Abstract
Tumors are among the leading causes of death worldwide. Cell-derived biomimetic functional materials have shown great promise in the treatment of tumors. These materials are derived from cell membranes, extracellular vesicles and bacterial outer membrane vesicles and may evade immune recognition, improve drug targeting and activate antitumor immunity. However, their use is limited owing to their low drug-loading capacity and complex preparation methods. Liposomes are artificial bionic membranes that have high drug-loading capacity and can be prepared and modified easily. Although they can overcome the disadvantages of cell-derived biomimetic functional materials, they lack natural active targeting ability. Lipids can be hybridized with cell membranes, extracellular vesicles or bacterial outer membrane vesicles to form lipid-hybrid cell-derived biomimetic functional materials. These materials negate the disadvantages of both liposomes and cell-derived components and represent a promising delivery platform in the treatment of tumors. This review focuses on the design strategies, applications and mechanisms of action of lipid-hybrid cell-derived biomimetic functional materials and summarizes the prospects of their further development and the challenges associated with it.
Keywords: Liposomes, Cell membranes, Extracellular vesicles, Bacterial outer membrane vesicles, Hybrid, Tumors therapy
Graphical abstract
Characterization and antitumor mechanisms of lipid-hybrid biomimetic functional materials of different cellular origins.
1. Introduction
Tumor is one of the leading causes of death among humans [1]. The most common treatment strategies for cancer include surgical resection and local radiation therapy, which are usually accompanied by systemic therapy for the eradication of unresectable and metastatic tumors [2]. Most anti-drugs have side effects when administered at high doses and have poor specificity in targeting tumors [3]. Nanoparticles (NPs) can alter the pharmacokinetic properties of drugs, and some NPs are already in use in the clinic [4]. Cell-derived biomimetic functional materials are novel agents that can be used as nanocarriers for active targeting of tumors and can activate the immune system to exert antitumor effects. For example, the CD47 protein on the surface of the erythrocyte membrane facilitates evasion of immune surveillance and enhances nanocarrier circulation [5], tumor-derived extracellular vesicles (EVs) aggregate more readily in tumor tissues when they are homologously targeted by integrins [6] and bacterial outer membrane vesicles (OMVs) stimulate antigen-presenting cells (APCs) and T cells to enhance antitumor immunity [7]. However, cell-derived biomimetic functional materials are difficult to prepare in large quantities and exhibit poor physical stability owing to the low yield and loss of functional properties [8]. Moreover, EVs have poor drug-loading capacity [9]. These limitations hinder the development and application of cell-derived biomimetic functional materials for the treatment of cancer.
In recent years, the combination of polymers, nanomaterials and liposomes with bacterial OMVs and tumor-derived EVs has emerged as a novel design strategy [[10], [11], [12]]. In particular, liposomes have a bilayer structure similar to biomembranes, and the formation of lipid-hybrid cell-derived biomimetic functional materials through the hybridization of liposomes with membrane vesicles has become a major focus of research on bionanomaterials. Liposomes are artificial bionic membranes with good biocompatibility and excellent drug-loading capacity. They can be used to encapsulate both hydrophilic and hydrophobic drugs and help to improve the drug-loading capacity of membrane vesicles [13]. In addition, the surface of liposomes can be easily modified to improve their drug-targeting ability [14,15]. Owing to their inherent properties, liposomes can overcome the disadvantages of cell-derived biomimetic functional materials in drug delivery and enhance their efficacy as anticancer agents. In addition, nanostructures derived from cells can induce active targeting ability in liposomes and help them evade immune recognition.
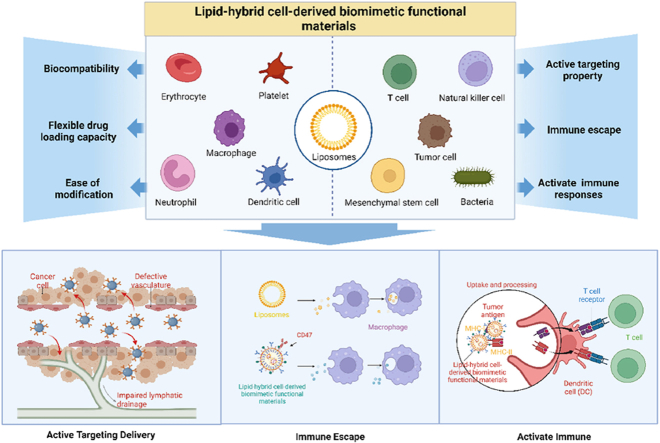
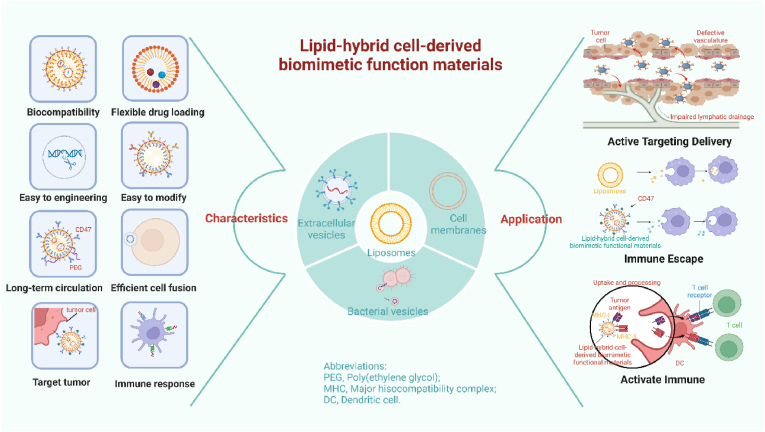
Given the substantial potential of lipid-hybrid cell-derived biomimetic functional materials in the treatment of tumors, biohybrid nanomaterials have been intensively investigated in the past decade [10,16]. Although some reviews have reported the research progress of one or several nanocarriers (liposomes, EVs or cell membrane nanocarriers), to the best of our knowledge, no review has comprehensively summarized the types, modification strategies and mechanisms of action of lipid-hybrid cell-derived biomimetic functional materials. This review highlights the recent research progress of lipid-hybrid cell-based primitive biomimetic functional materials used in cancer treatment. Fig. 1 illustrates the types and characteristics of different membrane-derived vesicles hybridized with liposomes to form lipid-hybrid cell-derived biomimetic functional materials. The preparation methods, loading strategies, applications and antitumor mechanisms of these functional materials are specifically described, indicating their prospective use for the development of novel therapeutic strategies for cancer in the future.
Fig. 1.
Characterization and antitumor mechanisms of lipid-hybrid biomimetic functional materials of different cellular origins. Created by BioRender. Com.
2. Design strategy of lipid-hybrid cell-derived biomimetic functional materials
2.1. Preparation of liposomes
Drug delivery systems based on liposomes have been established as potent delivery systems. At present, liposomes are one of the most widely used nanocarriers in anticancer drug development, with several formulations being clinically approved and more undergoing clinical trials [[17], [18], [19]].
Liposomes are synthetic membranes containing phospholipids and cholesterol as their main constituents. Phospholipids are the basis of the liposome membrane structure and can be folded into a bilayer to form a closed vesicular structure. The closed structure is stable owing to the amphiphilic nature of liposomes, with the hydrophilic head on one side and the hydrophobic tail on the other side [20]. A wide range of hydrophilic and lipophilic drugs can be encapsulated in liposomes [21,22]. Cholesterol plays a pivotal role in stabilizing the structure of liposomes under changing environmental conditions. The structure of liposomes is similar to that of the plasma membrane and has good physiological compatibility, which helps liposomes evade immune surveillance when they are used as a drug delivery system [20]. Furthermore, liposomes can be modified with various functional groups and hence perform a broader range of functions as synthetic membranes. For example, folic acid modification enhances tumor-targeting ability [23], and polyethylene glycol (PEG) modification prolongs the retention of liposomes in circulation [24]. With the development of technology, researchers have developed several techniques to prepare liposomes, mainly including the thin-film hydration method [25,26], reverse evaporation [27], double emulsion method [28,29], freeze drying [30] and ethanol injection method [31,32]. Because passive wrapping is ineffective for some amphiphilic drugs, active loading methods have been developed to reduce leakage while improving efficiency [33]. These methods are used to create transmembrane chemical gradients (e.g., pH) to stimulate drug diffusion [33,34].
Overall, liposomes can be easily prepared using various strategies and can be used to encapsulate different drugs. Some methods of preparing liposomes are summarized in Table 1.
Table 1.
Conventional preparation methods of liposomes and their characteristics.
| Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Thin-film hydration method | Simple and maneuverable, this method of production allows for quick and easy production |
|
[26,35] |
| Reverse evaporation | Because liposomes contain more aqueous phase, they are ideal for encapsulating proteins or large molecules like DNA, RNA, and other drugs. |
|
[27,36] |
| Double emulsion method | Multicapsule structures with a non-concentric structure are better suited to encapsulating water-soluble drugs to increase their encapsulation rate, and they have slow release characteristics. | The particle size of non-concentric multivesicular liposomes is larger than that of other types and their stability is lower than that of other liposome types. | [28] |
| Freeze drying method | Long-term storage is possible with the preparation as it is very stable. | Higher production costs due to long freeze-drying processes. | [30,37,38] |
| Ethanol injection method | Some unstable drugs can be loaded with this process because it is simple, fast, and reduces the exposure of lipid and encapsulants. |
|
[31,39] |
| Active loading methods | In situations where pH and ionic strength are strongly influencing the oil-water partition coefficient, this methods is ideal. | Liposomes can only contain weakly basic or weakly acidic drugs, and drugs must not have an electrical charge in order to be loaded into them. | [33,40] |
2.2. Preparation of cell-derived biomimetic functional materials
In recent years, nanostructures derived from cells, including cell membranes, EVs and bacterial OMVs, have been widely used for the delivery of various drugs owing to their specific bioactivity and flexibility. Cell membranes are composed of membrane structures that are obtained from different types of cells by special processes. They can be modified to form NPs that can evade immune surveillance or have active targeting ability [41,42] and can be functionalized with other ligands [43]. These cell-derived nanostructures can be prepared using ultrasonic fragmentation [44], repeated freeze‒thaw cycles [45] and hypotonic buffers [46].
In the extracellular space, cells secrete nano-to micrometer-sized particles called EVs. They contain proteins and genetic material for cellular communication at a local or systemic level [47,48]. EVs are classified into three major categories based on their particle size: exosomes, micro-vesicles and apoptotic bodies [49]. Similar to the cell membrane, EVs can target tumor-specific sites and remain in the body for a long period without being recognized by the immune system, thus resulting in effective delivery of drugs to the target site [50]. These characteristics enable the use of EVs as drug delivery vehicles. Exosomes, which contain proteins (growth factors, enzymes and receptors) and RNAs that mediate cell communication, have been intensively investigated [51]. The most common preparation and purification method for exosomes is density gradient centrifugation, which isolates them primarily based on their sedimentation coefficient. However, this method has a low yield [52,53]. In recent years, membrane vesicles with a uniform particle size and similar composition to exosomes have been developed using filter membranes with certain pore sizes [[54], [55], [56]]. This approach results in a high yield while retaining the relevant proteins of cellular origin.
Slightly different from EVs, OMVs are a class of spherical and bilayer proteolipids produced by gram-negative bacteria. They contain immunostimulatory proteins that induce an immune response [57]. At present, OMVs are used as immune adjuvants to activate antitumor immunity. They are usually extracted and purified from cultured cells via ultracentrifugation [58].
2.3. Preparation and characterization of lipid-hybrid cell-derived biomimetic functional materials
Lipid-hybrid cell-derived biomimetic functional materials are prepared by fusing membrane vesicles with liposomes. This fusion should be efficient and should not cause drug leakage or protein denaturation. Membrane extrusion [10,16,59,60] and freeze‒thaw cycles [61,62] are common methods of fusion. Membrane extrusion involves the sequential extrusion of samples (cell-derived biomimetic functional materials and liposomes) through pores of different sizes. Although this technique is effective, large-scale preparation is challenging. In the freeze‒thaw method, samples are repeatedly frozen and thawed to induce eventual fusion. This method is simpler than extrusion and is suitable for large-scale production; however, proteins present in membrane vesicles may become denatured during repeated freezing and thawing.
After the successful fusion of liposomes with cell-derived biomimetic functional materials, factors such as the protein content, chemical structure, morphology and physicochemical properties of membrane vesicles should be verified in vitro to evaluate the fusion efficiency. In this section, we discuss some physicochemical and biological properties of NPs that can be used to evaluate the performance of lipid-hybrid cell-derived biomimetic functional materials.
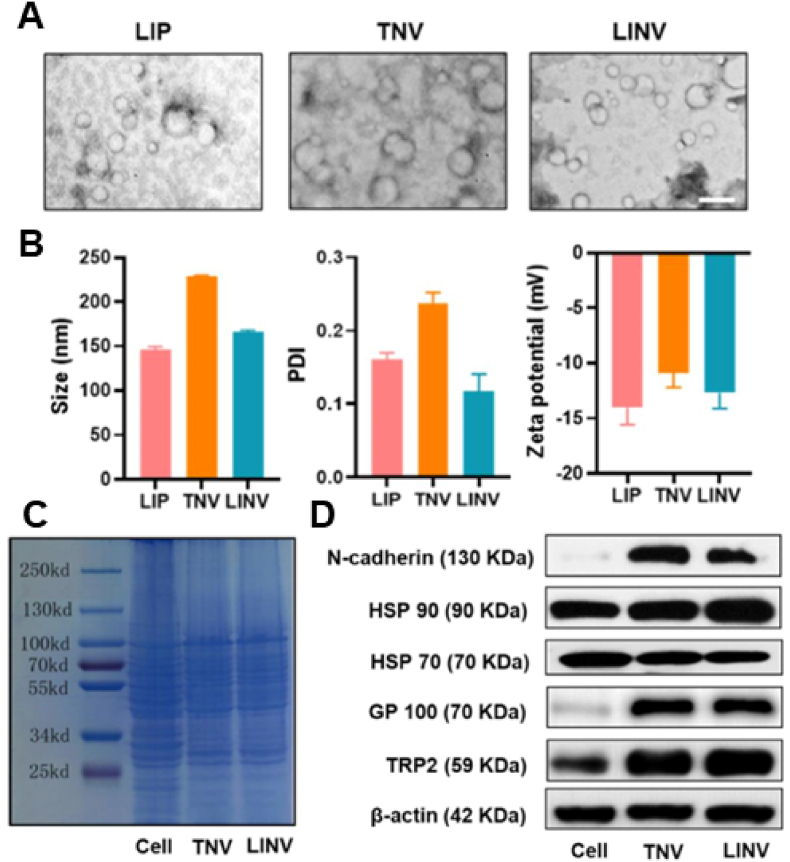
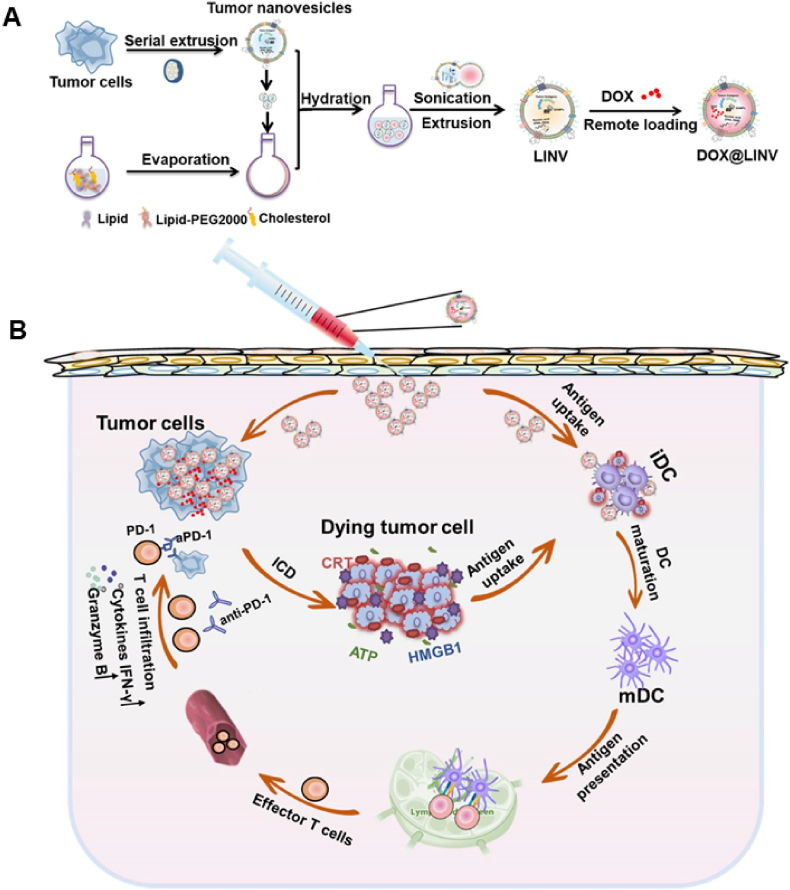
Transmission electron microscopy (TEM) is considered the gold-standard technique for the characterization of lipid-hybrid cell-derived biomimetic functional materials because membrane vesicles composed of lipids and proteins usually have different electron densities and are currently one of the most important tools for the morphological characterization of NPs. Fig. 2A [25] shows the morphology of liposomes (LIPs), tumor-derived nanovesicles (TNVs) and hybrid nanovesicles after fusion (LINVs) as analyzed via TEM, with image analysis demonstrating that the lipid-hybrid cell-derived biomimetic functional materials are more uniformly sized and spherical. Furthermore, NPs can be evaluated based on their average particle size, zeta potential and polydispersity index (PDI). Fig. 2B shows the particle size, zeta potential and PDI of LIPs, TNVs and LINVs. LINVs based on the nanoscale can accumulate massively within tumors owing to the enhanced permeation and retention (EPR) effect. Altogether, these characteristics demonstrate the physicochemical properties of lipid-hybrid cell-derived biomimetic functional materials and, more importantly, verify the biological activity of NPs. Sodium dodecyl sulfate‒polyacrylamide gel electrophoresis (SDS‒PAGE) and western blotting are widely used molecular biology techniques for monitoring the protein expression profile of cells and are therefore adopted as standard methods for characterizing membrane proteins. The protein expression profiles of original cells, extracted membrane vesicles and lipid-hybrid cell-derived biomimetic functional materials were initially compared via SDS‒PAGE. As shown in Fig. 2C, TNVs and LINVs have protein bands similar to those of the original cells, and the protein profiles of TNVs and LINVs closely match the total protein profile of whole cells, indicating that cellular proteins are adequately retained in the lipid-hybrid NPs [25]. As shown in Fig. 2D, representative images of western blotting demonstrate similar results [25].
Fig. 2.
Morphological and biological characterization of lipid-hybrid cell-derived biomimetic functional materials. (A) Transmission electron microscopy images of LIPs, TNVs and LINVs (scale bar = 0.2 μm). (B) Particle size distribution, polydispersity coefficient and zeta potential of LIPs, TNVs and LINVs (n = 3). (C) SDS‒PAGE of cells, TNVs and LINVs. (D) Western blotting of specific antigens on TNVs and LINVs. Reproduced with permission from Ref. [25], Copyright © 2021, American Chemical Society. (LIPs: liposomes; TNVs: tumor-derived nanovesicles; LINVs: lipid hybridization of cell-based primitive biomimetic functional materials.)
3. Characteristics of lipid-hybrid cell-derived biomimetic functional materials
As nanocarriers, liposomes are efficient drug delivery vehicles owing to their ease of preparation and good drug-loading capacity, but its inherent disadvantages prevent it from maximizing drug efficacy. Liposomes are taken up through endocytosis and bind to introns to take them into lysosomes [[63], [64], [65]]. Known as the “digestive organs” of the cell, lysosomes are responsible for breaking down macromolecules inside the cell, which hinders or prevents liposomes from delivering drugs. It was found that cell-derived biomimetic functional materials can be fused directly to the membrane, which would facilitate the release of drugs into cytoplasm rather than lysosomes [66]. As a result, cell-derived biomimetic functional materials would be beneficial in altering the uptake pathway of liposomes and improving the effective utilization of drugs in liposomes. In addition, various cell-derived biomimetic materials have specific biological activities, including active targeting of tumor tissues [67], participation in cellular communication [51,68], and regulation of immune cells [69]. The bioactive markers and functional properties of nanostructures derived from different cells are summarized in Table 2. However, cell-derived biomimetic functional materials have limitations in terms of their application in tumor therapy because of their complex purification process, low drug load, and poor stability [70,71]. Liposomes and cell-derived biomimetic functional materials can be fused by lipid exchange to form lipid-hybrid cell-derived biomimetic functional materials due to the similar bilayer structure [72,73]. The combination of these strengths will result in greater efficacy, including increased drug loading, natural active targeting, and immune system modulation, improving anti-tumor effects. In addition to the above, many engineering strategies can enhance tumor cell killing using such nanomaterials, including magnetic targeting [74], ligand-receptor binding [75], and bioorthogonal click chemistry [74].
Table 2.
Representative biomarkers of biomimetic functional materials of different cellular origins and their functions.
| Cell type | Representative biomarkers | Functions | References |
|---|---|---|---|
| Red blood cells | CD47 | Long-range looping | [41] |
| Platelets | P-selectin | Binding to CD44 on the tumor cell surface for active targeting | [76,77] |
| Macrophages | CD47 and tumor necrosis factor-α | Improve circulation time, target tumors and engage antitumor immunity | [78,79] |
| Neutrophils | Adhesion molecules (e.g. intercellular adhesion molecule-1) | Bind to inflamed cells and migrate to the site of inflammation | [80,81] |
| Dendritic cells | Major histocompatibility complex | Activation and maintenance of antigen-specific T cells by activating antigen-presenting cells and delivering peptide antigens | [82,83] |
| T cells | T-cell receptors | Localize and target tumors | [84] |
| Natural killer cells | Membrane proteins (e.g. RANKL or dNaM-1) | Induction of polarization of tumor-associated macrophages | [85,86] |
| Tumor cells | Plasma membrane proteins (including N-cadherin, Galectin 3 and adhesion molecules) | Immune escape and homologous targeting of tumor cells | [87,88] |
| Mesenchymal stem cells | CXC chemokine receptor 4 and hypoxia-inducible factor. | Tumor targeting and low immunogenicity | [89,90] |
| Bacteria | Pathogen-associated molecular patterns | Tumor targeting and activation of immunity | [7,91] |
3.1. Lipid-hybrid blood cell-derived biomimetic functional materials
Red blood cells (RBCs) and platelets are derived from hematopoietic stem cells and are a unique component of the circulatory system. They can circulate in the bloodstream for several days, which is beneficial for drug delivery, especially for some drugs that tend to cause immune reactions. Erythrocyte and platelet membranes have been used for coating nanomaterials encapsulating drugs.
