Abstract
Background:
Reliance on prehospital trauma triage guidelines misses patients with serious injury. Lactate is a biomarker capable of identifying high-risk trauma patients. Our objective is to compare prehospital point of care lactate (P-LAC) to systolic blood pressure (SBP) for predicting the need for resuscitative care (RC) in trauma patients transported by ground emergency medical services (EMS).
Methods:
A prospective observational study at 9 sites within the Resuscitation Outcomes Consortium conducted from March, 2011 to August, 2012. Lactate was measured on patients with a prehospital SBP ≤ 100 mmHg who were transported by EMS to a level I or II trauma center. Patients were followed up for need for RC, defined as any of the following within 6 hours of ED arrival: blood transfusion ≥5 units, intervention for hemorrhage including thoracotomy, laparotomy, pelvic fixation or interventional radiology (IR) embolization, or death.
Results:
387 patients had a lactate value and presented with SBP between 71–100 mmHg, and 70 (18%) required RC. Using a P-LAC decision rule (≥ 2.5 mmol/L) that yielded the same specificity as SBP ≤ 90 (48%), the observed sensitivities for RC were 93% (95% confidence interval [CI] 84–98%) for P-LAC ≥ 2.5 mmol/L and 67% (CI 55–78%) for SBP ≤ 90 mmHg (McNemar’s test [p<0.001]). P-LAC has an estimated area under the curve (AUC) of 0.78 (95% CI 0.73–0.83), which is statistically superior to that of SBP [0.59 (CI 0.53–0.66)] and shock index (heart rate / SBP) [0.66 (CI 0.60–0.74)].
Conclusion:
P-LAC obtained at the scene is associated with the need for RC. P-LAC is superior to other early surrogates for hypoperfusion (SBP and shock index) in predicting the need for RC in trauma patients with 70 < SBP ≤ 100 mmHg.
Level of Evidence:
III, Diagnostic Test Prospective
Keywords: Lactate, Prehospital, Trauma
Background:
Prehospital trauma triage guidelines rely primarily on vital signs, anatomic injury, mechanism, and provider judgment. Reliance on these guidelines has been reported to miss patients with serious injury1,2. Trauma system goals for under-triage range from 0 to 5% of patients requiring Level I or Level II trauma-center care while over-triage may approach 25%−50%3. Prospective research suggests that field hypotension (systolic blood pressure [SBP] ≤ 90 mmHg) lacks sensitivity and specificity for predicting patient outcomes.4,5. Despite this, systolic hypotension remains a common component of trauma triage guidelines, an indication for trauma center care and a surrogate for cellular hypoperfusion. Delayed identification of hypoperfusion could lead to triage of some patients away from specialized trauma centers, failure to recognize compensated shock, and delayed resuscitation, all of which are associated with an increase in infection, multiple organ dysfunction (MOD), and mortality6–9.
Lactate is a biomarker that serves as a predictive tool for identifying high-risk trauma patients and provides information beyond that of vital signs and mechanism of injury6. A byproduct of anaerobic metabolism, lactate is a circulating biomarker of organ perfusion failure, and is associated with mortality in patients with sepsis, myocardial infarction, and trauma 6,10–12. Previous studies have demonstrated that an elevated lactate is predictive of poor outcomes in the in-hospital setting 6,13–17. Trends in lactate levels are associated with the effectiveness of resuscitation, even in patients with normal vital signs11,18. Hospital and emergency department-based studies have validated hand-held point of care (P-LAC) devices in sepsis and suggest the utility of P-LAC testing among trauma patients7,8,19. P-LAC devices are now available for prehospital use and are operationally similar to glucometers with a per-patient cost of only a few dollars. Preliminary evidence from out-of-hospital studies suggests that P-LAC also predicts death, organ dysfunction, and the need for operative intervention independent of vital signs and GCS12,20,21. We hypothesized that P-LAC is superior to SBP in predicting the need for early resuscitative care following injury.
The objective of this study was to compare prehospital P-LAC to prehospital hypotension for predicting the need for early resuscitative care in trauma patients transported by ground EMS agencies across 9 geographic regions in North America.
Methods:
This was a prospective observational study at 8 regional clinical centers and 1 satellite center within the Resuscitation Outcomes Consortium (ROC). ROC is a network of 10 regional clinical centers and 7 satellite clinical centers across North America tasked to study prehospital resuscitation in severe traumatic injury and cardiac arrest. All sites were invited to participate and site leadership selected EMS agencies within the site. EMS agencies with historical data demonstrating a favorable ratio of eligible patients per prehospital vehicle were selected for participation so that the limited number of lactate meters could be most efficiently deployed. Patients were recruited from 23 ground-based, EMS agencies (air transport will be reported separately) serving a mix of urban, suburban and rural regions with a total catchment area of approximately 10 million people. The Institutional Review Boards and Research Ethics Boards of all the participating ROC institutions approved this study as minimal risk; informed consent was not required for participation.
Blinded P-LAC values were collected following IV placement in blunt or penetrating trauma patients transported by ground EMS agencies to a level I or II trauma center. Patients were included in the study and further data collection performed on those with a prehospital SBP ≤ 100 mmHg. Exclusion criteria included age younger than 15 years, obvious isolated penetrating head injury, drowning, asphyxia due to hanging, burns on more than 20% of total body surface area, or prisoner status.
In addition, patients presenting with systolic blood pressure ≤ 70 mmHg were excluded from the primary analyses a priori, as the probability of requiring resuscitative care is known to be greater than 50% in that cohort.4
A drop of blood obtained during intravenous line (IV) insertion (either with the first or second IV) was placed on a measurement strip for lactate testing by the hand-held, point of care (POC) device (Lactate Pro, Arkray, Japan). If a patient had already received fluid through an IV and an additional IV was required, the lactate sample was obtained from the second IV upon insertion. To avoid influencing prehospital care or transport decisions, EMS providers were blinded to the initial lactate and remained blinded until hospital arrival by obscuring the value displayed on the screen. The receiving care team was also blinded to the lactate value. Following arrival at the hospital, EMS recorded the lactate measurement, the time and location of blood sampling, and the qualifying blood pressure.
