Abstract
Background.
Sjögren syndrome (SS) is an autoimmune disease characterized by lymphocytic infiltration and diminished secretory function of the salivary glands. Dexamethasone (DEX) resolves dry mouth and lymphocytic infiltration; however, this treatment is difficult to maintain because of multiple adverse effects (eg, osteoporosis and skin thinning); likewise, aspirin-triggered resolvin D1 (AT-RvD1) increases saliva secretion but cannot eliminate lymphocytic infiltration. Previous studies showed that a combination of low-dose DEX with AT-RvD1 before disease onset prevents SS-like features in a mouse model; however, this is not clinically practical because there are no reliable indicators of SS before disease onset. Therefore, the authors applied the combined treatment at disease onset to show its efficacy and comparative lack of adverse effects, so that it may reasonably be maintained over a patient’s lifetime.
Methods.
NOD/ShiLtJ mice were treated with ethanol (vehicle control), high-dose DEX alone, AT-RvD1 alone, or a combination of low-dose DEX with AT-RvD1 at disease onset for 8 weeks. Then saliva flow rates were measured, and submandibular glands were harvested for histologic analyses.
Results.
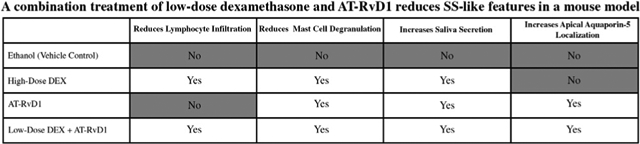
A combined treatment of low-dose DEX with AT-RvD1 significantly decreased mast cell degranulation and lymphocytic infiltration, increased saliva secretion, and restored apical aquaporin-5 expression in submandibular glands of NOD/ShiLtJ mice.
Conclusions.
Low-dose DEX combined with AT-RvD1 reduces the severity of SS-like manifestation and prevents the development of advanced and potentially irreversible damage, all in a form that can reasonably be administered indefinitely without the need to cease treatment because of secondary effects.
Keywords: Salivary glands, inflammation, resolvins, steroids, lipid mediators
Graphical Abstract
Introduction
Sjögren syndrome (SS) is a systemic autoimmune disease characterized by loss of saliva and tear secretion mediated by salivary and lacrimal glands, respectively.1 Specifically, the glandular inflammation induced by lymphocytic infiltration and mast cell degranulation results in a loss of epithelial acinar cells that mediate fluid secretion.1 Primary and secondary SS occur in the absence or presence of other rheumatic disorders such as lupus erythematosus, rheumatoid arthritis, or scleroderma.2 Genetic predisposition and environmental factors influence the development of SS; however, the specific causes and effective therapies are not known.3–7 Given the heterogeneity of the clinical manifestations in SS, early diagnosis is difficult, and patients are often only identified once saliva secretion has been significantly diminished.8
Dexamethasone (DEX) is a common treatment for SS because it reduces salivary gland inflammation, restores saliva secretion, and greatly diminishes lymphocytic infiltration9,10 that will inevitably lead to a reversal of treatment gains if left to proliferate; however, DEX treatment has significant issues. Specifically, treatments must be continued throughout the life span to maintain the desired effects, but significant adverse effects accrue that negatively affect a patient’s quality of life.11–14 To make DEX treatment for salivary gland inflammation more viable for lifelong use, we explore the use of a diminished dosage of DEX with aspirin-triggered resolvin D1 (AT-RvD1), which is reported to be effective in managing salivary gland inflammation, as detailed below. In so doing, we aim to preserve treatment gains achieved with higher doses of DEX but at a dosage threshold that will not discourage patients from continuing treatment indefinitely.
Resolution of inflammation is an actively regulated process mediated in part by a family of specialized proresolving lipid mediators (SPM), which include resolvins, maresins, lipoxins, and protectins as well as their AT forms, which are comparable in their properties to naturally occurring SPM15–22 but have a longer half-life.23 SPM and their AT forms can be an alternative for treating inflammatory diseases by limiting uncontrolled inflammation in response to injury or environmental challenges24–30 while promoting its termination and leading to tissue repair and functionality.31–34 SPM have been detected in human tears,35 plasma,35–39 milk,40 and saliva41 as well as in animal models of infection and chronic inflammation.36,42–46 Studies of SPM and AT forms within the salivary glands have been largely confined to AT-RvD1, one of many in the resolvin family, that has shown particular promise in treating some key features of hyposalivation.9,10 Previous studies showed that mouse and human salivary glands express SPM41,47 with their biosynthetic machinery24 and receptors.48 In salivary glands, the SPM family members, most notably RvD1 and its aspirin-triggered epimer AT-RvD1, have been shown to activate formyl peptide receptor 2 (ALX/FPR2) and promote prosurvival signals both in vitro and in vivo.49–53
Despite the promise shown by SPM in general and AT-RvD1 in particular for treating hyposalivation, a critical obstacle must be overcome for the potential use of this treatment in SS. Specifically, AT-RvD1 does not completely eradicate lymphocytic infiltration, which leads to the secretion of SS-associated proinflammatory cytokines that are known to disrupt epithelial integrity and invariably lead to loss of function.54,55 Subsequent to this observation, a previous study showed that coadministration of low-dose DEX with AT-RvD1 enhances saliva secretion and prevents lymphocytic infiltration in an SS-like mouse model when administered before disease onset.10 However, this treatment to date has been limited by a lack of screening techniques for SS. The disease has no reliable early genetic markers and is typically identified with a loss of saliva secretion not attributable to other causes.8,56 Therefore, we sought to extend prior findings to the early disease onset phase (ie, after lymphocytic infiltration and reduced saliva secretion) by determining whether combined treatment with low-dose DEX with AT-RvD1 reduces SS-like responses in the NOD/ShiLtJ mouse model of SS. Should our study prove successful, a later investigation will seek to extend these findings to cases of late-stage disease manifestation with the aim of reversing the most severe damage caused by SS.
