Abstract
Essential oils (EO) and natural bioactive compounds are well-known antibacterial and anti-inflammatory factors; however, little is known about their anticoccidial activity and mode of action. EO deriving from basil (BEO), garlic (GAR), oregano (OEO), thyme (TEO), and their main bioactive compounds were investigated for their anticoccidial proprieties and compared to salinomycin (SAL) and amprolium (AMP) in vitro. The invasion of Eimeria tenella sporozoites was studied on 2 cell models: Madin–Darby Bovine Kidney (MDBK) cells and primary chicken epithelial cells (cIEC). Invasion efficiency was evaluated at 2 and 24 h postinfection (hpi) with counts of extracellular sporozoites and by detection of intracellular E. tenella DNA by PCR. Results show that at both timepoints, the EO were most effective in preventing the invasion of E. tenella with an average reduction of invasion at 24 hpi by 36% in cIEC and 55% in MDBK. The study also examined cytokine gene expression in cIEC at 24 hpi and found that AMP, BEO, OEO, TEO, carvacrol (CAR), and thymol (THY) significantly reduced interleukin (IL)8 expression, with CAR also reducing expression of IL1β and IL6 compared to the infected control. In addition, this work investigated the morphology of E. tenella sporozoites treated with anticoccidial drugs and EO using a scanning electron microscope. All the treatments induced morphological anomalies, characterized by a reduction of area, perimeter and length of sporozoites. SAL had a significant impact on altering sporozoite shape only at 24 h, whereas CAR and THY significantly compromised the morphology already at 2 hpi, compared to the untreated control. OEO and GAR showed the most significant alterations among all the treatments. The findings of this study highlight the potential of EO as an alternative to traditional anticoccidial drugs in controlling E. tenella invasion and in modulating primary immune response.
Key words: Eimeria tenella, cIEC, essential oil, natural identical compound, in vitro
INTRODUCTION
Avian coccidiosis is one of the most threatening parasitic diseases in poultry production and the related economic losses go up to 13 billion dollars per year (Blake et al., 2020). The acute form of the disease is characterized by symptoms such as morbidity, severe diarrhea, and reduced feed consumption, leading to rapid weight loss and death. The subacute form is less obvious and thus it is less easy to diagnose and can predispose to secondary infections of other pathogens, like Clostridium perfringens (Blake et al., 2021). Among the 5 most prevalent species of the genus Eimeria, Eimeria tenella is one of the most pathogenic and widely studied, particularly through in vitro research (Felici et al., 2021).
Eimeria spp. have a multistage lifecycle, which alternates an exogenous and an endogenous phase. During the endogenous phase, the invading stage of the parasite, named sporozoite, enters inside the enterocytes and starts to develop. Usually, the invasion process is very fast and happens within 24 h after infection; afterward, the endogenous development of the parasites starts with the trophozoite stage (López-Osorio et al., 2020). Inhibition of invasion is a key strategy to prevent the detrimental effects of the disease, as lower amounts of the parasites in enterocytes usually mean a lower probability to develop the disease in clinical or subclinical form (Williams, 2005).
To constrain coccidiosis, the poultry industry relies on the use of vaccines and drugs, especially ionophores, like salinomycin (SAL), or chemicals, like amprolium (AMP) (Blake et al., 2021). Even though the use of mainstream anticoccidials is cost-effective and successful in most cases, the presence of drug resistance has raised many concerns (Muthamilselvan et al., 2016). Furthermore, these molecules could also interfere with cell proliferation and biochemical pathways, thus exhibiting a certain degree of toxicity in animals (Koutoulis et al., 2013; Kaushik et al., 2018; Noack et al., 2019). Limitations of anticoccidial drugs and the need to improve the current methods to control the disease have pushed research toward the investigation of alternative molecules (Soutter et al., 2021; Arias-Maroto et al., 2022).
Plant-derived compounds, like essential oils (EO) and their bioactive derivatives, have recently gained popularity as antimicrobial and anti-inflammatory compounds, and have increasingly been used to help ensure the intestinal health of animals (Muthamilselvan et al., 2016). Several EO have been suggested as alternatives to anticoccidials and some of them have shown good antiparasitic properties; among these, garlic, oregano, and thyme compounds (Muthamilselvan et al., 2016; Attree et al., 2021; Felici et al., 2021). In particular, EO derived from Oregano spp. and Allium spp. have been studied for their potential anticoccidial properties in chickens infected with Eimeria. These oils have shown promising results in soothing the clinical signs of coccidiosis and in reducing the output of oocysts following infection, with results comparable to anticoccidial drugs. Additionally, in certain cases, they have demonstrated improvements in the scoring of Eimeria-induced lesions in the gut (Mohiti-Asli and Ghanaatparast-Rashti, 2015; Sidiropoulou et al., 2020; Chang et al., 2021; Gordillo Jaramillo et al., 2021). Their biological properties have been attributed to bioactive molecules contained in their composition. Even though EO could be precious allies, they might have some limitations due to the high variability in terms of composition, concentration of molecules, and potential contaminants. Natural identical compounds (NIC) are botanical bioactive compounds originating from chemical synthesis characterized by a definite concentration and purity, so they could be used to by-pass the issues of EO (Rossi et al., 2020).
However, the efficacy and mode of action of botanical compounds are not well characterized and for this reason, these supplements are still far to replace anticoccidial drugs (Attree et al., 2021). Moreover, the techniques used to investigate the anticoccidial proprieties of novel molecules are often various and different in the literature, so results are challenging (Felici et al., 2021). Improvements to in vitro screening methods would offer the opportunity to characterize alleged anticoccidial molecules before moving to expensive and controversial studies in vivo (Blake et al., 2021; Arias-Maroto et al., 2022).
Invasion assays on Madin–Darby Bovine Kidney (MDBK) cells are a commonly used method to perform in vitro studies on anticoccidial molecules, allowing for relatively fast data collection on Eimeria spp. invasion in epithelial cells; however, these assays have limitations as they are not tissue and species-specific (Felici et al., 2021; Arias-Maroto et al., 2022).
Chicken intestinal epithelial cells (cIEC) have been recently developed and have already been used to understand the inflammatory response to microbial infections (Ghiselli et al., 2021, 2022). This study aimed to screen natural anticoccidial alternatives deriving form Allium sativum (garlic), Thymus vulgaris (thyme), Oregano heracleoticum (oregano), and Ocimum basilicum (basil) plants on MDBK cells and cIEC. The second model was also used to understand the inflammatory response after the challenge with E. tenella and treatments. Much effort has been done to establish avian enterocytes cultures in vitro but they have never been used to evaluate invasion efficiency during a coccidia challenge. The use of species-specific cell models would contribute to the baseline understanding of the pathogenic mechanisms and could eventually provide precious information for future therapies.
Further, the mode of action of these EO against E. tenella sporozoites, including morphological changes were assessed by digital imaging analysis after scanning electron microscopy.
