Abstract
The emergence of safe and functional eggs for consumer acceptance has gained focus. The production of carotenoid-enriched eggs has received attention due to its multifunctional biological properties. Nutritional modification of laying hens’ diet can be a strategy to produce such eggs. This review presents the chemistry of carotenoids in nature and eggs, the accumulation process of carotenoids into eggs, and the functions of carotenoids in eggs. Our findings showed that carotenoids can be deposited into the egg and contribute to improving its nutritive value. The biosynthesis, chemical structure, and metabolism pathways of carotenoids lead to the deposition of carotenoids into eggs in their original or metabolized forms. Also, some factors modulate the efficiency of carotenoids in fowls before accumulation into eggs. Carotenoid-enriched eggs may be promising, ensuring the availability of highly nutritive eggs. However, further studies are still needed to comprehend the full metabolism process and the extensive functions of carotenoids in eggs.
Keywords: Egg enrichment, Egg fortification, Carotenoid chemistry, Bioavailability, Metabolism, Function
1. Introduction
A fresh whole egg contains about 36% lipids in its whole weight. The lipids of the yolk are exclusively associated with lipoprotein aggregates. Composed of triglycerides, phospholipids, and cholesterol at 62%, 33%, and 5%, respectively, less than 1% of yolk lipids are carotenoids, which give the yolk its hue ranging from a very pale yellow to a dark bright orange (Abeyrathne et al., 2022; Anton, 2007). With the well-known functional properties of carotenoids such as antioxidant and anti-inflammation, fortification and enrichment of edible eggs with carotenoids can increase the content of carotenoids in eggs and improve the quality and nutritional value of eggs (Andersen, 2015; Zaheer, 2017). The so-called “designer egg” belongs to the “functional food” concept, which is any healthy food similar in appearance to traditional foods, eaten as part of a regular diet, and believed to provide physiological advantages beyond the essential role of delivering nutrition, such as health-promoting or disease-preventing properties (Lesnierowski and Stangierski, 2018; Rajasekaran and Kalaivani, 2013).
Carotenoid-enriched eggs have also gained interest because of the high bioavailability response in humans after egg consumption compared with carotenoid consumption from other sources. The natural micellar matrix (triglycerides, phospholipids, and cholesterol) that is found in egg yolk offers a vehicle for the effective absorption of egg-derived carotenoids in humans (Kelly et al., 2017). On the other hand, data have indicated that carotenoid-enriched eggs can not only be a good nutritional food but also a good vector for the distribution of other essential nutrients vital to human health. Moreover, carotenoid absorption from other carotenoid-rich meals, such as raw vegetable salad, has been improved by simultaneously eating eggs (Kim et al., 2015). Therefore, as a large source of natural antioxidants, carotenoid-enriched eggs may not be able to substitute fruit and vegetables but can greatly boost the diet's nutritional value, participating significantly in the daily consumption of carotenoids (Alagawany et al., 2019; Surai et al., 2000).
From the natural source to concentration in eggs, carotenoids undergo different processes that greatly modulate their form, structure, and functions in edible eggs. They may be isomerized or metabolized into more bioaccessible forms before deposition into eggs. In addition, the efficiency of carotenoids in fowls is subject to several factors similar to that described in mammals by Castenmiller and West (1998), summarized in the mnemonic “SLAMENGHI”. This is explained as species of carotenoids, linkages at the molecular level, amount of carotenoids consumed in a meal, the matrix in which the carotenoid is incorporated, effectors of absorption and bioconversion, nutrient status of the host, genetic factors, host-related factors, and interactions between these factors. Finally, carotenoids could have several positive effects on eggs before consumption by humans.
Despite the progress in research regarding carotenoids’ enrichment and fortification in eggs, there are still numerous aspects to explore. Therefore, it is important to review where the research stands. We hypothesized that carotenoid absorption in fowls, its metabolism, accumulation, and efficiency in eggs, as well as its biological functions in eggs, are still not fully understood. Therefore, we aimed to review the advances and gaps in the production of carotenoid-enriched eggs from a biochemistry perspective. We focused on carotenoid chemistry in nature and eggs, the absorption, transport, and accumulation of carotenoids in eggs as well as the different factors influencing them, and finally the functions of carotenoids to improve egg quality.
2. Overview of the chemistry of carotenoids
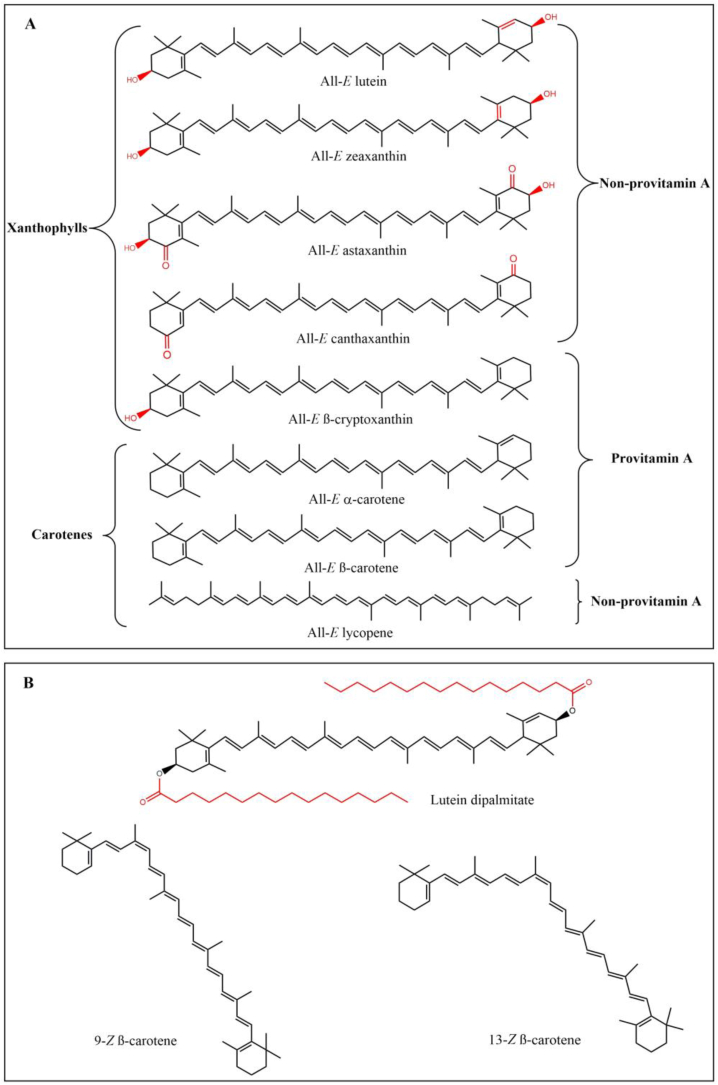
Over 700 carotenoids are found in nature, and they are generally categorized into two groups: carotenes and xanthophylls based on their chemical structures (Kopec and Failla, 2018; Kotake-Nara and Nagao, 2011). The chemical structures of the most common natural carotenoids are shown in Fig. 1A. The carotenes are nonpolar, highly conjugated C40 hydrocarbon chains, linear (lycopene) or cyclic at each end of the molecule (β-carotene, α-carotene). The β-carotene is the most common form of carotene in plants. The α-carotene differs from β-carotene in the position of the double bond in the cyclic group at one end (Higuera-Ciapara et al., 2006; Namitha and Negi, 2010). The xanthophylls are regarded as oxygenated carotene derivatives that are more polar than carotenes. These oxygenated derivatives are identified based on the presence of various oxygen-containing functional groups, e.g., hydroxyl (zeaxanthin, β-cryptoxanthin, and lutein) and carbonyl (astaxanthin, canthaxanthin, and capsanthin) (Mohd Hatta and Othman, 2020). Both zeaxanthin and lutein are isomeric, differing only by the conversion of one double bond, such that all double bonds in zeaxanthin are conjugated (Mohd Hatta and Othman, 2020). The β-cryptoxanthin has a bipolar structure with an electronegative hydroxyl group on one side and an unsubstituted β-ionone ring on the other end of the molecule (Liu et al., 2012). The structures of α-carotene, β-carotene, and β-cryptoxanthin allow them to generate vitamin A after cleavage during metabolism, hence they are considered pro-vitamin A (details in Section 4.2.1). In natural forms, xanthophylls can exist either in free form or esterified with fatty acids such as stearic, palmitic, myristic, or lauric at one or both hydroxyl groups. They can also form complexes with lipoproteins (carotenolipoproteins) or proteins (carotenoproteins) (Higuera-Ciapara et al., 2006; Rodriguez-Amaya, 2016). An example of such complexes is a xanthophyll ester known as lutein dipalmitate, shown in Fig. 1B.
Fig. 1.
Structural formula of free carotenoids, carotenoid esters, and carotenoid geometric isomers. (A) Structural formula of main natural carotenoids involved in egg enrichment and fortification. (B) Carotenoid esters represented by lutein dipalmitate and carotenoid cis isomers represented by 9-cis β-carotene and 13-cis β-carotene.
Each carotenoid can potentially form many geometrical isomers due to its many conjugated double bonds. However, the most common forms are the two configurations of every double bond from the polyene chain which lead to trans (E) or cis (Z) geometric isomers (Fig. 1B). In nature, the majority of carotenoids detected are primarily all-E isomers (Honda et al., 2020a; Yu and Liu, 2020). In addition, carotenoids can present optical configurations such as (3R,3′R), (3S,3′S), and (3R,3′S) (Honda et al., 2017).
3. Regulations and sources of carotenoids used for egg enrichment
Carotenoids are primarily used in the poultry industry to enhance yolk color to increase consumer acceptance. In this regard, several laws governing their use in animal feed have been enacted, which vary from one country to another. The regulation (EC) No. 1831/2003 of the European Parliament defines the use of additives in animal nutrition and establishes criteria for licensing and labeling of feed additives within the European Union (EU). According to this regulation, xanthophylls such as lutein, zeaxanthin, canthaxanthin, capsanthin, citranaxanthin, and ethyl ester of β-apo-8′-carotenoic acid (apo-ester) were authorized for use in chicken feed. The β-carotene is classified in the vitamins and provitamin A groups. In addition are saponified paprika extract (capsanthin), lutein-rich extract of Tagetes erecta, and lutein/zeaxanthin extract of Tagetes erecta (European Union Register of Feed Additives, 2016, 2020, 2023). Apo-ester has a maximum concentration of 5 mg/kg complete feed for laying hens and 15 mg/kg complete feed for chickens. Meanwhile, canthaxanthin has a maximum concentration of 8 mg/kg complete feed for laying hens and 25 mg/kg complete feed for chickens. On the other hand, saponified paprika extract (capsanthin) has maximum concentration of 40 mg/kg feed for laying hens and chickens (EFSA FEEDAP Panel, 2014, 2019, 2020). In the United States, the Food and Drug Administration (USFDA), an agency of the Department of Health and Human Services (HHS), is mainly responsible for enforcing food color laws under Title 21 of the Code of Federal Regulations (Code of Federal Regulations, 2016). The ingredients added to animal feed for meat, milk, or egg coloring purpose are included in the regulations for coloring additives, whereas colors used on packaging are considered color additives if they could migrate to the contents of the package. This is in contrast to the EU, where feed additives and food materials are regulated separately (Lehto et al., 2017). Xanthophyll supplements should not exceed 30 mg/lb (66.13 mg/kg) of solid or semisolid food or every pint of liquid food, and broiler chicken feed should not exceed 4.41 mg/kg (Abdel-Aal et al., 2017).
Improvement of egg functional properties has driven the exploration of various sources of carotenoids to achieve such nutritional targets. Carotenoids such as lutein, zeaxanthin, lycopene, β-carotene, β-cryptoxanthin, canthaxanthin, and astaxanthin have been mostly added to eggs for enrichment and biofortification. Carotenoids used for egg biofortification often exist as either naturally occurring products, biofortified plants, or synthetic carotenoids. Among natural products, carotenoids can be derived from algae such as Chlorella vulgaris, Vischeria helvetica, Trachydiscus minutus, Japonochytrium marinum, and Heamatococcus pluvialis (Ambati et al., 2019; Coudert et al., 2020; Henriquez et al., 2016; Jiru et al., 2021; Nwoba et al., 2020); yeasts (Phaffia rhodozyma, Rhodotorula rubra) (Akiba et al., 2000; Pârvu and Paraschivescu, 2014); bacteria (Paracoccus carotinifaciens) (Honda et al., 2020b); plants, leaves, and flowers (calendula (Calendula officinalis L.), basil (Ocimum basilicum L.), stevia (Stevia rebaudiana), marigold (Tagetes erecta), alfalfa (Medicago sativa), and spinach (Spinacia oleracea)) (Kljak et al., 2021a; Pirgozliev et al., 2022; Sunder et al., 2022; Yıldız et al., 2020); plant by-products (red pepper powder, tomato pomace, carrot derivatives, broccoli meal, grapeseed meal, sea buckthorn meal, chili pepper meal, rosehip meal, and paprika oleoresin) (Alay and Karadas, 2017; Arimboor et al., 2015; Hammershoj et al., 2010; Hu et al., 2011; Konca et al., 2021; Moeini et al., 2013; Reda et al., 2022; Sowbhagya, 2019; Vlaicu et al., 2021); and animal by-products (crab meals and golden snail egg powder) (Anderson et al., 2008; Coral-Hinostroza and Bjerkeng, 2002; Nusantoro et al., 2020). Regarding biofortified plants, commercial and biofortified maize containing a set of carotenoids (Burt et al., 2013; Kljak et al., 2021b; Liu et al., 2012; Moreno et al., 2016) were applied as well. Last are synthetic sources, used in the poultry industry to manipulate the carotenoid content of egg yolk. Except apo-ester exclusively present in synthetic form (EFSA FEEDAP Panel, 2009), other carotenoids are mainly chemically synthesized for commercial reasons and available from different companies. Among them are synthetic zeaxanthin (EFSA FEEDAP Panel, 2009), synthetic canthaxanthin (EFSA FEEDAP Panel, 2014), and synthetic lycopene (An et al., 2019).
Primarily, carotenoids from natural sources are on the increase as consumers are now raising health and safety concerns over the use of synthetic carotenoids in feeds and foods (Marounek and Pebriansyah, 2018). Therefore, in an organic egg production system the use of synthetic carotenoids and saponified extracts of carotenoids is not tolerated. As a result, the birds raised in outdoor areas obtain carotenoids from plants to which they are exposed in the process of feeding (Sunder et al., 2022).
4. Absorption, transport, metabolism, and depletion
4.1. Absorption and transport of carotenoids to egg yolks
Dietary carotenoids are absorbed by birds, like other animals, through the intestinal mucosa, where they are built into lipoprotein particles and transported via the blood to peripheral organs and tissues (McGraw et al., 2002). Therefore, the release of carotenoids, dispersion into the gastrointestinal tract, solubilization in mixed micelles, absorption in the gut, transit into circulatory systems, metabolism in the liver or site of deposition, and deposition into organs, integuments, and eggs are all separate physiological stages in the expression of carotenoids in birds (Toomey et al., 2017).
First, carotenoids are released from the food matrix by the digestive enzymes in the duodenum and dispersed into the gastrointestinal tract thanks to dietary lipids. Thereafter, the carotenoids are solubilized in mixed micelles (consisting of fatty acids, phospholipids, bile acids, cholesterol, and monoacylglycerols) for intestinal uptake and absorption (Desmarchelier and Borel, 2017). Two uptake mechanisms of carotenoids then take place: passive diffusion and active diffusion. According to the basic process of diffusion, the micelles transfer to the brush boundary membrane through the unstirred water layer, then the carotenoid moves from the micellar formation and diffuses into the enterocyte cytoplasm through the membrane (Yonekura and Nagao, 2007). When it comes to the active pathway, carotenoid absorption is facilitated by the scavenger receptor class B type 1 protein (SR-B1), which is partly responsible for transporting lipids and cholesterol from lipoproteins to tissues as well as from tissues to lipoproteins (Canene-Adams and Erdman, 2009). SR-B1 is a cell surface high-density lipoprotein (HDL) receptor that mediates HDL-phospholipids, -cholesterol ester, -phosphatidylserine, and -apoptotic cell uptake (Shen et al., 2018). In a study of the Wisconsin hypoalpha mutant (WHAM) chicken, the disruption of carotenoid transport function was identified as caused by a mutation in the SR-B1 gene in that breed. That implicates SR-B1 as a major modulator of carotenoid-based coloration in chicken (Toomey et al., 2017) and shows a relationship between carotenoid and lipid uptake. It is believed that carotenoid absorption modulation occurs on the apical side of intestinal cells where the SR-B1 is expressed (Shmarakov and Blaner, 2013). Because SR-B1 and the cluster of differentiation 36 (CD36) have similar lipid mobilization roles, it has been proposed that CD36 may also play a role in the absorption of carotenoids. Niemann-Pick C1 (NPC1), which is a major sterol carrier in the intestine, is a contender for carotenoid absorption as well. In human cells and mice, NPC1 is involved in intestinal absorption of α-carotene, β-carotene, β-cryptoxanthin, and lutein, but not lycopene and phytoene (Reboul, 2019). After intestinal absorption, carotenoids are incorporated in portomicrons (lipid-rich 90% triacylglycerol) released from the intestinal cells into the portal vein, for transport to the liver (Alvarenga et al., 2011; Surai et al., 2001). Fig. 2A summarizes the absorption and transport mechanisms of carotenoids to the liver.
