This cross-sectional study investigates the health claims made on the labels of fish oil supplements and the total daily dose of eicosapentaenoic acid and docosahexaenoic acid in commonly available fish oil supplements.
Key Points
Questions
What health claims are made on the labels of fish oil supplements, and what is the total daily dose of eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA) in commonly available fish oil supplements?
Findings
In this cross-sectional study of 2819 fish oil supplements, 73.9% possessed at least 1 health claim, usually related to heart health, followed by brain and joint health, and US Food and Drug Administration–approved qualified health claim language was infrequently used. The total daily dose of EPA plus DHA was highly variable between supplements.
Meaning
Results suggest that additional regulation of the claims made on fish oil supplement labels may be needed to prevent consumer misinformation.
Abstract
Importance
One in 5 US adults older than 60 years takes fish oil supplements often for heart health despite multiple randomized clinical trials showing no data for cardiovascular benefit for supplement-range doses. Statements on the supplement labels may influence consumer beliefs about health benefits.
Objectives
To evaluate health claims made on the labels of fish oil supplements in the US, and to examine doses of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in commonly available formulations.
Design, Setting, and Participants
This cross-sectional study used data from labels of on-market fish oil (and nonfish ω-3 fatty acid) supplements obtained from the National Institutes of Health Dietary Supplement Label Database. The study was conducted and data analyzed from February to June 2022.
Main Outcome and Measures
The frequency and types of health claims made on fish oil labels (US Food and Drug Administration [FDA]–reviewed qualified health claim vs a structure/function claim) and the organ system referenced were evaluated. The total daily doses of combined EPA and DHA (EPA+DHA) were assessed for supplements from 16 leading manufacturers and retailers.
Results
Across 2819 unique fish oil supplements, 2082 (73.9%) made at least 1 health claim. Of these, only 399 (19.2%) used an FDA-approved qualified health claim; the rest (1683 [80.8%]) made only structure/function claims (eg, “promotes heart health”). Cardiovascular health claims were the most common (1747 [62.0%]). Across 16 leading brands/manufacturers, 255 fish oil supplements were identified. Among these, substantial variability was found in the daily dose of EPA (median [IQR], 340 [135-647] mg/d), DHA (median [IQR], 270 [140-500] mg/d), and total EPA+DHA (median [IQR], 600 [300-1100] mg/d). Only 24 of 255 supplements (9.4%) evaluated contained a daily dose of 2 g or more EPA+DHA.
Conclusions
Results of this cross-sectional study suggest that the majority of fish oil supplement labels make health claims, usually in the form of structure/function claims, that imply a health benefit across a variety of organ systems despite a lack of trial data showing efficacy. Significant heterogeneity exists in the daily dose of EPA+DHA in available supplements, leading to potential variability in safety and efficacy between supplements. Increasing regulation of dietary supplement labeling may be needed to prevent consumer misinformation.
Introduction
Fish oil supplements are widely used; 20% of adults older than 60 years report taking fish oil, often due to a belief that it improves general health or provides cardiovascular benefit.1,2 The US Food and Drug Administration (FDA) is responsible for regulating health claims on supplement labels per the Dietary Supplement Health and Education Act of 1994. Fish oil supplements can contain 2 different health claim types: qualified health claims (QHCs) and structure/function claims. QHCs are statements regarding a supplement or food’s potential for disease treatment or prevention and undergo evidence review by the FDA. All QHCs include qualifying language reflecting lack of scientific consensus or uncertainty. Two FDA-approved (QHCs) exist for fish oil: 1 for coronary heart disease (CHD) and 1 for blood pressure (BP) (eTable 1 in Supplement 1). Structure/function claims “describe the role of a nutrient or dietary ingredient intended to affect the structure or function in humans”3 but cannot state that the supplement prevents, treats, or cures any disease. Two example structure/function claims are “calcium builds strong bones” or “fiber maintains bowel regularity.”3 Commonly used language in structure/function claims includes that the supplement “maintains,” “supports,” or “promotes” the function of certain organs.3 The degree to which supplements use approved QHCs vs structure/function claims is unknown.
Multiple randomized clinical trials have shown no cardiovascular benefit to fish oil supplements.4,5,6 At higher doses, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the active components of fish oil, have both potential benefits and risks. One randomized clinical trial found that 4 g per day of purified EPA reduced cardiovascular events, although concerns have been raised regarding potential harm of the placebo in the trial, and a subsequent trial of high-dose combination EPA+DHA found no cardiovascular benefit.7,8,9 At doses of 2 g or more per day, EPA and DHA can lower triglycerides in patients with hypertriglyceridemia.10 However, several studies have found that at this dose, EPA and DHA may also increase the risk of atrial fibrillation.11,12 Presently, manufacturers are not required to disclose a potential risk of atrial fibrillation at higher doses.
