Abstract
The aims of this study were to i) estimate the occurrence of pale, soft, and exudative (PSE) meat in modern commercial Ontario broiler flocks, ii) determine the effects of the chilling method (water vs. air) on PSE meat, and iii) investigate a new inexpensive colorimeter (10% of the price of traditional color meters), the Nix Color Sensor, as an objective color measurement of chicken meat. Between June 2019 to March 2020, a total of 17 different broiler flocks were processed. The color of 1,700 boneless skinless Pectoralis major muscles was randomly measured (100/flock), where 255 samples were also measured for pH, water-holding capacity (WHC), cooking loss, and penetration force. In addition, a traditional Minolta colorimeter was used to measure random 95 samples from a single water-chilled flock and subsequently compared the values obtained with the Nix Color Sensor. Strong correlations of L* values (rho = 0.75; P < 0.001), a* values (rho = 0.72; P < 0.001), and b* values (rho = 0.80; P < 0.001) were observed. When an L* value of 43 was used as the cut-off for the Nix, 12.5% of fillets were classified as PSE meat. Statistical differences (P < 0.05) were observed between the air and water-chill methods for L*, pH, and WHC. However, there were no significant differences observed between the 2 methods for cooking loss and penetration force values. The study indicated that PSE meat is still a challenge in Ontario broilers, and that the L*, pH, and WHC of breast meat (all indicate meat quality) are affected by the chilling method. In addition, the Nix was found to be an affordable, objective, and convenient sensor for measuring chicken meat color.
Key words: broiler breast chicken, color, pH, meat quality, water-holding capacity
INTRODUCTION
Poultry meat is the most consumed meat, accounting for 47% of the world's meat production, where broiler chicken meat accounts for approximately 92% of the world's poultry production (OECD/FAO, 2021). Pale, soft, and exudative (PSE) meat has a negative impact on meat quality due to its pale color and poor water-holding capacity (WHC) (Barbut et al., 2005; Barbut, 2009; Owens et al., 2009; Jiang et al., 2017; Zhao et al., 2017; Lee and Choi, 2021; Yang et al., 2022). It has been estimated that the occurrence of PSE meat in poultry ranges from 5 to 47% (Petracci et al., 2009). As consumers’ demand for quality meat has increased, the poultry meat industry has been investigating ways to reduce PSE meat (Barbut et al., 2008). Overall poultry consumption growth is estimated to increase by 16% from 2020 to 2031 (OECD/FAO, 2021), meaning that economic loss from poor quality PSE meat, if continues at the same levels, will also increase.
A few studies on the effects of chilling methods have been conducted in the United States, and there are no data on Canadian broiler flocks. For example, 2 studies have reported similar findings concerning the effects of chilling methods on the color of poultry meat. Huezo et al. (2007) demonstrated that raw fillets remained unaffected by chilling, whether it was air or immersion in ice water. Also, Demirok et al. (2013) reported that drip and cooking losses were not significantly different between air- and water-chill methods.
Color is one of the most important attributes for consumers’ acceptance of fresh meat which is sold in sealed plastic packages (Fletcher, 1999; Qiao et al., 2001). However, color perception varies between individuals. Thus, instrumental color measurements have been used to quantify consumers’ acceptance of meat and evaluate meat quality parameters (Holman and Hopkins, 2019; Swatland, 2021).
Traditionally, Minolta Chroma Meter and HunterLab colorimeter have been used to measure meat color which can be correlated to technological aspects such as WHC (Barbut, 2015). However, these instruments are fairly large and expensive compared to new devices such as the Nix Color Sensor (i.e., 10% of the cost and size of more traditional instruments), and it can also be easily operated via a mobile app (Stiglitz et al., 2016). Two recent studies revealed contradictory results related to color measurements by the 2 devices. Holman and Hopkins (2019) reported that the Nix did not provide comparable beef color values when compared to the HunterLab colorimeter due to higher L* values measured by the Nix. However, Schelkopf et al. (2019) reported that Nix provided comparable color measurements to the HunterLab colorimeter when used to assess beef L* values. In the case of poultry meat, only limited research has been reported comparing these devices.
Thus, the aims of this study were to i) estimate the occurrence of PSE meat in modern commercial Canadian broiler flocks, ii) investigate the effects of 2 chilling methods on the occurrence of PSE, and iii) investigate the capabilities of the Nix as an objective inexpensive color measurement device for poultry meat.
MATERIALS AND METHODS
Sample Selection
From June 2019 to March 2020, a total of 17 broiler flocks processed at 2 processing plants in Ontario, Canada, were monitored to assess the occurrence of PSE meat. One of the plants exclusively employed water chilling, while the other only used air chilling. The 2 processing plants were owned by the same company and utilized the same genetic material, feed, and husbandry practices. A total of 1,700 boneless and skinless Pectoralis major muscles (100/flock) from 17 flocks (8 air-chilled and 9 water-chilled) were selected using systematic random sampling: collecting each fifth sample passing on the deboning line.
