This randomized clinical trial investigates the effect of coronary venous pressure on myocardial resistances in patients with microvascular disease.
Key Points
Question
How does coronary venous pressure influence myocardial resistances in patients with microvascular disease?
Findings
In this blinded, physiology end point, sham-controlled randomized clinical trial that included 20 adults with microvascular disease, an increase in coronary venous pressure led to significantly decreased resting and hyperemic microvascular resistances.
Meaning
In patients with microvascular angina, the coronary venous circulation may be a novel therapy target.
Abstract
Importance
The role of the coronary venous circulation in regulating myocardial perfusion and its potential in treating microvascular angina is unexplored.
Objective
To evaluate whether an increase in coronary venous pressure modifies microvascular resistance in patients with microvascular angina.
Design, Setting, and Participants
This was a blinded, sham-controlled, crossover, randomized clinical trial that enrolled participants between November 2021 and January 2023. Participants for this physiology end point study were recruited from the Cardiology Center of the University of Medicine in Mainz, Germany. Patients with moderate/severe angina pectoris (Canadian Cardiovascular Society class 2-4) due to microvascular dysfunction (as defined by the thermodilution-based index of microvascular resistance >25 mm Hg × s). Exclusion criteria were epicardial coronary disease, second- and third-degree atrioventricular block, severe valvular heart disease, cardiomyopathy, and pulmonary or kidney disease.
Intervention
Inflation of an undersized balloon placed in the cardiac coronary sinus (CS), hereafter referred to as balloon and the deflated balloon in the right atrium, referred to as sham. Measurements were performed at rest and during maximal coronary hyperemia. Both patients and final assessors were blinded to the randomization sequence.
Main Outcomes and Measures
Hemodynamic parameters, including aortic (Pa) and distal (Pd) coronary pressure, coronary sinus pressure (Pcs), right atrial pressure (Pra), and the mean transit time (inverse of blood flow [Tmn]), were measured.
Results
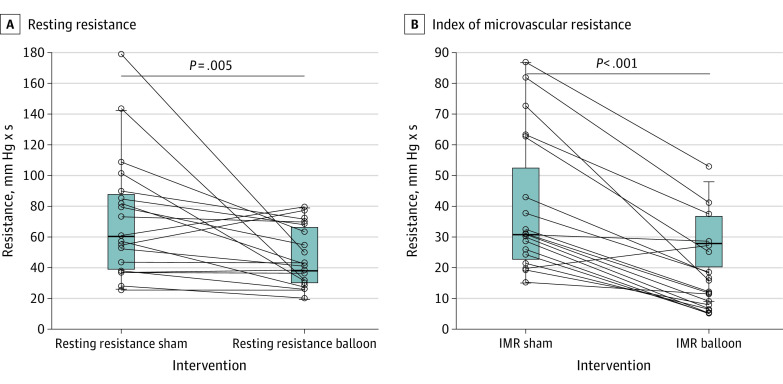
A total of 20 patients (median [IQR] age, 69 [64-75] years; 11 female [55.0%]) were included in the study. Two patients (10%) had diabetes, 6 (30%) had hypercholesterolemia, 15 (75%) had hypertension, and 3 (15%) were active smokers. The inflation of the CS balloon caused a significant increase in CS pressure at rest and during hyperemia (300% and 317% increase, respectively, compared with sham, both P < .001), a decrease in hyperemic distal coronary pressure (median [IQR], sham: 92 [80-100] mm Hg; balloon: 79 [75-93] mm Hg; P = .01) and mean transit time (sham: 0.39 [0.23-0.62] s; balloon: 0.26 [0.17-0.46] s; P = .008). As a result, CS occlusion led to a decrease in both resting coronary resistance (median [IQR], sham: 59 [37-87] mm Hg × s; balloon: 42 [31-67] mm Hg × s; P = .005) and the primary end point hyperemic coronary resistance (mean [IQR], sham: 31 [23-53] mm Hg × s; balloon: 14 [8-26] mm Hg × s; P < .001).
Conclusion and Relevance
Increased coronary venous pressure led to a reduction of microvascular resistances in patients with microvascular angina, a mechanism with potential implications for the therapy of this complex disease.
