Abstract
Background
Malnutrition is prevalent in patients with pulmonary tuberculosis (PTB) and is associated with a poor prognosis.
Objective
This study aims to assess the prevalence and risk factors of malnutrition in patients with PTB.
Methods
Studies related to the prevalence and risk factors of malnutrition in patients with PTB were searched through PubMed, Embase, Web of Science, and Cochrane Library databases from January 1990 to August 2022, and two researchers screened the literature, evaluated the quality, and extracted data independently. A random-effects model was used to pool the effect sizes and 95% confidence intervals. Subgroup analysis, meta-regression analysis, and sensitivity analysis were further performed to identify sources of heterogeneity and evaluate the stability of the results. Publication bias was assessed by Doi plot, Luis Furuya-Kanamori (LFK) asymmetry index, funnel plot, and Egger's tests.
Results
A total of 53 studies involving 48, 598 participants were identified in this study. The prevalence of malnutrition was 48.0% (95% CI, 40.9–55.2%). Subgroup analysis revealed that malnutrition was more common among male gender (52.3%), bacterial positivity (55.9%), family size over 4 (54.5%), drug resistance (44.1%), residing in rural areas (51.2%), HIV infection (51.5%), Asian (51.5%), and African (54.5%) background. The prevalence of mild, moderate, and severe malnutrition was 21.4%, 14.0%, and 29.4%, respectively. Bacterial positivity (OR = 2.08, 95% CI 1.26–3.41), low income (OR = 1.44, 95% CI 1.11–1.86), and residing in rural areas (OR = 1.51, 95% CI 1.20–1.89) were risk factors of malnutrition in patients with PTB. However, male (OR = 1.04, 95% CI 0.85–1.26) and drinking (OR = 1.17, 95% CI 0.81–1.69) were not risk factors for malnutrition in patients with PTB. Due to the instability of sensitivity analysis, HIV infection, age, family size, smoking, and pulmonary cavity need to be reevaluated. Meta-regression suggested that sample size was a source of heterogeneity of prevalence. The Doi plot and LFK asymmetry index (LFK = 3.87) indicated the presence of publication bias for prevalence, and the funnel plot and Egger's test showed no publication bias for risk factors.
Conclusion
This meta-analysis indicated that malnutrition was prevalent in patients with PTB, and bacterial positivity, low income, and those residing in rural areas were risk factors for malnutrition. Therefore, clinical workers should pay attention to screening the nutritional status of patients with PTB and identifying the risk factors to reduce the incidence of malnutrition and provide nutritional interventions early to improve the prognosis in patients with PTB.
Keywords: pulmonary tuberculosis, malnutrition, prevalence, risk factors, systematic review, meta-analysis
1. Introduction
Tuberculosis is a chronic infectious disease caused by infection with Mycobacterium tuberculosis (1). Global Tuberculosis Report 2022 indicates that there will be ~10.6 million cases of tuberculosis worldwide, 6.40 million new cases of tuberculosis, and up to 1.60 million deaths in 2021, which makes it rank second as a cause of death from a single infectious agent after COVID-19 (2). It was also discovered that ~50% of patients with tuberculosis were diagnosed delayed during COVID-19, resulting in more severe symptoms in individuals who were hospitalized (3). Despite some progress made in the prevention and treatment of pulmonary tuberculosis (PTB) under the guidance of the World Health Organization, PTB remains widespread and is difficult to treat due to high transmission rates, rising drug resistance, lengthy treatment regimens, delayed access, and poor treatment compliance (4–6). Thus, the disease burden of tuberculosis remains severe.
As a chronic infectious disease of the respiratory system, PTB often presents with complications as the disease progresses, such as malnutrition, anemia, bronchiectasis, pulmonary hypertension, and respiratory failure (7–9). Malnutrition is currently widely studied in patients with PTB, but the prevalence of malnutrition varies widely between studies, ranging from 1.04 to 92.60% (10, 11). Malnutrition is not only an outcome of tuberculosis but also an important risk factor for the development of tuberculosis. According to a study, malnutrition may have a significant role in the development of tuberculosis. Patients with malnutrition may be more susceptible to developing the disease due to impaired innate and adaptive immunity to Mycobacterium tuberculosis (12). Additionally, patients with PTB frequently experience elevated energy needs, reduced appetite, low dietary intake, and malabsorption, all of which are closely associated with malnutrition (13). Patients with tuberculosis are frequently associated with high mortality, length of time to convert sputum, risk of drug resistance, and liver damage from anti-tuberculosis medications, which has a significant impact on the outcome and clinical prognosis (10, 14–16). Therefore, there is a positive impact on improving the treatment outcome and clinical prognosis by evaluating the nutritional status and its risk factors in patients with PTB.
A meta-analysis has reported the prevalence of malnutrition in patients with PTB (17), but the study is limited by small sample size, lack of subgroup analysis of demographic characteristics, and restricted to the Ethiopian region. Second, the awareness of risk factors of malnutrition remains controversial in patients with PTB. For instance, Kornfeld et al. (18) and Hussien et al. (19) suggested that male, HIV infection, and the pulmonary cavity were risk factors for malnutrition in patients with PTB, whereas Magassoub et al. (20) did not. Thus, we conduct this meta-analysis to assess the prevalence and risk factors of malnutrition in order to prevent and treat malnutrition early and improve the prognosis in patients with PTB.
2. Materials and methods
This study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (21) (see PRISMA checklist in Supplementary material). This protocol was registered in PROSPERO (No. CRD 42022358772).
2.1. Inclusion and exclusion criteria
Inclusion criteria were as follows: (1) study designs: including cross-sectional, cohort studies, and case-control studies; (2) participants: patients with PTB; (3) articles with diagnostic criteria of PTB (including bacteriology as well as clinical diagnosis) and malnutrition [including Body Mass Index (BMI), Nutritional Risk Screening 2002 (NRS-2002), Patient-Generated Subjective Global Assessment (PG-SGA), Subjective Global Assessment (SGA), Mini Nutritional Assessment (MNA), Short-Form Mini-Nutritional Assessment (MNA-SF), Global Leadership Initiative on Malnutrition (GLIM) criteria, European Society for Clinical Nutrition and Metabolism (ESPEN) criteria, French criteria 2007, Malnutrition Screening Tool (MST), Malnutrition Universal Screening Tool (MUST), and Geriatric Nutritional Risk Index (GNRI)]; (4) outcomes: prevalence and risk factors of malnutrition; and (5) articles published in English.
Exclusion criteria were as follows: (1) duplicate studies, reviews, and animal studies; (2) relevant outcomes not available; (3) full text not available, conference abstract; and (4) studies with extrapulmonary tuberculosis.
2.2. Search strategy
Studies related to the prevalence and risk factors for malnutrition in patients with PTB published between January 1990 and August 2022 were searched through PubMed, Embase, Web of Science, and the Cochrane Library databases. We used a combination of Medical Subject Headings terms and free terms for the search. The detailed search strategy is shown in Supplementary Table 1.
2.3. Study selection, data extraction, and quality assessment
Two researchers (AL and S-YY) screened the literature and extracted relevant data independently, and any disagreements were resolved through discussion, and if unresolved, a final decision was made by a third person (N-NL). The information extracted from the studies included the study's first author, publication year, country, study design, sample size, the number of patients with malnutrition, the definition of malnutrition, and risk factors. The quality of cross-sectional studies was evaluated using the Agency for Healthcare Research and Quality with 0 to 3 being as low quality, 4 to 7 as moderate quality, and 8 to 11 as high quality. The Newcastle-Ottawa Scale (22) was used to assess the quality of cohort studies and case-control studies, with 0–3 being as low quality, 4–6 as moderate quality, and ≥7 as high quality.
2.4. Statistical analysis
Prevalence and risk factors were pooled when at least three and more studies reported the same outcome, and with the consideration of heterogeneity between studies, we used the random effects model to calculate the effect sizes of prevalence and risk factors with their 95% confidence intervals (95% CI) (23). Heterogeneity was assessed using Chi-square and I2 tests, and sources of heterogeneity were explored by subgroup analysis (including gender, malnutrition degree, age, bacteriology, family size, drug resistance, residence, HIV infection, and region) as well as meta-regression. Sensitivity analysis was conducted by the leave-one-out method. As the conventional funnel plot was not applicable for assessing publication bias of prevalence (24, 25), we used the Doi plot and the Luis Furuya-Kanamori (LFK) asymmetry index to assess publication bias (26), with LFK index within ±1, between ±1 and ±2, and above ±2 being considered as no asymmetry, minor asymmetry, and major asymmetry, respectively. Publication bias was tested by funnel plot and Egger's test when risk factors were pooled with more than 10 studies. P-values of < 0.05 was considered statistically significant. Meta-analyses were performed by using Stata 14.0 software.