Fig. 2.
Absorption and transport mechanism of carotenoids from the food matrix to the egg yolks. (A) In the gastrointestinal tract, carotenoids in the food matrix are first released under the effects of intestinal enzymes, then dispersed in lipids and solubilized in mixed micelles. The mixed micelles pass the unstirred water of the intestine for uptake to the enterocyte through passive diffusion or other lipid transporters such as the scavenger receptor class B type 1 protein (SR-B1), the cluster of differentiation 36 (CD36), or the Niemann-Pick C1 (NPC1). Once in the enterocytes, the carotenoids are incorporated into portomicrons for transport to the liver by the portal vein. (B) In the liver, the carotenoids and their metabolites are incorporated into different lipoproteins including the yolk-targeted very low-density lipoprotein (VLDLy) for transport to the oocytes. (C) The carotenoid containing VLDLy passes through the granulosa layer, binds to the LR8 receptor (a low-density lipoprotein receptor family member with eight ligand binding repeats) in the perivitelline layer without degradation, and is endocytosed in the oocyte.
In the liver, carotenoids and metabolites (the metabolism process is presented in Section 4.2) are incorporated into HDL, low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) to be transported to the target tissues and ovary by the bloodstream (Bortolotti et al., 2003; Grashorn, 2016). HDL is an important lipoprotein for the transport of carotenoids into the bloodstream. The deficiency of HDL in WHAM chickens was found to be the major cause of the low levels of plasma phospholipids in the mutant chickens and by extension limiting the level of carotenoids in serum, which was colorless (Attie et al., 2002). However, the role of HDL in the transport of carotenoids may be limited to some tissues. In particular, it was proven that the retinas of WHAM chicks acquire their lutein and zeaxanthin mainly from the HDL transport mechanism, whereas the heart and adipose tissue may derive their lutein mainly from LDL and VLDL, which are not suppressed in WHAM chicks (Connor et al., 2007). It is also suggested that in the feeding state, LDL and VLDL mainly drive the transport of carotenoids from the liver to organs, meanwhile, in the fasted state, carotenes are driven by LDL and xanthophylls by HDL and minorly by VLDL (Moreno et al., 2016). Oogenesis in birds requires a significant movement of cholesterol and other lipids, as well as carotenoids, from the liver into the oocytes, which is necessary for the embryo's growth and development (Toomey et al., 2017). Lipids and carotenoids are mostly delivered into the developing oocyte via VLDL. In laying hens, VLDL has certain particular characteristics. VLDL particles produced in the liver of laying chickens have a diameter of around 30 nm, which is substantially smaller than that in immature hens. In addition, these so-called yolk-targeted very low-density lipoprotein (VLDLy) are not susceptible to lipoprotein lipase (LPL)-mediated hydrolysis (Bortolotti et al., 2003; Moreno et al., 2016). Fig. 2B summarizes the incorporation of carotenoids into lipoproteins inside the liver and transport to the follicles.
The tiny size of VLDLy enables it to pass the ovarian follicle's basal lamina and granulosa cells and bind to the LR8 receptor (a low-density lipoprotein receptor family member with eight ligand binding repeats) present in the membrane that surrounds the oocyte (perivitelline layer). It is then endocytosed without modification to produce yolk. The ovarian follicle's basal lamina sieves out portomicrons carrying dietary fats, preventing them from passing through. Only lipids including carotenoids, delivered by the liver, are integrated into the yolk as a result of this combination of follicular ultrastructures and VLDLy size, allowing for regulation of the yolk's lipid and carotenoid properties (Alvarenga et al., 2011). Fig. 2C highlights the deposition mechanism of carotenoids and metabolites in the follicles.
The deposition of carotenoids into egg yolks generally occurs very quickly following ingestion. However, because the rapid phase of the follicular growth of the oocytes (mainly consisting of lipids deposition into the oocytes) lasts 6 to 14 days depending on the size of the egg yolk, carotenoid accumulation after feed supplementation needs a similar duration to be stabilized (Nys and Guyot, 2011). Considering the trend of egg yolk color, the maximum accumulation of carotenoid in laying hens’ egg yolk is reached on day 12 of an experiment with diets based on different corns (Ortiz et al., 2021). Similarly, experiments conducted by Nelson et al. (1990) have shown that egg yolk color change under the effect of canthaxanthin stabilized from day 10 to 12 with only a very slight change appearing after day 13. Furthermore, Walker et al. (2012) found that color change following astaxanthin supplementation reached a peak after 8 days of feeding and became stable over time. Similarly, Karadas et al. (2006) reported that the color of egg yolk stabilized within 7 to 9 days after ingestion by quail of diet supplemented with lucerne-, marigold-, and tomato-derived natural carotenoids. All these accumulation stabilizations were irrespective of the doses of supplementation.
4.2. Metabolic transformations and existential form of carotenoids in eggs
From ordinary corn-based feeding regimes, lutein and zeaxanthin are the most common carotenoids tested in eggs because of the high content of these carotenoids in maize (Zaheer, 2017). While comparing the carotenoid profile of egg yolk from different species of fowls, Sinanoglou et al. (2011) found that lutein was significantly higher in ostrich, turkey, and duck yolk, whereas zeaxanthin was higher in quail and goose yolk. Because of the designer egg concept, following incorporation of further carotenoids into the diet, carotenoids including lycopene (Honda et al., 2019), astaxanthin (Dansou et al., 2021a), β-carotene, canthaxanthin, β-cryptoxanthin, adonirubin, and adonixanthin (Honda et al., 2020b), as well as further lutein and zeaxanthin (Abdel-Aal et al., 2017) were detected in egg yolks. On the other hand, carotenoids in fowls may go through oxygenation, hydrogenation, esterification, de-esterification, enzymatic cleavage, or isomerization before deposition into eggs.
4.2.1. Enzymatic cleavage and apocarotenoids in eggs
Carotenoids such as β-carotene and β-cryptoxanthin are provitamin A. After absorption, β,β-carotene 15,15′-dioxygenase (BCO1) oxidatively cleaves these carotenoids at the central 15,15′ double bond to produce one or two retinal (vitamin A aldehyde) molecules. The retinal thus formed is reduced to retinoic acid or retinol (vitamin A) (Fig. 3A) (Dela Sena et al., 2016; Harrison and Kopec, 2018). Thenceforth, although provitamin A carotenoids may be present in egg yolk after supplementation, a huge amount of them is converted into vitamin A before deposition into egg yolks, thereby enhancing the content of vitamin A in eggs. Vitamin A is by far the main apocarotenoid derived from the enzymatic cleavage of provitamin A. However, the conversion rate is closely related to the vitamin A status in the diet. A high vitamin A status is assumed to decrease provitamin A carotenoid cleavage and conversion into vitamin A, and vice versa (Rodriguez-Concepcion et al., 2018). On the other hand, the formation of vitamin A may be more critical for β-carotene than β-cryptoxanthin. For instance, when laying hens were fed a diet based on either β-carotene biofortified maize or β-cryptoxanthin biofortified maize, egg yolks content in β-cryptoxanthin significantly increased, meanwhile, the content in β-carotene did not change. Meantime, there was an increase in the yellow color score of eggs enriched with β-carotene suggesting a deposition of β-carotene as well, although this may be very low (Liu et al., 2012).
Fig. 3.
Oxidative cleavage of carotenoids by β-carotene oxygenase 1 (BCO1) and β-carotene oxygenase 2 (BCO2). (A) BCO1 cleaves provitamin A carotenoids (β-carotene shown here) at the central 15,15′ double bond to produce retinal which yields vitamin A and retinoic acid after dehydrogenase. (B) BCO2 at the 9,10′ double bond breaks no-provitamin A carotenoid (lutein shown here) to yield either 3-hydroxy-β-apo-10′-carotenal or 3-hydroxy-α-apo-10′-carotenal. Also, BCO2 can cleave provitamin A carotenoid (β-carotene shown here) to produce β-apo-10′-carotenal which in turn is cleaved by BCO1 to produce retinal.
Alternatively to BCO1, β,β-carotene 9′,10′-oxygenase (BCO2) catalyzes the oxidative cleavage of the provitamin A carotenoids α-carotene, β-carotene, and β-cryptoxanthin but also the non-provitamin A carotenoids such as zeaxanthin and lutein at the 9,10 and 9′,10′ double bonds as demonstrated by purified recombinant chicken BCO2 (Fig. 3B) (Dela Sena et al., 2016). In chicken myoblasts, BCO1 was responsible for the catalytic conversion of β-carotene into retinoic acid to inhibit the cells’ proliferation. However, when BCO1 was deficient, the BCO2 gene was overexpressed, leading to a proliferation decrease and contributing to a residual response to β-carotene (Praud et al., 2017). On the other hand, the ability of chickens with a derived BCO2 genotype to allocate stored carotenoids to their eggs suggested a physiological benefit of the introgressed BCO2 in limiting any deficit of carotenoids during egg production (Fallahshahroudi et al., 2019). Thus, carotenoids in birds, like mammals, are broken down by two major recognized enzymes: the β-carotene oxygenase 1 (BCO1), specifically active on provitamin A carotenoids, and the β-carotene oxygenase 2 (BCO2), active on both pro- and non-provitamin A carotenoids. Because of the asymmetry of carotenoid molecules, cleavage by BCO2 can result in several apocarotenoids from a single molecule. Nonetheless, BCO2 was ineffective with lycopene and β-apocarotenoids, and the catalytic activity of BCO2 with β-carotene as the substrate was at least 10-fold lower than that of BCO1 (Dela Sena et al., 2016). In mice cells, BCO2 can asymmetrically cleave a full-length carotenoid at the 9,10 double bond to form an apocarotenoid which is then cleaved again at the 9′,10′ double bond site, resulting in the production of a di-apocarotenoid product (Bandara et al., 2021). However, neither chicken BCO2 nor BCO1 was effective on hydroxy apocarotenoids whereas the cleavage of no hydroxylated apocarotenoids was assured by BCO1 as represented in Fig. 3B (Dela Sena et al., 2016).
4.2.2. Isomerization and carotenoid isomers in eggs
During the transfer of carotenoids from the food matrix to eggs, they may be subject to isomerization. Factors such as acidic pH (Meléndez-Martínez et al., 2009), the presence of metal ions (Li et al., 2012), and heat treatment (Mezzomo and Ferreira, 2016) favor the isomerization of all-E carotenoids to Z isomers. Therefore, the inner temperature (up to 50 °C) (Sarra et al., 1992), the pH (2.5–7) (Mabelebele et al., 2013), and the different chemical and enzymatic reactions in the gastrointestinal tract of chicken may offer a good environment for isomerization of carotenoids. To illustrate this, lycopene content in egg yolk following supplementation of laying hens’ diets with all-E lycopene revealed that egg yolk contained more than 65% of Z isomers (Honda et al., 2019). Similarly, Honda et al. (2020b) found that 25% of astaxanthin content in egg yolk was present in Z isomers, meanwhile the feeding source, P. carotinifaciens, accumulated less than 5% of astaxanthin Z isomers. From our study on astaxanthin supplementation in the diet of laying hens, we also found a huge increase of Z isomers in egg yolks when compared with the feeding source (Dansou et al., 2021b). The total Z-isomer ratios of astaxanthin, adonirubin, and adonixanthin in P. carotinifaciens cell powder were 3.9%, 11.3%, and 5.0%, respectively, and those in the pulverized form were 4.2%, 11.6%, and 5.3%, respectively. However, contrary to both diets, the Z-isomer ratios of astaxanthin, adonirubin, and adonixanthin in egg yolk were approximately 25%, 20%, and 7%, respectively (Honda et al., 2020b). These results sustain the assumption of the isomerization of all-E isomers into Z isomers but also suggest that the accumulation of carotenoid Z isomers in egg yolk differs depending on the type of carotenoids.
4.2.3. Other metabolic reactions
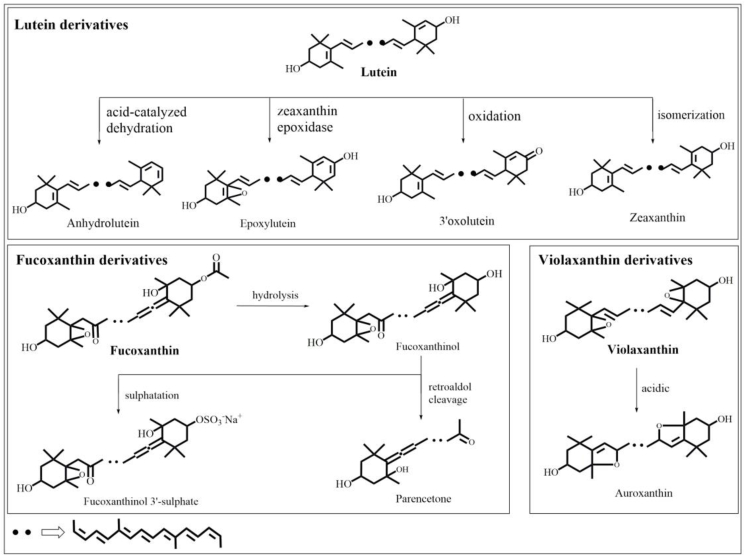
Following the chemical and enzymatic reactions occurring during the passage of carotenoids from the food matrix to depot sites, different metabolites of carotenoids were found in some birds' eggs. After feeding zebra finches with a diet enriched with lutein, cryptoxanthin, and zeaxanthin, anhydrolutein was found in the eggs and serum of the said birds (McGraw et al., 2002). In addition, supplementation of laying hens' diet with seaweed meal containing fucoxanthin, violaxanthin, and furanoid gave rise to the presence of fucoxanthinol, fucoxanthinol 3′-sulfate, and paracentrone (metabolites of fucoxanthin), and difuranoid auroxanthin (metabolite of violaxanthin/furanoid derivatives) in egg yolks, meanwhile neither fucoxanthin nor violaxanthin was detected in egg yolks (Strand et al., 1998). On the other hand, a metabolite of lutein, 3′-oxolutein (3-hydroxy-3′-oxo-β,ε-carotene), was detected in quail, turkey, and laying hen's eggs, following feeding with a diet containing lutein but free of 3′-oxolutein (Tyczkowski et al., 1986). Such ability of laying hens to metabolize oxycarotenoids and deposit the derivatives in the egg yolks was confirmed in another study involving laying hens fed a diet containing a huge amount of lutein, little lutein monoester and lutein diester, traces of epoxylutein, and an undetectable amount of 3′-oxolutein. More than 20 carotenoids such as lutein, lutein monoester, lutein diester, 3′-oxolutein, cryptoxanthin, zeaxanthin, β-carotene, and zeacarotene were detected after analysis of the egg yolks (Schaeffer et al., 1988). The chemical reactions involved in these metabolic transformations are presented in Fig. 4.
Fig. 4.
Possible reaction mechanisms for the metabolic conversion of carotenoids which metabolites were determined in eggs.