MATERIALS AND METHODS
Chemicals and Reagents
Anticoccidial drugs and all the natural identical compounds were purchased from Sigma-Aldrich (St. Louis, MO): SAL from S. albus (Cat.# S4526) and AMP hydrochloride (Cat.# A0542) were resuspended respectively with ethanol and water and used at a final concentration of 5 ppm.
The EO in this study were analyzed all at the same concentrations (40 ppm) in order to compare their efficacy; the NIC were tested at 20 ppm considering an average composition of 50% of the NIC inside the EO.
Basil essential oil (BEO) and oregano essential oil (OEO) were provided by Galen-N (Galen-N Ltd, Sofia, Bulgaria). Garlic oil (GAR) was purchased from Lluch Essence (Lluch Essence S.L.U, Barcelona, Spain), and thyme essential oil (TEO) from Grupo Indukern (Grupo Indukern, Madrid, Spain); all the stock solutions were prepared in ethanol and supplemented to cells with a final concentration of 40 ppm. In all cases, the final concentration of ethanol was less than 0.5% (v/v).
Carvacrol (CAR - Cat.# W224511, analytical grade 99%), diallyl-disulfide (DDS - Cat.# SMB00378, analytical grade ≥ 98%), linalool (LNL - Cat.# L2602, analytical grade 97%), and thymol (THY - Cat.# T0501, analytical grade ≥ 98.5%) were all diluted in ethanol to prepare stock solutions and used at a final concentration of 20 ppm.
Care and Use of Animals
Specific pathogen-free eggs were purchased from Valo-Biomedia (Osterholz-Scharmbeck, Germany) and incubated at 37.7°C, 48% relative humidity in a semiautomated incubator. On the 19th day of incubation, according to the AVMA guidelines and animal welfare, chick embryos were sacrificed by decapitation. As chicken embryos older than 14 d can experience pain, decapitation was recommended as a humane method of euthanasia. According to the Italian legislation (D.lgs. 26/2014, the act on the protection of animals used for scientific and educational purposes, which was passed in March 2014 and transposed Directive 2010/63/EU into current Italian legislation), avian embryos are not considered as “live vertebrate animals,” so the approval of Animal Ethics Commission was not required.
MDBK Cultivation and Maintenance
MDBK (Cat. #CCL-22—ATCC, Manassas, VA) cells were maintained in cell culture flasks (Cat.# 83.3911—Sarstedt AG & Co. KG, Nümbrecht, Germany) in complete medium (Supplementary Material Table 1).
For the invasion assays, MDBK cells were seeded (1×104 cells/well) on 96-well plates (Cat.# 351172 - Corning Incorporated, Corning, NY) and grown for 48 h at 37°C and 5% CO2 until complete confluency.
Isolation of cIEC and Growth
Chicken intestinal epithelial cells were isolated and maintained as described by Ghiselli et al. with some modifications (Ghiselli et al., 2021). Briefly, the intestinal tract of 19-day-old specific-pathogen-free chicken embryos was recovered and cleaned from excessive tissues. The intestinal tissues were digested for 50 min at 37°C with a collagenase and hyaluronidase-based digestive medium (Supplementary Material Table 2). Intestinal aggregates were then recovered and placed in 96-well plates coated with 0.8 mg/mL Matrigel (Cat. #356234—Corning Incorporated). Cells were cultured for the first 48 h with an isolation medium (Complete recipes and catalog numbers in Supplementary Material Table 2), containing as supplementing factors valproic acid (1 mM), Y27632 (10 μM), and CHIR990021 (3 μM). Afterward, the medium was changed to a medium containing as a supplement 50% L-WRN (AddexBio S0011002) conditioned medium and valproic acid (1 mM) (Vandussen et al., 2019). The cells were infected 5 d after seeding, when complete confluency was reached.
Invasion Assay with E. tenella
Eimeria tenella sporulated oocysts were stored in potassium dichromate 2.5% (Cat.# P5271, Sigma-Aldrich). Before each invasion assay, the oocysts were washed with water 2 times by centrifugation (10 min, 1,500 × g). To allow sterilization, the oocysts were resuspended in sodium hypochlorite for 20 min, washed, and then lysed with glass beads 1 mm for 2 min with Tyssue Lyser (Cat.# 85600—Qiagen, Hilden, Germany) to release sporocysts. These were counted and resuspended in excystation medium (Supplementary Material Table 3) at a concentration of 1 × 106 sporocysts/mL. Excystation occurred for 90 min at 41°C. The released sporozoites were purified with Pluristrainer 5 μm (Cat.#43-50005-13—PluriSelect Life Science, Leipzig, Germany) and washed by centrifugation (10 min, 500 × g). The sporozoites were counted and inoculated onto the cells at a concentration of 2 × 104 sporozoites/well in the absence (ET+) or presence of the described treatments.
The invasion occurred for 2 and 24 h, and afterward, the supernatant was collected and pooled 2 by 2 wells. The cells were washed 2 times with phosphate-buffered saline (PBS, Cat.# 806552—Sigma-Aldrich) and lysed with NucleoSpin Tri-prep lysis buffer (Cat.# 740966—Macherey-Nagel Inc., Bethlehem, PA) for nucleic acid extraction.
The extracellular sporozoites were counted using a Burker chamber as described previously (Felici et al., 2020). The data of the treatments are represented as a percentage relative to the 24 h postinfection (hpi) infected control.
qPCR and RT-PCR
Nucleic acid extraction was performed from cIEC samples harvested at 24 hpi. The Nucleospin Tri-prep extraction kit was used according to the manufacturer's protocol. DNA and RNA yield and quality were assessed spectrophotometrically by detecting absorbance at 260 and 280 nm with Varioskan LUX multimode microplate reader (Cat.# VL0000D0—Thermo Fisher Scientific, Waltham, MA). Samples with abnormal 260/280 absorbance values were excluded from the analysis.
The DNA was used to quantify copies of E. tenella DNA according to the number of copies of the internal transcribed spacer 1 (ITS-1) gene (Kawahara et al., 2008). With the serial dilution of the cloned sequence, a standard curve was built to allow absolute quantification of the E. tenella DNA in the samples.
The RNA was used to perform inflammatory cytokines gene expression analysis for interleukin (IL) 1β, IL6, IL8, and IL10. The total RNA was reverse transcribed with iScript cDNA Synthesis Kit (Cat. # 1708890—Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Last, real-time PCR reactions were performed in duplicate using CFX Connect Real-Time PCR System and iTaq Universal SYBR Green Supermix (Cat. # 1725120—Bio-Rad Laboratories) as previously described (Felici et al., 2020). Gene expression was reported as folds of change using the 2^ΔΔCt method (Livak and Schmittgen, 2001) using as housekeeping genes the 60S acidic ribosomal protein P0 (RPLP0) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). All the primers are listed in Table 1 and were purchased from Sigma-Aldrich.
Table 1.
