Summary
Background
Right ventricular dysfunction (RVD) is associated with adverse outcomes of acute pulmonary embolism (PE). However, there are no studies describing the long-term, full-spectrum right ventricular parameters on morphology, pressure and function at certain follow-up time points after PE onset. More exploration of right ventricular function would provide useful clues for long-term management of patients with PE.
Methods
For this systematic review and meta-analysis, we completed a literature search in Pubmed, EMBASE and WebofScience (from Jan 1st, 1998 to April 20th, 2023). Studies of patients with acute PE followed-up longer than 3 months with right ventricle assessment and written in English-language were included. Right ventricular function was assessed by either echocardiography or computed tomographic pulmonary angiography (CTPA). The primary outcome was structural and functional parameters of the right ventricle, and the secondary outcomes were functional assessments [New York Heart Association (NYHA) functional classification and 6-min walk test distance (6 MWD)], at each follow-up time points. Random effect meta-analyses were performed using R software (PROSPERO: CRD42023433332).
Findings
A total of 33 studies (3920 patients) were included in the final analysis. The 3-month, 6-month and 1-year prevalence of right ventricular dysfunction (RVD) was 0.34 [95% confidence interval (CI) 0.21–0.48, I2 = 96%], 0.26 (95% CI 0.17–0.36, I2 = 93%) and 0.34 (95% CI 0.19–0.48, I2 = 94%), respectively. Pooled tricuspid annulus plane systolic excursion (TAPSE), right ventricular to left ventricular diameter (RV/LV) ratio and pulmonary artery systolic pressure (PASP) at 1-year was 21.80 mm (95% CI 20.08–23.52, I2 = 93%), 0.64 (95% CI 0.48–0.81, I2 = 92%) and 27.33 mmHg (95% CI 18.88–35.78) (I2 = 96%), respectively. The proportion of NYHA III–IV was 0.06 (95% CI 0.0–0.12) and the pooled 6 MWD was 462.98 m (95% CI 447.55–478.41) over 1 year. Patients treated with thrombolysis had lower prevalence of RVD (1-year 0.17 and 0.07 in systemic thrombolysis and catheter-directed thrombolysis, respectively) than those treated with anticoagulation therapy alone (1-year 0.24) but the pooled risk ratio (RR) was not statistically significant.
Interpretation
Although the conclusion of this study may be limited by its high heterogeneity from varied study designs, inclusion criteria and definition of RVD of each study, our findings suggested that persistent RVD and functional impairment were of considerable high prevalence during long-term follow-up after acute PE. Treatment strategy may influence the prevalence of long-term RVD.
Funding
This study is supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-061). The National Key Research and Development Program of China (2016YFC0905600). National High Level Hospital Clinical Research Funding (2022-NHLHCRF-LX-01-02-03). CAMS Institute of Respiratory Medicine Grant for Young Scholars (2023-ZF-8).
Keywords: Pulmonary embolism, Long-term, Right ventricular function, Functional impairment, Meta-analysis
Research in context.
Evidence before this study
More assessments on right ventricular function and associated factors of persistent RVD could provide useful clues for long-term management of patients with pulmonary embolism (PE). We performed a systematic literature search in PubMed, EMBASE and Web of science evaluating studies published from Jan 1st, 1998 to April 20th, 2023. Medical terms “pulmonary embolism”, “right heart failure/dysfunction”, “follow-up” combined with Boolean Operators and truncation symbols were used. We found only one previous meta-analysis reported a RVD prevalence of 18.1% among patients with ambiguous follow-up time points. However, no studies described the long-term full-spectrum right ventricular parameters on morphology, pressure and function at certain follow-up time points after PE onset.
Added value of this study
To our knowledge, this study was the largest and most comprehensive meta-analysis that provided the long-term, full-spectrum description on right ventricular parameters after acute PE so far, by including 3920 patients from 33 studies. We revealed a considerable high prevalence of RVD during long-term follow-up and more than one third of the study population had RVD at 1 year after an acute episode. Nevertheless, functional parameters of the right ventricle, including TAPSE, PASP and RV/LV ratio, were approximately normal during long-term follow-up.
Implications of all the available evidence
The remarkable high prevalence of RVD reported by our study emphasises the necessity and urgency for further research on the mechanism of persistent RVD, especially for those with normotensive PE, to improve their prognoses. We suggest that more attention be paid on RVD and more specific methods, like cardiac magnetic resonance, should be applied for further investigation for mechanism of the maladaptation and more precise evaluation of long-term RVD. Further investigations are warranted. Moreover, identifying patients who could yield more benefits from thrombolysis therapy in long-term functional recovery of the right ventricle, would also be important.
Introduction
Acute pulmonary embolism (PE) is a leading cause of cardiovascular death throughout the world.1 Acute right ventricular dysfunction (RVD), defined as a rapidly progressive syndrome due to acute obstruction of pulmonary artery by thrombus resulting in impaired right ventricular filling and/or reduced right ventricular flow output, indicated by abnormal echocardiographic signs or elevated cardiac biomarkers has been shown as a critical determinant of short-term mortality in patients with PE, even in the absence of clinically evident haemodynamic compromise.1, 2, 3, 4 Acute RVD was reported an incidence of at least 34% at disease onset and is determined as one of the vital indicators for intermediate-risk PE.5, 6, 7
Acute PE, in spite of adequate anticoagulation for at least three months, has been reported that up to 40–60% survivors suffered from long-term functional, post-pulmonary embolism impairment. Functional impairment is also referred as post-pulmonary embolism syndrome (PPES), which is defined as new or progressive dyspnea, exercise intolerance and/or impaired functional or mental status.1 The algorithm for functional impairment and its assessment was firstly proposed by the 2019 guidelines of the European Society of Cardiology (ESC), calling for an additional focus for those patients who suffer from persisting functional limitation and reduced quality of life after PE.2 For instance, more than 20% patients with PE have been reported persistent RVD at long-term follow-up8; studies reported that residual perfusion defects by ventilation-perfusion lung scan can be detected in up to 50% patients after 6 months after the PE onset, and approximately 50% of patients suffered from persisting symptoms and cardiopulmonary functional limitations up to 1 year.6 Persistent RVD, due to background comorbidity or residual clot in pulmonary vasculature,9 is supposed to be one of the reasons of PPES and more explorations on the right ventricular function and associated factors of persistent RVD would provide useful clues for long-term management of patient with PE.