Measurements of Technological Properties
At the plant, the color of 100 skinless fillets from each flock was measured (approximately 105 min postmortem in the air-chilled plant, and 450 min postmortem in the water-chilled plant, adhering to the standard processing procedures performed at each of the plants) on the medial ventral skinless surface, in an area free of blood/bruising, using a Nix Color Sensor (Model Pro 2; NIX Sensor Ltd., Hamilton, ON, Canada). To observe a correlation between the color values determined at the plant and meat quality, 15 samples per flock were transported to our University of Guelph laboratory. The pH was measured on the same day the sampling occurred; cooking loss and penetration force were measured the day after sampling.
A Minolta Chroma Meter (Model CR-400; Konica Minolta, Mississauga, ON, Canada) was also used to evaluate 95 samples and compare the lightness (L*), redness (a*), and yellowness (b*) values to the NIX values. The random 95 samples were obtained from a single water-chilled flock and transported to our laboratory (within 90 min) in individual plastic bags. This was done because the NIX uses the D-50 illuminant and the Minolta uses the D-65 illuminant.
The pH was measured at the lateral medial part of the chicken fillets using a special piercing glass pH probe (Hanna Instruments, Newmarket, ON, Canada) which was inserted into the P. major muscle (255 samples, 15/flock; 8 air-chilled, 9 water-chilled).
WHC was determined by adding 16 mL of cold 0.6 M sodium chloride solution to 10 g samples of chopped meat (40 s at the speed 1 setting of a Braun's food processor, Frankfurt, Germany) which were incubated for 30 min at 4°C and then centrifuged (Avanti J-E, Beckman Coulter, Life Sciences, Indianapolis, IN) at 7,000 × g for 15 min (255 samples, 15/flock; 8 air-chilled, 9 water-chilled). Later, free water was removed, and remaining sample weighed. WHC (%) was calculated by the following equation.
where Sola is the solution added to the chopped meat sample, and Solr is the solution separated during the centrifugation. Samples run in duplicates.
For cooking loss determination, chopped meat samples (50 g meat from each of the 255 samples obtained from the air- and water-chilled flocks) were mixed with 25 g of water and 1.5 g of salt. They were then divided into two 30 g portions, stuffed into two 50 mL test tubes, and centrifuged at 47 × g for 1 min to pack the meat. Subsequently, test tubes were heated from 20°C to 72°C in a water bath (HAAKE, Thermo Scientific, Mississauga, ON, Canada) and then cooled in cold water to 30°C. A thermometer (OMEGA, St-Eustache, QC, Canada) was used to monitor the sample's core temperature. Cooking loss was measured by determining the amount of released liquid divided by the initial meat weight.
Following a 2 h of refrigeration period, penetration force (gel strength) was determined, employing a texture analyzer (TA-XT2, Texture Technologies Corp., Hamilton, MA), as the peak force (N) at the point of penetration, by plunging a 9 mm round, flat stainless steel probe at a speed of 100 mm/min into the cooked samples (placed in the 50 mL test tubes).
Statistical Analyses
The statistical analysis was performed using Stata 14 (Stata Corp., College Station, TX). The data set was tested for normality using the Shapiro-Wilk normality test. Correlations between variables (color, pH, WHC, cooking loss, and penetration force) were analyzed using Spearman's ρ correlation for nonparametric tests. The mean difference of variables was analyzed using the Kruskal-Wallis test, and Duncan's multiple range test was used for post hoc multiple comparisons. Linear regression analyses were used to determine the relationship between L* values and technological characteristics of pH, WHC, cooking loss, and penetration. The statistical unit used in this study refers to the individual chicken breast fillets that were monitored for the occurrence of PSE meat. Significance was set at P < 0.05.
RESULTS
Determination of PSE Meat
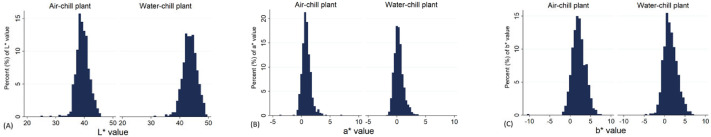
Samples were classified as PSE meat when L* > 43. The threshold for L* was based on the previously published data by Zhang and Barbut (2005a) with minor modification considering the different illumination settings of the Nix vs. the Minolta. The incidence of PSE meat was 12.5%. This can be seen in Figure 1 and will be further discussed below.
Figure 1.