A centralized, web-based data collection system was employed using standardized definitions, a manual of operations (including detailed instructions for data collection) and standard data entry formats. Data entry and quality were overseen and maintained by the ROC Data Coordinating Center (DCC). The DCC used rigorous quality assurance processes for data collection and processing, identical to those described for a previous observational trauma cohort in ROC22.
Variables collected included baseline demographics, injury characteristics, and care process data including age, sex, mechanism of injury, prehospital Glasgow Coma Scale (GCS), serial prehospital vital signs (heart rate [HR], systolic blood pressure [SBP], and respiratory rate [RR]), and revised trauma score (RTS). Outcomes collected included survival and need for resuscitative care. Also recorded were type and amount of fluid administered prior to lactate draw, total fluids, and date and time of procedures (laparotomy, thoracotomy, pelvic fixation, or angiographic hemorrhage control) in the first 6 hours after ED arrival. In addition, data regarding potential adverse events including transport delays, device issues, and documented protocol violations were collected. Following the patient’s evaluation, the Injury Severity Score (ISS) was calculated.
The primary endpoint was the need for resuscitative care (RC), defined as any of the following within 6 hours of ED arrival: blood transfusion ≥ 5 units, intervention for hemorrhage including thoracotomy, laparotomy, pelvic fixation or interventional radiology (IR) embolization, or death (including death prior to hospital arrival).
In the primary analysis, the sensitivity of P-LAC ≥ 2.5 mmol/L for identifying patients requiring early resuscitative care was compared to that of SBP ≤ 90 mmHg using McNemar’s test. The P-LAC cutoff of 2.5 mmol/L was selected by an a priori decision rule requiring the specificity to be the same as that of SBP ≤ 90 mmHg. SBP ≤ 90 mmHg was selected as it is one of the criteria used in the 2012 CDC Guidelines for Field Triage of Trauma Patients.23 In secondary analyses, the McNemar test was repeated on subgroups of interest defined by mechanism of injury (blunt vs. penetrating) and time to lactate measurement (within 15 minutes of dispatch to lactate sample vs. greater than 15 minutes). The receiver operating characteristic area under the curve (AUC) for the binary classification of patients as needing or not needing RC was estimated for P-LAC, SBP and Shock Index (SI = HR/SBP). The P-LAC AUC was compared to the AUCs of SBP and SI.24
Multivariable logistic regression was used to evaluate the predictive value of P-LAC above other clinically available variables to identify injured patients requiring early RC. Lactate was modeled as a linear spline with knots at 2.5 and 4.0 mmol/L and evaluated over and above the predictive ability of age, sex, mechanism of injury, prehospital vital signs (SBP, shock index, GCS), airway status, and regional site.
Sensitivity analyses were performed using two alternative definitions of RC: one in which the blood transfusion component was any blood within 6 hours of ED arrival rather than ≥ 5 units and one in which the death component was any prehospital or in-hospital death rather than death within 6 hours of ED arrival.
Analyses were conducted using R v3.01 and the following R libraries: pROC, epiR, mice, and exact2×2 25–28.
Results:
Of the 1,251 eligible patients who met the inclusion/exclusion criteria over an 18 month period, 514 had a lactate measurement attempted and 492 yielded a valid P-LAC measurement. Of these, 387 (79%) presented with SBP 71–100 and were included in the primary analyses; 70 (18%) required RC. Among the 105 excluded patients presenting with SBP ≤ 70, 56 (53%) required RC (Figure 1). The primary analysis sample was enrolled from the following sites (enrollment in parenthesis): Dallas-Fort Worth (105), Milwaukee (99), Seattle-King County (84), Birmingham, Alabama (51), Pittsburgh (23), Memphis (10), San Diego (10), and British Columbia (5).
Figure 1.