3. Results
3.1. Literature selection process
A total of 7, 607 publications were obtained from the initial search, and 53 studies were enrolled for meta-analysis after selecting according to inclusion and exclusion criteria. The flow diagram of the literature selection is shown in Figure 1.
Figure 1.
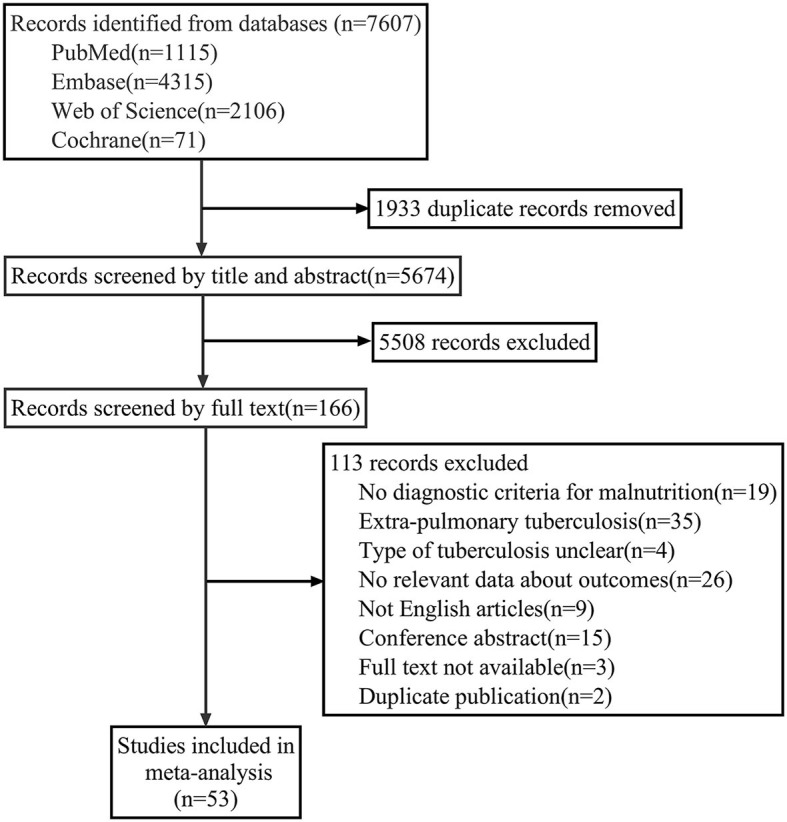
Flow diagram of literature selection.
3.2. Basic characteristics and quality assessment of the included studies
These studies included 34 cross-sectional studies and 19 cohort studies with a total of 48, 598 patients with PTB; 26 studies were from Asia, 20 from Africa, 6 from the Americas, and 1 from Europe. The prevalence of malnutrition was reported in 53 research studies, while risk factors for malnutrition (such as gender, age, smoking, HIV infection, and income level) have been evaluated in 27 studies. A total of 51 studies used the BMI, one used NRS-2002, and one used PG-SGA for assessing malnutrition. Table 1 presents the basic characteristics of the incorporated studies, and the quality assessment is shown in Supplementary Tables 2, 3.
Table 1.
Characteristics of the included studies.
| Study's first author | Publication year | Country | Study design | Simple size | Number of cases | Age | Definition of malnutrition | Risk factors | Study quality |
|---|---|---|---|---|---|---|---|---|---|
| Baluku et al. et al. (27) | 2022 | Uganda | Cross-sectional | 354 | 186 | ≥15 | BMI < 18.5 kg/m2 | - | 8 |
| Soeroto et al. (28) | 2022 | Indonesia | Cohort | 315 | 199 | ≥18 | BMI < 18.5 kg/m2 | Gender; age; smoking; cavity | 7 |
| Iqbal et al. (29) | 2022 | Pakistan | Cross-sectional | 252 | 169 | 31.97 ± 15.34 | BMI < 18.5 kg/m2 | - | 8 |
| Tao et al. (30) | 2021 | China | Cross-sectional | 9,970 | 2,391 | 49.8 ± 19.7 | BMI < 18.5 kg/m2 | - | 7 |
| Magassouba et al. (20) | 2021 | Guinea | Cohort | 218 | 141 | ≥18 | BMI < 18.5 kg/m2 | Gender; residence; HIV; cavity | 6 |
| Li et al. (31) | 2021 | China | Cross-sectional | 246 | 149 | ≥18 (24–65) | NRS-2002≥3 | - | 6 |
| Asemahagn (16) | 2021 | Ethiopia | Cohort | 282 | 198 | ≥15 | BMI < 18.5 kg/m2 | - | 5 |
| Sahile et al. (32) | 2021 | Ethiopia | Cohort | 330 | 159 | ≥18 | BMI < 18.5 kg/m2 | - | 7 |
| Singla et al. (33) | 2021 | India | Cohort | 250 | 207 | - | BMI < 16 kg/m2 (severe) | - | 5 |
| Hussien and Ameni (19) | 2021 | Ethiopia | Cross-sectional | 450 | 232 | Apr-81 | BMI < 18.5 kg/m2 | HIV; smoking; drinking; family size | 7 |
| Baluku et al. (34) | 2021 | Uganda | Cohort | 473 | 276 | 39.0 ± 14.0 | BMI < 18.5 kg/m2 | - | 6 |
| Song et al. (35) | 2021 | China | Cross-sectional | 8,957 | 2,121 | All | BMI < 18.5 kg/m2 | Gender; age; HIV; smoking; drinking | 8 |
| Kassa et al. (36) | 2021 | Ethiopia | Cross-sectional | 515 | 388 | 31.88 ± 12.18 | BMI < 18.5 kg/m2 | Bacteriology | 6 |
| Montes et al. (10) | 2021 | Guatemala | Cross-sectional | 3,945 | 41 | ≥18 | BMI < 18.5 kg/m2 | - | 7 |
| Kitonsa et al. (37) | 2020 | Uganda | Cross-sectional | 491 | 118 | ≥15 | BMI < 18.5 kg/m2 | Bacteriology | 6 |
| Shimouchi et al. (38) | 2020 | Japan | Cross-sectional | 192 | 69 | - | BMI < 18.5 kg/m2 | Bacteriology | 7 |
| Musuenge et al. (39) | 2020 | Saharan | Cross-sectional | 302 | 108 | ≥15 | BMI < 18.5 kg/m2 | Age; Gender; HIV; family size smoking; drinking; low income; residence | 6 |
| Ko et al. (40) | 2020 | Korea | Cross-sectional | 215 | 44 | ≥20 | BMI < 18.5 kg/m2 | Bacteriology | 6 |
| Kornfeld et al. (18) | 2020 | India | Cohort | 389 | 184 | 25–60 | BMI < 18.5 kg/m2 | Gender; low income; smoking; drinking; cavity | 6 |
| White et al. (9) | 2020 | Philippines | Cross-sectional | 634 | 232 | ≥18 | BMI < 18.5 kg/m2 | Residence | 6 |
| Campos et al. (41) | 2019 | Mexico | Cross-sectional | 39 | 6 | ≥15 | BMI < 18.5 kg/m2 | - | 4 |
| Kubiak et al. (42) | 2019 | India | Cross-sectional | 919 | 564 | ≥6 | BMI < 18.5 kg/m2 | - | 7 |
| Rashak et al. (43) | 2019 | Mexico | Cross-sectional | 4,954 | 724 | ≥18 | BMI < 18.5 kg/m2 | - | 6 |
| Hoyt et al. (44) | 2019 | India | Cross-sectional | 173 | 100 | ≥15 | BMI < 18.5 kg/m2 | Gender; smoking; drinking; cavity | 6 |
| Hussien et al. (45) | 2019 | Ethiopia | Cross-sectional | 372 | 235 | 31.55 ± 15.79 | BMI z-score < −2 (age < 18);BMI < 18.5 kg/m2 (age≥18) | Gender; HIV; smoking; drinking; family size; residence; cavity | 5 |