It may be worth mentioning some metabolic processes that occur in colorful birds and broilers as well, although not within the primary scope of this review. Birds can metabolically oxidize carotenes to form xanthophylls and differently colored forms, which are then absorbed into different tissues and organs (Anton, 2007; McGraw et al., 2002). Ingested carotenoids may also be metabolically modified before deposition, for example, by ketolase activity at the β- and ε-rings (Mendes-Pinto et al., 2012). Dehydrogenation, like 4-oxidation reactions, is a common metabolic conversion in colorful songbirds, especially those with yellow plumage. Such dehydrogenation involves the conversion of hydroxyl groups to carbonyls at the C3 position on the end rings of the carotenoid molecule (McGraw et al., 2003). Otherwise, red plumage colorants are formed following a series of oxidation reactions at the C4 or 4′ positions. One or two keto groups are then inserted into the β-ionone rings of a given dietary carotenoid molecule (McGraw et al., 2003). Enzymatic 4-oxidation of the β-end group appears to be the predominant metabolic pathway leading to the production of red pigments. Because xanthophylls are absorbed preferentially, adonirubin found in wild bullfinches might be formed by a double oxidation of β-cryptoxanthin, astaxanthin from zeaxanthin, and α-doradexanthin from lutein. Papilioeritrinone may be produced by oxidation of the allylic hydroxyl of the ε-end group in α-doradexanthin, whereas canthaxanthin may be derived from β-carotene in low concentrations (Morishita et al., 2001). In broiler chicks, canthaxanthin was found partly reduced to hydroxyechinenone, a more polar hydroxycarotenoid whose hydroxyl group was acylated in part to form an ester, less polar than canthaxanthin (Tyczkowski and Hamilton, 1986). In another study, a part of canthaxanthin was reduced to 4-hydroxyechinenone that in turn was reduced in part to isozeaxanthin (Tyczkowski et al., 1988). All those different reactions show the complexity of the mechanism of metabolism of carotenoids in birds, and probably before accumulation into eggs. Nonetheless, there is no evidence on whether all the above-mentioned metabolites can be stored in egg yolks.
4.2.4. Metabolism sites of carotenoids
Several studies suggested the liver as the main site of carotenoid metabolism. However, the metabolism sites still cannot be clearly identified. del Val et al. (2009) found that the liver may act as the main site for the synthesis of carotenoid metabolites in common crossbills. Schiedt et al. (1985) also reported that zeaxanthin was esterified and stored in the liver of laying hens. Similarly, Tyczkowski et al. (1986) reported that the oxidation of lutein might occur in the liver, from where 3′-oxolutein is partially released into the intestinal lumen via the bile and the residual 3′-oxolutein goes to the serum where it is transported to depot sites including eggs. However, these authors did not exclude the possibility of oxidation of lutein in the oviduct in opposition to Schaeffer et al. (1988) who assumed later that the metabolism site of lutein was not the ovary based on the concentrations and ratios of lutein and its metabolites in serum and yolk. Moreover, Wyss (2004) has reported that the conversion sites of carotenoids into vitamin A in chicken may be the duodenum, liver, kidney, and lungs because BCO1 was expressed in all these organs. Alternatively, Fallahshahroudi et al. (2019) showed that BCO2 expression was maintained in the intestine and liver while being downregulated in adipose tissues, muscle, and skin during carotenoid accumulation. Because VLDLy responsible for carotenoid transport to the yolk originated extensively from the liver, the latter could be considered as the main source of carotenoid metabolites present in eggs, although an oviduct source of metabolism cannot be rejected. However, because both liver and eggs serve as storage organs, some metabolites from other organs might reenter the circulatory system, be reimported by the liver, and then deposited into the yolk. Hence, the carotenoid metabolites in eggs may also originate from other organs where they are not used. Currently, there is no evidence on the site of the metabolism of carotenoids. Moreover, the different enzymatic and chemical reactions appearing in the animal that favor the metabolism of carotenoids are still uncovered. The accumulation of carotenoid metabolites in egg yolks requires more attention. Fig. 5 presents a proposed pathway for carotenoid metabolism in birds leading to the deposition of the metabolites in eggs.
Fig. 5.
Proposed pathway of carotenoid metabolism in birds leading to the deposition of the metabolites into eggs. Carotenoids (C), non-provitamin carotenoids (NPV-C), provitamin carotenoids (PV-C), apocarotenoids (Apo-C), β-carotene oxygenase 1 (BCO1), and the β-carotene oxygenase 2 (BCO2), bold blue arrows (known pathways), bold dash red arrows (uncertain pathways). Carotenoids in the intestine are subject to BCO1 and BCO2 enzymatic cleavage as well as other chemical reactions to form vitamin A, apocarotenoids, and other derivatives. The metabolites and non-converted carotenoids are transferred into the liver, the main site of metabolism, where they can be exposed to further enzymatic and chemical reactions before transfer to the ovary and peripheric organs. Unused carotenoids and metabolites in the peripheric organs could reenter the circulatory system, pass into the liver, and transfer to oocytes for accumulation. Meanwhile, carotenoids in the oocytes could be exposed to oxidative reactions during passage to the oviducts as well.
4.3. Depletion of carotenoids in eggs
A minimal dose of carotenoid content in eggs was achieved after 12 days of washout period, feeding laying hens with a diet depleted in carotenoid in an experiment by Moreno et al. (2020). Wu et al. (2009) applied a 14-day period of carotenoid deprivation to reach the carotenoid content in eggs lower than the method detection limit. However, the depletion of carotenoids from egg yolk may depend on the dose of supplementation in the feed. Depletion of carotenoid from high dose carotenoid-enriched eggs was found to be slower than that in moderate- or low-dose supplementation. It was reported that yolk pigmentation in a group of laying hens fed a diet supplemented with 1.5% R. rubra yeast began to diminish 2 days after removal and stabilized at the level of the control group. Meanwhile, the pigmentation of yolks in the group supplemented with 3.0% began to diminish 3 days after depletion shifting gradually for 7 days before eventually stabilizing at the level of the other groups (Pârvu and Paraschivescu, 2014).
5. Enrichment efficiency of carotenoids
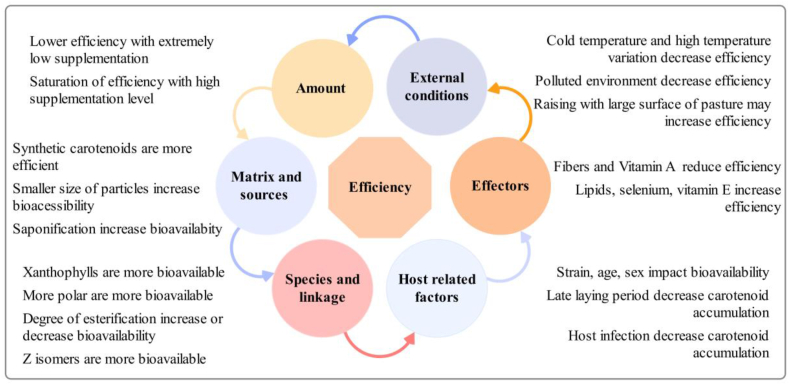
Production of efficient carotenoid-enriched eggs requires optimization of the use of carotenoids in birds. According to Surai (2012), overall, canthaxanthin is deposited at 30% to 45%, zeaxanthin at 25%, and astaxanthin at 14% in egg yolk. In their experiment, Burt et al. (2013) found that β-cryptoxanthin had a higher average efficiency of deposition in eggs (99%) than lutein (43%) and zeaxanthin (30%). On the other hand, Hammershoj et al. (2010) reported that lutein supplementation from various dietary sources resulted in concentrations of approximately 7–120 mg/kg of yolk, whereas the content of zeaxanthin was comparatively low, varying between 0.2 and 13 mg/kg yolk showing the large range of efficiency. Table 1 summarizes the efficiency of carotenoids to enrich table eggs as measured in different study conditions. It is worth mentioning that the comparison of efficiency inter- or intra-carotenoids from one study to another might be challenging due to the differences between animals and study conditions. Thus, the efficiency of carotenoids to enrich egg yolk cannot accurately be discussed without referring to the different beforehand processes and parameters influencing the deposition of carotenoids in eggs. Different parameters were revealed as key factors influencing the absorption, transport, metabolism, and deposition of carotenoids, and then their efficiency to enrich egg yolks. The most prevalent factors that affect the efficiency of carotenoids are summarized in Fig. 6.
Table 1.
Dose response and transfer efficiency of carotenoid in poultry as measured in different conditions of study.
| Carotenoids | Species | Sources | Carotenoid content in diet, mg/kg | Carotenoid content in egg, μg/g | Transfer efficiency, % 1 | References |
|---|---|---|---|---|---|---|
| α-Carotene | Laying hens | Bolero Orange | 34.2 | 0.58 | 0.2 | Hammershoj et al. (2010) |
| Laying hens | Rainbow Yellow | 5.6 | 0.16 | 0.5 | Hammershoj et al. (2010) | |
| Laying hens | Purple Haze Purple | 71.2 | 1.29 | 0.3 | Hammershoj et al. (2010) | |
| β-Carotene | Laying hens | Bolero Orange | 84.9 | 1.84 | 0.3 | Hammershoj et al. (2010) |
| Laying hens | Rainbow Yellow | 15.3 | 0.52 | 0.5 | Hammershoj et al. (2010) | |
| Laying hens | Purple Haze Purple | 121.2 | 3.39 | 0.5 | Hammershoj et al. (2010) | |
| Lutein | Quail | Marigold extract | 10.56 2 | 33.792 | 22.6 | Karadas et al. (2006) |
| Laying hens | Tagets premix | 128 and 518 | 1.43 and 1.65 mg/60 g egg3 | 10 and 3 | Leeson and Caston (2004) | |
| Laying hens | Bolero Orange | 1.7 | 8.25 | 19.5 | Hammershoj et al. (2010) | |
| Laying hens | Rainbow Yellow | 1.9 | 10.40 | 27.4 | Hammershoj et al. (2010) | |
| Laying hens | Purple Haze Purple | 6.5 | 12.23 | 18.8 | Hammershoj et al. (2010) | |
| Laying hens | Marigold extract | 191.33 and 388.70 | 107.41 and 118.86 | Grcevic et al. (2019b) | ||
| Laying hens | Tomato powder | 0.25, 0.5, and 0.75 | 13.2, 13.6, and 13.9 | Habanabashaka et al. (2014) | ||
| Laying hens | Lutein powder extract | 67.20 | 133.9 | Englmaierová et al. (2013) | ||
| Laying hens | Chlorella | 21.4 | 49 | Englmaierová et al. (2013) | ||
| Laying hens | Marigold flower extract | 3.9, 4.7, and 6.2 | 22.6, 26.2, and 29.8 | Skřivan et al. (2016) | ||
| Laying hens | Corn gluten meal | 6.90, 10.27, 13.61, and 16.95 | 91.9, 142.7, 163.2, 197.1, and 242.9 | Shin et al. (2016) | ||
| Laying hens | Corn distillers dried grains with solubles | 9.87, 14.57, 19.27, and 23.95 | 91.9, 119.9, 150.7, 176.6, and 211.9 | Shin et al. (2016) | ||
| Laying hens | Lutein | 250 | 1.04 mg/60 g3 | 14.0 | Skřivanová et al. (2017) | |
| Laying hens | Chlorella alga | 12,500 | 0.70 mg/60 g3 | 16.4 | Skřivanová et al. (2017) | |
| Zeaxanthin | Laying hens | Bolero Orange | 0.1 | 2.23 | 18.9 | Hammershoj et al. (2010) |
| Laying hens | Rainbow Yellow | 0.3 | 2.62 | 27.9 | Hammershoj et al. (2010) | |
| Laying hens | Purple Haze Purple | 1.0 | 2.15 | 20.1 | Hammershoj et al. (2010) | |
| Laying hens | Marigold flower extract | 3.0, 3.8, and 4.5 | 14.6, 17.0, and 19.2 | Skřivan et al. (2016) | ||
| Laying hens | Lutein | 250 | 0.96 mg/60 g3 | 13.7 | Skřivanová et al. (2017) | |
| Laying hens | Chlorella alga | 12,500 | 0.70 mg/60 g3 | 15.3 | Skřivanová et al. (2017) | |
| Canthaxanthin | Laying hens | Carophyll1 red, roche | 0.5, 1, 2, 4, 8, 16, 32, and 64 | 1.58, 2.92, 4.66, 9.92, 20.8, 41.2, 86.7, and 154.5 | 50.9, 45.8, 37.2, 40.3, 40.6, 38.6, 43.6, and 47.2 | Grashorn and Steinberg (2002) |
| Laying hens | Reagent-grade | 0.37, 0.75, 1.5 | 0.11, 0.33, 0.52 | Akiba et al. (2000) | ||
| Lycopene | Quail | Tomato powder | 2.26 | 1.48 | 5.8 | Karadas et al. (2006) |
| Laying hens | Roche lycopene (10%, water soluble) | 12 μg | 1.88 | 2 | Kang et al. (2003) | |
| Laying hens | DSM nutritional products Inc. | 10 and 20 | 0.80 and 0.88 | 1.06 | An et al. (2019) | |
| Laying hens | Tomato oleoresin | 100, 200, and 300 | 1.58, 1.67, and 1.71 | Honda et al. (2019) | ||
| Laying hens | Synthetic | 20 | 2.4 | Orhan et al. (2021) | ||
| Laying hens | Tomato powder | 20 | 2 | Orhan et al. (2021) | ||
| Laying hens | Tomato powder | 10.5, 21, and 31.5 | 0.5, 0.83, and 0.95 | Habanabashaka et al. (2014) | ||
| Astaxanthin | Laying hens | Heamatococcus pluvialis | 7.1, 14.2, 21.3, and 42.6 | 2.43, 6.65, 10.67, and 22.13 | 4.19, 5.54, 6.07, and 5.76 | Dansou et al. (2021b) |
| Laying hens | H. pluvialis | 25, 50, and 100 | 12.87, 21.06, and 44.20 | Gao et al. (2020) | ||
| Laying hens | Genetically-engineered maize BKT | 4.42 | 6.56 | Moreno et al. (2020) |
Some of the experiments did not report the efficiency value.
The data from the original research articles have been converted into milligrams per kilogram in order to be consistent.
The carotenoid contents were reported as milligrams per 60 g of egg.
Fig. 6.
Factors influencing the efficiency of carotenoids to enrich eggs.
5.1. Amount of carotenoids
A common feature of all carotenoid supplementation is the dose-dependent accumulation in egg yolks. Several studies demonstrated that the more carotenoids in the feed, the higher the concentration in eggs. This is consistent for different carotenoids, regardless of the source including fortified maize, algae supplementation, and by-product residual (Gao et al., 2020; Honda et al., 2019; Moreno et al., 2020; Weber et al., 2013). By contrast, the efficiency is generally decreased with an increase in supplementation dose. The lutein content in egg yolks improved remarkably when bird diets were supplemented with up to 500 ppm (500 mg/kg) of lutein, but the transfer efficiency was lowered as the dietary lutein increased. The transfer efficiency declined from 10% with 125 ppm (125 mg/kg) in the diet to 2% to 3% with a lutein level of 500 ppm (500 mg/kg) (Leeson and Caston, 2004). Similarly, the efficiency of lycopene diminished as the amount of supplementation increased (from 65 to 650 mg/kg diet) in an experiment on laying hens. Based on regression analysis, the optimum lycopene accumulation in egg yolk was expected to occur at 420 mg/kg in the diet (Olson et al., 2008). In addition, a limit on the deposition rate of canthaxanthin into egg yolks was recorded because laying hens fed a diet supplemented with 12 mg/kg diet of canthaxanthin yielded just 20% to 39% more canthaxanthin in eggs than those supplemented with just 6 mg/kg diet canthaxanthin (Johnson-Dahl et al., 2017). Higher supplementation of calendula and dandelion did not achieve a higher content of carotenoid in eggs (15.27 and 15.36 μg/g at 1%, respectively, compared with 21.76 and 22.8 μg/g at 3% supplementation level (Kljak et al., 2021a)). However, the same authors also reported that with marigold supplementation, the values were 33.96 μg/g at 1% and 66.95 μg/g at 3%. This suggests that the effect of carotenoid dose level in the feed also depends on the species of carotenoid. In our study, we also found that a very low supplementation of astaxanthin in the diet may result in a lower efficiency in eggs because laying hens might give priority to carotenoid in use as an antioxidant instead of storage in eggs (Dansou et al., 2021b). In conclusion, both very low and very high additions of carotenoid in the diet may affect its bioavailability and accumulation in eggs.
5.2. Matrix and sources of carotenoids
The matrix in which a carotenoid is embedded is another determining factor that affects its absorption and bioavailability (Kopec and Failla, 2018). Based on the pigmentation efficacy, different studies revealed that yellow synthetic carotenoids including apo-ester and canthaxanthin were more efficient than natural carotenoids including marigold and algae Chlorella (Balnave and Bird, 1996; Englmaierová et al., 2013a). Likewise, Sirri et al. (2007) reported that diets supplemented with the yellow pigment apo-ester caused a better accumulation of β-carotene in eggs than diets with marigolds. The efficiency of apo-ester to facilitate β-carotene accumulation in eggs was 50% and 55% for Hy-Line and ISA Brown, respectively, compared with 16% and 18% for Hy-Line and ISA Brown, respectively, when it comes to marigolds. Consequently, the efficiency ratio of apo-ester to marigold was about 3:1 which was also reflected in the coloration.