Histogram representing L*, a*, and b* value distribution in broiler flocks processed in air- and water-chilled plants using Nix Color Sensor. (A) Average L* at air-chill plant was 39.5 and 43.5 at water-chill plant (P < 0.001, Wilcoxon rank-sum test, n = 1,700); (B) Average a* at air-chill plant was 0.88 and 0.34 at water-chill plant (P < 0.001); (C) Average b* at air-chill plant: 2.14 and 1.46 at water-chill plant (P < 0.001). L* value greater than 43 using Nix was categorized as PSE meat.
Color Differences Between Chilling Methods
A significant difference in lightness (L*) values between chilling methods was detected (P < 0.001). The fillets from the air-chill plant had an average L* value of 39.5, whereas the average value from the water-chill plant was 43.5. Unlike L* values, both a* and b* values were significantly higher (P < 0.001) in the air-chill broilers (Figure 1).
Technological Properties
Breast fillet pH from the water-chilled plant was significantly lower than that of the air-chill plant (Table 1; P < 0.05). WHC of fillets from the water-chill plant was significantly higher than that of the air-chill plant (P < 0.05). However, differences in cooking loss and penetration force between the plants were not significant (Table 1).
Table 1.
Technological properties of broiler breast fillets obtained by different chilling methods.
| Trait | Mean ± SEM1 |
P value | |
|---|---|---|---|
| Air chill (n = 120) | Water chill (n = 135) | ||
| pH | 5.94 ± 0.03a | 5.79 ± 0.02b | <0.001 |
| Water-holding capacity (%) | 11.51 ± 0.68a | 14.11 ± 0.48b | 0.002 |
| Cooking loss (%) | 4.32 ± 0.41a | 4.76 ± 0.43a | 0.174 |
| Penetration force* (N) | 4.82 ± 0.14a | 4.91 ± 0.13a | 0.575 |
Standard error of the means.
Means within the same property in each row, with different superscripts are significantly different (P < 0.05).
Samples for penetration force were cooked. (N = newton).
Water-holding capacity and pH were evaluated by t test; cooking loss and penetration force by Wilcoxon rank-sum test.
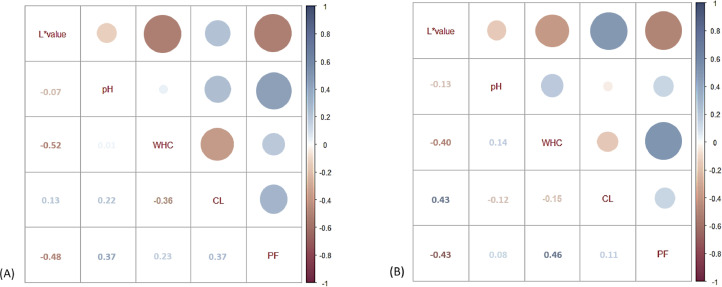
Figure 2A shows the correlation analysis of 120 breast fillets from the air-chill plant (8 flocks). L* values using Nix and WHC were negatively correlated (rho = −0.52; P < 0.001). L* values and penetration force were also negatively correlated (rho = −0.48; P < 0.001). The correlation between pH and penetration force was positive (rho = 0.37; P < 0.001), and the correlation between WHC and cooking loss was negative (rho = −0.36; P < 0.001). Cooking loss and penetration force also showed a positive correlation (rho = 0.37; P < 0.001). Figure 2B illustrates the correlation analysis of 135 breast fillets from the water-chill plant (9 flocks). The associations among technological properties were similar between the air- and water-chill plants. Negative correlations were observed between L* value using Nix and WHC (rho = −0.40; P < 0.001), and L* and penetration force (rho = −0.43, P < 0.001). Positive correlations were observed between L* and cooking loss (rho = 0.43, P < 0.001), and WHC and penetration force (rho = 0.46, P < 0.001).
Figure 2.
Correlations among broiler breast meat L*, pH, water-holding capacity (WHC, %), cooking loss (CL, %), and penetration force (PF, N) in air-chill (A, n = 120) and in water-chill (B, n = 135) processing plants.
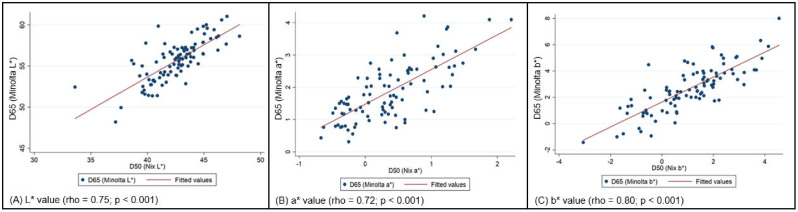
Figure 3 demonstrates the L* value results of 95 samples obtained by 2 different colorimeters, one employing D-50 (Nix) and another D-65 (Minolta) illuminants. A strong positive correlation (rho = 0.75; P < 0.001) of L* values (lightness) was observed between the 2 systems, a* values (rho = 0.72; P < 0.001), and b* value (rho = 0.80; P < 0.001).
Figure 3.