List of all primers used for standard quantification and cytokine gene expression.
| Gene | Primer sequence (5′→3′) | Product length (bp) | Accession N. | |
|---|---|---|---|---|
| Cytokines | IL1β | F: TGCCTGCAGAAGAAGCCTCG | 137 | NM_204524.1 |
| R: CTCCGCAGCAGTTTGGTCAT | ||||
| IL6 | F: GCAGGACGAGATGTGCAAGA | 84 | NM_204628.1 | |
| R: ACCTTGGGCAGGTTGAGGTT | ||||
| IL8 | F: AGCTGCTCTGTCGCAAGGTA | 124 | NM_205498.1 | |
| R: GCTTGGCGTCAGCTTCACATC | ||||
| IL10 | F: GTCACCGCTTCTTCACCTGC | 84 | NM_001004414.2 | |
| R: TCCCGTTCTCATCCATCTTCTCG | ||||
| Housekeeping | RPLP0 | F: TTGGGCATCACCACAAAGATT | 83 | NM_204987 |
| R: CCCACTTTGTCTCCGGTCTTAA | ||||
| GAPDH | F: ACTGTCAAGGCTGAGAACGG | 86 | NM_204305 | |
| R: CATTTGATGTTGCTGGGGTC | ||||
| E. tenella DNA marker | ITS1 | F: TGGAGGGGATTATGAGAGGA | 147 | AF026388.1 |
| R: CAAGCAGCATGTAACGGAGA |
Scanning Electron Microscopy (SEM) and Morphological Analysis
For the morphological assay, 5 × 104 freshly excysted sporozoites were incubated with the anticoccidial drugs and bioactive compounds for either 2 or 24 h in complete medium. At the end of incubation, the sporozoites were fixed in glutaraldehyde 2.5% (Cat.# 1.04239—Sigma-Aldrich) and stored at 4°C until further processing.
Before observation on scanning electron microscope, the samples were washed twice with PBS and twice with distilled water. Then, all the specimens were then mounted on aluminum stubs with double-stick carbon tape, sputter-coated with 5 nm gold using an Emitech K500 coater (Labtech International Ltd., Heathfield, UK), and observed with a Hitachi SEM 510 (Cat.# 9099478—Hitachi Ltd., Tokyo, Japan) at 25kV. Images were obtained with DISS5 (Point electronic GmbH, Halle, Germany) SEM acquisition System.
For each sample, 10 random fields were captured at 800× magnification and the parameters of area, perimeter, major, and minor length were measured with ImageJ software (https://imagej.net/ij).
Statistical Analysis
GraphPad Prism 9.4.1 was used to perform statistical analysis. For the invasion assays, data obtained by different experiments were normalized on the mean value of ET+ at 24 hpi of each trial. For the inflammatory cytokines’ expression assay, data obtained by different experiments were normalized on the mean value of ET+ of the same trial. Descriptive analysis of data was done, and normal distribution was assessed with the Shapiro–Wilk test (P > 0.05). Normally distributed data were analyzed with a parametric one-way ANOVA test, whereas not-normally distributed data were analyzed with Kruskal–Wallis tests. To determine significant differences among treatments post hoc multiple comparison analyses were done: the mean of each treatment was compared with the mean of every other treatment with Tukey's test for normally distributed data and with Dunn's test for non-normally distributed data. Differences were considered significant when the P-value was ≤ 0.05 and are represented as letters. Also, for a more targeted analysis of the effect of the single substance, the mean of each treatment was compared with the mean of the ET+ with Dunnett's test for normally-distributed data and with Dunn's test for non-normally distributed data. Differences were considered significant when P-value was ≤ 0.05 and are represented with asterisks.
RESULTS
Invasion Assay
Table 2 shows ET+ group invasion efficiencies in MDBK and cIEC cells at 2 and 24 hpi. E. tenella sporozoites successfully invaded both cell models. In MDBK cells, 44.67% of the inoculum invaded at 2 hpi, increasing to 74.98% at 24 hpi. In contrast, 71.78% of the inoculum invaded cIEC already at 2 hpi, whereas the highest invasion efficiency was observed in cIEC, with 93.66% of the inoculum successfully invading the cell monolayer at 24 hpi.
Table 2.
Invasion efficiencies of the infected controls at 2 and 24 h obtained by counts of extracellular sporozoites.
| MDBK |
CIEC |
|||
|---|---|---|---|---|
| Treatment | 2 hpi (%) | 24 hpi (%) | 2 hpi (%) | 24 hpi (%) |
| Control (ET+) | 44.67 ± 1.61a | 74.98 ± 1.18b | 71.78 ± 1.52b | 93.66 ± 2.06c |
Invasion assays were performed on MDBK and cIEC. Data are represented as a percentage of the total starting inoculum. Data are reported as mean with SEM (n = 12). Data were normally distributed and were analyzed with one-way ANOVA with Tukey's multiple comparison. Different letters represent significant differences (P ≤ 0.05).
Table 3 lists the invasion efficiencies of all the treatments at 2 and 24 hpi obtained by counts of extracellular sporozoites normalized on the respective infected control at 24 hpi.
Table 3.
Invasion efficiencies of all the treatments at 2 and 24 h obtained by counts of extracellular sporozoites.
| MDBK |
CIEC |
|||
|---|---|---|---|---|
| Treatment | 2 hpi (%) | 24 hpi (%) | 2 hpi (%) | 24 hpi (%) |
| ET+ | 59.66 ± 2.20 a | 100.00 ± 1.34 a | 76.76 ± 1.38 a | 100.00 ± 0.82 a |
| AMP 5 ppm | 37.93 ± 4.34 bc* | 63.13 ± 4.18 ab* | 65.11 ± 1.73 ab | 78.24 ± 1.58 ab |
| SAL 5 ppm | 40.58 ± 1.78 b* | 54.11 ± 2.06 bc* | 46.93 ± 7.09 bc* | 72.98 ± 1.01 ac |
| CAR 20 ppm | 24.59 ± 3.30 bd* | 56.83 ± 4.43 bc* | 59.24 ± 1.83 ac | 69.60 ± 2.19 bc* |
| DDS 20 ppm | 10.88 ± 3.42 d* | 48.81 ± 4.18 bc* | 44.73 ± 3.24 bc* | 62.35 ± 1.64 c* |
| LNL 20 ppm | 26.53 ± 3.85 bd* | 63.40 ± 2.78 ac* | 57.86 ± 4.56 ac | 72.71 ± 1.55 ac |
| THY 20 ppm | 25.11 ± 3.61 bd* | 68.19 ± 2.02 ac* | 63.39 ± 1.91 ac | 73.40 ± 1.64 ac |
| BEO 40 ppm | 25.23 ± 6.21 bd* | 38.90 ± 2.25 bc* | 37.10 ± 6.31 bc* | 63.64 ± 3.01 bc* |
| GAR 40 ppm | 10.27 ± 3.26 cd* | 33.22 ± 4.45 b* | 29.73 ± 3.36 c* | 52.83 ± 3.29 c* |
| OEO 40 ppm | 18.48 ± 4.58 cd* | 42.10 ± 4.70 bc* | 47.42 ± 5.46 bc* | 68.06 ± 2.07 bc* |
| TEO 40 ppm | 30.70 ± 3.58 bd* | 63.43 ± 3.03 bc* | 56.76 ± 4.24 ac | 71.01 ± 1.06 ac* |
AMP, amprolium; BEO, basil essential oil; CAR, carvacrol; DDS, diallyl-disulfide; ET+, Challenged control; GAR, garlic oil; LNL, linalool; OEO, oregano essential oil; SAL, salinomycin; TEO, thyme essential oil; THY, thymol.
