Res. 2017;77:6499–6512. doi: 10.1158/0008-5472.CAN-17-0360. [DOI] [PubMed] [Google Scholar]
- 57.Martinotti S., Ranzato E. Scratch wound healing assay. Methods Mol. Biol. 2020;2109:225–229. doi: 10.1007/7651_2019_259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available within the article.