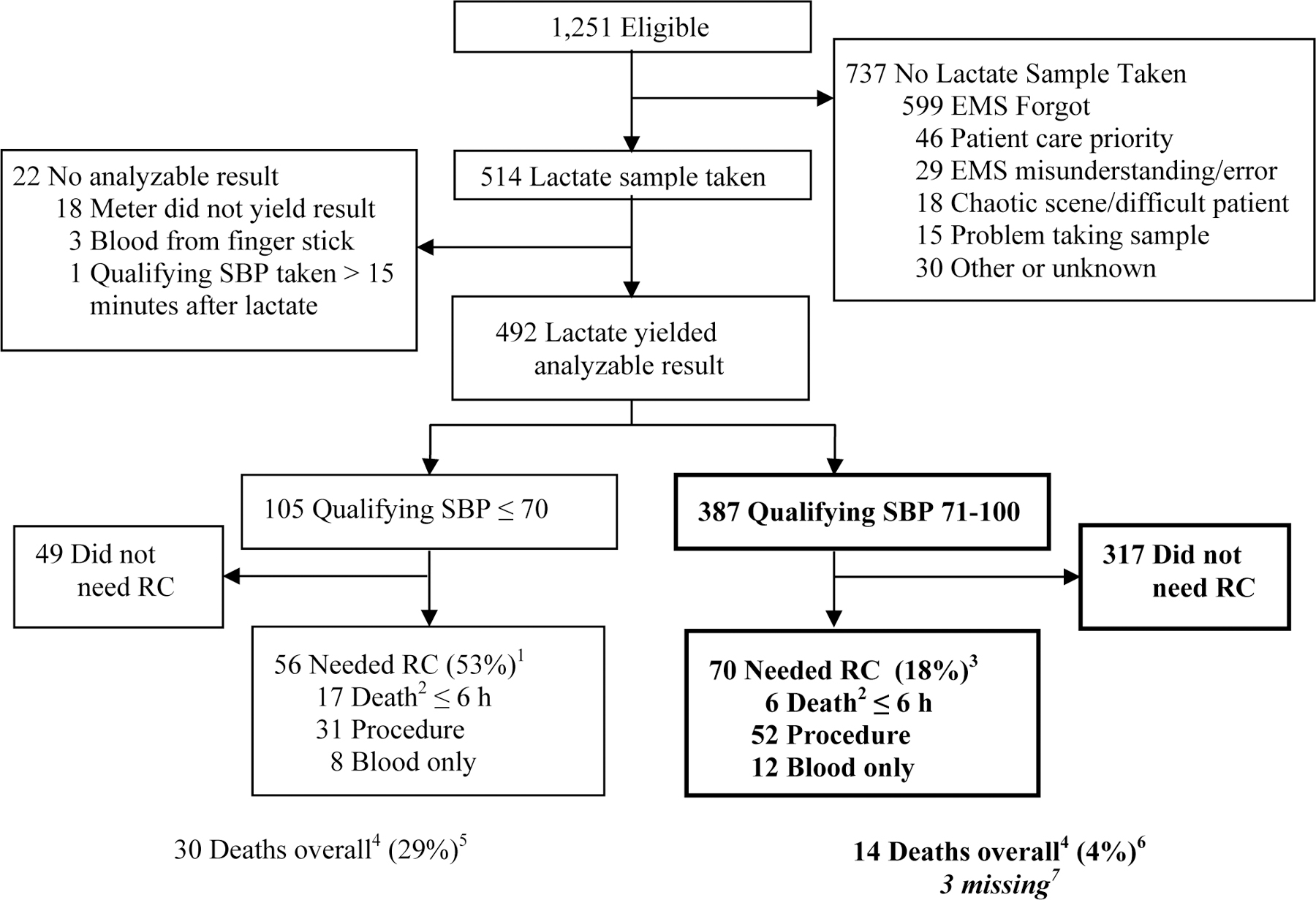
Patient Enrollment
Abbreviations: EMS, Emergency Medical Services; RC, Resuscitative Care; SBP, systolic blood pressure.
1. The percent is the portion of patients with qualifying SBP ≤ 70 that needed RC.
2. Patients in the “Death” category may have also had a procedure and/or ≥ 5 units of blood products. Patients with a procedure may have had ≥ 5 units of blood products.
3. The percent is the portion of patients with qualifying SBP between 71 and100 that needed RC.
4. Death here is “any death” and includes patients who died more than 6 hours after ED arrival. Those who died beyond 6 hours may have met the RC definition by getting a procedure or having ≥ 5 units of blood products before 6 hours.
5. The percent is the portion of patients with qualifying SBP ≤ 70 that died.
6. The percent is the portion of patients with qualifying SBP between 71 and 100 that died.
7. These are patients for whom hospital discharge status is unknown: one withdrew consent from another ROC study, one was transferred to a locked unit in the ICU (police custody), and one was transferred to a non-ROC hospital.
The demographic, injury, and prehospital care data of patients who required RC were compared to those who did not (Table 1). Those requiring RC were significantly more likely to be non-white or Hispanic and more likely to suffer a penetrating mechanism of injury. They also had a significantly higher mean ISS, lower initial SBP, higher initial heart rate, higher SI, lower RTS, and higher P-LAC. The populations did not differ significantly with regard to initial respiratory rate or initial GCS. The P-LAC cutpoint of 2.5 mmol/L was chosen to yield approximately the same estimated specificity as SBP ≤ 90 mmHg (49% vs. 48%).
TABLE 1.
Demographic, Injury and Prehospital Care Characteristics Among Patients with SBP 71–100
| Need for Resuscitative Care? | Difference (95% CI) |
||
|---|---|---|---|
| Yes (n=70) | No (n=317) | ||
| Age, Mean (SD), y | 35.9 (16.5) | 39.6 (17.9) | −3.6 (−8.0, 0.8) |
| Male, No. (%) | 53 (75.7) | 214 (67.5) | 8.2 (−4.0, 20.4) |
| Non-White or Hispanic1, No. (%) | 46 (73.0) | 168 (56.9) | 16.1 (2.8, 29.4) |
| Penetrating trauma, No. (%) | 42 (60.0) | 69 (21.8) | 38.2 (25.0, 51.4) |
| ISS2, Mean (SD) | 22.3 (14.8) | 9.8 (10.1) | 12.6 (8.8, 16.4) |
| > 15, No. (%) | 43 (64.2) | 76 (24.0) | 40.2 (26.9, 53.5) |
| Head AIS > 2, No. (%) | 7 (10.0) | 43 (13.6) | −3.6 (−12.4, 5.3) |
| Qualifying SBP, Mean (SD), mmHg | 87.5 (7.2) | 89.9 (7.9) | −2.4 (−4.3, −0.5) |
| ≤ 90, No. (%) | 47 (67.1) | 166 (52.4) | 14.8 (1.6, 27.9) |
| Shock index | 1.20 (0.33) | 1.01 (0.25) | 0.19 (0.11, 0.27) |
| Initial GCS, Mean (SD) | 12.7 (4.0) | 13.4 (3.0) | −0.7 (−1.7, 0.3) |
| Initial respiratory rate2, Mean (SD), breaths/m | 19.0 (6.8) | 19.6 (4.9) | −0.5 (−2.2, 1.2) |
| Initial heart rate2, Mean (SD), beats/m | 104.4 (24.7) | 91.1 (21.0) | 13.3 (7.0, 19.6) |
| Revised Trauma Score2, Mean (SD) | 6.79 (1.39) | 7.16 (0.90) | −0.38 (−0.72, −0.03) |
| Lactate, Mean (SD), mmol/L | 6.2 (3.7) | 3.7 (3.4) | 2.5 (1.6, 3.5) |
| ≥ 2.5, No. (%) | 65 (92.9) | 163 (51.4) | 41.4 (32.4, 50.5) |
| ≥ 2.5 and SBP 91–100, No. (%) | 21 (30.0) | 72 (22.7) | 7.3 (−5.3, 19.8) |
Abbreviations: AIS, Abbreviated Injury Scale; CI, confidence interval; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; SBP, systolic blood pressure.