Invasion assays were performed on MDBK and cIEC. Data are represented as a percentage on the respective challenged control at 24 hpi. Data are reported as mean with SEM (n = 6). Data were not normally distributed and were analyzed with Kruskal–Wallis test with Dunn's multiple comparisons. Different letters represent significant differences within the same column (same timepoint and same cell model) (P ≤ 0.05). The asterisks indicate significant differences with the ET+ group (P ≤ 0.05).
In MDBK cells, all treatments showed lower invasion efficiency compared to ET+. GAR and DDS had the lowest values with 10.27% and 10.88% of efficiency, respectively. The invasion efficiencies of CAR, LNL, and THY were comparable, with values of 24.59%, 26.53%, and 25.11%, respectively. BEO, OEO, and TEO also had similar invasion efficiencies, ranging from 18.48% to 30.70%. SAL and AMP prevented coccidia invasion compared to ET+ (P = 0.002 and P = 0.0003, respectively), but less efficiently than the other compounds, with recorded values of about 40%. At 24 hpi, a rise in invasion efficiency was observed in all groups. The most effective treatments were GAR, BEO, and OEO, for which a 33.22%, 38.90%, and 42.70% of invasion efficiency was recorded, corresponding to a reduction of about 60% compared to ET+ (P < 0.0001). TEO was less effective than the other oils with an invasion efficiency of 63.43% (P = 0.0034 compared to ET+). Among NIC, only CAR and DDS reduced invasion significantly with a 56.63% and 48.81% (P = 0.0026 and P < 0.0001, respectively), whereas for LNL and THY, a significant difference from ET+ was not detected. Regarding the anticoccidials, AMP and SAL confirmed their inhibiting effect compared to ET+ at 24 hpi (P = 0.032 and P = 0.0003, respectively).
In cIEC, the overall efficiencies of invasion were slightly higher for all the treatments compared to MDBK cells. Again at 2 hpi, the lowest value of invasion was recorded for GAR (29.73%, P < 0.0001). However, the other EO hindered invasion less markedly: BEO and OEO reduced invasion to 42% on average (P < 0.001), while TEO only to 56.76%, being not different from ET+. Concerning NIC, only DDS significantly inhibited invasion compared to ET+ (44.73%, P = 0.0002), whereas CAR, LNL, and THY only caused a numerical reduction to about 60%. Among the anticoccidials, only SAL significantly reduced invasion to 46.93% (P = 0.0014), whereas the reduction seen in AMP was not significant (65.11%). At 24 hpi, the invasion efficiencies rose in all groups on cIEC too. GAR confirmed its inhibitory action and was the most effective treatment, reducing invasion to 52.83% (P < 0.0001). The other groups showed efficiencies higher than 60%. Among oils, BEO e OEO inhibited invasion significantly (P < 0.0001 and P = 0.0011, respectively), whereas TEO inhibition was less important than the other compounds (P = 0.0251). Furthermore, all NIC reduced invasion similarly, with only CAR and DDS significantly lower than ET+ (P = 0.0064 and P < 0.0001, respectively). Last, both AMP and SAL showed a not-significant numerical reduction in invasion efficiency compared to ET+.
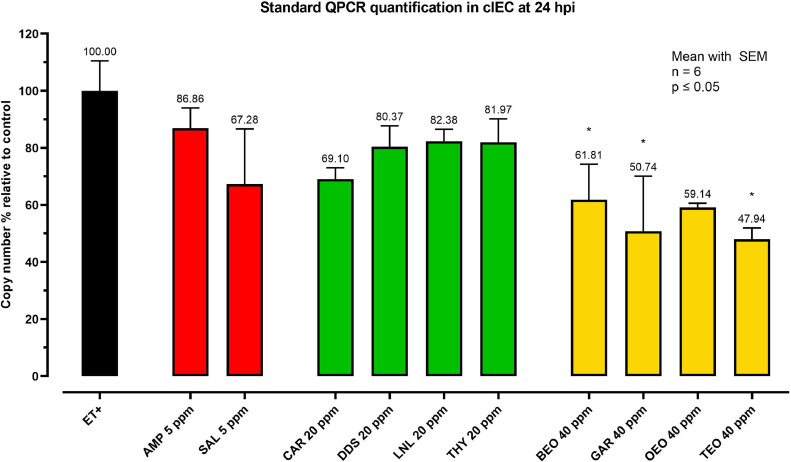
The data of invasion efficiencies obtained with the counts of extracellular sporozoites were then confirmed using standard qPCR as a quantification method detecting intracellular E. tenella DNA in cIEC at 24 hpi. Data were normalized on the number of copies quantified in ET+. As represented in Figure 1, the pattern of inhibition found by extracellular sporozoites counts was confirmed. Comparing the mean of each group with the mean of ET+, it was possible to spot a significant decrease of E. tenella ITS-1 copies inside cells in BEO (P = 0.0343), GAR (P = 0.0329), and TEO (P = 0.0028) groups, with relative copy numbers ranging from 61.81% (BEO) and 47.94% (TEO). Regarding NIC, not-significant numerical decreases of intracellular Eimeria DNA were recorded ranging from 30% to 17.62%. Similarly, anticoccidial drugs were statistically not different from ET+. SAL showed a numerical reduction of 32.72%, whereas AMP had the highest value of relative copy numbers (86.86%).
Figure 1.
E. tenella DNA quantification inside cIEC at 24 hpi. Data are represented as a percentage on the respective challenged control at 24 hpi. The color of the bars defines the category of each compound: red for anticoccidials, green for NIC and yellow for EO. Data are reported as mean with SEM (n = 6). Data were not normally distributed and were analyzed with Kruskal–Wallis test with Dunn's multiple comparisons. The asterisks indicate significant differences compared to ET+ group (P ≤ 0.05). Abbreviations: AMP, amprolium; BEO, basil essential oil; CAR, carvacrol; DDS, diallyl-disulfide; ET+, Challenged control; GAR, garlic oil; LNL, linalool; OEO, oregano essential oil; SAL, salinomycin; TEO, thyme essential oil; THY, thymol.
