Graphical abstract
Keywords: Tremella fuciformis, Degraded polysaccharides, Structural characterization, Antioxidant and stress resistance, Caenorhabditis elegans, Metabolites
Highlights
-
•
The activities of TFLPs prepared by different methods were evaluated.
-
•
TFLP-1 has in vivo antioxidant activity and stress resistance.
-
•
TFLP-1 can regulate the composition of metabolites in C. elegans.
Abstract
Different methods were used to degrade Tremella fuciformis polysaccharides (TFP) and prepare low molecular weight polysaccharides of Tremella fuciformis (TFLP) to improve their bioavailability. It was found that the TFLP prepared by ultrasonic-assisted H2O2-Vc method showed the highest level of antioxidant activity and stress resistance in C. elegans. The structural characteristics, in vivo antioxidant and stress resistance of TFLP-1 were evaluated after isolation and purification of TFLP, it was found that TFLP-1 was an acid polysaccharide with a molecular weight of 75770 Da, which mainly composed of mannose. Meanwhile, it could regulate the antioxidant activity and stress resistance in C. elegans by upregulating the transcription of fat-5, fat-7, acs-2, glp-1, hsf-1, hsp-1, mtl-1, nhr-49, skn-1 and sod-3 mRNA. The improvement effects were closely related to the significant regulation of galactose metabolism, alpha linolenic acid metabolism, and pantothenate and CoA biosynthesis metabolic pathways. These results provided insights into the high value application of Tremella fuciformis in the food industry and the development of antioxidant related functional foods.
1. Introduction
As a traditional and common edible fungus, studies have found that Tremella fuciformis (TF) is rich in nutrients and active substances such as polysaccharides, protein, cellulose and minerals, which means that it has benign nutritional value and pharmacological activity [1], [2]. As its characteristic active substance, the nutritional research of Tremella fuciformis polysaccharide (TFP) has received extensive attention [3]. Zhang et al. (2023) found that TF dietary fiber rich in polysaccharides could significantly regulate the level of lipid metabolism through increasing the level of HDL-C in hyperlipidemic mice, while decreasing the levels of TC, TG and LDL-C [2]. Xiao et al. (2021) found that TFP could significantly alleviate the symptoms of DSS-induced colitis in mice by improving weight loss, lengthening the length and reducing the thickness of the colon, and improving the intestinal mucosal barrier [4]. Ge et al. (2020) obtained a polysaccharide with the molecular weight of 1.14 × 103 kDa by isolation and purification of TFP, and it was found that the scavenging rates of this polysaccharide on superoxide anions and hydroxyl radicals were 69.84% and 71.00%, respectively [5]. Wang et al. (2015) treated TFP by carboxymethylation and found that the superoxide radical and hydroxyl radical scavenging activity of the modified polysaccharide was improved. [6]. Studies have indicated that there existed a close correlation between antioxidant activity and lipid metabolism, immune enhancement, and anti-inflammatory effects [7]. TFP was found to have extremely strong water holding capacity and swelling ability, which means that it often used as a natural thickener in the food industry [8]. However, it was difficult to be digested and absorbed by organisms due to the high swelling and water holding capacity of high molecular weight polysaccharides, indicating that the bioavailability is reduced [9]. The degradation of polysaccharides in different ways has received widespread attention in order to improve their nutritional value and bioavailability, making them more easily digested and absorbed by organisms [10], [11]. Therefore, the highest level of antioxidant activity preparation method for the low molecular weight polysaccharide of Tremella fuciformis (TFLP) was selected through activity evaluation methods and its structural characteristics after separation and purification were analyzed, which will provide an important foundation for improving the bioavailability of the TF and the development of functional foods.
The stable state of reactive oxygen species (ROS) is broken when the organism is stimulated by the external environment, and excessive accumulation causes oxidative damage to the cell, which is represented by malondialdehyde (MDA) generated by lipid peroxidation. In this process, a series of antioxidant enzymes, such as total superoxide dismutase (T-SOD), catalase (CAT) and glutathione peroxidase (GSH-Px), are produced by the biological system in order to fight against cell oxidation and damage caused by ROS overexpression [12], [13]. However, the secretion of endogenous antioxidant enzymes is not enough to resist the damage caused by ROS to cells and organism systems in some harsh environments, and other health problems appeared or aggravated, such as ageing, lipid metabolism disorder, gut microbiota imbalance, and low immunity when the organism is in the state of oxidation-antioxidation homeostasis imbalance for a long time [7], [14]. Therefore, it has become an effective measure to intake exogenous active substances in daily diet to improve the antioxidant activity of organisms and prevent related diseases, which means that the active substances from natural products have become a critical research target [15]. As an extremely important active substance from natural products, polysaccharides play an important role in anti-oxidation, improving immunity, anti-ageing, regulating lipid metabolism and the structure and function of gut microbiota [16], [17].
As a biological animal model, Caenorhabditis elegans has the advantages of easy observation, small range of motion, and short life cycle. Thus, C. elegans has been received widespread attention in food nutrition evaluation, especially in antioxidant and anti-ageing [18], [19]. Furthermore, it has been found that there existed 60–80% of genes homologous to humans, which suggests it has credibility by exploring changes in its gene transcription level [18], [19], [20]. In addition, C. elegans has a complete digestive and reproductive system, which is of reference significance in exploring the underlying mechanisms [18], [19], [20]. Polysaccharides was not easy to be digested and absorbed by organisms, but it could be fermented in the gut to produce a series of metabolic derivatives, which played an important role in maintaining health [21], [22]. Wu et al. (2022) found that TFP could significantly regulate the composition of gut microbiota and the level of short-chain fatty acids by increasing the relative abundance of Phascolarctacterium, Bacteroides and Lachnoclostridium to improve the health of the organism [23]. Therefore, the antioxidant and stress resistance of TFLP were investigated through C. elegans, and its mechanism was explored through changes in mRNA transcription levels and gut metabolites.
In this study, the preparation method of TFLPs with highest level of antioxidant activity was selected through comparing their antioxidant and anti-stress activity in C. elegans. Meanwhile, the structural characteristics and dose–effect relationship of antioxidant and stress resistance were analyzed by purified polysaccharides. The mechanism has been explored at transcription level and gut metabolites through modern biotechnology. The prebiotic effect of Tremella fuciformis low molecular weight polysaccharide is crucial for the development of TFLP as a functional food related to antioxidation.
2. Materials and methods
2.1. Preparation of TFLP
TFP was provided by Fujian Hongtailai Biotechnology Co., Ltd (Fuzhou, China). The preparation method of TFP was as follows: TF was dried, powdered, mixed with water (material and liquid ratio was 1:50), and heated at 85 ℃ for 5 h. The supernatant of the mixed solution was obtained after centrifugation. The supernatant was filtered through Ceramic membrane filter column (StarMem, Xiamen, China), and the intercepted solution was freeze-dried to obtain TFP. The molecular weight of TFP was 2, 238, 035 Da, and TFP was mainly composed of mannose, fucose, xylose, glucose and glucuronic acid with a molar ratio of 0.402: 0.259: 0.238: 0.059: 0.042. The preparation of TFLP mainly included pressure-heating method, enzymatic hydrolysis method, and ultrasonic-assisted H2O2-Vc method [24], [25], [26]. The detailed degradation methods are as follows:
-
(1)
Pressure-heating method: TFP (2 mg/mL) was subjected to pressure-heating treatment with a pressure of 100–150 kPa and a temperature of 115–130 ℃ for 20–30 min (TFLP-PH).
-
(2)
Enzymatic hydrolysis method: Pectinase (40 U/mg polysaccharide) was added to hydrolyze TFP (2 mg/mL) at pH 3, maintaining temperature at 50 ℃ for 2.5 h. Then, the pH was adjusted to neutral, and freeze-dried after dialysis to obtain TFLP (TFLP-P); Cellulase (40 U/mg polysaccharide) was added to hydrolyze TFP (2 mg/mL) at pH 5, and TFLP was obtained after enzymatic hydrolysis treatment (TFLP-C); Cellulase and pectinase (1:1 configuration of 40 U/mg polysaccharides based on enzyme activity) were added to hydrolyze TFP (2 mg/mL) at pH 3, and TFLP was obtained after enzymatic hydrolysis treatment (TFLP-CP). The conditions for preparing TFLP by enzymatic hydrolysis method were the same with the exception of the different types of enzymes and the pH value.
-
(3)
Ultrasonic-assisted H2O2-Vc method: H2O2 (30%, 0.03 mL) and ascorbic acid (0.0529 g) were added to TFP (2 mg/mL, 100 mL) and subjected to ultrasonic degradation at a power of 280 W and a temperature of 55 ℃ for 40 min, TFLP was obtained after freeze drying (TFLP-U).
2.2. Antioxidation and stress resistance in C. elegans
The wild isolate of C. elegans (N2) was purchased from Fujian Sunybiotech Co., Ltd. (Fuzhou, China). C. elegans at different stages were lysed by NaOH-HClO lysis solution for 5 min (The solution was prepared by 5 mol/L NaOH and 0.5% HClO solution in a volume ratio of 1: 2, 1 mL), and most of nematodes disappeared through microscope observation. After centrifugation, the supernatant was discarded, and the sediments (nematode eggs) were placed on a new nematode growth plate (NGM) containing Escherichia coli OP50 (OP50) and grown at 20 ℃ for 72 h to obtain synchronized nematodes (L4 stage) [27]. Synchronized nematodes were randomly grouped and transferred to NGM containing different TFLP-OP50 foods, which including the NFD group (OP50), TFLP-PH group (OP50-TFLP-PH solution, 50 μg/mL), TFLP-P group (OP50-TFLP-P solution, 50 μg/mL), TFLP-C group (OP50-TFLP-C solution, 50 μg/mL), TFLP-CP group (OP50-TFLP-CP solution, 50 μg/mL), TFLP-U group (OP50-TFLP-U solution, 50 μg/mL) [28]. Nematodes were collected for subsequent experiments after three days of TFLP supplementation.