3.1.1. Lipid-hybrid red blood cell-derived biomimetic functional materials
RBCs are the most abundant cells in the body, and their primary function is to transport oxygen throughout the body. RBCs have been extensively investigated for their potential use in drug delivery owing to their capability to circulate in and easily separate from the blood [92,93]. The RBC membrane can significantly improve some characteristics of nanocarriers. First, it protects the activity of the encapsulated substance, enables its longer and more controlled life cycle and prevents its immune clearance. The surface of the RBC membrane has high expression of the ‘do not eat me’ signal CD47, a receptor that interacts with signal-regulatory protein alpha present on macrophages to ensure that NPs escape the reticuloendothelial system, thus helping NPs evade immunogenic clearance and prolong their blood circulation time [92]. Although PEGylation is equally beneficial for prolonging the circulation time of nanocarriers, it hinders the penetration and internalization of liposomes into tumors to some extent [94,95]. In addition, RBC membrane-camouflaged nanostructures may have better blood retention than PEGylated preparations [96]. Second, RBCs are primarily involved in oxygen transport owing to the presence of large amounts of hemoglobin that bind to oxygen molecules and carry them to tissues throughout the body [97]. Influenced by the ability of erythrocytes to transport oxygen, researchers have designed erythrocyte membranes wrapped with oxygen-generating devices to mimic RBC function and inhibit tumor development by increasing the oxygen content in the tumor microenvironment (TME) or combined with photodynamic therapy to increase the efficiency of reactive oxygen species (ROS) production [98]. Third, RBC membranes can be completely degraded in vivo without resulting in toxic byproducts, demonstrating good biocompatibility and biodegradability for in vivo applications. In addition, RBC membrane-encapsulated nanocarriers can reduce toxicity and increase stability by reducing aggregation [99]. Therefore, nanocarriers coated with RBC membranes have several advantages in nanodrug delivery.
Liposomes are widely used as delivery vehicles for various drugs because of their excellent drug-loading properties [100]. Researchers have developed lipid NPs encapsulating 5-fluorouracil in RBC membranes. In addition to the high drug-loading capacity, NPs exhibit long-term drug release, which facilitates nanodrug penetration and function within tumor tissues [5]. Therefore, lipid NPs encapsulating drugs in RBC membranes can be used to deliver more effective drug doses to tumors because liposomes and RBC membranes synergistically increase the circulation time and drug-loading capacity of liposomes. However, this method of drug delivery primarily depends on the passive targeting ability of nanocarriers because cancer cells lack certain cell adhesion molecules on the erythrocyte membrane. Researchers have modified the RBC membrane to improve its ability to deliver drugs to tumor tissues by improving its structural properties [101]. CD44 is a class of proteins highly expressed on the surface of tumor cells, to which platelets can bind for active targeting [102]. To increase the accumulation of RBC membrane-coated nanocarriers in tumor tissues, hyaluronic acid, a CD44 ligand with good biocompatibility, biodegradability and non-immunogenicity, has been used to modify the RBC membrane [75]. This modification increases the accumulation of drug-encapsulated NPs at the tumor site, improving the effectiveness of the treatment. Altogether, the surface of natural nanocarriers can be modified to improve active targeting; however, direct modification of cell-derived biomimetic functional materials is difficult, which limits further modification of natural membrane vesicles. Liposomes are similar to the vesicles of cell membranes and can be easily modified to enhance the function of lipid-hybrid cell-derived biomimetic functional materials.
In conclusion, RBC membrane coating can increase the circulation time of nanocarriers, reduce the possibility of phagocytosis in circulating blood, enrich tumor tissues through the EPR effect and improve the stability of nanocarriers in the circulatory system. In addition, liposomes can be easily prepared and have high drug-loading capacity. Therefore, lipid-hybrid cell-derived biomimetic functional materials formed by hybridizing RBC membranes with liposomes can combine the advantages of the two components to increase drug accumulation in tumor tissues, achieve efficient targeting and improve the therapeutic effects of chemotherapy or phototherapy.
3.1.2. Lipid-hybrid platelet -derived biomimetic functional materials
Platelets are nonnucleated blood cells derived from the bone marrow [103]. Compared with RBCs, platelets have a more complex structure with multiple surface receptors, which enable them to communicate with the cellular microenvironment [104]. An in-depth understanding of the interactions of platelets with the TME and immune system may offer promising avenues for developing platelet-based drug delivery systems and functional platelet-targeting strategies for the effective treatment of cancer [105].
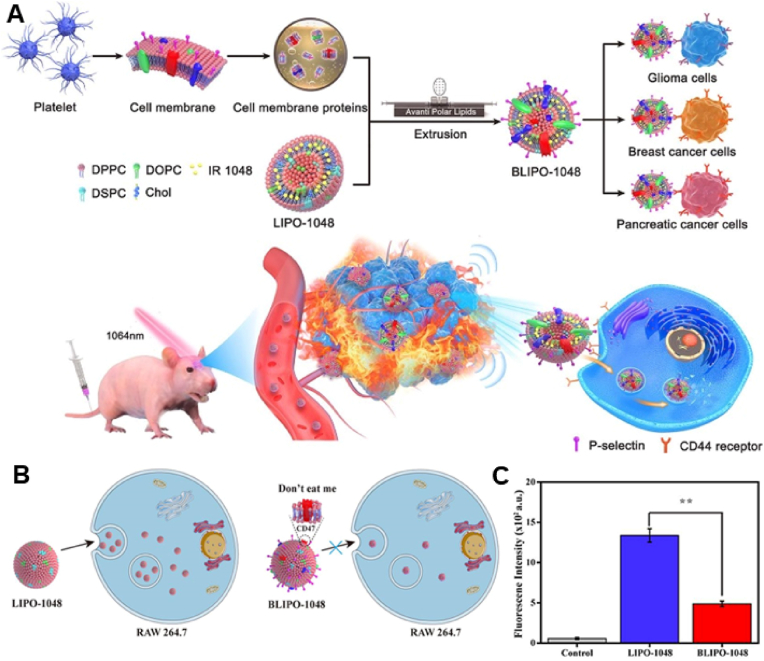
First, similar to RBC membranes, platelet membranes, which are also derived from blood cells, can actively send ‘do not eat me’ signals to macrophages, prolong the circulation time of nanocarriers and evade immune detection and clearance [76]. Second, P-selectin (P-sel) on platelet membranes can specifically bind to CD44 on the surface of tumor cells, which in turn increases drug enrichment at the tumor site [106]. Geng et al. [107] prepared NIR nanoprobes using platelet membranes (Fig. 3A). IR1048 was encapsulated in liposomes, which were modified with platelet membranes to form BLIPO-1048. The active targeting ability of BLIPO-1048 was evaluated using fluorescence and near-infrared photoacoustic imaging techniques. Platelet membrane-encapsulated liposomes had an enhanced ability to evade immune clearance owing to the expression of CD47 on platelet membranes (Fig. 3B and C). Furthermore, P-sel contributes to better tumor targeting. They were combined with NIR-II photothermal therapy (PTT) in an in situ glioma-bearing mouse model, resulting in good photothermal conversion efficiency. However, studies have demonstrated that platelets can protect circulating tumor cells (CTCs) and promote tumor metastasis through recognition of CD44 [108,109]. Based on the specific binding between platelets and CTCs, therapeutic strategies particularly targeting CTCs can be designed for inhibiting metastasis [110]. A study demonstrated that doxorubicin (DOX) loaded into platelets was specifically delivered to tumor cells in both in vivo and in vitro models, resulting in significant cytotoxicity [111]. Although platelet targeting of distal CTCs shows great promise, the encapsulation of drugs into platelets limits the application of this strategy. This treatment strategy primarily relies on the interaction between P-sel on the platelet membrane and CD44 on the surface of tumor cells; therefore, it is reasonable to speculate that lipid-hybrid platelet-derived biomimetic functional materials formed by hybridizing liposomes with platelet membranes have similar functionality, higher drug-loading capacity and more potent therapeutic efficacy.
Fig. 3.
Lipid-hybrid platelet -derived biomimetic functional materials for evade immune and active targeting. (A) Schematic diagram of lipid-hybrid platelet-derived biomimetic materials for active targeted delivery in different tumor models. (B) Schematic representation of differences in the uptake of liposomes and lipid-hybrid platelet-derived biomimetic functional materials by RAW264.7 cells. (C) Flow cytometric detection of the fluorescence intensity of liposomes and lipid-hybrid platelet-derived biomimetic functional materials after coincubation with RAW264.7 cells for 4 h (n = 3) (**, p < 0.01). Reproduced with permission from Ref. [107] Copyright © 2020, American Chemical Society. (BLIPO-1048: platelet membrane-embedded IR 1048 dye-loaded liposomes; LIPO-1048: IR 1048 dye-loaded liposomes.)
With continuous development, various stimulus-responsive liposomes that can achieve responsive drug release through changes in the external environment have been developed [112]. pH-responsive liposomes enable responsive drug release based on pH differences between the TME and normal tissues or between intracellular lysosomes and the cytoplasm [22,113]. These liposomes can be used to develop lipid-hybrid platelet-derived biomimetic functional materials with responsive drug release and active targeting ability to increase drug delivery efficiency. Liu et al. [106] used platelet membranes and pH-sensitive liposomes to prepare pH-responsive lipid-hybrid platelet-derived biomimetic functional materials that delivered specific drugs to tumors and released them when tumors were stimulated. These materials exhibited a strong affinity for tumor cells and allowed selective drug release in the acidic microenvironment of lysosomes. In addition, their antitumor effects were significantly better than those of pH-unresponsive lipid-hybrid cell-derived biomimetic functional materials and conventional pH-sensitive liposomes. Altogether, the pH-responsive lipid-hybrid platelet-derived biomimetic functional materials had improved active tumor-targeting ability and therapeutic efficacy.
Owing to the natural physiological characteristics of platelets, platelet membrane-coated drug delivery systems have high biocompatibility and active targeting ability and can evade phagocytosis, thereby representing more promising nanocarriers for cancer treatment. Lipid-hybrid platelet-derived biomimetic functional materials developed by hybridizing liposomes with platelet membranes have increased therapeutic efficacy and more diverse drug release forms. Although these materials have significant advantages, their translation to clinical applications remains challenging. On the one hand, the current state of the art is insufficient for large-scale production, supply and storage of platelet membranes; on the other hand, the interaction between platelets and tumor tissues remains unclear and warrants further investigation.
3.2. Lipid-hybrid immune cell-derived biomimetic functional materials
Immune cells are the largest blood cells that develop from the differentiation of hematopoietic stem cells in the bone marrow and primarily include macrophages, neutrophils (NEs), dendritic cells (DCs), natural killer (NK) cells and lymphocytes [114]. Inflammatory cells (e.g., macrophages, DCs, NEs and lymphocytes) significantly contribute to the development and progression of cancerous lesions. Given that tumor tissue is naturally an inflammatory site, immune cells such as T cells, macrophages and NEs can target and accumulate at tumor sites [115]. Immune cell-derived bionic functional materials are effective carriers for targeted delivery of drugs to tumors owing to the secondary phenomenon of inflammatory chemotaxis [115]. The membrane structure of immune cells is more complex than that of RBCs and platelets and contains specific proteins and glycans not found in other cell membranes. Therefore, immune cells can initiate active immune responses to eliminate inflammation and suppress tumorigenesis [92,115]. In this regard, lipid-hybrid immune cell-derived bionic functional materials hold great promise in the field of targeted therapeutics.
3.2.1. Lipid-hybrid macrophage-derived biomimetic functional materials
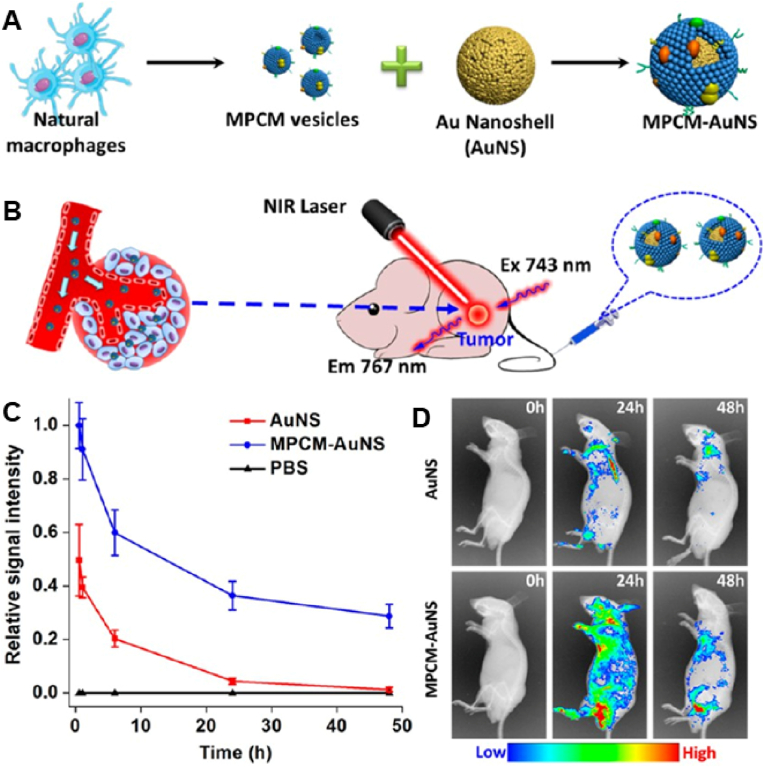
Macrophages can respond more actively to multiple mediators to enhance immune defense while maintaining a balance between antitumor and pro-tumor immune interactions. Therefore, macrophages are important cells for the development of therapeutic strategies. Macrophage membranes are considered particularly suitable for camouflaging NPs targeting tumors for two primary reasons [116]. On one hand, macrophages can target tumor cells. In a study, bionic nanocarriers that mimicked macrophages were developed by coating synthetic Fe3O4 NPs with macrophage membranes [117]. With robust tumor-targeting ability and acceptable biosafety, these nanocarriers were found to be suitable as a good photothermal conversion material for the treatment of tumors. On the other hand, macrophage membrane coating can significantly increase the circulation time of nanocarriers. Xuan et al. [78] coated macrophage membranes onto the surface of Au nanoshells (AuNSs) and investigated whether the biomimetic NPs inherited the long retention characteristics of macrophages through pharmacokinetic experiments (Fig. 4A and B). The results revealed that macrophage membrane-coated AuNSs exhibited a significantly longer blood retention time than pristine AuNSs at 48 h (Fig. 4C). In addition, macrophage membrane-coated AuNSs were detected in >30% of blood vessels at 48 h, whereas unmodified AuNSs were almost cleared from the blood at 24 h. This result was validated via in vivo fluorescence imaging (Fig. 4D). Altogether, macrophage membrane coating significantly enhanced the accumulation of AuNSs in tumors, as the extended in vivo circulating time promoted sustained systemic drug delivery through the passive EPR effect.
Fig. 4.
(A) Schematic diagram of the preparation of MPCM-AuNSs. (B) Schematic diagram of in vivo photothermal therapy. (C) Relative fluorescence intensity of nanoparticles loaded with Cy7 in blood after intravenous injection. (D) Cell membrane-encapsulated or nonencapsulated gold nanoshells were intravenously injected into nude mice within 48 h for in vivo fluorescence imaging. (n = 3). Reproduced with permission from Ref. [78] Copyright © 2016, American Chemical Society. (AuNSs: Au Nanoshells; MPCM-AuNSs: macrophage cell membrane-camouflaged AuNSs). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Therefore, bionic hybrid nanoplatforms based on macrophage membranes can actively target tumor cells and extend the circulation time of NPs. This strategy is of great significance for the prevention and treatment of tumors, especially tumor metastasis. Li et al. [118] encapsulated the DOX nanodrug in liposomes and coated them with macrophage membranes to form cell-like nanostructures loaded with pH-sensitive precursors. These nanostructures were biocompatible, enabled the release of DOX in a pH-responsive manner and inherited relevant characteristics of macrophage membranes, such as prolonged blood circulation time, active tumor-targeting ability and enhanced cellular internalization. Consequently, the nanostructures significantly inhibited lung metastasis of breast cancer and had fewer side effects. This strategy combines the good drug-loading ability of liposomes with the excellent active targeting ability and blood circulation time of macrophage membranes and represents an effective approach to inhibiting tumor metastasis.
The excellent drug-carrying properties of liposomes ensure adequate drug loading, and the natural active targeting of macrophage membranes to tumor cells ensures drug accumulation in tumor tissues and increases the circulation time of nanocarriers to prevent clearance during circulation. Therefore, lipid-hybrid macrophage-derived biomimetic functional materials appear to be more beneficial than the existing drug delivery platforms for targeted treatment of tumors. They can be used to effectively deliver drugs at the maximum dosing concentrations while improving the therapeutic efficacy and reducing adverse effects.
3.2.2. Lipid-hybrid neutrophil-derived biomimetic functional materials
NEs, the most abundant leukocytes in peripheral blood, are major participants in proinflammatory immune responses and play a key role in the immune response to pathogens [119]. Considering that carcinogenesis is essentially an inflammatory process, NEs have been widely used as targeting vectors in cancer treatment [[120], [121], [122]].
Glioblastoma is the most aggressive type of brain tumor and is characterized by a high mortality rate, shorter survival, poor prognosis and frequent recurrence [[123], [124], [125]]. In recent years, nanodrug delivery systems based on liposomes have been developed to cross the blood‒brain barrier (BBB) and the blood‒brain tumor barrier (BBTB) via passive diffusion or target-specific binding [60]. These delivery systems are designed to weaken the BBB or BBTB through either active ligand delivery or passive diffusion [126]. Although they are effective, they inevitably have some disadvantages, such as a short circulation period and the inability to deliver high drug concentrations. NEs can target tumors in a specific manner because of their intrinsic characteristics. After the tumor is surgically removed, local brain inflammation develops along with the release of inflammatory factors such as interleukin 8 [127,128] and tumor necrosis factor alpha [129], resulting in the migration of NEs to the inflamed brain region. Because the amplification of inflammatory signals of NEs enhances brain tumor targeting, NEs are considered an effective vehicle for targeting inflammation. Xue et al. [122] developed NE-based delivery vehicles targeting glioma by coincubating paclitaxel-encapsulated liposomes with NEs. This drug delivery system recognized postoperative inflammatory signals, unlike conventional NPs that passively target tumor aggregates [130]. Tumor resection causes tissue damage, triggering an inflammatory response that produces inflammatory cytokines. NE-based delivery vehicle complexes are directed from the circulation to brain tissues invaded by cancer cells through these cytokines. In a study, treatment with NE-based delivery vehicles effectively inhibited tumor recurrence but did not significantly inhibit tumor growth in mice with surgically treated glioma. This result indicates that postoperative amplification of inflammatory signals enhances the tumor-targeting ability and therapeutic effectiveness of NE-based delivery vehicles, highlighting the potential of NEs as a targeting vehicle for postoperative drug delivery.
Similar to living cells, biomimetic functional materials with NE components can target NEs to metastatic cancer cells or inflammatory sites [105]. NE membrane-encapsulated NPs have been developed for targeting tumors. These nanocarriers can inhibit tumor growth and metastasis in vitro and in vivo and induce apoptosis of premetastatic tumor cells and CTCs [121]. In addition, NE membrane-bound liposomes have been used for targeted drug delivery in pancreatic cancer [131]. Liposomes coated with NE membranes camouflage NEs and represent a more advantageous form of drug delivery than NPs prepared using live cells. Live cell-based delivery systems require a secondary NEs infusion step, which may damage NEs irreversibly, and the injection of live cells of extracorporeal origin can result in immune rejection and a lower survival rate. In addition, compared with live cells, liposomes can load a larger number of drugs. Although only a few studies have reported the use of lipid-hybrid NE-derived biomimetic functional materials in the treatment of tumors, we speculate that these nanocarriers can effectively penetrate the BBB, improve tumor targeting, deliver more drugs to tumor tissues and reduce adverse events associated with the use of live cells.
3.2.3. Lipid-hybrid dendritic cell-derived biomimetic functional materials
DCs, the sentinels of the immune system, initiate and regulate adaptive immune responses [132]. They are the most functional of all APCs and recognize pathogenesis-associated molecular patterns and danger-associated molecular patterns through pattern recognition receptors. To activate the immune system, DCs internalize and process antigens and present antigen-specific information to T cells [133]. Briefly, when activated by pathogens, DCs phagocytose and process antigens, forming antigenic determinant clusters, which migrate to lymphoid tissues and metastasize. T cells differentiate into regulatory T cells, helper T cells, cytotoxic T lymphocytes and activated cytotoxic T lymphocytes when they receive stimulatory signals in the lymph [133,134].
DC-based immunotherapy has great potential for the treatment of cancer, as DCs are considered a key player in immunotherapy [135,136]. DC membrane-based therapeutic approaches have shown some success in the treatment of tumors. DC membrane-encapsulated NPs can activate antigen-presenting cells, deliver peptide antigens and enable memory T cells to respond to antigens. Ochyl et al. [137] derived DC membrane vesicles (DC-MVs) from preactivated APCs. These DC-MVs efficiently carried antigenic peptides and successfully activated and amplified T cells in vitro by inducing DC maturation. They enhanced the proliferation of cytotoxic T lymphocytes in vivo, leading to the proliferation of more antigen-specific T cells. Several studies have demonstrated that DC membranes carrying tumor antigens can target lymph nodes to enhance immune activation. Liu et al. [138] used DC membrane-coated NPs to target lymph nodes and found that the NPs were highly effective in localizing lymph nodes and triggering acquired immunity. The abovementioned studies suggest that the DC membrane can target lymph nodes and maintain T-cell activation and retention through antigen presentation, thus playing an important role in activating the immune response.