Trial Registration
ClinicalTrials.gov Identifier: NCT05034224
Introduction
Microvascular angina pectoris (MVA) is a complex clinical condition in which functional and/or structural changes in the coronary microcirculation determine an increase in vascular tone compromising myocardial perfusion.1
Empirical observations suggest that implantation of a coronary sinus reducer may improve myocardial perfusion and decrease symptoms in patients with microvascular angina2 as it does in patients with refractory angina due to coronary artery disease.3 However, the physiologic mechanisms of this observation and the role of the coronary venous circulation in modulating (microvascular) hemodynamics remain unclear.
The study presented here aimed to investigate whether an increase in coronary sinus pressure may lead to a measurable change in coronary microvascular resistance in patients with microvascular dysfunction, which may pave the way for an interventional approach.
Methods
Study Design
This study was a sham-controlled, crossover, randomized clinical trial to investigate the effect of changes in coronary venous pressure on microvascular resistances. The protocol (described in detail elsewhere; Supplement 1)4 was approved by the University of Mainz ethics committee, and all patients provided written informed consent before enrollment. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Study Population
Patients had chronic angina (Canadian Cardiovascular Society 2-4) without epicardial stenosis, reversible ischemia on noninvasive testing, and evidence of microvascular dysfunction (index of microvascular resistance [IMR] ≥25 mm Hg × s). Main exclusion criteria were second- or third-degree atrioventricular block, any valvular heart disease, any cardiomyopathy, and pulmonary or kidney disease.
Procedures
Randomization and Intervention
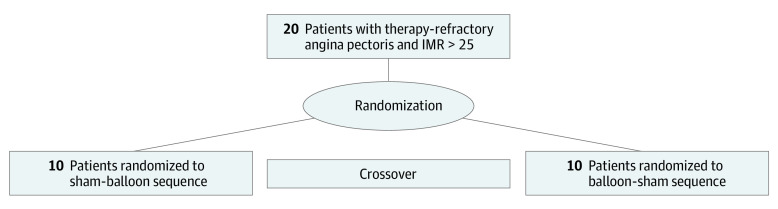
Vasoactive therapies were suspended for more than 12 hours before measurements were taken. Patients were randomly assigned 1:1 to 1 of 2 arms (sham/balloon or balloon/sham), with each patient undergoing invasive measurements at rest and during hyperemia in both conditions (Figure 1). For the measurements during balloon inflation, a 9-mm Swan-Ganz catheter was advanced into the cardiac coronary sinus (CS), and measurements were performed 1 minute after balloon inflation. The balloon was placed to obtain a stable approximately 80% CS lumen reduction throughout the measurements. For the sham measurements, the balloon was maintained in the deflated state. Ten minutes after the first set of rest/hyperemia measurements (balloon or sham), patients would cross over to the other condition (sham or balloon), and the measurements (rest/hyperemia) were repeated.
Figure 1. Study Protocol .
A total of 20 patients with microvascular angina were randomly assigned to treatment in this crossover, sham-controlled trial to assess the effect of inflation of a coronary sinus balloon on myocardial hemodynamic. IMR indicates index of microvascular resistance.
Hemodynamic Measurements
Coronary microvascular function assessments were performed with a wire-based thermodilution method using Pressure Wire X (Abbott Vascular) as per instructions for use; the same hyperemic agent was used for the 2 sets of measurements in each patient (sham and balloon). The mean transit time (Tmn), coronary flow reserve (CFR), and IMR were measured. The absence of drift was confirmed at the end of the measurements. Patients were blinded to the randomization sequence, and hemodynamic data were encoded in the CoroFlow software (Coroventis) and collected by blinded assessors. Coronary flow capacity was measured to identify 4 different regions of myocardial ischemia (eMethods in Supplement 2).5,6
Trial End Points
The study’s primary end point was the (hyperemic) IMR during the inflation of the CS balloon as compared with sham. IMR was calculated as [(Pd − Pcs) × Tmn] where Pd is the distal intracoronary pressure, Pcs the pressure in the coronary sinus, and Tmn the inverse of flow.