Scatterplots showing the correlation of color values between Nix Pro Color Sensor and Minolta Chroma Meter (n = 95), indicating a strong positive correlation (rho = 0.75; P < 0.001) of L* values (lightness). The a* values (redness) also demonstrated a strong correlation (r = 0.72; P < 0.001). Correlation was the highest for b* value (yellowness) (rho = 0.80; P < 0.001).
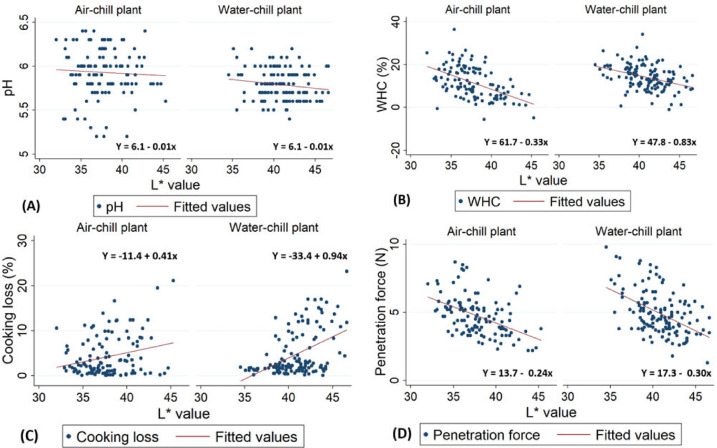
Figure 4 demonstrates the correlation between L* value and technological characteristics of samples obtained from the air-chill and water-chill plants. Panel A illustrates negative correlation between L* values and pH. For each 1-unit increase in L* value, pH was reduced by 0.01 pH, regardless of chilling methods. Panel B illustrates negative correlation between L* value and WHC. For each 1-unit increase in L* value, WHC was reduced by 0.33 (%) in the air-chill plant and 0.83 in the water-chill plant. Panel C illustrates positive correlation between L* value and cooking loss. For each 1-unit increase in L* value, cooking loss was increased by 0.41 (%) in the air-chill plant and 0.94 in the water-chill plant. Panel D illustrates negative correlation between L* value and penetration. For each 1-unit increase in L* value, penetration force was reduced by 0.24 (N) in the air-chill plant and 0.30 in the water-chill plant.
Figure 4.
The relationship between L* value and pH, water-holding capacity (WHC), cooking loss, and penetration force for chicken breast fillets processed at air-chill (n = 120) and water-chill (n = 135) processing plants.
DISCUSSION
PSE meat, which is characterized by its light color, soft texture, and low WHC, significantly impacts sensory attributes and reduces consumer demand (Barbut, 1997; Barbut et al., 2005; Zhang and Barbut, 2005b). Furthermore, the decreased yield and functionality of PSE meat can lead to substantial economic losses (Dong et al., 2020). To evaluate the prevalence of the PSE condition in poultry breast meat, lightness has been used as an indicator of poultry breast meat quality for further processing (Woelfel et al., 2002; Barbut, 2009). L* value, the measurement of lightness, is a useful and easy (e.g., fast and noncontact) method to differentiate PSE fillets from normal fillets due to its high sensitivity and high specificity as well as the ability to operate at a high speed in a broiler processing plant that processes 15,000 birds per h (Boulianne and King, 1995; Barbut and Leishman, 2022).
The results obtained for color values of PSE and normal meat—significantly higher L* values in PSE meat than in normal fillet—are in agreement with previous studies (Barbut, 1997; Laack et al., 2000; Woelfel et al., 2002; Petracci et al., 2004). PSE meat can be caused by various factors, including genetic predisposition, preslaughter stress, and poor handling or transportation of the birds (Guarnieri et al., 2004; Petracci et al., 2004; Barbut et al., 2005). These factors can lead to alterations in the metabolic rate of the poultry, resulting in a rapid postmortem pH decline. This decline can negatively impact meat quality, resulting in the pale, soft, and exudative characteristics associated with PSE meat (Ali et al., 2008).
It was also reported that color variations in chicken meat have a strong correlation with muscle pH: lighter muscles (i.e., higher L* values) had lower pH values (Fletcher, 1999; Berri et al., 2007; Karunanayaka et al., 2016). PSE meat has a lower WHC value because of the denaturation of myofibrillar and sarcoplasmic proteins during the rigor process (Hertog-Meischke et al., 1997). The current study also shows the negative correlation between L* value and WHC which is in line with previous studies (Qiao et al., 2001; Petracci et al., 2009; Karunanayaka et al., 2016). It should be noted that WHC is influenced by various factors, including the meat pH, breed and sex of the broilers, as well as chilling method (Hertog-Meischke et al., 1997).