Methods
Animals
Forty female 12-week-old NOD/ShiLtJ mice were randomly divided into 4 groups: ethanol (vehicle control) treated, high-dose DEX treated, AT-RvD1 treated, and a combination of low-dose DEX with AT-RvD1 treated. Specifically, animals were treated twice a week for 8 weeks via tail vein injection with ethanol (3.5% [vol/vol], vehicle control), high-dose DEX (8.25 mg/kg) (Sigma Aldrich), AT-RvD1 alone (0.1 mg/kg) (Cayman Chemical) or a low-dose of DEX (4.125 mg/kg) with AT-RvD1 (0.1 mg/kg). The doses of DEX and AT-RvD1 used in this study were chosen on the basis of a pilot study indicating that these compounds produce a significant downregulation of systemic inflammatory genes.9 In this study, only female NOD/ShiLtJ mice were used in light of the predominance of females affected by SS, with a 9:1 ratio compared with males.57,58 For submandibular gland (SMG) harvesting, mice were euthanized using carbon dioxide at 20 weeks of age, followed by abdominal exsanguination. SMGs were then removed and processed, as detailed in Figure 1. Animals were housed in cages in a room with a controlled environment (12-hour day/night cycles) and provided with a standard pellet diet and water. Moreover, mice were ear-tagged to minimize potential confounding variables, and group allocation at the different stages of the study was controlled by all the people who obtained experimental data. Finally, this study was performed using protocols approved by the Institutional Animal Care and Use Committee and the Animal Research: Reporting In Vivo Experiments guidelines.59
Figure 1.
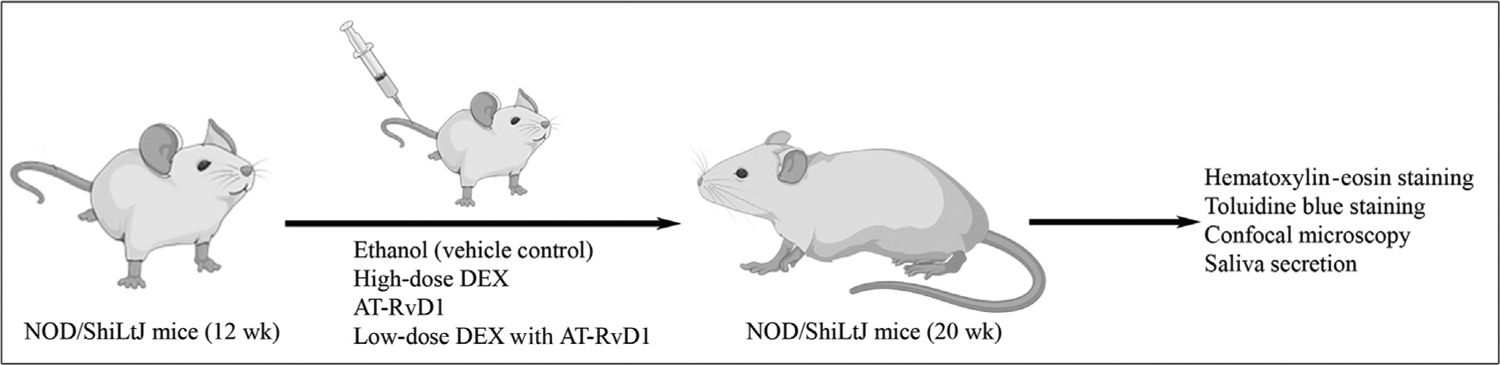
Diagram summarizing the treatments used in the study. Sjögren syndrome–like mice were randomly divided into 4 groups and treated twice a week for 8 weeks via tail vein injection: ethanol (vehicle control), high-dose dexamethasone (DEX), aspirin-triggered resolvin D1 (AT-RvD1), and low-dose DEX with AT-RvD1. After the indicated times, specimens were collected as described in the Methods section. The study was performed using protocols approved by the Institutional Animal Care and Use Committee and the Animal Research Reporting In Vivo Experiments guidelines,59 and figures were generated using biorender.com.