| Feleke and Feleke (46) | 2019 | Ethiopia | Cross-sectional | 1,297 | 735 | 27.78 ± 13.98 | BMI < 18.5 kg/m2 | - | 7 |
| Lazzari et al. (47) | 2018 | Brazil | Cross-sectional | 108 | 39 | ≥18 | BMI < 18.5 kg/m2 | - | 5 |
| Patsche et al. (48) | 2017 | Guinea | Cross-sectional | 141 | 71 | ≥15 | BMI < 18.5 kg/m2 | - | 7 |
| Hochberg et al. (49) | 2017 | India | Cross-sectional | 405 | 244 | 14–81 | BMI < 18.5 kg/m2 | Gender | 6 |
| Yen et al. (15) | 2017 | China | Cohort | 2,226 | 577 | ≥18 | BMI < 18.5 kg/m2 | - | 7 |
| Park et al. (50) | 2016 | Korea | Cohort | 218 | 53 | 41.7 ± 14.2 | BMI < 18.5 kg/m2 | Gender; bacteriology | 8 |
| Chung-Delgado et al. (51) | 2014 | Peru | Cohort | 201 | 53 | 33.6 ± 16.2 | BMI < 18.5 kg/m2 | - | 6 |
| Putri et al. (52) | 2014 | Indonesia | Cohort | 212 | 152 | 37.5 ± 11.9 | BMI < 18.5 kg/m2 | Gender | 7 |
| Bhargava et al. (53) | 2013 | India | Cohort | 1,523 | 1,358 | ≥18 | BMI < 18.5 kg/m2 | Gender | 7 |
| Mupere et al. (54) | 2012 | Uganda | Cohort | 747 | 310 | ≥18 | BMI < 18.5 kg/m2 | Gender; HIV; smoking; drinking | 6 |
| Podewils et al. (55) | 2011 | Latvia | Cohort | 995 | 199 | ≥18 | BMI < 18.5 kg/m2 | Gender; cavity; drinking | 8 |
| Kawai et al. (56) | 2011 | Tanzania | Cohort | 887 | 282 | 18–65 | BMI < 18.5 kg/m2 | Gender; HIV | 6 |
| Lawson et al. (57) | 2008 | Nigeria | Cross-sectional | 625 | 179 | ≥15 | BMI < 18.5 kg/m2 | HIV | 7 |
| Lettow et al. (58) | 2004 | Malawi | Cross-sectional | 779 | 456 | 18–59 | BMI < 18.5 kg/m2 | HIV | 6 |
| Madebo et al. (59) | 1997 | Ethiopia | Cross-sectional | 239 | 187 | ≥15 | BMI < 18.5 kg/m2 | - | 7 |
| Muchsin et al. (60) | 2019 | Indonesia | Cross-sectional | 116 | 64 | ≥18 | BMI < 18.5 kg/m2 | - | 3 |
| Kennedy et al. (61) | 1996 | Tanzania | Cohort | 148 | 106 | ≥18 | BMI < 18.5 kg/m2 | Gender | 5 |
| Swaminathan et al. (62) | 2008 | India | Cross-sectional | 174 | 82 | 31.1 ± 7.5 | BMI < 18.5 kg/m2 | Gender | 7 |
| Nandasena et al. (63) | 2019 | Sri Lanka | Cohort | 424 | 295 | All | BMI < 18.5 kg/m2 | - | 6 |
| Frediani et al. (64) | 2016 | Georgia | Cohort | 191 | 46 | ≥18 | BMI < 18.5 kg/m2 | - | 5 |
| Sari et al. (65) | 2019 | Indonesia | Cross-sectional | 39 | 13 | ≥16 | BMI < 18.5 kg/m2 | - | 6 |
| Abdus Salam (11) | 2018 | India | Cross-sectional | 54 | 50 | 20–50 | BMI < 18.5 kg/m2 | Bacteriology | 4 |
| PrayGod et al. (66) | 2011 | Tanzania | Cross-sectional | 355 | 203 | ≥15 | BMI < 18.5 kg/m2 | Gender | 6 |
| Cegielski et al. (67) | 2013 | Georgia | Cohort | 439 | 233 | ≥18 | BMI < 18.5 kg/m2 | - | 5 |
| Piva et al. (68) | 2013 | Brazi | Cross-sectional | 34 | 13 | 15–59 | BMI < 18.5 kg/m2 | - | 6 |
| Dodor (69) | 2008 | Ghana | Cross-sectional | 570 | 291 | ≥18 | BMI < 18.5 kg/m2 | - | 7 |
| Lin et al. (70) | 2021 | China | Cross-sectional | 117 | 59 | 70.7 | PG-SGA | Gender; family size; low income; cavity | 6 |
| Pakasi et al. (71) | 2009 | Indonesia | Cross-sectional | 121 | 103 | 18–55 | BMI < 18.5 kg/m2 | - | 4 |
3.3. The overall prevalence of malnutrition in patients with PTB
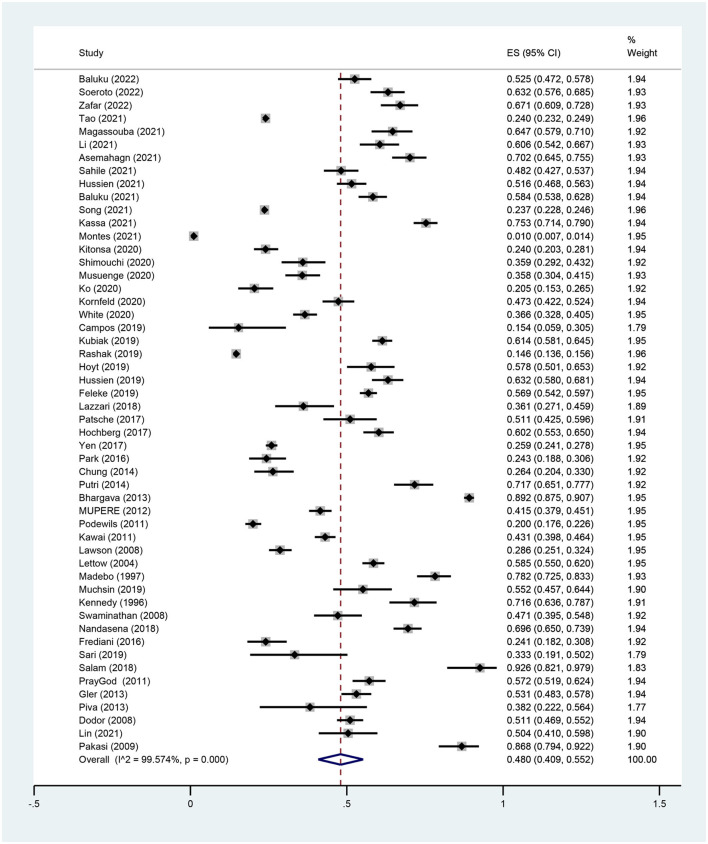
In total, 53 studies described the prevalence of malnutrition among PTB patients. Since Singla et al. (33) described only severe malnutrition, this study was not included to avoid any impact on the overall prevalence. The results showed that the overall prevalence of malnutrition was 48.0% (95% CI, 40.9–55.2%, P < 0.001) with significant heterogeneity between studies (I2 = 99.6%, P < 0.001) (Figure 2).
Figure 2.
Forest plot of the prevalence for malnutrition in patients with PTB.
3.4. Subgroup analysis of the prevalence of malnutrition in patients with PTB
The results of the subgroup analysis were as follows: the prevalence of malnutrition was slightly higher in male subjects than in female subjects (52.3 vs. 50.8%). The prevalence of mild, moderate, and severe malnutrition was 21.6, 14.0, and 29.4%, respectively. The prevalence of malnutrition was 49.6 and 33.4% among those aged < 65 and ≥65 years. The prevalence of malnutrition was higher among PTB patients with bacterial positivity, family size over 4, drug resistance, residing in rural areas, and HIV infection. The prevalence of malnutrition was significantly higher among PTB patients in Africa and Asia compared with the Americas, and it is not described in Europe as only one study was offered. The summary results of the subgroup analysis are shown in Table 2.
Table 2.