Retaining moisture is crucial when it comes to raw meat products, and its degree can be determined by the WHC of the myofibrillar proteins (Huff-Lonergan and Lonergan, 2005). Our study shows that WHC is significantly higher in water-chill broilers than air-chill broilers. By contrast, Zhuang et al. (2013) reported that cooking loss and drip loss were higher in immersion water chilling than that in air chilling. The effects of the chilling method on WHC can be influenced by other factors, and it is plausible that a different broiler type was used in our study compared to Zhuang et al. (2013).
Our results showed weak positive correlations between cooking loss and penetration force in both air- and water-chill plant samples. Cooking loss is used to evaluate the decrease in liquid content resulting from protein denaturation and cell membrane disruption during cooking (Mudalal et al., 2014). Penetration force, on the other hand, evaluates meat texture, which influences consumer preference and sensory quality (Barbut, 2015). Overall, our finding is consistent with a previous study reporting a strong positive correlation between cooking loss and the Warner-Bratzler shear force results of chicken breast meat (Murphy and Marks, 2000). Furthermore, within the temperature range of 130°C to 170°C, an increase in shear force was observed when employing air and air-steam cooking methods (Barbanti and Pasquini, 2005). It is important to note that the relationship between cooking loss and meat quality is complex and influenced by factors such as cooking temperature, rate of heating, and cooking method (Barbanti and Pasquini, 2005). Typically, as cooking loss increases, meat tenderness decreases. Overall, these findings highlight the complexity of the relationship between cooking loss and meat quality and emphasize the need to consider various factors in meat quality assessment.
The present study also shows that L*, a*, and b* values are affected by the chilling methods. This finding is contrary to a previous study by Huezo et al. (2007) which indicated that raw fillet was not affected by chilling method. This discrepancy in findings could be attributed to the fact that poultry meat color is influenced by various preslaughter and processing factors, such as genetics, feed, handling, stress, and stunning techniques (Mir et al., 2017; Cano et al., 2021; Tomasevic et al., 2021). These findings highlight the complex nature of the factors contributing to meat color and quality, underscoring the need to consider such factors in future research.
Maintaining optimal temperature levels during processing is crucial to ensuring the safety and quality of poultry products. Air chilling and water chilling are the 2 most frequently used methods globally (Belk et al., 2021). Our study found no significant differences in cooking loss between air and water chilling. Similarly, Demirok et al. (2013) observed no significant differences in cooking loss between breast fillets processed by air chilling and water chilling. Similarly, Bowker et al. (2014) reported no significant differences in cooking yield between air and immersion chilling. Our study also found no significant differences in penetration force between air and water chilling, which is consistent with previous studies (Zhuang et al., 2008; Jeong et al., 2011). However, 2 earlier studies reported that immersion chilling resulted in higher shear force compared to air chilling of breast fillets (Demirok et al., 2013; Bowker et al., 2014). As suggested by Bowker et al. (2014), discrepancies among studies may be attributed to factors such as the timing of rigor mortis onset and/or resolution, as well as differences in processing methods including stunning and deboning techniques.
Meat color is one of the most important qualities that affect consumer acceptance of meat (Fletcher et al., 2000), especially considering the current practice of selling fresh meat in closed plastic packages. Colorimeters, such as the Minolta Chroma Meter and HunterLab colorimeter, are widely used in product development and colorimetry to measure and quantify color. The Minolta Chroma Meter has been utilized in 60.0 and 67.5% of published papers in 1998 to 2007 and 2019 to 2020, respectively, while the HunterLab colorimeter has been utilized in 31.6 and 16.3% of published papers in 1998 to 2007 and 2019 to 2020, respectively (Tapp et al., 2011; Tomasevic et al., 2021).
Since the inexpensive and smaller Nix colorimeter has been introduced, Schelkopf et al. (2019) compared beef color using the HunterLab colorimeter and the Nix. Similarly, Holman et al. (2018) measured the precision of technical replicates on aged beef using the Nix. We measured color values using the Nix which is smaller and less expensive (10% of the cost) compared to the HunterLab colorimeter or the Minolta Chroma Meter. Our results show strong correlation of L*, a*, and b* values between the Minolta Chroma Meter and the Nix. Overall, we concluded that the Nix is affordable, convenient, and comparable to the traditional instruments when measuring skinless chicken meat color.
Our findings also demonstrate that the quality defect from light-colored (PSE) meat is still an issue for the broiler industry. When we set our cut-off L* values to 43 using the Nix, 12.5% of breast fillets were classified as PSE meat. Petracci et al. (2009) reported that PSE incidences between 1998 and 2006 ranged from 5 to 47% (depending on the study) in North America and Europe. Two later studies (Carvalho et al., 2014; Karunanayaka et al., 2016) reported 41.7% PSE in Brazil (Carvalho et al., 2014) and 70% in Sri Lanka (Karunanayaka et al., 2016). It was estimated that the economic loss from PSE meat in poultry is approximately $200 million a year in the United States alone (Owens et al., 2009; Paião et al., 2013). As chicken consumption in the world is increasing annually (OECD/FAO, 2021), the current loss from PSE meat is most probably higher. As color measurement is a good and easy indicator for assessing meat quality, it will be cost-effective to use as an inexpensive and small colorimeter in processing plants. This technology can also be used in line without an employee piercing and measuring (e.g., for pH determination) each sample manually.