To improve the transfer efficiency of carotenoids to egg yolks, some treatments are performed. Pulverization treatment of P. carotinifaciens powder increased the efficiency of carotenoid deposition in egg yolks (Honda et al., 2020b). Moreover, the smaller size of corn particles can increase surface contact with intestinal enzymes and carriers, favoring the enterohepatic absorption of carotenoids for the pigmentation of egg yolks, thereby allowing more effective absorption of carotenoids (Oliveira et al., 2019). In addition, the saponification of natural paprika and marigold pigmentation products resulted in increased pigmentation quality of these products. The pigmenting ability of saponified xanthophylls was a minimum of 1.5 times that of nonsaponified xanthophylls (Galobart et al., 2004).
5.3. Genetics and host-related factors
Limitations in carotenoid accumulation can arise after ingestion and may depend on the physiological demands in relationship with the health status, genetics, and nutritional state of each bird (Hargitai et al., 2006). The impact of sex was remarkable in passerine birds because they emphasize feather coloration for sexual attractiveness above deposition into eggs (McGraw, 2005). Therefore, feather coloration could affect the allocation of carotenoids to eggs. On the other hand, differences regarding interspecies variation in micelle, portomicron, or lipoprotein concentrations or affinities to xanthophyll carotenoids likely cause variations in carotenoid accumulation. Passerine birds had greater amounts of carotenoids in serum than gamebirds (McGraw, 2005). In table egg production, Kojima et al. (2022) found that zeaxanthin and lutein content in the egg yolk of Rhode Island Red and Silky Fowl hens was affected by breed. Silky Fowl hens had higher carotenoid contents than Rhode Island Red. In addition, the lutein but not zeaxanthin and astaxanthin content in egg yolk was affected by the interaction between breeds and diet type. Similarly, ISA Brown had a higher carotenoid deposition rate in eggs than Hy-Line (Sirri et al., 2007).
It is assumed that birds have different needs of carotenoids depending on their reproductive state and age. Bortolotti et al. (2003) found that the diet content of carotenoids at the end of the laying season of Red-Legged Partridge (Alectoris rufa) affected egg content but not plasma concentration. Later, Hammouda et al. (2014) reported no difference between plasma lutein levels in early-laying and late-laying females, but the egg yolk lutein content decreased in late laying compared with early-laying. The decrease in lutein transfer to eggs within the breeding season may reflect a change in the distribution of carotenoids between self-maintenance and reproduction. This could also support the observations from laying hens. Lutein and zeaxanthin contents in eggs from early-age laying hens (21.8 and 13.4 μg/g, respectively) were significantly higher than laying hens of intermediate- and late-age. Moreover, the carotenoid contents calculated on a per egg basis also showed the significantly highest content in eggs from early-age laying hens, in comparison to eggs from intermediate-age and late-age laying hens. An active metabolism of laying hens at an early age may be a possible reason behind the higher content at that stage as well (Ko et al., 2020). We also found that a long-term supplementation (24 weeks) of astaxanthin to laying hens led to a decrease in deposition in egg yolks probably due to egg yolk size and saturation in astaxanthin accumulation (Dansou et al., 2021b).
In addition, the absorption and deposition of carotenoids in organs may be impaired by parasites. The concentration of lutein in the eggs of Barn swallows whose immune system was threatened with an antigen that induced a humoral immune response was lower than that of the eggs of unchallenged females. This suggested that the use of carotenoids for health care was prioritized rather than accumulation in eggs (Saino et al., 2002). In conclusion, bird strain, laying order, reproduction output, age, and maternal infection all can modulate the absorption of carotenoids, then the deposition in eggs.
5.4. Species and molecular linkage of carotenoids
Fowls seem to discriminate against some carotenoids while favoring others during absorption. It was shown that xanthophylls were more effective in birds and laying hens than carotenes so that they are used more frequently than the latter (Blount et al., 2001; Møller et al., 2000). Laying hens fed a xanthophyll-enriched maize-based diet more efficiently accumulated violaxanthin in egg yolk at different proportions than β-carotene (Moreno et al., 2020). Similarly, in a study implying different carotenoid by-products supplementation to diet, lutein was preferentially and more effectively delivered to quail egg yolk than any other individual carotenoid, meanwhile, β-carotene presented the lowest efficiency (Karadas et al., 2006). When laying hens were fed diet supplemented with carrots (Daucus carota), the average deposition efficiency of lutein and zeaxanthin was around 25%, meanwhile, β-carotene was deposited quite ineffectively with an average of 0.5% (Hammershoj et al., 2010). In breeder hens, alfalfa extract supplementation resulted in eggs that are mainly enriched with lutein with some zeaxanthin. In the eggs laid by the supplemented hens, however, a small amount of β-carotene was retrieved, whereas no β-carotene was found in the eggs of the control birds (Karadas et al., 2005a).
Added to the conversion of β-carotene into vitamin A discussed in Section 4.2.1, the inability of laying hens to deposit β-carotene into the egg yolk may also be ascribed to the polarity effect. In fact, nonpolar carotenes are absorbed, transported, and incorporated less effectively than more polar xanthophylls (Hargitai et al., 2006). Polar carotenoids such as canthaxanthin and β-8-apocarotenoic acid ethyl ester affected egg yolk coloration following a week of supplementation whereas β-carotene did not (Na et al., 2004). The deposition efficiencies of lutein (on average 25.52%) and zeaxanthin (on average 26.05%) were higher than those of β-cryptoxanthin (on average 8.30%) and β-carotene (on average 5.65%) (Kljak et al., 2021b). In an experiment by Burt et al. (2013), the ratio of lutein:zeaxanthin in the diet as compared with egg yolk indicated that lutein is preferentially accumulated in the egg yolk compared with zeaxanthin for those corns. This ratio was even lower in egg yolk, especially considering the diet supplemented with marigolds. This also suggests a link between the type of carotenoid and the matrix to affect the carotenoid deposition into eggs.
Studies revealed that the physicochemical properties and additional molecular groups such as ester or aldehyde might affect the deposition of carotenoids into eggs. Before the absorption of carotenoid esters, the fatty acid group is cleaved by pancreatic carboxyl ester lipase to form free xanthophylls in the gastrointestinal tract (von Lintig et al., 2020). Thus, the degree of esterification is believed to decrease the absorption of carotenoids. In fishes, mice, and in vitro, the bioavailability of free astaxanthin and astaxanthin monoester was significantly higher than that of astaxanthin diester. Moreover, astaxanthin ester with short-chain fatty acids was more bioavailable than that with long-chain fatty acids. Meanwhile, low unsaturated fatty acids astaxanthin ester presented better bioavailability than their high unsaturated fatty acid counterparts (White et al., 2003; Yang et al., 2021). Nevertheless, there are controversial findings about the effect of esterification degree on carotenoid bioavailability. Mariutti and Mercadante (2018) have reported similar or even higher bioavailability of carotenoid esters with comparison to free carotenoids as analyzed in the plasma and serum of humans. Failla et al. (2019) also reported comparable bioavailability of esterified and free carotenoids. In laying hens, a quantitative study revealed equivalent plasma lutein and capsanthin concentrations regardless of whether free or esterified carotenoids were given (Breithaupt et al., 2003). Lutein concentrations in plasma between laying hens fed either unesterified or esterified lutein were not significantly different after 7 days of the test. However, the concentration was higher in laying hens fed unesterified lutein than in those fed esterified lutein on day 3 of the experiment (Wu et al., 2009). In fact, the hydrolyzation of carotenoid ester may delay the absorption of carotenoid but should not affect the total amount of carotenoid absorbed. The lower amount of carotenoid absorbed with esterified carotenoids in the aforementioned studies was mostly analyzed in plasma within 70 h after consumption. Few studies exist on the esterification effect on carotenoids' efficiency in laying hens’ eggs. The carotenoid contents in eggs from laying hens fed a diet supplemented with lutein, zeaxanthin, and meso-zeaxanthin diacetates were higher than the contents in eggs from laying hens fed unesterified carotenoids. Furthermore, the content of carotenoids in eggs from a diet supplemented with zeaxanthin diacetate and meso-zeaxanthin diacetate was higher than in those fed lutein (unesterified and diacetate) and a mixture of meso-zeaxanthin and lutein (diacetate) (Nolan et al., 2016). This may lead to even better bioavailability of carotenoid esters and deposition into eggs, but also revealed that the effect of esterification also depends on the type of carotenoid.
Finally, the geometrical configuration of carotenoids may also affect their efficiency. In fishes, crustaceans, and humans, a better bioavailability of Z isomers than all-E isomers or a preferential distribution of Z isomers into certain organs is also suspected (Yeum and Russell, 2002; Yu and Liu, 2020). An in vitro study by Yang et al. (2017) revealed that both all-E to Z and Z to all-E isomerizations of astaxanthin were possible. However, most importantly, their study demonstrated that the bioaccessibility of 13-Z isomers was higher than those of 9-Z and all-E. In laying hens, all-E to Z isomerization of carotenoids leading to a better accumulation of Z isomers in eggs is clearly established (Section 4.2.2). However, there is no evidence of the better absorption of Z isomers compared with all-E isomers. Further studies employing in vitro cells and stable isotopes may be valuable to elucidate this aspect.
5.5. Absorption effectors
Different dietary factors known as effectors may also influence the accumulation of carotenoids in egg yolk. In general, because carotenoids are lipophilic compounds, their efficiency is increased with incorporation of dietary lipids. Fat has three separate effects; it creates a hydrophobic milieu for the released carotenoids, stimulates bile salt production, and promotes the creation of micelles (Priyadarshani, 2017). Many authors have highlighted the importance of dietary fat in carotenoid absorption. However, the amount of dietary fat required for optimum absorption of carotenoids is unclear. Selvaraj et al. (2006) reported that higher dietary fat up to 6% did not increase tissue lutein levels and that 3% dietary fat may be enough to optimize lutein absorption up to 50 mg/kg feed. Besides, the saturation of fat has the potential to influence the bioavailability of xanthophylls. Feeding laying hens with diets enriched in saturated fatty acids and with a low dietary unsaturated/saturated ratio may reduce lutein, zeaxanthin, and total carotenoid levels in egg yolk during the early-laying period. In contrast, providing diets with a higher content of polyunsaturated fatty acids and fewer saturated fatty acids did not affect egg yolk carotenoids (Papadopoulos et al., 2019). Moreover, with the particular characteristics of xanthophyll esters such as higher fat solubility and de-esterification before absorption, their requirements for fat for better assimilation are much higher. Therefore, efficient absorption of xanthophyll esters at levels similar to or higher than their free-form counterparts required greater fat intake (Fernández-García et al., 2012).
By contrast, fibers are generally known to reduce the bioavailability of carotenoids in the intestine (Priyadarshani, 2017). For example, lutein retention in egg yolk was tested in laying hens fed either diet-based corn distillers' dried grains with soluble or corn gluten meal. The results showed a lower concentration of lutein in egg yolks from corn distillers’ dried grains (47.7 μg/g) than with corn gluten meal (77.4 μg/g) (Shin et al., 2016). Moreover, other nutritional factors were evaluated to be detrimental (aflatoxin, high amount of vitamin A) or beneficial (reproductive hormones, vitamin E, and other carotenoids) to the absorption of carotenoids. Zaghini et al. (2005) reported that aflatoxin B1 may interfere with lipid metabolism, carotenoid absorption, or yolk deposition, resulting in changes in certain color characteristics. Aflatoxin inhibited carotenoid absorption to a higher extent in low fat diets than in high fat diets, demonstrating the intricacy of mycotoxin interactions. In Japanese quail, GnRH association with a carotenoid supplementation diet favored the deposition of carotenoid into egg yolks. The concentrations of carotenoids and vitamins in yolks were significantly and positively correlated with testosterone concentration in egg yolks (Peluc et al., 2012). Vitamin E (α-tocopherol) efficiency to improve carotenoid absorption was evaluated and it was found that eggs from the group of laying hens supplemented with lutein ester associated with tocopherol accumulated more lutein (13.72 mg/kg), zeaxanthin (0.65 mg/kg), and α-tocopherol (297.40 mg/kg) than the group supplemented with only lutein ester (10.96, 0.55, and 205.20 mg/kg, respectively). Hence, α-tocopherol in diet may improve the bioavailability of lutein. Owing to the use of α-tocopherol as an antioxidant, better bioavailability may be due to increased absorption of lutein in the presence of tocopherol and/or greater stabilization of lutein/zeaxanthin (Islam et al., 2016).
5.6. External conditions
The metabolic activity of birds rises in colder weather, resulting in a greater rate of free radical generation and an increase in the antioxidant system's activity level. As a result of their elevated antioxidant demands, females may be unable to invest substantial amounts of carotenoids in their eggs in cold weather and carotenoid distribution to the eggs may be limited due to the females' increased antioxidant requirement. Following that, the concentration and profile of yolk carotenoids in the same bird population may change significantly between seasons and environmental conditions (Hargitai et al., 2006). Shuvra, Shorna, and Hy-Line white commercial layer strains were exposed to two different ambient temperatures (cyclic heat stress 25–35 °C and thermoneutral 20–21 °C). The results showed that cyclic heat stress generally decreased the yolk color score compared with thermoneutral temperature, the Hy-Line commercial layer strain presented the lowest yolk color score. There was also a pronounced correlation between the strain and temperature (Hassan et al., 2018). Furthermore, exposure to some parasites and toxins can also reduce the availability of carotenoids. It was found that lutein and vitamin D3 concentrations in yolks were lower in a polluted zone than in an unpolluted one. Thus, females in an unpolluted zone could have better access to lutein-rich food sources and can invest more of these nutrients in the yolk (Espin et al., 2016). Consequently, the rearing conditions (housing and temperature) of poultry could affect the bioaccessibility of carotenoids.
Though organic egg production requires more natural carotenoid addition than conventional egg production, researchers have demonstrated some promising results based on season and system management to improve considerably the carotenoid content of organic eggs. Therefore, organic-plus system raising (10 m2 per hen with access to pasture) of laying hens resulted in eggs with much higher amounts of carotenoids and tocopherols in yolks than eggs obtained from an organic system (4 m2 per hen with access to pasture) and standard housing system (hens kept in cages without access to pasture) in four seasons: winter, spring, summer, and autumn. In addition, the highest contents of total carotenoids in egg yolks were observed in the organic-plus hens during spring and autumn (Mugnai et al., 2014). On the other hand, lyophilized permanent pasture had 128, 115, 75, and 79 mg/kg of dry matter of lutein, zeaxanthin, α-tocopherol, and β-carotene, respectively. After hens grazed in combination with sequential feeding of whole wheat and balanced mixed diet, the egg yolks significantly accumulated more carotenoids than the eggs from the control group without grazing. The grazing enhanced egg concentrations of lutein, zeaxanthin, α-tocopherol, and β-carotene by 260, 174, 270, and 2 μg per egg, respectively (Skřivan and Englmaierová, 2014).
Finally, we could mention that the efficiency of carotenoids might be underestimated during analysis because the measurement of their metabolites is often neglected. Thenceforth, it may be important to do proper measurements and study the functional properties of the metabolites to maximize the efficiency of carotenoids in eggs.
6. Functions of carotenoids in eggs
6.1. Influence of carotenoids on the quality of eggs
The primary use of carotenoids in the poultry industry is the enhancement of meat and egg yolk color (Meléndez-Martínez et al., 2021). There are numerous studies on the effect of carotenoids on the coloration of egg yolks. Carotenoids such as β-cryptoxanthin, canthaxanthin, lycopene, and astaxanthin confer color to the egg yolk ranging in shade from orange to red, whereas lutein and zeaxanthin give egg yolk a yellow color (Fan et al., 2021; Liu et al., 2012; Orhan et al., 2021). With the color measurement CIE L∗a∗b∗ (the International Commission on Illumination where L∗, a∗, and b∗ stand for relative lightness, redness, and yellowness, respectively), an increase in yolk yellowness leads to a significant increase in the b∗ score (Skřivan et al., 2016). An increase in yolk redness leads to a significant increase in the a∗ score with a slight decrease in the L∗ score and b∗ score (Niu et al., 2008). Islam et al. (2017) explained this negative correlation between the a∗ score and L∗ score by the increase in darkness by certain carotenoids. However, this does not apply to all carotenoids because other carotenoids showed a positive or fair correlation between the different color measurement scores.