Subgroup analysis of the prevalence of malnutrition in pulmonary tuberculosis.
| Subgroups | No. of studies | Prevalence (95%CI) | Heterogeneity | |
|---|---|---|---|---|
| I2 | P-value | |||
| Gender | ||||
| Male | 18 | 52.3% (39.6%, 64.9%) | 99.3% | < 0.001 |
| Female | 18 | 50.8% (37.0%, 64.6%) | 98.6% | < 0.001 |
| Malnutrition degree | ||||
| Mild | 10 | 21.6% (18.3%, 25.0%) | 86.4% | < 0.001 |
| Moderate | 10 | 14.0% (11.7%, 16.5%) | 79.8% | < 0.001 |
| Severe | 16 | 29.4% (19.2%, 40.9%) | 98.9% | < 0.001 |
| Age | ||||
| ≥65 | 3 | 33.4% (19.7%, 48.5%) | 59.0% | < 0.001 |
| < 65 | 8 | 49.6% (33.4%, 65.8%) | 99.3% | < 0.001 |
| Bacteriology | ||||
| Positive | 27 | 55.9% (47.9%, 63.8%) | 99.1% | < 0.001 |
| Negative | 8 | 40.0% (21.8%, 59.8%) | 96.7% | < 0.001 |
| Family size | ||||
| ≤ 4 | 4 | 48.8% (41.8%, 55.8%) | 78.5% | < 0.001 |
| >4 | 4 | 54.5% (39.7%, 68.9%) | 93.9% | < 0.001 |
| Drug resistance | ||||
| Yes | 14 | 44.1% (32.3%, 56.3%) | 99.0% | < 0.001 |
| No | 4 | 20.5% (6.9%, 39.1%) | 99.9% | < 0.001 |
| Residence | ||||
| Rural | 3 | 51.2% (29.5%, 72.6%) | 95.2% | < 0.001 |
| Urban | 4 | 41.1% (32.1%, 50.4%) | 91.9% | < 0.001 |
| HIV infection | ||||
| Yes | 10 | 51.5% (42.9%, 60.1%) | 90.8% | < 0.001 |
| No | 9 | 47.7% (39.1%, 56.2%) | 95.4% | < 0.001 |
| Region | ||||
| Asia | 25 | 51.5% (42.2%, 60.7%) | 99.5% | < 0.001 |
| Africa | 20 | 54.1% (47.7%, 60.4%) | 97.4% | < 0.001 |
| America | 6 | 19.0% (7.2%, 34.4%) | 99.4% | < 0.001 |
3.5. Meta-regression
To further explore the sources of heterogeneity, a multivariate meta-regression analysis was performed based on the publication year, sample size, study design, and study quality, and the results showed that sample size was a possible source of heterogeneity (I2 = 93.58%, adjusted R2 = 36.30%, t = −2.05, P = 0.046) (Table 3). The studies were divided into two groups according to the median sample size (n = 342) and the results showed that both the small sample studies (I2 = 96.7%, P < 0.001) and the large sample studies (I2 = 99.8%, P < 0.001) did not reduce heterogeneity.
Table 3.
Multivariate meta-regression analysis of the prevalence for malnutrition in patients with PTB.
| Covariates | Coef. | Std. err. | t | P | 95% Conf. interval | |
|---|---|---|---|---|---|---|
| Publication year | −0.005 | 0.004 | −1.16 | 0.254 | −0.013 | 0.004 |
| Simple size | −0.265a | 0.129a | −2.05 | 0.046 | −0.526a | −0.004a |
| Study design | ||||||
| Cross–sectional study | −0.009 | 0.051 | −0.17 | 0.866 | −0.112 | 0.095 |
| Study quality | −0.014 | 0.027 | −0.52 | 0.605 | −0.068 | 0.040 |
| Region | ||||||
| Africa | 0.295 | 0.174 | 1.69 | 0.098 | −0.056 | 0.645 |
| Asia | 0.298 | 0.176 | 1.70 | 0.097 | −0.056 | 0.653 |
| America | −0.003 | 0.193 | −0.01 | 0.989 | −0.392 | 0.386 |
aOriginal data × 10000.
3.6. Sensitivity analysis
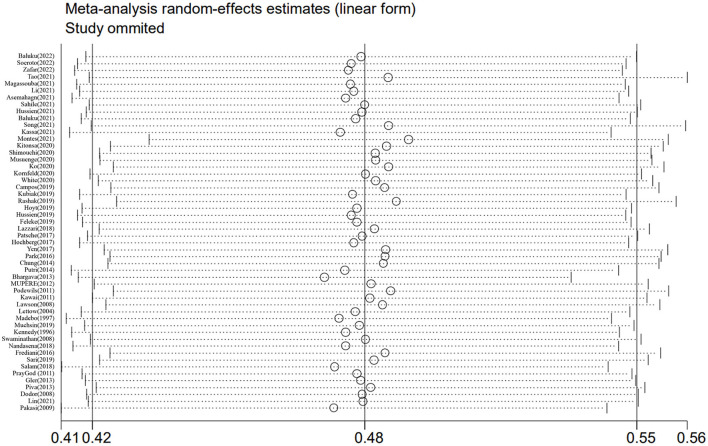
Sensitivity analysis was performed by excluding individual studies one by one, and the results showed that the prevalence of malnutrition ranged from 47.1 to 49.4%, with little difference from the overall prevalence, which suggested that this meta-analysis was stable and reliable (Figure 3).
Figure 3.
Sensitivity analysis of the prevalence of malnutrition in patients with PTB.
3.7. Risk factors for malnutrition in patients with PTB
3.7.1. Gender
A total of 18 studies reported the association between male and malnutrition in patients with PTB. The meta-analysis showed that male was not a risk factor for malnutrition (OR = 1.04, 95% CI 0.85–1.26, P = 0.720), and there was significant heterogeneity between studies (I2 = 75.4%, P < 0.001). The meta-regression analysis showed that publication year (βcoef=-0.008, P = 0.681), sample size (βcoef=-0.000, P = 0.963), study design (βcoef=0.236, P = 0.314), and study quality (βcoef=-0.102, P = 0.437) did not explain the source of heterogeneity. Sensitivity analysis showed that none of the studies had an effect on the overall effect size, which suggested that the results of the studies were reliable.
3.7.2. Bacterial positivity
A total of eight studies were enrolled, with seven moderate and one high quality studies. The study revealed that bacterial positivity was a risk factor for malnutrition (OR = 2.08, 95% CI 1.26–3.41, P = 0.004), with significant heterogeneity between studies (I2 = 79.1%, P < 0.001). Meta-regression was not performed as the number of studies was < 10. In addition to the restriction to cross-sectional studies (I2 = 81.9%, P < 0.001), heterogeneity was not reduced and the combined effect sizes remained significant. Sensitivity analysis showed that the result was stable and reliable.
3.7.3. HIV infection
A total of eight studies were included, all being moderate quality for the studies. The study indicated that HIV infection was not a risk factor for malnutrition (OR = 1.37, 95% CI 0.97–1.93, P = 0.070). Heterogeneity between studies was observed (I2 = 79.1%, P < 0.001). Heterogeneity was still not reduced when limited to cross-sectional studies (I2 = 77.0%, P = 0.002); however, the pooled results were altered (OR = 1.80, P = 0.002). Sensitivity analysis showed that the result was unstable.
3.7.4. Low income
A total of three studies were enrolled and the results showed that low income was a risk factor for malnutrition (OR = 1.44, 95% CI 1.11–1.86, P = 0.005), with no heterogeneity between studies (I2 = 0.0%, P = 0.418). Sensitivity analysis suggested that the result was stable.
3.7.5. Residing in rural areas
A total of four studies were excluded and the results revealed that residing in rural areas was a risk factor for malnutrition (OR = 1.51, 95% CI 1.20–1.89, P < 0.001), with no heterogeneity between studies (I2 = 0.0%, P = 0.690). The sensitivity analysis was stable and reliable.
3.7.6. Smoking
A total of eight studies were included and the results suggested that smoking was a risk factor for malnutrition (OR = 1.40, 95% CI 1.02–1.92, P = 0.039) with significant heterogeneity between studies (I2 = 76.6%, P < 0.001). Heterogeneity was significantly lower when restricted to cross-sectional studies (I2 = 27.0%, P = 0.241) and high-quality studies (I2 = 0.0%, P = 0.515); however, the pooled results were not statistically significant. Sensitivity analysis revealed that the result was unstable.
3.7.7. Pulmonary cavity
Six studies were enrolled and the results showed that the pulmonary cavity was a risk factor for malnutrition (OR = 1.59, 95% CI 1.04–2.43, P = 0.033) with heterogeneity between studies (I2 = 84.4%, P < 0.001). When applying restriction to cohort studies, there was no heterogeneity between studies (I2 = 0.0%, P = 0.417), and the pooled result was still significant (OR = 1.91, P < 0.001). However, when applying restriction to high-quality studies, the pooled effect size was not statistically significant (OR = 1.45, P = 0.219) and heterogeneity between studies still existed (I2 = 90.0%, P < 0.001). Sensitivity analyses revealed the result was unstable.
3.7.8. age
A total of three studies were enrolled and the results showed that age ≥65 years was not associated with malnutrition (OR = 1.26, 95% CI 0.54–2.96, P = 0.598) with heterogeneity between studies (I2 = 70.6%, P = 0.033). The sensitivity analysis was unstable.
3.7.9. Family size
Four studies were enrolled for this meta-analysis and the results showed that family size over 4 was a risk factor for malnutrition (OR = 1.33, 95% CI 1.08–1.62, P = 0.006) with heterogeneity between studies (I2 = 49.8%, P = 0.113). The sensitivity analysis was unstable.