However, the previous long-term follow-up studies of RVD were heterogeneous in diagnosis, population, sample size and prevalence. Although a meta-analysis reported a RVD prevalence of 18.1% among patients with median follow-up of 6 months,10 there were no studies describing the long-term full-spectrum right ventricular parameters on morphology, pressure and function at certain follow-up time points after PE onset. In this meta-analysis, we included studies that reported functional parameters of the right ventricle over 3 months after an acute onset of PE that at certain time points of follow-up, with the aims to 1) investigate the pooled prevalence of RVD of 3, 6-months and over 1-year after acute PE, 2) reveal the characteristics of other parameters of the right ventricle, 3) provide underlying factors that associate with long-term RVD, in order to achieve a comprehensive understanding of long-term prognosis among patients with PE.
Methods
Study design
This systematic review and meta-analysis were performed in accordance with the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 This analysis was registered in International prospective register of systematic reviews (PROSPERO registration number CRD42023433332). All studies involved in the analysis were approved by relevant ethical committees and informed consents were acquired for all participants.
Search strategy and selection criteria
We performed a systematic literature search in PubMed, EMBASE and Web of science evaluating studies published from Jan 1st, 1998 to April 20th, 2023. Medical terms “pulmonary embolism”, “right heart failure/dysfunction”, “follow-up” combined with Boolean Operators and truncation symbols were used. Detailed search strategy is listed in Supplementary Table S1. An additional manual search of potentially eligible studies within references of the included studies, international guidelines and relevant (systematic) reviews was conducted. Articles in the search were limited to English language involving humans. Search results were screened independently by two reviewers (X.Z and D.W) for the relevance of titles/abstracts and full texts of the studies fulfilling the inclusion criteria. Potential disagreements were solved by a third reviewer (G.F).
Studies were considered eligible if they fulfilled all the following criteria:
-
(i)
PE with acute onset was objectively confirmed by imaging tests (ii) patients received follow-up visits at least 3 months after the episode (iii) right ventricular function evaluated by echocardiology or computed tomographic pulmonary angiography (CTPA) with/without 6-min walk test distance (6 MWD), New York Heart Association (NYHA) functional classification or other quality-of-life evaluation were available. Studies were excluded if the entire study population had one of the following characteristics: (i) Sample size is less than 15 (ii) without exact timepoint of follow-up (iii) lack of outcome parameters.
Data extraction and quality assessment
Three researchers (X.Z., L.X. and Y.C.) independently extracted the following information from each study: surname of the first author, publication year, country, participant characteristics, measurement of right ventricular function and follow-up duration. The Newcastle–Ottawa Scale (NOS) was used to assess the risk of bias in observational studies12 and Jadad scale was applied for randomized clinical trials (RCTs).13 The procedure was conducted by two independent reviewers and disagreement was resolved by consensus. A cut-off of less than 5 points was considered as high-risk bias. The scores of all included studies are provided in Supplementary Table S2.
Outcome definition
Primary outcomes of this review included RVD reported by each individual study and functional parameters of the right ventricle including tricuspid annulus plane systolic excursion (TAPSE), right ventricular to left ventricular diameter (RV/LV) ratio, pulmonary artery systolic pressure (PASP). Secondary outcomes included NYHA functional classification and 6 MWD.
The definitions of RVD varied across time and studies. In this analysis, we classified RVD into reported RVD and determinate RVD: if the RVD was clearly defined and reported, the prevalence of RVD was obtained directly as reported RVD according to the original research. The detailed definitions of RVD reported by authors are listed in the Supplementary Table S3. Determinate RVD was considered when RVD was not defined by the original article but could be estimated from explicit measurement by calculation (see details in Statistical analysis) and then defined through any of the following guidelines: 2019 ESC Guidelines1, the British Society of Echochardiograpy14, the Guidelines for the echocardiographic assessment of the right heart in adults15 or the definitions of RVD in Pulmonary Embolism Thrombolysis (PEITHO) trial.16 Functional capacity was assessed with the NYHA functional classification, comprised of four categories of increasing functional impairment: no limitation of physical activity by shortness of breath (class I, no symptoms); dyspnea with ordinary physical activity (class II, mild symptoms); dyspnea with less than ordinary activity but none at rest (class III, moderate symptoms); and symptoms of cardiac insufficiency at rest and inability to carry out any physical activity without discomfort (class IV, severe symptoms). The 6-min walk test measures the distance walked over a 6-min period and serves as an indicator of submaximal aerobic capacity.
Statistical analysis
To pool continuous data, reported medians and ranges were translated to means and standard deviations (SDs) according to previous recommendations using the following website: http://www.math.hkbu.edu.hk/∼tongt/papers/median2mean.html. A random-effects model was used to pool the prevalence of RVD, values of parameters (TAPSE, PASP and RV/LV ratio) and degree of functional limitation at different follow-up time points (baseline, 3 months, 6 months, ≥1 year), respectively. The heterogeneity between studies was assessed by the inconsistency index I2 statistic (ranging from 0 to 100%) on the basis of the Cochrane Q test. Heterogeneity is considered to be low between studies if I2 ranges from 0% to 25%, moderate from 25% to 75% and high from 75% to 100%. Meta regressions were performed in order to figure out the high heterogeneity according to type of PE risk in long-term follow-up. Pooled estimates risk ratio (RR) with 95% confidence interval was generated using Mantel-Haenszel transformation under the random effects models to evaluate association between intervention and RVD prevalence at different time point.