CONCLUSIONS
This study shows that PSE meat is frequently occurring and causing a quality loss in Ontario broiler flocks, and results in a portion of the meat exhibiting lower quality. Furthermore, chilling method can affect the color and meat quality (e.g., the WHC value) differently. Lastly, as the use of the Nix colorimeter is affordable, convenient, and comparable to the traditional instruments, the colorimeter can help the meat industry monitor the situation and react to improve meat quality; long-term solution—removing birds susceptible to PSE from the breeding flocks, and short term—identify and direct PSE meat to certain streamlines.
ACKNOWLEDGMENTS
The authors thank the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) (054855) for providing financial support. The technical assistance by Chaoyue Wang, Michelle Yahiro, Alex Leacy, and Elana Huong is greatly appreciated.
DISCLOSURES
The authors declare that there are no conflicts of interest with respect to the publication of this manuscript.
REFERENCES
- Ali M.S., Kang G.H., Joo S.T. A review: influences of pre-slaughter stress on poultry meat quality. Asian-Australas. J. Anim. Sci. 2008;21:912–916. [Google Scholar]
- Barbanti D., Pasquini M. Influence of cooking conditions on cooking loss and tenderness of raw and marinated chicken breast meat. LWT. 2005;38:895–901. [Google Scholar]
- Barbut S. Problem of pale soft exudative meat in broiler chickens. Br. Poult. Sci. 1997;38:355–358. doi: 10.1080/00071669708418002. [DOI] [PubMed] [Google Scholar]
- Barbut S. Pale, soft, and exudative poultry meat - reviewing ways to manage at the processing plant. Poult. Sci. 2009;88:1506–1512. doi: 10.3382/ps.2009-00118. [DOI] [PubMed] [Google Scholar]
- Barbut S., Evaluating texture and sensory attributes, Pages 1–36 in The Science of Poultry and Meat Processing, 2015, Free download. Accessed Jan. 2023. www.poultryandmeatprocessing.com.
- Barbut S., Leishman E.M. Quality and processability of modern poultry meat. Animals. 2022;12 doi: 10.3390/ani12202766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbut S., Sosnicki A.A., Lonergan S.M., Knapp T., Ciobanu D.C., Gatcliffe L.J., Huff-Lonergan E., Wilson E.W. Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Sci. 2008;79:46–63. doi: 10.1016/j.meatsci.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Barbut S., Zhang L., Marcone M. Effects of pale, normal, and dark chicken breast meat on microstructure, extractable proteins, and cooking of marinated fillets. Poult. Sci. 2005;84:797–802. doi: 10.1093/ps/84.5.797. [DOI] [PubMed] [Google Scholar]
- Belk A.D., Duarte T., Quinn C., Coil D.A., Belk K.E., Eisen J.A., Quinn J.C., Martin J.N., Yang X., Metcalf J.L. Air versus water chilling of chicken: a pilot study of quality, shelf-life, microbial ecology, and economics. mSystems. 2021;6:e00912–20. doi: 10.1128/mSystems.00912-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berri C., Bihan-Duval E.L., Debut M., Santé-Lhoutellier V., Baéza E., Gigaud V., Jégo Y., Duclos M.J. Consequence of muscle hypertrophy on characteristics of Pectoralis major muscle and breast meat quality of broiler chickens. J. Anim. Sci. 2007;85:2005–2011. doi: 10.2527/jas.2006-398. [DOI] [PubMed] [Google Scholar]
- Boulianne M., King A.J. Biochemical and color characteristics of skinless boneless pale chicken breast. Poult. Sci. 1995;74:1693–1698. [Google Scholar]
- Bowker B.C., Zhuang H., Buhr R.J. Impact of carcass scalding and chilling on muscle proteins and meat quality of broiler breast fillets. LWT. 2014;59:156–162. [Google Scholar]
- Cano C., Meneses Y., Chaves B.D. Application of peroxyacetic acid for decontamination of raw poultry products and comparison to other commonly used chemical antimicrobial interventions: a review. Acta Med. Port. 2021;84:1772–1783. doi: 10.4315/JFP-21-107. [DOI] [PubMed] [Google Scholar]
- Carvalho R.H., Soares A.L., Honorato D.C.B., Guarnieri P.D., Pedrão M.R., Paião F.G., Oba A., Ida E.I., Shimokomaki M. The incidence of pale, soft, and exudative (PSE) turkey meat at a Brazilian commercial plant and the functional properties in its meat product. LWT - Food Sci. Technol. 2014;59:883–888. [Google Scholar]