Given the widespread use of fish oil supplements and commonly believed potential health benefits, we sought to (1) systematically evaluate health claim types made on fish oil supplement labels and (2) quantify the doses of EPA and DHA in fish oil supplements sold by leading retailers.
Methods
Data were obtained from the Dietary Supplement Label Database maintained by the National Institutes of Health Office of Dietary Supplements. Manufacturers voluntarily submit labels for supplements sold in the US, including label text and a photo of each label. We identified on-market fish oil supplements, including non–fish-derived ω-3 fatty acid alternatives such as algal- or seaweed-derived formulations (henceforth referred to as fish oil), as of June 22, 2022. We first identified labels with relevant terms on the label including name, ingredient list, or other text. We then manually reviewed (primary review by J.D. with confirmation by A.N.) supplement names and ingredient lists to identify primarily fish oil supplements and exclude multivitamins and multicomponent supplements, supplements lacking EPA or DHA, and prenatal and pediatric-targeted supplements. Text string searches were performed on the label text to identify QHCs and structure/function claims (eTable 2 in Supplement 1) and categorize claims by organ system; claims were then verified with manual review.
Manual data extraction was used to determine the total daily dose of EPA, DHA, and total EPA+DHA from each supplement based on manufacturer-recommended serving size for fish oil supplements made by the 9 largest manufacturers by US market share and the top 7 retailers in the US by worldwide retail sales13,14 (eTable 3 in Supplement 1). When labels contained ranges for dose, the range midpoint was used. If daily serving size varied (eg, 1-2 tablets daily), we used the higher end of the range.
Statistical Analysis
Descriptive statistics were used to quantify the proportion of health claims on available supplement labels. The dose analysis is summarized using median (IQR). We also evaluated the proportion of supplements with 2000 mg or more of EPA. Data extraction was done using R software, version 4.1.2 (R Project for Statistical Computing), and data curation, manual review, and string searches were performed in Excel (Microsoft). Data analyses were performed in Stata/SE, version 17.0 (StataCorp) and Prism 9 (GraphPad).
Results
Label Health Claims
We initially identified 23 247 supplements containing at least 1 keyword on the label indicating a possible fish oil supplement. We then excluded 20 036 that were not fish oil (most were identified because they contained the word “algae”), 93 DHA/EPA-containing multivitamins, 168 prenatal formulations, and 131 pediatric supplements (eFigure in Supplement 1).
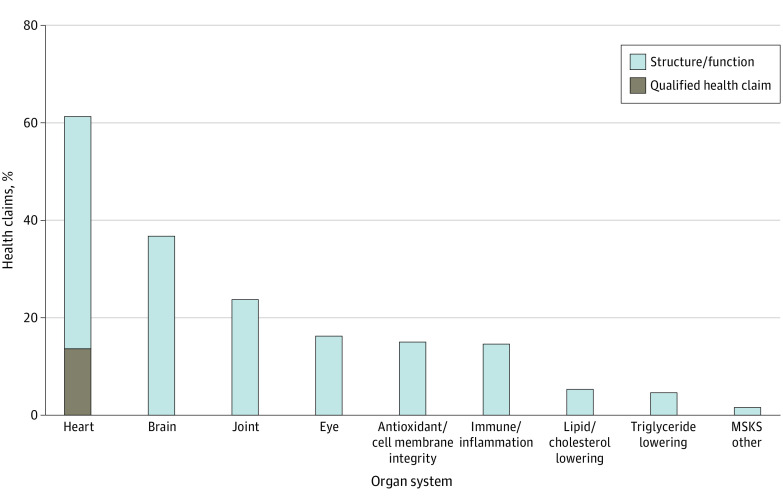
Our final sample included 2819 on-market fish oil supplements, of which 2082 (73.9%) included at least 1 health-related label statement. Of these, only 399 (19.2%) used an FDA-approved QHC. Most labels with a health-related statement (1683 [80.8%]) included only structure/function claims. Only 394 labels (18.9%) used the FDA-approved QHC for coronary heart disease, 3 (0.14%) used a QHC for blood pressure, and 2 (0.09%) used both QHCs.