Subgroup analyses were conducted for the following considerations: 1) reported or determinate RVD, 2) patients with different risk stratification at acute onset, 3) reported clearly with adequate anticoagulation therapy for 3 or 6 months 4) treated with anticoagulation vs thrombolysis (either systemic or catheter directed thrombolysis), 5) only RCTs were included.
The filled funnel plot was used to visually present the presence of publication bias which is inevitable due to the unpublished studies with negative results or extremely deviation from previous results. Meanwhile, the Egger’s test, a weighted regression test helping justify the asymmetry of funnel plots, was performed to assess the statistical evidence of publication bias, with a significance level defined as P < 0.1. A two-sided P value of less than 0.05 was considered statistically significant. All statistical analyses were performed in R version 4.2.2 (package “meta” (Schwarzer, 2007; Balduzzi et al., 2019).
Role of the funding source
D.W, G.F, X.Z and Z.Z had full access to dataset and decision to submit for publication. The funding sources had no role for this study.
Results
Description of studies
The literature search identified 900 records in Pubmed, 3447 in Web of Science, and 4251 in EMBASE, leaving 5969 records after duplicate removing. Eventually, 33 studies (references for include studies were listed in Supplementary materials) with long-term right ventricular functional assessment (3920 patients) were selected (Fig. 1), including 29 cohort studies (25 prospective cohorts and 4 retrospective cohorts) and 4 RCTs. 32 studies used echocardiography for right ventricular assessment and one used CTPA during follow-up. Among those studies, 18 (54.5%) studies with 2211 patients reported defined RVD and 15 studies (45.5%) reported determinate RVD. TASPE, RV/LV ratio and PASP during follow-up were reported in 13, 6 and 10 studies, respectively. Characteristics of the included studies were listed in Table 1 and Supplementary Table S4.
Fig. 1.
Flow-chart of literature research and study selection. Abbreviations: TTE, transthoracic echocardiography; RVD, right ventricular dysfunction.
Table 1.
Characteristics of the included articles.
| Characteristics | Number (%) |
|---|---|
| Publication year (n = 33) | |
| 2020–2023 | 9 (27.3%) |
| 2015–2020 | 5 (15.2%) |
| 2010–2015 | 14 (42.4%) |
| before 2010 | 5 (15.2%) |
| Study design (n = 33) | |
| Prospective | 25 (75.8%) |
| Retrospective | 4 (12.1%) |
| RCT | 4 (12.1%) |
| Population characteristics (n = 3920) | |
| Male | 1994 (50.9%) |
| PE risk stratification at baseline (n = 33) | |
| Normotensive | 11 (33.3%) |
| Intermediate and high | 6 (18.2%) |
| High | 1 (3.0%) |
| Unspecified | 15 (45.5%) |
| RVD definition (n = 33) | |
| Reported RVD | 18 (54.5%) |
| Determinate RVD | 15 (45.5%) |
| Studies with specific echocardiographic parameters (n = 18) | 54.5% |
| TAPSE | 13 (72.2%) |
| RV/LV ratio | 6 (33.3%) |
| PASP | 10 (55.6%) |
RCT, randomized controlled trial; PE, pulmonary embolism; RVD, right ventricular dysfunction; TAPSE, tricuspid annulus plane systolic excursion; RV/LV ratio, right ventricular to left ventricular diameter; PASP, pulmonary artery systolic pressure.
Prevalence of RVD during follow-up
The pooled prevalence of RVD at baseline (25 studies included 2417 patients) was 0.69 (95% CI 0.59, 0.78, I2 = 99%). The prevalence among normotensive, intermediate and high-risk patient with PE was 0.66 (95% CI 0.55, 0.78, I2 = 99%) and 0.91 (95% CI 0.80, 1.00, I2 = 88%), respectively. The prevalence RVD in studies that unstratified PE risk was 0.64 (95% CI 0.42, 0.87, I2 = 99%) (Fig. 2).
Fig. 2.
Pooled prevalence of reported and determinate RVD in 3-month, 6-month and over-1-year follow up after acute PE. Abbreviations: RVD, right ventricular dysfunction; CI, confidence interval; PE, pulmonary embolism.
At 3-months after acute onset, 10 studies with 938 patients were evaluated. The pooled prevalence of RVD was 0.34 (95% CI 0.21, 0.48, I2 = 96%). The prevalence among normotensive and unspecified risk of patients with PE was 0.36 (95% CI 0.22, 0.49, I2 = 90%) and 0.31 (95% CI 0.00, 0.82, I2 = 98%), respectively.
At around 6 months after acute PE, 14 studies include 1386 patients had a pooled RVD prevalence of 0.26 (95% CI 0.17, 0.36, I2 = 93%). The prevalence of RVD among normotensive patient with PE (10 studies) were 0.22 (95% CI 0.11, 0.33, I2 = 93%), with unspecified risk stratification (3 studies) was 0.36 (95% CI 0.17, 0.56, I2 = 92%).
Over 1-year after acute PE, the pooled prevalence of RVD was 0.34 (95% CI 0.19, 0.48, I2 = 94%) in 10 studies with 871 patients. The prevalence of RVD among normotensive (4 studies) were 0.34 (95% CI 0.12, 0.56, I2 = 96%), with intermediate and high risk (2 studies) was 0.25 (95% CI 0.00, 0.50, I2 = 89%), with unspecified risk stratification (3 studies) was 0.29 (95% CI 0.00, 0.62, I2 = 92%). Separated analysis for reported and determinate RVD is listed in Supplementary Figs. S1 and S2.