- Demirok E., Veluz G., Stuyvenberg W.V., Castañeda M.P., Byrd A., Alvarado C.Z. Quality and safety of broiler meat in various chilling systems. Poult. Sci. 2013;92:1117–1126. doi: 10.3382/ps.2012-02493. [DOI] [PubMed] [Google Scholar]
- Dong M., Chen H., Zhang Y., Xu Y., Han M., Xu X., Zhou G. Processing properties and improvement of pale, soft, and exudative-like chicken meat: a review. Food Bioprocess Technol. 2020;13:1280–1291. [Google Scholar]
- Fletcher D.L. Broiler breast meat color variation, pH, and texture. Poult. Sci. 1999;78:1323–1327. doi: 10.1093/ps/78.9.1323. [DOI] [PubMed] [Google Scholar]
- Fletcher D.L., Qiao M., Smith D.P. The relationship of raw broiler breast meat color and pH to cooked meat color and pH. Poult. Sci. 2000;79:784–788. doi: 10.1093/ps/79.5.784. [DOI] [PubMed] [Google Scholar]
- Guarnieri P., Soares A., Olivo R., Schneider J., Macedo R., Ida E., Shimokomaki M. Preslaughter handling with water shower spray inhibits PSE (pale, soft, exudative) broiler breast meat in a commercial plant. Biochemical and ultrastructural observations. J. Food Biochem. 2004;28:269–277. [Google Scholar]
- Hertog-Meischke M.J.A., van Laack R.J.L.M., Smulders F.J.M. The water-holding capacity of fresh meat. Vet. Q. 1997;19:175–181. doi: 10.1080/01652176.1997.9694767. [DOI] [PubMed] [Google Scholar]
- Holman B.W.B., Collins D., Kilgannon A.K., Hopkins D.L. The effect of technical replicate (repeats) on Nix Pro Color SensorTM measurement precision for meat: a case-study on aged beef colour stability. Meat Sci. 2018;135:42–45. doi: 10.1016/j.meatsci.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Holman B.W.B., Hopkins D.L. A comparison of the Nix Colour Sensor ProTM and HunterLab MiniScanTM colorimetric instruments when assessing aged beef colour stability over 72 h display. Meat Sci. 2019;147:162–165. doi: 10.1016/j.meatsci.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Huezo R., Northcutt J.K., Smith D.P., Fletcher D.L. Effect of chilling method and deboning time on broiler breast fillet quality. J. Appl. Poult. Res. 2007;16:537–545. [Google Scholar]
- Huff-Lonergan E., Lonergan S.M. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Jeong J.Y., Janardhanan K.K., Booren A.M., Karcher D.M., Kang I. Moisture content, processing yield, and surface color of broiler carcasses chilled by water, air, or evaporative air. Poult. Sci. 2011;90:687–693. doi: 10.3382/ps.2010-00980. [DOI] [PubMed] [Google Scholar]
- Jiang H., Yoon S.C., Zhuang H., Wang W., Yang Y. Evaluation of factors in development of Vis/NIR spectroscopy models for discriminating PSE, DFD and normal broiler breast meat. Br. Poult. Sci. 2017;58:673–680. doi: 10.1080/00071668.2017.1364350. [DOI] [PubMed] [Google Scholar]
- Karunanayaka D.S., Jayasena D.D., Jo C. Prevalence of pale, soft, and exudative (PSE) condition in chicken meat used for commercial meat processing and its effect on roasted chicken breast. J. Anim. Sci. Technol. 2016;58:1–8. doi: 10.1186/s40781-016-0110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laack R.L.J.M., Liu C.H., Smith M.O., Loveday H.D. Characteristics of pale, soft, exudative broiler breast meat. Poult. Sci. 2000;79:1057–1061. doi: 10.1093/ps/79.7.1057. [DOI] [PubMed] [Google Scholar]
- Lee B., Choi Y.M. Comparison of histochemical characteristics, chicken meat quality, and heat shock protein expressions between PSE-like condition and white-stripping features of Pectoralis major muscle. Poult. Sci. 2021;100:1–7. doi: 10.1016/j.psj.2021.101260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudalal S., Babini E., Cavani C., Petracci M. Quantity and functionality of protein fractions in chicken breast fillets affected by white striping. Poult. Sci. 2014;93:2108–2116. doi: 10.3382/ps.2014-03911. [DOI] [PubMed] [Google Scholar]
- Murphy R.Y., Marks B.P. Effect of meat temperature on proteins, texture, and cook loss for ground chicken breast patties. Poult. Sci. 2000;79:99–104. doi: 10.1093/ps/79.1.99. [DOI] [PubMed] [Google Scholar]