3.7.10. Drinking
With eight studies included, the meta-analysis showed that drinking was not a risk factor for malnutrition (OR = 1.17, 95% CI 0.81–1.69, P = 0.389), with apparent heterogeneity between studies (I2 = 85.4%, P < 0.001). When limited to high-quality studies (I2 = 0.0%, P = 0.512) and cross-sectional studies (I2 = 35.9%, P = 0.182), heterogeneity was significantly lower and the pooled results were similar to the total effect size. The sensitivity analysis was stable and reliable.
3.8. Publication bias
Publication bias assessment was conducted for overall prevalence and the Doi plot revealed major asymmetry (LFK index = 3.87), which suggested that smaller studies may report a higher prevalence for malnutrition (Supplementary Figure 1). Among the risk factors, only the number of studies for “gender” was above 10. The funnel plot showed a slight asymmetry, but Egger's test was not statistically significant (t = −0.31, P = 0.759), which indicated that there was no publication bias (Supplementary Figure 2).
4. Discussion
This study consolidated the current evidence of studies related to the prevalence and risk factors for malnutrition in patients with PTB. A total of 53 studies were included and the pooled prevalence for malnutrition was 48.0% among PTB patients, slightly lower than the 50.8% reported by Wondmieneh et al. (17), which may be related to the small sample size included in his study. The meta-regression revealed that sample size was a source of heterogeneity for overall prevalence with prevalence decreasing with increasing sample size, which was consistent with the Doi plot and suggested that smaller studies may report a higher prevalence for malnutrition. Therefore, a subgroup analysis was carried out and showed a prevalence of 51.5% for the small sample studies, which was similar to the results reported by Wondmieneh et al. (17). However, the reason why the prevalence was higher in the small sample studies may be that most of them included were from hospitalized patients, and studies have demonstrated that patients who were hospitalized tended to have poorer nutritional status (72).
Although the study noted a higher prevalence of PTB among men (73), there was no significant difference in the prevalence of malnutrition between male and female patients with PTB. The meta-analysis showed that male was not associated with malnutrition, which further confirmed that gender was not a risk factor for malnutrition in patients with PTB. Currently, this study showed that the prevalence of severe malnutrition was higher than mild and moderate in patients with PTB, the possible reasons for this are as follows: (1) the studies included were mainly from Africa and Asia, an area with a larger population in rural, lack of medical resources, and poor health awareness, where patients were often not treated adequately and timely due to delayed access to care (4, 74), which may lead to an increased risk for malnutrition and a higher rate for severe malnutrition, and our study also demonstrated that the prevalence for malnutrition was significantly higher in Asia and Africa than America. (2) In addition, a previous study showed a high proportion of drug resistance in Asia and Africa (75), which makes it harder to treat, longer treatment cycles, and poor adherence to treatment among these patients, with a higher risk for severe malnutrition due to the persistence of active PTB (76, 77).
In this study, the prevalence of malnutrition in patients with bacterial positivity was significantly higher than bacteria negative, and bacterial positivity was a risk factor for malnutrition. Walker et al. (78) observed that patients with bacterial positivity had significantly higher levels of inflammatory factors, such as matrix metalloproteinase 1 and matrix metalloproteinase 8, than negative patients, and the stronger the inflammatory response, the more active the catabolism, the greater the energy expenditure, and the greater the risk for malnutrition. Moreover, malnutrition can also lead to delayed sputum conversion (16). Although patients with bacterial positivity were prone to malnutrition and poor outcomes, a study showed that nutritional support not only reduced the risk for malnutrition by suppressing the inflammatory response but also improved the sputum smear or culture conversion rate of PTB (79). Therefore, it was beneficial for the treatment of patients with PTB by nutritional support early.
In our study, the prevalence of malnutrition was significantly higher among patients with pulmonary cavities than those without cavities. A study suggested that patient with a pulmonary cavity on chest radiograph was associated with higher bacterial positivity and a higher bacterial load with thick-walled cavities in patients (80, 81); at the same time, patients with pulmonary cavities always had lower success rates of treatment and longer cycles of treatment (82, 83), which made patients who suffered from PTB with cavities more likely to develop malnutrition. Although this meta-analysis revealed that the pulmonary cavity was a risk factor for malnutrition, sensitivity analysis showed that the results were unstable. Therefore, the conclusions need to be interpreted with caution. One study showed that the prevalence of malnutrition increased with age (84). However, our study concluded the opposite, and age was not a risk factor for malnutrition in patients with PTB. Through the analysis of the included studies, we noted that the same three studies were included both in the older and the younger age groups, and the prevalence of malnutrition was higher in the older than that in the younger group. Because fewer studies were included in the older age group and the number difference between the two groups was large, it could not be assumed that the prevalence of malnutrition was lower in the older age group and the result needed to be reassessed.
This meta-analysis revealed that malnutrition was more prevalent among PTB patients with low income, residing in rural areas, and larger family sizes. According to a meta-analysis, the social economic status has a significant impact on a patient's prognosis for tuberculosis, and low-income patients are more likely to experience treatment failure and multidrug resistance (85). Patients with low income and residing in rural areas had difficulty accessing healthcare and often experienced delayed access, which caused malnutrition due to the persistence of active PTB (69, 86). Whereas, the family size affected the educational status of family members and the distribution of food to a certain extent for less economically developed areas (45, 87), which made patients more vulnerable to malnutrition due to poor health awareness and inadequate intake. This study also showed a significant association between low income and residing in rural areas and malnutrition in PTB, which further confirmed that low income and residing in rural areas were important risk factors for malnutrition. Although the family size was associated with malnutrition in patients with PTB, the results of sensitivity analysis were unstable, so the relationship between them needed to be explored further.
In addition, malnutrition was more prevalent with HIV infection among PTB patients. Patients with HIV infection often had various degrees of immune deficiency and were prone to occurring opportunistic infections, which lead to more energy expenditure and increased nutritional requirements (88, 89). At the same time, people with HIV infection may suffer from oral candidiasis, anorexia, intestinal mucosal damage, and diarrhea, with resulting deficiencies in intake and impairment of absorption (89, 90). Therefore, patients with HIV infection were more likely to be malnourished. However, this study was unclear whether HIV infection was a risk factor for malnutrition in patients with PTB due to the instability of sensitivity analysis, but the outcome suggested that HIV infection tended to increase malnutrition. Furthermore, Montes et al. (10) showed that HIV infection was significantly associated with poor prognosis in patients with PTB, thus it was important to screen for HIV and provide antiviral treatment timely. We also assessed the relationship between smoking and drinking and malnutrition. The meta-analysis showed that smoking increased the risk of malnutrition, but the sensitivity analysis was unstable. In contrast, drinking was not a risk factor for malnutrition; although it was not associated with malnutrition, it was necessary to avoid drinking to prevent exacerbating liver damage during anti-tuberculosis drug treatment (91).
Malnutrition is strongly associated with clinical outcomes in patients with tuberculosis. Several prospective cohort studies have shown that malnutrition is a risk factor for treatment failure, relapse, and death in patients with tuberculosis (92–94). Therefore, it is important to focus on the nutritional status and the risk factors of malnutrition in patients with PTB in clinical management.
4.1. Strengths and limitations
There were several advantages to the systematic review and meta-analysis. The first benefit of this study is the consolidated data on the prevalence of malnutrition and risk factors in PTB patients around the world, which will help to improve the management of PTB patients. Second, this meta-analysis displayed a larger sample size, a wider range of research, and more reliable results compared to earlier research. Finally, we searched multiple databases to minimalize the omission of relevant studies. However, there were some limitations to our study. First, some indicators were characterized by high heterogeneity between studies, a small number of studies, and an unstable sensitivity analysis, which suggests that more prospective cohort studies are needed to further evaluate the relationship among HIV infection, age, family size, smoking, pulmonary cavity, and malnutrition in the future, so the results should be explained with caution. Second, as the majority of the studies were retrospective observational studies with an unclear sequence of exposure and outcome, the results should be treated with caution. Third, only one study was from Europe, which made it impossible to provide an accurate assessment of the prevalence of malnutrition among PTB patients in Europe. Finally, we only included studies published in English, which may cause bias in the results due to missing important studies published in other languages.