Right ventricular functional parameters during follow-up
According to the 10 studies with 344 patients reported baseline TAPSE, the mean TAPSE was 18.36 mm (95% CI 16.27, 20.46, I2 = 98%); at 3-months after acute onset, the pooled mean TAPSE of 6 studies with 227 patients was 21.38 mm (95% CI 19.60, 23.16, I2 = 89%); at 6-months, the pooled mean TAPSE of 4 studies with 138 patients was 22.59 mm (95% CI 20.18, 25.01, I2 = 94%); over 1 year, the pooled mean from 5 studies with 368 patients was 21.80 mm (95% CI 20.08, 23.52, I2 = 93%). Our meta-analysis showed approximately normal TASPE during both acute and follow-up period (Fig. 3).
Fig. 3.
Pooled value of TAPSE in 3-month, 6-month and over-1-year follow up after acute PE. Abbreviations: TAPSE, tricuspid annular plane systolic excursion; SD, standard deviance; MRAW, untransformed raw means; CI, confidence interval; PE, pulmonary embolism.
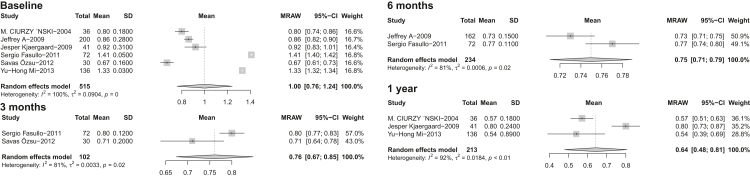
Considering RV/LV ratio, the pooled results were 1.00 (95% CI 0.76, 1.24, I2 = 100%) at baseline, 0.76 (95% CI 0.67, 0.85, I2 = 81%) at 3-months, 0.75 (95% CI 0.71 0.79, I2 = 81%) at 6-months and 0.64 (95% CI 0.48 0.81, I2 = 92%) over 1 year (Fig. 4).
Fig. 4.
Pooled value of RV/LV ratio in 3-month, 6-month and over-1-year follow up after acute PE. Abbreviations: RV/LV, right ventricle to left ventricle; SD, standard deviance; MRAW, untransformed raw means; CI, confidence interval; PE, pulmonary embolism.
Pooled results of PASP of available studies were: 42.83 mmHg (95% CI 35.84, 49.81, I2 = 96%) at baseline, 30.77 mmHg (95% CI 29.66, 31.87) (I2 = 9%) at 3-months, 24.89 mmHg (95% CI 19.90, 29.88) (I2 = 96%) at 6-month and 27.33 mmHg (95% CI 18.88–35.78, I2 = 96%) over 1 year (Fig. 5).
Fig. 5.
Pooled value of PASP in 3-month, 6-month and over-1-year follow up after acute PE. Abbreviations: PASP, pulmonary arterial systolic pressure; SD, standard deviance; MRAW, untransformed raw means; CI, confidence interval; PE, pulmonary embolism.
Functional impairment during long-term follow-up
A pooled prevalence of moderate or several functional impairments (NYHA III–IV) was 0.09 (95% CI 0.05, 0.13, I2 = 85%). The proportion was 0.11 (95% CI 0.04, 0.17, I2 = 80%) and 0.06 (95% CI 0.0, 0.12, I2 = 92%) at 6-months and over 1-year, respectively (Supplementary Fig. S3). The 6 MWD at 3-months and over 1-year after acute PE was 393.17 m (95% CI 370.04, 416.30, I2 = 47%) and 462.98 m (95% CI 447.55, 478.41, I2 = 0%), respectively (Supplementary Fig. S4).
Subgroup analysis
The results of subgroup analyses are shown in Table 2. The 1-year prevalence of reported and determinate RVD were similar: 0.34 (95% CI 0.12, 0.55, I2 = 96%) and 0.34 (95% CI 0.13, 0.55, I2 = 94%), respectively that indicated a consistency of our main result (Supplementary Fig. S1).
Table 2.
Subgroup analyses of the prevalence of right ventilation dysfunction among different subgroups of patients.