Several studies have reported that carotenoids do not affect the physical quality of eggs such as egg weight, shell thickness, albumen height, Haugh Unit (Honda et al., 2019; Hsu et al., 2015; Shahsavari, 2014; Walker et al., 2012). Nonetheless, some positive effects of carotenoids were reported. Egg and egg yolk weights from hens fed a diet supplemented with either purified lycopene (62.80 and 18.66 g for egg and egg yolk, respectively) or tomato powder (63.01 and 18.75 g), were greater than those of hens fed the control diet (60.50 and 16.49 g) (Orhan et al., 2021). In addition, astaxanthin from the bacterium Paracoccus marcusii contributed to egg weight increase compared with the control group (Conradie et al., 2018). Moreover, there were linear changes in egg weight and yolk color as tomato powder content increased in laying hens’ diet (Akdemir et al., 2012). On the other hand, some research presented positive effects on the internal quality of eggs. For instance, the dietary inclusion of lycopene contributed to enhancing the albumen width of eggs (Honda et al., 2019). Besides, the inclusion levels of Dunaliella salina biomass had a linear correlation with the egg weight, yolk weight, and yolk index increase, as well as physicochemical parameters of eggs such as increase in total carotenoids and decrease in thiobarbituric acid reactive substances (TBARS) (Fernandes et al., 2020). Eggshell thickness was influenced by a diet supplemented with 6% flaxseed meal as a source of n-3 polyunsaturated fatty acids (PUFA) and either 2% dehydrated kapia peppers, 2% dehydrated sea buckthorn pomace, or 2% dehydrated carrots as a rich source of carotenoids. The highest eggshell thickness was recorded in the dehydrated sea buckthorn pomace group, which was significantly higher than the two other groups (Panaite et al., 2021). In fresh eggs, the dietary addition of canthaxanthin has improved the resistance of egg yolk vitelline membrane as well (Damaziak et al., 2018).
Despite numerous studies reporting the beneficial effects of carotenoids' inclusion on egg quality, evidence cannot be found on the real implication of carotenoids in these improvements. Egg yolk weight is extensively related to bird intrinsic factors and diet composition, including lipid content. The lipid content of egg yolk ranged from 22.9% to 34.0% of its total weight, and 60.7% to 64.7% of its dry matter in different chicken genotypes (Antova et al., 2019). Even though it is not possible to change the global lipid content of yolks, studies have established that the profile of fatty acids in egg yolk is strongly dependent on diet, and the proper balance between linoleic acid and oleic acid in the hen's feed was proven to increase the retention of other lipids and then increase the weight of the yolks (Ahmad et al., 2019; Nys et al., 2011). On the other hand, lipid metabolism may be impaired in laying hens because hepatic lipogenesis and lipidemia are increased, which could lead to fatty liver hemorrhagic syndrome. Carotenoids are known to decrease fatty liver hemorrhagic syndrome in laying hens, mainly through the restoration of mitochondrial function which normalizes lipid metabolism by improving the balance between lipolysis and fat synthesis and improves β-oxidation of lipids and energy generation (Arroyave-Ospina et al., 2021; Clugston, 2020). Therefore, a hypothesis for the egg weight increase by carotenoids may be that carotenoids improve hepatic lipid metabolism, which regulates the synthesis and disposal of specific fatty acids contributing to the increase of egg yolk weight and then the whole egg weight. Another explanation may be that the different matrixes in which the carotenoids are imbedded presumably contain other nutritional compounds, that might have impacted the eggs as well. This consideration should be taken into account when studying the effect of carotenoids on egg physical quality.
6.2. Influence of carotenoids on the nutritional value of eggs
Apart from the increase of total carotenoids content, which is an important aspect of the nutritional value of eggs, carotenoids were used for many other purposes in eggs such as the alleviation of cholesterol levels or regulation of triglycerides and fatty acids content (Puertas and Vazquez, 2019). In laying hens, dietary supplementation with red yeast Sporidiobolus pararoseus resulted in egg yolks with considerably lower cholesterol and triglycerides (Tapingkae et al., 2018). While evaluating the efficacy of two different sources of lycopene, purified lycopene was more effective in increasing the concentration of lycopene in egg yolk, whereas tomato powder was more effective in lowering the cholesterol concentrations in egg yolk (Orhan et al., 2021). Similarly, lycopene contributed to the reduction of triglycerides in yolks as the triglycerides content of yolks in the group of bacterial lycopene and commercial lycopene supplements was slightly lower than in the control group (Hsu et al., 2015). At 6% and 9% inclusion levels of tomato waste meal, yolk total lipids were at the lowest levels (6.94 and 6.38 mg/mL) compared with the control group (8.38 mg/mL). Similar results were observed for total cholesterol, free fatty acids, phospholipids, and triglycerides (Habanabashaka et al., 2014). In terms of fatty acids, marine by-product meal was found to be a better choice for enhancing the composition of egg yolk by increasing n-3 highly unsaturated fatty acids (n-3 HUFA) compared with fish meal. This is possible due to the carotenoid content in marine by-products which reduces the oxidation of n-3 HUFA (Toyes-Vargas et al., 2018). Likewise, rapeseed oil combined with algae (Nannochloropsis oculate) in diets improved the nutritional benefit of egg yolk as n-3 PUFA was enhanced following supplementation of the algae in the diet (Fredriksson et al., 2006). Moreover, there was a positive correlation between the inclusion of alfalfa and marigold in quail breeder diets and the increase of retinyl palmitate and total vitamin A in egg yolks. Vitamin A was present as retinol (52%–62%), retinyl linoleate (9%–11%), retinyl stearate (4%), retinyl oleate (11%–15%), and retinyl palmitate (13%–22%) (Karadas et al., 2005b).
Carotenoids are also used to decrease lipid peroxidation in fresh eggs. Dietary astaxanthin strengthened the antioxidant potential and lipid profile in laying hens. Thus, in the plasma, liver, and egg yolks, total antioxidant capacity, superoxide dismutase, and glutathione peroxidase activities were elevated (Gao et al., 2020). Moreover, antioxidant activity evaluation revealed that bacterial lycopene had a 100% scavenging capacity of 1,1-diphenyl-2-picrylhydrazyl (DPPH) at 4.65 mg/mL concentration, which was more efficient than butylated hydroxytoluene (BHT) and industrial lycopene (Hsu et al., 2015). In a study including diets supplemented with tomato powder, concentrations of serum and egg yolk lycopene, lutein, β-carotene, and vitamin A improved, whereas malondialdehyde (MDA) decreased linearly with the increase of supplementary tomato powder concentrations (Akdemir et al., 2012).
6.3. Influence of carotenoids on the storage capacity of eggs
One of the most significant criteria that determine consumer demand and choice is the freshness of the egg. However, during storage, this freshness diminishes and is associated with a reduction in Haugh Units, albumen weight proportion, and an increase in egg weight loss, yolk weight proportion, albumen pH, yolk pH, and change in yolk lipid (Akter et al., 2014; Grcevic et al., 2019a). Biochemical changes in the composition and function of egg membranes are responsible for this process. The pH of the freshly laid yolk is normally around 6, but the pH rises steadily to a value between 6.4 and 6.9. During storage, the balance between chemical compounds that regulate the albumen pH level is disrupted causing a rise in the pH from 7.6 to 9.7 (Sunwoo and Gujral, 2014). According to Obianwuna et al. (2022a), natural products are efficient to improve albumen quality because of their high bioavailability, antioxidant properties, and function to improve gut microflora, which is crucial for animal health and biofortification of animal products. Plant-derived antioxidants contain bioactive compounds including carotenoids that can pass into eggs. As a result, the key benefit of using natural antioxidants comes from their ability to inhibit oxidation processes and limit oxidative products in animal and food systems (Obianwuna et al., 2022b).
To maintain the quality of eggs during storage, carotenoid functions were evaluated. The main effect of carotenoids was found to be a significant reduction of egg yolk's pH and the improvement of oxidation in egg yolk. For instance, lycopene was tested in laying hens, and it was found that after 30 days of storage at room temperature, the combined use of lycopene (800 mg/kg of diet) and minerals reduces yolk pH. At refrigerator temperature, the use of 800 mg/kg lycopene of feed stabilized the yolk color intensity during storage (Cruz et al., 2015). Similarly, astaxanthin-derived H. pluvialis was found to inhibit egg weight loss and maintain yolk color during storage at 4 °C and 25 °C (Heng et al., 2020). Moreover, the use of marigold extract has been proven effective in reducing egg weight loss and oxidation, and strengthening eggshells during 28 days storage of eggs. With 2 g/kg dietary inclusion of marigold extract, egg weight was 59.02 g and eggshell thickness 0.387 mm, being better than the values of the control group of 58.43 g and 0.380 mm, respectively (Grcevic et al., 2019a). Dietary carotenoids supplementation lowered the n-6:n-3 ratio in egg yolk and cholesterol content of the egg yolk, and enhanced yolk pH and yolk oxidative stability after 28 days storage at 4 °C (Panaite et al., 2021). We also found that during storage, the carotenoid level of eggs could significantly decrease. On days 28 and 42 of storage, the amount of astaxanthin in the egg had dropped by 25.08% and 40.94%, respectively. However, astaxanthin contributed to improving the stability of DHA-enriched eggs during storage by inhibiting the further oxidation of nonesterified PUFA, which is a key role in improving the storage stability of DHA. The enrichment of astaxanthin in yolk favored hydroxy radical inhibition, hence lowered oxidation products, including 4-hydroxy-2-hexenal (HHE), 4-hydroxy-2-nonenal (HNE), and MDA (Wang et al., 2022).
7. Conclusions and perspectives
Carotenoids are investigated due to their importance in the food chain. Naturally found in plants, microorganisms, and some wild animals, they can also be chemically synthesized and incorporated into edible eggs through feeding, providing specific functions. Studies revealed that carotenoids were different in structures and sources, which combined with human handling and individual factors related to the animal could affect the transfer efficiency to eggs. Present in different forms in eggs following enzymatic and chemical transformation in fowls, they can contribute to the improvement of egg quality, enhancement of other nutrients in eggs, and stability of eggs during storage. However, few recent studies exist regarding the metabolism of carotenoids in birds, and among the existing literature, few works reported the fate of carotenoids in laying hens, the principal species involved in the production of table eggs. With the increasing use of novel carotenoids such as astaxanthin in the poultry industry, more work should address the fate of these carotenoids and their specific functions in eggs as well. Moreover, limited studies have focused on the measurement of the metabolites of carotenoids in eggs. Consideration could be given to the metabolites to measure the efficiency of carotenoids more effectively. In addition, the role of carotenoids in egg quality improvement is not well defined because the matrix in which the carotenoids are embedded might greatly affect the egg quality and then lead to misinterpretation of the carotenoid functions. Further studies are required to clarify these points. The carotenoid-enriched egg is an emerging industry that requires knowledge investment by researchers.
Author contributions
Dieudonné M. Dansou: Conceptualization, Methodology, Investigation and Writing-original draft. Huiyan Zhang: Methodology, Validation, Writing-review and editing. Yanan Yu: Methodology, Writing-review and editing. Hao Wang: Methodology and Investigation. Chaohua Tang: Project administration, Writing-review and editing. Qingyu Zhao: Supervision and Project administration. Yuchang Qin: Funding acquisition and Resources. Junmin Zhang: Conceptualization, Project administration and Funding acquisition.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by the China Agriculture Research Systems (CARS-40-K11), the Beijing Innovation Consortium of Agriculture Research System (BAIC06-2022-G05), the National Key R&D Program of China (No. 2022YFD1600105), and the Chinese Academy of Agricultural Science and Technology Innovation Project (ASTIP-IAS-12).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Yuchang Qin, Email: qinyuchang@caas.cn.
Junmin Zhang, Email: zhangjunmin@caas.cn.