Race or ethnicity is not reported for 29 patients.
ISS is missing for 3 patients; Respiratory rate and Revised Trauma Score are missing for 2 patients; and heart rate is missing for 1 patient.
The observed sensitivities associated with these cut points were 93% (95% CI 84–98%) for P-LAC ≥ 2.5 mmol/L and 67% (CI 55–78%) for SBP ≤ 90 mmHg. This difference in sensitivity is statistically significant by McNemar’s test (p < 0.001). In addition, P-LAC < 2.5 mmol/L has a negative predictive value (NPV) of 97% (CI 93–99%) compared to 87% (CI 81–91%) for a SBP > 90 mmHg and the estimated area under the curve (AUC) associated with all possible P-LAC cut points in these data is 0.78 (95% CI 0.73–0.83), which is statistically significantly superior to that of SBP [0.59 (CI 0.53–0.66)] and SI [0.66 (CI 0.60–0.74)] (Figure 2). The 2×2 table in Figure 2 presents a cross of the P-LAC ≥ 2.5 mmol/L and SBP ≤ 90 mmHg decision rules among the patients who needed RC. The P-LAC rule identified 21 of the patients not identified by the SBP rule as needing RC while the SBP rule only identified 3 patients that the P-LAC rule did not identify. In sensitivity analyses exploring alternative definitions of RC, one in which in-hospital deaths beyond 6 hours after ED arrival were added to the composite endpoint (RC + later death) and one in which patients receiving blood transfusions < 5 units within 6 hours of ED arrival were added to the composite endpoint (RC + any blood), there were no qualitative differences in results for the McNemar test or the comparison of AUC.
Figure 2.
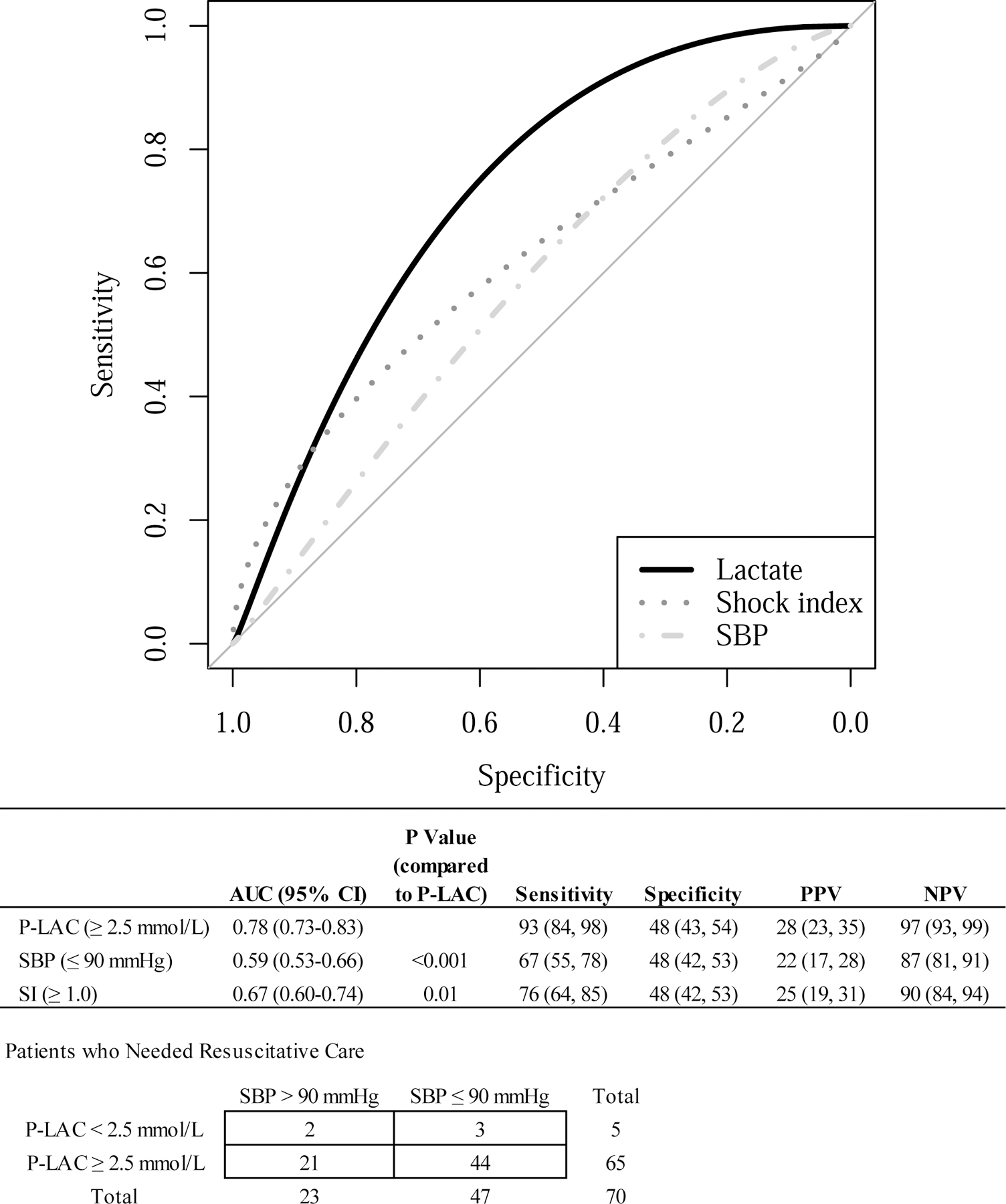
Receiver Operating Characteristics for Lactate, Shock Index, and SBP
Abbreviations: P-LAC, prehospital point of care lactate; SBP, systolic blood pressure; SI, shock index.