| Baseline |
3 months |
6 months |
over 1 year |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroups | N. of studies | N. of participants | pooled prevalence (95% CI) | I2 (%) | N. of studies | N. of participants | pooled prevalence (95% CI) | I2 (%) | N. of studies | N. of participants | pooled prevalence (95% CI) | I2 (%) | N. of studies | N. of participants | pooled prevalence (95% CI) | I2 (%) |
| Study design | ||||||||||||||||
| RCTs | 1 | 72 | 1 (0.95, 1.0) | – | 3 | 233 | 0.34 (0.14, 0.54) | 92 | 1 | 72 | 0.19 (0.11, 0.30) | – | 1 | 285 | 0.40 (0.35, 0.46) | – |
| Cohort studies | 24 | 2345 | 0.67 (0.58, 0.77) | 99 | 7 | 705 | 0.35 (0.16, 0.53) | 95 | 13 | 1314 | 0.25 (0.15, 0.35) | 93 | 9 | 586 | 0.33 (0.17, 0.49) | 92 |
| Dignostic approach | ||||||||||||||||
| Reported RVD | 14 | 1739 | 0.65 (0.53, 0.77) | 99 | 5 | 752 | 0.28 (0.10, 0.45) | 95 | 8 | 920 | 0.25 (0.13, 0.37) | 93 | 4 | 514 | 0.34 (0.12, 0.55) | 96 |
| Determinate RVD | 10 | 657 | 0.73 (0.56, 0.90) | 98 | 5 | 186 | 0.41 (0.21, 0.62) | 93 | 5 | 445 | 0.34 (0.18, 0.50) | 90 | 6 | 357 | 0.34 (0.13, 0.55) | 94 |
| Risk stratificaton | ||||||||||||||||
| Normotensive | 16 | 1479 | 0.66 (0.55, 0.78) | 99 | 8 | 378 | 0.36 (0.22, 0.49) | 90 | 10 | 1068 | 0.22 (0.11, 0.33) | 93 | 4 | 535 | 0.34 (0.12, 0.56) | 96 |
| Intermediate and high | 3 | 169 | 0.91 (0.80, 1.00) | 98 | 0 | 0 | – | – | 1 | 20 | 0.45 (0.23, 0.68) | – | 2 | 149 | 0.25 (0.00, 0.50) | 89 |
| High | 0 | 0 | – | 0 | 0 | 0 | – | – | 3 | 298 | 0.36 (0.17, 0.56) | 92 | 1 | 20 | 0.70 (0.46, 0.88) | – |
| Unspecified | 6 | 769 | 0.64 (0.42, 0.87) | 99 | 2 | 560 | 0.31 (0.00, 0.82) | 98 | 0 | 0 | – | – | 3 | 167 | 0.29 (0.00, 0.62) | 92 |
| Patient undertaken adequate anticoagulation for 3 to 6 months | 14 | 1285 | 0.71 (0.58, 0.83) | 99 | 5 | 197 | 0.47 (0.34, 0.59) | 70 | 9 | 1013 | 0.18 (0.09, 0.27) | 90 | 4 | 265 | 0.33 (0.11, 0.55) | 91 |
| Patients with first episode of PE | 12 | 655 | 0.76 (0.65, 0.88) | 96 | 4 | 186 | 0.48 (0.34, 0.62) | 76 | 7 | 502 | 0.28 (0.14, 0.42) | 90 | 4 | 170 | 0.47 (0.26, 0.67) | 89 |
| Treatment | ||||||||||||||||
| Anticoagulation | 5 | 380 | 0.79 (0.56, 1.00) | 99 | 3 | 113 | 0.45 (0.17, 0.74) | 92 | 2 | 179 | 0.22 (0.15, 0.28) | 2 | 3 | 257 | 0.24 (0.11, 0.37) | 85 |
| Systemic thrombolysis | 7 | 509 | 0.86 (0.71, 1.00) | 93 | 3 | 324 | 0.25 (0.10, 0.41) | 85 | 2 | 55 | 0.13 (0.04, 0.23) | 6 | 3 | 234 | 0.17 (0.00, 0.44) | 97 |
| Catheter directed thrombolysis | 3 | 244 | 0.83 (0.70, 0.96) | 90 | 1 | 46 | 0.07 (0.01, 0.18) | – | 0 | 0 | – | – | 1 | 43 | 0.07 (0.01, 0.19) | – |
RCT, randomized controlled trial; PE, pulmonary embolism; RVD, right ventricular dysfunction; CI, confidence interval.
For patients who were clearly reported been conducted adequate anticoagulation therapy for 3 or 6 months (14 studies at baseline), the prevalence of RVD was 0.71 (95% CI 0.58, 0.83, I2 = 99%), 0.47 (95% CI 0.34, 0.59, I2 = 70%), 0.18 (95% CI 0.09, 0.27, I2 = 90%) and 0.33 (95% CI 0.11, 0.55, I2 = 91%) at baseline, 3-months, 6-months and over 1 year after acute onset (Supplementary Fig. S6). For the four RCTs included, the pooled prevalence of RVD at 3-months was 0.34 (95% CI 0.14, 0.54, I2 = 92%) (Supplementary Fig. S7).
For the relationship between treatment and residual RVD, patients undertaken either systemic thrombolysis or catheter directed thrombolysis had lower prevalence of RVD (0.17 and 0.07 over 1-year, respectively) than those treated with anticoagulants alone (0.24 at 1-year) (Supplementary Fig. S10) but the pooled risk ratios (RRs) of thrombolysis therapy to anticoagulation were not statistically significant RVD (3-month: RR 0.51, 95% CI 0.21, 1.25, I2 = 77%; 6-month: RR 0.67, 95% CI 0.23, 1.90, I2 = 55%; 1-year: RR 0.64, 95% CI 0.27, 1.54, I2 = 74%) (Supplementary Fig. S5).
Study quality and risk of bias assessment
Supplementary Table S2 showed that all included studies with NOS scores (29 studies) receive 5 or more stars (11 with 9 stars, 5 with 7–8 stars, 13 with 5–6 stars) and that studies with Jadad scales (4 studies) receive 3 or more points (2 with 5 points, 1 with 4 points, 1 with 3 points), indicating low or medium risk of bias. Funnel plot analysis (Supplementary Fig. S11) was used to assess the evidence of possible publication bias for pooled prevalence of RVD on each follow-up time point. Prevalences of RVD reported in each study at 6 months and more than 1 year follow-up is distributed symmetrically, and there is no significant bias from small studies (Egger’s test P = 0.6361 and 0.7008 respectively). However, at 3 months follow-up, the effect size is distributed asymmetrically (Egger’s test P = 0.0007). The observed asymmetry in funnel plots suggested the potential publication bias existed among studies.
Discussion
To our knowledge, this study is so far the largest and most comprehensive meta-analysis that provided the long-term, full-spectrum description on right ventricular parameters after acute PE, by including 3920 patients from 33 studies. We revealed a considerable high prevalence of RVD during long-term follow-up and more than one third of the study population had RVD at 1 year after an acute episode. Nevertheless, functional parameters of the right ventricle, including TAPSE, PASP and RV/LV ratio, were approximately normal during long-term follow-up.