DC membrane-based immunotherapy can be combined with other therapies to produce synergistic antitumor effects. Sun et al. [139] developed intelligent nano-DCs (iDCs) by coating mature DC films with polymer NPs encapsulated with the photosensitizer IR-797. Given that the DC membrane maintains the antigen-presenting function and T-cell-activating capability of native DCs, these iDCs entered lymph nodes and activated T cells. T cells activated by iDCs decreased the expression of heat shock proteins in tumor cells, increasing their heat sensitivity. Tumors are more likely to be ablated thermally with the photosensitizer IR-797 under activated irradiation. In addition, the interaction between dying tumor cells and surviving immune cells can induce immunogenic cell death (ICD), reactivate antitumor immunity and contribute to synergistic antitumor effects. These therapeutic effects can be achieved through drug delivery platforms based on the combination of liposomes and cell-derived biomimetic functional materials. Altogether, DC membrane-encapsulated NPs can effectively target lymph nodes and activate T-cell responses, and liposomes can have good loading capacity for various drugs (both hydrophilic and hydrophobic). Novel nanocarriers developed using DC membrane-encapsulated liposomes can realize the combined application of chemotherapy/phototherapy and immunotherapy or other therapeutic strategies to achieve stronger antitumor effects.
Although lipid-hybrid DC-derived biomimetic functional materials have not yet been reported, they can be considered potential nanocarriers. DC-derived membrane vesicles can be directed to lymph nodes for targeted drug delivery, which has greater potential in the treatment of lymphatic metastasis of tumors. In addition, DC membranes can activate antigen-presenting cells and deliver peptide antigens, enabling the activation and maintenance of antigen-specific T cells, which in combination with immune checkpoint inhibitors can achieve more sensitive and durable antitumor immunity.
3.2.4. Lipid-hybrid T-cell-derived biomimetic functional materials
T cells directly kill tumor and virus-infected cells and coordinate the entire process of elimination [140]. They originate in the bone marrow and differentiate into several types of effector cells as they proliferate and differentiate. They also transform into memory cells, which participate in a second immune response against foreign substances. The T-cell receptor (TCR) is a specific receptor present on the surface of T cells that initiates first transduction signals by specifically recognizing and binding antigenic peptides presented by the major histocompatibility complex (MHC) on the surface of APCs, thereby inducing T-cell activation and performing adaptive immune functions [84]. Given that TCR complexes expressed on T cells can be actively targeted through antigens of tumor origin, T-cell membranes can be used to modify nanocarriers for targeted delivery of drugs in the treatment of cancer. Yaman et al. [141] delivered trametinib to tumors using NPs encapsulated within T-cell membranes. These biocompatible NPs reduced the potential risks of conventional drug carriers. Compared with pristine NPs, the membrane-coated NPs exhibited superior targeting ability, with twice the uptake and concentration of tumor drugs in the in vivo and in vitro models of melanoma, respectively. A major reason for this phenomenon is that TCRs specifically target the gp100 protein.
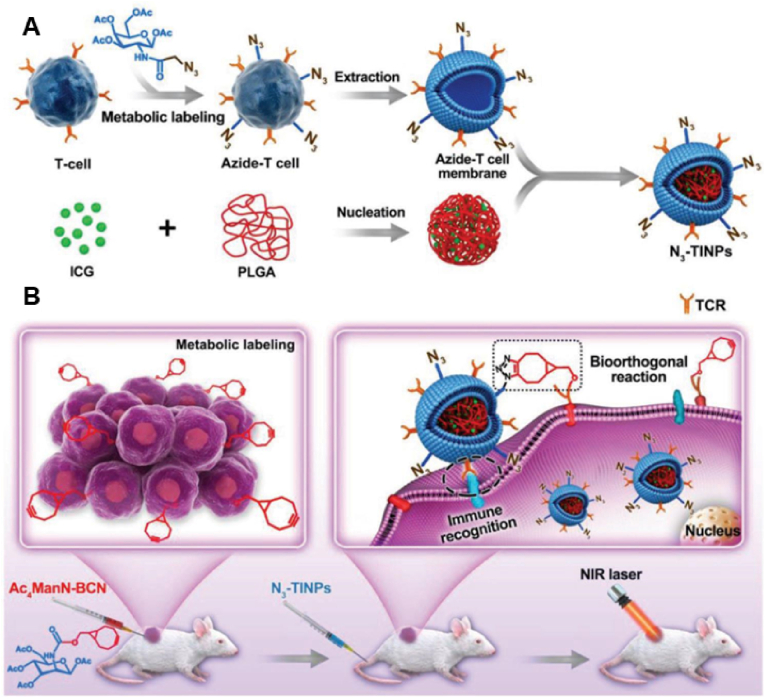
Owing to the presence of TCRs on the cell surface, T-cell membrane-encapsulated NPs are promising drug carriers for targeted treatment of tumors. The high heterogeneity of tumors, however, may reduce the detection capability of the vector when natural and unmodified T-cell membranes (i.e., single targeting strategy) are used. Researchers have reported that fusing complementary molecules with T-cell membranes (a dual-targeting strategy) is a more effective way of delivering nanodrugs [142]. Metabolic glycoengineering techniques can be used to introduce various chemical groups into the cellular glycoconjugate chain through the treatment of nonnatural monosaccharides, which can artificially generate bioorthologous groups on tumors as artificial ‘receptor’ targets for selective targeting in complex environments [143,144]. Targeting strategies developed using the combination of bioorthogonal chemistry and metabolic glycoengineering have received substantial attention in recent years [142]. Researchers have identified a novel bicyclo [6.1.0]nonarginine (BCN)-modified unnatural sugar that can efficiently and nondestructively integrate into the sugar chains on the surface of tumor cells [145]. Through bioorthogonal click chemistry, the BCN motif has been identified as a useful targeting label for azide (N3)-modified T cells that enhance tumor recognition. Han et al. used this technique in combination with T-cell membrane recognition to enhance the tumor-targeting ability of nanocarriers [146] (Fig. 5A). Specifically, activated T-cell membranes were labeled with N3 groups via glycolysis and used to modify NPs loaded with the photosensitizer indocyanine green (ICG). T-cell membrane-encapsulated NPs had improved tumor-targeting ability. The bioorthogonal reaction between N3 and BCN moieties enhanced the anchoring of NPs to tumor cells (Fig. 5B). With dual targeting (immune recognition and bioorthogonal chemical recognition), the accumulation of photosensitizers (PSs) effectively cleared tumor cells through ICG-mediated photothermal effects.
Fig. 5.
Schematic diagram of N3-TINPs with a dual targeting mechanism for efficient photothermal therapy. (A) Synthesis of N3-TINPs. (B) After pretreatment with Ac4ManN-BCN, BCN-expressing T cells were labeled. N3-TINPs targeted tumors through immune recognition of T-cell membranes and bio-orthogonal reaction between BCN and N3 moieties and effectively cleared tumors in mice through ICG-mediated photothermal effects. Reproduced with permission from Ref. [146] (N3: azide; N3-TINPs: N3-labeled T-cell membrane; BCN, l bicyclo [6.1.0] nonyne; Ac4ManN-BCN: BCN-modified unnatural sugars; ICG: indocyanine green.). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Overall, T-cell-derived bionic functional materials are biocompatible and can be used to improve the targeting of tumors through TCRs, thus enabling precise drug delivery. However, it is necessary to develop more precise dosing regimens to improve the effectiveness of therapeutic measures against immune evasion of tumors. Liposomes can be easily modified in various ways to achieve active targeting [147] and have a high drug-loading capacity. Therefore, lipid-hybrid T-cell-derived biomimetic functional materials can precisely deliver drugs owing to their increased accumulation in tumor tissues. Although these hybrid materials have not yet been reported, we speculate that the combination of liposomes and T-cell-derived membrane vesicles can be used for precise treatment of tumors.
3.2.5. Lipid-hybrid NK cell-derived biomimetic functional materials
NK cells are important cells involved in innate immunity [148]. With receptors on the membrane, these cells can recognize abnormal cells, including tumor cells [149]. They participate in immune regulation mainly by killing target cells directly [86], secreting cytokines [150] and contributing to the maturation of antigen-presenting cells [86,151].
NK cell-based immunotherapy holds great promise for clinical application. The role of recruitment and infiltration of NK cells in tumors is currently being investigated to improve treatment [152]. To enhance the direct killing effect, targeted strategies that promote the accumulation of NK cells at the tumor site have been proposed. In a study, dopamine-coated magnetic Fe3O4 NPs were found to be internalized by NK cells through phagocytosis. With the assistance of a magnetic field, NK cells aggregated in the tumor tissue and significantly inhibited the growth of tumor cells [153]. Given that NK cells have the natural ability to target tumor cells, especially those with stem cell characteristics [154], studies have investigated the potential of NK cell membrane-encapsulated NPs for tumor targeting [155]. The natural tumor-targeting ability of NK cells may be attributed to the action of certain proteins on the cell membrane. In a study, DOX liposomes were fused with NK cell membrane vesicles to form a membrane camouflage delivery system called ‘NKsomes’, and the homing ability and antitumor effects of NKsomes were examined in an MCF-7-induced solid tumor model [156]. The results revealed that ‘NKsomes’ were nonimmunogenic and efficiently carried the chemotherapeutic drug DOX for targeted therapy. In addition, the effectiveness of NKsomes in targeting tumor cells relied entirely on the membrane properties of NK cells. Altogether, NKsomes exerted good antitumor effects against MCF-7 human breast cancer cells both in vitro and in vivo.
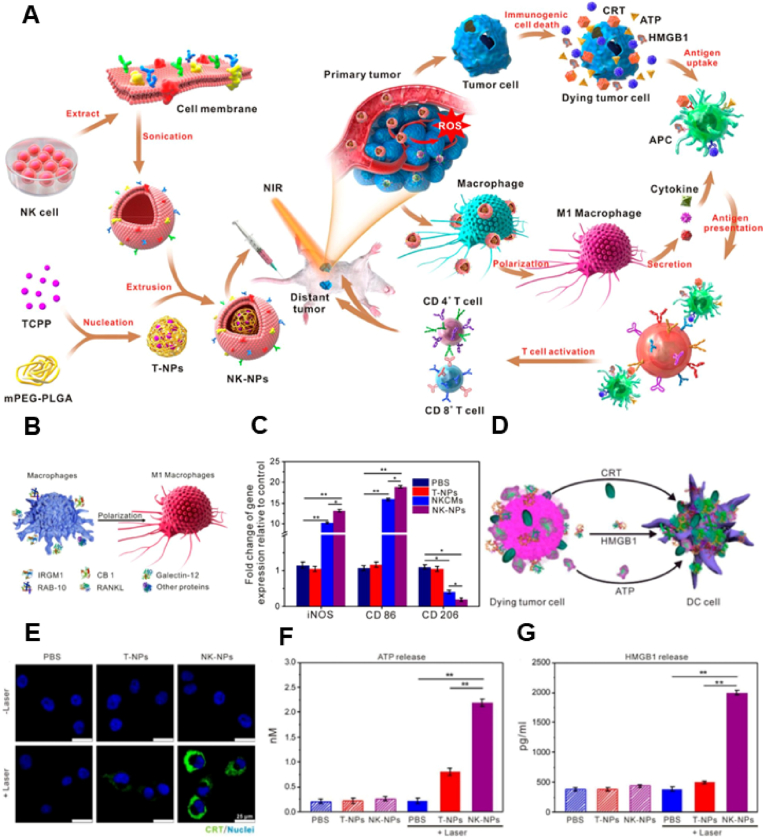
Proteins on NK cell membranes promote the polarization of M1-type macrophages and facilitate tumor targeting [85,157,158]. Deng et al. modified NPs to resemble NK cell membranes and used them for combined phototherapy and immunotherapy, which significantly improved the antitumor effects [86] (Fig. 6A). NK cells can effectively target tumors and improve antitumor immune responses. On the one hand, transformation of TAMs reverses the suppressive tumor immune microenvironment and provides favorable conditions for antitumor immunity (Fig. 6B and C). On the other hand, photodynamic therapy directly kills tumor cells, induces ICD and enhances antigen presentation, thereby augmenting the immune response to tumors (Fig. 6D–G). Consequently, phototherapy and immunotherapy produce synergistic antitumor effects, significantly improving therapeutic efficacy.
Fig. 6.
(A) Schematic diagram of NK cell membrane-encapsulated nanoparticles for PDT-enhanced cell membrane-based immunotherapy. (B) Proteins on the NK cell membrane polarize M1 macrophages. (C) Gene markers associated with macrophage polarization in vitro. (D) Dying tumor cells release immunogenic cell death-related signaling molecules to promote the maturation of dendritic cells. (E–G) Effects of various drugs on the release of CRT, ATP and HMGB-1 (n = 3 per group; single asterisks indicate p < 0.05, and double asterisks indicate p < 0.01). Reproduced with permission from Ref. [86] Copyright © 2018, American Chemical Society.
NK cell-derived membrane vesicles have significant potential for targeting tumors and reversing the phenotype of TAMs. The suppressive tumor immune microenvironment results in a low antitumor immune response, which can be addressed by regulating the polarization of TAMs [159]. Biomimetic functional materials derived from NK cells can induce the conversion of TAMs to the M1 type, which may lead to the transformation of ‘cold tumors’ to ‘hot tumors’. NK cell-derived membrane vesicles can target tumors and reverse the suppressive immune microenvironment. Although lipid-hybrid NK cell-derived biomimetic functional materials have not been extensively investigated, they may play an important role in the treatment of cancer. For example, ICD-inducible chemotherapeutic agents can be loaded in liposomes, and hybridization of liposomes with NK cell membrane vesicles can be used to make lipid-hybrid NK cell-derived biomimetic functional materials. These hybrid nanocarriers can target tumor tissues to induce ICD and activate the antitumor immune response. In addition, NK cell-derived membrane vesicles can reverse the polarization of TAMs, creating a favorable environment for the aggregation, activation and proliferation of immune cells in tumor tissues. Altogether, this combination delivery strategy can target tumor tissues and exert more sensitive tumor-killing effects.
3.3. Lipid-hybrid tumor cell-derived biomimetic functional materials
Cancer cells exhibit numerous characteristics that distinguish them from normal cells, including unlimited proliferation, homotypic adhesion and immune escape [160]. These characteristics have prompted the development of tumor cell-based nanostructures for the treatment of tumors. Lipid-hybrid tumor cell-derived biomimetic functional materials prepared by embedding the membrane of glioma cells in liposomes can cross the BBB and are enriched in cancer cells after crossing the BBB [161]. Tumor cell membranes enhance the active targeting ability of NPs because of homotypic binding between cancer cells and NPs [11]. In a nude mouse model of MCF-7 tumors, the accumulation of tumor cell membrane-modified liposomes at the tumor site was found to be > 5-fold higher than that of unmodified liposomes [162]. Some functional proteins (e.g., CD47) and polysaccharides on tumor cell membranes can promote immune escape and prolong circulation time, which can effectively assist NPs in evading immune recognition in the blood [59]. Xu et al. [59] used tumor cell membranes modified with liposomes loaded with ICG and verified the uptake of NPs by RAW264.7 cells. The results revealed that the uptake rate of ICG liposomes encapsulated in tumor cell membranes was lower than that of unmodified ICG liposomes, suggesting that tumor cell membrane-modified liposomes have a longer circulation time in the blood. Because cancer cell membrane-encapsulated NPs can target homologous cancer cells and have reduced potential for uptake in the circulating blood, they are ideal vehicles for targeted delivery of antitumor drugs [163].
Tumor vaccines stimulate antigen-presenting cells to activate and promote specific killing of tumor cells by the immune system by extracting or synthesizing tumor-associated antigens [132]. Cancer vaccines have been developed using cancer cell membranes as tumor-specific antigens because cytotoxic T lymphocytes typically recognize cancer cells by binding to receptors on their membranes. Kroll et al. [164] demonstrated that tumor cell membranes can function as tumor vaccines, presenting antigens and activating the immune system against tumors. Compared with tumor-specific peptides, proteins, DNA and mRNA, tumor vaccines exploit differences in the expression of different proteins between tumor and normal cells, resulting in higher specificity and relatively fewer side effects [165]. Cell membrane-encapsulated drug-loaded NPs have been developed to enhance antitumor immunity. A study reported that nano-vaccines prepared using tumor cell membranes encapsulated with polymeric NPs containing the immune adjuvant R837 exhibited stronger immune stimulation upon uptake by antigen-presenting cells [166]. Although some functional proteins on tumor cell membranes (e.g., CD47) promote immune escape and prolong the circulation time of NPs, they are not beneficial for the delivery of signaling molecules through antigen-presenting cells to activate T-cell responses, and the inefficiency of the vaccine delivery system hinders effective and long-lasting antitumor immune responses. To address this issue, CRISPR‒Cas9 gene editing technology was used to knockdown tumor phagocytosis checkpoints in an in vitro study [167]. In addition, this strategy was combined with mitoxantrone to induce ICD of tumor cells in vitro, resulting in the activation of a large number of tumor-specific CD8+ T cells to kill tumors.
EVs derived from tumor cells contain tumor antigens and endogenous danger signals. These signals stimulate DC maturation and produce subsequent antitumor immune responses [168]. Tumor cell-derived EVs can deliver antigens directly into the cytoplasm of DCs and effectively activate cytotoxic T lymphocytes to generate antitumor immunity through antigen cross-presentation [169]. However, non-individualized tumor vaccines do not induce immune responses against autologous tumors owing to the high heterogeneity of tumor cells. Zhao et al. [170] developed polymer-modified liposomes containing PSs. These liposomes had a bioactive surface and high structural stability and recruited tumor-associated antigens (TAAs), allowing for the combined application of phototherapy and immunotherapy. Photodynamic therapy (PDT) induces ICD and produces TAAs, and cationic polymers confer the ability to recruit TAAs and enhance antigen presentation through the proton-sponge effect. In this strategy, autoantigens are used to activate the immune system without the risk of a poor immune response owing to the specificity of tumor cells.
Overall, nanostructures based on tumor cell sources hold great promise as novel nanocarriers for drug delivery. They can evade immune surveillance, aggregate in tumor tissues and activate antitumor immunity as tumor antigens. Despite these beneficial characteristics, tumor cell-based nanocarriers have some drawbacks, including low drug-loading capacity, poor stability and loss of immunogenicity as a result of tumor antigen degradation. Lipid-hybrid tumor cell-derived bionic functional materials can significantly overcome the abovementioned challenges, and the combined use of liposomes and tumor cell components can complement their strengths and result in synergistic therapeutic effects [25,73,171]. Tumor cell-derived biomimetic functional materials enhance the circulation time of liposomes in vivo and target tumor sites, whereas liposomes enhance the drug-loading capability, stability and antitumor effects of tumor cell-derived bionic functional materials by carrying drug/immune adjuvants.
3.4. Lipid-hybrid mesenchymal stem cell cell-derived biomimetic functional materials
Mesenchymal stem cells (MSCs) are multipotent tissue stem cells that can differentiate into various cell types, including osteoblasts, adipocytes, chondrocytes and myofibroblasts [172], and are widely used for wound healing [173], chondrogenesis [174] and nerve regeneration [175]. During the past few years, studies have reported a close relationship between MSCs and tumorigenesis [176]. Moreover, tumors are considered ‘unhealable wounds’ because of the similarities between them and healing wounds [177]. Cancer growth and wound healing are associated with processes such as angiogenesis, fibroblast activation and remodeling of the extracellular matrix [178]. MSCs are capable of homing to inflamed or injured tissues [179]. Although the specific underlying mechanisms remain unclear, the tumor-homing ability of MSCs is mainly mediated by the synergistic effects of cytokines, chemokines and other functional molecules [180]. First, cytokines are involved in the adhesion of circulating MSCs to the vascular endothelium. Tumor necrosis factor alpha, IL-6, IL-1β and interferon gamma are involved in this process [[181], [182], [183]]. Second, chemokines can stimulate chemotaxis in cells, causing them to migrate to a target site or tissue [184]. For example, stromal cell-derived factor-1 (SDF1) is highly expressed on the surface of tumor cells [185]. MSCs express CXC chemokine receptor 4 (CXCR4), which is part of the SDF1 receptor family. The CXCR4-SDF1 axis is an important pathway for the migration of MSCs to tumors [89]. Third, other functional molecules, such as growth factors and hypoxia-inducible factor (HIF), contribute to the tumor-homing function of MSCs. Growth factors such as platelet-derived growth factor (PDGF) can affect the migratory ability of MSCs [186], and HIF induces the expression of its cognate receptor CXC chemokine receptor 3 (CXCR3) on MSCs and breast cancer cells [90]. Although the mechanisms underlying MSC migration remain unclear, tumor tropism has been used to develop more specific and effective anticancer therapies [187].
MSCs can influence cancer development in several ways [188]. On one hand, MSCs participate in the development and progression of cancer. They produce high levels of angiogenesis-stimulating growth factors and cytokines, such as VEGF, β-FGF, PDGF, IL-6, IL-8, TGFβ and angiopoietin, which promote tumor angiogenesis and metastasis [189]. On the other hand, MSCs inhibit tumor growth and progression. They have been demonstrated to induce apoptosis and inhibit the proliferation of glioma cells through inhibition of the phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) pathway [190]. In addition, direct contact between MSCs and tumor cells may inhibit tumor growth. A study reported that coculturing tumor stem cells with MSCs significantly reduced the proliferation rate of tumor cells [191].
Owing to their inherent tumor-targeting properties, MSCs can be used to deliver therapeutic agents to tumor sites. However, the precise role of MSCs in tumorigenesis should be intensively investigated to assess whether they pose certain risks, such as the development of metastasis [192]. Compared with MSCs, mesenchymal cell membranes (MSCMs) are considered safer platforms for drug delivery. MSCMs retain the natural targeting ability of MSCs and have low immunogenicity. MSCM-encapsulated NPs have attracted substantial interest owing to their inherent tumor-targeting ability [193]. Yang et al. [194] used MSCMs to modify PLGA NPs. The uptake efficiency of membrane-coated NPs was three times higher than that of pristine NPs. In addition, membrane-coated NPs had stronger tumor-targeting ability, which improved the elimination of tumor cells. Altogether, the study added to the knowledge of MSC-derived nanostructures with significant tumor-targeting ability.