2.2.1. Heat stress resistance
Thirty nematodes were randomly selected from NGM supplemented with different TFLP for three days and placed in a new NGM. Heat stress resistance studies were completed in an incubator at 37 ℃. The number of nematode deaths was recorded every 2 h until all of nematodes died. Each experimental group was set up with three parallels [27].
2.2.2. Acute oxidative stress resistance
Thirty nematodes were randomly selected from NGM supplemented with different TFLP for three days and placed in a NGM containing H2O2. Acute oxidative stress resistance study was completed at 20 ℃. The number of nematode deaths was recorded every 1 h until all nematodes were dead. Each experimental group was set three parallels [27].
2.2.3. Antioxidant related biochemical indexes
Nematodes were collected after different TFLP supplemented for 3 d and washed with M9 buffer. Western and IP cell lysing solutions (1 mL) (Beyotime Biotechnology, Shanghai, China) were added to the nematode collection tube and cracked on ice. The supernatant was collected after centrifuging the nematode lysate and its protein concentration was measured by using a BCA protein assay kit (Lablead, Beijing, China). The levels of MDA, T-SOD, GSH-Px, CAT and ROS were determined by the kit methods (Jiancheng Bioengineering, Nanjing, China) [27].
2.3. Isolation, purification, identification, and antioxidant analysis of TFLP
2.3.1. Isolation and purification of TFLP
7The preparation method of TFLP with the highest antioxidant activity was screened, and the TFLP was subjected to isolation and purification process. Ultrapure water was used as the mobile phase, TFLP (20 mg/mL) was sequentially passed through DEAE cellulose DE-52 and Sephadex G-200 to obtain the purified polysaccharide component TFLP-1.
2.3.2. Structural identification of TFLP-1
The determination of monosaccharide components and molecular weight were followed the method of Li et al [27]. In briefly, TFLP-1 solution (2 mg/mL) was configured with NaCl solution (0.2 mol/L) as solvent. After centrifugation, the supernatant of the TFLP-1 solution was obtained, and the molecular weight of TFLP-1 was determined by high performance liquid gel chromatography [28].
The supernatant of TFLP-1 was obtained through hydrolysis, nitrogen blowing, dissolution, vortex mixing, dilution, centrifugation, and the monosaccharide components of TFLP-1 were determined by the method of ion chromatography (IC). Monosaccharide standards including fucose (Fuc), rhamnose (Rha), arabinose (Ara), galactose (Gal), glucose (Glc), xylose (Xyl), mannose (Man), fructose (Fru), ribose (Rib), galactose uronic acid (GalA), glucuronic acid (GlcA), glucosamine hydrochloride (GlcN), galactosamine hydrochloride (GalN), N-acetyl-D-glucosamine (GlcNAc), guluronic acid (GulA), mannuronic acid (ManA) [28].
TFLP-1 was mixed in spectral grade KBr and pressed to form a thin sheet. The thin sheet was placed in a sample bin, and its changes in the wavelength range of 500–4000 cm−1 were recorded and analyzed through the fourier transform infrared (FT-IR) spectroscopy to identify the structure of TFLP-1 [27].
2.3.3. Antioxidant and stress resistance of TFLP-1 in vivo
After synchronization treatment, nematodes were divided into four groups: normal group (NFD); TFLP-1 low dose group (TFLP-1 was dissolved in OP50 solution to form 50 μg/mL TFLP-1-OP50 solution) (TFLP-1L); TFLP-1 medium dose group (TFLP-1 was dissolved in OP50 solution to form 100 μg/mL TFLP-1-OP50 solution) (TFLP-1 M) and TFLP-1 high dose group (TFLP-1 was dissolved in OP50 solution to form 200 μg/mL TFLP-1-OP50 solution) (TFLP-1H). The antioxidant and stress resistance of TFLP-1 was evaluated by exploring the levels of heat stress resistance, acute oxidative stress resistance and biochemical indicators in C. elegans according to the method in section 2.2 [27].
2.4. Real-time PCR
Nematodes (about 300) treated with different concentration of TFLP-1 were collected and cleaned with M9 buffer. The lysate was added to the nematode collection tubes and the nematode RNA was extracted according to the kit instructions. Total RNA was reversed and transcribed into a first-strand cDNAs through the method of a cDNAs synthesis kit (Novoprotein, Shanghai, China). The relative transcription of mRNA was measured by amplification of cDNA using the ABI 7300 PCR system. β-actin was an internal reference gene, and the experimental results were calculated and analyzed using 2-ΔΔCT method. The sequence of the target gene was shown in Table 1 [27].
Table 1.
Primer sequences used in qPCR.
| Genes | Forward primers | Reverse primers |
|---|---|---|
| act-1 | ATCCATCGTTCACCGCAAGT | TAAGGACAAAAATGGGGCGG |
| daf-1 | CCCAGAAACCGAAAGACGAGTCAC | TCCCGCCAATTTTCCTGCTCATC |
| daf-2 | CGGTTGTTGTTCGTGTTCGG | CCGTTGTTGTTGCTCACTGC |
| daf-16 | TACATTGCTCGAAGTGCCGA | TCAGATGGTAGCGGCGAATC |
| fat-5 | GCCCTCTTCCGTTACTGCTTCAC | CTTCTCCGACTGCCGCAATAGATG |
| fat-6 | TCGGAGAGGGAGGTCACAACTTC | CGGTCGTAGACAAGTCCAAGAGC |
| fat-7 | ATCGTTGCCATCACAAGTGGACTG | TTACGCACAAGAAGCCATCCCATG |
| acs-2 | TCGCTGATGCTCATGTCGTC | GCCTTCTTAATGTGCTCCGC |
| age-1 | GCTCTTCCACGCAGTCAAAC | GGTGAAAGATACGGCGGGTT |
| akt-1 | CGTTCTTGCCGGATACCTTCAC | TCGGCTCGTCATTCAGATTCTTCATC |
| ddl-1 | ACCACCGCCACACCATCATC | TTCCACTGTTTCCTCCACGATTTG |
| glp-1 | AGATGTAACTGCCCCAACGG | ACTCCTCCGTTCTTGCATGG |
| hsf-1 | AGGAGGACCATCTACATCATCTTCGG | TTCTATTCACCTGAGCCATTTGCCTAG |
| hsp-1 | CATGGTCAACGAAGCTGAGA | TTCCAAATCCTTCTGTTGGTG |
| mod-1 | ACCCCTGATTGAAGAGATGCG | AGTGCCACCAGTAGACAAGA |
| mtl-1 | TGCAAGTGCGGAGACAAATG | GTTCCCTGGTGTTGATGGGT |
| nhr-49 | ATGGACTACTTTCTTGATGCTTCT | CGGCGGAAGAATCCCTTACA |
| skn-1 | TCTACTCTTTCCTCCCTTCGG | ACAAGGGGATGGGGATCAGA |
| sod-3 | CCACCTGTGCAAACCAGGAT | TGCAAGTAGTAGGCGTGCTC |
2.5. Untargeted metabolomics study
The C. elegans supplemented with different concentrations of TFLP-1 (0 and 200 μg/mL) were collected, the supernatant of the sample for detection was obtained after cleaning, grinding, lysing and centrifugation. Agilent 1290 ultra-high performance liquid phase was used for capturing and obtaining metabolomics data of untargeted metabolomics. After filtering and normalizing the obtained data, principal component analysis (PCA), orthogonal partial least squares discriminant analysis (OPLS-DA), and permutation experimental analysis were performed. The criteria for determining significant differences in metabolites were VIP > 1 and p < 0.05 and (Fold Change < 0.67 or Fold Change > 1.5). The database KEGG (https://www.kegg.jp) and MetaboAnalyst (https://www.metaboanalyst.ca/) was used for the analysis and judgment of metabolic pathways.
2.6. Statistical analysis
All the date of experiments were repeated at least three times, and the experimental results were expressed as means ± SD. One-way analysis of variance (ANOVA) for comparisons of data obtained from experiments and the production of corresponding pictures were performed using IBM SPSS Statistics 26 software and Origin 2019b 32Bit software. *p < 0.05 and **p < 0.01, compared to the NFD group. Spearman’s method was used to analyze the correlation between biochemical indicators and metabolites.