Previous studies reported an overall RVD incidence of 34%–37.8% at baseline in haemodynamically stable patient with PE4,6 and a prevalence of 18.1% for long-term RVD at a median follow-up time of 6 months.10 Our study found a higher pooled prevalence of acute or persistent RVD at baseline or follow-up period among studies with normotensive patient with PE, and the overall pooled prevalence was even higher after pooling all studies together regardless of risk classification. Currently, RVD at baseline or acute phase had been considered as an immediate damage to the right ventricle, including subendocardial ischaemia and shear-mediated ultrastructural damage to myocytes. Large increase in the expression of the chemokine monocyte chemoattractant protein (MCP-1) in the right ventricles (but not the left ventricles) of rats with experimental PE was found.17 Wadate et al.18 observed large numbers of CD68-staining cells on histological examination of right ventricle tissue from humans who died from PE. However, the PEITHO-2 trial failed to provide underlying risk factors for the unrecovered right ventricular function19 and the mechanism of long-term decompensation of the right ventricle, including remodeling and fibrosis has been seldom studied. Previous study supposed that persistent RVD may be due to the presence of pre-existing chronic PE/CTEPH at the time of acute PE and a persistent RVD already existed at baseline may also indicate a high risk of persistent RVD,20 whilst long-term RVD remained at a high level of prevalence in our subgroup analysis that excluded patients with previous PE. The remarkable high prevalence of RVD reported by our study emphasised the necessity and urgency for further research on the mechanism of persistent RVD, especially for those with normotensive PE, to improve their prognoses.
We innovatively summarised particular parameters to describe the long-term conditions of the right ventricle from a more comprehensive aspects: TASPE, RV/LV ratio and PASP represented contraction ability, morphological changes and pressure load of the right ventricle, respectively. According to our results, the decompensation of the right ventricle was significant at acute phase. Despite the high prevalence of RVD, these parameters above were all at normal range at each time points during long-term follow-up. Of note, it has been newly reported that the parameters were predictive for poor prognoses of acute PE than RVD alone in accuracy: for each unit increase in RV/LV ratio, the odds of all-cause mortality increased by over 2.5 fold, every 1 mm decrease in TAPSE increased the odds of combined adverse events by 1.3-fold, and for every 10 mmHg increase in PASP the odds of a combined adverse event increased by 1.5-fold.3 However, dynamic changes of the parameters, the underlying mechanism, their relationship with long-term structural and functional changes of the right ventricle and outcomes were less discussed. Further investigation are warranted.
Long-term sequela of acute PE, such as chronic thromboembolic pulmonary hypertension (CTEPH), chronic thromboembolic pulmonary disease (CTEPD), RVD, post-PE impairment (PPEI) and PPES, etc. have been increasingly emphasised in recent years.21 Prospective studies reported CTEPH incidence of 2.3%–5.25% and CTEPD of 5.75% at 2-years after acute PE.22, 23, 24, 25 Remarkably, RVD was much higher in prevalence than CTEPH after acute PE, which has been inferred that residual clot due to incomplete thrombus resolution lead to a poor recovery of right ventricular function,26 while incomplete thrombus resolution occurs in 25–50% of patients after acute PE despite adequate anticoagulation.9
Recently, Alizadehasl et,al27 demonstrated that patients with a higher RV/LV ratio, lower TASPE and lower right ventricular free wall strain at discharge were more likely to develop PPEI at 6-months, indicating the relationship between long-term condition of the right ventricle and symptoms of functional impairment. Also, apart from the high incidence, incomplete recovery of the right ventricle had also a high adjusted OR (12.5, 95% CI 3.33–45.45) for predicting an intermediate-to-high probability of pulmonary hypertension. The PEITHO trial reported that the incomplete recovery of echocardiographic parameters at 6-months strongly predicted PPEI or CTEPH.19 PPES is defined as new or progressive dyspnea, exercise intolerance and/or impaired functional or mental status after at least 3 months of adequate anticoagulation following acute PE9 and had been reported a general prevalence of 40–60% of PE survivors. An incidence of PPEI/PPES was 16.0% at two-year after acute PE.23 A prevalence of NYHA III–IV (moderate to severe impairment of heart function) was reported 11.3% at a median follow-up time of 9 months,8 which was consistent with our results. The relationship between persistent RVD and functional impairment has not been fully demonstrated. More studies should explore the relationship between persistent RVD, long-term functional status, exercise tolerance, and mortality. Considering the high prevalence of RVD, relevant parameters in our study and its relation to long-term prognosis, a lower threshold for echocardiographic follow-up in patients with PE might be reasonable.
The adequacy of treatment may heavily impact the prognosis of acute PE. In our study, a lower prevalence of long-term RVD was found among patients undertaken adequate anticoagulation therapy. Thrombolysis therapy for patients at intermediate risk has been argued for years but remains controversial. A meta-analysis revealed a benefit on all-cause mortality (OR 0.53, 95% CI 0.32–0.88) and higher risks of major bleeding (OR 2.73, 95% CI 1.92–3.91).28 According to PEITHO and other clinical trials, thrombolysis therapy, including catheter-directed thrombolysis, may improve long-term right ventricular function for intermediate-to-high risk patients with PE in acceptable rates of bleeding events.29, 30, 31 A possible explanation for the potential benefit of thrombolysis therapy was that the rapid clearance of thrombus releases the afterload of the right ventricle, preventing the maladaptive cardiopulmonary remodeling. However, from our analysis, the prevalence of long-term RVD was numerically lower in thrombolysis group (either systemic or catheter directed) than anticoagulation alone (mostly among patients with intermediate risk) but the RRs were not statistically significant in any subgroup. Even in subgroup analysis among normotensive patients, thrombolysis did not significantly reduce the rate of long-term RVD. This result probably due to the high heterogeneity of participants included varied in age, clot burden and clinical condition. Nevertheless, identifying patients who could yield more benefits from thrombolysis therapy, especially in long-term functional recovery, would be important for further clinical practice.