Lipid-hybrid MSC-derived biomimetic functional materials have been reported in previous studies. Sonodynamic therapy is a noninvasive antitumor approach that generates ROS through the simultaneous interaction of acoustic sensitizers, molecular oxygen and low-intensity ultrasound waves, leading to apoptosis and necrosis of cancer cells while minimizing damage to adjacent normal tissues [195]. However, most acoustic sensitizers have drawbacks, including poor solubility in water, low bioavailability and non-tumor specificity. Liposomes can improve the stability and bioavailability of acoustic sensitizers. However, the presence of biological barriers in tumors, such as low oxygen concentration, high interstitial fluid pressure and poor tumor penetration, makes it difficult for liposomes to achieve precise targeted delivery to tumor sites. Sun et al. [196] wrapped MSCMs with liposomes loaded with acoustic sensitizers and oxygenators to form lipid-hybrid MSCM-based biomimetic functional materials and used them for the treatment of oral cancer. In vivo experiments demonstrated that these functional materials selectively accumulated in and penetrated oral tumor tissues in situ, alleviated hypoxia, effectively inhibited tumor growth and prolonged survival time in mice under ultrasonic stimulation. Altogether, the study verified that lipid-hybrid MSC-derived biomimetic functional materials have great potential for targeted drug delivery in the treatment of tumors.
MSC-derived nanostructures have emerged as promising drug delivery vehicles owing to their inherent properties, such as tumor affinity, extended circulation time and low immunogenicity [197]. However, they have low drug-loading efficiency and are more often used for modifying other drug-encapsulated NPs to enhance their targeting ability. The use of liposomes is considered optimal for enhancing the tumor-targeting ability because they have a phospholipid layer similar to biological membranes and are highly compatible with cell-derived membrane vesicles. Therefore, MSC-derived nanostructures can be hybridized with liposomes to form lipid-hybrid MSC-derived biomimetic functional materials that synergistically target tumor tissues for drug delivery, thus enabling precision treatment.
3.5. Lipid-hybrid bacteria-derived biomimetic functional materials
The use of bacteria as therapeutic agents, either alone or in combination with traditional anticancer therapies, has shown promising results in reducing tumor recurrence and inhibiting metastasis [198,199]. Many bacteria, such as Salmonella typhimurium and [200,201] Escherichia coli (E. coli) [202], not only exhibit inherent tumor-targeting activity and antitumor effects but also have the potential to be genetically engineered to target and deliver anticancer drugs [203]. As a noninvasive treatment strategy, high-intensity focused ultrasound (HIFU) ablates tumors and promotes the controlled release of drugs in the treatment of benign and malignant cancers [204]. However, the propagation of ultrasound energy decreases with increasing distance, which results in the poor efficacy of HIFU in the treatment of deep-seated tumors. Researchers have used genetic engineering techniques to develop transgenic E. coli that can carry bubbles. E. coli is a typical parthenogenic anaerobic bacterium that can sufficiently colonize a hypoxic environment and achieve tumor targeting [205]. It can be coloaded with cationic liposomes containing chemotherapeutic agents to achieve targeted drug delivery. This approach has been demonstrated to enhance the therapeutic effects of HIFU and promote the synergistic effects of HIFU and chemotherapy to improve the killing of 4T1 cells and suppress breast cancer progression.
Although bacteriotherapy-based treatment strategies have demonstrated better results in mouse models, these strategies do not trigger tumor regression and induce several adverse effects, especially at higher doses [206,207]. Antitumor activity is not observed because bacteria are effectively internalized by host cells and destroyed through cellular autophagy. Inhibition of autophagy promotes apoptotic activity in cancer cells through bacteria-mediated killing [208]. Hydroxychloroquine (HCQ) is a widely used inhibitor of autophagy that disrupts lysosomal function by increasing lysosomal pH, thereby reducing bacterial degradation [209]. However, the nonspecific distribution of HCQ limits its therapeutic efficacy in cancer cells while potentially damaging normal cells. The EPR effect of liposomes allows tumor-targeted delivery of HCQ through enhanced liposome accumulation [210]. Wang et al. [91] used HCQ-encapsulated liposomes in combination with tumor-targeting Salmonella VNP20009 for antitumor therapy. HCQ-encapsulated liposomes accumulated in tumors through the EPR effect and triggered apoptosis and necrosis of tumor cells, overcoming the autophagic barrier to bacterial treatment. Therefore, modulation of tumor cell autophagy through delivery of autophagy inhibitors may represent a promising approach to enhancing the therapeutic effects of bacteria-based therapies in various cancer types.
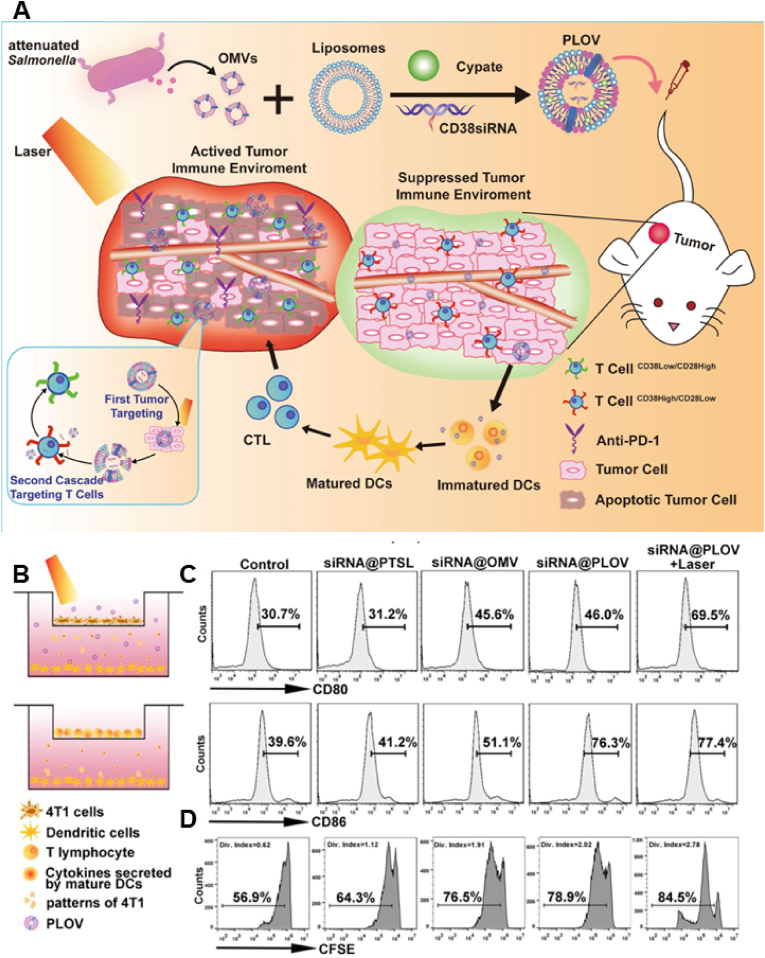
Bacterial therapy has received substantial attention owing to the tumor-targeting ability of tumors. However, clearance of bacteria from tumors after they have been targeted is challenging. In recent years, the focus of bacterial research has shifted to OMVs secreted by gram-negative bacteria. Owing to the presence of numerous pathogen-associated molecular patterns, OMVs can be used as natural immune adjuvants for activating immune responses and inherently have similar tumor-targeting effects [211,212]. Although OMVs inhibit tumor growth, they produce severe inflammatory responses in vivo [213,214]. Park et al. [58] synthesized bacterial vesicles to replace OMVs and minimize their toxic effects. They obtained fragmented bacterial debris through lysozyme-mediated degradation and sonication. In addition, they used the ionic detergent sarkosyl to remove the bacterial inner membrane and induced high-pH conditions to eliminate the cytoplasmic component of the debris. This method resulted in a high yield, with the minimal presence of cytoplasmic components and the absence of RNAs or DNAs. Unlike naturally released OMVs, these bacterial vesicles did not elicit a systemic proinflammatory cytokine response in mice, indicating that they can be used as good drug carriers for the development of novel nano-formulations [215]. However, their low drug-loading capacity, similar to that of EVs, limits their application in drug delivery. The combined use of bacterial outer vesicles and liposomes to form lipid-hybrid cell-derived biomimetic functional materials can retain the biological properties of bacterial vesicles while enhancing their drug-loading capacity [216]. Owing to their tumor-targeting ability and immunogenicity, bacterial vesicles are considered excellent drug delivery vehicles. In addition, when bacterial vesicles are hybridized with liposomes to form lipid-hybrid bacteria-derived biomimetic functional materials, the production of severe systemic immune responses is reduced. Zhai et al. [216] used thermosensitive liposomes encapsulated with the photosensitizer Cypate and CD38 siRNA to develop bacterial-derived biomimetic functional materials (Fig. 7A). These tumor-targeting nanocarriers migrated to the tumor site and destroyed tumor cells through thermal ablation upon laser irradiation. PTT enhanced antigen exposure of tumor cells by inducing ICD, promoted antigen presentation and activated antitumor immunity [217]. In addition, inhibition of CD38 enhanced the role of T cells in the tumor environment (Fig. 7B–D). This therapeutic strategy used the combination of PTT and an improved immune microenvironment to enhance antitumor effects, indicating the advantages of the combined application of liposomes and bacterial vesicles.
Fig. 7.
(A) PLOVs are formed by fusing OMVs and PTSLs. PLOVs promote immune cell maturation and improve the tumor immune microenvironment owing to the self-regulation of OMVs and the thermal effect of PTSLs in response to NIR radiation. Inhibition of CD38 enhances the role of T cells in the tumor microenvironment. (B) The transwell system is illustrated in the schematic diagram. (C) Flow cytometry analyses of CD80 and CD86 expression on DCs. (D) CFSE T-cell proliferation assay. Reproduced with permission from Ref. [216] (PLOVs, lipid-hybrid bacterial-derived biomimetic functional materials).
Overall, OMVs are potential drug carriers because they have inherent tumor-targeting ability and can activate pathogen-associated molecular patterns. However, the severe inflammatory response triggered by OMVs in vivo limits their application. Liposomes have a bilayer structure similar to that of biological membranes. The development of biomimetic functional materials by hybridizing liposomes with bacterial components is a major focus of research on biomimetic nano-vaccines. The combination of liposomes and bacterial components has not been reported to reduce the inflammatory response induced by OMVs. However, given that the combined use of liposomes and tumor cell-derived EVs can reduce the strong toxic response produced by whole tumor lysate vaccines, we speculate that the combined use of liposomes and bacterial EVs may have a better safety profile than individual bacterial OMVs [72]. In addition, binding confers active tumor-targeting ability to liposomes to deliver a large amount of drugs to tumor tissues and reduce their accumulation in non-tumor regions.
4. Applications of lipid-hybrid cell-derived biomimetic functional materials
4.1. Chemotherapy
Chemotherapy is used as both a primary and adjuvant treatment strategy for cancer in clinical practice. However, its efficacy is limited by innate and acquired chemoresistance, which may result in more side effects [218,219]. With the recent development of nanomedicines, targeted therapy has been primarily used to improve the efficacy of chemotherapy and reduce adverse effects [220,221]. Nanocarriers cannot easily penetrate micro-vessel walls in normal tissues because their endothelial gap is dense and structurally intact. In contrast, tumor tissues have abundant blood vessels and wide gaps in the vessel wall, which increases their permeability and extends the retention of nanocarriers [222]. Nano-formulations have higher tumor-targeting properties than conventional drugs. However, their effects have not been verified as satisfactory in some clinical studies [223]. Therefore, developing nano-formulations with enhanced tumor-targeting properties is necessary.
Although the tumor mesenchyme accumulation of nanodrugs is high owing to the EPR effect, they have a lower penetration capacity in tissues compared with small-molecule drugs [224]. The higher fluid pressure in tumor tissues severely hinders the diffusion of NPs in vascular endothelial cells and deep tumor regions and results in inefficient drug delivery owing to the barrier formed by the overproduced extracellular matrix [225]. Cell-derived biomimetic functional materials exhibit biomimetic properties that improve the penetration of nanocarriers into tumor tissues. Tumor cell-derived exosomes exhibit enhanced penetration into the BBB, possibly owing to their ability to downregulate proteins involved in lysosomal biogenesis, thereby increasing the transcytosis of endothelial cells [226]. A study demonstrated that the intratumor delivery efficiency of T-cell membrane-encapsulated NPs was significantly increased after low-dose local irradiation pretreatment owing to the upregulation of ICAM-1 expression in the tumor vasculature [227].
Liposomes are highly efficient drug carriers. Lipid-hybrid cell-derived biomimetic functional materials confer active targeting ability to NPs and reduce their immune clearance during circulation [228]. Liposomes and cell-derived membrane vesicles have been used to develop stimulus-responsive strategies that allow membrane rupture or disruption of covalent bonds triggered by signals from tumor-related factors [229,230]. In a study, conventional polyethylene glycol phospholipids were replaced with light-sensitive lipid-cholesterol-o-nitrobenzyl-polyethylene glycol in long-term circulating liposomes to achieve light-responsive polyethylene glycol nanoparticles and selectively reveal targeting ligands in tumor tissues [231]. In addition, liposomes can be modified with or grafted onto some compounds or peptides to improve their active targeting ability [232,233]. The tumor-targeting peptide cyclic arginine–glycine–aspartate (cRGD) specifically targets the cell attachment receptor integrin αvβ3 and is widely used as a target for the diagnosis and treatment of tumors owing to its overexpression in various tumor cells [234]. Therefore, liposomes can be used to actively target tumor cells by modifying cRGD [235].
Although liposomes and cell-derived biomimetic functional materials can be used for targeted delivery of drugs to tumors, some concerns associated with the use of single drug carriers should be addressed. Because cell-derived biomimetic functional materials have low drug-loading capacity, they cannot deliver sufficient amounts of drugs to tumor tissues and cells. Moreover, the easy clearance of liposomes by the immune system limits their application in drug delivery. The hybridization of liposomes with cell-derived biomimetic functional materials can combine the advantages of both and improve the penetration rate of bionic hybrid NPs in tumor tissues, accelerating the development of nanomedicine in tumor therapy [10]. For example, tumor-derived exosomes can highly express CD47, and the binding of this protein to signal-regulated protein α (Sirpα) acts as a ‘do not eat me’ signal, thus evading the phagocytic effects of the mononuclear phagocyte system (MPS) [236]. Therefore, the fusion of tumor-derived exosomes with liposomes modified with the targeting peptide cRGD reduces the possibility of nanocarrier phagocytosis in circulation and improves the active targeting ability of nanocarriers, eventually allowing more drugs to accumulate at tumor sites. Li et al. [237] prepared triptolide liposomes and fused them with tumor cell-derived exosomes, followed by the adsorption of microRNA-497 onto the surface of the nanocarriers. Tumor cell-derived exosomes and modified cRGD synergistically enhanced the tumor-targeting ability of lipid-hybrid biomimetic functional materials. Furthermore, CD47 on the exosome surface reduces nanocarrier clearance, resulting in increased transport of nanocarriers to tumor sites. Lipid-hybrid biomimetic functional materials loaded with TP and miRNA-497 can synergistically overcome drug resistance by inhibiting the PI3K/AKT/mTOR pathway and regulating the polarization of TAMs. Lai et al. [238] proposed a phototriggered efficient sequential drug delivery method based on lipid-hybrid cell-derived biomimetic functional materials for multimodal combination therapy. First, DOX, a photosensitizer, and lipid components were used to develop drug-encapsulated liposomes using the thin-film hydration method, and lipid-hybrid cell-derived biomimetic functional materials were obtained via coextrusion with erythrocyte membranes. Finally, the antiangiogenic drug sunitinib was encapsulated in the nanocarrier using an active drug delivery method. The nanocarriers could circulate efficiently because the erythrocyte membrane was hybridized with liposomes, which reduced immune clearance and allowed for effective drug delivery. After the nanocarriers reached tumor tissues, the PS was activated via laser irradiation, resulting in the penetration of the nanocarriers into tissues. Consequently, sunitinib was rapidly released and entered endothelial cells to exert antiangiogenic effects. Subsequently, NPs containing DOX entered tumor cells, exerting antitumor effects and inducing immune cell death. DOX-induced immunogenic stimulation exerted synergistic antitumor effects with sunitinib-mediated inhibition of myeloid-derived suppressor cells. Altogether, these lipid-hybrid cell-derived biomimetic functional materials effectively targeted drugs to tumor tissues and stimulated sequential drug release under specific conditions (e.g., laser irradiation) to exert antitumor effects on different targets.
4.2. Radiotherapy
Radiotherapy involves the use of ionizing radiation from high-energy rays to kill cancer cells. It is one of the three major strategies for the treatment of tumors, including surgery and chemotherapy [239,240]. In radiotherapy, high-energy X-rays or gamma rays are used to induce DNA damage or ROS production [241]. However, certain types of tissues or organs can be irreversibly damaged based on the dose and area of exposure [242]. Moreover, the high expression of glutathione (GSH) in the TME limits the efficacy of radiotherapy [243]. Researchers have developed various methods to reduce GSH levels to enhance the efficacy of radiotherapy [244,245]. Among these methods, the conversion of GSH to its oxidized form is commonly used to deplete GSH in the TME [246]. Wu et al. developed MoS2 nanomaterials that effectively oxidized GSH without releasing metal ions that are harmful to humans [247]. Yin et al. used palmitoyl ascorbate micelles to deplete GSH in tumors and improve oxidation-based treatment strategies for cancer [246].
Delivering the maximum dose of GSH-depleting oxides to tumor tissues and cells is difficult owing to the lack of tumor-targeting and immune evasion capabilities. Studies have reported the use of cell-derived biomimetic functional materials for depleting GSH and enhancing the efficacy of radiotherapy. Huang et al. [248] used tumor cell-derived exosomes to encapsulate pyrite (FeS2). The results showed that the introduction of tumor cell-derived exosomes improved the ability of FeS2 to evade immune recognition and target tumors, reduced ROS consumption by depleting GSH and improved the efficacy of radiotherapy.
Although lipid-hybrid cell-derived biomimetic functional materials have not yet been used in combination with radiotherapy, we speculate that these materials have the potential to improve the effects of radiotherapy, which may expand their future applications.
4.3. Phototherapy
At present, the primary treatment strategies for tumors include surgery, chemotherapy and radiotherapy. However, each of these treatment strategies has its drawbacks; for example, surgery cannot completely remove tumor cells from the body, whereas chemotherapy and radiotherapy have side effects [249]. Because phototherapy is simple and highly effective and does not require the use of drugs, it is a viable strategy for treating tumors [250]. The principle of phototherapy is that light energy is converted into chemical or thermal energy by PSs, resulting in a series of chemical reactions in the body that kill tumor cells. With regard to safety, phototherapy is less damaging to normal cells and tissues than chemotherapy and radiotherapy. In PDT, PSs are irradiated with specific wavelengths of light to generate ROS. Currently, PDT is being used to treat cohorts of patients with cancer [[251], [252], [253]]. PTT works by absorbing light energy and converting it into heat, thereby increasing the temperature of the lesion site and killing cancer cells [254]. However, conventional PSs do not exhibit tumor-targeting capability, which limits their therapeutic efficacy. Although the advent of nanotechnology has improved the ability to deliver PSs, challenges such as immune recognition, blood clearance and poor targeting remain unaddressed.
In recent years, researchers have developed various nanocarriers that deliver PSs to tumor tissues and cells through biomimetic materials. Modification of ICG liposomes with tumor cell membranes prevents the immune escape of liposomes and prolongs their circulation time [59]. These lipid-hybrid cell-derived biomimetic functional materials combine the high drug-carrying capacity of liposomes with the immune evasion capability of tumor cell membranes expressing CD47. In addition, lipid-hybrid NE-derived biomimetic functional materials have been reported to deliver PSs effectively to tumor tissues for improved therapeutic effects [255].
Overall, lipid-hybrid cell-derived biomimetic functional materials play an important role in evading immune recognition and actively targeting tumor tissues for more efficient delivery of PSs to tumor tissues and cells, thereby improving the effectiveness of phototherapy and resulting in more precise tumor cell killing. In addition, the combination of PTT and PDT has potential synergistic effects and is more beneficial to tumor cell elimination than the individual use of PTT or PDT. On the one hand, ROS generated by PDT can disrupt the activity of heat shock proteins, thus inhibiting their protective effects on tumor cells during PTT. On the other hand, PTT increases the transport of PSs to tumor tissues and improves blood flow and oxygenation, thereby increasing the therapeutic efficacy of PDT [256].
4.4. Immunotherapy
Immunotherapy has received significant attention in recent years. It involves the use of the immune system of patients to identify and destroy cancer cells. Therefore, it can effectively eradicate tumors and prevent their recurrence with high specificity and minimal toxicity [257,258]. Tumor vaccines use TAAs to trigger the immune system, resulting in an immune response to the tumor, with the eventual goal of activating the autoimmune system to recognize and destroy tumor cells [259]. However, traditional cancer vaccines are not very effective with regard to immunity and treatment outcomes. On the one hand, high tumor heterogeneity and mutation rates make cancer vaccines based on a single TAAs vulnerable to a build-up of immune resistance [164]. On the other hand, although vaccine preparations based on cancer cell lysates have a complete antigenic profile, numerous nonimmunogenic proteins are simultaneously retained, reducing the effectiveness of cancer vaccination [260]. Tumor cell membranes or tumor cell-derived vesicles contain tumor antigens and endogenous danger signals that process DC maturation and trigger immune responses against tumors [25]. Therefore, tumor-derived EVs are an ideal candidate for cell-free immunotherapeutic vaccines that can induce antitumor immunity and prevent tumor growth [261,262]. Kimura et al. [169] demonstrated that tumor cell-derived EVs delivered antigens directly to DCs and effectively activated antitumor immunity through antigen cross-presentation.
The main challenge associated with the use of vaccines designed to induce CD8+ T-cell responses is that CD8+ T-cell activation requires MHC-I delivery of antigens; however, exogenous antigens are usually delivered by MHC-II. The lack of antigen cross-presentation ability makes it difficult for tumor vaccines to activate CD8+ T cells through DCs to promote antitumor immunity [263]. Autophagy promotes the presentation of antigens on MHC-II class molecules while preventing their presentation on MHC-I class molecules [264]. Zhou et al. [33] were the first to report the efficacy of doxycycline in inhibiting autophagy. They prepared lipid-hybrid tumor cell-derived biomimetic functional materials containing doxycycline. These materials inhibited tumor cell autophagy and increased the expression of MHC-1 on the cell surface, which facilitated the recognition and killing of tumor cells by cytotoxic T lymphocytes. Furthermore, lipid-hybrid cell-derived biomimetic functional materials can enhance antitumor immune responses by inducing ICD. Hu et al. [25] prepared lipid-hybrid cell-derived biomimetic functional materials using tumor-derived EVs and liposomes and encapsulated the ICD-inducible drug DOX into nanocarriers (Fig. 8A). These nanocarriers had the following advantages: 1) nanocarriers containing liposomes encapsulated with sufficient amounts of chemotherapeutic agents directly killed tumor cells and induced ICD; 2) tumor cell-derived EVs targeted tumor tissues for drug delivery and stimulated the immune system to generate antitumor immune responses; and 3) the chemotherapeutic drug DOX effectively induced ICD to regulate the immunosuppressed TME and improve the immunogenicity of tumors (Fig. 8B). Altogether, the lipid-hybrid cell-derived biomimetic functional materials effectively enhanced antigen presentation and promoted antitumor immune responses. In addition, they enabled the synergistic antitumor effects of anti-PD-1 antibodies.