5. Conclusion
In summary, this meta-analysis revealed that the prevalence for malnutrition was quite prevalent in patients with PTB, and bacterial positivity, low income, and residing in rural areas were significantly associated with malnutrition in patients with PTB. Therefore, we urge clinicians and TB patients to pay attention to the screening and prevention of malnutrition. Early identification of malnutrition and its risk factors in patients with PTB facilitates timely nutritional support treatment for patients with PTB, which will prevent further development and deterioration of PTB.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Study design: AL and N-nL. Data search and extraction: AL and S-yY. Data analysis and writing: AL, S-yY, Q-gL, and J-xL. Manuscript revision: X-yY and N-nL. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1173619/full#supplementary-material
References
- 1.Gutierrez MC, Brisse S, Brosch R, Fabre M, Omais B, Marmiesse M, et al. Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog. (2005) 1:e5. 10.1371/journal.ppat.0010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Tuberculosis Report 2022 . (2022). https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed October 27, 2022).
- 3.Di Gennaro F, Gualano G, Timelli L, Vittozzi P, Di Bari V, Libertone R, et al. Increase in tuberculosis diagnostic delay during first wave of the COVID-19 pandemic: data from an italian infectious disease referral hospital. Antibiotics. (2021) 10:272. 10.3390/antibiotics10030272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owolabi OA, Jallow AO, Jallow M, Sowe G, Jallow R, Genekah MD, et al. Delay in the diagnosis of pulmonary tuberculosis in The Gambia, West Africa: a cross-sectional study. Int J Infect Dis. (2020) 101:102–06. 10.1016/j.ijid.2020.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Zhao G, Wu L, Lu M, Liu W, Wu Y, et al. Prevalence and patterns of drug resistance among pulmonary tuberculosis patients in Hangzhou, China. Antimicrob Resist Infect Control. (2018) 7:61. 10.1186/s13756-018-0348-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AlSahafi AJ, Shah H, AlSayali MM, Mandoura N, Assiri M, Almohammadi EL, et al. High non-compliance rate with anti-tuberculosis treatment: a need to shift facility-based directly observed therapy short course (DOTS) to community mobile outreach team supervision in Saudi Arabia. BMC Public Health. (2019) 19:1168. 10.1186/s12889-019-7520-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev. (2018) 27:1–14. 10.1183/16000617.0077-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh KF, Lui JK. Post-tuberculosis pulmonary hypertension: a case of global disparity in health care. Lancet Glob Health. (2022) 10:e476. 10.1016/S2214-109X(22)00042-0 [DOI] [PubMed] [Google Scholar]
- 9.White LV, Edwards T, Lee N, Castro MC, Saludar NR, Calapis RW, et al. Patterns and predictors of co-morbidities in Tuberculosis: A cross-sectional study in the Philippines. Sci Rep. (2020) 10:1–27. 10.1038/s41598-020-60942-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montes K, Atluri H, Silvestre Tuch H, Ramirez L, Paiz J, Hesse Lopez A, et al. Risk factors for mortality and multidrug resistance in pulmonary tuberculosis in Guatemala: a retrospective analysis of mandatory reporting. J Clin Tuberc Other Mycobact Dis. (2021) 25:100287. 10.1016/j.jctube.2021.100287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdus Salam PO. Nutritional status in sputum positive and sputum negative cases of pulmonary tuberculosis. Nat J Physiol Pharm Pharmacol. (2017). 10.5455/njppp.2018.8.124751812201712607337 [DOI] [Google Scholar]
- 12.Sinha P, Davis J, Saag L, Wanke C, Salgame P, Mesick J, et al. Undernutrition and Tuberculosis: Public Health Implications. J Infect Dis. (2019) 219:1356–63. 10.1093/infdis/jiy675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadesse F, Mitiku H, Girma S, Kenay A. Magnitude of undernutrition and associated factors among adult tuberculosis patients attending public health facilities in Haramaya District, Eastern Ethiopia. Bmc Pulm Med. (2023) 23:42. 10.1186/s12890-023-02318-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song JH, Yoon SY, Park TY, Heo EY, Kim DK, Chung HS, et al. The clinical impact of drug-induced hepatotoxicity on anti-tuberculosis therapy: a case control study. Respir Res. (2019) 20:283. 10.1186/s12931-019-1256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen YF, Tung FI, Ho BL, Lai YJ. Underweight increases the risk of early death in tuberculosis patients. Br J Nutr. (2017) 118:1052–60. 10.1017/S0007114517003166 [DOI] [PubMed] [Google Scholar]
- 16.Asemahagn MA. Sputum smear conversion and associated factors among smear-positive pulmonary tuberculosis patients in East Gojjam Zone, Northwest Ethiopia: a longitudinal study. BMC Pulm Med. (2021) 21:83. 10.1186/s12890-021-01483-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wondmieneh A, Gedefaw G, Getie A, Demis A. Prevalence of undernutrition among adult tuberculosis patients in Ethiopia: a systematic review and meta-analysis. J Clin Tuberc Other Mycobact Dis. (2021) 22:100211. 10.1016/j.jctube.2020.100211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornfeld H, Sahukar SB, Procter-Gray E, Kumar NP, West K, Kane K, et al. Impact of diabetes and low body mass index on tuberculosis treatment outcomes. Clin Infect Dis. (2020) 71:e392–98. 10.1093/cid/ciaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussien B, Ameni G. A cross-sectional study on the magnitude of undernutrition in tuberculosis patients in the oromia region of Ethiopia. J Multidiscip Healthc. (2021) 14:2421–28. 10.2147/JMDH.S326233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magassouba AS, Toure AA, Diallo BD, Camara LM, Toure D, Conte N, et al. Malnutrition prevalence and associated biochemical factors among drug-resistance tuberculosis (DR-TB) patients at key treatment sites in Conakry City, Republic of Guinea. Pan Afr Med J. (2021) 38:279. 10.11604/pamj.2021.38.279.27270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. (2009) 339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–05. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 23.Chen N, Li Y, Fang J, Lu Q, He L. Risk factors for malnutrition in stroke patients: A meta-analysis. Clin Nutr. (2019) 38:127–35. 10.1016/j.clnu.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 24.Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E, et al. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. (2021) 21:189. 10.1186/s12874-021-01381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. (2014) 67:897–903. 10.1016/j.jclinepi.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Furuya-Kanamori L, Barendregt JJ, Doi S. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. (2018) 16:195–203. 10.1097/XEB.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 27.Baluku JB, Mayinja E, Mugabe P, Ntabadde K, Olum R, Bongomin F. Prevalence of anaemia and associated factors among people with pulmonary tuberculosis in Uganda. Epidemiol Infect. (2022) 150:e29. 10.1017/S0950268822000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soeroto AY, Nurhayati RD, Purwiga A, Lestari BW, Pratiwi C, Santoso P, et al. Factors associated with treatment outcome of MDR/RR-TB patients treated with shorter injectable based regimen in West Java Indonesia. PLoS ONE. (2022) 17:e263304. 10.1371/journal.pone.0263304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iqbal Z, Khan MA, Aziz A, Nasir SM. Time for culture conversion and its associated factors in multidrug-resistant tuberculosis patients at a tertiary level hospital in Peshawar, Pakistan. Pak J Med Sci. (2022) 38:1009–15. 10.12669/pjms.38.4.5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao N, Li Y, Song W, Liu J, Zhang Q, Xu T, et al. Risk factors for drug-resistant tuberculosis, the association between comorbidity status and drug-resistant patterns: a retrospective study of previously treated pulmonary tuberculosis in Shandong, China, during 2004–2019. BMJ Open. (2021) 11:e44349. 10.1136/bmjopen-2020-044349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Yang F, Zhou H, Shu L, Wang R, Zhao C. Clinical application of NRS-2002 in nutritional risk screening of tuberculosis inpatients. Ann Palliat Med. (2021) 10:5322–28. 10.21037/apm-21-610 [DOI] [PubMed] [Google Scholar]
