Highlights
-
•
A new waikavirus identified in carrots putatively named CPBAV.
-
•
The psyllid Bactericera trigonica Hodkinsonis is the vector for CPBAV transmission.
-
•
CPBAV localized in the phloem replicated in carrots but not in the psyllid vector.
-
•
CPBAV infected Datura innoxia belonging to the Solanaceae family.
Keywords: Bactericera trigonica, Carrot yellows disease, Phloem-limited
Abstract
Carrots collected from the Western Negev region in Israel during the winter of 2019 showed disease symptoms of chlorosis, leaf curling, a loss of apical dominance, and multiple lateral roots that were not associated with known pathogens of the carrot yellows disease. Symptomatic carrots were studied for a possible involvement of plant viruses in disease manifestations using high throughput sequencing analyses. The results revealed the presence of a waikavirus, sharing a ∼70% nucleotide sequence identity with Waikavirus genus members. Virions purified from waikavirus-positive carrots were visualized by transmission electron microscopy, showing icosahedral particle diameter of ∼28 nm. The genome sequence was validated by overlapping amplicons by designed 12 primer sets. A complete genome sequence was achieved by rapid amplification of cDNA ends (RACE) for sequencing the 5′ end, and RT-PCR with oligo dT for sequencing the 3′ end. The genome encodes a single large ORF, characteristic of waikaviruses. Aligning the waikavirus-deduced amino-acid sequence with other waikavirus species at the Pro-Pol region, a conserved sequence between the putative proteinase and the RNA-dependent RNA polymerase, showed a ∼40% identity, indicating the identification of a new waikavirus species. The amino-acid sequence of the three coat proteins and cleavage sites were experimentally determined by liquid chromatography-mass spectrometry. A phylogenetic analysis based on the Pro-Pol region revealed that the new waikavirus clusters with persimmon waikavirus and actinidia yellowing virus 1. The new waikavirus genome was localized in the phloem of waikavirus-infected carrots. The virus was transmitted to carrot and coriander plants by the psyllid Bactericera trigonica Hodkinson (Hemiptera: Triozidae).
Graphical abstract
1. Introduction
Waikaviruses of the Secoviridae family (order Picornavirales) are phloem-limited vector-transmitted viruses known to be transmitted by aphids or leafhoppers in a semi-persistent manner (Sanfaçon et al., 2009). Waikavirus transmission could involve a virus-encoded helper protein that is abundant in the host phloem (Hunt et al., 1988). Several of the virus species belonging to the Waikavirus genus cause damage to crops, such as the maize chlorotic dwarf virus (MCDV), which causes a disease in corn (Nault et al., 1973). Some waikaviruses are helper viruses such as the rice tungro spherical virus (RTSV), which associates with rice tungro bacilliform virus (RTBV), which together cause the rice tungro disease (Hibino, 1983). Several newly reported waikaviruses have no known pathogenic effect, such as brassica napus RNA virus 1 (BnRV1), identified in a transcriptome of Brassica napus plants (Park and Hahn, 2019). The host range of the virus genus currently includes seven families: Poaceae (Maclot et al., 2021; Shen et al., 1992; Thole and Hull, 1996), Campanulaceae (Seo et al., 2015), Fabaceae (Koloniuk and Fránová, 2018), Ebenaceae (Ito and Sato, 2020), Brassicaceae (Park and Hahn, 2019), Grossulariaceae (Thekke-Veetil et al., 2020) and Apiaceae (Murant and Roberts, 1977).
Carrot crops (Daucus carota L.) of the Apiaceae family, primarily grown for consumption, are among the top ten important vegetable crops in the world. Carrot cultivation in Israel is on the rise, with the production of approximately 35,000 tons per year, of which 60% are designated to export, according to the Israeli Ministry of Agriculture and Rural Development https://www.gov.il/en/departments/ministry_of_agriculture_and_rural_development/govil-landing-page (accessed on 28.3.2023). Carrot yellows disease in Israel was associated with several disease-causing agents. Phytoplasma (Orenstein et al., 1999), spiroplasma (Gera et al., 2011) and a viral pathogen carrot red leaf virus, a polerovirus belonging to the Solemoviridae family (Marco, 1993), were identified as causal agents. However, in the recent years, new disease symptoms in carrots emerged, which were not associated with known carrot yellows disease-causing agents. The disease symptoms included leaf curling, chlorosis, aberrant coloring, a loss of apical dominance and multiple lateral roots. A tight association was found between Candidatus Liberibacter solanacearum, established in carrot plots since 2015, and the carrot yellows disease (Mawassi et al., 2018). Since 2019, we have detected symptomatic carrots that were negative for Ca. L. solanacearum in open fields in the Western Negev region of Israel, particularly during the winter months (October-January). The possibility that additional pathogens were involved in disease manifestations was studied. Analyses of symptomatic carrots subjected to high throughput sequencing (HTS) revealed the presence of a new virus in the Waikavirus genus. We focused on characterizing the new carrot waikavirus and studied the involvement of an insect vector in virus transmission.
2. Materials and methods
2.1. Collection of symptomatic carrot plants, local vegetation, and insects
Symptomatic carrot plants manifesting symptoms of curled leaves, chlorosis, abnormal coloring, a loss of apical dominance and multiple lateral roots were collected from commercial carrot plots in the Western Negev, Israel during 2019. Whole carrot plants and the local vegetation were uprooted and replanted in a net house in Volcani centre, agricultural research organization (ARO) for diagnostic work. Psyllids were captured in symptomatic carrot plots and their vicinity. The collected psyllids (∼50 individuals in each of seven sampled populations) were kept at −20 °C for further analyses.
2.2. Viral RNA extractions from plant and insect samples and RT-PCR
Fresh carrot leaf blades and petioles (∼10 g) and 5–7 individual psyllids, from each population, were subjected to viral RNA extractions. All samples were treated with the AccuPrep® Viral RNA extraction kit (Bioneer, Daejeon, Korea) according to the manufacturer's specifications. RNA concentrations were measured using the NanoDrop™ Spectrophotometer (Thermo Scientific, USA). From each sample, 400–500 ng of RNA were used as a reverse transcriptase (RT) reaction template, performed with the qPCRBIO cDNA Synthesis kit (PCR Biosystems, London, UK). The complementary DNA (cDNA) was used for subsequent amplification by PCR using PCRBIO HS VeriFi™ Mix Red (PCR Biosystems, London, UK). For regular diagnosis of plant and insect samples, primer set 10 (with an amplicon of 1066 bp) (Supplementary Table 1) was used: F 5′ GCAAAGTGACAAGTGCCCTA 3′ and R 5′ CTCCAGAGTATCTACCCAT 3′.
The amplification products were separated on a 1% agarose gel along with 1 KB plus DNA Ladder RTU (Bio-Helix, New Taipei, Taiwan). DNA was extracted with the Zymoclean Gel DNA Recovery™ kit (Zymo Research, California, USA) and sequenced by Sanger analysis (Hylabs, Rehovot, Israel).
2.3. HTS analyses
Viral RNA extractions were prepared from symptomatic carrot plants, virions purified from virus-positive carrots (see below), and field-collected psyllids. The extracted viral RNA was sequenced using Illumina Hiseq2500 (50 cycles) (Technion Genome Center, Haifa, Israel). Low-quality sequences were filtered and trimmed using Trimmomatic version 0.39 (Bolger et al., 2014). Obtained clean reads were searched for matched viral sequences using VirusDetect software version 1.7 (Zheng et al., 2017). VirusDetect software employed a pipeline that combined de novo assembly using Velvet software (Zerbino, 2010) with mapping to plant viral references from Genbank utilizing Velvet and the Burrows-Wheeler Alignment tool (BWA) (Houtgast et al., 2018) for mapping the reads. To compare results, we also used Trinity software (Haas et al., 2013) for de novo assembly of the reads, and then Diamond Blastx searches (Buchfink et al., 2015) against the NCBI protein non-redundant database to annotate the assembly (Geer et al., 2010). The depth coverage of the viral contigs was calculated using Bowtie2 alignment (Langmead and Salzberg, 2012) and Samtools version 1.7 (Danecek et al., 2021).