3. Results
3.1. Effects of TFLP on antioxidant and stress resistance in C. elegans
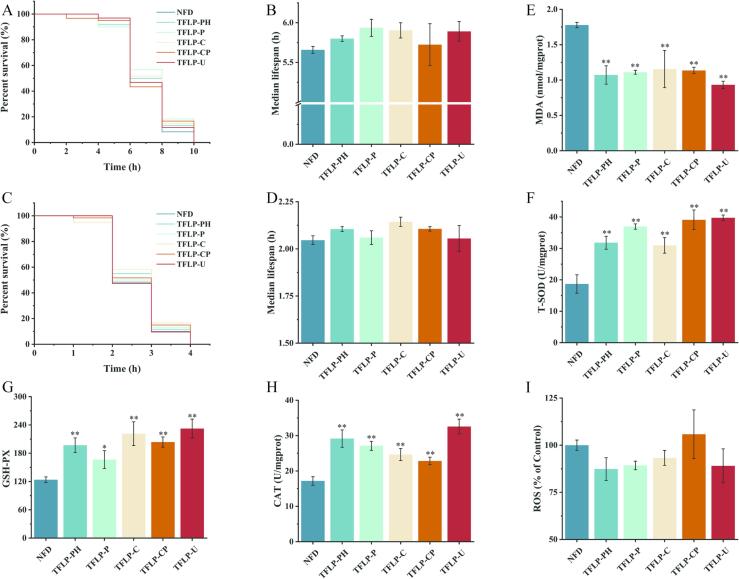
TFLPs prepared by different methods have affected the antioxidant activity and stress resistance of C. elegans (Fig. 1) (Table 2). When the organism is suffered from strong external stimuli, it can trigger a series of stress reactions, which have a malignant impact on health and lifespan [29]. Therefore, it is the most intuitive method through exploring the stress resistance of TFLP by comparing the survival time of C. elegans supplemented TFLP in harsh environments [30]. Compared with the NFD group, there was no significant difference in the maximum and median lifespan of nematodes between different TFLP supplementation groups in heat and acute oxidative stress environments (Fig. 1A-D). However, there existed an increasing trend in the TFLP-PH, TFLP-P, TFLP-C and TFLP-U groups of nematodes under heat stress, the median lifespan of the TFLP-PH and TFLP-C groups of nematodes in acute oxidative stress environments showed an increasing trend compared with the NFD group (Fig. 1A-D). The improvement of stress resistance is inevitably accompanied by an increase in the secretion of antioxidant enzymes in the organism and a decrease in the level of harmful substances [31]. T-SOD, GSH-Px, and CAT are typical antioxidant enzymes. MDA is a substance secreted by excessive accumulation of ROS that has a malignant effect on organism protein metabolism and lipid metabolism [32]. Therefore, exploring the changes in these biochemical indicators could provide a basis for elucidating the in vivo antioxidant capacity of TFLP. Compared with the NFD group, supplementation with different TFLPs significantly improved the antioxidant activity related biochemical indicators in C. elegans, especially MDA, T-SOD, GSH-Px and CAT (p < 0.01) (Fig. 1E-H). In this study, it was found that the TFLP-U group showed the highest improvement of antioxidant activity in C. elegans. Specifically, the MDA level in C. elegans decreased by 0.87 nmol/mg prot, T-SOD enzyme activity increased by 21.07 U/mg prot, GSH-Px enzyme activity increased by 108.48, and CAT enzyme activity increased by 15.40 U/mg prot compared to the NFD group (p < 0.01) (Fig. 1E-H). However, there was no significant difference in the level of ROS in C. elegans supplemented with different TFLP compared with the NFD group, but there was a varying degree of decrease (Fig. 1F). In general, it was screened that TFLP obtained by ultrasonic-assisted H2O2-Vc method that had relatively strong antioxidant and stress resistance by comparing the in vivo antioxidant and stress resistance of TFLP prepared by degrading TFP using different methods.
Fig. 1.
Effects of TFLP obtained from different methods of degrading TFP on stress resistance and antioxidant activity related biochemical indicators in C. elegans. Lifespan curve (A) and median lifespan (B) in C. elegans supplemented with different TFLP at 37 ℃; Lifespan curve (C) and median lifespan (D) in C. elegans supplemented with different TFLP at H2O2-induced acute oxidative stress environment; The level of MDA (E), T-SOD (F), GSH-Px (G), CAT (H) and ROS (I) in C. elegans supplemented with different TFLP for 3 d. *P < 0.05, **P < 0.01, compared to the NFD group.
Table 2.
Effects of different TFLP on antioxidant activity and stress resistance of C. elegans.
| Median lifespan at 37 ℃ (h) | Median lifespan atacute oxidative stress (h) | MDA (nmol/mgprot) | T-SOD (U/mgprot) | GSH-Px |
CAT (U/mgprot) |
ROS (% of control) |
|
|---|---|---|---|---|---|---|---|
| NFD | 5.66 ± 0.04 a | 2.04 ± 0.02 a | 1.78 ± 0.04 a | 18.68 ± 2.92 d | 123.78 ± 5.98c | 17.18 ± 1.21 d | 1.00 ± 0.02 a |
| TFLP-PH | 5.80 ± 0.04 a | 2.10 ± 0.01 a | 1.07 ± 0.13b | 31.80 ± 2.06 bc | 197.02 ± 15.48 ab | 29.16 ± 2.45b | 0.87 ± 0.06 a |
| TFLP-P | 5.94 ± 0.11 a | 2.06 ± 0.04 a | 1.11 ± 0.03b | 36.96 ± 0.85 ab | 166.50 ± 18.92b | 27.12 ± 1.26b | 0.89 ± 0.02 a |
| TFLP-C | 5.90 ± 0.10 a | 2.14 ± 0.02 a | 1.16 ± 0.26b | 30.97 ± 2.48c | 221.47 ± 25.27 a | 24.66 ± 1.71c | 0.93 ± 0.04 a |
| TFLP-CP | 5.72 ± 0.26 a | 2.11 ± 0.01 a | 1.14 ± 0.04b | 39.10 ± 3.13 a | 203.73 ± 10.83 ab | 22.85 ± 1.06c | 1.06 ± 0.13 a |
| TFLP-U | 5.89 ± 0.12 a | 2.06 ± 0.07 a | 0.93 ± 0.05b | 39.75 ± 0.87 a | 232.26 ± 20.15 a | 32.58 ± 2.10 a | 0.89 ± 0.09 a |
3.2. Isolation, purification and structural identification of TFLP
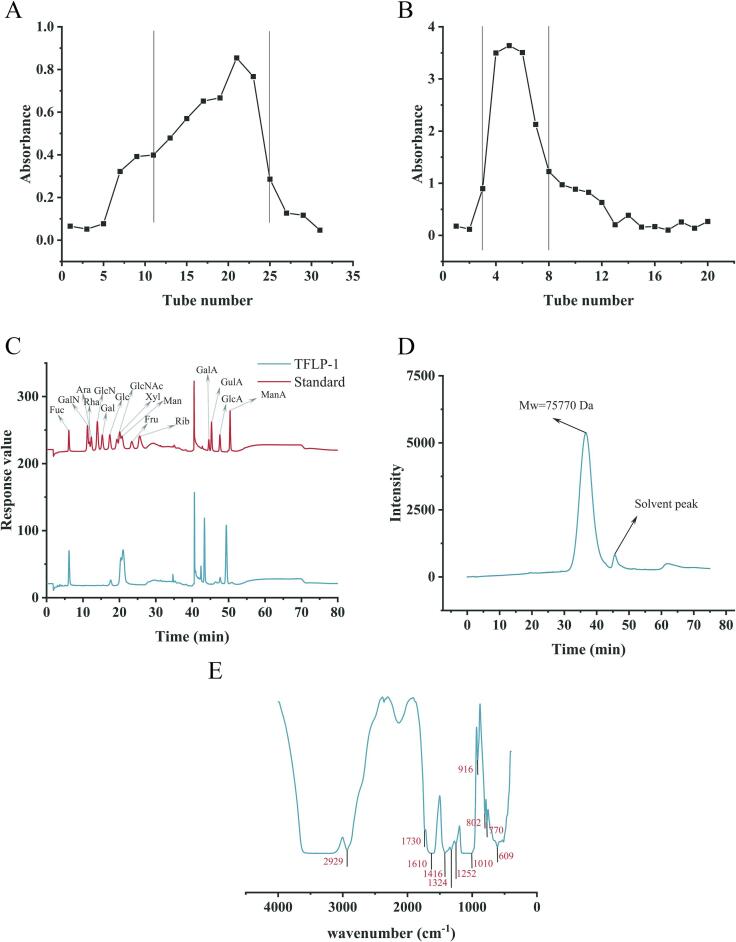
The TFLP that degrade from TFP by the ultrasonic-assisted H2O2-Vc method was isolated and purified, and its monosaccharide components, molecular weight, and structure were identified. TFLP was purified by DEAE-52 cellulose column and G-200 gel column to obtain TFLP-1 (Fig. 2A, B). It was found that TFLP-1 was a pure polysaccharide mainly composed of Man through the identification of monosaccharide components of TFLP-1, and its monosaccharide components included Man, Fuc, Xyl, Glc and GlcA with a molar ratio of 0.433: 0.257: 0.219: 0.050: 0.042 (Fig. 2C). The molecular weight of TFLP-1 was determined by high performance gel permeation chromatography (HPGPC), and it was found that the weight average molecular weight (Mw) of TFLP-1 was 75, 770 Da under the analysis conditions of lgMw-RT correction curve equation was y = -0.1766x + 11.348 and R2 = 0.9981 (Fig. 2D).
Fig. 2.
TFLP-1 was obtained after TFLP-U purified by DEAE-52 cellulose column (A) and G-200 gel column (B); Monosaccharide composition (C), molecular weight (D), and FT-IR spectra (E) of TFLP-1.