Figure 1 shows the distribution of health claims by organ system referenced. The Table shows examples of structure/function claims made by organ system and FDA QHCs found in our sample. Heart/cardiovascular claims (including QHCs) were the most common (1747 [62.0%] of all labels), followed brain health (1057 [37.5%]) and joint-related claims (684 [24.3%]). No labels mentioned a potential risk of atrial fibrillation.
Figure 1. Proportion of Fish Oil Labels With Structure/Function and Qualified Health Claims by Organ System Referenced.
The proportion of all on-market fish oil/ω-3 supplement labels that contain at least 1 health-related statement on the label is shown, including structure/function claims and qualified health claims, stratified by organ system reference. Blue bars represent labels that include only structure and function claims, whereas the brown bar represents labels that include a US Food and Drug Administration–approved qualified health claim for fish oil. MSKS indicates musculoskeletal system.
Table. Frequency and Examples of Specific and Nonspecific Health-Related Structure/Function Claims Made on the Labels of Fish Oil/ω-3 Supplements.
| Organ system | Percentage of labels (N = 2819), No. (%) | Examples |
|---|---|---|
| Heart and cardiovascular health | 1747 (62.0) | “Promotes heart health” |
| “Supports heart, mind and mood” | ||
| “Omega-3 fatty acids are considered some of the ‘good fats’ important for cellular, heart and metabolic health” | ||
| Brain, neurologic, and mental health | 1057 (37.5) | “DHA is one of the good fats that helps keep our brains running optimally as we age” |
| “Helps protect against normal cognitive decline as we age” | ||
| “Supports cognitive health” | ||
| “Brain support” | ||
| Joint-related health | 684 (24.3) | “Promotes joint comfort & mobility” |
| “Omega-3 fatty acids are necessary for many vital functions in the body and support heart health and healthy skin and joint function.” | ||
| “Help support healthy joints” | ||
| “Joint health and mobility” | ||
| Eye health | 476 (16.9) | “Maintains eye health and normal vision” |
| “Promotes brain, vision, joint and heart health” | ||
| “Vision support” | ||
| Antioxidant protection/cell membrane integrity | 436 (15.5) | “Powerful antioxidant support for healthy aging and vitality.” |
| “Superior antioxidant protection for healthy aging” | ||
| “Potent antioxidant” | ||
| “Broad-spectrum cellular health formula” | ||
| Immune/ inflammation |
425 (15.1) | “Omega-3 fatty acids are important for cardiovascular, immune and nervous system health.” |
| “Immune system support” | ||
| “Advanced cellular support & immune activation” | ||
| Lipid/cholesterol lowering | 161 (5.7) | “Omega-3 FAs, especially when combined with exercise, can help to support the maintenance of normal blood lipids” |
| “Supports healthy lipid metabolism and cardiovascular function” | ||
| “Supports healthy cholesterol and blood pressure levels” | ||
| Triglyceride lowering | 141 (5.0) | “Helps maintain healthy triglycerides” |
| “Clinically proven to maintain healthy triglyceride levels” | ||
| “Omega-3 fatty acids also help maintain triglyceride levels already in the normal range.” | ||
| FDA QHC statement | ||
| Coronary heart disease | 394 (18.9) | “Supportive but not conclusive research shows that consumptions of EPA and DHA omega-3 fatty acids may reduce the risk of coronary heart disease” |
| Blood pressure | 3 (0.1) | “Consuming EPA and DHA combined may help lower blood pressure in the general population and reduce the risk of hypertension. However, FDA has concluded that the evidence is inconsistent and inconclusive.” |
Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FA, fatty acid; FDA, US Food and Drug Administration; QHC, qualified health claim.