2.4. Confirmation of cDNA ends
To confirm the 3′ UTR sequence obtained by the HTS analysis (excluding the poly-A sequence at the 3′ end), RT was conducted using a Maxima Reverse Transcriptase cDNA Kit (Thermo Fisher Scientific) and an oligo dT primer. The RT reaction product was used as a template for RT-PCR using Phusion High-Fidelity DNA Polymerase (Thermo Fisher Scientific), an oligo dT, and the viral-specific primer: F-11,439 (Supplementary Table 1). The resulting amplicon was Sanger sequenced using F-11,439 and oligo dT primers (HyLabs, Rehovot, Israel).
For the 5′ UTR amplification, a touchdown reaction was performed with the SMARTer® rapid amplification of cDNA ends (RACE) 5′/3′ Kit (Takara, California, USA) according to the manufacturer's manual, using three genome-specific primers R-946, R-435, and R-324 (Supplementary Table 1). The products, separated on a 1% agarose gel, were extracted, and following a blunt-end truncation, ligated into the pJET1.2 vector. The plasmid was cloned into DH5-alpha-competent cells to create a DNA library. Based on the length of the PCR products, colonies were screened by PCR with the pJET1.2 specific primers to find colonies that contained the inserts. Positive colonies were sent for Sanger sequencing. Virus-positive plants' viral RNA was then tested by RT-PCR using primer set 1 (Supplementary Table 1) to validate the 5′ end amplicons. Based on the HTS sequence and 5′ and 3′ UTR sequences, specific primers were designed to amplify overlapping genome segments (Supplementary Table 1). The RT-PCR products were Sanger sequenced, and the viral genome was assembled.
2.5. Genome organization and conserved motif mapping
The assembled viral genome was analysed using the NCBI ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) to identify open reading frames (ORFs). A single large ORF that starts at nt 557 and ends at nt 11,209, 10,653 nts long, 3550 amino acids (aa) was identified, typical of waikaviruses. The deduced ORF aa sequence was aligned with similar sequences of closely related viruses: bellflower vein chlorosis virus (BVCV) (NCBI accession No. NC_027915) and RTSV (NCBI accession No. MK655459.1) to confirm the selection of the correct ORF. The locations of coat-proteins (CPs) were experimentally determined by aa sequencing (see below).
Conserved motifs, homologous to picorna-like plant viruses (Chaouch et al., 2004; Gorbalenya et al., 1989; Momoeda et al., 1994; Poch et al., 1989; Walker et al., 1982), were searched in the deduced aa sequence of the new virus and other waikaviruses. The sequences were aligned using the MEGA-X (Version 10.2.2) software, and the motifs were mapped.
2.6. Virion purification and transmission electron microscopy (TEM)
To purify virions, 140 g of fresh waikavirus-positive carrot roots were pulverized with 150 ml of 0.1 M potassium phosphate buffer (pH=7) and 0.2 g of sodium sulfite. The suspension was stirred, and 30 ml each of chloroform and n‑butyl alcohol added. The suspension was stirred at 4 °C for 1 hour. The suspension was then centrifuged for 20 min at 6 °C at a speed of 12,000 rpm (22,000 g). The supernatant was filtered once through Miracloth (Calbiochem, California, USA) and centrifuged using SW 55 Ti rotor (Beckman Coulter, California, USA) for 2.5 h at 6 °C at 45,000 rpm (246,000 g). The pellets were re-suspended in a total volume of 360 µL, which was micro-centrifuged at 7000 rpm for 5 min. The virus suspension was collected. The virion suspension was subjected to viral RNA extraction using AccuPrep® Viral RNA extraction kit and tested by RT-PCR with primer set 10 (Supplementary Table 1) to confirm the presence of the waikavirus genome. The purified virions were subjected to TEM visualization. The suspension (5 µL) was loaded on a 300-mesh carbon-coated copper grid and incubated for 3 min. Excess liquid was absorbed using Whatman paper and samples were washed with a drop of distilled water. The sample was then fixed with 2% paraformaldehyde and stained with a drop of 1% phosphotungstic acid for 30 s. After air-drying samples were visualized under a Tecani T12 transmission electron microscope (Thermo FisherFEI-Philips). The lengths of viral particles were measured by stretching a line from end to end.
2.7. Characterization of the coat proteins and amino acid sequence determination
Purified virion preparation was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) to separate the coat proteins and estimate their molecular mass. The separation was done on a 15% polyacrylamide gel along with ExcelBand™ 3-color Pre-Stained Protein Ladder (Smobio Technology, Hsinchu, Taiwan) size marker, using the Mini-PROTEAN 3 Dodeca electrophoresis chamber (Bio-Rad, California, USA). Protein staining was done using Coomassie brilliant blue (R-250, Sigma-Aldrich). Separated protein bands were cut out and sent for aa sequencing by liquid chromatography-mass spectrometry (LC-MS) (The Smoler Protein Research Center, Haifa, Israel). The obtained peptides were compared with the deduced aa sequence of the waikavirus ORF1.
2.8. Fluorescence in situ hybridization (FISH)
For FISH analysis, a specific fluorophore cyanine-Cy3 probe was designed based on the specific reverse complementary primer R-11,876 (Supplementary Table 1). The probe was used to detect viral localization in plant tissues using FISH. A leaf petiole of a waikavirus-positive carrot plant was thinly sliced, longitudinally and transversally. Similar cuts were conducted with an uninfected plant tissue as a negative control. FISH analysis was performed as previously described (Shargil et al., 2015). The samples were hybridized with the probe for 16 h before visualization by a Leica SP8 laser-scanning microscope (Leica, Wetzler, Germany).
2.9. Waikavirus replication assay
Detection of replication of the new waikavirus was conducted with viral RNA extracted from either the waikavirus-positive carrots or field-collected viruliferous psyllids (confirmed by RT-PCR using primer set 10, Supplementary Table 1). Three different cDNAs were prepared by RT reactions: (1) with primer TagF-10,000, which tagged the cDNA of the minus RNA strand; (2) with primer R-10,507; (3) with no added primers to detect self-priming. The reactions were conducted using RevertAid (Thermo Fisher Scientific Inc. USA) at 50 °C to prevent self-priming (Zhang et al., 2015). A sample from each of the three preparations was subjected to digestion by Exonuclease I, which digests ssDNA, before the PCR assay to digest the tagged primers and prevent nonspecific false positive PCR amplification (Craggs et al., 2001; de Miranda et al., 2013). Exonuclease I-digestion was performed at 37 °C for 30 min, followed by inactivation at 80 °C for 10 min. The samples were kept at 4 °C. Four different PCR assays were conducted (Table 1): (1) with the primer set TagF and R-10,507; (2) with the primer set F-10,000 and TagR-10,507; (3) with the primer set F-10,000 and R-10,507; (4) with one primer R-10,507 to test the effectivity of the Exonuclease I-digestion and the presence of any self-priming (primer sequences are detailed in Supplementary Table 1).
Table 1.
Waikavirus replication reactions and expected results.
|
*RT PCR |
1.TagF-10,000 |
2.R-10,507 |
3. No primer |
|||
|---|---|---|---|---|---|---|
| ⁎⁎-Exo | +Exo | -Exo | +Exo | -Exo | +Exo | |
| 1. TagF + R-10,507 | Amplifies minus strand | Amplifies minus strand | No amplification | No amplification | No amplification | No amplification |
| 2. F-10,000 + TagR-10,507 | Amplifies minus strand & ⁎⁎⁎could amplify plus strand | Amplifies minus strand & ⁎⁎⁎could amplify plus strand | Amplifies plus strand & ⁎⁎⁎could amplify minus strand | Amplifies plus strand & ⁎⁎⁎could amplify minus strand | No amplification | No amplification |
| 3. F-10,000 + R-10,507 | Amplifies minus strand & ⁎⁎⁎could amplify plus strand | Amplifies minus strand & ⁎⁎⁎could amplify plus strand | Amplifies plus strand & ⁎⁎⁎could amplify minus strand | Amplifies plus strand & ⁎⁎⁎could amplify minus strand | No amplification | No amplification |
| 4. R-10,507 | Amplifies minus strand & ⁎⁎⁎could amplify plus strand | No amplification | No amplification | No amplification | No amplification | No amplification |
RT, reverse transcription.