The subgroup analyses also indicated that patients with adequate anticoagulation might be associated with lower prevalence of RVD in 6 months and 1 year, with a reduction of heterogeneity. The results of subgroup analyses also provided some clues that higher risk-stratification at PE onset and inadequate anticoagulation therapy may be underlying risk factors for long-term RVD. Therefore, to identify patients at higher risk of long-term RVD would be a future point for research.
There are some strengths of the present study, e.g., large number of the including studies, the comprehensive analysis method and description of right ventricular function, etc. However, the following limitations should be claimed. First, a considerable heterogeneity across involved studies may impact the accuracy of our conclusions. The potential sources of heterogeneity may be from the varied inclusion criteria and strategy of risk stratification in each cohort, the different guidelines applied during the past decades, and the large difference in sample size among involved studies. We also acknowledged that the differences in study design, level of healthcare facilities, adequacy of anticoagulation therapy and recurrent PE also contributed to heterogeneity, and subgroup and subgroup analyses may help explore the heterogeneity to some extent. Second, there lacks a unified definition of RVD till now. Thus, we estimated “determinate RVD”, based on the information from the original article with the most widely accepted definitions of RVD. Third, in this meta-analysis, it was inappropriate to describe a dynamic change of right ventricular condition over time because the data were derived from different studies with high heterogeneity and inconsistent follow-up time points. Therefore, the pooled prevalence and other parameters of RVD were calculated at different time points respectively. To provide more precise results of long-term outcomes of acute PE, an individual meta-analysis is necessary. Forth, we aimed to provide more practical information for clinical practice in long-term management of acute PE, so studies without follow up at certain time points of visit were excluded. This may influence the pooled result of RVD. Even so, the high prevalence in our results were remarkable enough in emphasizing the management of long-term RVD and call for more attention. Fifth, we found the publication bias was relatively high in our study, considering that the variation of medical service across countries would influence the prevalence of RVD and studies with higher rates of RVD were more likely to be published. Moreover, in this study, we found the prevalence of RVD is much higher than CTPEH after acute PE, but in recent studies focused on long-term follow up of acute PE, CTEPH is considered as most common endpoint. We suggest that more attention to be paid on RVD and more specific method, like cardiac magnetic resonance, should be applied for further investigation for mechanism of the maladaptation and more precise evaluation of long-term RVD.
To conclude, persistent RVD and functional impairment were of considerable high prevalence during long-term follow-up after acute PE. Treatment strategy may influence the prevalence of long-term RVD.
Due to the high prevalence of long-term RVD revealed by our analysis, it is of great necessity to regularly monitor the right ventricular function after an acute episode of PE, for the purpose of thorough assessment of PPES. Management that improves heart function or inhibit myocardial remodeling might be benefit. Further investigation is required for the identification of risk factors, underlying mechanism and potential management for long-term RVD. Treatment strategy at acute phase might have impact on long-term recovery of the right ventricle. Potential candidates for reperfusion therapy remain to be determined.
Contributors
D.W. and Z.Z., designed the study. X.Z., L.X., and Y.C., searched the database and screened the involved studies. G.F. and X.Z., analyzed the data. G.F., D.W., and X.Z., drafted the manuscript. D.W., G.F., and X.Z., had verified the underlying data. A.L., provided consultation of echocardiology of study. D.W., G.F., X.Z., and Z.Z., had access to dataset and decision to submit for publication. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data sharing statement
Since this study is a meta-analysis, all available data were derived from involved studies. There were no additional original data needing to be shared in this study.
Declaration of interests
The authors have no conflict of interest or financial relationships to disclose. No form of payment was given to anyone to produce the manuscript.
Acknowledgements
We thank all the medical professionals who offered precious suggestions to this study. This study is supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-061). The National Key Research and Development Program of China (2016YFC0905600). National High Level Hospital Clinical Research Funding (2022-NHLHCRF-LX-01-02-03). CAMS Institute of Respiratory Medicine Grant for Young Scholars (2023-ZF-8).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102153.