The structure of TFLP-1 was determined and analyzed by FT-IR in the wavelength range of 400–4000 cm−1 (Fig. 2E). The peak observed at 2929 cm−1 was speculated to be the stretching vibration of alkyl C-H [33]. The characteristic absorption peak appeared at 1730 cm−1 proved that the polysaccharide contained C = O [34]. The absorption peak at 1416 cm−1 was considered to be the deformation vibration of the C-H bond [35]. The absorption peak appeared at 916 cm−1 indicated that TFLP-1 was β-type glycosidic linkage occurred [36]. The absorption peaks at 1252 cm−1 and 1010 cm−1 were speculated to be a stretching motion between C-O-H and C-O-C [36]. Meanwhile, the absorption peak at 802 cm−1 was confirmed to have residual mannose, which was consistent with the identification results of monosaccharide components [37]. The peaks observed in a series of wavebands between 1300 and 500 cm−1 (770 and 609 cm−1) were speculated to the stretching motion of the C-O-C and S-O-S sulfate, which means that TFLP-1 is an acidic polysaccharide [27]. These results demonstrated that TFLP-1 was an acidic polysaccharide mainly composed of mannose.
3.3. Effects of TFLP-1 on antioxidant and stress resistance in C. elegans
The antioxidant and stress resistance of TFLP-1 novel polysaccharide was determined in C. elegans. It was found that the nematode supplemented with TFLP-1 had no significant effect on the maximum lifespan and median lifespan of acute oxidative stress, but it significantly increased the median lifespan of nematodes in thermal emergency environments (Fig. 3A-D). Compared with the NFD group, the median lifespan of nematodes in the TFLP-1L and TFLP-1H groups increased by 0.30 and 0.32 h, respectively (p < 0.05) (Fig. 3B). The MDA level of nematodes in the TFLP-1L group significantly decreased by 21% compared with the NFD group (p < 0.01), the relative enzyme activities of T-SOD and CAT significantly increased by 3.38 and 0.51 times (p < 0.01), and the GSH-Px activity increased by 0.50 times (p < 0.05) (Fig. 3E-H). Moreover, the MDA level of nematodes in the TFLP-1 M group significantly decreased by 57% (p < 0.01), the ROS level significantly decreased by 25% (p < 0.05), the relative enzyme activities of T-SOD and GSH-Px significantly increased by 5.55 and 0.83 times (p < 0.01), and the CAT activity increased by 0.28 times (p < 0.05) (Fig. 3E-I). The MDA level of nematodes in the TFLP-1H group significantly decreased by 68% (p < 0.01), the relative enzyme activities of T-SOD significantly increased by 5.13 (p < 0.01) (Fig. 3E, F). In summary, TFLP-1 could exert antioxidant and stress resistance by increasing the activity of antioxidant enzymes (such as T-SOD, GSH-Px, and CAT) in C. elegans, reducing MDA and ROS levels, and enhancing median lifespan in heat stress environments.
Fig. 3.
Effects of different concentrations of TFLP-1 (0, 50, 100 and 200 μg/mL) on stress resistance and antioxidant activity related biochemical indicators in C. elegans. Lifespan curve (A) and median lifespan (B) in C. elegans supplemented with different concentrations of TFLP-1 at 37 ℃; Lifespan curve (C) and median lifespan (D) in C. elegans supplemented with different concentrations of TFLP-1 at H2O2-induced acute oxidative stress environment; The level of MDA (E), T-SOD (F), GSH-Px (G), CAT (H) and ROS (I) in C. elegans supplemented with different concentrations of TFLP-1 for 3 d. *P < 0.05, **P < 0.01, compared to the NFD group.
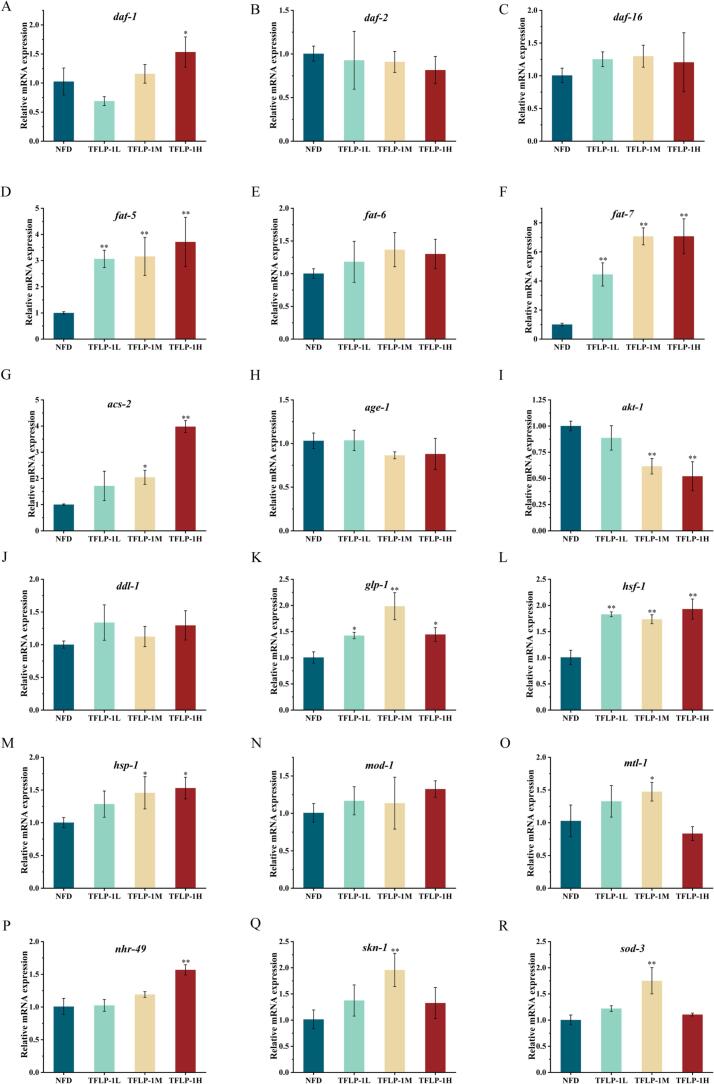
3.4. Effect of TFLP-1 on mRNA transcription in C. elegans
The transcriptional levels of signaling factors were investigated to elucidate the mechanism by which TFLP-1 enhances the antioxidant activity and stress resistance of C. elegans (Fig. 4). The insulin signaling pathway, as a key signaling pathway in the research process of antioxidant and stress resistance in C. elegans, mainly investigated the effect of TFLP-1 on the transcription levels of daf-2, age-1, ddl-1, hsf-1, daf-16, akt-1, mtl-1, and sod-3 mRNA in this study [38]. Furthermore, studies have found that the regulation of lipid metabolism pathways could affect stress resistance and antioxidant activity. Therefore, the relative transcription levels of fat-5, fat-6, fat-7, mod-1, acs-2, hsp-1, nhr-49, and daf-1 mRNA have been investigated [39]. Compared with the NFD group, the transcription levels of fat-5, fat-7, acs-2, glp-1, hsf-1, hsp-1, mtl-1, nhr-49, skn-1, and sod-3 mRNA in C. elegans supplemented with TFLP-1 were significantly upregulated, while the transcription level of akt-1 mRNA was significantly downregulated. Additionally, it demonstrated an excellent dose–effect relationship between the concentration of TFLP-1 and the transcription of fat-5, fat-7, acs-2, akt-1, and nhr-49 mRNA in C. elegans, although there was no significant difference between the TFLP-1L, TFLP-1 M, and TFLP-1H groups. However, it was found that the transcription levels of glp-1, mtl-1, skn-1 and sod-3 mRNA were upregulated relatively higher in the TFLP-1 M group compared to other groups.
Fig. 4.
Effects of different concentrations (0, 50, 100 and 200 μg/mL) of TFLP-1 on mRNA transcription levels related to stress resistance and antioxidant activity in C. elegans. The relative transcription levels of daf-1 (A), daf-2 (B), daf-16 (C), fat-5 (D), fat-6 (E), fat-7 (F), acs-2 (G), age-1 (H), akt-1 (I), ddl-1 (J), glp-1 (K), hsf-1 (L), hsp-1 (M), mod-1 (N), mtl-1 (O), nhr-49 (P), skn-1 (Q) and sod-3 (R) mRNA.