Exo, Exonuclease I.
under conditions of incomplete reverse transcriptase inactivation.
2.10. Insect transmission
Insect-proof net cages were utilized for the insect transmission experiment. In each cage, a collection of plants was placed: three seed-grown carrot plants, one plant of coriander (Coriandrum sativum; Apiaceae), one plant of parsley (Petroselinum crispum; Apiaceae), and one Datura stramonium plant (Solanaceae). All plants were confirmed to be negative for the virus using RT-PCR, primer set 10 (Supplementary Table 1) before the experiment. Three similar cages were set up, and a population of Bactericera trigonica Hodkinson (Hemiptera: Triozidae) from three different infected carrot plots (Nir-Yitzhak, Urim and Ein-HaShlosha) was housed in each cage. Each population of B. trigonica (∼ 50 individuals) was sampled and confirmed to be viruliferous by RT-PCR before the experiment, using primer set 10 (Supplementary Table 1). At 45–80 days following the introduction of the viruliferous insects, the plants were tested for waikavirus acquisition using RT-PCR with primer set 10 (Supplementary Table 1).
2.11. Mechanical transmission experiments
For mechanical transmission analyses, the following plants were tested (15 plants per each species): Nicotiana glutinosa, N. benthamiana, D. innoxia, D. stramonium and carrots. All plants tested negative by RT-PCR (using primer set 10, Supplementary Table 1) before the experiment. Sap inoculum pastes were prepared from approximately ten positively tested carrot leaves, submerged in 0.01 M sodium phosphate buffer (pH=7), and crushed to a homogenous paste. The same procedure was repeated with 0.05 M sodium phosphate buffer (pH=7) and 0.1% sodium sulfite. Each inoculum mixture was applied onto the leaf surface of five uninfected plants and rubbed into the leaf tissue with carborundum powder. The treated leaves were tagged. As a negative control, five plants of each species were treated in a similar method with carborundum and buffer only. The treated plants were tested by RT-PCR twice, five weeks and eight weeks after treatment, using primer set 10 (Supplementary Table 1).
A second mechanical transmission experiment was conducted to introduce the virions directly into the phloem tissue. Carrot plants grown from seed were tested by RT-PCR (primer set 10, Supplementary Table 1) to confirm that they were negative for the virus. Four solutions were prepared: (1) 25 µL of purified virions in 1 ml sodium phosphate buffer (0.01 M, pH=7.0). (2) 25 µL of purified virions in 1 ml of sodium phosphate buffer (0.05 M, pH=7.0) supplemented with 0.1% Na2SO3. (3) 2.5 g of waikavirus-positive carrot leaves (tested by RT-PCR, primer set 10, Supplementary Table 1), crushed in sodium phosphate buffer (0.01 M, pH=7.0). (4) 2.5 g of waikavirus-positive carrot leaves (tested by RT-PCR, primer set 10, Supplementary Table 1) crushed in sodium phosphate buffer (0.05 M, pH=7.0), supplemented with 0.1% Na2SO3. A mature leaf of each plant was marked. The leaf was cut transversally with a sterile blade, and a drop of the solution was pipetted onto the cut surface. Each treatment was repeated ten times, and control plants were treated with the control buffers. Twenty-five days post-inoculation, a new leaf was sampled from each plant and tested by RT-PCR using primer set 10 (Supplementary Table 1).
2.12. A phylogenetic tree analysis
Rooted phylogenetic trees were constructed using the maximum-likelihood method based on the aa sequence of the CPs and the conserved Pro-Pol region. The Pro-Pol region that serves as a species demarcation criterion in the Picornavirales order, is delineated between the GxCG motif of the proteinase and the GDD motif of the polymerase. Parsnip yellow fleck virus (PYFV), belonging to the Sequivirus genus in the Secoviridae family, served as an out-group. The trees were created with the MEGA-X (Version 10.2.2) software, based on Jones-Taylor-Thornton (JTT) model using the parameter of 1000 bootstraps.
3. Results
3.1. A waikavirus was identified in infected carrot plants
Two different viral RNA samples from carrots were subjected to HTS analyses. The first sample was viral RNA extracted from symptomatic carrot leaves (Fig. 1). The number of total HiSeq reads was 18,269,522, and the number of clean reads was 17,648,933, allowing the assembly of a single contig of 2573 nt. The contig covered the genome of the persimmon waikavirus (PWaiV) (NCBI accession No. LC488189.1) from 9374 nt to 11,960 nt, excluding the poly-A tail (Fig. 2A). The HTS contig of 2573 nts from symptomatic carrots shared a 65.19%−78.48% nucleotide sequence identity with viruses belonging to the Waikavirus genus, when compared to the GenBank database, using the Basic Local Alignment Search Tool (BLAST) (Supplementary Table 2).
Fig. 1.
Carrot yellows disease symptoms. (A) An uninfected asymptomatic carrot plant (B) A carrot plant showing yellowing leaves and multiple lateral roots. (C) A yellowing stem and leaves. (D) A carrot plant showing yellowing and curled leaves and multiple lateral roots. (E) A carrot plant showing yellowing leaves, a loss of apical dominance and aberrant coloring.
Fig. 2.
High throughput sequencing (HTS) analyses and genome sequencing of the carrot waikavirus. (A) HTS read coverage and depth of a waikavirus genome identified in symptomatic carrots. (B) HTS read coverage and depth of a waikavirus genome identified in virions purified from waikavirus-infected carrots, as determined by RT-PCR using primer set 10 (Supplementary Table 1). (C) HTS read coverage and depth of a waikavirus genome identified in the field-collected psyllids Bactericera trigonica Hodkinson (Hemiptera: Triozidae). (D) Sequencing of the carrot waikavirus using 12 primer sets designing overlapping amplicons (Supplementary Table 1). (E-H) RT-PCR of the waikavirus overlapping amplicons that were Sanger sequenced. (I) RT-PCR of the waikavirus in the field collected psyllids B. trigonica. UTR, untranslated region; M, molecular size marker; ntc, negative template control; pc, positive control.
The second carrot sample subjected to the HTS analysis was viral RNA extracted from purified virions, prepared from waikavirus-positive carrots as determined by RT-PCR using primer set 10 (Supplementary Table 1) followed by Sanger sequencing. The number of the total obtained HiSeq reads was 25,031,563. The number of the obtained clean reads was 622,910, allowing the assembly of a single contig of 9573 nts, covering the genome of PWaiV (NCBI accession No. LC488189.1) from 100 nt to 9672 nt (Fig. 2B). Based on the HTS-derived sequences, 12 overlapping sets of primers were designed to amplify overlapping amplicons (Supplementary Table 1), which were Sanger sequenced and aligned with the HTS contig. The 5′ end was amplified by RACE reaction, and the 3′ end was confirmed by RT-PCR using an oligo dT primer. Both amplification products were Sanger sequenced, and the whole genome was assembled by alignment of the overlapping regions of the amplicons. A final sequence of 11,960 nts was obtained, excluding the poly (A) tail (Fig. 2D-H).
3.2. Carrot purified virions showing icosahedral morphology and coat proteins characteristic of waikaviruses
The presence of the waikavirus in the purified virions was confirmed by RT-PCR test using primer set 10 (Supplementary Table 1). Visualization by TEM revealed icosahedral particles of 26.22–28.41 nm in diameter, a characteristic of waikaviruses (Fig. 3A, B).
Fig. 3.