- OECD/FAO . OECD Publishing; Paris: 2021. OECD-FAO Agricultural Outlook 2021-2030. [Google Scholar]
- Owens C.M., Alvarado C.Z., Sams A.R. Research developments in pale, soft, and exudative turkey meat in North America. Poult. Sci. 2009;88:1513–1517. doi: 10.3382/ps.2009-00008. [DOI] [PubMed] [Google Scholar]
- Paião F.G., Ferracin L.M., Pedrão M., Kato T., Shimokomaki M. Skeletal muscle calcium channel ryanodine and the development of pale, soft, and exudative meat in poultry. Genet. Mol. Res. 2013;12:3017–3027. doi: 10.4238/2013.August.20.3. [DOI] [PubMed] [Google Scholar]
- Petracci M., Betti M., Bianchi M., Cavani C. Color variation and characterization of broiler breast meat during processing in Italy. Poult. Sci. 2004;83:2086–2092. doi: 10.1093/ps/83.12.2086. [DOI] [PubMed] [Google Scholar]
- Petracci M., Bianchi M., Cavani C. The European perspective on pale, soft, exudative conditions in poultry. Poult. Sci. 2009;88:1518–1523. doi: 10.3382/ps.2008-00508. [DOI] [PubMed] [Google Scholar]
- Qiao M., Fletcher D.L., Smith D.P., Northcutt J.K. The effect of broiler breast meat color on pH, moisture, water-holding capacity, and emulsification capacity. Poult. Sci. 2001;80:676–680. doi: 10.1093/ps/80.5.676. [DOI] [PubMed] [Google Scholar]
- Schelkopf C., Swenson J., Hess A., Belk K.E., Nair M.N. Nix pro color sensor provides comparable color measurements to HunterLab colorimeter. Meat Muscle Biol. 2019;3 doi: 10.1007/s13197-021-05077-6. 178–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiglitz R., Mikhailova E., Post C., Schlautman M., Sharp J. Evaluation of an inexpensive sensor to measure soil color. Comput. Electron. Agric. 2016;121:141–148. [Google Scholar]
- Swatland H.J. An explanation of subsurface optical pathways through food myosystems and their effect on colorimetry. Asian J. Agric. Food Sci. 2021;9 [Google Scholar]
- Tapp W.N., Yancey J.W.S., Apple J.K. How is the instrumental color of meat measured? Meat Sci. 2011;89:1–5. doi: 10.1016/j.meatsci.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Tomasevic I., Djekic I., Font-i-Furnols M., Terjung N., Lorenzo J.M. Recent advances in meat color research. Curr. Opin. Food Sci. 2021;41:81–87. [Google Scholar]
- Woelfel R.L., Owens C.M., Hirschler E.M., Martinez-Dawson R., Sams A.R. The characterization and incidence of pale, soft, and exudative broiler meat in a commercial processing plant. Poult. Sci. 2002;81:579–584. doi: 10.1093/ps/81.4.579. [DOI] [PubMed] [Google Scholar]
- Yang T., Liu R., Yang L., Yang W., Li K., Qin M., Ge Q., Yu H., Wu M., Zhou X. Improvement strategies for quality defects and oxidation of pale, soft and exudative (PSE)-like chicken meat: effects of domestic cooking and core temperature. RSC Adv. 2022;12:7485–7496. doi: 10.1039/d2ra00392a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Barbut S. Effects of regular and modified starches on cooked pale, soft, and exudative; normal; and dry, firm, and dark breast meat batters. Poult. Sci. 2005;84:789–796. doi: 10.1093/ps/84.5.789. [DOI] [PubMed] [Google Scholar]
- Zhang L., Barbut S. Rheological characteristics of fresh and frozen PSE, normal and DFD chicken breast meat. Br. Poult. Sci. 2005;46:687–693. doi: 10.1080/00071660500391516. [DOI] [PubMed] [Google Scholar]
- Zhao X., Xing T., Chen X., yi Han M., lian Xu X., hong Zhou G. Yield, thermal denaturation, and microstructure of proteins isolated from pale, soft, exudative chicken breast meat by using isoelectric solubilization/precipitation. Process Biochem. 2017;58:167–173. [Google Scholar]
- Zhuang H., Bowker B.C., Buhr R.J., Bourassa D.V., Kiepper B.H. Effects of broiler carcass scalding and chilling methods on quality of early-deboned breast fillets. Poult. Sci. 2013;92:1393–1399. doi: 10.3382/ps.2012-02814. [DOI] [PubMed] [Google Scholar]
- Zhuang H., Savage E.M., Smith D.P., Berrang M.E. Effect of dry-air chilling on Warner-Bratzler shear force and water-holding capacity of broiler breast meat deboned four hours postmortem. Int. J. Poult. Sci. 2008;7:743–748. [Google Scholar]