Tissue processing
SMGs were fixed in 10% (vol/vol) formalin for 24 hours at room temperature and then transferred to 70% (vol/vol) ethanol. Next, SMGs were dehydrated through a series of graded ethanol washes (30%, 50%, 70%, twice at 95%, and 3 times at 100%), embedded in paraffin, and cut into 5-μm sections. Paraffin-embedded slides were then deparaffinized by washing 3 times for 5 minutes in 100% (vol/vol) xylene. Slides were washed for 5 minutes in xylene:ethanol (1:1), twice for 5 minutes in 100% (vol/vol) ethanol, followed by 5 minute washes in 95%, 80%, 70%, and 50% ethanol, then twice in distilled water.
Hematoxylin-eosin staining
Deparaffinized and rehydrated tissue sections were stained with hematoxylin for 10 minutes, washed twice for 5 minutes each with tap water, then destained with 0.3% hydrogen chloride for 3 seconds and rinsed twice for 1 minute each with tap water. Next, sections were washed with 95% (vol/vol) ethanol for 1 minute, stained with eosin for 10 minutes, and washed 3 times with 95% (vol/vol) ethanol for 1 minute. Subsequently, samples were rinsed 3 times with 100% (vol/vol) ethanol, cleared in xylene, and mounted with a xylene-based mounting medium (Poly-sciences). Finally, to evaluate histopathologic features, samples were examined using a Leica DMI6000B inverted microscope (Leica Microsystems), and lymphocytic foci size was divided by the total SMG area using ImageJ (National Institutes of Health).
Toluidine blue staining
Deparaffinized and rehydrated tissue sections were stained with toluidine blue working solution (VitroView) for 3 minutes. Next, specimens were washed 3 times with distilled water, and tissue sections were dehydrated by washing 3 times for 3 minutes each in 95% and 100% (vol/vol) alcohol. Then, specimens were washed twice for 3 minutes in xylene and mounted with a xylene-based mounting medium. Finally, to assess mast cell degranulation, samples were examined using a Leica DMI6000B inverted microscope.
Confocal microscopy analyses
Deparaffinized tissue sections were incubated with sodium citrate buffer (10 mM sodium citrate, 0.05% [vol/vol] polyethylene glycol sorbitan monolaurate [Tween 20, Sigma-Aldrich], pH 6.0) at 95 °C for 30 minutes for antigen retrieval. Next, samples were rinsed twice with distilled water and permeabilized with 0.1% (vol/vol) t-octylphenoxypolyethoxyethanol (Triton X-100, Sigma-Aldrich) in phosphate-buffered saline (PBS) at room temperature for 45 minutes. Sections were then blocked with 5% (vol/vol) goat serum in PBS at room temperature for 1 hour and incubated with rabbit–anti-mouse aquaporin-5 (1:100 [ab78486; Abcam]) and rabbit–anti-mouse chymase (1:100 [ab233103; Abcam]) antibodies at 4 °C overnight. Then, specimens were washed 3 times with PBS and incubated with Alexa Fluor 488-conjugated anti-rabbit immunoglobulin G (1:500 [A-11008; ThermoFisher]) at room temperature for 1 hour. Finally, sections were washed 3 times with PBS, nuclei were counterstained with 4′,6-diamidino-2-phenylindole at room temperature for 15 minutes (1:1,000 dilution), and images were analyzed using a confocal Stellaris 5 microscope (Leica Microsystems). The positive area of chymase was calculated by measuring positive pixels using ImageJ in each tissue section.
Measurement of saliva flow rate
Mice were anesthetized with 100 mg/kg of ketamine and 5 mg/kg of xylazine followed by intraperitoneal injection of 50 mg/kg of pilocarpine-hydrogen chloride (Sigma) and 0.5 mg/kg of isoproterenol (Sigma) in PBS to stimulate saliva secretion. Then, the whole saliva was collected for 5 minutes using a 200-μL pipette, and the saliva flow rate was calculated by dividing the total amount of stimulated saliva (μL) by the product of the mouse body weight (g) and the collection time (5 min).
Statistical analyses
Data are presented as mean (SD) from 3 or more determinations. Prism software (GraphPad) was used for statistical analyses by t test or 1-way analysis of variance. P ≤ 0.05 represents significant differences between experimental groups.
Results
A results summary describing treatment effects by groups is provided in the graphical abstract.
Treatment with low-dose DEX with AT-RvD1 reduces lymphocytic infiltration in SMG of SS-like mice
To determine the degree of lymphocytic infiltration in SMG from NOD/ShiLtJ SS-like mice, tissues were stained with hematoxylin-eosin, and histopathologic analysis was performed as described in the Methods section. As shown in Figure 2A, SMG from ethanol (vehicle control) and AT-RvD1–treated mice showed extensive lymphocytic infiltration. Moreover, lymphocytic foci covered more than 40% of the glandular tissue, indicating that systemic AT-RvD1 treatment alone has no appreciable impact on reducing immune cell infiltration or expansion. In contrast, SMG from mice treated with high-dose DEX alone or low-dose DEX with AT-RvD1 showed a significant reduction in lymphocytic foci size per glandular area (Figure 2B).