- 32.Sahile Z, Tezera R, Haile Mariam D, Collins J, Ali JH. Nutritional status and TB treatment outcomes in Addis Ababa, Ethiopia: an ambi-directional cohort study. PLoS ONE. (2021) 16:e247945. 10.1371/journal.pone.0247945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singla R, Raghu B, Gupta A, Caminero JA, Sethi P, Tayal D, et al. Risk factors for early mortality in patients with pulmonary tuberculosis admitted to the emergency room. Pulmonology. (2021) 27:35–42. 10.1016/j.pulmoe.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 34.Baluku JB, Namiiro S, Nabwana M, Muttamba W, Kirenga B. Undernutrition and treatment success in drug-resistant tuberculosis in Uganda. Infect Drug Resist. (2021) 14:3673–81. 10.2147/IDR.S332148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song W, Guo J, Xu T, Li S, Liu J, Tao N, et al. Association between body mass index and newly diagnosed drug-resistant pulmonary tuberculosis in Shandong, China from 2004 to 2019. BMC Pulm Med. (2021) 21:1–14. 10.1186/s12890-021-01774-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kassa GM, Merid MW, Muluneh AG, Fentie DT. Sputum smear grading and associated factors among bacteriologically confirmed pulmonary drug-resistant tuberculosis patients in Ethiopia. BMC Infect Dis. (2021) 21:1–7. 10.1186/s12879-021-05933-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitonsa PJ, Nalutaaya A, Mukiibi J, Nakasolya O, Isooba D, Kamoga C, et al. Evaluation of underweight status may improve identification of the highest-risk patients during outpatient evaluation for pulmonary tuberculosis. PLoS ONE. (2020) 15:e243542. 10.1371/journal.pone.0243542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimouchi A, Tsuda Y, Komukai J, Matsumoto K, Yoshida H, Ohkado A. Characteristics of individuals with tuberculosis in an urban, poor population in Osaka City, Japan — a case-control study. Western Pacific Surv Resp J. (2020) 11:20–6. 10.5365/wpsar.2018.9.1.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musuenge BB, Poda GG, Chen P. Nutritional status of patients with tuberculosis and associated factors in the health centre region of burkina faso. Nutrients. (2020) 12:2540. 10.3390/nu12092540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ko Y, Kim C, Park YB, Mo E, Moon J. Changes in nutritional status in pulmonary tuberculosis: longitudinal changes in BMI according to acid-fast bacilli smear positivity. J Clin Med. (2020) 9:4082. 10.3390/jcm9124082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campos-Gongora E, Lopez-Martinez J, Huerta-Oros J, Arredondo-Mendoza GI, Jimenez-Salas Z. Nutritional status evaluation and nutrient intake in adult patients with pulmonary tuberculosis and their contacts. J Infect Dev Ctries. (2019) 13:303–10. 10.3855/jidc.11267 [DOI] [PubMed] [Google Scholar]
- 42.Kubiak RW, Sarkar S, Horsburgh CR, Roy G, Kratz M, Reshma A, et al. Interaction of nutritional status and diabetes on active and latent tuberculosis: a cross-sectional analysis. BMC Infect Dis. (2019) 19:1–4. 10.1186/s12879-019-4244-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rashak HA, Sánchez-Pérez HJ, Abdelbary BE, Bencomo-Alerm A, Enriquez-Ríos N, Gómez-Velasco A, et al. Diabetes, undernutrition, migration and indigenous communities: tuberculosis in Chiapas, Mexico. Epidemiol Infect. (2019) 147:e61. 10.1017/S0950268818003461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoyt KJ, Sarkar S, White L, Joseph NM, Salgame P, Lakshminarayanan S, et al. Effect of malnutrition on radiographic findings and mycobacterial burden in pulmonary tuberculosis. PLoS ONE. (2019) 14:e214011. 10.1371/journal.pone.0214011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussien B, Hussen MM, Seid A, Hussen A. Nutritional deficiency and associated factors among new pulmonary tuberculosis patients of Bale Zone Hospitals, southeast Ethiopia. Bmc Res Notes. (2019) 12:1–16. 10.1186/s13104-019-4786-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feleke BE, Feleke TE, Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm Med. (2019) 19:843. 10.1186/s12890-019-0953-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazzari TK, Forte GC, Silva DR. Nutrition status among HIV-positive and HIV-negative inpatients with pulmonary tuberculosis. Nutr Clin Pract. (2018) 33:858–64. 10.1002/ncp.10006 [DOI] [PubMed] [Google Scholar]
- 48.Patsche CB, Rudolf F, Mogensen SW, Sifna A, Gomes VF, Byberg S, et al. Low prevalence of malnourishment among household contacts of patients with tuberculosis in Guinea-Bissau. Int J Tuberc Lung Dis. (2017) 21:664–69. 10.5588/ijtld.16.0673 [DOI] [PubMed] [Google Scholar]
- 49.Hochberg NS, Sarkar S, Horsburgh CR, Knudsen S, Pleskunas J, Sahu S, et al. Comorbidities in pulmonary tuberculosis cases in Puducherry and Tamil Nadu, India: opportunities for intervention. PLoS ONE. (2017) 12:e183195. 10.1371/journal.pone.0183195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park H, Kim S, Moon S, Byun J, Kim J, Lee C, et al. Association between body mass index and sputum culture conversion among south korean patients with multidrug resistant tuberculosis in a tuberculosis referral hospital. Inf Chemother. (2016) 48:317. 10.3947/ic.2016.48.4.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung-Delgado K, Revilla-Montag A, Guillén-Bravo S, Bernabe-Ortiz A. Weight variation over time and its relevance among multidrug-resistant tuberculosis patients. Int J Infect Dis. (2014) 23:20–4. 10.1016/j.ijid.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 52.Putri FA, Burhan E, Nawas A, Soepandi PZ, Sutoyo DK, Agustin H, et al. Body mass index predictive of sputum culture conversion among MDR-TB patients in Indonesia. The Int J Tuberc Lung Disease. (2014) 18:564–70. 10.5588/ijtld.13.0602 [DOI] [PubMed] [Google Scholar]
- 53.Bhargava A, Chatterjee M, Jain Y, Chatterjee B, Kataria A, Bhargava M, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS ONE. (2013) 8:e77979. 10.1371/journal.pone.0077979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mupere E, Malone L, Zalwango S, Chiunda A, Okwera A, Parraga I, et al. Lean tissue mass wasting is associated with increased risk of mortality among women with pulmonary tuberculosis in urban Uganda. Ann Epidemiol. (2012) 22:466–73. 10.1016/j.annepidem.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Podewils LJ Holtz T, Riekstina V, Skripconoka V, Zarovska E, Kirvelaite G, et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect. (2011) 139:113–20. 10.1017/S0950268810000907 [DOI] [PubMed] [Google Scholar]
- 56.Kawai K, Villamor E, Mugusi FM, Saathoff E, Urassa W, Bosch RJ, et al. Predictors of change in nutritional and hemoglobin status among adults treated for tuberculosis in Tanzania. The Int J Tub Lung Disease. (2011) 15:1380–89. 10.5588/ijtld.10.0784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lawson L, Yassin MA, Thacher TD, Olatunji OO, Lawson JO, Akingbogun TI, et al. Clinical presentation of adults with pulmonary tuberculosis with and without HIV infection in Nigeria. Scand J Infect Dis. (2009) 40:30–5. 10.1080/00365540701509899 [DOI] [PubMed] [Google Scholar]
- 58.van Lettow M, Harries AD, Kumwenda JJ, Zijlstra EE, Clark TD, Taha TE, et al. Micronutrient malnutrition and wasting in adults with pulmonary tuberculosis with and without HIV co-infection in Malawi. BMC Infect Dis. (2004) 4:61. 10.1186/1471-2334-4-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madebo T, Nysaeter G, Lindtjorn B. HIV infection and malnutrition change the clinical and radiological features of pulmonary tuberculosis. Scand J Infect Dis. (1997) 29:355–59. 10.3109/00365549709011830 [DOI] [PubMed] [Google Scholar]
- 60.Muchsin M, Siregar FA, Sudaryati E. The influence of nutritional status and ventilation on the incidence of pulmonary tuberculosis at Langsa. Open Access Maced J Med Sci. (2019) 7:3421–24. 10.3889/oamjms.2019.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kennedy N, Ramsay A, Uiso L, Gutmann J, Ngowi FI, Gillespie SH. Nutritional status and weight gain in patients with pulmonary tuberculosis in Tanzania. Trans R Soc Trop Med Hyg. (1996) 90:162–66. 10.1016/S0035-9203(96)90123-6 [DOI] [PubMed] [Google Scholar]
- 62.Swaminathan S, Padmapriyadarsini C, Sukumar B, Iliayas S, Kumar SR, Triveni C, et al. Nutritional status of persons with HIV infection, persons with HIV infection and tuberculosis, and HIV-Negative individuals from Southern India. Clin Infect Dis. (2008) 46:946–49. 10.1086/528860 [DOI] [PubMed] [Google Scholar]