McNemar tests comparing P-LAC to SBP were performed on subgroups defined by mechanism of injury (blunt, n=276 vs. penetrating, n=111) and time to lactate (within 15 minutes of dispatch, n=84 vs. greater than 15 minutes, n=302). A P-LAC decision rule with comparable specificity to SBP ≤ 90 has a substantially higher estimated sensitivity than SBP ≤ 90 for patients with blunt injury: 96% (CI 82–100%) vs. 64% (CI 44–81%) (p = 0.01). The estimated difference in sensitivity for patients experiencing penetrating trauma is smaller: 79% (CI 63–90%) for the P-LAC decision rule compared to 69% (CI: 53–82%) for SBP ≤ 90 (p = 0.42).
P-LAC sensitivity (100%, CI 73–100%) exceeds that of SBP (59%, CI 33–82%) for both early P-LAC, defined as time less than 15 minutes from 911 call to the lactate measure, and late P-LAC, defined as time greater than 15 minutes [P-LAC 89% (CI 77–96%) vs. SBP 70% (CI 56–82%)]. All patients in the short time group who required RC had a P-LAC ≥ 3.4.
To explore further the relationship between lactate level and the need for RC, we examined a multiple logistic model including lactate as a linear spline with knots at 2.5 and 4.0 mmol/L, cutpoints selected from previous studies10. The adjusted odds ratios relating RC to a 1 mmol/L difference in P-LAC within the ranges <2.5, 2.5–3.9, and ≥ 4.0 mmol/L were 1.76 (95% CI 0.41–12.93), 3.61 (CI 1.67–8.35) and 0.97 (CI 0.87–1.07), respectively (Table 2). These results indicate that the adjusted linear association between P-LAC and RC is primarily evident in the 2.5–3.9 mmol/L range and that beyond 4.0 mmol/L, higher levels of lactate are not associated with additional increases in probability of RC. This is consistent with the unadjusted association (Figure 3), in which the greatest increase in the probability of RC is in the range of 2–4 mmol/L, with an apparent leveling-off beyond this range. Because of the sparsity of data at higher P-LAC levels and the concern that only a few patients’ data might have a disproportionate effect on the parameter estimates, the model in Figure 3 excludes the five patients for whom P-LAC was greater than 18 mmol/L29.
TABLE 2.
Multivariable Logistic Regression Model
| Unadjusted | Adjusted | |
|---|---|---|
| Model variables1 | Odds ratio (95% CI) | Odds ratio (95% CI) |
| Lactate2 (per mmol/L in range) | ||
| < 2.5 (n=159) | 2.96 (0.62, 14.18) | 1.76 (0.41, 12.93) |
| 2.5–3.9 (n=98) | 4.51 (2.25, 9.05) | 3.61 (1.67, 8.35) |
| ≥ 4.0 (n=130) | 0.98 (0.90, 1.06) | 0.97 (0.87, 1.07) |
| Age3 | ||
| per 5-year increment for age < 45 | 0.94 (0.81, 1.08) | 1.02 (0.85, 1.23) |
| per 5-year increment for age ≥ 45 | 0.95 (0.79, 1.14) | 1.15 (0.91, 1.43) |
| Male | 1.51 (0.83, 2.73) | 0.72 (0.33, 1.58) |
| Penetrating injury | 5.47 (3.16, 9.47) | 4.82 (2.17, 11.31) |
| SBP (per 5 mmHg) | 0.82 (0.69, 0.96) | 0.92 (0.73, 1.15) |
| Shock index (per increment of 0.1) | 1.28 (1.16, 1.41) | 1.21 (1.06, 1.38) |
| Initial GCS (per increment of 1) | 0.94 (0.88, 1.01) | 1.01 (0.90, 1.13) |
| Any airway/BVM attempted | 4.41 (2.32, 8.38) | 4.55 (1.40, 15.43) |
Abbreviations: BVM, bag valve mask; CI, confidence interval; GCS, Glasgow Coma Scale; SBP, systolic blood pressure.
Site adjusted for as a fixed effect but not presented. Two patients who were missing shock index were excluded from the model.
Modeled as linear spline with knots at 2.5 and 4.0 mmol/L. The estimate for a given lactate range is the ratio of odds for need for resuscitative care between two patients who both have lactates within the same range (e.g. 2.5–3.9) and have the same covariate data except that their lactates differ by 1 mmol/L.
Age modeled as spline with one knot specified at 45 years.
Figure 3.