Figure 2.
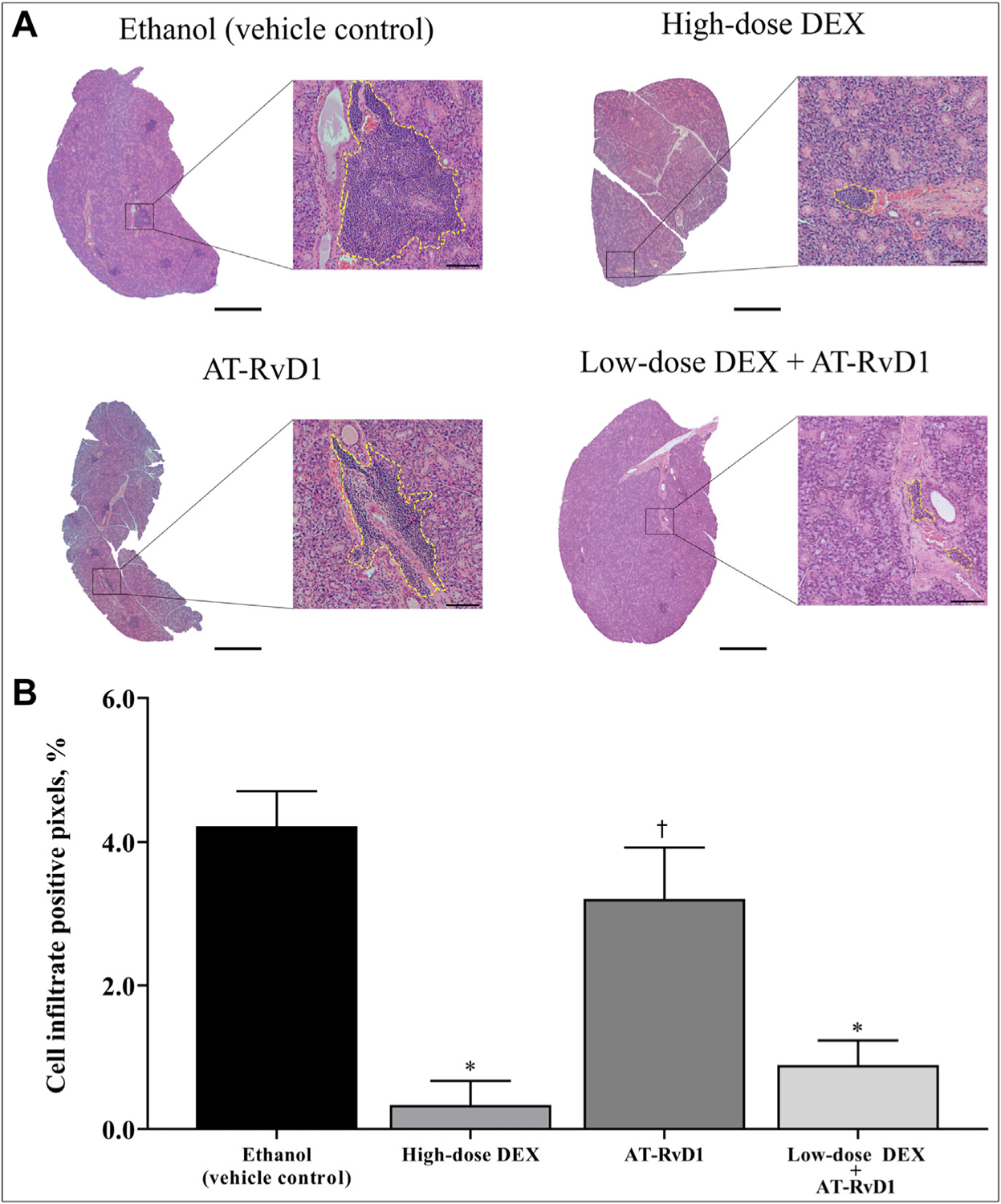
Combined treatment with low-dose dexamethasone (DEX) with aspirin-triggered resolvin D1 (AT-RvD1) reduces submandibular gland lymphocytic infiltration in submandibular glands of Sjögren syndrome–like mice. Mice were treated as described in the Methods section, and submandibular glands were harvested, sectioned, and stained with hematoxylin-eosin. A. Lymphocytic foci are shown within yellow dotted lines in which scale bars in low and high magnification images are 500 μm and 50 μm, respectively. B. Lymphocytic foci were quantified, and data were expressed as mean (SD), in which * indicates P = .001 and † indicates not significant.