Inflammatory Cytokines Gene Expression
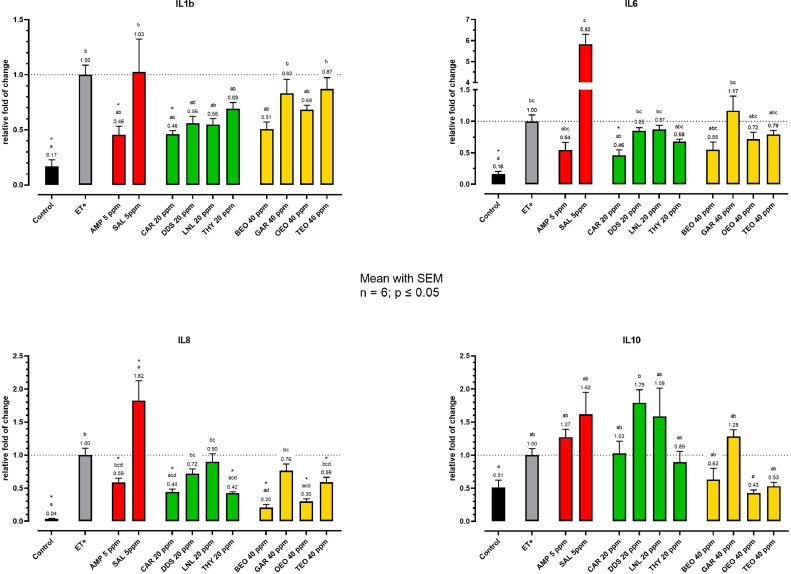
Figure 2 shows the inflammatory cytokine gene expression of cIEC at 24 hpi. All the tested pro-inflammatory cytokines were significantly upregulated by E. tenella invasion: IL1β and IL6 increased 6-fold compared to the unchallenged control (P < 0.0001). IL8 had the highest increase (25-fold, P < 0.0001), whereas IL10 increased only numerically (almost 2-fold).
Figure 2.
Cytokine gene expression of cIEC after 24 h of invasion. Data are represented as expression relative to the challenged control. The color of the bars defines the category of each compound: red for anticoccidials, green for NIC and yellow for EO. Data are reported as mean with SEM (n = 6). Different letters indicate significant differences among treatments (one-way ANOVA with Tukey's multiple comparisons for normally distributed data and Kruskal–Wallis with Dunn's multiple comparisons for non-normal data), asterisks represent significant differences compared to ET+ (one-way ANOVA with Dunnett's multiple comparisons for normally distributed data and Kruskal–Wallis with Dunn's multiple comparisons for non-normal data; P ≤ 0.05). Abbreviations: AMP, amprolium; BEO, basil essential oil; CAR, carvacrol; DDS, diallyl-disulfide; ET+, Challenged control; GAR, garlic oil; LNL, linalool; OEO, oregano essential oil; SAL, salinomycin; TEO, thyme essential oil; THY, thymol.
Interestingly, SAL increased the expression of IL8 (P < 0.0001), whereas AMP and most of the botanical compounds were able to reduced it.
CAR significantly reduced the expression of IL1β (P = 0.0086), IL6 (P = 0.0337), and IL8 (P = 0.0013) by 50% compared to the ET+ values. BEO, OEO, THY, and TEO also significantly decreased the mRNA levels of IL8 compared to ET+, with reductions of about 5- (P < 0.0001), 3- (P < 0.0001), 2- (P = 0.0003), and 1.7-fold (P = 0.0172), respectively.
BEO almost halved the expression of IL1β and IL6, whereas OEO reduced them by one quarter compared to the ET+ expression. All NIC reduced the expression of IL1β by over a third and THY also decreased the expression of IL6 by 0.30-fold compared to ET+.
OEO and TEO decreased IL10 expression the most, but GAR was similar to ET+. Among NIC, none altered IL10 expression significantly compared to ET+. DDS increased IL10 expression by almost 2-fold, but it was still similar to ET+.
Morphological Analysis
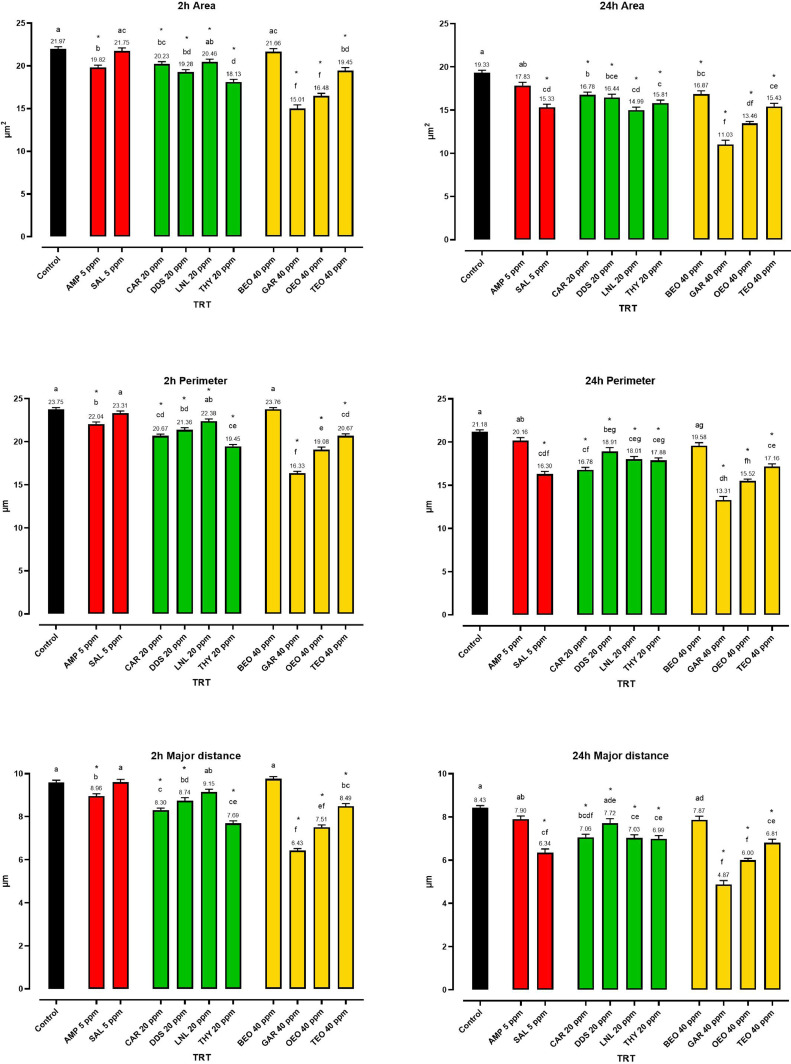
Sporozoites were observed through a scanning electron microscope to study the impact of anticoccidial drugs, NIC, and EO on the area, perimeter, and major length of sporozoites. Untreated E. tenella sporozoites appeared as comma-shaped cells with an area of 21.97 μm2, a perimeter of 23.75 μm, and a length of 9.60 μm on average. It was observed that the untreated sporozoites underwent normal aging effects during the 24 h of observation, which led to a decrease in their dimensions by 12%, 10%, and 12% in area, perimeter, and length respectively. This resulted in an average area of 19.33 μm2, perimeter of 21.18 μm, and length of 8.43 μm after 24 h. Almost all the treatments reduced the size of E. tenella sporozoites, making them appear as oval objects (Figure 3).