Fig. 8.
Schematic representation of DOX@LINV for tumor suppression based on the synergistic effects of immunogenic tumor nanoparticles (TNVs) and DOX-induced ICD effects. (A) Cofusion of TNVs with artificial liposomes to prepare DOX@LINV. (B) Mechanisms of tumor inhibition by DOX@LINVs. Reproduced with permission from Ref. [25] Copyright © 2021, American Chemical Society. (LIPs, liposomes; TNVs, tumor-derived nanovesicles; LINV, lipid hybridization of cell-based primitive biomimetic functional materials.)
Cell-derived biomimetic functional materials can activate immune responses as tumor vaccines or immune adjuvants. Lipid-hybrid cell-derived biomimetic functional materials can combine drug/immune adjuvants and tumor antigens to enhance antitumor immune responses to tumor-based vaccines. In addition, certain immune cell-derived biomimetic functional materials have been found to improve the immune microenvironment of tumors. For example, some proteins present on the NK cell membrane can induce the polarization of M1 macrophages and target tumor cells [86]. Reversing the suppressive tumor immune microenvironment enhances the ability of the immune system to recognize and kill tumor cells. In most cases, this strategy is combined with other therapeutic strategies to improve their efficacy and exert synergistic antitumor effects. Lipid-hybrid cell-derived bionic functional materials can reverse the suppressive tumor immune microenvironment and enhance the sensitivity of tumor cells to antitumor drugs and immune cells by combining multiple therapies, resulting in more efficient killing of tumor cells.
5. Conclusion and outlook
This review summarizes lipid-hybrid cell-derived biomimetic functional materials that have been developed for the treatment of cancer. Cell-derived biomimetic functional materials have good biocompatibility and some specific bioactivities. These special bioactivities facilitate the delivery of drugs to tumor tissues for effective treatment. For example, cell membranes/EVs can be used to develop nanocarriers with prolonged circulation time in vivo and increased aggregation in tumor tissues. OMVs can be functionalized to target tumor tissues and act as immune adjuvants to activate antitumor immunity, but their development is hampered by drawbacks such as the complexity of preparation, low drug-loading capacity and inflexibility to modification. Liposomes can be used for drug delivery owing to their high drug-loading capacity, ease of modification and simple preparation. However, they lack active targeting ability and cannot activate immune activity owing to the lack of endogenous proteins on the surface. In recent years, the combined use of liposomes and cell-derived biomimetic functional materials has received substantial attention. The advantages of liposomal and cell-derived biomimetic functional materials can be inherited by hybrid materials, including their higher drug-loading capacity, flexibility for modification and natural targeting capability. Liposomes have flexible preparation methods. For example, 1-myristoyl-2-stearoyl-sn-glycero-3-phosphocholine can be used as a membrane material for the preparation of temperature-sensitive liposomes using the thin-film hydration method [61]. Different compositions of PEG can improve the stability of liposomes in circulation and their lymphatic drainage efficiency [263,265,266]. Furthermore, the surface of liposomes can be modified with folic acid or peptides such as cRGD to induce active targeting ability [[267], [268], [269]]. Cationic polymers modified on liposome surfaces can enhance liposome recruitment to cell membrane vesicles. Biomimetic functional materials of different cellular origins have different characteristics. For example, RBC membrane-coated NPs have a long circulation time, tumor cell membrane-encapsulated NPs have good homologous targeting ability and promote activation of antitumor immunity, NK cell-derived membrane vesicles can regulate the polarization of TAMs, and OMVs have natural tumor-targeting ability and can activate antitumor immunity. The combination of different liposomes and cell-derived biomimetic functional materials can achieve the superposition of multiple active functions, thereby promoting the targeted delivery of drugs and regulating the TME to achieve antitumor efficacy from multiple perspectives.
Lipid-hybrid cell-derived biomimetic functional materials have been used to treat tumors owing to the following characteristics: 1) Tumor targeting: Because most anticancer drugs have higher toxicity and drug resistance is readily developed, traditional drug therapy cannot effectively kill tumor cells and often leads to several toxic effects. Biomimetic materials drive targeted drug delivery for the treatment of tumors. Biomimetic functional materials derived from cellular components (e.g., tumor and immune cells) have natural tumor-targeting properties, which can effectively improve tumor-targeting ability, enhance antitumor effects and reduce adverse effects. 2) Immune escape: The immune system can recognize and eliminate NPs carrying drugs. CD47 is expressed on RBC membranes, platelet membranes and tumor cell membranes and can bind to SIRPα on phagocytes, thereby inhibiting phagocytosis and reducing the risk of NP clearance by MPS in circulation. 3) Activation of antitumor immunity: Tumor vaccines can activate antigen-presenting cells through specific antigens associated with tumor cells, thus generating an immune response to specifically kill tumor cells, which is characterized by high specificity and fewer side effects [132]. Tumor cell-derived biomimetic functional materials can be used as tumor vaccines to activate antitumor immune responses, resulting in precise tumor cell killing [115,270]. OMVs can serve as immune adjuvants that enhance antitumor effects [58]. 4) High drug-loading capacity: Although cell-derived biomimetic functional materials have natural activities such as tumor targeting, evasion of immune recognition and activation of antitumor immunity, their inherently low drug-loading capacity limits their application in drug delivery strategies. Liposomes are artificial biomimetic membranes with good biocompatibility and flexible drug-loading capability. The combined liposomes with cell-derived biomimetic functional materials can increase drug-loading capacity, resulting in more efficient tumor targeting, immune recognition and antitumor immune responses.
Moreover, combined liposomes with cell-derived biomimetic functional materials can also be applied to other diseases based on the characteristics of both materials. For example, the cargos in liposomes are broken down in the lysosome as a result of endocytosis, which limits the effectiveness of drugs. It was found that cell-derived biomimetic functional materials can fuse directly with cell membranes to release drugs in the cytoplasm while avoiding endocytosis pathways that may affect drug stability [271]. The modification of cell membranes to liposomes attenuates the body's response to liposomes, protecting the body from drug-loaded lysosomes and enabling greater efficacy [66,271,272]. Furthermore, some cell-derived biomimetic functional materials have also been reported to reduce inflammation [273], stimulate cell proliferation and migration [274,275], regulate immunity [276,277], and initiate chondrogenesis [275]. As a result, the application areas for lipid-hybrid cell-derived biomimetic functional materials will be expanded further. Currently, lipid-hybrid cell-derived biomimetic functional materials are also being used in the treatment of other diseases, including inflammation [66], regenerative medicine [171,278,279], pulmonary fibrosis [280], and osteogenesis [281,282].
Overall, the combination of liposomes and cell-derived biomimetic functional materials can leverage their respective advantages to achieve more efficient antitumor effects. With further research on bionanomaterials, lipid-hybrid cell-derived biomimetic functional materials will play an important role in the treatment of tumors. However, the following challenges should be overcome for further development of lipid-hybrid cell-derived biomimetic functional materials: 1) The existing preparation methods for cell-derived biomimetic functional materials are relatively complex, which impedes large-scale preparation and preservation of these materials; 2) In-depth safety assessment and toxicity reduction: Biomimetic functional materials derived from allogeneic cells should be carefully characterized and modified to achieve better safety; 3) Although the preparation methods of liposomes are well developed, the interaction between lipids and cell-derived biomimetic functional materials and the development of bionic materials for improved therapeutic efficacy warrant further investigation; 4) Lipid-hybrid cell-derived biomimetic functional materials should have the potential to be tailored to meet the requirements of different patients and tumor types.
Author contributions
Wen-Shang Liu: Writing – original draft, Resources. Li-li Wu: Investigation, Writing – original draft. Cui-Min Chen: Investigation, Writing – original draft. Hao Zheng: Resources. Jie Gao: Writing – review & editing, Conceptualization, Supervision. Zheng-Mao Lu: Writing – review & editing, Conceptualization. Meng Li: Writing – review & editing, Supervision, Funding acquisition. All authors have approved the final article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (grant number: 82003331), the National Natural Science Foundation of China (grant number: 81903233), the National Natural Science Foundation of China (grant number: 81874250) and the National Natural Science Foundation of China (grant number: 82072051).
Contributor Information
Jie Gao, Email: jmsx2021@shu.edu.cn.
Zheng-Mao Lu, Email: luzhengmao1982@163.com.
Meng Li, Email: lemonlives_dr@163.com.
Abbreviations:
- APCs
antigen-presenting cells
- AuNSs
Au nanoshells
- BBB
blood‒brain barrier
- BBTB
blood‒brain tumor barrier
- CTCs
circulating tumor cells
- CXCR3
CXC chemokine receptor 3
- CXCR4
CXC chemokine receptor 4
- cRGD
cyclic arginine–glycine–aspartate
- DC-MVs
DC membrane vesicles
- DCs
dendritic cells
- DOX
doxorubicin
- EPR
enhanced permeation and retention
- EVs
extracellular vesicles
- HIFU
focused ultrasound
- GSH
glutathione
- MHC
histocompatibility complex
- LINVs
hybrid nanovesicles after fusion
- HCQ
Hydroxychloroquine
- HIF
hypoxia-inducible factor
- ICD
immunogenic cell death
- iDCs
intelligent nano-DCs
- LIPs
liposomes
- MSCMs
mesenchymal cell membranes
- MSCs
Mesenchymal stem cells
- MPS
mononuclear phagocyte system
- NPs
Nanoparticles
- NK cells
natural killer cells
- Nes
neutrophils
- OMVs
outer membrane vesicles
- PI3K/AKT
phosphatidylinositol-3-kinase/protein kinase B
- PDT
Photodynamic therapy
- PSs
photosensitizers
- PDGF
platelet-derived growth factor
- PDI
polydispersity index
- PEG
polyethylene glycol
- P-sel
P-selectin
- FeS2
pyrite
- ROS
reactive oxygen species
- RBCs
Red blood cells
- Sirpα
signal-regulated protein α
- SDS‒PAGE
Sodium dodecyl sulfate‒polyacrylamide gel electrophoresis
- SDF1
stromal cell-derived factor-1
- TCR
T-cell receptor
- TEM
transmission electron microscopy
- TME
tumor microenvironment
- TAAs
tumor-associated antigens
- TNVs
tumor-derived nanovesicles
Data availability
No data was used for the research described in the article.
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Stribbling S.M., Ryan A.J. The cell-line-derived subcutaneous tumor model in preclinical cancer research. Nat. Protoc. 2022;17(9):2108–2128. doi: 10.1038/s41596-022-00709-3. [DOI] [PubMed] [Google Scholar]
- 3.Ryu J.H., Yoon H.Y., Sun I.C., Kwon I.C., Kim K. Tumor-targeting glycol chitosan nanoparticles for cancer heterogeneity. Adv. Mater. 2020;32(51) doi: 10.1002/adma.202002197. [DOI] [PubMed] [Google Scholar]
- 4.Thi T.T.H., Suys E.J.A., Lee J.S., Nguyen D.H., Park K.D., Truong N.P. Lipid-based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines. 2021;9(4) doi: 10.3390/vaccines9040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AlQahtani S.A., Harisa G.I., Badran M.M., AlGhamdi K.M., Kumar A., Salem-Bekhit M.M., Ahmad S.F., Alanazi F.K. Nano-erythrocyte membrane-chaperoned 5-fluorouracil liposomes as biomimetic delivery platforms to target hepatocellular carcinoma cell lines. Artif. Cell Nanomed. Biotechnol. 2019;47(1):989–996. doi: 10.1080/21691401.2019.1577887. [DOI] [PubMed] [Google Scholar]
- 6.Xie X., Hu X., Li Q., Yin M., Song H., Hu J., Wang L., Fan C., Chen N. Unraveling cell-type-specific targeted delivery of membrane-camouflaged nanoparticles with plasmonic imaging. Nano Lett. 2020;20(7):5228–5235. doi: 10.1021/acs.nanolett.0c01503. [DOI] [PubMed] [Google Scholar]
- 7.Prior J.T., Davitt C., Kurtz J., Gellings P., McLachlan J.B., Morici L.A. Bacterial-derived outer membrane vesicles are potent adjuvants that drive humoral and cellular immune responses. Pharmaceutics. 2021;13(2) doi: 10.3390/pharmaceutics13020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trenkenschuh E., Richter M., Heinrich E., Koch M., Fuhrmann G., Friess W. Enhancing the stabilization potential of lyophilization for extracellular vesicles. Advanced healthcare materials. 2022;11(5) doi: 10.1002/adhm.202100538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016;106(Pt A):148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Rayamajhi S., Nguyen T.D.T., Marasini R., Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–494. doi: 10.1016/j.actbio.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 11.Liu L., Pan D., Chen S., Martikainen M.V., Kårlund A., Ke J., Pulkkinen H., Ruhanen H., Roponen M., Käkelä R., Xu W., Wang J., Lehto V.P. Systematic design of cell membrane coating to improve tumor targeting of nanoparticles. Nat. Commun. 2022;13(1):6181. doi: 10.1038/s41467-022-33889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L., Xie H., Li J. Red blood cell membrane-camouflaged gold nanoparticles for treatment of melanoma. Journal of oncology. 2022;2022 doi: 10.1155/2022/3514984. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Tamam H., Park J., Gadalla H.H., Masters A.R., Abdel-Aleem J.A., Abdelrahman S.I., Abdelrahman A.A., Lyle L.T., Yeo Y. Development of liposomal gemcitabine with high drug loading capacity. Mol. Pharm. 2019;16(7):2858–2871. doi: 10.1021/acs.molpharmaceut.8b01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbasi H., Rahbar N., Kouchak M., Khalil Dezfuli P., Handali S. Functionalized liposomes as drug nanocarriers for active targeted cancer therapy: a systematic review. J. Liposome Res. 2022;32(2):195–210. doi: 10.1080/08982104.2021.1903035. [DOI] [PubMed] [Google Scholar]
- 15.Mojarad-Jabali S., Farshbaf M., Walker P.R., Hemmati S., Fatahi Y., Zakeri-Milani P., Sarfraz M., Valizadeh H. An update on actively targeted liposomes in advanced drug delivery to glioma. Int. J. Pharm. 2021;602 doi: 10.1016/j.ijpharm.2021.120645. [DOI] [PubMed] [Google Scholar]
- 16.Piffoux M., Silva A.K.A., Wilhelm C., Gazeau F., Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12(7):6830–6842. doi: 10.1021/acsnano.8b02053. [DOI] [PubMed] [Google Scholar]
- 17.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update. Bioengineering & translational medicine. 2019;4(3) doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu L.M., Ju R.J., Liu R., Bu Y.Z., Zhang J.Y., Li X.Q., Zeng F., Lu W.L. Dual-functional drug liposomes in treatment of resistant cancers. Adv. Drug Deliv. Rev. 2017;115:46–56. doi: 10.1016/j.addr.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Jensen G.M., Hodgson D.F. Opportunities and challenges in commercial pharmaceutical liposome applications. Adv. Drug Deliv. Rev. 2020;154–155:2–12. doi: 10.1016/j.addr.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 20.De Leo V., Maurelli A.M., Giotta L., Catucci L. Liposomes containing nanoparticles: preparation and applications, Colloids and surfaces. B, Biointerfaces. 2022;218 doi: 10.1016/j.colsurfb.2022.112737. [DOI] [PubMed] [Google Scholar]
- 21.Łukawski M., Dałek P., Borowik T., Foryś A., Langner M., Witkiewicz W., Przybyło M. New oral liposomal vitamin C formulation: properties and bioavailability. J. Liposome Res. 2020;30(3):227–234. doi: 10.1080/08982104.2019.1630642. [DOI] [PubMed] [Google Scholar]
- 22.Dos Reis S.B., de Oliveira Silva J., Garcia-Fossa F., Leite E.A., Malachias A., Pound-Lana G., Mosqueira V.C.F., Oliveira M.C., de Barros A.L.B., de Jesus M.B. Mechanistic insights into the intracellular release of doxorubicin from pH-sensitive liposomes. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2021;134 doi: 10.1016/j.biopha.2020.110952. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J., Du J., Wang J., An N., Zhou K., Hu X., Dong Z., Liu Y. Folic acid and poly(ethylene glycol) decorated paclitaxel nanocrystals exhibit enhanced stability and breast cancer-targeting capability. ACS Appl. Mater. Interfaces. 2021;13(12):14577–14586. doi: 10.1021/acsami.1c00184. [DOI] [PubMed] [Google Scholar]
- 24.Mare R., Da H., Fresta M., Cosco D., Awasthi V. Anchoring property of a novel hydrophilic lipopolymer, HDAS-SHP, post-inserted in preformed liposomes. Nanomaterials. 2019;9(9) doi: 10.3390/nano9091185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu M., Zhang J., Kong L., Yu Y., Hu Q., Yang T., Wang Y., Tu K., Qiao Q., Qin X., Zhang Z. Immunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer immunochemotherapy. ACS Nano. 2021;15(2):3123–3138. doi: 10.1021/acsnano.0c09681. [DOI] [PubMed] [Google Scholar]
- 26.Bao P., Zheng Z.T., Ye J.J., Zhang X.Z. Apoptotic body-mediated intracellular delivery strategy for enhanced STING activation and improved tumor immunogenicity. Nano Lett. 2022;22(6):2217–2227. doi: 10.1021/acs.nanolett.1c03996. [DOI] [PubMed] [Google Scholar]
- 27.Liu C., Liu Y.Y., Chang Q., Shu Q., Shen N., Wang H., Xie Y., Deng X. Pressure-controlled encapsulation of graphene quantum dots into liposomes by the reverse-phase evaporation method. Langmuir : the ACS journal of surfaces and colloids. 2021;37(48):14096–14104. doi: 10.1021/acs.langmuir.1c02338. [DOI] [PubMed] [Google Scholar]
- 28.Michelon M., Huang Y., de la Torre L.G., Weitz D.A., Cunha R.L. Single-step microfluidic production of W/O/W double emulsions as templates for β-carotene-loaded giant liposomes formation. Chem. Eng. J. 2019;366:27–32. [Google Scholar]
- 29.Stucki A., Jusková P., Nuti N., Schmitt S., Dittrich P.S. Synchronized reagent delivery in double emulsions for triggering chemical reactions and gene expression. Small Methods. 2021;5(8) doi: 10.1002/smtd.202100331. [DOI] [PubMed] [Google Scholar]
- 30.Liu T., Zhu W., Han C., Sui X., Liu C., Ma X., Dong Y. Preparation of glycyrrhetinic acid liposomes using lyophilization monophase solution method: preformulation, optimization, and in vitro evaluation. Nanoscale Res. Lett. 2018;13(1):324. doi: 10.1186/s11671-018-2737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maritim S., Boulas P., Lin Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021;592 doi: 10.1016/j.ijpharm.2020.120051. [DOI] [PubMed] [Google Scholar]
- 32.Duong T.T., Isomäki A., Paaver U., Laidmäe I., Tõnisoo A., Yen T.T.H., Kogermann K., Raal A., Heinämäki J., Pham T.M. Nanoformulation and evaluation of oral berberine-loaded liposomes. Molecules. 2021;26(9) doi: 10.3390/molecules26092591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F., Gao J., Tang Y., Zou Z., Jiao S., Zhou Z., Xu H., Xu Z.P., Yu H., Xu Z. Engineering chameleon prodrug nanovesicles to increase antigen presentation and inhibit PD-L1 expression for circumventing immune resistance of cancer. Advanced materials (Deerfield Beach, Fla. 2021;33(43) doi: 10.1002/adma.202102668. [DOI] [PubMed] [Google Scholar]
- 34.Odeh F., Nsairat H., Alshaer W., Alsotari S., Buqaien R., Ismail S., Awidi A., Al Bawab A. Remote loading of curcumin-in-modified β-cyclodextrins into liposomes using a transmembrane pH gradient. RSC Adv. 2019;9(64):37148–37161. doi: 10.1039/c9ra07560g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Amin M.D., Bellato F., Mastrotto F., Garofalo M., Malfanti A., Salmaso S., Caliceti P. Dexamethasone loaded liposomes by thin-film hydration and microfluidic procedures: formulation challenges. Int. J. Mol. Sci. 2020;21(5) doi: 10.3390/ijms21051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo-Takahashi Y., Kurokawa R., Sato K., Takizawa N., Katagiri F., Hamano N., Suzuki R., Maruyama K., Nomizu M., Takagi N., Negishi Y. Ternary complexes of pDNA, neuron-binding peptide, and PEGylated polyethyleneimine for brain delivery with nano-bubbles and ultrasound. Pharmaceutics. 2021;13(7) doi: 10.3390/pharmaceutics13071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franzé S., Selmin F., Samaritani E., Minghetti P., Cilurzo F. Lyophilization of liposomal formulations: still necessary, still challenging. Pharmaceutics. 2018;10(3) doi: 10.3390/pharmaceutics10030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain M.T., Forbes N., Perrie Y., Malik K.P., Duru C., Matejtschuk P. Freeze-drying cycle optimization for the rapid preservation of protein-loaded liposomal formulations. Int. J. Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118722. [DOI] [PubMed] [Google Scholar]
- 39.Du G., Sun X. Ethanol injection method for liposome preparation, methods in molecular biology (clifton. N. J. 2023;2622:65–70. doi: 10.1007/978-1-0716-2954-3_5. [DOI] [PubMed] [Google Scholar]