A combination treatment with low-dose DEX with AT-RvD1 reduces mast cell degranulation in SMG of SS-like mice
Given that mast cells are involved with SS initiation and progression by releasing inflammatory mediators (eg, prostaglandin and leukotrienes),60–63 the effect of combination low-dose DEX with AT-RvD1 treatment on mast cell degranulation was determined. As shown in Figure 3A, SMG from ethanol (vehicle control) showed extensive mast cell degranulation, as evidenced by the presence of toluidine blue-stained granules (ie, purple) outside the mast cells. In contrast, treatment with high-dose DEX alone or low-dose DEX combined with AT-RvD1 significantly diminished mast cell degranulation. To confirm and quantify these results, confocal analysis using a selective antibody for mast cells (ie, rabbit–anti-mouse chymase antibody) was performed as described in the Methods section. Results show that mice treated with high-dose DEX alone, AT-RvD1 alone, or low-dose DEX with AT-RvD1 all displayed a significant reduction of mast cell degranulation compared with ethanol (vehicle control) (Figure 3B, C).
Figure 3.
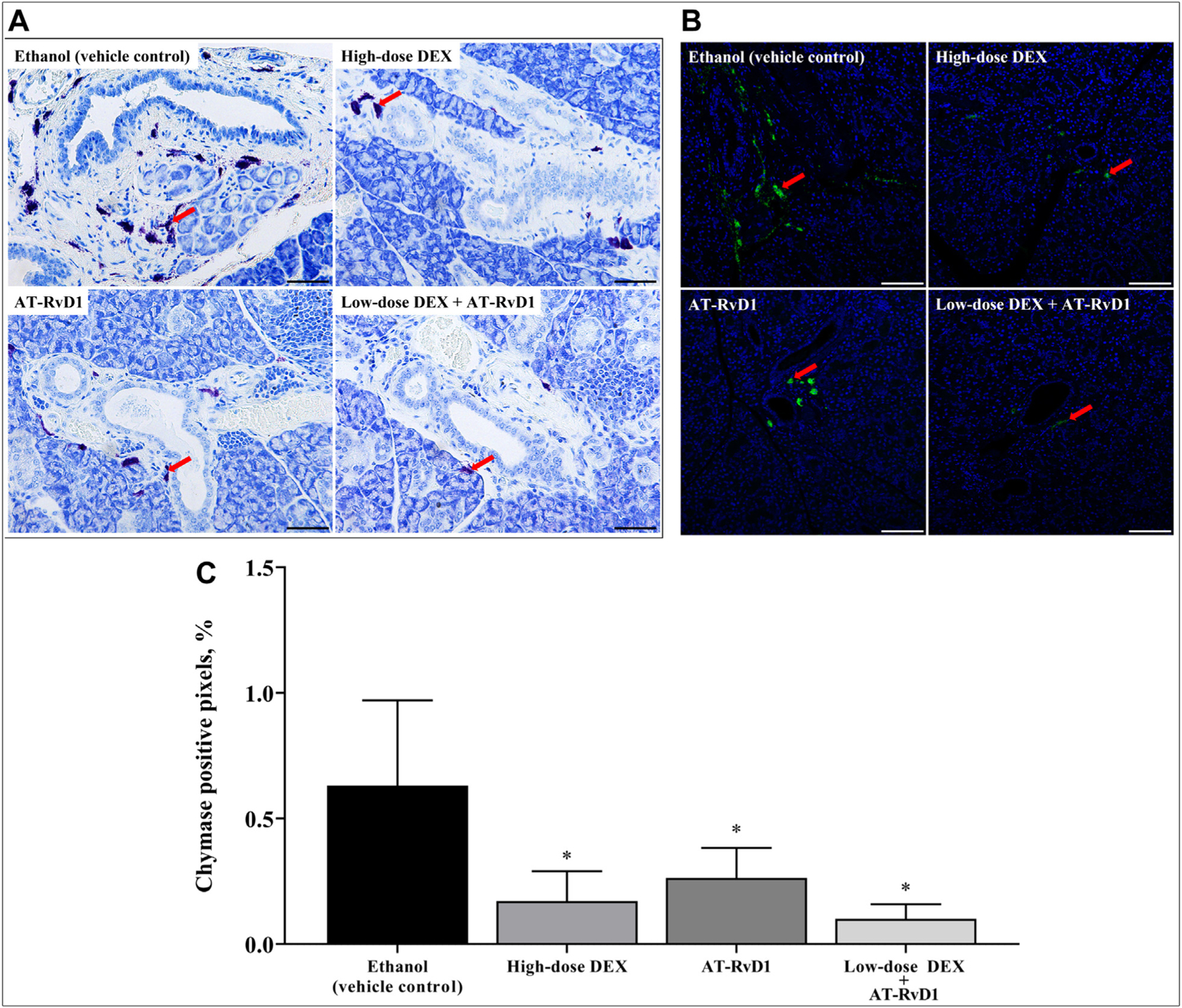
Combined treatment with low-dose dexamethasone (DEX) with aspirin-triggered resolvin D1 (AT-RvD1) reduces mast cell degranulation in submandibular glands of Sjögren syndrome–like mice. Submandibular glands were harvested, formalin-fixed, paraffin-embedded, and sectioned. A. Mast cell degranulation in submandibular glands was observed using toluidine blue staining (red arrows), in which scale bars represent 50 μm. B. Chymase was detected with rabbit–anti-mouse chymase antibody (green; red arrows), and nuclei were stained for nucleic acids with 4′,6-diamidino-2-phenylindole (blue) with images analyzed using confocal microscopy. Representative fluorescence images are shown from 4 samples, in which scale bars represent 100 μm. C. Mast cell degranulation was quantified and expressed as mean (SD), in which * indicates P = .05.