Statistical Analysis
Randomization was performed by a computer-generated random sequence with permuted blocks. The power calculation was based on previous articles investigating the effect of acute administration of fasudil and enalaprilat on IMR.7,8 Assuming a mean (SD) IMR of 33 (13) mm Hg × s, an effect size of approximately 0.69, and a power of 80%, 19 patients were needed for the analysis.
The primary analysis was performed as a within-participant comparison of the primary parameter using a linear mixed-regression model with a patient random intercept, condition, and period as well as their interaction as fixed factors and age and sex as covariates. Secondary end points were analyzed using the Wilcoxon test. Data are presented as median (IQR). All P values were 2-sided, and P < .05 was considered statistically significant. Data were analyzed using MedCalc software, version 15 (MedCalc Ltd).
Results
A total of 20 patients (median [IQR] age, 69 [64-75] years; 11 female [55.0%]; 9 male [45%]) were enrolled in this physiology end point study (eTable in Supplement 2). Two patients (10%) had diabetes, 6 (30%) had hypercholesterolemia, 15 (75%) had hypertension, and 3 (15%) were active smokers. Ten patients were randomly assigned to the sham-balloon sequence and 10 to the balloon-sham sequence. The median (IQR) IMR measured before inclusion in the study was 36 (33-71) mm Hg × s (normal <25 mm Hg × s).
Hemodynamic data are presented in the Table. Inflation of the CS balloon caused a significant increase in CS pressure both at rest (300% vs sham; P < .001) and during hyperemia (317%; P < .001), but it was not associated with a change in right atrial pressure. A decrease in hyperemic distal coronary pressure was also observed (median [IQR], sham: 92 [80-100] mm Hg; balloon: 79 [75-93] mm Hg; P = .01) along with a decrease in Tmn (median [IQR], sham: 0.39 [0.23-0.62] s; balloon: 0.26 [0.17-0.46] seconds, P = .008). Balloon inflation provoked a decrease in both resting coronary resistance (median [IQR], sham: 59 [37-87] units; balloon: 42 [31-67] mm Hg × s; P = .005) and hyperemic coronary resistance (median [IQR] primary end point, sham: 31 [23-53] mm Hg × s; balloon: 14 [8-26] mm Hg × s; P < .001) (Figure 1). The estimated adjusted between-conditions difference (sham-balloon) was 21 (95% CI, 14-28 mm Hg × s; P < .001). Balloon inflation also caused a decrease in fractional flow reserve (median [IQR], sham: 0.94 [0.88-0.94]; balloon: 0.87 [0.82-0.94]; P = .003).
Table. Hemodynamic Variables and Effects of Balloon Inflation at Rest and During Hyperemiaa.
| Hemodynamic variable | Sham | Balloon | P valueb |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Primary end point | |||
| IMR, mm Hg × s | 31 (23-53) | 14 (8-26) | <.001 |
| Secondary end points | |||
| Rest | |||
| Pa, mm Hg | 103 (93-110) | 101 (89-111) | .28 |
| Pd, mm Hg | 98 (85-101) | 89 (84-102) | .21 |
| Tmn, s | 0.69 (0.43-1.14) | 0.58 (0.44-0.82) | .37 |
| Pcs, mm Hg | 5 (2-9) | 20 (13-29) | <.001 |
| Pra, mm Hg | 4 (2-7) | 3 (2-8) | .63 |
| Resistances, mm Hg × s | 59 (37-87) | 42 (31-67) | .005 |
| Hyperemia | |||
| Pa, mm Hg | 98 (88-110) | 89 (84-102) | .05 |
| Pd, mm Hg | 92 (80-100) | 79 (75-93) | .01 |
| Tmn, s | 0.39 (0.23-0.62) | 0.26 (0.17-0.46) | .008 |
| Pcs, mm Hg | 6 (3-9) | 25 (13-36) | <.001 |
| Pra, mm Hg | 6 (3-8) | 5 (3-8) | >.99 |
| FFR | 0.94 (0.88-0.94) | 0.87 (0.82-0.94) | .003 |
| CFR | 1.70 (1.4-2.3) | 2.1 (1.3-4.1) | .18 |
| MRR | 2.0 (1.4-2.7) | 2.7 (1.4-5.3) | .06 |
Abbreviations: CFR, coronary flow reserve; FFR, fractional flow reserve; IMR, index of microvascular resistance; MRR, microvascular resistance reserve; Pa, mean aortic pressure; Pcs, mean pressure in the coronary sinus; Pd, mean distal coronary pressure; Pra, mean pressure in the right atrium; Tmn, mean transit time.