Visualization of the waikavirus virions using transmission electron microscopy (TEM) and LC-MS analyses of SDS-PAGE-separated coat proteins (CPs) comprising the CPs in the waikavirus assembled genome. (A, B) TEM visualization of the carrot-isolated waikavirus virions. (C) An SDS-PAGE analysis of carrot-purified virions separating viral CPs. (D) A LC-MS analysis of the gel-dissected proteins aligned with the putative aa sequence of the waikavirus CPs. (E) A scheme of genome organization of the new waikavirus isolated from carrots. M, molecular size marker; UTR, untranslated region; ORF, open reading frame; NTP, nucleoside triphosphate; RdRp, RNA-dependent RNA polymerase.
Purified virions run on SDS-PAGE showed three bands of ∼28 kDa, ∼25 kDa, and ∼24 kDa (Fig. 3C). Amino acid sequencing by two LC-MS reactions of the three isolated proteins detected 455 unknown peptides that matched 95.63% of the deduced viral coat protein aa. No other known viral peptides were sequenced in the reactions. After removing duplicates, forty-four unique peptides were selected (Supplementary Table 3). The unique peptides were aligned with the deduced aa sequence of the waikavirus, using overlapping regions to locate the coat proteins within the ORF1-deduced aa sequence. Gaps between the peptides could indicate potential cleavage sites between the CPs. The protein molecular mass was compared with the molecular mass of the putative waikavirus-encoded CPs (Fig. 3D). Experimentally confirmed and putative cleavage sites were determined based on the LC-MS results and formerly reported cleavage sites of other waikaviruses (Fig. 3D, E).
3.3. Genome organization and conserved motifs
Using the NCBI ORF Finder, an ORF search identified a large ORF of 3550 aa starting at 557 nt and ending at 11,209 nt. UTRs at each end are characteristic of the waikavirus genome organization. The 5′ UTR is 557 nts and the 3′ UTR is 751 nts. The conserved context of the AUG codon was adenine at −3 and +4 sites, relative to adenine of the AUG codon. A smaller ORF (ORFX) (Firth and Atkins, 2008) predicted in a + 1 reading frame is positioned between 867 nt and 1148 nt, putatively encoding a 90 aa protein. The context of the AUG codon of ORFX was A at −3 nt and C at +4 nt, relative to the adenine of the AUG codon. The large ORF of the waikavirus encodes a putative polyprotein of 402.16 kDa that is comprised of three CPs, a helicase, a 3C-like cysteine proteinase and an RNA-dependent RNA polymerase (RdRp) (Fig. 3E). In the putative helicase domain, a nucleoside triphosphate-binding site conserved motifs A and B, where a hydrophobic aa precedes site B, were identified. Motif A GxxxxGK(T/S) is located at aa 1806–1813: GKPGCGKS. Motif B (D/E)(D/E) and the preceding hydrophobic aa sequences are located at aa 1854–1858: VLYDD (Gorbalenya et al., 1989; Momoeda et al., 1994; Walker et al., 1982). In the putative 3C-like cysteine proteinase domain, the conserved catalytic triad H,E/D,C is putatively located at aa 2745, 2782, and 2877: H,D,C. The proteinase GxCG motif is located at aa 2875–2879: GSCG. (Fernando Bazan and Fletterickt, 1988; Gorbalenya et al., 1989). In the putative RdRp domain, the following conserved motifs were identified: motif (A) DYSxFDGxxxP is located at aa 3828–3839: DYAKFDGISDP; motif (B) (S/T)GxxxTxxxN(S/T) is located at aa 3291–3301: SGFPMTALFNS; motif (C) GDD is located at aa 3340–3342, with the preceding hydrophobic residue Y at aa 3339 and motif (D) FLKR is located at aa 3393–3396 (Chaouch et al., 2004; Choi, 2008; Poch et al., 1989). The aa sequences of the conserved motifs in the putative helicase domain, the 3C-like cysteine proteinase domain, and the RdRp domain were compared between identified waikaviruses (Supplementary Table 4).
3.4. Deduced amino acid sequence indicated the identification of a new waikavirus
Amino acid sequence identity was compared between the identified waikavirus and known waikaviruses. The Pro-Pol region, delineated between the GxCG motif of the proteinase and the GDD motif of the polymerase, was compared between waikaviruses (Table 2). According to ICTV species demarcation criteria, identity lower than 80% at the Pro-Pol region indicated a different species (Thompson et al., 2017). The experimentally determined sequences of the CPs were also compared with published waikavirus' CPs (Table 3). According to ICTV species demarcation criteria, identity lower than 75% of total CP aa sequence indicated a different species (Thompson et al., 2017). Therefore, we have identified a new waikavirus provisionally named carrot psyllid-borne associated virus (CPBAV) (see below), which was submitted to GenBank (NCBI accession no. OM801008, Supplementary file 1).
Table 2.
Amino acid sequence identity of CPBAV and other waikaviruses at the Pro-Pol region.
| Virus | Abbrev. | Accession number | % Identity |
|---|---|---|---|
| Bellflower vein chlorosis virus | BVCV | KT238881 | 40.38 |
| Maize chlorotic dwarf virus | MCDV | U67839 | 41.51 |
| Rice tungro spherical virus | RTSV | M95497 | 40.51 |
| Actinidia yellowing virus 1 | AcYV1 | MN180070 | 44.68 |
| Blackcurrant waikavirus A | BCWVA | MN701059 | 40.17 |
| Brassica napus RNA Virus 1 | BnRV1 | MH844554 | 41.31 |
| Poaceae liege virus 1 | PoLV1 | MW289237 | 43.37 |
| Persimmon waikavirus | PWaiV | LC488189 | 47.44 |
| Red clover associated virus | RCaV1 | MH325329 | 40.21 |
Table 3.
A percent identity comparison between the experimentally determined coat protein aa sequences of CPBAV and coat protein aa sequences of other waikavirues.
| %identity | Accession number | Abbrev. | Virus |
|---|---|---|---|
| 40.36 | BBL52501.1 | PWaiV | Persimmon waikavirus |
| 38.59 | UFX17410.1 | CamVA | Camellia virus A |
| 28.37 | NP_619,716.1 | MCDV | Maize chlorotic dwarf virus |
| 23.89 | AIZ76642.1 | BCWVA | Blackcurrant waikavirus A |
| 25.67 | UAT11553.1 | SRCTaV | Sweetbriar rose curly top-associated virus |
| 25.63 | QSM07376.1 | LWaiV1 | Lettuce waikavirus 1 |
| 25.00 | AAB17089.1 | RTSV | Rice tungro spherical virus |
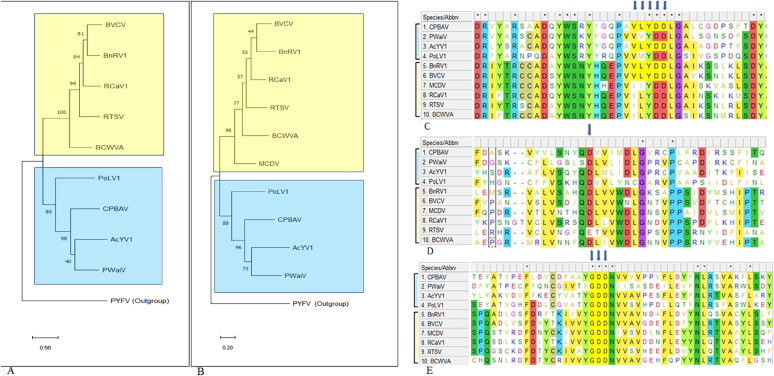
Rooted phylogenetic trees were constructed based on the CPs and the Pro-Pol conserved sites (Fig. 4A, B). The results showed a division into two main branches. One branch was comprised of six viruses, and the second was comprised of four viruses. This two-branch division was also observed in alignments of the waikaviruses at three additional conserved sites: the conserved VLYDD motif site of the putative helicase (Fig. 4C); the conserved catalytic aspartate 'D' site of the putative 3C-like cysteine proteinase (Fig. 4D) and the conserved YGDD motif site of the putative RdRp (Fig. 4E). CPBAV was clustered with PWaiV and AcYV1 in the branch containing four waikaviruses (Fig. 4A, B).