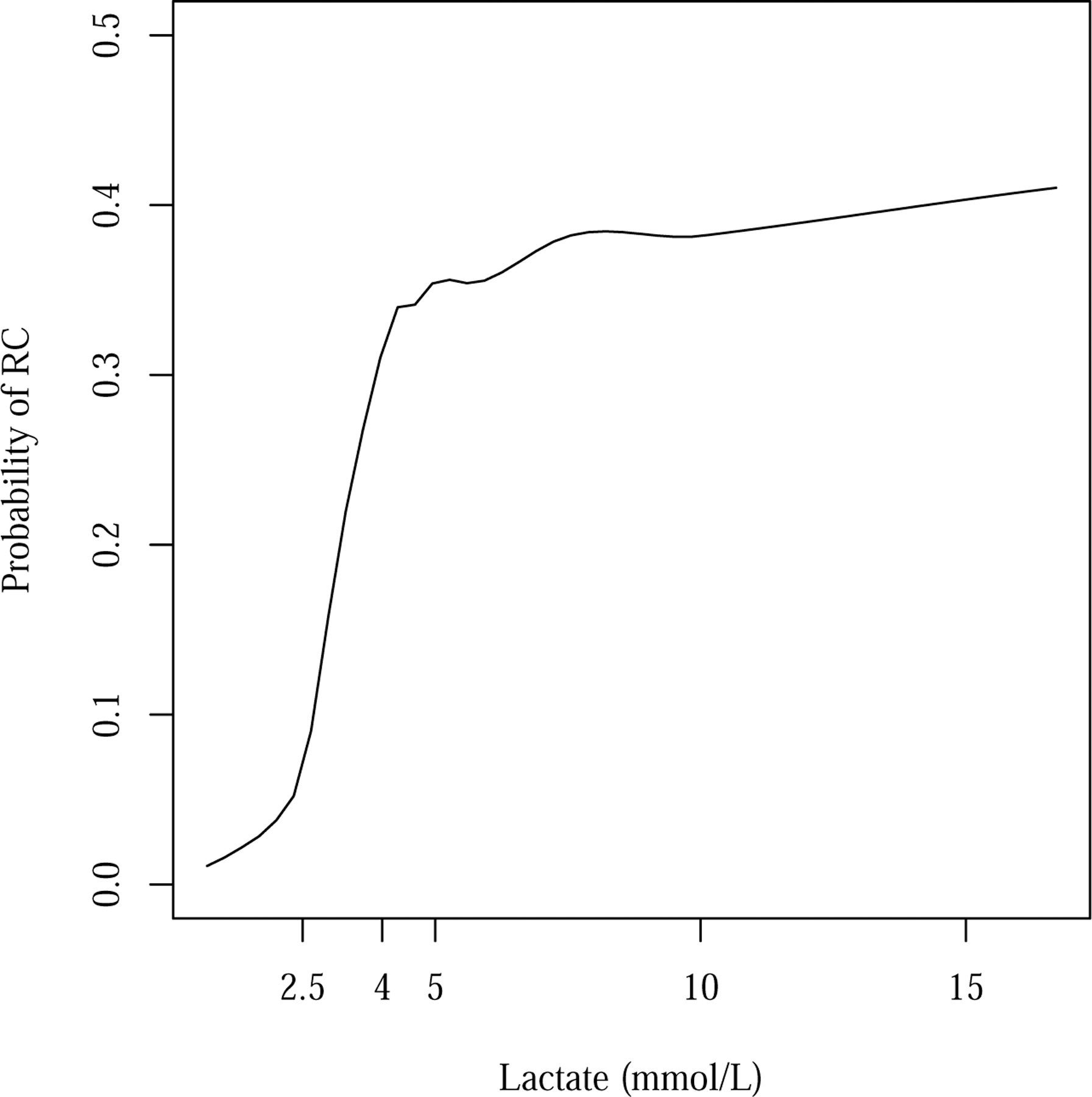
Estimated Probability of Need for Resuscitative Care (RC) as a Function of Prehospital Lactate
Loess smoother29 with span of 0.5 and degree of 1.
Discussion:
Compared to field vital signs and other injury related information, P-LAC is more useful in the recognition of trauma patients in need of RC.1–3 Consistent with previous studies, as P-LAC increases so does the need for RC.12,20 We selected a cut off point of 2.5 mmol/L for lactate to have the same observed specificity as a systolic blood pressure (SBP) ≤90 mmHg since this categorization is one of the key physiologic criteria used in the CDC guidelines for trauma triage.23 Using a lactate cut point of 2.5 mmol/L is also consistent with previous studies looking to identify severe injury in trauma patients.13–15 In our sample, a decision rule based on P-LAC ≥2.5 mmol/L was more sensitive than a SBP ≤90 mmHg for identifying the need for RC within 6 hours. When included in a multivariable model accounting for differences in age, sex, SBP, shock index, GCS, airway status, injury type, and regional site, prehospital P-LAC remained a significant independent predictor of the need for RC.
The prehospital recognition of shock in trauma patients is often inadequate leading to underestimation of severity and potential delays to definitive care7,8,30. Despite over-triage rates estimated to be 25–50%31, more than 1.8 million trauma patients a year may be inappropriately under-triaged, increasing the time to definitive care and resulting in increased morbidity and mortality3. Current physiologic criteria are limited in their ability to predict shock and miss many severely injured patients due to population variation, effects of medication, associated comorbid illness, and presence of underlying intoxicants. Other surrogates for hypoperfusion, such as near infrared spectroscopy (NIRs) and base deficit, are not easily interpreted or available. P-LAC, which more directly represents tissue perfusion, appears to be an important adjunct to existing physiologic criteria.
Hyperlactemia, as a result of lactic acidosis, is a marker of cellular hypoxia. P-LAC measurements allow for an early assessment of global perfusion, which is useful for the recognition of underlying severe traumatic injury and may also aid in prognosis as well as management of these through frequent reassessment. P-LAC may also prevent inappropriate treatment of patients whose baseline pressure is low by providing another data point at the time of assessment. Furthermore, P-LAC may also be important in secondary triage decisions, which are necessary when patients are transferred from community hospitals to trauma centers.