The estimated adjusted between-conditions difference (sham-balloon) was 21 (95% CI, 14-28) mm Hg × s (P < .001).
All P values are calculated with the Wilcoxon test.
Randomization sequence, resting resistances, sex, and age did not affect the change in IMR caused by balloon inflation. The decrease in IMR after balloon inflation was largest in patients with the highest sham IMR (R2 = 0.67; P < .001) (eFigure 2 in Supplement 2).
Coronary flow capacity was improved by balloon inflation (Figure 2 and eFigure 1 in Supplement 2). Average coronary flow capacity (CFC) went from moderate myocardial ischemia (sham: CFC = 1.7; 1/Tmn = 2.56 s) to the nonischemic region (balloon: CFR = 2.1; 1/Tmn = 3.89 s).
Figure 2. The Primary End Point of the Trial .
Balloon inflation caused a decrease in resting and hyperemic microvascular resistances. IMR indicates index of microvascular resistance.
Discussion
Many patients undergoing diagnostic coronary angiography for angina have microvascular disease.8 These patients rarely receive a definitive diagnosis in clinical routine and often remain symptomatic.9,10 To date, there is little evidence of the possibility of modulating microvascular resistances using pharmacologic or mechanical approaches. Small case series support the concept that occlusion of the CS may improve symptoms in these patients, but the mechanism is unknown.2 We show here that an acute increase in CS pressure leads to a decrease in microvascular resistance (with a more significant effect during hyperemia) and an increase in blood flow.
Animal models in the peripheral circulation show that total vascular resistance within the coronary circulation is determined by vascular resistance and viscosity, with the latter playing a multiplicative role during hyperemia when the capillary bed determines up to 75% of the total myocardial vascular resistance.11 In the healthy circulation, increases in venous blood pressure elicit myogenic vasoconstrictor response of precapillary vessels and capillary derecruitment.12 In patients with severe vascular disorders, however, an increase in venous pressure has been associated with a rise in capillary (transmural) pressure, leading to an increase in capillary diameter and density (and therefore total blood volume) and a reduction in viscosity.13 Based on the Poiseuille law, volume flow rate is directly proportional to the fourth power of the vessel’s radius and inversely proportional to dynamic viscosity. Following an increase in CS pressure, even small increases in capillary recruitment/diameter and viscosity may, therefore, result in relevant improvements in microvascular resistance in patients with MVA, with a proportionally larger effect during hyperemia (eFigure 3 in Supplement 2).
Limitations
This study has some limitations. Of note, a mechanistic study is not designed or powered to assess clinical benefit, and we only studied the effect of a single acute increase in CS pressure in patients with microvascular dysfunction. Whether similar effects occur over chronic periods and in patients with epicardial (as compared with microvascular) disease will have to be investigated. Also, the mechanisms hypothesized are based on evidence from animal models or human in vivo models of the extracardiac circulation.11,12,13
Conclusions
In this crossover randomized clinical trial, the current findings that an acute increase in CS pressure led to a decrease in microvascular resistance and an increase in blood flow allow hypothesizing that the venous vasculature may be leveraged to improve coronary resistances in patients with MVA. This may have implications for therapy with a coronary sinus reducer.
Trial Protocol
eTable. Patient Characteristics
eMethods.
eFigure 1. Impact of Balloon Expansion on Coronary Flow Capacity
eFigure 2. Correlation Between the Decrease in IMR Following Balloon Inflation and Sham (Resting) IMR
eFigure 3. Proposed Mechanism
eReferences.