Fig. 4.
Rooted phylogenetic tree analyses and alignment of the CPBAV-deduced aa sequences and other waikaviruses at three conserved motif sites. (A) A phylogenetic tree based on experimentally identified CPs. (B) A phylogenetic tree based on the Pro-Pol conserved region located between GSCG site of the 3C-like proteinase and the GDD site of the RdRp. Parsnip yellow fleck virus (PYFV) belonging to the genus Sequivirus of the Secoviridae was used as an outgroup. A maximum-likelihood phylogenetic tree based on JTT model was constructed using 1000 bootstraps parameter. (C-E) Alignment of waikavirus-deduced aa sequences of ten waikaviruses at three conserved motif sites. (C) 'VLYDD' conserved motif site B of the helicase. (D) The conserved aspartate 'D' in the catalytic site of 3C-like cysteine proteinase. (E) The conserved 'GDD' site of the RNA dependent RNA polymerase (RdRp). CPBAV (OM801008), carrot psyllid-borne associated virus; PWaiV (BBL52501), persimmon waikavirus; AcYV1 (QJD14915), actinidia yellowing virus; PoLV1 (QTJ95918), poaceae liege virus 1; BnRV1 (YP_009552078), brassica napus RNA virus 1; BVCV (YP_009165498), bellflower vein chlorosis virus; MCDV (NP_619,716), maize chlorotic dwarf virus; RCaV1 (AXU44617), red clover associated virus 1; RTSV (NP_042507), rice tungro spherical virus and BCWVA (QMU95531), blackcurrant waikavirus A.
3.5. CPBAV localized to carrot phloem and replicated in carrot plants
Carrot plants positive for the virus, as confirmed by RT-PCR using primer set 10 (Supplementary Table 1), were tested for the presence of CPBAV in plant tissues using FISH. Dissected carrot leaves showed that the waikavirus genome was localized in the phloem only in the plants positive for the new waikavirus (Fig. 5). A study of viral replication showed that the waikavirus apparently replicates in carrots but does not replicate in the psyllid vector (see below) (Fig. 6). Waikavirus replication in carrot plants was observed when cDNA was synthesized from the minus RNA strand in RT reaction 1 with TagF-10,000 primer, followed by Exonuclease I-digestion and PCR assay 1, using the primer pair TagF and R-10,507 (Fig. 6A1, PCR1 lane 1Ex, C). The higher amplification observed in lane 1, compared to lane 1Ex, indicated that under conditions of incomplete reverse transcriptase inactivation the presence of TagF-10,000 primer before Exonuclease I-digestion, could allow cDNA synthesis during PCR amplification as well (Craggs et al., 2001; de Miranda et al., 2013) (Fig. 6A1, PCR1 lanes 1, 1Ex). PCR assay 1 (primer pair TagF and R-10,507) could not amplify untagged cDNA synthesized from positive RNA strand using primer R-10,507 in RT reaction 2 (Fig. 6D) and there was no self-priming of the RNA as observed in RT reaction 3 using no primers (Fig. 6A1, PCR1, lanes 2–3Ex). Results of PCR assays 2 and 3, using primer sets F-10,000+TagR-10,507 and F-10,000+R-10,507, respectively, on cDNA synthesized in RT reaction 1 (using TagF-10,000 primer) showed the amplification of the negative strand. Amplification of the positive strand could also occur under conditions of incomplete reverse transcriptase inactivation resulting in cDNA synthesis during the PCR (Fig. 6A1, A2, PCR2 and 3, lanes 1,1Ex). PCR assays 2 and 3 conducted on cDNA prepared from the positive RNA strand using RT reaction 2 (with R-10,507 primer) showed amplification of the positive strand and could also show amplification of the negative strand, the latter under conditions of incomplete reverse transcriptase inactivation (Fig. 6A1, A2, PCR2 and 3, lanes 2, 2Ex). PCR assay 4, using one primer R-10,507, could amplify cDNA synthesized from the negative strand in RT reaction 1 (using TagF-10,000 primer) only in the presence of the forward primer before the Exonuclease I-digestion (Fig. 6A2, PCR4, lane 1). The effectiveness of the Exonuclease I-digestion was observed in undetectable amplification levels when using PCR assay 4 (with one primer R-10,507) on cDNA prepared in RT reaction 1 (using TagF-10,000 primer) following Exonuclease I-digestion (Fig. 6A2, PCR4, lane 1Ex, E).
Fig. 5.
Fluorescence in-situ hybridization (FISH) visualization of cyanine-Cy3-bound waikavirus genome in carrot stems. (A-C) A longitudinal section of uninfected carrot stems. (D-F) A longitudinal section of a waikavirus-infected carrot stem, as tested by RT-PCR using primer set 10 (Supplementary Table 1). (G-I) A transversal section of the waikavirus-infected carrot stems. (A, D, G) Images using a bright channel. (B, E, H) Images using bright and red channels. (C, F, I) Images using the red channel.
Fig. 6.
Waikavirus replication test. (A1, A2) Viral replication test conducted on carrot-extracted viral RNA. (B1, B2) Viral replication test conducted on psyllid-extracted viral RNA. Lanes 1 and 4 represent cDNA synthesis using RT reaction 1 with TagF-10,000 primer, tagging the minus RNA strand. Lanes 2 and 5 represent cDNA synthesis using RT reaction 2 with R-10,507 primer. Lanes 3 and 6 represent cDNA synthesis using RT reaction 3 with no primers. Ex, Exonuclease I treatment before PCR test; M, molecular size marker; NTC, non-template control. (C) A scheme of PCR 1, using TagF and R-10,507 primers, on samples of RT reaction 1 with TagF-10,000 primer, tagging the minus RNA strand, followed by Exonuclease I digestion (PCR1, lanes 1+Ex and 4+Ex). (D) A scheme of PCR 1, using TagF and R-10,507 primers, on samples of RT reaction 2 with R-10,507 primer followed by Exonuclease I digestion (PCR1, lanes 2+Ex and 5+Ex). (E) A scheme of PCR 4, using one primer R-10,507 on samples of RT reaction 1 with TagF-10,000 primer followed by Exonuclease I digestion (PCR4, lanes 1+Ex and 4+Ex). Primer details are in Supplementary Table 1.
3.6. CPBAV was transmitted by psyllids but did not replicate in the vector
Field-collected viruliferous B. trigonica individuals were subjected to a viral RNA extraction and detection of the waikavirus genome by RT-PCR and an HTS analysis. In the HTS results, total reads were 38,485,460. Clean reads were 572,085, and 111 reads were mapped to the virus spanning the nucleotide area between 9216 and 11,960 (Fig. 2C, I). The viruliferous psyllids were placed in three cages, each containing the following tested plants: three seed-grown carrots, a coriander, a parsley and a D. stramonium. Eighty days after occupying the cages, the plants were tested by RT-PCR using primer set 10 (Supplementary Table 1). Consistent results were obtained in all cages. All carrot and coriander plants were positive for CPBAV, whereas parsley and D. stramonium were negative for the waikavirus. The viruliferous B. trigonica transmitted the virus to the plants but symptoms were not apparent (Table 4). In the psyllids, CPBAV seemed not to replicate, as was observed in PCR assay 1 (using the primer pair TagF and R-10,507) of the cDNA prepared in RT reaction 1 with TagF-10,000 primer used to tag the negative strand (Fig. 6B1, PCR1, lanes 4, 4Ex). The absence of tagged cDNA was also apparent in PCR assay 2 (using the primer pair F-10,000 and TagR-10,507) of the cDNA prepared in RT reaction 1 with TagF-10,000 primer (Fig. 6B1, PCR2, lanes 4, 4Ex). PCR assay 3 (using the primer pair F-10,000 and R-10,507) on cDNA prepared in RT reaction 1 (using TagF-10,000 primer) showed residual amplification that could be of the positive RNA strand under conditions of incomplete reverse transcriptase inactivation and a concurrent RT and PCR reactions (Fig. 6B2, PCR3, lanes 4,4Ex). PCR assay 4, using one primer R-10,507 conducted on cDNA prepared in RT reaction 1 (with TagF-10,000 primer) showed no amplification when the TagF-10,000 primer was still in the reaction mixture before Exonuclease I-digestion (Fig. 6B2, PCR4, lane 4).