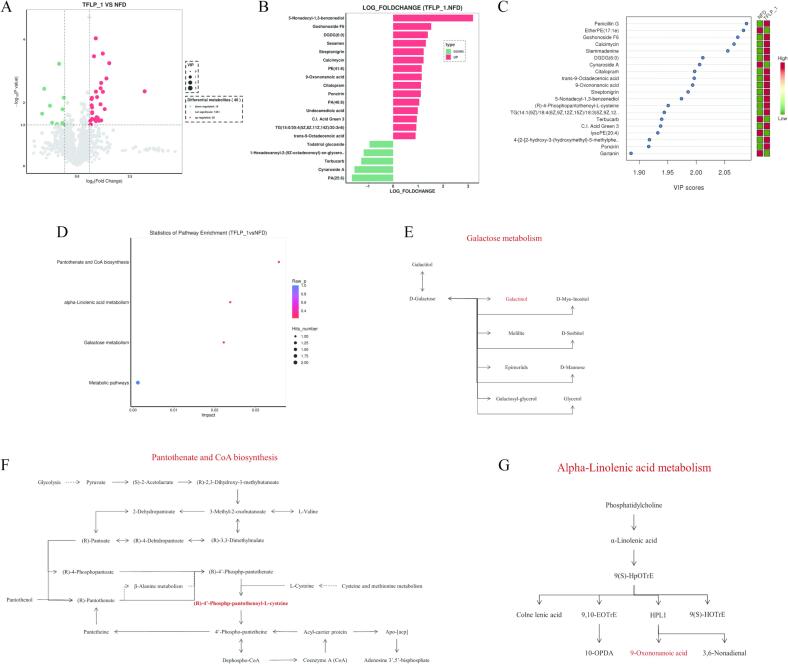
3.5. Effect of TFLP-1 on metabolites in C. elegans
The antioxidant and stress resistance were significantly enhanced in C. elegans supplemented with TFLP-1, which was not only influenced by changes in the transcription level of related mRNAs, but also affected by changes in metabolites [40], [41]. Therefore, the changes in metabolites were investigated by comparing the supplementation of different concentrations of TFLP-1 (0 and 200 μg/mL) in C. elegans. It was found that 40 metabolites (a total of 1351 metabolites) group were significantly improved in C. elegans of the TFLP-1 group compared with the NFD group, with 10 metabolites significantly decreased and 30 metabolites significantly upregulated (Fig. 5A). Based on the results of Log Fold change and VIP scores, it was found that the co-downregulated metabolites included cynaroside A and terbucarb, while the co-upregulated metabolites included trans-9-Octadecenoic acid, C.I. Acid Green 3, Poncirin, Citalopram, 9-Oxononanoic acid, Calcimycin, Streptonigrin, DGDG (6:0), Goshonoside F6 and 5-Nonadecyl-1,3-benzenediol (Fig. 5B, C). In addition, it was found that three pathways were significantly affected, which were galactose metabolism, alpha linolenic acid metabolism, and pantothenate and CoA biosynthesis metabolic pathways in C. elegans supplemented with TFLP-1 (Fig. 5D). The metabolites with significant differences of these pathways were labeled and upstream and downstream metabolites were identified (Fig. 5E-G). These results elucidated the mechanism of antioxidant activity and stress resistance in vivo of TFLP-1 at the metabolite level.
Fig. 5.
Metabolite changes in C. elegans supplemented with different concentrations of TFLP-1 (0 and 200 μg/mL). A: Volcano plot; B: The column chart of differential metabolites obtained from log (fold change) analysis, red represented upregulation and green represented downregulation. C: Differential metabolite map obtained from VIP value analysis; D: Enrichment map of differential metabolite KEGG metabolic pathway. E-G: Integration diagram of metabolic pathways associated with differential metabolites, with significant changes in metabolites shown in red.
3.6. The correlation analysis between biochemical indicators and metabolites
The impact of different differential metabolites on each indicator was explored by analyzing the correlation between antioxidant related biochemical indicators and differential metabolites, providing a reference for elucidating the mechanism of TFLP-1 in improving the antioxidant activity in C. elegans (Fig. 6). It was found that there was a significant negative correlation between T-SOD and metabolites such as todatrol glucose and terbucarb, and a significant positive correlation with metabolites such as p-Lacto N-hexose, C.I. acid green 3, calcymycin, 5-Nonadecyl-1,3-benzenediol and Penicillin G. There was a significant positive correlation between CAT and metabolites such as trans-9-octadecenoic acid and stemmadenine. GSH-Px was negatively correlated with metabolites gartanin, and positively correlated with Goshonoside F6. The significant changes of MDA were positively correlated with metabolites terbucarb), and negatively correlated with trans-9-octadecenoic acid and (R)-4-Phosphopantothenoyl-L-cysteine. In conclusion, these results indicated that different biochemical indicators were regulated by different metabolites, and elucidated the mechanism by which TFLP-1 enhanced antioxidant activity and stress resistance in C. elegans.
Fig. 6.
Analysis of the correlation between biochemical indicators related to antioxidant activity and metabolites. A: Heatmap; B: Network diagram, the data was filtered to achieve the criteria of a positive correlation threshold of ≥ 0.5, a negative correlation threshold of ≤ -0.5, and a p-value threshold of < 0.05.
4. Discussion
4.1. The degradation of high molecular weight polysaccharides could significantly improve their bioactivity
Polysaccharides extracted from natural products had large molecular weight and tight glycosidic bond, which led to active groups being surrounded and hidden, relatively low bioavailability and nutritional activity [42]. Therefore, macromolecular polysaccharides were degraded by different methods to break their glycosidic bond, expose active groups and improve their nutritional activity [43]. Generally, the degradation methods of polysaccharides included acidolysis, enzymolysis, high pressure and high temperature method, and ultrasonic assisted composite method [10], [44], [45], [46]. Although strong acidic degradation could break the glycosidic bond and reduce the molecular weight of polysaccharides, it had the disadvantage of strong corrosivity and unstable degradation, which was not considered in this study [47]. The ultrasonic-assisted H2O2-Vc method was selected as the highest level of antioxidant activity preparation method by comparing the in vivo antioxidant and stress resistance of C. elegans compared to other methods. As a strong oxidant of active oxygen source, H2O2 plays an important role in the degradation of polysaccharides [48]. At the same time, it was found that adding strong reducing substances (metal ions or Vc) to the system of hydrogen peroxide degradation of polysaccharides could activate hydrogen peroxide to generate hydroxyl radicals, formed a fenton system with stronger oxidation capacity, broke the glycosidic bond of high molecular weight polysaccharides, and accelerated the degradation of polysaccharides [26], [48]. The reason for the relatively low antioxidant activity of the polysaccharide degraded by enzymolysis in this study might be that pectinase mainly hydrolyzed pectin polysaccharide (galactose uronic acid was the main monosaccharide component), and cellulase mainly hydrolyzed cellulose polysaccharide (glucose was the main monosaccharide component), and this was consistent with the result that TFLP-1 was mainly composed of mannose and fucose through the analysis of the monosaccharide composition [49], [50]. Li et al. (2020) conducted a combined ultrasound and hydrogen peroxide treatment on TFP and found that the degraded TFP exhibited excellent antioxidant activity in vitro, which was consistent with the results in this study [51]. The molecular weight of polysaccharides decreased after degradation and their antioxidant activity increased, indicating an increase of functional activities (such as anti-ageing, immune regulation, anti-inflammatory). Antioxidation was closely related to improving health, which suggested that the multi-dimensional nutritional activity of TFLP was worth exploring [22].
4.2. TFLP-1 improved the antioxidant activity and stress resistance of C. elegans by regulating its mRNA transcription level
The molecular mechanism of TFLP-1 regulating antioxidant and stress resistance in C. elegans has been explored and discussed in this study. The insulin like signaling pathway played an important role in the antioxidant and anti-ageing activities in C. elegans [52], [53]. Moreover, daf-16, as a key signaling factor, might be a homologous gene of human FKHRL1, FKHR, and AFX [52]. It was inversely regulated by upstream signaling factors such as daf-2 and age-1, and positively affects downstream signaling factors such as sod-3 and mtl-1, played an important regulatory role in stress resistance [27]. Skn-1 was a highly conserved signaling factor related to human NRF1/2, primarily phosphorylated by the MAPK signaling pathway to activate skn-1 for antioxidant and anti-ageing activities [54]. It was found that TFLP-1 could significantly improve the transcription of skn-1 mRNA, exerted antioxidant activity and stress resistance, but there was no significant change in the transcription of daf-16 mRNA in this study, and it was consistent with the molecular mechanism research on antioxidant and anti-ageing activity in C. elegans supplemented with Paecilomyces variotii extract by Wang et al. (2022) [55]. Some studies have found that the enhancement of antioxidant activity in C. elegans was positively regulated by both daf-16 related signaling factors and skn-1 related signaling factors [28]. However, it was pointed that activation of skn-1 negatively regulated the transcription of daf-16, thereby exerted antioxidant and anti-ageing effects [55], [56], [57]. These two viewpoints are controversial and need to be verified. It was found that glucagon like peptide-1 (glp-1), as a meso-endogenous insulin stimulating hormone, had significant effects on regulating blood glucose and antioxidant, and its expression and secretion of related substances could play a neuroprotective role by forming synapses, synthesizing related proteins and autophagy [58]. There existed a close correlation between antioxidant activity and lipid metabolism regulation. Therefore, it was found that TFLP-1 could regulate the transcription levels of fat-5 and fat-7 mRNA to exert antioxidant activity, indicating the potential role of TFLP-1 in lipid metabolism regulation [59]. The increase of acs-2 mRNA transcription not only affected the antioxidant activity in C. elegans, but also might be related to lipid lowering, normal reproductive behavior and the increase of fecundity [60]. Meanwhile, akt-1 could negatively regulate cell apoptosis caused by DNA damage, which was independent of the transcription of daf-16 mRNA [61]. This confirmed that the increase in antioxidant activity was closely related to the decrease in akt-1 mRNA transcription, but not significantly related to daf-16 in this study. Under long-term mild heat stress, the lifespan of C. elegans would be shortened, and their reproductive and motor abilities would be weakened, which was related to the inhibitory transcription of hsp-1 and hsf-1 mRNA, and TFLP-1 could significantly increase the transcription levels of hsf-1 and hsp-1, exerted anti-stress activation [62]. The exploration of these gene expressions provided a theoretical basis for the molecular mechanism of TFLP-1 in enhancing the antioxidant and stress resistance of C. elegans. In addition, it highlighted potential in some related activities such as lipid metabolism regulation, proliferation promoting activity, and anti-ageing activity.