Data Sharing Statement
References
- 1.Beltrame JF, Crea F, Kaski JC, et al. ; Coronary Vasomotion Disorders International Study Group (COVADIS) . International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38(33):2565-2568. [DOI] [PubMed] [Google Scholar]
- 2.Giannini F, Baldetti L, Ielasi A, et al. First experience with the coronary sinus reducer system for the management of refractory angina in patients without obstructive coronary artery disease. JACC Cardiovasc Interv. 2017;10(18):1901-1903. doi: 10.1016/j.jcin.2017.06.062 [DOI] [PubMed] [Google Scholar]
- 3.Verheye S, Jolicœur EM, Behan MW, et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N Engl J Med. 2015;372(6):519-527. doi: 10.1056/NEJMoa1402556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ullrich H, Olschewski M, Münzel T, Gori T. Randomized, crossover, controlled trial on the modulation of cardiac coronary sinus hemodynamics to develop a new treatment for microvascular disease: protocol of the MACCUS trial. Front Cardiovasc Med. 2023;10:1133014. doi: 10.3389/fcvm.2023.1133014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamaya R, Yonetsu T, Kanaji Y, et al. Diagnostic and prognostic efficacy of coronary flow capacity obtained using pressure-temperature sensor-tipped wire-derived physiological indices. JACC Cardiovasc Interv. 2018;11(8):728-737. doi: 10.1016/j.jcin.2018.01.249 [DOI] [PubMed] [Google Scholar]
- 6.van de Hoef TP, Echavarría-Pinto M, van Lavieren MA, et al. Diagnostic and prognostic implications of coronary flow capacity: a comprehensive cross-modality physiological concept in ischemic heart disease. JACC Cardiovasc Interv. 2015;8(13):1670-1680. doi: 10.1016/j.jcin.2015.05.032 [DOI] [PubMed] [Google Scholar]
- 7.Mangiacapra F, Peace AJ, Di Serafino L, et al. Intracoronary Enalaprilat to Reduce Microvascular Damage During Percutaneous Coronary Intervention (ProMicro) study. J Am Coll Cardiol. 2013;61(6):615-621. doi: 10.1016/j.jacc.2012.11.025 [DOI] [PubMed] [Google Scholar]
- 8.Suda A, Takahashi J, Hao K, et al. Coronary functional abnormalities in patients with angina and nonobstructive coronary artery disease. J Am Coll Cardiol. 2019;74(19):2350-2360. doi: 10.1016/j.jacc.2019.08.1056 [DOI] [PubMed] [Google Scholar]
- 9.Ford TJ, Stanley B, Good R, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: the CorMicA trial. J Am Coll Cardiol. 2018;72(23 Pt A):2841-2855. doi: 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 10.Rahman H, Corcoran D, Aetesam-Ur-Rahman M, Hoole SP, Berry C, Perera D. Diagnosis of patients with angina and non-obstructive coronary disease in the catheter laboratory. Heart. 2019;105(20):1536-1542. doi: 10.1136/heartjnl-2019-315042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pries AR, Secomb TW, Gessner T, Sperandio MB, Gross JF, Gaehtgens P. Resistance to blood flow in microvessels in vivo. Circ Res. 1994;75(5):904-915. doi: 10.1161/01.RES.75.5.904 [DOI] [PubMed] [Google Scholar]
- 12.Iida N, Mitamura Y. Effects of venous pressure elevation on myogenic vasoconstrictive responses to static and dynamic arterial pressures. Jpn J Physiol. 1989;39(6):811-823. doi: 10.2170/jjphysiol.39.811 [DOI] [PubMed] [Google Scholar]
- 13.Ubbink DT, Reneman RS, Jacobs MJ. Effects of venous pressure and posture on skin capillary perfusion. Eur J Clin Invest. 1999;29(9):737-743. doi: 10.1046/j.1365-2362.1999.00523.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Patient Characteristics
eMethods.
eFigure 1. Impact of Balloon Expansion on Coronary Flow Capacity
eFigure 2. Correlation Between the Decrease in IMR Following Balloon Inflation and Sham (Resting) IMR
eFigure 3. Proposed Mechanism
eReferences.
Data Sharing Statement