Table 4.
Host range of CPBAV that were either collected from commercial carrot plots or identified in the insect transmission test.
| Scientific name | Common name | Family | *RT-PCR results ⁎⁎F, ⁎⁎⁎Ex, (+/-) |
|---|---|---|---|
| Daucus carota | Carrot | Apiaceae | +F,+ Ex |
| Coriandrum sativum | Coriander | Apiaceae | +Ex |
| Datura innoxia | Prickly burr | Solanaceae | +F |
| Datura stramonium | Jimsonweed | Solanaceae | -Ex |
| Solanum nigrum | Black Nightshade | Solanaceae | -F |
| Convolvulus arvensis | Bindweed | Convolvulaceae | -F |
| Malva nicaeensis | Bull Mallow | Malvaceae | -F |
| Apium graveolens | Celery | Apiaceae | -F |
| Petroselinum crispum | Parsley | Apiaceae | -Ex |
RT-PCR using primer set 10 (Supplementary Table 1) confirmed virus infection.
F, field collected plants.
Ex, plants subjected to insect transmission experiment.
3.7. CPBAV was mechanically non-transmissible
Mechanical transmission experiments conducted on seed-grown carrot plants negative for the virus and N. glutinosa, N. benthamiana, D. innoxia and D. stramonium plants were analysed by RT-PCR using primer set 10 (Supplementary Table 1). Experimental rubbing of viral sap, prepared from infected carrot leaves with carborundum powder, was tested five and eight weeks after mechanical transmission. Experiments of pipetting virion preparations and infected-carrot sap into leaf veins were analysed after twenty-five days. Regardless of plant species or transmission method, no plants were found positive for the virus by an RT-PCR test (using primer set 10, Supplementary Table 1). Hence, we concluded that the virus was not mechanically transmitted.
4. Discussion
In recent years a carrot yellows disease tightly associated with the bacterium Ca. L. solanacearum was established in Israel (Mawassi et al., 2018). The disease has spread in carrots with symptoms of chlorosis, leaf curling, a loss of apical dominance and multiple lateral roots. However, since 2019 disease symptoms were observed in carrots negative for Ca. L. solanacearum. We have initiated our study of viruses that could be associated with the disease by testing symptomatic carrots that lack other possible causative agents such as Ca. L. solanacearum and carrot red leaf virus. In our work, a new waikavirus species was identified in symptomatic carrot plants subjected to an HTS analysis, and the assembled complete genome sequence was submitted to the Genbank under the proposed name carrot psyllid-borne associated virus (CPBAV).
Waikaviruses are single-stranded, positive sense RNA viruses with a poly-A tail and a presumed 5′-linked VPg (viral protein genome-linked). The viral genome of ∼12 kb has an ORF1 that encodes a polyprotein, which is processed by a virus-encoded 3C-like proteinase (Sanfaçon et al., 2009). The specific cleavage sites of the 3C-like proteinase differ between genera, and in waikaviruses they contain the following putative cleavage sites: a glutamine 'Q' at −1 position of the dipeptides with one of the following aa: an alanine 'A', a serine 'S', a methionine 'M' and a valine 'V' (Gorbalenya et al., 1989; Thompson et al., 2017). At the N- terminal region of ORF1, three coat proteins (CP 1–3) are encoded, which comprise a subunit in the encapsulated non-enveloped icosahedral particles of ∼30 nm in diameter (Sanfaçon et al., 2020). The replication-associated proteins are encoded at the C-terminal region: the nucleoside triphosphate binding protein (NTB) of the helicase, the 3C-like proteinase, and the RNA-dependent RNA polymerase. At the N-terminus, immediately upstream of the coat proteins, a protein with an unknown function is encoded, and a silencing suppressor was identified in waikaviruses such as MCDV (Stewart et al., 2017). Small ORFs at the 5′ end (ORFX), overlapping the polyprotein (Firth and Atkins, 2008), and at the 3′ end were predicted, but had no known functions (Thompson et al., 2017).
The new waikavirus species was identified in our work due to the unbiased HTS technology that has brought about a sharp rise in the number of new viral species discovered recently (Pecman et al., 2017; Wylie et al., 2012). The HTS-retrieved results revealed long reads that led to the assembly of a 9573 nt long-sequence, indicative of clean samples and successful sequencing (Abdel-Latif and Osman, 2017; Tamari et al., 2013). Testing the presence of the sequenced virus in the symptomatic carrots was done by RT-PCR followed by Sanger sequencing, confirming the validity of the HTS results (Aird et al., 2011; Koboldt et al., 2010; Ledergerber and Dessimoz, 2011). The assembled genome showed 65.70–78.48% nucleotide sequence identity with other waikaviruses. The AUG start codon of the large ORF1 was amid the consensus nucleotide context of adenine at −3 and +4 sites, relative to the adenine of the AUG codon (Joshi et al., 1997). Genome analyses of CPBAV showed in the large ORF1 three coat-proteins at the N-terminal region. At the C-terminal region, the replication-associated proteins showed the conserved motifs of the helicase, the 3C-like proteinase, and the polymerase. The three coat proteins of ∼28 kDa, ∼25 kDa, and ∼24 kDa, experimentally identified, were isolated and aa sequenced using LC-MS. Alignment of the obtained aa sequences with the deduced aa sequence of the assembled genome allowed the identification of Q699/M700 and Q1,197/S1,198 cleavage sites, characteristic of waikaviruses (Koloniuk and Fránová, 2018). However, a Q1,409/N1,410 cleavage site was identified at the C-terminal area of the ∼24 kDa CP (Fig. 3). A segment of 17 aa between ∼25 kDa CP and ∼24 kDa CP (aa residues 1178–1194) was not revealed by the LC-MS sequencing, but the Q1,167/S1,168 site preceding the segment was in a context of peptides that were not cleaved (peptide 28, Supplementary Table 3). This has led us to suggest that Q1,197/S1,198 was the putative cleavage site between the two coat proteins (Fig. 3). An ORFX of 90 aa (9.36 kDa) was predicted from a + 1 reading frame, overlapping ORF1 (Firth and Atkins, 2008) (Fig. 3). The +1 AUG codon, located at 876 nt, has the nucleotide context of A at −3 nt and C at +4 nt relative to the adenine of the AUG codon, indicating an un-preferred translation initiation site (Joshi et al., 1997).
We have shown that the psyllid B. trigonica acted as a vector for CPBAV transmission. The field-collected psyllids (∼50 individuals from each of seven populations) were sampled and confirmed by RT-PCR and HTS to contain the waikavirus. All field collected populations were viruliferous although the percentage of infected individuals in each sample was not determined. The HTS-obtained clean reads covered only a waikavirus segment of 2744 nt between 9216 nt and 11,960 nt, indicating that most of the viral RNA genome failed to survive in the psyllid. This result and the fact that no replication of CPBAV was observed in the psyllids (Fig. 6) rule out the possibility of a persistent propagative manner of transmission and may support a semi-persistent transmission manner characteristic of other waikaviruses transmitted by aphids or leafhoppers (Sanfaçon et al., 2009). CPBAV transmission experiments using field-collected viruliferous psyllids caused infection of carrots and coriander. Visualization of CPBAV in carrot-dissected tissues, using the FISH test, localized the virus in the phloem (Fig. 5), and the virus replicated in carrots (Fig. 6). As far as we know, this is a second report of a psyllid acting as a plant virus vector (Salazar, 2006; Tenorio et al., 2003).