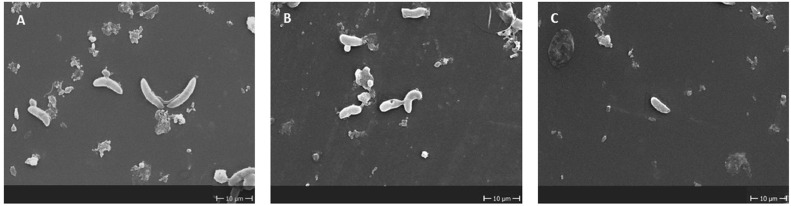
Figure 3.
E. tenella sporozoites captured by scanning electron microscope at 2,000×. (A) Control without treatments after 2 h of incubation. (B) Abnormal sporozoites corresponding to thymol 20 ppm (2 h of treatment). (C) Abnormal sporozoite corresponding to thymol 20 ppm (24 h of treatment).
Figure 4 reports the measurement of area, perimeter and major length after 2 and 24 h of treatment with the various compounds. AMP significantly reduced sporozoite's area, perimeter, and length down to 19.82 μm2 (P < 0.0001), 22.04 μm (P < 0.0001), and 8.96 μm (P = 0.0031), respectively in 2 h time, but at 24 h, it was not different to the untreated control. After 2 h of incubation with SAL, there was no reduction in the dimensions of the sporozoites. However, after 24 h, a marked decrease was observed in the area, perimeter, and length of the sporozoites, reaching 15.33 μm2, 16.30 μm, and 6.23 μm respectively (P < 0.0001).
Figure 4.
Shape measurements of E. tenella sporozoites. The color of the bars defines the category of each compound: red for anticoccidials, green for NIC and yellow for EO. Data are reported as mean with SEM (n = 6). Different letters indicate significant differences among treatments (Kruskal–Wallis with Dunn's multiple comparisons). The asterisks represent significant differences compared to ET+ (Kruskal–Wallis with Dunn's multiple comparisons, P ≤ 0.05). Abbreviations: AMP, amprolium; BEO, basil essential oil; CAR, carvacrol; DDS, diallyl-disulfide; GAR, garlic oil; LNL, linalool; OEO, oregano essential oil; SAL, salinomycin; TEO, thyme essential oil; THY, thymol.
Regarding NIC, CAR and THY were particularly effective, causing major reductions in all parameters at 2 h, but most effectively after 24 h of incubation. At 2 h, DSS significantly decreased the overall dimension of sporozoites (P < 0.001); however, this decrease was less pronounced at 24 h, with DDS having higher values compared to other treatments.
Among EO, BEO was not as effective in reducing the dimensions of sporozoites compared to the control. Only a small reduction in area was observed after 24 h of treatment. On the other hand, all other oils, including GAR and OEO, were effective in altering the dimensions of the sporozoites. GAR significantly reduced the area, perimeter and major length of sporozoites after 2 h but especially after 24, with an overall decrease of almost 40% (P < 0.0001). TEO also reduced all parameters, but to a lesser extent than GAR and OEO (P < 0.0001).
DISCUSSION
Invasion assays traditionally use nonintestinal models like MDBK cells to examine the inhibitory properties of anticoccidials (Felici et al., 2021). The current study assessed the ability of 2 traditional anticoccidials, some EO, and their main active ingredients to prevent coccidial infections in vitro using cIEC for the first time. This approach allowed the measurement of E. tenella invasion in the species-specific cellular target and the identification of the initial inflammatory response 24 h after invasion in vitro. cIEC responded to E. tenella by significantly increasing expression of IL1β and IL6 and IL8, consistently to previous in vivo findings (Zhang et al., 2012). Additionally, a numerical increase in the expression of IL10 was observed in E. tenella-positive samples, suggesting a potential immune evasion strategy by Eimeria spp. (Arendt et al., 2019).
To further investigate the effects of EO and NIC on E. tenella sporozoites, a novel method to study the anticoccidial power via ultrastructural analysis was used in this study. The treatments that inhibited invasion also caused clear morphological aberrations, characterized by a decrease in area, perimeter and major length of sporozoites. Similar findings have already been reported with 18 ppm of curcumin or with heat (55°C for 60 min), that like the botanicals tested hereby, disrupt cell membranes and alter permeability, leading to morphological changes followed by a decrease in infectivity (Khalafalla et al., 2010; Tyagi et al., 2015; Schneiders et al., 2020).
For this study, MDBK cells and cIEC were used to perform invasion assays with timepoints set at 2 and 24 h. cIEC were found to be more susceptible to Eimeria internalization than MDBK, and 2 h of incubation are enough to let in more than 70% of the total invading sporozoites, whereas MDBK cells only allow the 45%. This could be due to a major affinity of E. tenella to chicken enterocytes, which represent the natural host. Lately, research has provided growing evidence that invasion involves both parasite and host cells structures, especially the cytoskeleton proteins (Liu et al., 2022). For instance, it was recently found that host vimentin has an inhibitory action on E. tenella invasion process (Liu et al., 2022). Ben-Ze'ev reported that MDBK cells express high amounts of this protein that is not present in enterocytes, like the ones used in this work (Ben-Ze'ev, 1984; Ghiselli et al., 2021). This difference could explain the results found in our study. However, the detailed mechanisms of Eimeria interaction with target cells are still obscure and need further elucidations.
This study compared 2 commonly used anticoccidial drugs with natural compounds. Salinomycin, an ionophore drug, and amprolium, a thiamine uptake blocker, exhibit distinct modes of action (James, 1980; Felici et al., 2021). Salinomycin was more efficient in preventing invasion and caused more evident morphological abnormalities on sporozoites after 24 h than amprolium. However, ionophores can have toxic effects on host cells, including the inhibition of Wnt-driven proliferation pathway, which is essential for enterocyte expansion and survival. Impairment of this pathway could hinder the ability of the intestine to recover from damage during coccidial infections (Dewangan et al., 2017).
Salinomycin treatment resulted in a pro-inflammatory effect on cIEC in vitro, increasing the expression of cytokines similarly to other findings on broilers in vivo (Munir et al., 2009). While an enhanced immune response may aid in combating Eimeria spp., excessive cytokine production could be detrimental to animal health. The underlying mechanisms require further investigation to develop effective treatment strategies that prevent invasion without triggering excessive immune responses.
Essential oils, especially garlic oil, demonstrated a significant inhibition of invasion and caused morphological alterations in sporozoites. These results are in agreement with other similar studies: Sidiropoulou et al. already observed a significant reduction of invasion 24 hpi of garlic oil-treated sporozoites in MDBK cells (Sidiropoulou et al., 2020). Garlic oil is rich in reactive sulfur species, such as allicin. These are able to oxidize thiols in protein residues, leading to changes in structures and the subsequent loss of functions (Borlinghaus et al., 2014). This could explain the reduction in parasite invading ability and the observed shape aberrations. Alnassan et al. recorded inhibition of replication of E. tenella in MDBK by allicin at very low dosages, showing that this compound is very effective as anticoccidial (Alnassan et al., 2015). However, allicin is a very unstable molecule and it is rapidly decomposed mainly to diallyl-disulfide losing the thiosulfinate group in aqueous solutions (Singh and Singh, 2008). In this study, diallyl-disulfide was tested and showed a less marked anticoccidial effect, meaning that garlic oil is more effective and suggesting that the thiosulfinate group might be a key effector for the anticoccidial ability is important in disrupting the function of coccidia (Borlinghaus et al., 2014).