- 63.Nandasena S, Senavirathna C, Munasinghe C, Wijesena C, Sucharitharathna R. Characteristics and sputum conversion of tuberculosis (TB) patients in Kalutara, Sri Lanka. Indian J Tub. (2019) 66:76–80. 10.1016/j.ijtb.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 64.Frediani JK, Sanikidze E, Kipiani M, Tukvadze N, Hebbar G, Ramakrishnan U, et al. Macronutrient intake and body composition changes during anti-tuberculosis therapy in adults. Clin Nutr. (2016) 35:205–12. 10.1016/j.clnu.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sari DK, Mega JY, Harahap J. Nutrition status related to clinical improvement in AFB-positive pulmonary tuberculosis patients in primary health centres in Medan, Indonesia. Open Access Maced J Med Sci. (2019) 7:1621–27. 10.3889/oamjms.2019.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.PrayGod G, Range N, Faurholt-Jepsen D, Jeremiah K, Faurholt-Jepsen M, Aabye MG, et al. Weight, body composition and handgrip strength among pulmonary tuberculosis patients: a matched cross-sectional study in Mwanza, Tanzania. Trans R Soc Trop Med Hyg. (2011) 105:140–47. 10.1016/j.trstmh.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 67.Cegielski P, Gler MT, Guilatco R, Johnson JL, Caoili JC, Ershova J. Weight gain and response to treatment for multidrug-resistant tuberculosis. Am J Trop Med Hyg. (2013) 89:943–49. 10.4269/ajtmh.13-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piva SGN, Costa MDCN, Barreto FR, Pereira SM. Prevalence of nutritional deficiency in patients with pulmonary tuberculosis. J Bras Pneumol. (2013) 39:476–83. 10.1590/S1806-37132013000400012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dodor EA. Evaluation of nutritional status of new tuberculosis patients at the effia-nkwanta regional hospital. Ghana Med J. (2008) 42:22–8. [PMC free article] [PubMed] [Google Scholar]
- 70.Lin H, Lin M, Chi C, Ye J, Hsieh C. Nutrition assessment and adverse outcomes in hospitalized patients with tuberculosis. J Clin Med. (2021) 10:2702. 10.3390/jcm10122702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pakasi TA, Karyadi E, Dolmans WM, van der Meer JW, van der Velden K. Malnutrition and socio-demographic factors associated with pulmonary tuberculosis in Timor and Rote Islands, Indonesia. Int J Tuberc Lung Dis. (2009) 13:755–59. [PubMed] [Google Scholar]
- 72.Leij-Halfwerk S, Verwijs MH, van Houdt S, Borkent JW, Guaitoli PR, Pelgrim T, et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults ≥65 years: a systematic review and meta-analysis. Maturitas. (2019) 126:80–9. 10.1016/j.maturitas.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 73.Moyo S, Ismail F, Van der Walt M, Ismail N, Mkhondo N, Dlamini S, et al. Prevalence of bacteriologically confirmed pulmonary tuberculosis in South Africa, 2017-19: a multistage, cluster-based, cross-sectional survey. Lancet Infect Dis. (2022) 22:1172–80. 10.1016/S1473-3099(22)00149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arja A, Godana W, Hassen H, Bogale B. Patient delay and associated factors among tuberculosis patients in Gamo zone public health facilities, Southern Ethiopia: an institution-based cross-sectional study. PLoS ONE. (2021) 16:e255327. 10.1371/journal.pone.0255327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kundu S, Marzan M, Gan SH, Islam MA. Prevalence of antibiotic-resistant pulmonary tuberculosis in Bangladesh: a systematic review and meta-analysis. Antibiotics. (2020) 9:710. 10.3390/antibiotics9100710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cubillos-Angulo JM, Nogueira B, Arriaga MB, Barreto-Duarte B, Araujo-Pereira M, Fernandes CD, et al. Host-directed therapies in pulmonary tuberculosis: updates on anti-inflammatory drugs. Front Med. (2022) 9:970408. 10.3389/fmed.2022.970408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tiberi S, Munoz-Torrico M, Duarte R, Dalcolmo M, D'Ambrosio L, Migliori GB. New drugs and perspectives for new anti-tuberculosis regimens. Pulmonology. (2018) 24:86–98. 10.1016/j.rppnen.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 78.Walker NF, Karim F, Moosa M, Moodley S, Mazibuko M, Khan K, et al. Elevated plasma matrix metalloproteinase 8 associates with sputum culture positivity in pulmonary tuberculosis. J Infect Dis. (2022) 226:928–32. 10.1093/infdis/jiac160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haiqing C, Chen L, Yin C, Liao Y, Meng X, Lu C, et al. The effect of micro-nutrients on malnutrition, immunity and therapeutic effect in patients with pulmonary tuberculosis: a systematic review and meta-analysis of randomised controlled trials. Tuberculosis. (2020) 125:101994. 10.1016/j.tube.2020.101994 [DOI] [PubMed] [Google Scholar]
- 80.Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, Jain SK, Bishai WR. Cavitary tuberculosis: the gateway of disease transmission. Lancet Infect Dis. (2020) 20:e117–28. 10.1016/S1473-3099(20)30148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, et al. A simple, valid, numerical score for grading chest x-ray severity in adult smear-positive pulmonary tuberculosis. Thorax. (2010) 65:863–69. 10.1136/thx.2010.136242 [DOI] [PubMed] [Google Scholar]
- 82.Soeroto AY, Pratiwi C, Santoso P, Lestari BW. Factors affecting outcome of longer regimen multidrug-resistant tuberculosis treatment in West Java Indonesia: A retrospective cohort study. PLoS ONE. (2021) 16:e246284. 10.1371/journal.pone.0246284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wen Y, Zhang Z, Li X, Xia D, Ma J, Dong Y, et al. Treatment outcomes and factors affecting unsuccessful outcome among new pulmonary smear positive and negative tuberculosis patients in Anqing, China: a retrospective study. BMC Infect Dis. (2018) 18:104. 10.1186/s12879-018-3019-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El-Sherbiny NA, Younis A, Masoud M. A comprehensive assessment of the physical, nutritional, and psychological health status of the elderly populace in the Fayoum Governorate (Egypt). Arch Gerontol Geriatr. (2016) 66:119–26. 10.1016/j.archger.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 85.Di Gennaro F, Pizzol D, Cebola B, Stubbs B, Monno L, Saracino A, et al. Social determinants of therapy failure and multi drug resistance among people with tuberculosis: a review. Tuberculosis. (2017) 103:44–51. 10.1016/j.tube.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 86.Soeroto AY, Lestari BW, Santoso P, Chaidir L, Andriyoko B, Alisjahbana B, et al. Evaluation of Xpert MTB-RIF guided diagnosis and treatment of rifampicin-resistant tuberculosis in Indonesia: a retrospective cohort study. PLoS One. (2019) 14:e213017. 10.1371/journal.pone.0213017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shetty N, Shemko M, Vaz M, D'Souza G. An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int J Tuberc Lung Dis. (2006) 10:80–6. [PubMed] [Google Scholar]
- 88.Gunda DW, Maganga SC, Nkandala I, Kilonzo SB, Mpondo BC, Shao ER, et al. Prevalence and risk factors of active TB among adult HIV patients receiving art in northwestern Tanzania: a retrospective cohort study. Can J Infect Dis Med Microbiol. (2018) 2018:1346104. 10.1155/2018/1346104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alebel A, Demant D, Petrucka P, Sibbritt D. Effects of undernutrition on opportunistic infections among adults living with HIV on ART in Northwest Ethiopia: Using inverse-probability weighting. PLoS ONE. (2022) 17:e264843. 10.1371/journal.pone.0264843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Pee S, Semba RD. Role of nutrition in HIV infection: review of evidence for more effective programming in resource-limited settings. Food Nutr Bull. (2010) 31:S313–44. 10.1177/15648265100314S403 [DOI] [PubMed] [Google Scholar]
- 91.Zhang R, Tang Z, Xu W, Ding Y, Zhang M, Guan Q, et al. Risk factors and protective factors for alcohol-related liver disease: a systematic review and meta-analysis. Alcohol Clin Exp Res. (2022) 46:2128–36. 10.1111/acer.14951 [DOI] [PubMed] [Google Scholar]
- 92.Sinha P, Ponnuraja C, Gupte N, Prakash BS, Cox SR, Sarkar S, et al. Impact of undernutrition on tuberculosis treatment outcomes in India: a multicenter, prospective, cohort analysis. Clin Infect Dis. (2023) 76:1483–91. 10.1093/cid/ciac915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma JJ, Guo YJ Li Z, Chen Y, He H, Li WM. Prevalence and prognostic significance of malnutrition risk in patients with pulmonary tuberculosis: a hospital-based cohort study. Front Public Health. (2022) 10:1039661. 10.3389/fpubh.2022.1039661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu X, Zhu H, Cai L, Zhu X, Wang H, Liu L, et al. Malnutrition is associated with an increased risk of death in hospitalized patients with active pulmonary tuberculosis: a propensity score matched retrospective cohort study. Infect Drug Resist. (2022) 15:6155–64. 10.2147/IDR.S382587 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.