In contrast to previous studies of prehospital lactate, this cohort of patients was sampled from disparate geographic regions within the ROC consortium. Previous studies involved single centers and highly selected subpopulations12,20. In addition, this study was designed to compare P-LAC to data available at the time of triage by EMS. This may help us further define the role of P-LAC in primary and secondary triage in a way that is generalizable for all EMS systems. In addition to the potential role of P-LAC in destination triage, P-LAC may also assist in prehospital activation of trauma teams thereby allowing a more appropriate level of activation and judicious use of resources. Many trauma centers employ a tiered level of response reserving a full response of resources and personnel for patients with abnormal vital signs.32 Given the association between elevated P-LAC and the need for RC, informing the trauma team may result in an appropriate response and a reduced time to definitive care.
The data in this study are derived from enrollments across nine regions in North America representing a mix of rural, suburban and urban EMS systems transporting to both level one and two trauma centers. Qualifying blood pressures were obtained by both manual sphygmomanometry and automated blood pressure cuffs integrated into cardiac monitors, we are limited by the accuracy of both techniques. However, we were limited to enrolling patients with SBP≤100 mmHg, which may have prevented sampling from some patients with higher blood pressures and occult shock. Sampling of all patients would have yielded important data regarding overtriage but at an order of magnitude of additional cost. In addition, these cut points are only used to allow comparison of the fixed specificity of the current “gold standard” for prehospital assessment of hypoperfusion (blood pressure). The cut points are not intended to be clinical decision points and may result in over-triage if they are extended to a patient population that does not meet our eligibility criteria. The sampling of P-LAC was restricted to a venous sample from an IV line that was placed as part of standard clinical care. As a result many patients were excluded due to the inability to obtain a venous sample. Prehospital providers also failed to obtain P-LAC when they perceived the scene to be dangerous. This may have been related to the patient’s severity of injury or concerns for crew safety. In many cases the provider simply forgot to perform the lactate (Figure 1). This may have been related to the relatively low frequency of eligible patients. In a prior study where all trauma patients were enrolled, compliance was 66%.12 Review of available data on missed patients indicates that they have slightly higher blood pressures on average (90.6 vs. 89.5 mmHg). While we do not have reason to believe that there was selection bias that would significantly impact our results or conclusions, we do not have data available that would rule this out. Although blinded to the P-LAC, the trauma teams were not restricted from seeing values obtained in the trauma bay. It is possible that knowledge of an elevated lactate may have biased the trauma team towards intervention, though a recent study has suggested that when other data such as imaging is available, blood lactate has little impact on hospital course17. PLAC can be falsely elevated in the context of seizure, dehydration, and excessive alcohol consumption, which may further confound our interpretation of these data.
POC lactate obtained at the scene of injury is strongly associated with the need for RC. P-LAC is superior to other early surrogates for hypoperfusion (SBP and SI) in predicting the need for RC in trauma patients with 70 < SBP ≤ 100 mmHg. Prospective validation of P-LAC as a triage tool and trigger for protocolized trauma resuscitation is warranted.
Funding/Support:
The ROC is supported by a series of cooperative agreements to nine regional clinical centers and one Data Coordinating Center (5U01 HL077863-University of Washington Data Coordinating Center, HL077866-Medical College of Wisconsin, HL077867-University of Washington, HL077871-University of Pittsburgh, HL077873-Oregon Health and Science University, HL077881-University of Alabama at Birmingham, HL077887-University of Texas SW Medical Ctr/Dallas, HL077908-University of California San Diego) from the National Heart, Lung and Blood Institute in partnership with the U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defense Research and Development Canada, the Heart, Stroke Foundation of Canada and the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Footnotes
The authors do not have any conflicts of interest.
References
- 1.McGee S, Abernethy WB, 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA Mar 17 1999;281(11):1022–1029. [DOI] [PubMed] [Google Scholar]
- 2.Brasel KJ, Guse C, Gentilello LM, Nirula R. Heart rate: is it truly a vital sign? J Trauma Apr 2007;62(4):812–817. [DOI] [PubMed] [Google Scholar]
- 3.Committee on Trauma of the American College of Surgeons. Resources for the optimal care of the injured patient Chicago IL: American College of Surgeons; 2006. [Google Scholar]
- 4.Bulger EM, Jurkovich GJ, Nathens AB, Copass MK, Hanson S, Cooper C, Liu PY, Neff M, Awan AB, Warner K, Maier RV. Hypertonic resuscitation of hypovolemic shock after blunt trauma: a randomized controlled trial. Arch Surg Feb 2008;143(2):139–148; discussion 149. [DOI] [PubMed] [Google Scholar]
- 5.Newgard CD, Rudser K, Hedges JR, Kerby JD, Stiell IG, Davis DP, Morrison LJ, Bulger E, Terndrup T, Minei JP, et al. A critical assessment of the out-of-hospital trauma triage guidelines for physiologic abnormality. J Trauma 2010;68(2):452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandromme MJ, Griffin RL, Weinberg JA, Rue LW 3rd, Kerby JD. Lactate is a better predictor than systolic blood pressure for determining blood requirement and mortality: could prehospital measures improve trauma triage? J Am Coll Surg 2010;210(5):861–867, 867–869. [DOI] [PubMed] [Google Scholar]