4.3. The enhancement of antioxidant activity and stress resistance of C. elegans by TFLP-1 was related to galactose metabolism, alpha linolenic acid metabolism, and pantothenate and CoA biosynthesis metabolic pathways
The ability of TFLP-1 that enhanced the antioxidant activity and stress resistance of C. elegans was confirmed in this study, and it was found that the improvement of its nutritional activity was related to galactose metabolism, alpha linolenic acid metabolism, and pantothenate and CoA biosynthesis metabolic pathways through metabolomics. The disorder of galactose metabolism led to the postpone of aging and antioxidant capacity. Therefore, the regulation of galactose metabolism could delay aging and improve related diseases [63], [64]. The improvement of galactose metabolic pathway had excellent relevance to nonalcoholic fatty liver disease, lipid metabolism disorder and inflammation [65], [66]. Alpha linolenic acid was an active substance with multiple nutritional activities, and research has found that it had significant effects in antioxidant, lipid metabolism regulation, anti-inflammatory, memory improvement, and immune enhancement, which meant that significant improvements in metabolic pathways highly correlated with the active substance had the potential for related nutritional activities [67]. In addition to the alpha linolenic acid metabolic pathway that found in this study, which was a critical metabolic pathway for antioxidant and stress resistance, Fu et al. (2023) found that Schisandra polysaccharides could significantly improve Aβ-induced cognitive impairment in rats that was significantly regulated by the alpha linolenic acid metabolite pathway [68]. Lv et al. (2022) found that the complex of Ganoderma lucidum polysaccharides and chromium had a significant impact on the regulation of glucose and lipid metabolism in high-fat and high-sugar diet rats, which was significantly influenced by the alpha linolenic acid metabolism pathway [69]. Besides, it was found that there existed a close correlation between alpha linolenic acid metabolism disorder and hyperuricemia and bone necrosis [70], [71]. Pantothenate and CoA biosynthesis metabolic pathways were found that it had a close relationship with diabetes, nephropathy and iron deficiency anemia [72], [73], [74]. In conclusion, it was found that TFLP-1 not only had significant effects on enhancing antioxidant activity and stress resistance, but also had nutritional development potential for lipid metabolism regulation, anti-inflammatory, antiviral, and immune enhancement through discussions on metabolites and metabolic pathways.
4.4. Limitations of this study and future directions for continuation
In general, the ultrasonic-assisted H2O2-Vc method was used to degrade TFP, and the TFLP-1 obtained after separation and purification had excellent in vivo antioxidant activity and stress resistance. However, the preparation methods of low molecular weight active sugars need to be explored and exploit to develop a green, environmentally friendly, low energy consumption, industrial application advantages, and stable degradation of samples. Monosaccharide components, molecular weight, and FT-IR were used for structural analysis and speculation, and modern biotechnology methods should be applied to explore the structure of TFLP and explore their structure–activity relationship with antioxidant activity. In this study, the C. elegans model was selected for exploring antioxidant activity, and the related activity of TFLP in other biological models should be explored to fully confirm its nutritional value. Meanwhile, the mechanism of TFLP enhancing the antioxidant activity and stress resistance of C. elegans was explored at the molecular level in this study, but the changes in mRNA could not fully represent the changes in protein expression. Therefore, the focus of subsequent study is to explore the effect of TFLP on the protein expression of C. elegans, and clarify its mechanism through gene deficient C. elegans. In this study, it was found that it had potential effects on lipid metabolism regulation in the mechanism antioxidant enhancing effect in C. elegans at the molecular level of TFLP. Therefore, it is necessary to discuss its nutritional value in multiple dimensions and directions to increase the added value of its products. The development of related functional foods should be carried out simultaneously. The credibility of the results should be ensured by verifying their nutritional activity through various biological models. The characteristic metabolites screened for untargeted metabolomics should be validated for their antioxidant activity through C. elegans and other biological models.
5. Conclusion
The in vivo antioxidant activities of TFLPs obtained by degrading TFP through different methods were compared through C. elegans in order to improve the high bioavailability and nutritional activity of TFP, and the most excellent preparation method for in vivo antioxidant activity and stress resistance was selected as ultrasonic-assisted H2O2-Vc method. It was found that TFLP-1 was an acidic polysaccharide with a molecular weight of 75770 Da, which mainly composed of mannose after the polysaccharide was isolated, purified, and structurally identified. At the same time, it was found that the polysaccharide had benign antioxidant activity and stress resistance in C. elegans. The in vivo antioxidant mechanism of TFLP-1 has been explored through gene expression and untargeted metabolomic analysis. TFLP-1 enhanced the antioxidant activity and stress resistance of C. elegans by significantly upregulating the transcription of fat-5, fat-7, acs-2, glp-1, hsf-1, hsp-1, mtl-1, nhr-49, skn-1 and sod-3 mRNA, and significantly downregulating the transcription of akt-1 mRNA. Additionally, it was found that galactose metabolism, alpha linolenic acid metabolism, and pantothenate and CoA biosynthesis metabolic pathways were key metabolic pathways for enhancing antioxidant and stress resistance in C. elegans supplemented with TFLP-1. These results provided a reference for the development of TFLP as an active substance in functional foods.
CRediT authorship contribution statement
Quancen Lee: Methodology, Investigation, Writing – original draft. Xianjing Han: Formal analysis. Mingfeng Zheng: Supervision. Feng Lv: Supervision. Bin Liu: Supervision. Feng Zeng: Supervision, Conceptualization, Writing – review & editing, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project was funded by Science and Technology Planning Project of Fujian Province (2021L3007).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2023.106555.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Gao L., Wang Y., Zhang F., Li S., Zhao J., Zhang Q., Ye J., Ma Y., Wang Z., Chen W. A standardized method for the quantification of polysaccharides: an example of polysaccharides from tremella fuciformis. LWT. 2022;167 [Google Scholar]
- 2.Zhang S., Xu X., Cao X., Liu T. The structural characteristics of dietary fibers from Tremella fuciformis and their hypolipidemic effects in mice. Food Sci. Human Wellness. 2023;12(2):503–511. [Google Scholar]
- 3.Yuan H., Dong L., Zhang Z., He Y., Ma X. Production, structure, and bioactivity of polysaccharide isolated from tremella fuciformis. Food Sci. Human Wellness. 2022;11(4):1010–1017. [Google Scholar]
- 4.Xiao H., Li H., Wen Y., Jiang D., Zhu S., He X., Xiong Q., Gao J., Hou S., Huang S., He L., Liang J. Tremella fuciformis polysaccharides ameliorated ulcerative colitis via inhibiting inflammation and enhancing intestinal epithelial barrier function. Int. J. Biol. Macromol. 2021;180:633–642. doi: 10.1016/j.ijbiomac.2021.03.083. [DOI] [PubMed] [Google Scholar]
- 5.Ge X., Huang W., Xu X., Lei P., Sun D., Xu H., Li S. Production, structure, and bioactivity of polysaccharide isolated from tremella fuciformis xy. Int. J. Biol. Macromol. 2020;148:173–181. doi: 10.1016/j.ijbiomac.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Zhang Z., Zhao M. Carboxymethylation of polysaccharides from tremella fuciformis for antioxidant and moisture-preserving activities. Int. J. Biol. Macromol. 2015;72:526–530. doi: 10.1016/j.ijbiomac.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 7.Naliyadhara N., Kumar A., Kumar Gangwar S., Nair Devanarayanan T., Hegde M., Alqahtani M.S., Abbas M., Sethi G., Kunnumakkara A. Interplay of dietary antioxidants and gut microbiome in human health: what has been learnt thus far? J. Funct. Food. 2023;100 [Google Scholar]