A combination treatment with low-dose DEX and AT-RvD1 restores saliva secretion in SS-like mice
To determine the effects of DEX with AT-RvD1 treatment on saliva flow rates in the NOD/ShiLtJ SS-like mouse, mice were treated as described in the Methods section. Results show that mice treated with high-dose DEX alone, AT-RvD1 alone, or low-dose DEX with AT-RvD1 all showed a significant increase in saliva flow rates compared with vehicle controls (Figure 4).
Figure 4.
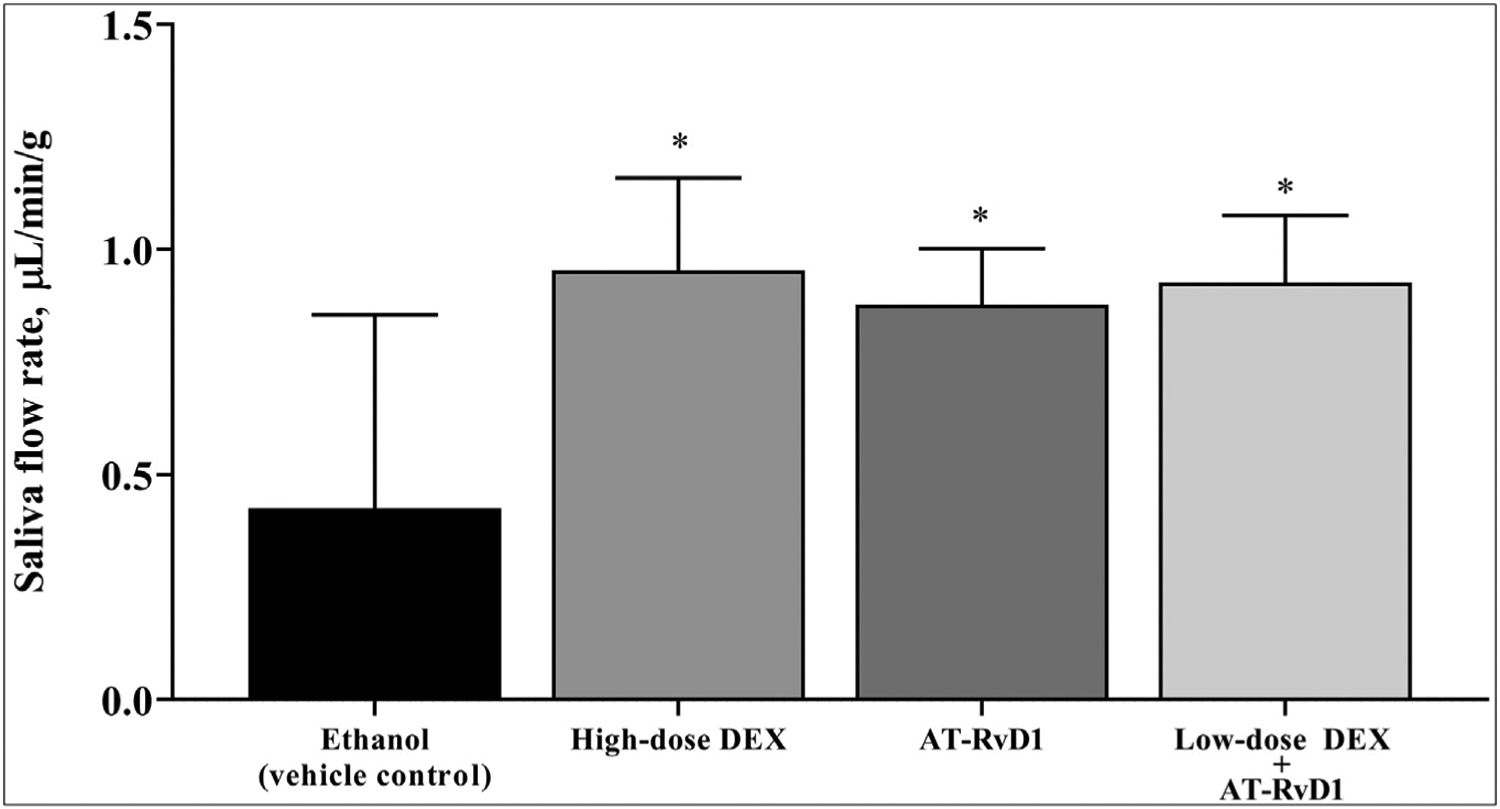
Combined treatment with low-dose dexamethasone (DEX) with aspirin-triggered resolvin D1 (AT-RvD1) increases saliva secretion in Sjögren syndrome–like mice. Mice were treated as described in Methods. Then, saliva was collected after intraperitoneal injection with pilocarpine-hydrogen chloride (50 mg/kg) and isoproterenol (0.5 mg/kg). Results are representative of 5 mice per condition, and data are expressed as mean (SD) in which * indicates P = .01.