References
- Abdel-Aal E.S.M., Akhtar H., Chambers J.R., Zaheer K. In: Egg innovations and strategies for improvements. Hester P.Y., editor. Elsevier Inc.; 2017. Lutein and zeaxanthin carotenoids in eggs; pp. 199–206. [DOI] [Google Scholar]
- Abeyrathne E., Nam K.C., Huang X., Ahn D.U. Egg yolk lipids: separation, characterization, and utilization. Food Sci Biotechnol. 2022;31(10):1243–1256. doi: 10.1007/s10068-022-01138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S., Yousaf M., Kamran Z., Sohail M.U., Tahir M.N., Koutoulis K.C., Manzoor A. Effects of supplementing various linoleic to α-linolenic acid ratios and vitamin A on production performance and egg characteristics of laying hens during summer months. JZR. 2019;1(1):1–9. doi: 10.30564/jzr.v1i1.112. [DOI] [Google Scholar]
- Akdemir F., Orhan C., Sahin N., Sahin K., Hayirli A. Tomato powder in laying hen diets: effects on concentrations of yolk carotenoids and lipid peroxidation. Br Poultry Sci. 2012;53(5):675–680. doi: 10.1080/00071668.2012.729142. [DOI] [PubMed] [Google Scholar]
- Akiba Y., Sato K., Takahashi K., Takahashi Y., Furuki A., Konashi S., Nishida H., Tsunekawa H., Hayasaka Y., Nagao H. Pigmentation of egg yolk with yeast Phaffia rhodozyma containing high concentration of astaxanthin in laying hens fed on a low carotenoid diet. Jpn Poult Sci. 2000;37(2):77–85. [Google Scholar]
- Akter Y., Kasim A., Omar H., Sazili A.Q. Effect of storage time and temperature on the quality characteristics of chicken eggs. J Food Agric Environ. 2014;12(3–4):87–92. [Google Scholar]
- Alagawany M., Farag M.R., Dhama K., Patra A. Nutritional significance and health benefits of designer eggs. World’s Poult Sci J. 2019;74(2):317–330. doi: 10.1017/s0043933918000041. [DOI] [Google Scholar]
- Alay T., Karadas F. The effects of carotenoids in quail breeder diets on egg yolk pigmentation and breeder performance. Acta Agric Scand A Anim Sci. 2017;66(4):206–214. doi: 10.1080/09064702.2017.1330360. [DOI] [Google Scholar]
- Alvarenga R.R., Zangeronimo M.G., Pereira L.J., Rodrigues P.B., Gomide E.M. Lipoprotein metabolism in poultry. World’s Poult Sci J. 2011;67(3):431–440. doi: 10.1017/s0043933911000481. [DOI] [Google Scholar]
- Ambati R.R., Gogisetty D., Aswathanarayana R.G., Ravi S., Bikkina P.N., Bo L., Yuepeng S. Industrial potential of carotenoid pigments from microalgae: current trends and future prospects. Crit Rev Food Sci Nutr. 2019;59(12):1880–1902. doi: 10.1080/10408398.2018.1432561. [DOI] [PubMed] [Google Scholar]
- An B.K., Choo W.D., Kang C.W., Lee J., Lee K.W. Effects of dietary lycopene or tomato paste on laying performance and serum lipids in laying hens and on malondialdehyde content in egg yolk upon storage. J Poultry Sci. 2019;56(1):52–57. doi: 10.2141/jpsa.0170118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen C.J. Bioactive egg components and inflammation. Nutrients. 2015;7(9):7889–7913. doi: 10.3390/nu7095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D.M., MacIssac J.L., Daniel M.A., MacKinnon T.L., Budgell K.L. Evaluating the effects of crab meal, Carophyll Red®, and Carophyll Yellow® in laying hen diets on egg yolk pigmentation and production performance. Can J Anim Sci. 2008;88(4):637–640. doi: 10.4141/cjas08063. [DOI] [Google Scholar]
- Anton M. In: Bioactive egg compounds. Huopalahti R., López-Fandinno R., Anton M., Schade R., editors. Springer; 2007. Composition and structure of hen egg yolk; pp. 1–6. [DOI] [Google Scholar]
- Antova G.A., Gerzilov V.T., Petkova Z.Y., Boncheva V.N., Bozhichkova I.N., St Penkov D., Petrov P.B. Comparative analysis of nutrient content and energy of eggs from different chicken genotypes. J Sci Food Agric. 2019;99(13):5890–5898. doi: 10.1002/jsfa.9863. [DOI] [PubMed] [Google Scholar]
- Arimboor R., Natarajan R.B., Menon K.R., Chandrasekhar L.P., Moorkoth V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: analysis and stability-a review. J Food Sci Technol. 2015;52(3):1258–1271. doi: 10.1007/s13197-014-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyave-Ospina J.C., Wu Z., Geng Y., Moshage H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy. Antioxidants. 2021;10(2):174. doi: 10.3390/antiox10020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attie A.D., Hamon Y., Brooks-Wilson A.R., Gray-Keller M.P., MacDonald M.L., Rigot V., Tebon A., Zhang L.H., Mulligan J.D., Singaraja R.R., Bitgood J.J., Cook M.E., Kastelein J.J., Chimini G., Hayden M.R. Identification and functional analysis of a naturally occurring E89K mutation in the ABCA1 gene of the WHAM chicken. J Lipid Res. 2002;43(10):1610–1617. doi: 10.1194/jlr.m200223-jlr200. [DOI] [PubMed] [Google Scholar]
- Balnave D., Bird J.N. Relative efficiencies of yellow carotenoids for egg yolk pigmentation. Asian-Australas J Anim Sci. 1996;9(5):515–518. doi: 10.5713/ajas.1996.515. [DOI] [Google Scholar]
- Bandara S., Thomas L.D., Ramkumar S., Khadka N., Kiser P.D., Golczak M., von Lintig J. The structural and biochemical basis of apocarotenoid processing by beta-carotene oxygenase-2. ACS Chem Biol. 2021;16(3):480–490. doi: 10.1021/acschembio.0c00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount J.D., Surai P.F., Houston D.C., Møller A.P. The relationship between dietary and yolk carotenoid composition in a wild bird: a supplemental feeding study of lesser black-backed gulls (Larus fuscus) Br Poultry Sci. 2001;42(sup001):84–85. doi: 10.1080/00071660152681683. [DOI] [Google Scholar]
- Bortolotti G.R., Negro J.J., Surai P.F., Prieto P. Carotenoids in eggs and plasma of red-legged partridges: effects of diet and reproductive output. Physiol Biochem Zool. 2003;76(3):367–374. doi: 10.1086/375432. [DOI] [PubMed] [Google Scholar]
- Breithaupt DE Weller P., Grashorn M.A. Quantification of carotenoids in chicken plasma after feeding free or esterified lutein and capsanthin using high-performance liquid chromatography and liquid chromatography-mass spectrometry analysis. Poultry Sci. 2003;82(3):395–401. doi: 10.1093/ps/82.3.395. [DOI] [PubMed] [Google Scholar]
- Burt A.J., Caston L., Leeson S., Shelp B.J., Lee E.A. Development and utilization of high carotenoid maize germplasm: proof of concept. Crop Sci. 2013;53(2):554–563. doi: 10.2135/cropsci2012.02.0069. [DOI] [Google Scholar]
- Canene-Adams K., Erdman J.W.J. In: Carotenoids volume 5: nutrition and health. Britton G., Pfander H., Liaaen-Jensen S., editors. Birkhäuser Verlag Basel; 2009. Absorption, transport, distribution in tissues and bioavailability; pp. 115–148. [DOI] [Google Scholar]
- Castenmiller J.J., West C.E. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr. 1998;18:19–38. doi: 10.1146/annurev.nutr.18.1.19. [DOI] [PubMed] [Google Scholar]
- Clugston R.D. Carotenoids and fatty liver disease: current knowledge and research gaps. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(11) doi: 10.1016/j.bbalip.2019.158597. [DOI] [PubMed] [Google Scholar]
- European Union Register of Feed Additives. Commission implementing regulation (EU) 2020/1418 of 6 October 2020 concerning the authorisation of saponified paprika (Capsicum annuum) extract (capsanthin) as a feed additive for chickens for fattening, minor poultry species for fattening, laying hens and minor poultry species for laying. Official Journal of the European Union. 2020:L326. [Google Scholar]
- Code of Federal Regulations . Office of the Federal Register & Government Publications Office; 2016. National archives and records administration.https://www.ecfr.gov/current/title-21/chapter-I/subchapter-E/part-570 [Google Scholar]
- Connor W.E., Duell P.B., Kean R., Wang Y. The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Invest Ophthalmol Vis Sci. 2007;48(9):4226–4231. doi: 10.1167/iovs.06-1275. [DOI] [PubMed] [Google Scholar]
- Conradie T.A., Pieterse E., Jacobs K. Application of Paracoccus marcusii as a potential feed additive for laying hens. Poultry Sci. 2018;97(3):986–994. doi: 10.3382/ps/pex377. [DOI] [PubMed] [Google Scholar]
- Coral-Hinostroza G.N., Bjerkeng B. Astaxanthin from the red crab langostilla (Pleuroncodes planipes): optical R/S isomers and fatty acid moieties of astaxanthin esters. Comp Biochem Physiol B Biochem Mol Biol. 2002;133(3):437–444. doi: 10.1016/s1096-4959(02)00186-0. [DOI] [PubMed] [Google Scholar]
- Coudert E., Baéza E., Berri C. Use of algae in poultry production: a review. World’s Poult Sci J. 2020;76(4):767–786. doi: 10.1080/00439339.2020.1830012. [DOI] [Google Scholar]
- Cruz FKd, Garcia ERdM., Ferraz A.L.J., Souza KMRd, Feliciano W.B., Rohod R.V. Quality and stability of eggs from laying hens fed with organic minerals and lycopene. Ciência Rural. 2015;46(1):157–162. doi: 10.1590/0103-8478cr20150594. [DOI] [Google Scholar]
- Damaziak K., Marzec A., Riedel J., Szeliga J., Koczywas E., Cisneros F., Michalczuk M., Lukasiewicz M., Gozdowski D., Siennicka A., Kowalska H., Niemiec J., Lenart A. Effect of dietary canthaxanthin and iodine on the production performance and egg quality of laying hens. Poultry Sci. 2018;97(11):4008–4019. doi: 10.3382/ps/pey264. [DOI] [PubMed] [Google Scholar]
- Dansou D.M., Wang H., Nugroho R.D., He W., Zhao Q., Tang C., Zhang H., Zhang J. Effects of duration and supplementation dose with astaxanthin on egg fortification. Poultry Sci. 2021;100(9) doi: 10.1016/j.psj.2021.101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansou D.M., Wang H., Nugroho R.D., He W., Zhao Q., Zhang J. Assessment of response to moderate and high dose supplementation of astaxanthin in laying hens. Animals. 2021;11(4) doi: 10.3390/ani11041138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Val E., Senar J.C., Garrido-Fernandez J., Jaren M., Borras A., Cabrera J., Negro J.J. The liver but not the skin is the site for conversion of a red carotenoid in a passerine bird. Sci Nat. 2009;96(7):797–801. doi: 10.1007/s00114-009-0534-9. [DOI] [PubMed] [Google Scholar]
- Dela Sena C., Sun J., Narayanasamy S., Riedl K.M., Yuan Y., RWJr Curley, Schwartz S.J., Harrison E.H. Substrate specificity of purified recombinant chicken beta-carotene 9',10'-oxygenase (BCO2) J Biol Chem. 2016;291(28):14609–14619. doi: 10.1074/jbc.M116.723684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarchelier C., Borel P. Overview of carotenoid bioavailability determinants: from dietary factors to host genetic variations. Trends Food Sci Technol. 2017;69:270–280. doi: 10.1016/j.tifs.2017.03.002. [DOI] [Google Scholar]
- EFSA FEEDAP Panel. Safety and efficacy of saponified paprika extract, containing capsanthin as main carotenoid source, for poultry for fattening and laying (except turkeys) EFSA J. 2020;18(2):6023. doi: 10.2903/j.efsa.2020.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel. Safety of ethyl ester of beta-apo-8’-carotenoic acid as a feed additive for poultry for fattening and poultry for laying. EFSA J. 2019;17(12):5911. doi: 10.2903/j.efsa.2019.5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel. Scientific opinion on the safety and efficacy of canthaxanthin as a feed additive for poultry and for ornamental birds and ornamental fish. EFSA J. 2014;12(1):3527. doi: 10.2903/j.efsa.2014.3527. [DOI] [Google Scholar]
- EFSA FEEDAP Panel. Safety of use of colouring agents in animal nutrition - Part III: ß-apo-8’-carotenal, ethyl ester of ß-apo-8’-carotenoic acid, lutein, zeaxanthin and concluding remarks. EFSA J. 2009;7(5):1098. doi: 10.2903/j.efsa.2009.1098. [DOI] [Google Scholar]
- Englmaierová M., Skřivan M., Bubancová I. A comparison of lutein, spray-dried Chlorella, and synthetic carotenoids effects on yolk colour, oxidative stability, and reproductive performance of laying hens. Czech J Anim Sci. 2013;58(9):412–419. doi: 10.17221/6941-cjas. [DOI] [Google Scholar]
- Espin S., Ruiz S., Sanchez-Virosta P., Salminen J.P., Eeva T. Effects of experimental calcium availability and anthropogenic metal pollution on eggshell characteristics and yolk carotenoid and vitamin levels in two passerine birds. Chemosphere. 2016;151:189–201. doi: 10.1016/j.chemosphere.2016.02.074. [DOI] [PubMed] [Google Scholar]
- European Union Register of Feed Additives . Edition 241. 2016. European union register of feed additives pursuant to regulation (EC) No 1831/2003. Annex 1. [Google Scholar]
- European Union Register of Feed Additives Food and Feed Information Portal Database Version 1.3. https://ec.europa.eu/food/food-feed-portal/screen/feed-additives/search [Accessed 20 July 2023]
- Failla M.L., Rodrigues D.B., Chitchumroonchokchai C. In: Carotenoid esters in foods: physical, chemical and biological properties. Mercadante A.Z., editor. Royal Society of Chemistry; 2019. Bioavailability and metabolism of carotenoid esters; pp. 390–420. [DOI] [Google Scholar]
- Fallahshahroudi A., Sorato E., Altimiras J., Jensen P. The domestic BCO2 allele buffers low-carotenoid diets in chickens: possible fitness increase through species hybridization. Genetics. 2019;212(4):1445–1452. doi: 10.1534/genetics.119.302258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G.J., Shih B.L., Lin H.C., Lee T.T., Lee C.F., Lin Y.F. Effect of dietary supplementation of Sargassum meal on laying performance and egg quality of Leghorn layers. Anim Biosci. 2021;34(3):449–456. doi: 10.5713/ajas.20.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes R.T.V., Gonçalves A.A., Arruda AMVd. Production, egg quality, and intestinal morphometry of laying hens fed marine microalga. Rev Bras Zootec. 2020;49 doi: 10.37496/rbz4920200011. [DOI] [Google Scholar]
- Fernández-García E., Carvajal-Lérida I., Jarén-Galán M., Garrido-Fernández J., Pérez-Gálvez A., Hornero-Méndez D. Carotenoids bioavailability from foods: from plant pigments to efficient biological activities. Food Res Int. 2012;46(2):438–450. doi: 10.1016/j.foodres.2011.06.007. [DOI] [Google Scholar]
- Fredriksson S., Elwinger K., Pickova J. Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chem. 2006;99(3):530–537. doi: 10.1016/j.foodchem.2005.08.018. [DOI] [Google Scholar]
- Galobart J., Sala R., Rincón-Carruyo X., Manzanilla E.G., Vilà B., Gasa J. Egg yolk color as affected by saponification of different natural pigmenting sources. J Appl Poultry Res. 2004;13(2):328–334. doi: 10.1093/japr/13.2.328. [DOI] [Google Scholar]
- Gao S., Li R., Heng N., Chen Y., Wang L., Li Z., Guo Y., Sheng X., Wang X., Xing K., Ni H., Qi X. Effects of dietary supplementation of natural astaxanthin from Haematococcus pluvialis on antioxidant capacity, lipid metabolism, and accumulation in the egg yolk of laying hens. Poultry Sci. 2020;99(11):5874–5882. doi: 10.1016/j.psj.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashorn M. In: Handbook on natural pigments in food and beverages. Carle R., Schweiggert R.M., editors. Woodhead Publishing; 2016. Feed additives for influencing chicken meat and egg yolk color; pp. 283–302. [DOI] [Google Scholar]
- Grashorn M.A., Steinberg W. Deposition rates of canthaxanthin in egg yolks. Arch fur Geflugelkunde. 2002;66(6):258–262. [Google Scholar]
- Grcevic M., Kralik Z., Kralik G., Galovic D., Radisic Z., Hanzek D. Quality and oxidative stability of eggs laid by hens fed marigold extract supplemented diet. Poultry Sci. 2019;98(8):3338–3344. doi: 10.3382/ps/pez134. [DOI] [PubMed] [Google Scholar]
- Grcevic M., Kralik Z., Kralik G., Galovic O. Effects of dietary marigold extract on lutein content, yolk color and fatty acid profile of omega-3 eggs. J Sci Food Agric. 2019;99(5):2292–2299. doi: 10.1002/jsfa.9425. [DOI] [PubMed] [Google Scholar]
- Habanabashaka M., Sengabo M., Oladunjoye I.O. Effect of tomato waste meal on lay performance, egg quality, lipid profile and carotene content of eggs in laying hens. Iran J Appl Anim Sci. 2014;4:555–559. [Google Scholar]
- Hammershoj M., Kidmose U., Steenfeldt S. Deposition of carotenoids in egg yolk by short-term supplement of coloured carrot (Daucus carota) varieties as forage material for egg-laying hens. J Sci Food Agric. 2010;90(7):1163–1171. doi: 10.1002/jsfa.3937. [DOI] [PubMed] [Google Scholar]