- 8.Xu J., Zou Y., Guo L., Lin J., Jiang Z., Zheng Q. Rheological and microstructural properties of polysaccharide obtained from the gelatinous Tremella fuciformis fungus. Int. J. Biol. Macromol. 2023;228:153–164. doi: 10.1016/j.ijbiomac.2022.12.214. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Zhou X., Sheng L., Zhang D.i., Zheng X., Pan Y., Yu X., Liang X., Wang Q.i., Wang B., Li N.a. Effect of ultrasonic degradation on the structural feature, physicochemical property and bioactivity of plant and microbial polysaccharides: a review. Int. J. Biol. Macromol. 2023;236:123924. doi: 10.1016/j.ijbiomac.2023.123924. [DOI] [PubMed] [Google Scholar]
- 10.Chen X., Sun-Waterhouse D., Yao W., Li X., Zhao M., You L. Free radical-mediated degradation of polysaccharides: mechanism of free radical formation and degradation, influence factors and product properties. Food Chem. 2021;365 doi: 10.1016/j.foodchem.2021.130524. [DOI] [PubMed] [Google Scholar]
- 11.Yiasmin M.N., Islam M.S., Easdani M.d., Yang R., Yanjun T., Hua X. Fermentability of maitake polysaccharides processed by various hydrothermal conditions and fermented with probiotic (Lactobacillus) Int. J. Biol. Macromol. 2022;209:1075–1087. doi: 10.1016/j.ijbiomac.2022.04.084. [DOI] [PubMed] [Google Scholar]
- 12.Liochev S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Sharma V., Mehdi M.M. Oxidative stress, inflammation and hormesis: the role of dietary and lifestyle modifications on aging. Neurochem. Int. 2023;164 doi: 10.1016/j.neuint.2023.105490. [DOI] [PubMed] [Google Scholar]
- 14.Amir Aslani B., Ghobadi S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016;146:163–173. doi: 10.1016/j.lfs.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Duan H., Pan J., Guo M., Li J., Yu L., Fan L. Dietary strategies with anti-aging potential: dietary patterns and supplements. Food Res. Int. 2022;158 doi: 10.1016/j.foodres.2022.111501. [DOI] [PubMed] [Google Scholar]
- 16.Zeng F., Yao Y., Wang L., Li W. Polysaccharides as antioxidants and prooxidants in managing the double-edged sword of reactive oxygen species. Biomed. Pharmacother. 2023;159 doi: 10.1016/j.biopha.2023.114221. [DOI] [PubMed] [Google Scholar]
- 17.Yarley O.P.N., Kojo A.B., Zhou C., Yu X., Gideon A., Kwadwo H.H., Richard O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021;183:2262–2271. doi: 10.1016/j.ijbiomac.2021.05.181. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Wang Y., Li P., Zhou Q., Zheng X., Gu Q. Caenorhabditis elegans: a nature present for advanced food science. Curr. Opin. Food Sci. 2023;49 [Google Scholar]
- 19.Mudd N., Liceaga A.M. Caenorhabditis elegans as an in vivo model for food bioactives: a review. Curr. Res. Food Sci. 2022;5:845–856. doi: 10.1016/j.crfs.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q., Xiao M., Li N.a., Cai W., Zhao C., Liu B., Zeng F. Application of Caenorhabditis elegans in the evaluation of food nutrition: A review. eFood. 2023;4(1):e68. [Google Scholar]
- 21.Li Q., Li N., Cai W., Xiao M., Liu B., Zeng F. Fermented natural product targeting gut microbiota regulate immunity and anti-inflammatory activity: a possible way to prevent Covid-19 in daily diet. J. Funct. Food. 2022;97 doi: 10.1016/j.jff.2022.105229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang D., Liu J., Cheng H., Wang H., Tan Y., Feng W., Peng C. Interactions between polysaccharides and gut microbiota: a metabolomic and microbial review. Food Res. Int. 2022;160 doi: 10.1016/j.foodres.2022.111653. [DOI] [PubMed] [Google Scholar]
- 23.Wu D., An L., Liu W., Hu Y., Wang S., Zou L. In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food Res. Int. 2022;156 doi: 10.1016/j.foodres.2022.111185. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X., Liu Y., Chen X., Aweya J.J., Cheong K. Catabolism of Saccharina japonica polysaccharides and oligosaccharides by human fecal microbiota. LWT. 2020;130 [Google Scholar]
- 25.T. Fang, X. Zhang, S. Hu, Y. Yu, X. Sun, N. Xu, Enzymatic degradation of Gracilariopsis lemaneiformis polysaccharide and the antioxidant activity of its degradation products. 19 (2021) 270. [DOI] [PMC free article] [PubMed]
- 26.Yan S., Pan C., Yang X., Chen S., Qi B., Huang H. Degradation of Codium cylindricum polysaccharides by H2O2-Vc-ultrasonic and H2O2-Fe2+-ultrasonic treatment: structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2021;182:129–135. doi: 10.1016/j.ijbiomac.2021.03.193. [DOI] [PubMed] [Google Scholar]
- 27.Li N., Li Q., He X., Gao X., Wu L., Xiao M., Cai W., Liu B., Zeng F. Antioxidant and anti-aging activities of Laminaria japonica polysaccharide in Caenorhabditis elegans based on metabonomic analysis. Int. J. Biol. Macromol. 2022;221:346–354. doi: 10.1016/j.ijbiomac.2022.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Wang S., Yang Y., Xiao D., Zheng X., Ai B., Zheng L., Sheng Z. Polysaccharides from banana (Musa spp.) Blossoms: isolation, identification and anti-glycation effects. Int. J. Biol. Macromol. 2023;236 doi: 10.1016/j.ijbiomac.2023.123957. [DOI] [PubMed] [Google Scholar]
- 29.Kim J., Kim S., Park S. Selenocysteine modulates resistance to environmental stress and confers anti-aging effects in C. elegans. Clinics. 2017;72(8):491–498. doi: 10.6061/clinics/2017(08)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q., Wu J., Huang J., Yang L., Tao J., Nie J., Zhao J., Wang Y. Cremastra appendiculata polysaccharides improve stress resistance and prolong the lifespan of Caenorhabditis elegans via daf-16 in the insulin signaling pathway. Int. J. Biol. Macromol. 2023;229:496–506. doi: 10.1016/j.ijbiomac.2022.12.234. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Sun Y., Wang X., Wang Y., Liao L., Zhang Y., Fang B., Fu Y. Novel antioxidant peptides from yak bones collagen enhanced the capacities of antiaging and antioxidant in Caenorhabditis elegans. J. Funct. Food. 2022;89 [Google Scholar]
- 32.Zhang Y., Han Y., He J., Ouyang K., Zhao M., Cai L., Zhao Z., Meng W., Chen L., Wang W. Digestive properties and effects of Chimonanthus nitens oliv polysaccharides on antioxidant effects in vitro and in immunocompromised mice. Int. J. Biol. Macromol. 2021;185:306–316. doi: 10.1016/j.ijbiomac.2021.06.114. [DOI] [PubMed] [Google Scholar]
- 33.Hu T., Zhu W., Yu Y., Zou B., Xu Y., Xiao G., Wu J. The variation on structure and immunomodulatory activity of polysaccharide during the Longan pulp fermentation. Int. J. Biol. Macromol. 2022;222:599–609. doi: 10.1016/j.ijbiomac.2022.09.195. [DOI] [PubMed] [Google Scholar]
- 34.Wang K., Yang C., Yan S., Wang H., Cao X., Cheng Y. Dendrobium hancockii polysaccharides, structure characterization, modification, antioxidant and antibacterial activity. Ind. Crop Prod. 2022;188 [Google Scholar]
- 35.Hojjati M., Noshad M., Sorourian R., Askari H., Feghhi S. Effect of gamma irradiation on structure, physicochemical and functional properties of bitter vetch (vicia ervilia) seeds polysaccharides. Radiat. Phys. Chem. 2023;202 [Google Scholar]
- 36.Gan T., Feng C., Lan H., Yang R., Zhang J., Li C., Li W. Comparison of the structure and immunomodulatory activity of polysaccharides from fresh and dried longan. J. Funct. Food. 2021;76 [Google Scholar]
- 37.Feng J., Tian H., Chen X.u., Cai X., Shi X., Wang S. Interaction between fish gelatin and tremella polysaccharides from aqueous solutions to complex coacervates: structure and rheological properties. Food Hydrocoll. 2023;138:108439. [Google Scholar]
- 38.Xiao Y., Zhang L., Zhu X., Qin Y., Yu C., Jiang N., Li S., Liu F., Liu Y. Luteolin promotes pathogen resistance in Caenorhabditis elegans via daf-2/daf-16 insulin-like signaling pathway. Int. Immunopharmacol. 2023;115 doi: 10.1016/j.intimp.2023.109679. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Shi J., Liu K., Wang Y., Xu Y., Liu Y. Metabolomics and gene expression levels reveal the positive effects of teaseed oil on lifespan and aging process in Caenorhabditis elegans. Food Sci. Human Wellness. 2023;12(4):1391–1401. [Google Scholar]
- 40.Huang Z., Chen M., Guo W., Li T., Liu B., Bai W., Ai L., Rao P., Ni L., Lv X. Monascus purpureus-fermented common buckwheat protects against dyslipidemia and non-alcoholic fatty liver disease through the regulation of liver metabolome and intestinal microbiome. Food Res. Int. 2020;136 doi: 10.1016/j.foodres.2020.109511. [DOI] [PubMed] [Google Scholar]
- 41.Zong S., Ye H., Ye Z., He Y., Zhang X., Ye M. Polysaccharides from lachnum sp. Inhibited colitis-associated colon tumorigenesis in mice by modulating fecal microbiota and metabolites. Int. Immunopharmacol. 2022;108 doi: 10.1016/j.intimp.2022.108656. [DOI] [PubMed] [Google Scholar]
- 42.Su Y., Li H., Hu Z., Zhang Y., Guo L., Shao M., Man C., Jiang Y. Research on degradation of polysaccharides during Hericium erinaceus fermentation. LWT. 2023;173 [Google Scholar]
- 43.Deng C., Zhao M.X., Zhao Q., Zhao L.M. Advances in green bioproduction of marine and glycosaminoglycan oligosaccharides. Carbohydr. Polym. 2023;300 doi: 10.1016/j.carbpol.2022.120254. [DOI] [PubMed] [Google Scholar]