- 40.Wu H., Yu M., Miao Y., He S., Dai Z., Song W., Liu Y., Song S., Ahmad E., Wang D., Gan Y. Cholesterol-tuned liposomal membrane rigidity directs tumor penetration and anti-tumor effect. Acta Pharm. Sin. B. 2019;9(4):858–870. doi: 10.1016/j.apsb.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng B.D., Xiao M.T. Red blood cell membrane nanoparticles for tumor phototherapy. Colloids Surf., B. 2022;220 doi: 10.1016/j.colsurfb.2022.112895. [DOI] [PubMed] [Google Scholar]
- 42.Wang H., Liu Y., He R., Xu D., Zang J., Weeranoppanant N., Dong H., Li Y. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater. Sci. 2020;8(2):552–568. doi: 10.1039/c9bm01392j. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z., Wang F., Liu X., Sang Y., Zhang L., Ren J., Qu X. Cell membrane-camouflaged liposomes for tumor cell-selective glycans engineering and imaging in vivo. Proc. Natl. Acad. Sci. U.S.A. 2021;118(30) doi: 10.1073/pnas.2022769118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang D., Dong H., Li M., Cao Y., Yang F., Zhang K., Dai W., Wang C., Zhang X. Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 2018;12(6):5241–5252. doi: 10.1021/acsnano.7b08355. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L., Zhu Y., Wei X., Chen X., Li Y., Zhu Y., Xia J., Huang Y., Huang Y., Wang J., Pang Z. Nanoplateletsomes restrain metastatic tumor formation through decoy and active targeting in a preclinical mouse model. Acta Pharm. Sin. B. 2022;12(8):3427–3447. doi: 10.1016/j.apsb.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzounakas V.L., Anastasiadi A.T., Dzieciatkowska M., Karadimas D.G., Stamoulis K., Papassideri I.S., Hansen K.C., D'Alessandro A., Kriebardis A.G., Antonelou M.H. Proteome of stored RBC membrane and vesicles from heterozygous beta thalassemia donors. Int. J. Mol. Sci. 2021;22(7) doi: 10.3390/ijms22073369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science (New York, N.Y.) 2020;367(6478) doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter M., Vader P., Fuhrmann G. Approaches to surface engineering of extracellular vesicles. Adv. Drug Deliv. Rev. 2021;173:416–426. doi: 10.1016/j.addr.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Than U.T.T., Guanzon D., Broadbent J.A., Leavesley D.I., Salomon C., Parker T.J. Differential expression of keratinocyte-derived extracellular vesicle mirnas discriminate exosomes from apoptotic bodies and microvesicles. Front. Endocrinol. 2018;9:535. doi: 10.3389/fendo.2018.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chitti S.V., Nedeva C., Manickam R., Fonseka P., Mathivanan S. Extracellular vesicles as drug targets and delivery vehicles for cancer therapy. Pharmaceutics. 2022;14(12) doi: 10.3390/pharmaceutics14122822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tastan B., Tarakcioglu E., Birinci Y., Park Y., Genc S. Role of exosomal MicroRNAs in cell-to-cell communication, methods in molecular biology (clifton. N. J. 2022;2257:269–292. doi: 10.1007/978-1-0716-1170-8_14. [DOI] [PubMed] [Google Scholar]
- 52.Yong T., Zhang X., Bie N., Zhang H., Zhang X., Li F., Hakeem A., Hu J., Gan L., Santos H.A., Yang X. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019;10(1):3838. doi: 10.1038/s41467-019-11718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei F., Li M., Crawford R., Zhou Y., Xiao Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019;86:480–492. doi: 10.1016/j.actbio.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Kim H.Y., Kim T.J., Kang L., Kim Y.J., Kang M.K., Kim J., Ryu J.H., Hyeon T., Yoon B.W., Ko S.B., Kim B.S. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243 doi: 10.1016/j.biomaterials.2020.119942. [DOI] [PubMed] [Google Scholar]
- 55.Lee J.R., Park B.W., Kim J., Choo Y.W., Kim H.Y., Yoon J.K., Kim H., Hwang J.W., Kang M., Kwon S.P., Song S.Y., Ko I.O., Park J.A., Ban K., Hyeon T., Park H.J., Kim B.S. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci. Adv. 2020;6(18) doi: 10.1126/sciadv.aaz0952. eaaz0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wen Y., Fu Q., Soliwoda A., Zhang S., Zheng M., Mao W., Wan Y. Cell-derived nanovesicles prepared by membrane extrusion are good substitutes for natural extracellular vesicles. Extracellular vesicle. 2022;1 doi: 10.1016/j.vesic.2022.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toyofuku M., Nomura N., Eberl L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019;17(1):13–24. doi: 10.1038/s41579-018-0112-2. [DOI] [PubMed] [Google Scholar]
- 58.Park K.S., Svennerholm K., Crescitelli R., Lässer C., Gribonika I., Lötvall J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J. Extracell. Vesicles. 2021;10(9) doi: 10.1002/jev2.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu H.L., Shen B.X., Lin M.T., Tong M.Q., Zheng Y.W., Jiang X., Yang W.G., Yuan J.D., Yao Q., Zhao Y.Z. Homing of ICG-loaded liposome inlaid with tumor cellular membrane to the homologous xenografts glioma eradicates the primary focus and prevents lung metastases through phototherapy. Biomater. Sci. 2018;6(9):2410–2425. doi: 10.1039/c8bm00604k. [DOI] [PubMed] [Google Scholar]
- 60.Zhao Y.Z., Shen B.X., Li X.Z., Tong M.Q., Xue P.P., Chen R., Yao Q., Chen B., Xiao J., Xu H.L. Tumor cellular membrane camouflaged liposomes as a non-invasive vehicle for genes: specific targeting toward homologous gliomas and traversing the blood-brain barrier. Nanoscale. 2020;12(28):15473–15494. doi: 10.1039/d0nr04212a. [DOI] [PubMed] [Google Scholar]
- 61.Cheng L., Zhang X., Tang J., Lv Q., Liu J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120964. [DOI] [PubMed] [Google Scholar]
- 62.Sato Y.T., Umezaki K., Sawada S., Mukai S.A., Sasaki Y., Harada N., Shiku H., Akiyoshi K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016;6 doi: 10.1038/srep21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lőrincz P., Juhász G. Autophagosome-lysosome fusion. J. Mol. Biol. 2020;432(8):2462–2482. doi: 10.1016/j.jmb.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 64.Lancaster C.E., Fountain A., Dayam R.M., Somerville E., Sheth J., Jacobelli V., Somerville A., Terebiznik M.R., Botelho R.J. Phagosome resolution regenerates lysosomes and maintains the degradative capacity in phagocytes. J. Cell Biol. 2021;220(9) doi: 10.1083/jcb.202005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takáts S., Nagy P., Varga Á., Pircs K., Kárpáti M., Varga K., Kovács A.L., Hegedűs K., Juhász G. Autophagosomal Syntaxin17-dependent lysosomal degradation maintains neuronal function in Drosophila. J. Cell Biol. 2013;201(4):531–539. doi: 10.1083/jcb.201211160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farid M., Faber T., Dietrich D., Lamprecht A. Cell membrane fusing liposomes for cytoplasmic delivery in brain endothelial cells. Colloids Surf., B. 2020;194 doi: 10.1016/j.colsurfb.2020.111193. [DOI] [PubMed] [Google Scholar]
- 67.Villa A., Garofalo M., Crescenti D., Rizzi N., Brunialti E., Vingiani A., Belotti P., Sposito C., Franzè S., Cilurzo F., Pruneri G., Recordati C., Giudice C., Giordano A., Tortoreto M., Beretta G., Stefanello D., Manenti G., Zaffaroni N., Mazzaferro V., Ciana P. Transplantation of autologous extracellular vesicles for cancer-specific targeting. Theranostics. 2021;11(5):2034–2047. doi: 10.7150/thno.51344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maheshwari S., Singh A.K., Arya R.K., Pandey D., Singh A., Datta D. Exosomes: emerging players of intercellular communication in tumor microenvironment. Discoveries. 2014;2(3):e26. doi: 10.15190/d.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katsiougiannis S. Extracellular vesicles: evolving contributors in autoimmunity. Forum Immunopathol. Dis. Ther. 2015;6(3–4):163–170. doi: 10.1615/ForumImmunDisTher.2016016491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng Y., Qiu Y., Jiang W., Shen J., Yao X., He X., Li L., Fu B., Liu X. Biological features of extracellular vesicles and challenges. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.816698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Claridge B., Lozano J., Poh Q.H., Greening D.W. Development of extracellular vesicle therapeutics: challenges, considerations, and opportunities. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.734720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bompard J., Rosso A., Brizuela L., Mebarek S., Blum L.J., Trunfio-Sfarghiu A.M., Lollo G., Granjon T., Girard-Egrot A., Maniti O. Membrane fluidity as a new means to selectively target cancer cells with fusogenic lipid carriers. Langmuir : the ACS journal of surfaces and colloids. 2020;36(19):5134–5144. doi: 10.1021/acs.langmuir.0c00262. [DOI] [PubMed] [Google Scholar]
- 73.Ding Y., Wang L., Li H., Miao F., Zhang Z., Hu C., Yu W., Tang Q., Shao G. Application of lipid nanovesicle drug delivery system in cancer immunotherapy. J. Nanobiotechnol. 2022;20(1):214. doi: 10.1186/s12951-022-01429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang K., Zhang Y., Zhou X., Yu Y., Zhu N., Cheng J., Yi Q., Wu Y. Hybrid extracellular vesicles-liposomes camouflaged magnetic vesicles cooperating with bioorthogonal click chemistry for high-efficient melanoma circulating tumor cells enrichment. Advanced healthcare materials. 2023;12(15) doi: 10.1002/adhm.202202825. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Y., Xia Q., Wu T., He Z., Li Y., Li Z., Hou X., He Y., Ruan S., Wang Z., Sun J., Feng N. A novel multi-functionalized multicellular nanodelivery system for non-small cell lung cancer photochemotherapy. J. Nanobiotechnol. 2021;19(1):245. doi: 10.1186/s12951-021-00977-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu Q., Sun W., Wang J., Ruan H., Zhang X., Ye Y., Shen S., Wang C., Lu W., Cheng K., Dotti G., Zeidner J.F., Wang J., Gu Z. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nature biomedical engineering. 2018;2(11):831–840. doi: 10.1038/s41551-018-0310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mammadova-Bach E., Zigrino P., Brucker C., Bourdon C., Freund M., De Arcangelis A., Abrams S.I., Orend G., Gachet C., Mangin P.H. Platelet integrin α6β1 controls lung metastasis through direct binding to cancer cell-derived ADAM9. JCI insight. 2016;1(14) doi: 10.1172/jci.insight.88245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xuan M., Shao J., Dai L., Li J., He Q. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl. Mater. Interfaces. 2016;8(15):9610–9618. doi: 10.1021/acsami.6b00853. [DOI] [PubMed] [Google Scholar]
- 79.Bhattacharyya S., Ghosh S.S. Transmembrane TNFα-expressed macrophage membrane-coated chitosan nanoparticles as cancer therapeutics. ACS Omega. 2020;5(3):1572–1580. doi: 10.1021/acsomega.9b03531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y.Z., ZhuGe D.L., Tong M.Q., Lin M.T., Zheng Y.W., Jiang X., Yang W.G., Yao Q., Xiang Q., Li X.K., Xu H.L. Ulcerative colitis-specific delivery of keratinocyte growth factor by neutrophils-simulated liposomes facilitates the morphologic and functional recovery of the damaged colon through alleviating the inflammation. J. Contr. Release : official journal of the Controlled Release Society. 2019;299:90–106. doi: 10.1016/j.jconrel.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 81.Feng L., Dou C., Xia Y., Li B., Zhao M., Yu P., Zheng Y., El-Toni A.M., Atta N.F., Galal A., Cheng Y., Cai X., Wang Y., Zhang F. Neutrophil-like cell-membrane-coated nanozyme therapy for ischemic brain damage and long-term neurological functional recovery. ACS Nano. 2021;15(2):2263–2280. doi: 10.1021/acsnano.0c07973. [DOI] [PubMed] [Google Scholar]
- 82.Man K., Brunet M.Y., Jones M.C., Cox S.C. Engineered extracellular vesicles: tailored-made nanomaterials for medical applications. Nanomaterials. 2020;10(9) doi: 10.3390/nano10091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greening D.W., Gopal S.K., Xu R., Simpson R.J., Chen W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015;40:72–81. doi: 10.1016/j.semcdb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 84.Greenbaum U., Dumbrava E.I., Biter A.B., Haymaker C.L., Hong D.S. Engineered T-cell receptor T cells for cancer immunotherapy. Cancer immunology research. 2021;9(11):1252–1261. doi: 10.1158/2326-6066.CIR-21-0269. [DOI] [PubMed] [Google Scholar]
- 85.Tian L., Li W., Yang L., Chang N., Fan X., Ji X., Xie J., Yang L., Li L. Cannabinoid receptor 1 participates in liver inflammation by promoting M1 macrophage polarization via RhoA/NF-κB p65 and ERK1/2 pathways, respectively, in mouse liver fibrogenesis. Front. Immunol. 2017;8:1214. doi: 10.3389/fimmu.2017.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng G., Sun Z., Li S., Peng X., Li W., Zhou L., Ma Y., Gong P., Cai L. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12(12):12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 87.Meng X., Wang J., Zhou J., Tian Q., Qie B., Zhou G., Duan W., Zhu Y. Tumor cell membrane-based peptide delivery system targeting the tumor microenvironment for cancer immunotherapy and diagnosis. Acta Biomater. 2021;127:266–275. doi: 10.1016/j.actbio.2021.03.056. [DOI] [PubMed] [Google Scholar]
- 88.Ren X., Yang S., Yu N., Sharjeel A., Jiang Q., Macharia D.K., Yan H., Lu C., Geng P., Chen Z. Cell membrane camouflaged bismuth nanoparticles for targeted photothermal therapy of homotypic tumors. J. Colloid Interface Sci. 2021;591:229–238. doi: 10.1016/j.jcis.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 89.Shi M., Li J., Liao L., Chen B., Li B., Chen L., Jia H., Zhao R.C. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92(7):897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 90.Chaturvedi P., Gilkes D.M., Wong C.C., Luo W., Zhang H., Wei H., Takano N., Schito L., Levchenko A., Semenza G.L. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J. Clin. Invest. 2013;123(1):189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Zhou Z., Chen W., Qin M., Zhang Z., Gong T., Sun X. Potentiating bacterial cancer therapy using hydroxychloroquine liposomes. J. Contr. Release : official journal of the Controlled Release Society. 2018;280:39–50. doi: 10.1016/j.jconrel.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 92.Xia Q., Zhang Y., Li Z., Hou X., Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm. Sin. B. 2019;9(4):675–689. doi: 10.1016/j.apsb.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glassman P.M., Hood E.D., Ferguson L.T., Zhao Z., Siegel D.L., Mitragotri S., Brenner J.S., Muzykantov V.R. Red blood cells: the metamorphosis of a neglected carrier into the natural mothership for artificial nanocarriers. Adv. Drug Deliv. Rev. 2021;178 doi: 10.1016/j.addr.2021.113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li S.D., Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J. Contr. Release : official journal of the Controlled Release Society. 2010;145(3):178–181. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maldiney T., Richard C., Seguin J., Wattier N., Bessodes M., Scherman D. Effect of core diameter, surface coating, and PEG chain length on the biodistribution of persistent luminescence nanoparticles in mice. ACS Nano. 2011;5(2):854–862. doi: 10.1021/nn101937h. [DOI] [PubMed] [Google Scholar]
- 96.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. U.S.A. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richardson K.J., Kuck L., Simmonds M.J. Beyond oxygen transport: active role of erythrocytes in the regulation of blood flow. Am. J. Physiol. Heart Circ. Physiol. 2020;319(4):H866–h872. doi: 10.1152/ajpheart.00441.2020. [DOI] [PubMed] [Google Scholar]
- 98.Wang H., Li J., Wang Y., Gong X., Xu X., Wang J., Li Y., Sha X., Zhang Z. Nanoparticles-mediated reoxygenation strategy relieves tumor hypoxia for enhanced cancer therapy. J. Contr. Release : official journal of the Controlled Release Society. 2020;319:25–45. doi: 10.1016/j.jconrel.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 99.Fang R.H., Kroll A.V., Gao W., Zhang L. Cell membrane coating nanotechnology. Adv. Mater. 2018;30(23) doi: 10.1002/adma.201706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Juhairiyah F., de Lange E.C.M. Understanding drug delivery to the brain using liposome-based strategies: studies that provide mechanistic insights are essential. AAPS J. 2021;23(6):114. doi: 10.1208/s12248-021-00648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vijayan V., Uthaman S., Park I.K. Cell membrane coated nanoparticles: an emerging biomimetic nanoplatform for targeted bioimaging and therapy. Adv. Exp. Med. Biol. 2018;1064:45–59. doi: 10.1007/978-981-13-0445-3_3. [DOI] [PubMed] [Google Scholar]
- 102.Xu H., Niu M., Yuan X., Wu K., Liu A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020;9(1):36. doi: 10.1186/s40164-020-00192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang S., Yuan J., Yang J., Li N., Liu R., Luan J., Ye D. Advancement of platelet-inspired nanomedicine. Platelets. 2018;29(7):690–694. doi: 10.1080/09537104.2018.1475633. [DOI] [PubMed] [Google Scholar]
- 104.Li Z., Hu S., Cheng K. Platelets and their biomimetics for regenerative medicine and cancer therapies. J. Mater. Chem. B. 2018;6(45):7354–7365. doi: 10.1039/C8TB02301H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y., Liu G., Wei J., Nie G. Platelet membrane-based and tumor-associated platelettargeted drug delivery systems for cancer therapy. Front. Med. 2018;12(6):667–677. doi: 10.1007/s11684-017-0583-y. [DOI] [PubMed] [Google Scholar]
- 106.Liu G., Zhao X., Zhang Y., Xu J., Xu J., Li Y., Min H., Shi J., Zhao Y., Wei J., Wang J., Nie G. Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv. Mater. 2019;31(32) doi: 10.1002/adma.201900795. [DOI] [PubMed] [Google Scholar]
- 107.Geng X., Gao D., Hu D., Liu Q., Liu C., Yuan Z., Zhang X., Liu X., Sheng Z., Wang X., Zheng H. Active-targeting NIR-II phototheranostics in multiple tumor models using platelet-camouflaged nanoprobes. ACS Appl. Mater. Interfaces. 2020;12(50):55624–55637. doi: 10.1021/acsami.0c16872. [DOI] [PubMed] [Google Scholar]
- 108.Hu Q., Sun W., Qian C., Wang C., Bomba H.N., Gu Z. Anticancer platelet-mimicking nanovehicles. Advanced materials (Deerfield Beach, Fla. 2015;27(44):7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haemmerle M., Stone R.L., Menter D.G., Afshar-Kharghan V., Sood A.K. The platelet lifeline to cancer: challenges and opportunities. Cancer Cell. 2018;33(6):965–983. doi: 10.1016/j.ccell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu Y., Hu Q., Jiang C., Gu Z. Platelet for drug delivery. Curr. Opin. Biotechnol. 2019;58:81–91. doi: 10.1016/j.copbio.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 111.Xu P., Zuo H., Chen B., Wang R., Ahmed A., Hu Y., Ouyang J. Doxorubicin-loaded platelets as a smart drug delivery system: an improved therapy for lymphoma. Sci. Rep. 2017;7 doi: 10.1038/srep42632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zangabad P.S., Mirkiani S., Shahsavari S., Masoudi B., Masroor M., Hamed H., Jafari Z., Taghipour Y.D., Hashemi H., Karimi M., Hamblin M.R. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnol. Rev. 2018;7(1):95–122. doi: 10.1515/ntrev-2017-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim Y., Oh K.T., Youn Y.S., Lee E.S. pH-sensitive twin liposomes containing quercetin and laccase for tumor therapy. Biomacromolecules. 2022;23(9):3688–3697. doi: 10.1021/acs.biomac.2c00571. [DOI] [PubMed] [Google Scholar]
- 114.Leone R.D., Powell J.D. Metabolism of immune cells in cancer, Nature reviews. Cancer. 2020;20(9):516–531. doi: 10.1038/s41568-020-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oroojalian F., Beygi M., Baradaran B., Mokhtarzadeh A., Shahbazi M.A. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small. 2021;17(12) doi: 10.1002/smll.202006484. [DOI] [PubMed] [Google Scholar]
- 116.He Z., Zhang Y., Feng N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: a review, Materials science & engineering. C, Materials for biological applications. 2020;106 doi: 10.1016/j.msec.2019.110298. [DOI] [PubMed] [Google Scholar]
- 117.Meng Q.F., Rao L., Zan M., Chen M., Yu G.T., Wei X., Wu Z., Sun Y., Guo S.S., Zhao X.Z., Wang F.B., Liu W. Macrophage membrane-coated iron oxide nanoparticles for enhanced photothermal tumor therapy. Nanotechnology. 2018;29(13) doi: 10.1088/1361-6528/aaa7c7. [DOI] [PubMed] [Google Scholar]
- 118.Li Y., Yan T., Chang W., Cao C., Deng D. Fabricating an intelligent cell-like nano-prodrug via hierarchical self-assembly based on the DNA skeleton for suppressing lung metastasis of breast cancer. Biomater. Sci. 2019;7(9):3652–3661. doi: 10.1039/c9bm00630c. [DOI] [PubMed] [Google Scholar]
- 119.Mayadas T.N., Cullere X., Lowell C.A. The multifaceted functions of neutrophils. Annual review of pathology. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao X., Hu Y., Luo S., Wang Y., Gong T., Sun X., Fu Y., Zhang Z. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm. Sin. B. 2019;9(3):575–589. doi: 10.1016/j.apsb.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kang T., Zhu Q., Wei D., Feng J., Yao J., Jiang T., Song Q., Wei X., Chen H., Gao X., Chen J. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11(2):1397–1411. doi: 10.1021/acsnano.6b06477. [DOI] [PubMed] [Google Scholar]
- 122.Xue J., Zhao Z., Zhang L., Xue L., Shen S., Wen Y., Wei Z., Wang L., Kong L., Sun H., Ping Q., Mo R., Zhang C. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017;12(7):692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 123.Liu E.K., Sulman E.P., Wen P.Y., Kurz S.C. Novel therapies for glioblastoma. Curr. Neurol. Neurosci. Rep. 2020;20(7):19. doi: 10.1007/s11910-020-01042-6. [DOI] [PubMed] [Google Scholar]