The bacterium Ca. L. solanacearum, an important carrot pathogen in Israel, is also transmitted by B. trigonica (Mawassi et al., 2018). This raises a question regarding a possible relationship between the two pathogens in the manifestation of disease symptoms apparent in carrots grown in the field. Because waikaviruses are also known as helper viruses (Hibino, 1983), viral co-infections might be necessary for severe disease symptom manifestations. We have focused our research on studying the new waikavirus on pot plants. However, field experiments would be required to reveal interactions between CPBAV and other known or unidentified disease-causing agents associated with the severe symptoms observed only under field growing conditions. In addition, screening wild plants that grew in the field adjacent to the symptomatic carrots revealed a new host plant, D. innoxia, of the Solanaceae family (Table 4). Identifying new host plants that could serve as an inoculum source for the virus might explain the preservation of the virus between carrot-growing cycles and could affect relationships between the vector's life cycle and disease symptom emergence in the field due to host-vector interactions (Mauck et al., 2018).
5. Conclusion
We have identified a new waikavirus species in field-collected symptomatic carrots manifesting yellows disease. The new waikavirus, putatively named CPBAV, was transmitted by viruliferous field-collected B. trigonica psyllids to carrot and coriander plants. Field-collected D. innoxia plants belonging to the Solanaceae family were infected by CPBAV. Our results suggest a possible contribution of CPBAV to carrot yellows disease upon co-infections with other disease-causing agents transmitted by the psyllids, such as the bacterium Ca. L. solanacearum.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We would like to thank Dr.Victor Gaba for reviewing our manuscript. We like to acknowledge Shahar Pincovici and Ziv Mey-tal from Israeli Agricultural Extension Services for contributing field input to the research.
Funding
The research was funded by the Israeli Carrot Growers' Board.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2023.199192.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Abdel-Latif A., Osman G. Comparison of three genomic DNA extraction methods to obtain high DNA quality from maize. Plant Methods. 2017;13(1) doi: 10.1186/s13007-016-0152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird D., Ross M.G., Chen W.S., Danielsson M., Fennell T., Russ C., Jaffe D.B., Nusbaum C., Gnirke A. Analyzing and minimizing PCR amplification bias in Illumina sequencing libraries. Genome Biol. 2011;12(2) doi: 10.1186/gb-2011-12-2-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchfink B., Xie C., Huson D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015;12(1):59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- Chaouch R., Redinbaugh M.G., Marrakchi M., Hogenhout S.A. Genomic of the severe isolate of Maize chlorotic dwarf virus. Plant Protect. Sci. 2004;40(4):113–119. doi: 10.17221/469-PPS. [DOI] [Google Scholar]
- Choi I.R. Elsevier; 2008. Sequiviruses. In Encyclopedia of Virology; pp. 546–551. [DOI] [Google Scholar]
- Craggs J.K., Ball J.K., Thomson B.J., Irving W.L., Grabowska A.M. Development of a strand-specific RT-PCR based assay to detect the replicative form of hepatitis C virus RNA. J. Virol. Methods. 2001;94(1–2):111–120. doi: 10.1016/S0166-0934(01)00281-6. [DOI] [PubMed] [Google Scholar]
- Danecek P., Bonfield J.K., Liddle J., Marshall J., Ohan V., Pollard M.O., Whitwham A., Keane T., McCarthy S.A., Davies R.M., Li H. Twelve years of SAMtools and BCFtools. Gigascience. 2021;10(2) doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miranda J.R., Bailey L., Ball B.V., Blanchard P., Budge G.E., Chejanovsky N., Chen Y.P., Gauthier L., Genersch E., de Graaf D.C., Ribière M., Ryabov E., De Smet L., van der Steen J.J.M. Standard methods for virus research in Apis mellifera. J. Apic. Res. 2013;52(4):1–56. doi: 10.3896/IBRA.1.52.4.22. [DOI] [Google Scholar]
- Fernando Bazan J., Fletterickt R.J. Proc. Natl. Acad. Sci. USA. 1988. Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications (picornavirus/protein structure prediction/sequence alignment) (Vol. 85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth A.E., Atkins J.F. Bioinformatic analysis suggests that a conserved ORF in the waikaviruses encodes an overlapping gene. Arch. Virol. 2008;153(7):1379–1383. doi: 10.1007/s00705-008-0119-5. [DOI] [PubMed] [Google Scholar]
- Geer L.Y., Marchler-Bauer A., Geer R.C., Han L., He J., He S., Liu C., Shi W., Bryant S.H. The NCBI BioSystems database. Nucleic Acids Res. 2010;38(suppl_1):D492–D496. doi: 10.1093/nar/gkp858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gera A., Maslenin L., Weintraub P.G., Mawassi M. Phytoplasma and spiroplasma diseases in open-field crops in Israel. Bull. Insectol. 2011;64:53–54. www.bulletinofinsectology.org [Google Scholar]
- Gorbalenya A.E., Blinov V.M., Donchenko A.P., Koonin E.V. An NTP-binding motif is the most conserved sequence in a highly diverged monophyletic group of proteins involved in positive strand RNA viral replication. J. Mol. Evol. 1989;28(3):256–268. doi: 10.1007/BF02102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B.J., Papanicolaou A., Yassour M., Grabherr M., Blood P.D., Bowden J., Couger M.B., Eccles D., Li B., Lieber M., MacManes M.D., Ott M., Orvis J., Pochet N., Strozzi F., Weeks N., Westerman R., William T., Dewey C.N.…Regev A. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013;8(8):1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H. Transmission of Two Rice Tungro-Associated Viruses and Rice Waika Virus from Doubly or Singly Infected Source Plants by Leafhopper Vectors. Plant Dis. 1983;67(7):774. doi: 10.1094/PD-67-774. [DOI] [Google Scholar]
- Houtgast E.J., Sima V.M., Bertels K., Al-Ars Z. Hardware acceleration of BWA-MEM genomic short read mapping for longer read lengths. Comput. Biol. Chem. 2018;75:54–64. doi: 10.1016/j.compbiolchem.2018.03.024. [DOI] [PubMed] [Google Scholar]
- Hunt R.E., Nault L.R., Gingery R.E. Evidence for infectivity of maize chlorotic dwarf virus and for a helper component in its leafhopper transmission. Phytopathology. 1988;78:499–504. [Google Scholar]
- Ito T., Sato A. Three novel viruses detected from Japanese persimmon 'Reigyoku' associated with graft-transmissible stunt. Eur. J. Plant Pathol. 2020;158(1):163–175. doi: 10.1007/s10658-020-02063-0. [DOI] [Google Scholar]
- Joshi C.P., Zhou H., Huang X., Chiang V.L. Context sequences of translation initiation codon in plants. Plant Mol. Biol. 1997;35(6):993–1001. doi: 10.1023/A:1005816823636. [DOI] [PubMed] [Google Scholar]
- Koboldt D.C., Ding L., Mardis E.R., Wilson R.K. Challenges of sequencing human genomes. Brief. Bioinformatics. 2010;11(5):484–498. doi: 10.1093/bib/bbq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloniuk I., Fránová J. Complete nucleotide sequence and genome organization of red clover associated virus 1 (RCaV1), a putative member of the genus Waikavirus (family Secoviridae, order Picornavirales) Arch. Virol. 2018;163(12):3447–3449. doi: 10.1007/s00705-018-4005-5. [DOI] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9(4):357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledergerber C., Dessimoz C. Base-calling for next-generation sequencing platforms. Brief. Bioinformatics. 2011;12(5):489–497. doi: 10.1093/bib/bbq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclot F.J., Debue V., Blouin A.G., Fontdevila Pareta N., Tamisier L., Filloux D., Massart S. Identification, molecular and biological characterization of two novel secovirids in wild grass species in Belgium. Virus Res. 2021;298 doi: 10.1016/j.virusres.2021.198397. [DOI] [PubMed] [Google Scholar]
- Marco, S. (1993). Carrot Red Leaf Virus in Israel.