- 7.Claridge JA, Crabtree TD, Pelletier SJ, Butler K, Sawyer RG, Young JS. Persistent occult hypoperfusion is associated with a significant increase in infection rate and mortality in major trauma patients. J Trauma 2000;48(1):8–14; discussion 14–15. [DOI] [PubMed] [Google Scholar]
- 8.Crowl AC, Young JS, Kahler DM, Claridge JA, Chrzanowski DS, Pomphrey M. Occult hypoperfusion is associated with increased morbidity in patients undergoing early femur fracture fixation. J Trauma 2000;48(2):260–267. [DOI] [PubMed] [Google Scholar]
- 9.Lipsky AM, Gausche-Hill M, Henneman PL, Loffredo AJ, Eckhardt PB, Cryer HG, de Virgilio C, Klein SL, Bongard FS, Lewis RJ. Prehospital hypotension is a predictor of the need for an emergent, therapeutic operation in trauma patients with normal systolic blood pressure in the emergency department. J Trauma Nov 2000;65(5):1228–1233. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med 2005;45(5):524–528. [DOI] [PubMed] [Google Scholar]
- 11.Jansen TC, van Bommel J, Mulder PG, Rommes JH, Schieveld SJ, Bakker J. The prognostic value of blood lactate levels relative to that of vital signs in the pre-hospital setting: a pilot study. Crit Care 2008;12(6):R160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyette F, Suffoletto B, Castillo JL, Quintero J, Callaway C, Puyana JC. Prehospital serum lactate as a predictor of outcomes in trauma patients: a retrospective observational study. J Trauma 2011;70(4):782–786. [DOI] [PubMed] [Google Scholar]
- 13.Regnier MA, Raux M, Le Manach Y, Asencio Y, Gaillard J, Devilliers C, Langeron O, Riou B. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology Dec 2012;117(6):1276–1288. [DOI] [PubMed] [Google Scholar]
- 14.Odom SR, Howell MD, Silva GS, Nielsen VM, Gupta A, Shapiro NI, Talmor D. Lactate clearance as a predictor of mortality in trauma patients. J Trauma Acute Care Surg Apr 2013;74(4):999–1004. [DOI] [PubMed] [Google Scholar]
- 15.Salottolo KM, Mains CW, Offner PJ, Bourg PW, Bar-Or D. A retrospective analysis of geriatric trauma patients: venous lactate is a better predictor of mortality than traditional vital signs. Scand J Trauma Resusc Emerg Med 2013;21:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouellet JF, Roberts DJ, Tiruta C, Kirkpatrick AW, Mercado M, Trottier V, Dixon E, Feliciano DV, Ball CG. Admission base deficit and lactate levels in Canadian patients with blunt trauma: are they useful markers of mortality? J Trauma Acute Care Surg 2012;72(6):1532–1535. [DOI] [PubMed] [Google Scholar]
- 17.Vohra T, Paxton J. Abnormal arterial blood gas and serum lactate levels do not alter disposition in adult blunt trauma patients after early computed tomography. West J Emerg Med 2013;14(3):212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abramson D, Scalea TM, Hitchcock R, Trooskin SZ, Henry SM, Greenspan J. Lactate clearance and survival following injury. J Trauma 1993;35(4):584–588; discussion 588–589. [DOI] [PubMed] [Google Scholar]
- 19.Coats TJ, Smith JE, Lockey D, Russell M. Early increases in blood lactate following injury. J R Army Med Corps 2002;148(2):140–143. [DOI] [PubMed] [Google Scholar]
- 20.Shah A, Guyette F, Suffoletto B, Schultz B, Quintero J, Predis E, King C. Diagnostic accuracy of a single point-of-care prehospital serum lactate for predicting outcomes in pediatric trauma patients. Pediatr Emerg Care Jun 2013;29(6):715–719. [DOI] [PubMed] [Google Scholar]
- 21.Asimos AW, Gibbs MA, Marx JA, Jacobs DG, Erwin RJ, Norton HJ, Thomason M. Value of point-of-care blood testing in emergent trauma management. J Trauma 2000;48(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 22.Newgard CD, Sears GK, Rea TD, Davis DP, Pirrallo RG, Callaway CW, Atkins DL, Stiell IG, Christenson J, Minei JP, Williams CR, Morrison LJ; ROC Investigators. The Resuscitation Outcomes Consortium Epistry-Trauma: design, development, and implementation of a North American epidemiologic prehospital trauma registry. Resuscitation 2008;78(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasser SM, Hunt RC, Sullivent EE, Wald MM, Mitchko J, Jurkovich GJ, Henry MC, Salomone JP, Wang SC, Galli RL, et al. ; National Expert Panel on Field Triage, Centers for Disease Control and Prevention (CDC). Guidelines for field triage of injured patients. Recommendations of the National Expert Panel on Field Triage. MMWR Recomm Rep 2009;58(RR-1):1–35. [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology Sep 1983;148(3):839–843. [DOI] [PubMed] [Google Scholar]
- 25.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson M, Nunes T, Sanchez J, et al. epiR: An R package for the analysis of epidemiological data. R package 2013;version 0.9–48. [Google Scholar]
- 27.R Core Team. R: A language and environment for statistical computing 2013.
- 28.Fay M Two-sided Exact Tests and Matching Confidence Intervals for Discrete Data. R Journal 2010;2(1):53–58. [Google Scholar]
- 29.Chambers JM, Hastie T. Statistical Methods in S: Wadsworth and Brooks; 1992. [Google Scholar]
- 30.Eastridge BJ, Salinas J, McManus JG, Blackburn L, Bugler EM, Cooke WH, Convertino VA, Wade CE, Holcomb JB. Hypotension begins at 110 mm Hg: redefining “hypotension” with data. J Trauma 2007;63(2):291–297; discussion 297–299. [DOI] [PubMed] [Google Scholar]
- 31.Lowe DK, Oh GR, Neely KW, Peterson CG. Evaluation of injury mechanism as a criterion in trauma triage. Am J Surg 1986;152(1):6–10. [DOI] [PubMed] [Google Scholar]
- 32.Eastes LS, Norton R, Brand D, Pearson S, Mullins RJ. Outcomes of patients using a tiered trauma response protocol. J Trauma May 2001;50(5):908–913. [DOI] [PubMed] [Google Scholar]