- 124.Tang L., Feng Y., Gao S., Mu Q., Liu C. Nanotherapeutics overcoming the blood-brain barrier for glioblastoma treatment. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.786700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vengoji R., Ponnusamy M.P., Rachagani S., Mahapatra S., Batra S.K., Shonka N., Macha M.A. Novel therapies hijack the blood-brain barrier to eradicate glioblastoma cancer stem cells. Carcinogenesis. 2019;40(1):2–14. doi: 10.1093/carcin/bgy171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oroojalian F., Charbgoo F., Hashemi M., Amani A., Yazdian-Robati R., Mokhtarzadeh A., Ramezani M., Hamblin M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Contr. Release : official journal of the Controlled Release Society. 2020;321:442–462. doi: 10.1016/j.jconrel.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 127.Salmaggi A., Eoli M., Frigerio S., Silvani A., Gelati M., Corsini E., Broggi G., Boiardi A., Intracavitary V.E.G.F. bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. Journal of neuro-oncology. 2003;62(3):297–303. doi: 10.1023/a:1023367223575. [DOI] [PubMed] [Google Scholar]
- 128.Brat D.J., Bellail A.C., Van Meir E.G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7(2):122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nabors L.B., Suswam E., Huang Y., Yang X., Johnson M.J., King P.H. Tumor necrosis factor alpha induces angiogenic factor up-regulation in malignant glioma cells: a role for RNA stabilization and HuR. Cancer Res. 2003;63(14):4181–4187. [PubMed] [Google Scholar]
- 130.De Filippo K., Dudeck A., Hasenberg M., Nye E., van Rooijen N., Hartmann K., Gunzer M., Roers A., Hogg N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121(24):4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 131.Zhao J., Lu H., Xu D., Sun R., Fang C., Zhao Q., He C., Pan Y., Xu F., Jiang T. Neutrophil membrane-coated nanoparticles for enhanced nanosecond pulsed electric field treatment of pancreatic cancer. Int. J. Hyperther. : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2022;39(1):1026–1035. doi: 10.1080/02656736.2022.2093994. [DOI] [PubMed] [Google Scholar]
- 132.Wculek S.K., Cueto F.J., Mujal A.M., Melero I., Krummel M.F., Sancho D. Dendritic cells in cancer immunology and immunotherapy, Nature reviews. Immunology. 2020;20(1):7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y., Xiang Y., Xin V.W., Wang X.W., Peng X.C., Liu X.Q., Wang D., Li N., Cheng J.T., Lyv Y.N., Cui S.Z., Ma Z., Zhang Q., Xin H.W. Dendritic cell biology and its role in tumor immunotherapy. J. Hematol. Oncol. 2020;13(1):107. doi: 10.1186/s13045-020-00939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fliervoet L.A.L., Mastrobattista E. Drug delivery with living cells. Adv. Drug Deliv. Rev. 2016;106(Pt A):63–72. doi: 10.1016/j.addr.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 135.Saxena M., Balan S., Roudko V., Bhardwaj N. Towards superior dendritic-cell vaccines for cancer therapy. Nature biomedical engineering. 2018;2(6):341–346. doi: 10.1038/s41551-018-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cancel J.C., Crozat K., Dalod M., Mattiuz R. Are conventional type 1 dendritic cells critical for protective antitumor immunity and how? Front. Immunol. 2019;10:9. doi: 10.3389/fimmu.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ochyl L.J., Moon J.J. Dendritic cell membrane vesicles for activation and maintenance of antigen-specific T cells. Advanced healthcare materials. 2019;8(4) doi: 10.1002/adhm.201801091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu W.L., Zou M.Z., Liu T., Zeng J.Y., Li X., Yu W.Y., Li C.X., Ye J.J., Song W., Feng J., Zhang X.Z. Expandable immunotherapeutic nanoplatforms engineered from cytomembranes of hybrid cells derived from cancer and dendritic cells. Advanced materials (Deerfield Beach, Fla. 2019;31(18) doi: 10.1002/adma.201900499. [DOI] [PubMed] [Google Scholar]
- 139.Sun Z., Deng G., Peng X., Xu X., Liu L., Peng J., Ma Y., Zhang P., Wen A., Wang Y., Yang Z., Gong P., Jiang W., Cai L. Intelligent photothermal dendritic cells restart the cancer immunity cycle through enhanced immunogenic cell death. Biomaterials. 2021;279 doi: 10.1016/j.biomaterials.2021.121228. [DOI] [PubMed] [Google Scholar]
- 140.Hui E. Understanding T cell signaling using membrane reconstitution. Immunol. Rev. 2019;291(1):44–56. doi: 10.1111/imr.12767. [DOI] [PubMed] [Google Scholar]
- 141.Yaman S., Ramachandramoorthy H., Oter G., Zhukova D., Nguyen T., Sabnani M.K., Weidanz J.A., Nguyen K.T. Melanoma peptide MHC specific TCR expressing T-cell membrane camouflaged PLGA nanoparticles for treatment of melanoma skin cancer. Front. Bioeng. Biotechnol. 2020;8:943. doi: 10.3389/fbioe.2020.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van der Schans J.J., van de Donk N., Mutis T. Dual targeting to overcome current challenges in multiple myeloma CAR T-cell treatment. Front. Oncol. 2020;10:1362. doi: 10.3389/fonc.2020.01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoon H.Y., Koo H., Kim K., Kwon I.C. Molecular imaging based on metabolic glycoengineering and bioorthogonal click chemistry. Biomaterials. 2017;132:28–36. doi: 10.1016/j.biomaterials.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 144.Layek B., Sadhukha T., Prabha S. Glycoengineered mesenchymal stem cells as an enabling platform for two-step targeting of solid tumors. Biomaterials. 2016;88:97–109. doi: 10.1016/j.biomaterials.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 145.Li W., Pan H., He H., Meng X., Ren Q., Gong P., Jiang X., Liang Z., Liu L., Zheng M., Shao X., Ma Y., Cai L. Bio-orthogonal T cell targeting strategy for robustly enhancing cytotoxicity against tumor cells. Small. 2019;15(4) doi: 10.1002/smll.201804383. [DOI] [PubMed] [Google Scholar]
- 146.Han Y., Pan H., Li W., Chen Z., Ma A., Yin T., Liang R., Chen F., Ma Y., Jin Y., Zheng M., Li B., Cai L. T cell membrane mimicking nanoparticles with bioorthogonal targeting and immune recognition for enhanced photothermal therapy. Adv. Sci. 2019;6(15) doi: 10.1002/advs.201900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kumar P., Huo P., Liu B. Formulation strategies for folate-targeted liposomes and their biomedical applications. Pharmaceutics. 2019;11(8) doi: 10.3390/pharmaceutics11080381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chiossone L., Dumas P.Y., Vienne M., Vivier E. Natural killer cells and other innate lymphoid cells in cancer, Nature reviews. Immunology. 2018;18(11):671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 149.Niehrs A., Garcia-Beltran W.F., Norman P.J., Watson G.M., Hölzemer A., Chapel A., Richert L., Pommerening-Röser A., Körner C., Ozawa M., Martrus G., Rossjohn J., Lee J.H., Berry R., Carrington M., Altfeld M. A subset of HLA-DP molecules serve as ligands for the natural cytotoxicity receptor NKp44. Nat. Immunol. 2019;20(9):1129–1137. doi: 10.1038/s41590-019-0448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Garber K. Natural killer cells blaze into immuno-oncology. Nat. Biotechnol. 2016;34(3):219–220. doi: 10.1038/nbt0316-219. [DOI] [PubMed] [Google Scholar]
- 151.Hu W., Wang G., Huang D., Sui M., Xu Y. Cancer immunotherapy based on natural killer cells: current progress and new opportunities. Front. Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yamasaki K., Horiguchi S., Kurosaki M., Kunii N., Nagato K., Hanaoka H., Shimizu N., Ueno N., Yamamoto S., Taniguchi M., Motohashi S., Nakayama T., Okamoto Y. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clinical immunology (Orlando, Fla. 2011;138(3):255–265. doi: 10.1016/j.clim.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 153.Wu L., Zhang F., Wei Z., Li X., Zhao H., Lv H., Ge R., Ma H., Zhang H., Yang B., Li J., Jiang J. Magnetic delivery of Fe(3)O(4)@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater. Sci. 2018;6(10):2714–2725. doi: 10.1039/c8bm00588e. [DOI] [PubMed] [Google Scholar]
- 154.Ames E., Canter R.J., Grossenbacher S.K., Mac S., Chen M., Smith R.C., Hagino T., Perez-Cunningham J., Sckisel G.D., Urayama S., Monjazeb A.M., Fragoso R.C., Sayers T.J., Murphy W.J. NK cells preferentially target tumor cells with a cancer stem cell phenotype. Journal of immunology (Baltimore, Md. 2015;195(8):4010–4019. doi: 10.4049/jimmunol.1500447. 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fregni G., Perier A., Avril M.F., Caignard A. NK cells sense tumors, course of disease and treatments: consequences for NK-based therapies. OncoImmunology. 2012;1(1):38–47. doi: 10.4161/onci.1.1.18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pitchaimani A., Nguyen T.D.T., Aryal S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials. 2018;160:124–137. doi: 10.1016/j.biomaterials.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 157.Fridman W.H., Pagès F., Sautès-Fridman C., Galon J. The immune contexture in human tumours: impact on clinical outcome, Nature reviews. Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 158.Huang R., Wang X., Zhou Y., Xiao Y. RANKL-induced M1 macrophages are involved in bone formation. Bone research. 2017;5 doi: 10.1038/boneres.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muraoka D., Seo N., Hayashi T., Tahara Y., Fujii K., Tawara I., Miyahara Y., Okamori K., Yagita H., Imoto S., Yamaguchi R., Komura M., Miyano S., Goto M., Sawada S.I., Asai A., Ikeda H., Akiyoshi K., Harada N., Shiku H. Antigen delivery targeted to tumor-associated macrophages overcomes tumor immune resistance. J. Clin. Invest. 2019;129(3):1278–1294. doi: 10.1172/JCI97642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xu C.H., Ye P.J., Zhou Y.C., He D.X., Wei H., Yu C.Y. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020;105:1–14. doi: 10.1016/j.actbio.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 161.Jia Y., Wang X., Hu D., Wang P., Liu Q., Zhang X., Jiang J., Liu X., Sheng Z., Liu B., Zheng H. Phototheranostics: active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano. 2019;13(1):386–398. doi: 10.1021/acsnano.8b06556. [DOI] [PubMed] [Google Scholar]
- 162.Nie D., Dai Z., Li J., Yang Y., Xi Z., Wang J., Zhang W., Qian K., Guo S., Zhu C., Wang R., Li Y., Yu M., Zhang X., Shi X., Gan Y. Cancer-cell-membrane-coated nanoparticles with a yolk-shell structure augment cancer chemotherapy. Nano Lett. 2020;20(2):936–946. doi: 10.1021/acs.nanolett.9b03817. [DOI] [PubMed] [Google Scholar]
- 163.Li S.Y., Cheng H., Qiu W.X., Zhang L., Wan S.S., Zeng J.Y., Zhang X.Z. Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials. 2017;142:149–161. doi: 10.1016/j.biomaterials.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 164.Kroll A.V., Fang R.H., Jiang Y., Zhou J., Wei X., Yu C.L., Gao J., Luk B.T., Dehaini D., Gao W., Zhang L. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv. Mater. 2017;29(47) doi: 10.1002/adma.201703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ye Y., Wang C., Zhang X., Hu Q., Zhang Y., Liu Q., Wen D., Milligan J., Bellotti A., Huang L., Dotti G., Gu Z. A melanin-mediated cancer immunotherapy patch. Science immunology. 2017;2(17) doi: 10.1126/sciimmunol.aan5692. [DOI] [PubMed] [Google Scholar]
- 166.Yang R., Xu J., Xu L., Sun X., Chen Q., Zhao Y., Peng R., Liu Z. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12(6):5121–5129. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 167.Liu S., Wu J., Feng Y., Guo X., Li T., Meng M., Chen J., Chen D., Tian H. CD47KO/CRT dual-bioengineered cell membrane-coated nanovaccine combined with anti-PD-L1 antibody for boosting tumor immunotherapy. Bioact. Mater. 2023;22:211–224. doi: 10.1016/j.bioactmat.2022.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mehanny M., Lehr C.M., Fuhrmann G. Extracellular vesicles as antigen carriers for novel vaccination avenues. Adv. Drug Deliv. Rev. 2021;173:164–180. doi: 10.1016/j.addr.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 169.Kimura S., Maeda K., Nagashima R., Miura K., Arakawa M., Ebina H., Tanaka N., Morita E. Efficient immunogenic peptide antigen delivery to dendritic cells using an ESCRT-mediated extracellular vesicle formation method. Vaccine. 2021;39(22):2976–2982. doi: 10.1016/j.vaccine.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 170.Zhao Y., Chen Z., Li Q., Cao X., Huang Q., Shi L., Liu Y. Polymer‐reinforced liposomes amplify immunogenic cell death‐associated antitumor immunity for photodynamic‐immunotherapy. Adv. Funct. Mater. 2022 [Google Scholar]
- 171.Evers M.J.W., van de Wakker S.I., de Groot E.M., de Jong O.G., Gitz-François J.J.J., Seinen C.S., Sluijter J.P.G., Schiffelers R.M., Vader P. Functional siRNA delivery by extracellular vesicle-liposome hybrid nanoparticles. Advanced healthcare materials. 2022;11(5) doi: 10.1002/adhm.202101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li P., Gong Z., Shultz L.D., Ren G. Mesenchymal stem cells: from regeneration to cancer. Pharmacol. Ther. 2019;200:42–54. doi: 10.1016/j.pharmthera.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Born L.J., Chang K.H., Shoureshi P., Lay F., Bengali S., Hsu A.T.W., Abadchi S.N., Harmon J.W., Jay S.M. HOTAIR-loaded mesenchymal stem/stromal cell extracellular vesicles enhance angiogenesis and wound healing. Advanced healthcare materials. 2022;11(5) doi: 10.1002/adhm.202002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Contentin R., Demoor M., Concari M., Desancé M., Audigié F., Branly T., Galéra P. Comparison of the chondrogenic potential of mesenchymal stem cells derived from bone marrow and umbilical cord blood intended for cartilage tissue engineering. Stem cell reviews and reports. 2020;16(1):126–143. doi: 10.1007/s12015-019-09914-2. [DOI] [PubMed] [Google Scholar]
- 175.Lin Y.J., Lee Y.W., Chang C.W., Huang C.C. 3D spheroids of umbilical cord blood MSC-derived schwann cells promote peripheral nerve regeneration. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.604946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lan T., Luo M., Wei X. Mesenchymal stem/stromal cells in cancer therapy. J. Hematol. Oncol. 2021;14(1):195. doi: 10.1186/s13045-021-01208-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dvorak H.F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 178.Liu T., Zhou L., Li D., Andl T., Zhang Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front. Cell Dev. Biol. 2019;7:60. doi: 10.3389/fcell.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Szydlak R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J. Stem Cell. 2021;13(6):619–631. doi: 10.4252/wjsc.v13.i6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sun Z., Wang S., Zhao R.C. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J. Hematol. Oncol. 2014;7:14. doi: 10.1186/1756-8722-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu S., Ginestier C., Ou S.J., Clouthier S.G., Patel S.H., Monville F., Korkaya H., Heath A., Dutcher J., Kleer C.G., Jung Y., Dontu G., Taichman R., Wicha M.S. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71(2):614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Teo G.S., Ankrum J.A., Martinelli R., Boetto S.E., Simms K., Sciuto T.E., Dvorak A.M., Karp J.M., Carman C.V. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cell. 2012;30(11):2472–2486. doi: 10.1002/stem.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Uchibori R., Tsukahara T., Mizuguchi H., Saga Y., Urabe M., Mizukami H., Kume A., Ozawa K. NF-κB activity regulates mesenchymal stem cell accumulation at tumor sites. Cancer Res. 2013;73(1):364–372. doi: 10.1158/0008-5472.CAN-12-0088. [DOI] [PubMed] [Google Scholar]
- 184.Stone M.J., Hayward J.A., Huang C., Z E.H., Sanchez J. Mechanisms of regulation of the chemokine-receptor network. Int. J. Mol. Sci. 2017;18(2) doi: 10.3390/ijms18020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lau T.T., Wang D.A. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expet Opin. Biol. Ther. 2011;11(2):189–197. doi: 10.1517/14712598.2011.546338. [DOI] [PubMed] [Google Scholar]
- 186.Salha S., Gehmert S., Brébant V., Anker A., Loibl M., Prantl L., Gehmert S. PDGF regulated migration of mesenchymal stem cells towards malignancy acts via the PI3K signaling pathway. Clin. Hemorheol. Microcirc. 2018;70(4):543–551. doi: 10.3233/CH-189319. [DOI] [PubMed] [Google Scholar]
- 187.Dührsen L., Hartfuß S., Hirsch D., Geiger S., Maire C.L., Sedlacik J., Guenther C., Westphal M., Lamszus K., Hermann F.G., Schmidt N.O. Preclinical analysis of human mesenchymal stem cells: tumor tropism and therapeutic efficiency of local HSV-TK suicide gene therapy in glioblastoma. Oncotarget. 2019;10(58):6049–6061. doi: 10.18632/oncotarget.27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Melzer C., Yang Y., Hass R. Interaction of MSC with tumor cells, Cell communication and signaling. CCS. 2016;14(1):20. doi: 10.1186/s12964-016-0143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Timaner M., Tsai K.K., Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin. Cancer Biol. 2020;60:225–237. doi: 10.1016/j.semcancer.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 190.Lu L., Chen G., Yang J., Ma Z., Yang Y., Hu Y., Lu Y., Cao Z., Wang Y., Wang X. Bone marrow mesenchymal stem cells suppress growth and promote the apoptosis of glioma U251 cells through downregulation of the PI3K/AKT signaling pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;112 doi: 10.1016/j.biopha.2019.108625. [DOI] [PubMed] [Google Scholar]
- 191.Bajetto A., Pattarozzi A., Corsaro A., Barbieri F., Daga A., Bosio A., Gatti M., Pisaturo V., Sirito R., Florio T. Different effects of human umbilical cord mesenchymal stem cells on glioblastoma stem cells by direct cell interaction or via released soluble factors. Front. Cell. Neurosci. 2017;11:312. doi: 10.3389/fncel.2017.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yan C., Chang J., Song X., Qi Y., Ji Z., Liu T., Yu W., Wei F., Yang L., Ren X. Lung cancer-associated mesenchymal stem cells promote tumor metastasis and tumorigenesis by induction of epithelial-mesenchymal transition and stem-like reprogram. Aging. 2021;13(7):9780–9800. doi: 10.18632/aging.202732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fan L., Wei A., Gao Z., Mu X. Current progress of mesenchymal stem cell membrane-camouflaged nanoparticles for targeted therapy. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2023;161 doi: 10.1016/j.biopha.2023.114451. [DOI] [PubMed] [Google Scholar]
- 194.Yang N., Ding Y., Zhang Y., Wang B., Zhao X., Cheng K., Huang Y., Taleb M., Zhao J., Dong W.F., Zhang L., Nie G. Surface functionalization of polymeric nanoparticles with umbilical cord-derived mesenchymal stem cell membrane for tumor-targeted therapy. ACS Appl. Mater. Interfaces. 2018;10(27):22963–22973. doi: 10.1021/acsami.8b05363. [DOI] [PubMed] [Google Scholar]
- 195.Zhang L., Yi H., Song J., Huang J., Yang K., Tan B., Wang D., Yang N., Wang Z., Li X. Mitochondria-targeted and ultrasound-activated nanodroplets for enhanced deep-penetration sonodynamic cancer therapy. ACS Appl. Mater. Interfaces. 2019;11(9):9355–9366. doi: 10.1021/acsami.8b21968. [DOI] [PubMed] [Google Scholar]
- 196.Sun L., Xu Y., Zhang X., Gao Y., Chen J., Zhou A., Lu Q., Wang Z., Shao K., Wu H., Ning X. Mesenchymal stem cells functionalized sonodynamic treatment for improving therapeutic efficacy and compliance of orthotopic oral cancer. Advanced materials (Deerfield Beach, Fla. 2020;32(48) doi: 10.1002/adma.202005295. [DOI] [PubMed] [Google Scholar]
- 197.Wu H.H., Zhou Y., Tabata Y., Gao J.Q. Mesenchymal stem cell-based drug delivery strategy: from cells to biomimetic. J. Contr. Release : official journal of the Controlled Release Society. 2019;294:102–113. doi: 10.1016/j.jconrel.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 198.Wang Y., Tang Y., Du Y., Lin L., Zhang Z., Ou X., Chen S., Wang Q., Zou J. Genetically engineered bacteria-mediated multi-functional nanoparticles for synergistic tumor-targeting therapy. Acta Biomater. 2022;150:337–352. doi: 10.1016/j.actbio.2022.07.056. [DOI] [PubMed] [Google Scholar]
- 199.Zhou S., Gravekamp C., Bermudes D., Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer. 2018;18(12):727–743. doi: 10.1038/s41568-018-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guo Y., Chen Y., Liu X., Min J.J., Tan W., Zheng J.H. Targeted cancer immunotherapy with genetically engineered oncolytic Salmonella typhimurium. Cancer Lett. 2020;469:102–110. doi: 10.1016/j.canlet.2019.10.033. [DOI] [PubMed] [Google Scholar]
- 201.Chen W., Wang Y., Qin M., Zhang X., Zhang Z., Sun X., Gu Z. Bacteria-driven hypoxia targeting for combined biotherapy and photothermal therapy. ACS Nano. 2018;12(6):5995–6005. doi: 10.1021/acsnano.8b02235. [DOI] [PubMed] [Google Scholar]
- 202.Zheng D.W., Chen Y., Li Z.H., Xu L., Li C.X., Li B., Fan J.X., Cheng S.X., Zhang X.Z. Optically-controlled bacterial metabolite for cancer therapy. Nat. Commun. 2018;9(1):1680. doi: 10.1038/s41467-018-03233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Du Y., Lin L., Zhang Z., Tang Y., Ou X., Wang Y., Zou J. Drug-loaded nanoparticles conjugated with genetically engineered bacteria for cancer therapy. Biochem. Biophys. Res. Commun. 2022;606:29–34. doi: 10.1016/j.bbrc.2022.03.049. [DOI] [PubMed] [Google Scholar]