A combination treatment with low-dose DEX with AT-RvD1 induces apical localization of the SMG water channel aquaporin-5
Given the increased saliva secretion in all treatment groups, specific effects on aquaporin-5 apical localization were investigated. Specifically, aquaporin-5 is the major water channel involved in polarized fluid secretion64–66 and is known to abnormally translocate from the apical to the basolateral membrane (ie, loss of polarity) in salivary glands of SS patients.9 For our study, confocal analysis using a selective antibody for aquaporin-5 (ie, rabbit–anti-mouse aquaporin-5 antibody) was performed as described in the Methods section. Our data show that treatment with AT-RvD1 alone or low-dose DEX with AT-RvD1 results in apical aquaporin-5 localization in SMG compared with mice treated with high-dose DEX or ethanol (vehicle control) (Figure 5). Although high-dose DEX alone did increase saliva flow rate (Figure 4), this treatment failed to induce aquaporin-5 apical localization (Figure 5), thereby suggesting damage to this secretory protein that may compromise saliva secretion in the long term. These results indicate that treatment with both AT-RvD1 alone and low-dose DEX with AT-RvD1 ensures an apical expression of aquaporin-5, the main conduit of saliva secretion in SMG.
Figure 5.
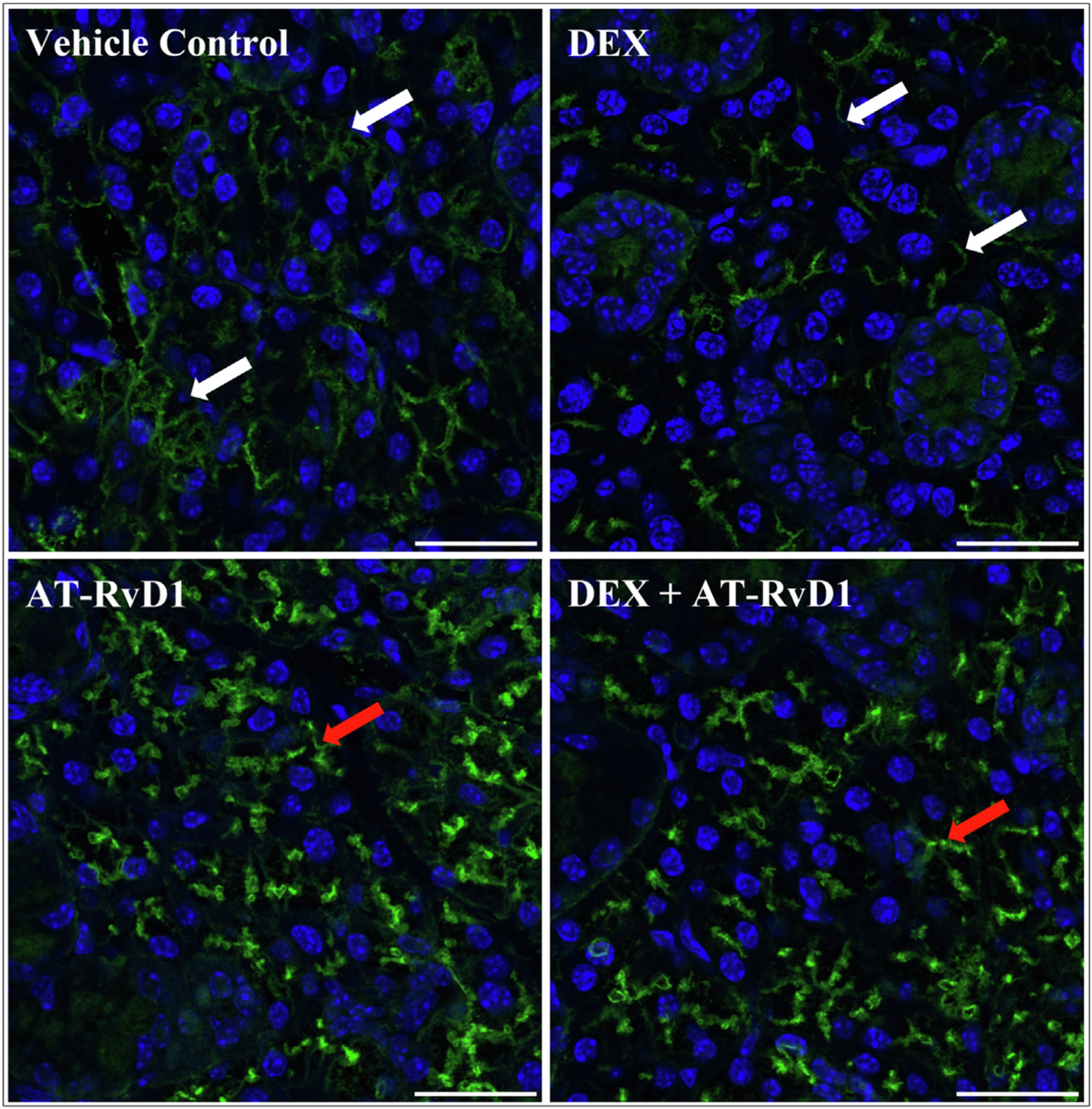
Combined treatment with low-dose dexamethasone (DEX) with aspirin-triggered resolvin D1 (AT-RvD1) enhances the expression and apical distribution of aquaporin-5 in submandibular glands of Sjögren syndrome–like mice. Submandibular glands were harvested, formalin-fixed, paraffin-embedded, and sectioned. Aquaporin-5 staining was detected with rabbit–anti-mouse aquaporin-5 (green), nuclei were stained with 4′,6-diamidino-2-phenylindole (blue), and images were analyzed using confocal microscopy. Representative fluorescence images from 4 samples, in which scale bars represent 25 μm. White arrows indicate basolateral staining, and red arrows indicate apical staining for aquaporin-5.