Thymol, and its isomer carvacrol, are monoterpenes found mainly in thyme EO and oregano EO. They are pore-forming compounds with reported antimicrobial, anti-inflammatory and antioxidant properties (Gholami-Ahangaran et al., 2022). Previous in vitro studies have also identified them as potential anticoccidials. Burt et al. found that carvacrol 20 ppm reduced the number of E. tenella sporozoites in MDBK after 2 h of treatment (Burt et al., 2013). A recent study by Sidiropoulou et al. found that a pretreatment of 1 h with thyme EO or oregano EO 100 ppm can inhibit invasion by over 80% (Sidiropoulou et al., 2022). In this work, oregano EO reduced invasion significantly by over 30% at 40 ppm and also remarkably reduced Eimeria dimensions after 2 h of treatment, highlighting its rapid action.
Additionally, thymol and carvacrol showed remarkable anticoccidial effects, but, these effects were more pronounced with EO compared to the pure compounds, suggesting that even if thymol and carvacrol alone can drive the anticoccidial activity, a mixture of them could boost their effects. This hypothesis is supported by a precedent study that found that thymol, carvacrol, and saponins act synergistically on Eimeria spp. sporozoites, increasing their anticoccidial power (Felici et al., 2020).
Also, a reduction of mRNA levels of pro-inflammatory cytokines, especially IL8, was observed in this study suggesting that all of them could mitigate the immune response after invasion.
Basil EO and its bioactive compound linalool resulted to be effective in reducing invasion and altering sporozoites’ morphology. Previously, Jitvitiyanon et al. observed a reduction of invading ability by almost half at 40 ppm with Ocimum basilicum EO in MDBK cells (Jitviriyanon et al., 2016). Linalool is a monoterpene produced by many plants that is gaining importance as pore-forming and antioxidant agent, and at the time of the study it had never been evaluated in vitro against E. tenella (Rosa et al., 2003; Kamatou and Viljoen, 2008). Concerning the effects on inflammation, linalool and basil EO reduced IL8 by over 80% but had less effects on the other markers.
In summary, this study evaluated the effectiveness of anticoccidial drugs, EO and NIC against E. tenella using in vitro invasion assays on chicken enterocytes and ultrastructural analysis, finding that plant-derived compounds can be valuable allies in counteracting coccidiosis and mitigating the inflammatory response following infection. Given the growing demand for new anticoccidial molecules, there is a pressing need for in vitro studies on invasion and immune response. These findings contribute to understand the effects and mode of action of potential alternatives to anticoccidial drugs, laying the groundwork for further testing of new natural anticoccidials.
ACKNOLEDGMENTS
This work was supported by a grant from Vetagro S.p.A. (Reggio Emilia, Italy). Funders participated in the study design, collection analysis interpretation of data, and writing of the manuscript.
DISCLOSURES
Ester Grilli reports financial support was provided by Vetagro S.p.A. Andrea Piva reports a relationship with Vetagro S.p.A. that includes: board membership. Andrea Piva reports a relationship with University of Bologna that includes: employment. Ester Grilli reports a relationship with Vetagro Inc. that includes: board membership. Ester Grilli reports a relationship with University of Bologna that includes: employment.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102898.
Appendix. Supplementary materials
REFERENCES
- Alnassan A.A., Thabet A., Daugschies A., Bangoura B. In vitro efficacy of allicin on chicken Eimeria tenella sporozoites. Parasitol. Res. 2015;114:3913–3915. doi: 10.1007/s00436-015-4637-2. [DOI] [PubMed] [Google Scholar]
- Arendt M., Elissa J., Schmidt N., Michael E., Potter N., Cook M., Knoll L.J. Investigating the role of interleukin 10 on Eimeria intestinal pathogenesis in broiler chickens. Vet. Immunol. Immunopathol. 2019;218 doi: 10.1016/j.vetimm.2019.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Maroto S., Aguiar-Martins K., Regidor-Cerrillo J., Ortega-Mora L., Marugan-Hernandez V. Reduction of chickens use to perform in vitro pre-screening of novel anticoccidials by miniaturisation and increased throughput of the current Eimeria tenella compound-screening model. F1000 Res. 2022;11:1135. [Google Scholar]
- Attree E., Sanchez-Arsuaga G., Jones M., Xia D., Marugan-Hernandez V., Blake D., Tomley F. Controlling the causative agents of coccidiosis in domestic chickens; an eye on the past and considerations for the future. CABI Agric. Biosci. 2021;2:1–16. doi: 10.1186/s43170-021-00056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Differential control of cytokeratins and vimentin synthesis by cell-cell contact and cell spreading in cultured epithelial cells. J. Cell Biol. 1984;99:1424–1433. doi: 10.1083/jcb.99.4.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.P., Knox J., Dehaeck B., Huntington B., Rathinam T., Ravipati V., Ayoade S., Gilbert W., Adebambo A.O., Jatau I.D., Raman M., Parker D., Rushton J., Tomley F.M. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:1–14. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.P., Marugan-Hernandez V., Tomley F.M. Spotlight on avian pathology: Eimeria and the disease coccidiosis. Avian Pathol. 2021;50:209–213. doi: 10.1080/03079457.2021.1912288. [DOI] [PubMed] [Google Scholar]
- Borlinghaus J., Albrecht F., Gruhlke M.C.H., Nwachukwu I.D., Slusarenko A.J. Allicin: chemistry and biological properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt S.A., Tersteeg-Zijderveld M.H.G., Jongerius-Gortemaker B.G.M., Vervelde L., Vernooij J.C.M. In vitro inhibition of Eimeria tenella invasion of epithelial cells by phytochemicals. Vet. Parasitol. 2013;191:374–378. doi: 10.1016/j.vetpar.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Chang L.Y., Di K.Q., Xu J., Chen Y.F., Xi J.Z., Wang D.H., Hao E.Y., Xu L.J., Chen H., Zhou R.Y. Effect of natural garlic essential oil on chickens with artificially infected Eimeria tenella. Vet. Parasitol. 2021;300 doi: 10.1016/j.vetpar.2021.109614. [DOI] [PubMed] [Google Scholar]
- Dewangan J., Srivastava S., Rath S.K. Salinomycin: a new paradigm in cancer therapy. Tumor Biol. 2017;39:1–12. doi: 10.1177/1010428317695035. [DOI] [PubMed] [Google Scholar]