- 204.Mai X., Chang Y., You Y., He L., Chen T. Designing intelligent nano-bomb with on-demand site-specific drug burst release to synergize with high-intensity focused ultrasound cancer ablation. J. Contr. Release : official journal of the Controlled Release Society. 2021;331:270–281. doi: 10.1016/j.jconrel.2020.09.051. [DOI] [PubMed] [Google Scholar]
- 205.Yu X., Lin C., Yu J., Qi Q., Wang Q. Bioengineered Escherichia coli Nissle 1917 for tumour-targeting therapy. Microb. Biotechnol. 2020;13(3):629–636. doi: 10.1111/1751-7915.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Toso J.F., Gill V.J., Hwu P., Marincola F.M., Restifo N.P., Schwartzentruber D.J., Sherry R.M., Topalian S.L., Yang J.C., Stock F., Freezer L.J., Morton K.E., Seipp C., Haworth L., Mavroukakis S., White D., MacDonald S., Mao J., Sznol M., Rosenberg S.A. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 2002;20(1):142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nemunaitis J., Cunningham C., Senzer N., Kuhn J., Cramm J., Litz C., Cavagnolo R., Cahill A., Clairmont C., Sznol M. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10(10):737–744. doi: 10.1038/sj.cgt.7700634. [DOI] [PubMed] [Google Scholar]
- 208.Nakano K., Masui T., Yogo A., Uchida Y., Sato A., Kasai Y., Nagai K., Anazawa T., Kawaguchi Y., Uemoto S. Chloroquine induces apoptosis in pancreatic neuroendocrine neoplasms via endoplasmic reticulum stress. Endocr. Relat. Cancer. 2020;27(7):431–439. doi: 10.1530/ERC-20-0028. [DOI] [PubMed] [Google Scholar]
- 209.Gies V., Bekaddour N., Dieudonné Y., Guffroy A., Frenger Q., Gros F., Rodero M.P., Herbeuval J.P., Korganow A.S. Beyond anti-viral effects of chloroquine/hydroxychloroquine. Front. Immunol. 2020;11:1409. doi: 10.3389/fimmu.2020.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Maruyama K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011;63(3):161–169. doi: 10.1016/j.addr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 211.Fitzgerald K.A., Kagan J.C. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gerritzen M.J.H., Martens D.E., Wijffels R.H., van der Pol L., Stork M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017;35(5):565–574. doi: 10.1016/j.biotechadv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 213.Go G., Lee J., Choi D.S., Kim S.S., Gho Y.S. Extracellular vesicle-mimetic ghost nanovesicles for delivering anti-inflammatory drugs to mitigate gram-negative bacterial outer membrane vesicle-induced systemic inflammatory response syndrome. Advanced healthcare materials. 2019;8(4) doi: 10.1002/adhm.201801082. [DOI] [PubMed] [Google Scholar]
- 214.Svennerholm K., Park K.S., Wikström J., Lässer C., Crescitelli R., Shelke G.V., Jang S.C., Suzuki S., Bandeira E., Olofsson C.S., Lötvall J. Escherichia coli outer membrane vesicles can contribute to sepsis induced cardiac dysfunction. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-16363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu H., Zhang Q., Wang S., Weng W., Jing Y., Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: advances and perspectives. Bioact. Mater. 2022;14:169–181. doi: 10.1016/j.bioactmat.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhai Y., Ma Y., Pang B., Zhang J., Li Y., Rui Y., Xu T., Zhao Y., Qian Z., Gu Y., Li S. A cascade targeting strategy based on modified bacterial vesicles for enhancing cancer immunotherapy. J. Nanobiotechnol. 2021;19(1):434. doi: 10.1186/s12951-021-01193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen Q., Hu Q., Dukhovlinova E., Chen G., Ahn S., Wang C., Ogunnaike E.A., Ligler F.S., Dotti G., Gu Z. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Advanced materials (Deerfield Beach, Fla. 2019;31(23) doi: 10.1002/adma.201900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Crunkhorn S. Overcoming chemoresistance, Nature reviews. Drug discovery. 2019;18(8):584. doi: 10.1038/d41573-019-00109-5. [DOI] [PubMed] [Google Scholar]
- 219.Lohan-Codeço M., Barambo-Wagner M.L., Nasciutti L.E., Ribeiro Pinto L.F., Meireles Da Costa N., Palumbo A., Jr. Molecular mechanisms associated with chemoresistance in esophageal cancer. Cell. Mol. Life Sci. : CM. 2022;79(2):116. doi: 10.1007/s00018-022-04131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Emmings E., Mullany S., Chang Z., Landen C.N., Jr., Linder S., Bazzaro M. Targeting mitochondria for treatment of chemoresistant ovarian cancer. Int. J. Mol. Sci. 2019;20(1) doi: 10.3390/ijms20010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Si Y., Chen K., Ngo H.G., Guan J.S., Totoro A., Zhou Z., Kim S., Kim T., Zhou L., Liu X. Targeted EV to deliver chemotherapy to treat triple-negative breast cancers. Pharmaceutics. 2022;14(1) doi: 10.3390/pharmaceutics14010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Park J., Choi Y., Chang H., Um W., Ryu J.H., Kwon I.C. Alliance with EPR effect: combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics. 2019;9(26):8073–8090. doi: 10.7150/thno.37198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Petersen G.H., Alzghari S.K., Chee W., Sankari S.S., La-Beck N.M. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Contr. Release : official journal of the Controlled Release Society. 2016;232:255–264. doi: 10.1016/j.jconrel.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 224.Zhao Z., Ukidve A., Kim J., Mitragotri S. Targeting strategies for tissue-specific drug delivery. Cell. 2020;181(1):151–167. doi: 10.1016/j.cell.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 225.He B., Sui X., Yu B., Wang S., Shen Y., Cong H. Recent advances in drug delivery systems for enhancing drug penetration into tumors. Drug Deliv. 2020;27(1):1474–1490. doi: 10.1080/10717544.2020.1831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Morad G., Carman C.V., Hagedorn E.J., Perlin J.R., Zon L.I., Mustafaoglu N., Park T.E., Ingber D.E., Daisy C.C., Moses M.A. Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano. 2019;13(12):13853–13865. doi: 10.1021/acsnano.9b04397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhang L., Li R., Chen H., Wei J., Qian H., Su S., Shao J., Wang L., Qian X., Liu B. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer. Int. J. Nanomed. 2017;12:2129–2142. doi: 10.2147/IJN.S126016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li Y., Wu Y., Federzoni E.A., Wang X., Dharmawan A., Hu X., Wang H., Hawley R.J., Stevens S., Sykes M., Yang Y.G. CD47 cross-dressing by extracellular vesicles expressing CD47 inhibits phagocytosis without transmitting cell death signals. Elife. 2022;11 doi: 10.7554/eLife.73677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Naziris N., Saitta F., Chrysostomou V., Libera M., Trzebicka B., Fessas D., Pispas S., Demetzos C. pH-responsive chimeric liposomes: from nanotechnology to biological assessment. Int. J. Pharm. 2020;574 doi: 10.1016/j.ijpharm.2019.118849. [DOI] [PubMed] [Google Scholar]
- 230.Darwish W.M., Bayoumi N.A., El-Kolaly M.T. Laser-responsive liposome for selective tumor targeting of nitazoxanide nanoparticles. Eur. J. Pharmaceut. Sci. : official journal of the European Federation for Pharmaceutical Sciences. 2018;111:526–533. doi: 10.1016/j.ejps.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 231.Kong L., Chen Q., Campbell F., Snaar-Jagalska E., Kros A. Light-triggered cancer cell specific targeting and liposomal drug delivery in a zebrafish xenograft model. Advanced healthcare materials. 2020;9(6) doi: 10.1002/adhm.201901489. [DOI] [PubMed] [Google Scholar]
- 232.Tie Y., Zheng H., He Z., Yang J., Shao B., Liu L., Luo M., Yuan X., Liu Y., Zhang X., Li H., Wu M., Wei X. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct. Targeted Ther. 2020;5(1):6. doi: 10.1038/s41392-020-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.d'Avanzo N., Torrieri G., Figueiredo P., Celia C., Paolino D., Correia A., Moslova K., Teesalu T., Fresta M., Santos H.A. LinTT1 peptide-functionalized liposomes for targeted breast cancer therapy. Int. J. Pharm. 2021;597 doi: 10.1016/j.ijpharm.2021.120346. [DOI] [PubMed] [Google Scholar]
- 234.Song Y., Guo X., Fu J., He B., Wang X., Dai W., Zhang H., Zhang Q. Dual-targeting nanovesicles enhance specificity to dynamic tumor cells in vitro and in vivo via manipulation of αvβ3-ligand binding. Acta Pharm. Sin. B. 2020;10(11):2183–2197. doi: 10.1016/j.apsb.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.AlSawaftah N.M., Paul V., Kosaji D., Khabbaz L., Awad N.S., Husseini G.A. Ultrasound-sensitive cRGD-modified liposomes as a novel drug delivery system, Artificial cells. nanomedicine, and biotechnology. 2022;50(1):111–120. doi: 10.1080/21691401.2022.2074439. [DOI] [PubMed] [Google Scholar]
- 236.Pan S., Zhang Y., Huang M., Deng Z., Zhang A., Pei L., Wang L., Zhao W., Ma L., Zhang Q., Cui D. Urinary exosomes-based engineered nanovectors for homologously targeted chemo-chemodynamic prostate cancer therapy via abrogating EGFR/AKT/NF-kB/IkB signaling. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120946. [DOI] [PubMed] [Google Scholar]
- 237.Li L., He D., Guo Q., Zhang Z., Ru D., Wang L., Gong K., Liu F., Duan Y., Li H. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J. Nanobiotechnol. 2022;20(1):50. doi: 10.1186/s12951-022-01264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lai X., Liu X.L., Pan H., Zhu M.H., Long M., Yuan Y., Zhang Z., Dong X., Lu Q., Sun P., Lovell J.F., Chen H.Z., Fang C. Light-Triggered Efficient Sequential Drug Delivery of Biomimetic Nanosystem for Multimodal Chemo-, Antiangiogenic, and Anti-MDSC Therapy in Melanoma, Advanced materials (Deerfield Beach, Fla. 2022;34(10) doi: 10.1002/adma.202106682. [DOI] [PubMed] [Google Scholar]
- 239.Guckenberger M., Saur G., Wehner D., Sweeney R.A., Thalheimer A., Germer C.T., Flentje M. Comparison of preoperative short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer, Strahlentherapie und Onkologie. Organ der Deutschen Rontgengesellschaft ... [et al. 2012;188(7):551–557. doi: 10.1007/s00066-012-0131-2. [DOI] [PubMed] [Google Scholar]
- 240.Deshmukh A.A., Shirvani S.M., Lal L., Swint J.M., Cantor S.B., Smith B.D., Likhacheva A. Cost-effectiveness analysis comparing conventional, hypofractionated, and intraoperative radiotherapy for early-stage breast cancer. J. Natl. Cancer Inst. 2017;109(11) doi: 10.1093/jnci/djx068. [DOI] [PubMed] [Google Scholar]
- 241.Song G., Cheng L., Chao Y., Yang K., Liu Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv. Mater. 2017;29(32) doi: 10.1002/adma.201700996. [DOI] [PubMed] [Google Scholar]
- 242.Wang K.X., Cui W.W., Yang X., Tao A.B., Lan T., Li T.S., Luo L. Mesenchymal stem cells for mitigating radiotherapy side effects. Cells. 2021;10(2) doi: 10.3390/cells10020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lyu M., Zhu D., Kong X., Yang Y., Ding S., Zhou Y., Quan H., Duo Y., Bao Z. Glutathione-depleting nanoenzyme and glucose oxidase combination for hypoxia modulation and radiotherapy enhancement. Advanced healthcare materials. 2020;9(11) doi: 10.1002/adhm.201901819. [DOI] [PubMed] [Google Scholar]
- 244.Huang C., Ding S., Jiang W., Wang F.B. Glutathione-depleting nanoplatelets for enhanced sonodynamic cancer therapy. Nanoscale. 2021;13(8):4512–4518. doi: 10.1039/d0nr08440a. [DOI] [PubMed] [Google Scholar]
- 245.Wu H., Liu L., Song L., Ma M., Gu N., Zhang Y. Enhanced tumor synergistic therapy by injectable magnetic hydrogel mediated generation of hyperthermia and highly toxic reactive oxygen species. ACS Nano. 2019;13(12):14013–14023. doi: 10.1021/acsnano.9b06134. [DOI] [PubMed] [Google Scholar]
- 246.Yin W., Li J., Ke W., Zha Z., Ge Z. Integrated nanoparticles to synergistically elevate tumor oxidative stress and suppress antioxidative capability for amplified oxidation therapy. ACS Appl. Mater. Interfaces. 2017;9(35):29538–29546. doi: 10.1021/acsami.7b08347. [DOI] [PubMed] [Google Scholar]
- 247.Wu S., Liu X., Ren J., Qu X. Glutathione depletion in a benign manner by MoS(2) -based nanoflowers for enhanced hypoxia-irrelevant free-radical-based cancer therapy. Small. 2019;15(51) doi: 10.1002/smll.201904870. [DOI] [PubMed] [Google Scholar]
- 248.Huang C., Liu Z., Chen M., Du L., Liu C., Wang S., Zheng Y., Liu W. Tumor-derived biomimetic nanozyme with immune evasion ability for synergistically enhanced low dose radiotherapy. J. Nanobiotechnol. 2021;19(1):457. doi: 10.1186/s12951-021-01182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chin V., Nagrial A., Sjoquist K., O'Connor C.A., Chantrill L., Biankin A.V., Scholten R.J., Yip D. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst. Rev. 2018;3(3) doi: 10.1002/14651858.CD011044.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yang S., Chen C., Qiu Y., Xu C., Yao J. Paying attention to tumor blood vessels: cancer phototherapy assisted with nano delivery strategies. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120562. [DOI] [PubMed] [Google Scholar]
- 251.Deng Y., Wang X., Liu Y., Xu Y., Zhang J., Huang F., Li B., Miao Y., Sun Y., Li Y. Dual-light triggered metabolizable nano-micelles for selective tumor-targeted photodynamic/hyperthermia therapy. Acta Biomater. 2021;119:323–336. doi: 10.1016/j.actbio.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 252.Li X., Lovell J.F., Yoon J., Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020;17(11):657–674. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 253.Mahvi D.A., Liu R., Grinstaff M.W., Colson Y.L., Raut C.P. Local cancer recurrence: the realities, challenges, and opportunities for new therapies. CA A Cancer J. Clin. 2018;68(6):488–505. doi: 10.3322/caac.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Doughty A.C.V., Hoover A.R., Layton E., Murray C.K., Howard E.W., Chen W.R. Nanomaterial applications in photothermal therapy for cancer. Materials. 2019;12(5) doi: 10.3390/ma12050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Xiong X., Li J., Gao D., Sheng Z., Zheng H., Liu W. Cell-membrane biomimetic indocyanine green liposomes for phototheranostics of echinococcosis. Biosensors. 2022;12(5) doi: 10.3390/bios12050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liu J., Shi J., Nie W., Wang S., Liu G., Cai K. Recent progress in the development of multifunctional nanoplatform for precise tumor phototherapy. Advanced healthcare materials. 2021;10(1) doi: 10.1002/adhm.202001207. [DOI] [PubMed] [Google Scholar]
- 257.Liu H., Pan C., Song W., Liu D., Li Z., Zheng L. Novel strategies for immuno-oncology breakthroughs with cell therapy. Biomarker research. 2021;9(1):62. doi: 10.1186/s40364-021-00316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Xu W., Atkinson V.G., Menzies A.M. vol. 127. European journal of cancer; Oxford, England: 2020. pp. 1–11. (Intratumoural Immunotherapies in Oncology). 1990. [DOI] [PubMed] [Google Scholar]
- 259.Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J. Clin. Invest. 2015;125(9):3335–3337. doi: 10.1172/JCI83871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lokhov P.G., Balashova E.E. Cellular cancer vaccines: an update on the development of vaccines generated from cell surface antigens. J. Cancer. 2010;1:230–241. doi: 10.7150/jca.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhang H., Tang K., Zhang Y., Ma R., Ma J., Li Y., Luo S., Liang X., Ji T., Gu Z., Lu J., He W., Cao X., Wan Y., Huang B. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer immunology research. 2015;3(2):196–205. doi: 10.1158/2326-6066.CIR-14-0177. [DOI] [PubMed] [Google Scholar]
- 262.Wolfers J., Lozier A., Raposo G., Regnault A., Théry C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T., Angevin E., Amigorena S., Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 263.Jiang H., Wang Q., Sun X. Lymph node targeting strategies to improve vaccination efficacy. J. Contr. Release : official journal of the Controlled Release Society. 2017;267:47–56. doi: 10.1016/j.jconrel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 264.Germic N., Frangez Z., Yousefi S., Simon H.U. Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019;26(4):715–727. doi: 10.1038/s41418-019-0297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nunes S.S., Fernandes R.S., Cavalcante C.H., da Costa César I., Leite E.A., Lopes S.C.A., Ferretti A., Rubello D., Townsend D.M., de Oliveira M.C., Cardoso V.N., de Barros A.L.B. Influence of PEG coating on the biodistribution and tumor accumulation of pH-sensitive liposomes. Drug delivery and translational research. 2019;9(1):123–130. doi: 10.1007/s13346-018-0583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Saw P.E., Park J., Lee E., Ahn S., Lee J., Kim H., Kim J., Choi M., Farokhzad O.C., Jon S. Effect of PEG pairing on the efficiency of cancer-targeting liposomes. Theranostics. 2015;5(7):746–754. doi: 10.7150/thno.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Alavi M., Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug metabolism and personalized therapy. 2019;34(1) doi: 10.1515/dmpt-2018-0032. [DOI] [PubMed] [Google Scholar]
- 268.Cintra E.R., Hayasaki T.G., Sousa-Junior A.A., Silva A.C.G., Valadares M.C., Bakuzis A.F., Mendanha S.A., Lima E.M. Folate-targeted PEGylated magnetoliposomes for hyperthermia-mediated controlled release of doxorubicin. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.854430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Qiu M., Wang J., Bai J., Li X., Tian C., Liu Z., Zheng C., Clark A.R., Cheng X., Liao X., Wu S., Lee R.J., Zhou X. Dual-ligand-functionalized liposomes based on glycyrrhetinic acid and cRGD for hepatocellular carcinoma targeting and therapy. Mol. Pharm. 2023;20(4):1951–1963. doi: 10.1021/acs.molpharmaceut.2c00842. [DOI] [PubMed] [Google Scholar]
- 270.Gong P., Wang Y., Zhang P., Yang Z., Deng W., Sun Z., Yang M., Li X., Ma G., Deng G., Dong S., Cai L., Jiang W. Immunocyte membrane-coated nanoparticles for cancer immunotherapy. Cancers. 2020;13(1) doi: 10.3390/cancers13010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yang J., Bahreman A., Daudey G., Bussmann J., Olsthoorn R.C., Kros A. Drug delivery via cell membrane fusion using lipopeptide modified liposomes. ACS Cent. Sci. 2016;2(9):621–630. doi: 10.1021/acscentsci.6b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yang L., Lin X., Zhou J., Hou S., Fang Y., Bi X., Yang L., Li L., Fan Y. Cell membrane-biomimetic coating via click-mediated liposome fusion for mitigating the foreign-body reaction. Biomaterials. 2021;271 doi: 10.1016/j.biomaterials.2021.120768. [DOI] [PubMed] [Google Scholar]
- 273.Robbins P.D., Dorronsoro A., Booker C.N. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J. Clin. Invest. 2016;126(4):1173–1180. doi: 10.1172/JCI81131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Huang M., Liu M., Huang D., Ma Y., Ye G., Wen Q., Li Y., Deng L., Qi Q., Liu T., Liu X., Chen M., Ye W., Zhang D. Tumor perivascular cell-derived extracellular vesicles promote angiogenesis via the Gas6/Axl pathway. Cancer Lett. 2022;524:131–143. doi: 10.1016/j.canlet.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 275.Pang L., Jin H., Lu Z., Xie F., Shen H., Li X., Zhang X., Jiang X., Wu L., Zhang M., Zhang T., Zhai Y., Zhang Y., Guan H., Su J., Li M., Gao J. Treatment with mesenchymal stem cell-derived nanovesicle-containing gelatin methacryloyl hydrogels alleviates osteoarthritis by modulating chondrogenesis and macrophage polarization. Advanced healthcare materials. 2023;12(17) doi: 10.1002/adhm.202300315. [DOI] [PubMed] [Google Scholar]
- 276.Xia W., Liu Y., Jiang X., Li M., Zheng S., Zhang Z., Huang X., Luo S., Khoong Y., Hou M., Zan T. Lean adipose tissue macrophage derived exosome confers immunoregulation to improve wound healing in diabetes. J. Nanobiotechnol. 2023;21(1):128. doi: 10.1186/s12951-023-01869-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jayaramayya K., Mahalaxmi I., Subramaniam M.D., Raj N., Dayem A.A., Lim K.M., Kim S.J., An J.Y., Lee Y., Choi Y., Raj A., Cho S.G., Vellingiri B. Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem-cell-derived exosomes for COVID-19 treatment. BMB reports. 2020;53(8):400–412. doi: 10.5483/BMBRep.2020.53.8.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tang J., Su T., Huang K., Dinh P.U., Wang Z., Vandergriff A., Hensley M.T., Cores J., Allen T., Li T., Sproul E., Mihalko E., Lobo L.J., Ruterbories L., Lynch A., Brown A., Caranasos T.G., Shen D., Stouffer G.A., Gu Z., Zhang J., Cheng K. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nature biomedical engineering. 2018;2:17–26. doi: 10.1038/s41551-017-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Koçak P., Unsal N., Canikyan S., Kul Y., Cohen S.R., Tiryaki T., Duncan D., Schlaudraff K.U., Ascher B., Tiryaki T.E. The Effect of Hybrosome (Umbilical Cord Blood Exosome-Liposome Hybrid Vesicles) on Human Dermal Cells In Vitro, Aesthetic surgery journal. Open forum. 2023;5 doi: 10.1093/asjof/ojad039. ojad039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sun L., Fan M., Huang D., Li B., Xu R., Gao F., Chen Y. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials. 2021;271 doi: 10.1016/j.biomaterials.2021.120761. [DOI] [PubMed] [Google Scholar]
- 281.Kang M., Lee C.S., Lee M. Bioactive scaffolds integrated with liposomal or extracellular vesicles for bone regeneration. Bioengineering. 2021;8(10) doi: 10.3390/bioengineering8100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hu Y., Li X., Zhang Q., Gu Z., Luo Y., Guo J., Wang X., Jing Y., Chen X., Su J. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact. Mater. 2021;6(9):2905–2913. doi: 10.1016/j.bioactmat.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.