- Hammouda A., Selmi S., Pearce-Duvet J. Patterns of within-clutch variation in yolk lutein in the Yellow-legged Gull Larus michahellis: the effects of egg laying order and laying date. J Ornithol. 2014;155(4):1009–1015. doi: 10.1007/s10336-014-1087-z. [DOI] [Google Scholar]
- Hargitai R., Matus Z., Hegyi G., Michl G., Toth G., Torok J. Antioxidants in the egg yolk of a wild passerine: differences between breeding seasons. Comp Biochem Physiol B Biochem Mol Biol. 2006;143(2):145–152. doi: 10.1016/j.cbpb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Harrison E.H., Kopec R.E. In: Physiology of the gastrointestinal tract. Said H.M., editor. Elsevier Inc; 2018. Digestion and intestinal absorption of dietary carotenoids and vitamin A; pp. 1133–1151. [DOI] [Google Scholar]
- Hassan M.R., Rabbani M.A.G., Sultana S., Sarker N.R. Effects of strains and temperature on production performance, egg qualities and physiological response of laying hens. Asian J Anim Vet Adv. 2018;13(3):253–262. doi: 10.3923/ajava.2018.253.262. [DOI] [Google Scholar]
- Heng N., GaoS GuoY., Chen Y., Wang L., Sheng X., Wang X., Xing K., Xiao L., Ni H., Qi X. Effects of supplementing natural astaxanthin from Haematococcus pluvialis to laying hens on egg quality during storage at 4 degrees C and 25 degrees C. Poultry Sci. 2020;99(12):6877–6883. doi: 10.1016/j.psj.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez V., Escobar C., Galarza J., Gimpel J. Carotenoids in microalgae. Subcell Biochem. 2016;79:219–237. doi: 10.1007/978-3-319-39126-7_8. [DOI] [PubMed] [Google Scholar]
- Higuera-Ciapara I., Felix-Valenzuela L., Goycoolea F.M. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46(2):185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- Honda M., Ishikawa H., Hayashi Y. Alterations in lycopene concentration and Z-isomer content in egg yolk of hens fed all-E-isomer-rich and Z-isomer-rich lycopene. Anim Sci J. 2019;90(9):1261–1269. doi: 10.1111/asj.13276. [DOI] [PubMed] [Google Scholar]
- Honda M., Kageyama H., Hibino T., Sowa T., Kawashima Y. Efficient and environmentally friendly method for carotenoid extraction from Paracoccus carotinifaciens utilizing naturally occurring Z-isomerization-accelerating catalysts. Process Biochem. 2020;89:146–154. doi: 10.1016/j.procbio.2019.10.005. [DOI] [Google Scholar]
- Honda M., Kawashima Y., Hirasawa K., Uemura T., Jinkun S., Hayashi Y. Possibility of using astaxanthin-rich dried cell powder from Paracoccus carotinifaciens to improve egg yolk pigmentation of laying hens. Symmetry. 2020;12(6):923. doi: 10.3390/sym12060923. [DOI] [Google Scholar]
- Honda M., Murakami K., Watanabe Y., Higashiura T., Fukaya T., Wahyudiono K.H., Goto M. The E/Z isomer ratio of lycopene in foods and effect of heating with edible oils and fats on isomerization of (all-E)-lycopene. Eur J Lipid Sci Technol. 2017;119(8):1600389. doi: 10.1002/ejlt.201600389. [DOI] [Google Scholar]
- Hsu W.T., Chiang C.J., Chao Y.P., Chang C.H., Lin L.J., Yu B., Lee T.T. Effects of recombinant lycopene dietary supplement on the egg quality and blood characteristics of laying quails. J Biosci Bioeng. 2015;120(5):539–543. doi: 10.1016/j.jbiosc.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Hu C.H., Zuo A.Y., Wang D.G., Pan H.Y., Zheng W.B., Qian Z.C., Zou X.T. Effects of broccoli stems and leaves meal on production performance and egg quality of laying hens. Anim Feed Sci Technol. 2011;170(1–2):117–121. doi: 10.1016/j.anifeedsci.2011.07.019. [DOI] [Google Scholar]
- Islam K.M., Khalil M., Manner K., Raila J., Rawel H., Zentek J., Schweigert F.J. Effect of dietary alpha-tocopherol on the bioavailability of lutein in laying hen. J Anim Physiol Anim Nutr. 2016;100(5):868–875. doi: 10.1111/jpn.12464. [DOI] [PubMed] [Google Scholar]
- Islam K.M.S., Khalil M., Manner K., Raila J., Rawel H., Zentek J., Schweigert F.J. Lutein specific relationships among some spectrophotometric and colorimetric parameters of chicken egg yolk. J Poultry Sci. 2017;54(4):271–277. doi: 10.2141/jpsa.0160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiru M., Stranska-Zachariasova M., Kohoutkova J., Schulzova V., Krmela A., Revenco D., Koplik R., Kastanek P., Fulin T., Hajslova J. Potential of microalgae as source of health-beneficial bioactive components in produced eggs. J Food Sci Technol. 2021;58(11):1–10. doi: 10.1007/s13197-020-04896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Dahl M.L., Zuidhof M.J., Korver D.R. The effect of maternal canthaxanthin supplementation and hen age on breeder performance, early chick traits, and indices of innate immune function. Poultry Sci. 2017;96(3):634–646. doi: 10.3382/ps/pew293. [DOI] [PubMed] [Google Scholar]
- Kang D.K., Kim S.I., Cho C.H., Yim Y.H., Kim H.S. Use of lycopene, an antioxidant carotinoid, in laying hens for egg yolk pigmentation. Asian-Australas J Anim Sci. 2003;16(12):1799–1803. doi: 10.5713/ajas.2003.1799. [DOI] [Google Scholar]
- Karadas F., Grammenidis E., Surai P.F., Acamovic T., Sparks N.H. Effects of carotenoids from lucerne, marigold and tomato on egg yolk pigmentation and carotenoid composition. Br Poultry Sci. 2006;47(5):561–566. doi: 10.1080/00071660600962976. [DOI] [PubMed] [Google Scholar]
- Karadas F., Pappas A.C., Surai P.F., Speake B.K. Embryonic development within carotenoid-enriched eggs influences the post-hatch carotenoid status of the chicken. Comp Biochem Physiol B Biochem Mol Biol. 2005;141(2):244–251. doi: 10.1016/j.cbpc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Karadas F., Surai P.F., Sparks N.H., Grammenidis E. Effects of maternal dietary supplementation with three sources of carotenoids on the retinyl esters of egg yolk and developing quail liver. Comp Biochem Physiol Mol Integr Physiol. 2005;140(4):430–435. doi: 10.1016/j.cbpb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kelly D., Nolan J.M., Howard A.N., Stack J., Akuffo K.O., Moran R., Thurnham D.I., Dennison J., Meagher K.A., Beatty S. Serum and macular response to carotenoid-enriched egg supplementation in human subjects: the Egg Xanthophyll Intervention clinical Trial (EXIT) Br J Nutr. 2017;117(1):108–123. doi: 10.1017/S0007114516003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Gordon S.L., Ferruzzi M.G., Campbell W.W. Effects of egg consumption on carotenoid absorption from co-consumed, raw vegetables. Am J Clin Nutr. 2015;102(1):75–83. doi: 10.3945/ajcn.115.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kljak K., Carović-Stanko K., Kos I., Janječić Z., Kiš G., Duvnjak M., Safner T., Bedeković D. Plant carotenoids as pigment sources in laying hen diets: effect on yolk color, carotenoid content, oxidative stability and sensory properties of eggs. Foods. 2021;10(4):721. doi: 10.3390/foods10040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kljak K., Duvnjak M., Bedeković D., Kiš G., Janječić Z., Grbeša D. Commercial corn hybrids as a single source of dietary carotenoids: effect on egg yolk carotenoid profile and pigmentation. Sustainability. 2021;13(21) doi: 10.3390/su132112287. [DOI] [Google Scholar]
- Ko E.Y., Saini R.K., Keum Y.S., An B.K. Age of laying hens significantly influences the content of nutritionally vital lipophilic compounds in eggs. Foods. 2020;10(1):22. doi: 10.3390/foods10010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S., Koizumi S., Kawami Y., Shigeta Y., Osawa A. Effect of dietary carotenoid on egg yolk color and singlet oxygen quenching activity of laying hens. J Poultry Sci. 2022;59(2):137–142. doi: 10.2141/jpsa.0210032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konca Y., Kaliber M., Uzkulekci H.H., Cimen B., Yalcin H. The effect of rosehip (rosa canina L.) supplementation to diet on the performance, egg and meat quality, antioxidant activity in laying quail. Sains Malays. 2021;50(12):3617–3629. doi: 10.17576/jsm-2021-5012-13. [DOI] [Google Scholar]
- Kopec R.E., Failla M.L. Recent advances in the bioaccessibility and bioavailability of carotenoids and effects of other dietary lipophiles. J Food Compos Anal. 2018;68:16–30. doi: 10.1016/j.jfca.2017.06.008. [DOI] [Google Scholar]
- Kotake-Nara E., Nagao A. Absorption and metabolism of xanthophylls. Mar Drugs. 2011;9(6):1024–1037. doi: 10.3390/md9061024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson S., Caston L. Enrichment of eggs with lutein. Poultry Sci. 2004;83(10):1709–1712. doi: 10.1093/ps/83.10.1709. [DOI] [PubMed] [Google Scholar]
- Lehto S., Buchweitz M., Klimm A., Strassburger R., Bechtold C., Ulberth F. Comparison of food colour regulations in the EU and the US: a review of current provisions. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017;34(3):335–355. doi: 10.1080/19440049.2016.1274431. [DOI] [PubMed] [Google Scholar]
- Lesnierowski G., Stangierski J. What's new in chicken egg research and technology for human health promotion? - a review. Trends Food Sci Technol. 2018;71:46–51. doi: 10.1016/j.tifs.2017.10.022. [DOI] [Google Scholar]
- Li X.X., Han L.J., Xiao W.H. Effect of Cu2+on the stability of xanthophylls in acetone. Int J Food Prop. 2012;15(3):471–480. doi: 10.1080/10942912.2010.491930. [DOI] [Google Scholar]
- Liu Y.Q., Davis C.R., Schmaelzle S.T., Rocheford T., Cook M.E., Tanumihardjo S.A. beta-Cryptoxanthin biofortified maize (Zea mays) increases beta-cryptoxanthin concentration and enhances the color of chicken egg yolk. Poultry Sci. 2012;91(2):432–438. doi: 10.3382/ps.2011-01719. [DOI] [PubMed] [Google Scholar]
- Mabelebele M., Alabi O.J., Ng`ambi J.W., Norris D., Ginindza M.M. Comparison of gastrointestinal tracts and pH values of digestive organs of ross 308 broiler and indigenous Venda chickens fed the same diet. Asian J Anim Vet Adv. 2013;9(1):71–76. doi: 10.3923/ajava.2014.71.76. [DOI] [Google Scholar]
- Mariutti L.R.B., Mercadante A.Z. Carotenoid esters analysis and occurrence: what do we know so far? Arch Biochem Biophys. 2018;648:36–43. doi: 10.1016/j.abb.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Marounek M., Pebriansyah A. Use of carotenoids in feed mixtures for poultry: a review. Agric trop subtrop. 2018;51(3):107–111. doi: 10.2478/ats-2018-0011. [DOI] [Google Scholar]
- McGraw K.J. Interspecific variation in dietary carotenoid assimilation in birds: links to phylogeny and color ornamentation. Comp Biochem Physiol B Biochem Mol Biol. 2005;142(2):245–250. doi: 10.1016/j.cbpb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- McGraw K.J., Adkins-Regan E., Parker R.S. Anhydrolutein in the zebra finch: a new, metabolically derived carotenoid in birds. Comp Biochem Physiol B Biochem Mol Biol. 2002;132(4):811–818. doi: 10.1016/s1096-4959(02)00100-8. [DOI] [PubMed] [Google Scholar]
- McGraw K.J., Hill G.E., Parker R.S. Carotenoid pigments in a mutant cardinal: implications for the genetic and enzymatic control mechanisms of carotenoid metabolism in birds. Condor. 2003;105(3):587–592. doi: 10.1650/7281. [DOI] [Google Scholar]
- Meléndez-Martínez A.J., Escudero-Gilete M.L., Vicario I.M., Heredia F.J. Effect of increased acidity on the carotenoid pattern and colour of orange juice. Eur Food Res Technol. 2009;230(3):527–532. doi: 10.1007/s00217-009-1190-1. [DOI] [Google Scholar]
- Melendez-Martinez A.J., Mandic A.I., Bantis F., Bohm V., Borge G.I.A., Brncic M., Bysted A., Cano M.P., Dias M.G., Elgersma A., Fikselova M., Garcia-Alonso J., Giuffrida D., Goncalves V.S.S., Hornero-Mendez D., Kljak K., Lavelli V., Manganaris G.A., Mapelli-Brahm P., O'Brien N. A comprehensive review on carotenoids in foods and feeds: status quo, applications, patents, and research needs. Crit Rev Food Sci Nutr. 2021;62(8):1999–2049. doi: 10.1080/10408398.2020.1867959. [DOI] [PubMed] [Google Scholar]
- Mendes-Pinto M.M., LaFountain A.M., Stoddard M.C., Prum R.O., Frank H.A., Robert B. Variation in carotenoid-protein interaction in bird feathers produces novel plumage coloration. J R Soc Interface. 2012;9(77):3338–3350. doi: 10.1098/rsif.2012.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzomo N., Ferreira S.R.S. Carotenoids functionality, sources, and processing by supercritical technology: a review. J Chem. 2016;2016:1–16. doi: 10.1155/2016/3164312. [DOI] [Google Scholar]
- Moeini M., Ghazi S., Sadegh S., Malekizadeh M. The effect of red pepper (capsicum annuum) and marigold flower (tageteserectus) powder on egg production, egg yolk color and some blood metabolites of laying hens. Iran J Appl Anim Sci. 2013;3(2):301–305. http://www.scopemed.org/fulltextpdf.php?mno=169246 [Google Scholar]
- Mohd Hatta F.A., Othman R. In: Carotenoids: properties, processing and applications. Galanakis C.M., editor. Elsevier Inc.; 2020. Carotenoids as potential biocolorants: a case study of astaxanthin recovered from shrimp waste; pp. 289–325. [DOI] [Google Scholar]
- Møller A.P., Biard C., Blount J.D., Houston D.C., Ninni P., Saino N., Surai P.F. Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poultry Biol Rev. 2000;11(3):137–159. [Google Scholar]
- Moreno J.A., Díaz-Gómez J., Fuentes-Font L., Angulo E., Gosálvez L.F., Sandmann G., Portero-Otin M., Capell T., Zhu C., Christou P., Nogareda C. Poultry diets containing (keto)carotenoid-enriched maize improve egg yolk color and maintain quality. Anim Feed Sci Technol. 2020;260 doi: 10.1016/j.anifeedsci.2019.114334. [DOI] [Google Scholar]
- Moreno J.A., Diaz-Gomez J., Nogareda C., Angulo E., Sandmann G., Portero-Otin M., Serrano J.C., Twyman R.M., Capell T., Zhu C., Christou P. The distribution of carotenoids in hens fed on biofortified maize is influenced by feed composition, absorption, resource allocation and storage. Sci Rep. 2016;6 doi: 10.1038/srep35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita T.Y., Mertins J.W., Baker D.G., Monahan C.M., Brooks D.L. Occurrence and species of lice on free-living and captive raptors in California. J Avian Med Surg. 2001;15(4):288–292. doi: 10.1647/1082-6742(2001)015[0288:Oasolo]2.0.Co;2. [DOI] [Google Scholar]
- Mugnai C., Sossidou E.N., Dal Bosco A., Ruggeri S., Mattioli S., Castellini C. The effects of husbandry system on the grass intake and egg nutritive characteristics of laying hens. J Sci Food Agric. 2014;94(3):459–467. doi: 10.1002/jsfa.6269. [DOI] [PubMed] [Google Scholar]
- Na J.C., Song J.Y., Lee B.D., Lee S.J., Lee C.Y., An G.H. Effect of polarity on absorption and accumulation of carotenoids by laying hens. Anim Feed Sci Technol. 2004;117(3–4):305–315. doi: 10.1016/j.anifeedsci.2004.08.012. [DOI] [Google Scholar]
- Namitha K.K., Negi P.S. Chemistry and biotechnology of carotenoids. Crit Rev Food Sci Nutr. 2010;50(8):728–760. doi: 10.1080/10408398.2010.499811. [DOI] [PubMed] [Google Scholar]
- Nelson D.S., Janky D.M., Harms R.H. Research note: a thirteen-day assay for use in pigmentation evaluation of egg yolks. Poultry Sci. 1990;69(9):1610–1613. doi: 10.3382/ps.0691610. [DOI] [Google Scholar]
- Niu Z., Fu J., Gao Y., Liu F. Influence of paprika extract supplement on egg quality of laying hens fed wheat-based diet. Int J Poultry Sci. 2008;7(9):887–889. doi: 10.3923/ijps.2008.887.889. [DOI] [Google Scholar]
- Nolan J.M., Meagher K.A., Howard A.N., Moran R., Thurnham D.I., Beatty S. Lutein, zeaxanthin and meso-zeaxanthin content of eggs laid by hens supplemented with free and esterified xanthophylls. J Nutr Sci. 2016;5:e1. doi: 10.1017/jns.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusantoro S., Rouf A., Wulandari S., Nurkholis, Kustiawan E., Awaludin A., Utami M.M.D. The use of Golden snail (Pomacea canaliculata) egg as source of carotenoid for improvement of Arabic Chicken egg quality. IOP Conf Ser Earth Environ Sci. 2020;411(1) doi: 10.1088/1755-1315/411/1/012038. [DOI] [Google Scholar]