- Felici M., Tugnoli B., Ghiselli F., Massi P., Tosi G., Fiorentini L., Piva A., Grilli E. In vitro anticoccidial activity of thymol, carvacrol, and saponins. Poult. Sci. 2020;99:5350–5355. doi: 10.1016/j.psj.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felici M., Tugnoli B., Piva A., Grilli E. In vitro assessment of anticoccidials: methods and molecules. Animals. 2021;11:1–19. doi: 10.3390/ani11071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli F., Giovagnoni G., Felici M., Tugnoli B., Piva A., Grilli E. A mixture of organic acids and thymol protects primary chicken intestinal epithelial cells from Clostridium perfringens infection in vitro. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli F., Rossi B., Felici M., Parigi M., Tosi G., Fiorentini L., Massi P., Piva A., Grilli E. Isolation, culture, and characterization of chicken intestinal epithelial cells. BMC Mol. Cell Biol. 2021;22:1–15. doi: 10.1186/s12860-021-00349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholami-Ahangaran M., Ahmadi-Dastgerdi A., Azizi S., Basiratpour A., Zokaei M., Derakhshan M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022;8:267–288. doi: 10.1002/vms3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo Jaramillo F.X., Kim D.H., Lee S.H., Kwon S.K., Jha R., Lee K.W. Role of oregano and citrus species-based essential oil preparation for the control of coccidiosis in broiler chickens. J. Anim. Sci. Biotechnol. 2021;12:1–9. doi: 10.1186/s40104-021-00569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. Thiamine uptake in isolated schizonts of Eimeria tenella and the inhibitory effects of amprolium. Parasitology. 1980;80:313–322. doi: 10.1017/s0031182000000779. [DOI] [PubMed] [Google Scholar]
- Jitviriyanon S., Phanthong P., Lomarat P., Bunyapraphatsara N., Porntrakulpipat S., Paraksa N. In vitro study of anti-coccidial activity of essential oils from indigenous plants against Eimeria tenella. Vet. Parasitol. 2016;228:96–102. doi: 10.1016/j.vetpar.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Kamatou G.P., Viljoen A.M. Linalool- a review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008;3:1183–1192. [Google Scholar]
- Kaushik V., Yakisich J.S., Kumar A., Azad N., Iyer A.K.V. Ionophores: potential use as anticancer drugs and chemosensitizers. Cancers (Basel) 2018;10:1–21. doi: 10.3390/cancers10100360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara F., Taira K., Nagai S., Onaga H., Onuma M., Nunoya T. Detection of five avian Eimeria species by species-specific real-time polymerase chain reaction assay. Avian Dis. 2008;52:652–656. doi: 10.1637/8351-050908-Reg.1. [DOI] [PubMed] [Google Scholar]
- Khalafalla R.E., Müller U., Shahiduzzaman M., Dyachenko V., Desouky A.Y., Alber G., Daugschies A. Effects of curcumin (diferuloylmethane) on Eimeria tenella sporozoites in vitro. Parasitol. Res. 2010;108:879–886. doi: 10.1007/s00436-010-2129-y. [DOI] [PubMed] [Google Scholar]
- Koutoulis K.C., Kefalas G., Minos E. Salinomycin toxicosis in broiler breeders and turkeys: report of the first case. Am. J. Anim. Vet. Sci. 2013;8:190–196. [Google Scholar]
- Liu Z., Geng X., Zhao Q., Zhu S., Han H., Yu Y., Huang W., Yao Y., Huang B., Dong H. Effects of host vimentin on Eimeria tenella sporozoite invasion. Parasit. Vectors. 2022;15:1–11. doi: 10.1186/s13071-021-05107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- López-Osorio S., Chaparro-Gutiérrez J.J., Gómez-Osorio L.M. Overview of poultry Eimeria life cycle and host-parasite interactions. Front. Vet. Sci. 2020;7:1–8. doi: 10.3389/fvets.2020.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prev. Vet. Med. 2015;120:195–202. doi: 10.1016/j.prevetmed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Munir K., Muneer M.A., Tiwari A., Masaoud E., Chaudhry R.M. Effects of salinomycin on cell-mediated immunity of broiler chickens against hydropericardium syndrome and Newcastle disease viruses. Poult. Sci. 2009;88:86–91. doi: 10.3382/ps.2008-00345. [DOI] [PubMed] [Google Scholar]
- Muthamilselvan T., Kuo T.F., Wu Y.C., Yang W.C. Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. Evidence-based complement. Altern. Med. 2016;2016 doi: 10.1155/2016/2657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack S., Chapman H.D., Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019;118:2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M.do S.S., Mendonça-Filho R.R., Bizzo H.R., de A. Rodrigues I., Soares R.M.A., Souto-Padrón T., Alviano C.S., Lopes A.H.C.S. Antileishmanial activity of a linalool-rich essential oil from Croton cajucara. Antimicrob. Agents Chemother. 2003;47:1895–1901. doi: 10.1128/AAC.47.6.1895-1901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi B., Toschi A., Piva A., Grilli E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr. Res. Rev. 2020;33:218–234. doi: 10.1017/S0954422420000013. [DOI] [PubMed] [Google Scholar]
- Schneiders G.H., Foutz J.C., Fuller A.L., Nelson J., Rekaya R., Aggrey S.E. The effect of increased temperatures on viability, morphology, infectivity, and development of Eimeria tenella. J. Parasitol. 2020;106:428–437. doi: 10.1645/19-17. [DOI] [PubMed] [Google Scholar]
- Sidiropoulou E., Marugán-Hernández V., Skoufos I., Giannenas I., Bonos E., Aguiar-Martins K., Lazari D., Papagrigoriou T., Fotou K., Grigoriadou K., Blake D.P., Tzora A. In vitro antioxidant, antimicrobial, anticoccidial, and anti-inflammatory study of essential oils of oregano, thyme, and Sage from Epirus, Greece. Life. 2022;12:1783. doi: 10.3390/life12111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiropoulou E., Skoufos I., Marugan-Hernandez V., Giannenas I., Bonos E., Aguiar-Martins K., Lazari D., Blake D.P., Tzora A. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Vet. Sci. 2020;7:1–11. doi: 10.3389/fvets.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.K., Singh D.K. Pharmacological effects of garlic (Allium sativum L.) Annu. Rev. Biomed. Sci. 2008;10:6–26. [Google Scholar]
- Soutter F., Werling D., Kim S., Pastor-Fernández I., Marugán-Hernández V., Tomley F.M., Blake D.P. Impact of Eimeria tenella oocyst dose on parasite replication, lesion score and cytokine transcription in the caeca in three breeds of commercial layer chickens. Front. Vet. Sci. 2021;8:1–9. doi: 10.3389/fvets.2021.640041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi P., Singh M., Kumari H., Kumari A., Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandussen K.L., Sonnek N.M., Stappenbeck T.S. L-WRN conditioned medium for gastrointestinal epithelial stem cell culture shows replicable batch-to-batch activity levels across multiple research teams. Stem Cell Res. 2019;37 doi: 10.1016/j.scr.2019.101430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Zhang L., Liu R., Ma L., Wang Y., Pan B., Cai J., Wang M. Eimeria tenella: expression profiling of toll-like receptors and associated cytokines in the cecum of infected day-old and three-week old SPF chickens. Exp. Parasitol. 2012;130:442–448. doi: 10.1016/j.exppara.2012.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.