Dosing Information
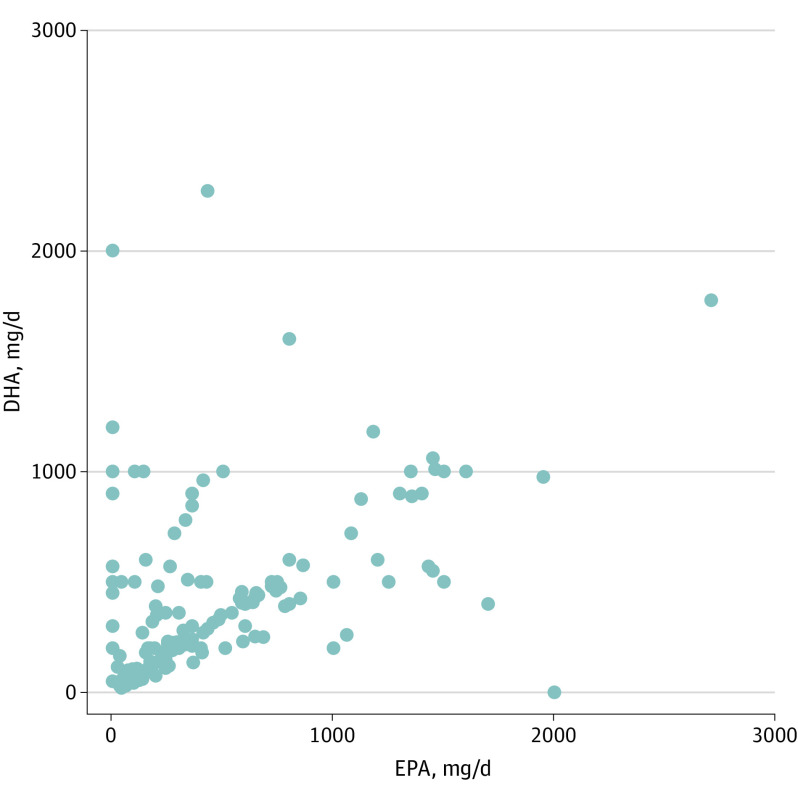
From 16 manufacturers and retailers included in the dose analysis, 282 unique supplement labels were identified. Of these, 27 were excluded due to lack of sufficient dose information for a final sample of 255 labels. Of these, 242 (91.2%) contained both EPA and DHA, 1 (0.4%) contained EPA only, and 12 (4.5%) contained DHA only. The median (IQR) dose was 340 (135-647) mg per day for EPA, 270 (140-500) mg per day for DHA, and 600 (300-1100) mg per day for total combination EPA+DHA (eTable 3 in Supplement 1). Substantial variability was seen in the daily dose of EPA, DHA, and combined EPA+DHA across supplements (Figure 2) as well as between supplements made by the same manufacturer (eTable 4 in Supplement 1). Only 9.4% of supplements (24 of 255) evaluated had a combined EPA+DHA content of 2000 mg or greater.
Figure 2. Total Daily Dose of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) in Commonly Available Fish Oil/ω-3 Fatty Acid Supplements.
The total daily dose is shown of EPA and/or DHA among 227 on-market fish oil/ω-3 fatty acid supplements distributed by 16 brands with the largest potential retail market share. Each dot represents 1 supplement.
Discussion
Most on-market fish oil supplement labels include at least 1 structure/function claim that implies a wide range of health benefits across a variety of organ systems. Relative to structure/function claims, the use of FDA-approved QHCs for fish oil is low. Doses of EPA and DHA in on-market supplements vary greatly, although most contain insufficient DHA and EPA to lower triglycerides.
Cardiovascular or heart health–related statements were the most common structure/function claims. Since the FDA first approved a QHC for fish oil and CHD in 2004, randomized clinical trials have failed to show cardiovascular benefit of fish oil supplements.4,5,6,8,15 This QHC has not subsequently been revisited by the FDA. However, even if the FDA revoked fish oil’s CHD QHC, most labels would remain unaffected, as the QHC for CHD is infrequently used. Rather, most labels use structure/function claims to imply cardiovascular benefit (eg, supports heart health), a claim type that does not require any mitigating language regarding potential scientific uncertainty of the statement. Many fish oil supplements also make claims implying benefit to other organ systems including brain/mental health, joint health, and eye health despite a lack of randomized clinical trial data supporting benefit. Given the pervasiveness of structure/function claims, more research is urgently needed to understand consumers’ interpretation of them. Ultimately, more regulation of this claim type may be necessary to prevent consumer misinformation.
Improving consumer understanding of the potential benefits and risks of fish oil supplements should also consider consumer awareness regarding variability in doses of EPA and DHA in on-market supplements. We observed substantial heterogeneity in the dose of EPA and DHA delivered in on-market supplements. Only a small minority of supplements contained 2 g per day of combination EPA+DHA, which may be recommended for triglyceride lowering but can also increase risk of atrial fibrillation.11 No supplements included potential warnings about this risk, although this is not presently required by the FDA.
Limitations
We acknowledge several limitations to our study. Manufacturers submit labels voluntarily; therefore, there are likely other fish oil supplements on the market that were not included. Next, our analysis weighed all supplement labels despite differences in market share. Third, we evaluated health claims only on the labels; other advertising or promotional materials were not covered. Finally, we only evaluated supplements made by the 16 largest potential brands.