- 44.Zheng L., Ma Y., Zhang Y., Meng Q., Yang J., Wang B., Liu Q., Cai L., Gong W., Yang Y., Shi J. Increased antioxidant activity and improved structural characterization of sulfuric acid-treated stepwise degraded polysaccharides from Pholiota nameko pn-01. Int. J. Biol. Macromol. 2021;166:1220–1229. doi: 10.1016/j.ijbiomac.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Qian W.W., Yang S.Q., Hu S.M., Wang X.L., Zhu Y.Z., Zhou T. Enzymatic degradation, antioxidant and immunoregulatory activities of polysaccharides from brown algae Sargassum fusiforme. J. Food Meas. Charact. 2021;15(2):1960–1972. [Google Scholar]
- 46.Wu Q., Qin D.D., Cao H.X., Bai Y. Enzymatic hydrolysis of polysaccharide from Auricularia auricula and characterization of the degradation product. Int. J. Biol. Macromol. 2020;162:127–135. doi: 10.1016/j.ijbiomac.2020.06.098. [DOI] [PubMed] [Google Scholar]
- 47.Hong Y., Wu Y.-R. Acidolysis as a biorefinery approach to producing advanced bioenergy from macroalgal biomass: a state-of-the-art review. Bioresour. Technol. 2020;318:124080. doi: 10.1016/j.biortech.2020.124080. [DOI] [PubMed] [Google Scholar]
- 48.Xu K., Jinfeng Dou C.W., Fan G., Li X., Sun W., Suo A., Li Z., Zhang L. Effects of ultrasound-assisted fenton treatment on structure and hypolipidemic activity of apricot polysaccharides. Food Biosci. 2022;50 [Google Scholar]
- 49.Zha X.Q., Li X.L., Zhang H.L., Cui S.H., Liu J., Wang J.H., Pan L.H., Luo J.P. Pectinase hydrolysis of Dendrobium huoshanense polysaccharide and its effect on protein nonenzymatic glycation. Int. J. Biol. Macromol. 2013;61:439–447. doi: 10.1016/j.ijbiomac.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Qing Q., Wyman C.E. Hydrolysis of different chain length xylooliogmers by cellulase and hemicellulase. Bioresour. Technol. 2011;102(2):1359–1366. doi: 10.1016/j.biortech.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Li M., Ma F., Li R., Ren G., Yan D., Zhang H., Zhu X., Wu R., Wu J. Degradation of Tremella fuciformis polysaccharide by a combined ultrasound and hydrogen peroxide treatment: process parameters, structural characteristics, and antioxidant activities. Int. J. Biol. Macromol. 2020;160:979–990. doi: 10.1016/j.ijbiomac.2020.05.216. [DOI] [PubMed] [Google Scholar]
- 52.R.Y. Lee, J. Hench, G. Ruvkun, Regulation of C. Elegans daf-16 and its human ortholog fkhrl1 by the daf-2 insulin-like signaling pathway, Current biology: CB 11 (24) (2001) 1950-1957. [DOI] [PubMed]
- 53.Li Y.H., Zhang G.G. Towards understanding the lifespan extension by reduced insulin signaling: bioinformatics analysis of daf-16/foxo direct targets in Caenorhabditis elegans. Oncotarget. 2016;7(15):19185–19192. doi: 10.18632/oncotarget.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C., Karakuzu O., Garsin D.A. Tribbles pseudokinase nipi-3 regulates intestinal immunity in Caenorhabditis elegans by controlling skn-1/Nrf activity. Cell Rep. 2021;36(7):109529. doi: 10.1016/j.celrep.2021.109529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y., Liu H., Fu G., Li Y., Ji X., Zhang S., Qiao K. Paecilomyces variotii extract increases lifespan and protects against oxidative stress in Caenorhabditis elegans through skn-1, but not daf-16. Arab. J. Chem. 2022;15(9):104073. [Google Scholar]
- 56.Havermann S., Humpf H., Wätjen W. Baicalein modulates stress-resistance and life span in C. elegans via skn-1 but not daf-16. Fitoterapia. 2016;113:123–127. doi: 10.1016/j.fitote.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Deng J.H., Dai Y.X., Tang H.Q., Pang S.S. Skn-1 is a negative regulator of daf-16 and somatic stress resistance in Caenorhabditis elegans. G3-Genes Genomes Genet. 2020;10(5):1707–1712. doi: 10.1534/g3.120.401203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhalla S., Mehan S., Khan A., Rehman M.U. protective role of igf-1 and glp-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 2022;142 doi: 10.1016/j.neubiorev.2022.104896. [DOI] [PubMed] [Google Scholar]
- 59.Chaturvedi S., Gupta P. Functional components in extracts of beta vulgaris (chukandar) parts for antioxidant effect and antiobesity potential with lipase inhibition. Food Biosci. 2021;41 [Google Scholar]
- 60.Zhang J., Bakheet R., Parhar R.S., Huang C.H., Hussain M.M., Pan X.Y., Siddiqui S.S., Hashmi S. Regulation of fat storage and reproduction by kruppel-like transcription factor klf3 and fat-associated genes in Caenorhabditis elegans. J. Mol. Biol. 2011;411(3):537–553. doi: 10.1016/j.jmb.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quevedo C., Kaplan D.R., Derry W.B. Akt-1 regulates DNA-damage-induced germline apoptosis in C. elegans. Curr. Biol. 2007;17(3):286–292. doi: 10.1016/j.cub.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 62.Plagens R.N., Mossiah I., Guisbert K., Guisbert E. Chronic temperature stress inhibits reproduction and disrupts endocytosis via chaperone titration in Caenorhabditis elegans. BMC Biol. 2021;19(1) doi: 10.1186/s12915-021-01008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen H., Dong L., Chen X., Ding C., Hao M., Peng X., Zhang Y., Zhu H., Liu W. Anti-aging effect of phlorizin on d-galactose–induced aging in mice through antioxidant and anti-inflammatory activity, prevention of apoptosis, and regulation of the gut microbiota. Exp. Gerontol. 2022;163 doi: 10.1016/j.exger.2022.111769. [DOI] [PubMed] [Google Scholar]
- 64.Zhou X., Sun H., Tan F., Yi R., Zhou C., Deng Y., Mu J., Zhao X. Anti-aging effect of lactobacillus plantarum hfy09-fermented soymilk on d-galactose-induced oxidative aging in mice through modulation of the nrf2 signaling pathway. J. Funct. Food. 2021;78 [Google Scholar]
- 65.Li T., Bai J., Du Y., Tan P., Zheng T., Chen Y., Cheng Y., Cai T., Huang M., Fu W., Wen J. Thiamine pretreatment improves endotoxemia-related liver injury and cholestatic complications by regulating galactose metabolism and inhibiting macrophage activation. Int. Immunopharmacol. 2022;108 doi: 10.1016/j.intimp.2022.108892. [DOI] [PubMed] [Google Scholar]
- 66.Wang R., Wang L., Wu H., Zhang L., Hu X., Li C., Liu S. Noni (Morinda citrifolia L.) Fruit phenolic extract supplementation ameliorates NAFLD by modulating insulin resistance, oxidative stress, inflammation, liver metabolism and gut microbiota. Food Res. Int. 2022;160 doi: 10.1016/j.foodres.2022.111732. [DOI] [PubMed] [Google Scholar]
- 67.Kim K., Nam Y.A., Kim H.S., Hayes A.W., Lee B. α-linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014;70:163–178. doi: 10.1016/j.fct.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Fu J., Li J.X., Sun Y.Z., Liu S., Song F.R., Liu Z.Y. An integrated study on the comprehensive mechanism of schisandra chinensis polysaccharides mitigating alzheimer's disease in rats using a uplc-q-tof-ms based serum and urine metabolomics strategy. Food Funct. 2023;14(2):734–745. doi: 10.1039/d2fo02842e. [DOI] [PubMed] [Google Scholar]
- 69.Lv X.C., Wu Q., Yuan Y.J., Li L., Guo W.L., Lin X.B., Huang Z.R., Rao P.F., Ai L.Z., Ni L. Organic chromium derived from the chelation of ganoderma lucidum polysaccharide and chromium (iii) alleviates metabolic syndromes and intestinal microbiota dysbiosis induced by high-fat and high-fructose diet. Int. J. Biol. Macromol. 2022;219:964–979. doi: 10.1016/j.ijbiomac.2022.07.211. [DOI] [PubMed] [Google Scholar]
- 70.Qin N., Jiang Y., Shi W., Wang L., Kong L., Wang C., Guo Y., Zhang J., Ma Q., Lee H. High-throughput untargeted serum metabolomics analysis of hyperuricemia patients by uplc-q-tof/ms. Evid. -Based Complement Altern. Med. 2021;2021:1–15. doi: 10.1155/2021/5524772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ren X.N., Shao Z.X., Fan W., Wang Z.X., Chen K.Y., Yu X.F. Untargeted metabolomics reveals the effect of lovastatin on steroid-induced necrosis of the femoral head in rabbits. J. Orthop. Surg. Res. 2020;15(1) doi: 10.1186/s13018-020-02026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma T., Liu T., Xie P., Jiang S., Yi W., Dai P., Guo X. Uplc-ms-based urine nontargeted metabolic profiling identifies dysregulation of pantothenate and coa biosynthesis pathway in diabetic kidney disease. Life Sci. 2020;258:118160. doi: 10.1016/j.lfs.2020.118160. [DOI] [PubMed] [Google Scholar]
- 73.Tian C.Y., Qiu M.Y., Lv H.Z., Yue F., Zhou F.F. Preliminary serum and fecal metabolomics study of spontaneously diabetic cynomolgus monkeys based on lc-ms/ms. J. Med. Primatol. 2022;51(6):355–366. doi: 10.1111/jmp.12610. [DOI] [PubMed] [Google Scholar]
- 74.He H., An F., Huang Q., Kong Y., He D., Chen L., Song H. Metabolic effect of aos-iron in rats with iron deficiency anemia using lc-ms/ms based metabolomics. Food Res. Int. 2020;130:108913. doi: 10.1016/j.foodres.2019.108913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.