- Nwoba E.G., Ogbonna C.N., Ishika T., Vadiveloo A. In: Microalgae biotechnology for food, health and high value products. Alam M.A., Xu J.L., Wang Z., editors. Springer Nature Singapore Pte Ltd.; 2020. Microalgal pigments: a source of natural food colors; pp. 81–123. [DOI] [Google Scholar]
- Nys Y., Guyot N. In: Improving the safety and quality of eggs and egg products. Nys Y., Bain M., Immerseel F.V., editors. Woodhead Publishing Ltd.; 2011. Egg formation and chemistry; pp. 83–132. [Google Scholar]
- Obianwuna U.E., Oleforuh-Okoleh V.U., Wang J., Zhang H.-J., Qi G.-H., Qiu K., Wu S.-G. Natural products of plants and animal origin improve albumen quality of chicken eggs. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.875270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obianwuna U.E., Oleforuh-Okoleh V.U., Wang J., Zhang H.J., Qi G.H., Qiu K., Wu S.G. Potential implications of natural antioxidants of plant origin on oxidative stability of chicken albumen during storage: a review. Antioxidants. 2022;11(4):630. doi: 10.3390/antiox11040630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira V.R.M., Arruda A.M.V., Melo A.S., Souza D.H., Souza-Junior J.B.F., Fernandes R.T.V., Queiroz J. Coarse corn particles cause a negative effect on eggshell quality of semi-heavy laying hens. Anim Nutr. 2019;5(4):432–434. doi: 10.1016/j.aninu.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J.B., Ward N.E., Koutsos E.A. Lycopene incorporation into egg yolk and effects on laying hen immune function. Poultry Sci. 2008;87(12):2573–2580. doi: 10.3382/ps.2008-00072. [DOI] [PubMed] [Google Scholar]
- Orhan C., Kucuk O., Sahin N., Tuzcu M., Sahin K. Lycopene supplementation does not change productive performance but lowers egg yolk cholesterol and gene expression of some cholesterol-related proteins in laying hens. Br Poultry Sci. 2021;62(2):227–234. doi: 10.1080/00071668.2020.1839017. [DOI] [PubMed] [Google Scholar]
- Ortiz D., Lawson T., Jarrett R., Ring A., Scoles K.L., Hoverman L., Rocheford E., Karcher D.M., Rocheford T. Biofortified orange corn increases xanthophyll density and yolk pigmentation in egg yolks from laying hens. Poultry Sci. 2021;100(7) doi: 10.1016/j.psj.2021.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaite T.D., Nour V., Saracila M., Turcu R.P., Untea A.E., Vlaicu P.A. Effects of linseed meal and carotenoids from different sources on egg characteristics, yolk fatty acid and carotenoid profile and lipid peroxidation. Foods. 2021;10(6):1246. doi: 10.3390/foods10061246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos G.A., Chalvatzi S., Kopecky J., Arsenos G., Fortomaris P.D. Effects of dietary fat source on lutein, zeaxanthin and total carotenoids content of the egg yolk in laying hens during the early laying period. Br Poultry Sci. 2019;60(4):431–438. doi: 10.1080/00071668.2019.1614526. [DOI] [PubMed] [Google Scholar]
- Pârvu M., Paraschivescu M.T. Feeding Rhodotorula rubra yeast in egg yolk pigmentation (II) Rom Biotechnol Lett. 2014;19(6):9959–9963. [Google Scholar]
- Peluc S.I., Reed W.L., McGraw K.J., Gibbs P. Carotenoid supplementation and GnRH challenges influence female endocrine physiology, immune function, and egg-yolk characteristics in Japanese quail (Coturnix japonica) J Comp Physiol B. 2012;182(5):687–702. doi: 10.1007/s00360-011-0638-3. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V.R., Whiting I.M., Kljak K., Mansbridge S.C., Atanasov A.G., Rose S.P., Enchev S.B. Stevia (Stevia rebaudiana) improves carotenoid content in eggs when fed to laying hens. Foods. 2022;11(10):1418. doi: 10.3390/foods11101418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praud C., Al Ahmadieh S., Voldoire E., Le Vern Y., Godet E., Courousse N., Graulet B., Le Bihan Duval E., Berri C., Duclos M.J. Beta-carotene preferentially regulates chicken myoblast proliferation withdrawal and differentiation commitment via BCO1 activity and retinoic acid production. Exp Cell Res. 2017;358(2):140–146. doi: 10.1016/j.yexcr.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Priyadarshani A.M. A review on factors influencing bioaccessibility and bioefficacy of carotenoids. Crit Rev Food Sci Nutr. 2017;57(8):1710–1717. doi: 10.1080/10408398.2015.1023431. [DOI] [PubMed] [Google Scholar]
- Puertas G., Vazquez M. Advances in techniques for reducing cholesterol in egg yolk: a review. Crit Rev Food Sci Nutr. 2019;59(14):2276–2286. doi: 10.1080/10408398.2018.1448357. [DOI] [PubMed] [Google Scholar]
- Rajasekaran A., Kalaivani M. Designer foods and their benefits: a review. J Food Sci Technol. 2013;50(1):1–16. doi: 10.1007/s13197-012-0726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul E. Mechanisms of carotenoid intestinal absorption: where do we stand? Nutrients. 2019;11(4):838. doi: 10.3390/nu11040838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., Madkour M., El-Azeem N.A., Aboelazab O., Ahmed S.Y.A., Alagawany M. Tomato pomace as a nontraditional feedstuff: productive and reproductive performance, digestive enzymes, blood metabolites, and the deposition of carotenoids into egg yolk in quail breeders. Poultry Sci. 2022;101(4) doi: 10.1016/j.psj.2022.101730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Amaya D.B. 1st ed. John Wiley & Sons, Ltd.; West Sussex: 2016. Food carotenoids: chemistry, biology, and Technology. [Google Scholar]
- Rodriguez-Concepcion M., Avalos J., Bonet M.L., Boronat A., Gomez-Gomez L., Hornero-Mendez D., Limon M.C., Melendez-Martinez A.J., Olmedilla-Alonso B., Palou A., Ribot J., Rodrigo M.J., Zacarias L., Zhu C. A global perspective on carotenoids: metabolism, biotechnology, and benefits for nutrition and health. Prog Lipid Res. 2018;70:62–93. doi: 10.1016/j.plipres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Saino N., Bertacche V., Ferrari R.P., Martinelli R., Moller A.P., Stradi R. Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc Biol Sci. 2002;269(1501):1729–1733. doi: 10.1098/rspb.2002.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarra P.G., Morelli L., Bottazzi V. In: The lactic acid bacteria. Wood B.J.B., editor. Elsevier Science Publishers Ltd.; 1992. The lactic microflora of fowl; pp. 3–19. [DOI] [Google Scholar]
- Schaeffer J.L., Tyczkowski J.K., Parkhurst C.R., Hamilton P.B. Carotenoid composition of serum and egg yolks of hens fed diets varying in carotenoid composition. Poultry Sci. 1988;67(4):608–614. doi: 10.3382/ps.0670608. [DOI] [PubMed] [Google Scholar]
- Schiedt K., Leuenberger F.J., Vecchi M., Glinz E. Absorption, retention and metabolic transformations of carotenoids in rainbow trout, salmon and chicken. Pure Appl Chem. 1985;57(5):685–692. doi: 10.1351/pac198557050685. [DOI] [Google Scholar]
- Selvaraj R.K., Koutsos E.A., Calvert C.C., Klasing K.C. Dietary lutein and fat interact to modify macrophage properties in chicks hatched from carotenoid deplete or replete eggs. J Anim Physiol Anim Nutr. 2006;90(1–2):70–80. doi: 10.1111/j.1439-0396.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Shahsavari K. Influence of different sources of natural pigmenting on egg quality and performance of laying hens. JJAS. 2014;10(4):786–796. doi: 10.12816/0031772. [DOI] [Google Scholar]
- Shen W.J., Asthana S., Kraemer F.B., Azhar S. Scavenger receptor B type 1: expression, molecular regulation, and cholesterol transport function. J Lipid Res. 2018;59(7):1114–1131. doi: 10.1194/jlr.R083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.S., Kim J.W., Lee D.G., Lee S., Kil D.Y. Bioavailability of lutein in corn distillers dried grains with solubles relative to lutein in corn gluten meal based on lutein retention in egg yolk. J Sci Food Agric. 2016;96(10):3401–3406. doi: 10.1002/jsfa.7520. [DOI] [PubMed] [Google Scholar]
- Shmarakov I.O.Y., Jason J., Blaner W.S. In: Carotenoids and human health. Tanumihardjo S.A., editor. Springer; 2013. Carotenoid metabolism and enzymology; pp. 29–56. [Google Scholar]
- Sinanoglou V.J., Strati I.F., Miniadis-Meimaroglou S. Lipid, fatty acid and carotenoid content of edible egg yolks from avian species: a comparative study. Food Chem. 2011;124(3):971–977. doi: 10.1016/j.foodchem.2010.07.037. [DOI] [Google Scholar]
- Sirri F., Iaffaldano N., Minelli G., Meluzzi A., Rosato M.P., Franchini A. Comparative pigmentation efficiency of high dietary levels of apo-ester and marigold extract on quality traits of whole liquid egg of two strains of laying hens. J Appl Poultry Res. 2007;16(3):429–437. doi: 10.1093/japr/16.3.429. [DOI] [Google Scholar]
- Skřivan M., Englmaierová M. The deposition of carotenoids and α-tocopherol in hen eggs produced under a combination of sequential feeding and grazing. Anim Feed Sci Technol. 2014;190:79–86. doi: 10.1016/j.anifeedsci.2014.01.009. [DOI] [Google Scholar]
- Skřivan M., Englmaierová M., Skřivanová E., Bubancová I. Increase in lutein and zeaxanthin content in the eggs of hens fed marigold flower extract. Czech J Anim Sci. 2016;60(3):87–96. doi: 10.17221/8073-cjas. [DOI] [Google Scholar]
- Skřivanová V., Englmaierová M., Bendová M., Skřivan M. Effect of the source and level of carotenoids in diets on their retention in eggs. Czech J Anim Sci. 2017;62(8):323–330. doi: 10.17221/17/2017-cjas. [DOI] [Google Scholar]
- Sowbhagya H.B. Value-added processing of by-products from spice industry. Food Qual Saf. 2019;3(2):73–80. doi: 10.1093/fqsafe/fyy029. [DOI] [Google Scholar]
- Strand A., Herstad O., Liaaen-Jensen S. Fucoxanthin metabolites in egg yolks of laying hens. Comp Biochem Physiol Mol Integr Physiol. 1998;119(4):963–974. doi: 10.1016/s1095-6433(98)00011-7. [DOI] [PubMed] [Google Scholar]
- Sunder A., Wilkens M., Bohm V., Liebert F. Egg yolk colour in organic production as affected by feeding - consequences for farmers and consumers. Food Chem. 2022;382 doi: 10.1016/j.foodchem.2021.131854. [DOI] [PubMed] [Google Scholar]
- Sunwoo H.H., Gujral N. In: Handbook of food chemistry. Cheung P.C.K., Mehta B.M., editors. Springer-Verlag; 2014. Chemical composition of egg and egg products; pp. 331–363. [DOI] [Google Scholar]
- Surai P.F. The antioxidant properties of canthaxanthin and its potential effects in the poultry eggs and on embryonic development of the chick. Part 2. World’s Poult Sci J. 2012;68(4):717–726. doi: 10.1017/s0043933912000840. [DOI] [Google Scholar]
- Surai P.F., MacPherson A., Speake B.K., Sparks N.H. Designer egg evaluation in a controlled trial. Eur J Clin Nutr. 2000;54(4):298–305. doi: 10.1038/sj.ejcn.1600939. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Speake B.K., Sparks N.H.C. Carotenoids in avian nutrition and embryonic development. 1. Absorption, availability and levels in plasma and egg yolk. J Poultry Sci. 2001;38:1–27. [Google Scholar]
- Tapingkae W., Panyachai K., Yachai M., Doan H.V. Effects of dietary red yeast (Sporidiobolus pararoseus) on production performance and egg quality of laying hens. J Anim Physiol Anim Nutr. 2018;102(1):e337–e344. doi: 10.1111/jpn.12751. [DOI] [PubMed] [Google Scholar]
- Toomey M.B., Lopes R.J., Araujo P.M., Johnson J.D., Gazda M.A., Afonso S., Mota P.G., Koch R.E., Hill G.E., Corbo J.C., Carneiro M. High-density lipoprotein receptor SCARB1 is required for carotenoid coloration in birds. Proc Natl Acad Sci USA. 2017;114(20):5219–5224. doi: 10.1073/pnas.1700751114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyes-Vargas E., Ortega-Perez R., Espinoza-Villavicencio J.L., Arellano-Perez M., Civera R., Palacios E. Effect of marine by-product meals on hen egg production parameters, yolk lipid composition and sensory quality. J Anim Physiol Anim Nutr. 2018;102(2):462–473. doi: 10.1111/jpn.12769. [DOI] [PubMed] [Google Scholar]
- Tyczkowski J.K., Hamilton P.B. Canthaxanthin as a model for the study of utilization of oxycarotenoids by chickens. Poultry Sci. 1986;65(7):1350–1356. doi: 10.3382/ps.0651350. [DOI] [PubMed] [Google Scholar]
- Tyczkowski J.K., Schaeffer J.L., Parkhurst C., Hamilton P.B. 3'-Oxolutein, a metabolite of lutein in chickens. Poultry Sci. 1986;65(11):2135–2141. doi: 10.3382/ps.0652135. [DOI] [PubMed] [Google Scholar]
- Tyczkowski J.K., Yagen B., Hamilton P.B. Metabolism of canthaxanthin, a red diketocarotenoid, by chickens. Poultry Sci. 1988;67(5):787–793. doi: 10.3382/ps.0670787. [DOI] [PubMed] [Google Scholar]
- Vlaicu P.A., Panaite T.D., Turcu R.P. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-00343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lintig J., Moon J., Lee J., Ramkumar S. Carotenoid metabolism at the intestinal barrier. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(11) doi: 10.1016/j.bbalip.2019.158580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.A., Wang T., Xin H., Dolde D. Supplementation of laying-hen feed with palm tocos and algae astaxanthin for egg yolk nutrient enrichment. J Agric Food Chem. 2012;60(8):1989–1999. doi: 10.1021/jf204763f. [DOI] [PubMed] [Google Scholar]
- Wang H., He W., Dansou D.M., Zhang H., Nugroho R.D., Tang C., Guo X., Yu Y., Zhao Q., Qin Y., Zhang J. Astaxanthin improved the storage stability of docosahexaenoic acid-enriched eggs by inhibiting oxidation of non-esterified poly-unsaturated fatty acids. Food Chem. 2022;38 doi: 10.1016/j.foodchem.2022.132256. [DOI] [PubMed] [Google Scholar]
- Weber G.M., Machander V., Schierle J., Aureli R., Roos F., Pérez-Vendrell A.M. Tolerance of poultry against an overdose of canthaxanthin as measured by performance, different blood variables and post-mortem evaluation. Anim Feed Sci Technol. 2013;186(1–2):91–100. doi: 10.1016/j.anifeedsci.2013.09.005. [DOI] [Google Scholar]
- White D.A., Moody A.J., Serwata R.D., Bowen J., Soutar C., Young A.J., Davies S.J. The degree of carotenoid esterification influences the absorption of astaxanthin in rainbow trout, Oncorhynchus mykiss (Walbaum) Aquacult Nutr. 2003;9(4):247–251. doi: 10.1046/j.1365-2095.2003.00250.x. [DOI] [Google Scholar]
- Wu L., Huang X., Shi K., Tan R. Bioavailability comparison of free and esterified lutein for layer hens. Braz J Poultry Sci. 2009;11(2):95–98. [Google Scholar]
- Wyss A. Carotene oxygenases: a new family of double bond cleavage enzymes. J Nutr. 2004;134(1):246S–250S. doi: 10.1093/jn/134.1.246S. [DOI] [PubMed] [Google Scholar]
- Yang C., Zhang L., Zhang H., Sun Q., Liu R., Li J., Wu L., Tsao R. Rapid and efficient conversion of all-E-astaxanthin to 9Z- and 13z-isomers and assessment of their stability and antioxidant activities. J Agric Food Chem. 2017;65(4):818–826. doi: 10.1021/acs.jafc.6b04962. [DOI] [PubMed] [Google Scholar]
- Yang L., Qiao X., Gu J., Li X., Cao Y., Xu J., Xue C. Influence of molecular structure of astaxanthin esters on their stability and bioavailability. Food Chem. 2021;343 doi: 10.1016/j.foodchem.2020.128497. [DOI] [PubMed] [Google Scholar]
- Yeum K.J., Russell R.M. Carotenoid bioavailability and bioconversion. Annu Rev Nutr. 2002;22:483–504. doi: 10.1146/annurev.nutr.22.010402.102834. [DOI] [PubMed] [Google Scholar]
- Yıldız A.Ö., Şentürk E.T., Olgun O. Use of alfalfa meal in layer diets – a review. World’s Poult Sci J. 2020;76(1):134–143. doi: 10.1080/00439339.2020.1708839. [DOI] [Google Scholar]
- Yonekura L., Nagao A. Intestinal absorption of dietary carotenoids. Mol Nutr Food Res. 2007;51(1):107–115. doi: 10.1002/mnfr.200600145. [DOI] [PubMed] [Google Scholar]
- Yu W., Liu J. Astaxanthin isomers: selective distribution and isomerization in aquatic animals. Aquaculture. 2020;520 doi: 10.1016/j.aquaculture.2019.734915. [DOI] [Google Scholar]
- Zaghini A., Martelli G., Roncada P., Simioli M., Rizzi L. Mannanoligosaccharides and aflatoxin B1 in feed for laying hens: effects on egg quality, aflatoxins B1 and M1 residues in eggs, and aflatoxin B1 levels in liver. Poultry Sci. 2005;84(6):825–832. doi: 10.1093/ps/84.6.825. [DOI] [PubMed] [Google Scholar]
- Zaheer K. Hen egg carotenoids (lutein and zeaxanthin) and nutritional impacts on human health: a review. CyTA - J Food. 2017;15(3):474–487. doi: 10.1080/19476337.2016.1266033. [DOI] [Google Scholar]