Conclusions
Results of this cross-sectional study suggest that fish oil supplement labels frequently include health claims in the form of structure/function claims that imply health benefits across a wide range of organ systems, increasing potential for consumer misinformation. Significant heterogeneity exists in the daily dose of EPA and DHA in available supplements, leading to potential variability in safety and efficacy between supplements.
eTable 1. Qualified Health Claims for Fish Oil Supplements
eFigure. Flow Diagram for Supplement Label Sample
eTable 2. String Search Terms to Augment Health Claim Label Statement Categorization
eTable 3. Names of Manufacturer and Retailer Fish Oil/Omega-3 Supplements Included in Dosing Analysis
eTable 4. EPA, DHA, and EPA+DHA Doses in Fish Oil and Omega-3 Supplements by Manufacturer/Retailer
Data Sharing Statement
References
- 1.Mishra S, Stierman B, Gahche JJ, Potischman N. Dietary supplement use among adults: US, 2017–2018. Accessed May 17, 2022. https://www.cdc.gov/nchs/products/databriefs/db399.htm
- 2.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173(5):355-361. doi: 10.1001/jamainternmed.2013.2299 [DOI] [PubMed] [Google Scholar]
- 3.Shalala DE, Henney JE. Regulations on statements made for dietary supplements concerning the effect of the product on the structure or function of the body. Accessed June 12, 2022. https://www.govinfo.gov/content/pkg/FR-2000-01-06/pdf/00-53.pdf
- 4.Aung T, Halsey J, Kromhout D, et al. ; Omega-3 Treatment Trialists’ Collaboration . Associations of ω-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225-234. doi: 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman L, Mafham M, Wallendszus K, et al. ; ASCEND Study Collaborative Group . Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N Engl J Med. 2018;379(16):1540-1550. doi: 10.1056/NEJMoa1804989 [DOI] [PubMed] [Google Scholar]
- 6.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11-22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 8.Nicholls SJ, Lincoff AM, Garcia M, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324(22):2268-2280. doi: 10.1001/jama.2020.22258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker PM, Rifai N, MacFadyen J, et al. Effects of randomized treatment with icosapent ethyl and a mineral oil comparator on interleukin-1β, interleukin-6, c-reactive protein, oxidized low-density lipoprotein cholesterol, homocysteine, lipoprotein(a), and lipoprotein-associated phospholipase a2: a REDUCE-IT biomarker substudy. Circulation. 2022;146(5):372-379. doi: 10.1161/CIRCULATIONAHA.122.059410 [DOI] [PubMed] [Google Scholar]
- 10.Skulas-Ray AC, Wilson PWF, Harris WS, et al. ; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology . Ω-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140(12):e673-e691. doi: 10.1161/CIR.0000000000000709 [DOI] [PubMed] [Google Scholar]
- 11.Curfman G. Ω-3 fatty acids and atrial fibrillation. JAMA. 2021;325(11):1063. doi: 10.1001/jama.2021.2909 [DOI] [PubMed] [Google Scholar]
- 12.Kalstad AA, Myhre PL, Laake K, et al. ; OMEMI Investigators . Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized, controlled trial. Circulation. 2021;143(6):528-539. doi: 10.1161/CIRCULATIONAHA.120.052209 [DOI] [PubMed] [Google Scholar]
- 13.Grand View Research . Ω-3 Supplements Market Size, Share & Trends Analysis Report by Source (Fish, Krill Oil), by Form (Soft Gels, Capsules), by End User (Adults, Infants), by Functionality, by Distribution Channel, and Segment Forecasts, 2020-2028. Grand View Research; 2021. [Google Scholar]
- 14.National Retail Federation . Top 100 retailers 2022 list. Accessed June 30, 2022. https://nrf.com/resources/top-retailers/top-100-retailers/top-100-retailers-2022-list
- 15.Hubbard WK. Letter responding to health claim petition dated June 23, 2003 (Wellness petition): ω-3 fatty acids and reduced risk of coronary heart disease. Accessed May 5, 2022. http://wayback.archive-it.org/7993/20171114183649/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072936.htm [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Qualified Health Claims for Fish Oil Supplements
eFigure. Flow Diagram for Supplement Label Sample
eTable 2. String Search Terms to Augment Health Claim Label Statement Categorization
eTable 3. Names of Manufacturer and Retailer Fish Oil/Omega-3 Supplements Included in Dosing Analysis
eTable 4. EPA, DHA, and EPA+DHA Doses in Fish Oil and Omega-3 Supplements by Manufacturer/Retailer
Data Sharing Statement