- Mauck, K.E., Chesnais, Q., & Shapiro, L.R. (2018). Evolutionary Determinants of Host and Vector Manipulation by Plant Viruses (pp. 189–250). 10.1016/bs.aivir.2018.02.007. [DOI] [PubMed]
- Mawassi M., Dror O., Bar-Joseph M., Piasezky A., Sjölund J.M., Levitzky N., Shoshana N., Meslenin L., Haviv S., Porat C., Katsir L., Kontsedalov S., Ghanim M., Zelinger-Reichert E., Arnsdorf Y.M., Gera A., Bahar O. Candidatus Liberibacter solanacearum" is tightly associated with carrot yellows symptoms in Israel and transmitted by the prevalent psyllid vector bactericera trigonica. Phytopathology. 2018;108(9):1056–1066. doi: 10.1094/PHYTO-10-17-0348-R. [DOI] [PubMed] [Google Scholar]
- Momoeda M., Wong S., Kawase M., Young N.S., Kajigaya S. A putative nucleoside triphosphate-binding domain in the nonstructural protein of B19 parvovirus is required for cytotoxicity. J. Virol. 1994;68(12):8443–8446. doi: 10.1128/jvi.68.12.8443-8446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murant A.F., Roberts I.M. Virus-like particles in phloem tissue of chervil Anthriscus cerefolium infected with anthriscus yellows virus. Ann. Appl. Biol. 1977;85(3):403–406. doi: 10.1111/j.1744-7348.1977.tb01926.x. [DOI] [Google Scholar]
- Nault L.R., Styer W.E., Knoke J.K., Pitre H.N. Semipersistent Transmission of Leafhopper-Borne Maize Chlorotic Dwarf Virus1. J. Econ. Entomol. 1973;66(6):1271–1273. doi: 10.1093/jee/66.6.1271. [DOI] [Google Scholar]
- Orenstein S., Franck A., Kuznetzova L., Sela I., Tanne E. Association of phytoplasmas with a yellows disease of carrot in Israel. In Source: J. Plant Pathol. 1999;81(3) https://www.jstor.org/stable/41998973 [Google Scholar]
- Park D., Hahn Y. A novel Waikavirus (the family Secoviridae) genome sequence identified in rapeseed (Brassica napus) Acta Virol. 2019;63(2):211–216. doi: 10.4149/av_2019_205. [DOI] [PubMed] [Google Scholar]
- Pecman A., Kutnjak D., Gutiérrez-Aguirre I., Adams I., Fox A., Boonham N., Ravnikar M. Next generation sequencing for detection and discovery of plant viruses and viroids: comparison of two approaches. Front. Microbiol. 2017;8(OCT) doi: 10.3389/fmicb.2017.01998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. Identification of four conserved motifs among the RNAdependent polymerase encoding elements. EMBO J. 1989;8(12):3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar L.F. Emerging and Re-emerging Potato Diseases in the Andes. Potato Res. 2006;49(1):43–47. doi: 10.1007/s11540-006-9005-2. [DOI] [Google Scholar]
- Sanfaçon H., Dasgupta I., Fuchs M., Karasev A.v., Petrzik K., Thompson J.R., Tzanetakis I., van der Vlugt R., Wetzel T., Yoshikawa N. Proposed revision of the family Secoviridae taxonomy to create three subgenera, "Satsumavirus", "Stramovirus" and "Cholivirus", in the genus Sadwavirus. Arch. Virol. 2020;165(2):527–533. doi: 10.1007/s00705-019-04468-7. [DOI] [PubMed] [Google Scholar]
- Sanfaçon H., Wellink J., le Gall O., Karasev A., van der Vlugt R., Wetzel T. Secoviridae: a proposed family of plant viruses within the order Picornavirales that combines the families Sequiviridae and Comoviridae, the unassigned genera Cheravirus and Sadwavirus, and the proposed genus Torradovirus. Arch. Virol. 2009;154(5):899–907. doi: 10.1007/s00705-009-0367-z. [DOI] [PubMed] [Google Scholar]
- Seo J.K., Kwak H.R., Lee Y., Kim J., Kim M.K., Kim C.S., Choi H.S. Complete genome sequence of bellflower vein chlorosis virus, a novel putative member of the genus Waikavirus. Arch. Virol. 2015;160(12):3139–3142. doi: 10.1007/s00705-015-2606-9. [DOI] [PubMed] [Google Scholar]
- Shargil D., Zemach H., Belausov E., Lachman O., Kamenetsky R., Dombrovsky A. Development of a fluorescent in situ hybridization (FISH) technique for visualizing CGMMV in plant tissues. J. Virol. Methods. 2015;223:55–60. doi: 10.1016/j.jviromet.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Shen P., Kaniewska M., Smith C., Beachy R.N. Nucleotide Sequence and Genomic Organization of Rice Tungro Spherical Virus. Virology. 1992;193:621–630. doi: 10.1006/viro.1993.1170. [DOI] [PubMed] [Google Scholar]
- Stewart L.R., Jarugula S., Zhao Y., Qu F., Marty D.M. Identification of a maize chlorotic dwarf virus silencing suppressor protein. Virology. 2017;504:88–95. doi: 10.1016/j.virol.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Tamari F., Hinkley C.S., Ramprashad N. A comparison of DNA extraction methods using Petunia hybrida tissues. J. Biomol. Tech. 2013;24(3):113–118. doi: 10.7171/jbt.13-2403-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio J., Chuquillanqui C., Garcia A., Guillén M., Chavez R., Salazar L.F. Sintomatología y efecto en el rendimiento de papa de un nuevo virus transmitido por el psílido Russelliana solanicola. Fitopatología. 2003;38:32–36. [Google Scholar]
- Thekke-Veetil T., Ho T., Postman J.D., Tzanetakis I.E. Blackcurrant waikavirus A, a new member of the genus Waikavirus, and its phylogenetic and molecular relationship with other known members. Eur. J. Plant Pathol. 2020;157(1):59–64. doi: 10.1007/s10658-020-01978-y. [DOI] [Google Scholar]
- Thole V., Hull R. Rice Tungro Spherical Virus: nucleotide Sequence Of The 3′ Genomic Half And Studies On The Two Small 3′ Open Reading Frames. Virus Genes. 1996;13(3):239–246. doi: 10.1007/BF00366984. [DOI] [PubMed] [Google Scholar]
- Thompson J.R., Dasgupta I., Fuchs M., Iwanami T., Karasev A.V., Petrzik K., Sanfaçon H., Tzanetakis I., van der Vlugt R., Wetzel T., Yoshikawa N. ICTV Virus Taxonomy Profile: secoviridae. J. Gen. Virol. 2017;98(4):529–531. doi: 10.1099/jgv.0.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J.E., Saraste M., Runswick M.J., Gay N.J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie S.J., Luo H., Li H., Jones M.G.K. Multiple polyadenylated RNA viruses detected in pooled cultivated and wild plant samples. Arch. Virol. 2012;157(2):271–284. doi: 10.1007/s00705-011-1166-x. [DOI] [PubMed] [Google Scholar]
- Zerbino D.R. Using the Velvet de novo Assembler for Short-Read Sequencing Technologies. Curr. Protoc. Bioinform. 2010;31(1) doi: 10.1002/0471250953.bi1105s31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu H.N., Zhang Y.Q., Shen J.G., Li W.M. Self-priming on the plant viral RNAs during reverse transcription-PCR. Acta Virol. 2015;59(1):92–97. doi: 10.4149/av_2015_01_92. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Gao S., Padmanabhan C., Li R., Galvez M., Gutierrez D., Fuentes S., Ling K.S., Kreuze J., Fei Z. VirusDetect: an automated pipeline for efficient virus discovery using deep sequencing of small RNAs. Virology. 2017;500:130–138. doi: 10.1016/j.virol.2016.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.