Appendix ASupplementary data
References
- 1.Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41(4):543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 2.Houston B.A., Brittain E.L., Tedford R.J. Right ventricular failure. N Engl J Med. 2023;388(12):1111–1125. doi: 10.1056/NEJMra2207410. [DOI] [PubMed] [Google Scholar]
- 3.Prosperi-Porta G., Ronksley P., Kiamanesh O., Solverson K., Motazedian P., Weatherald J. Prognostic value of echocardiography-derived right ventricular dysfunction in haemodynamically stable pulmonary embolism: a systematic review and meta-analysis. Eur Respir Rev. 2022;31(166) doi: 10.1183/16000617.0120-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barco S., Mahmoudpour S.H., Planquette B., Sanchez O., Konstantinides S.V., Meyer G. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2019;40(11):902–910. doi: 10.1093/eurheartj/ehy873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becattini C., Maraziti G., Vinson D.R., et al. Right ventricle assessment in patients with pulmonary embolism at low risk for death based on clinical models: an individual patient data meta-analysis. Eur Heart J. 2021;42(33):3190–3199. doi: 10.1093/eurheartj/ehab329. [DOI] [PubMed] [Google Scholar]
- 6.Chornenki N.L.J., Poorzargar K., Shanjer M., et al. Detection of right ventricular dysfunction in acute pulmonary embolism by computed tomography or echocardiography: a systematic review and meta-analysis. J Thromb Haemostasis. 2021;19(10):2504–2513. doi: 10.1111/jth.15453. [DOI] [PubMed] [Google Scholar]
- 7.Andrade I., Mehdipoor G., Le Mao R., et al. Prognostic significance of computed tomography-assessed right ventricular enlargement in low-risk patients with pulmonary embolism: systematic review and meta-analysis. Thromb Res. 2021;197:48–55. doi: 10.1016/j.thromres.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Klok F.A., Ageno W., Ay C., et al. Optimal follow-up after acute pulmonary embolism: a position paper of the European society of Cardiology working group on pulmonary circulation and right ventricular function, in collaboration with the European society of Cardiology working group on atherosclerosis and vascular biology, endorsed by the European respiratory society. Eur Heart J. 2022;43(3):183–189. doi: 10.1093/eurheartj/ehab816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller K., Tesche C., Gerhold-Ay A., et al. Quality of life and functional limitations after pulmonary embolism and its prognostic relevance. J Thromb Haemostasis. 2019;17(11):1923–1934. doi: 10.1111/jth.14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sista A.K., Miller L.E., Kahn S.R., Kline J.A. Persistent right ventricular dysfunction, functional capacity limitation, exercise intolerance, and quality of life impairment following pulmonary embolism: systematic review with meta-analysis. Vasc Med. 2017;22(1):37–43. doi: 10.1177/1358863X16670250. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;21(339):b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells G., Shea B.J., O'Connell D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2018. http://wwwohrica/programs/clinical_epidemiology/oxford
- 13.Jadad A.R., Ra M., Carroll D., et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Zaidi A., Knight D.S., Augustine D.X., et al. Echocardiographic assessment of the right heart in adults: a practical guideline from the British Society of Echocardiography. Echo Res Pract. 2020;7(1):G19–G41. doi: 10.1530/ERP-19-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudski L.G., Lai W.W., Afilalo J., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Meyer G., Vicaut E., Danays T., et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 17.Watts J.A., Zagorski J., Gellar M.A., et al. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol. 2006;41(2):296–307. doi: 10.1016/j.yjmcc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Iwadate K., Doi M., Tanno K., et al. Right ventricular damage due to pulmonary embolism: examination of the number of infiltrating macrophages. Forensic Sci Int. 2003;8(134):21. doi: 10.1016/s0379-0738(03)00138-5. [DOI] [PubMed] [Google Scholar]
- 19.Barco S., Russo M., Vicaut E., et al. Incomplete echocardiographic recovery at 6 months predicts long-term sequelae after intermediate-risk pulmonary embolism. A post-hoc analysis of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Clin Res Cardiol. 2018;108(7):772–778. doi: 10.1007/s00392-018-1405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guérin L., Couturaud F., Parent F., et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost. 2014;112(3):598–605. doi: 10.1160/TH13-07-0538. [DOI] [PubMed] [Google Scholar]
- 21.Yuriditsky E., Horowitz J.M., Lau J.F. Chronic thromboembolic pulmonary hypertension and the post-pulmonary embolism (PE) syndrome. Vasc Med. 2023 doi: 10.1177/1358863X231165105. 1358863X231165105. [DOI] [PubMed] [Google Scholar]
- 22.Held M., Pfeuffer-Jovic E., Wilkens H., et al. Frequency and characterization of CTEPH and CTEPD according to the mPAP threshold >20 mm Hg: retrospective analysis from data of a prospective PE aftercare program. Respir Med. 2023;210 doi: 10.1016/j.rmed.2023.107177. [DOI] [PubMed] [Google Scholar]
- 23.Valerio L., Mavromanoli A.C., Barco S., et al. Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: the FOCUS study. Eur Heart J. 2022;43(36):3387–3398. doi: 10.1093/eurheartj/ehac206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang W., Zhang Z., Wang Z., et al. Higher incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism in asians than in Europeans: a meta-analysis. Front Med. 2021;8 doi: 10.3389/fmed.2021.721294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luijten D., Talerico R., Barco S., et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: an updated systematic review and meta-analysis. Eur Respir J. 2023;62 doi: 10.1183/13993003.00449-2023. LID - 2300449 [pii] LID - 10.1183/13993003.00449-2023 [doi] FAU - Luijten, Dieuwke. (1399-3003 (Electronic)) [DOI] [PubMed] [Google Scholar]
- 26.Becattini C., Giustozzi M., Cerda P., Cimini L.A., Riera-Mestre A., Agnelli G. Risk of recurrent venous thromboembolism after acute pulmonary embolism: role of residual pulmonary obstruction and persistent right ventricular dysfunction. A meta-analysis. J Thromb Haemostasis. 2019;17(8):1217–1228. doi: 10.1111/jth.14477. [DOI] [PubMed] [Google Scholar]
- 27.Alizadehasl A., Farrashi M., Naghsbandi M., et al. Post-pulmonary embolism impairment six months after acute pulmonary embolism: a prospective registry. Vasc Endovascular Surg. 2023 doi: 10.1177/15385744231165152. 15385744231165152. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee S., Chakraborty A., Weinberg I., et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311(23):2414–2421. doi: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 29.Matusov Y., Yaqoob M., Karumanchi A., et al. Long term recovery of right ventricular function after treatment of intermediate and high risk pulmonary emboli. Thromb Res. 2023;225:57–62. doi: 10.1016/j.thromres.2023.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Konstantinides S.V., Vicaut E., Danays T., et al. Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol. 2017;69(12):1536–1544. doi: 10.1016/j.jacc.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 31.Sadeghipour P., Jenab Y., Moosavi J., et al. Catheter-directed thrombolysis vs anticoagulation in patients with acute intermediate-high-risk pulmonary embolism: the CANARY randomized clinical trial. JAMA Cardiol. 2022;7(12):1189–1197. doi: 10.1001/jamacardio.2022.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.