Discussion
A previous study showed that the potent anti-inflammatory drug DEX when administered alone and at high strength, significantly reduces lymphocytic infiltration in SMG of NOD/ShiLtJ mice when administered at the predisease stage,10 and our study extends these findings to disease onset. However, DEX has major limitations, including multiple secondary effects such as hyperglycemia, obesity, hypertension, osteoporosis, cataract formation, and striatal and skin thinning.10 Moreover, treatment with high-dose DEX cannot maintain apical expression of aquaporin-5 in salivary glands, consistent with a previous study.67 Similarly, although a clinical study showed that the use of corticosteroids relieves oral symptoms in SS patients (eg, dry mouth, increased water drinking frequency, sticky sensations, and lip dryness),68 such beneficial effects are not sustained when the drug is no longer taken,69 and long-term use of DEX at high doses has been shown to in fact lead to loss of saliva secretion in both humans and mice.14,70
Considerable benefits were likewise noted with AT-RvD1. Specifically, a previous study showed that activation of ALX/FPR2 with RvD1 blocks proinflammatory signals caused by tumor necrosis factor-α while enhancing salivary gland epithelial integrity in the rat parotid Par-C10 cell line.50 Moreover, previous studies confirmed that ALX/FPR2 is expressed in primary salivary gland epithelial cells.37,49 Activating AT-RvD1 increases a diverse set of intracellular prosurvival signaling pathways, such as calcium ion, Erk1/2, and Akt, which block TNF-α–mediated caspase-3 activation.49 A subsequent pilot study showed that AT-RvD1 treatment at 0.1 mg/kg significantly reduces SS-associated proinflammatory genes in SMG from NOD/ShiLtJ compared with vehicle-treated mice.9 Next, a preclinical study showed that AT-RvD1 treatment administered at disease onset reduced the number of T helper 17 cells in SMG and successfully restored salivary gland function in NOD/ShiLtJ mice.71 Together, these reports indicate that treatment with AT-RvD1 alone achieves proresolving effects by reestablishing normal tissue architecture and functionality in salivary gland epithelium while reducing proinflammatory signals in the NOD/ShiLtJ SS-like mouse model.9,10,49–53,71 However, treatment with AT-RvD1 alone does not reduce lymphocytic infiltration in SMG. T lymphocytes can develop into ectopic lymphoid structures, whereas B lymphocytes become hyperactive and form autoantibodies, lymphoepithelial lesions, and SS-related mucosa-associated lymphoid tissue lymphoma, all of which alter salivary epithelial integrity.72–78 Thus, AT-RvD1 has proven insufficient as a stand-alone treatment given its inability to reduce lymphocytic infiltration.
In light of the clear benefits of both of these candidates for the treatment of SS-like features (ie, DEX with AT-RvD1) as well as their significant deficits (adverse effects for long-term high strength DEX use and inability to impact lymphocytic infiltration for AT-RvD1), a combination of the 2 was used in this study, with results indicating a significant treatment effect that could reasonably be continued throughout the life span of the SS patient. Specifically, our findings indicate that low-dose DEX combined with AT-RvD1 is highly effective for blocking lymphocytic infiltration and mast cell degranulation (benefits previously seen with high-dose DEX9,10) while also increasing apical aquaporin-5 expression and saliva secretion in SMG of SS-like NOD/ShiLtJ mice (benefits previously seen with AT-RvD1 alone9,10), all without the significant adverse effects consistently seen with the higher dosage of DEX. Given that the proposed treatment combining low-dose DEX with AT-RvD1 is intended to be administered indefinitely to maintain treatment gains, we would highlight the overriding importance of reducing secondary effects associated with high-dose DEX.
The limitations of this study include a lack of experiments to prove that lymphocytic infiltration will lead to glandular dysfunction; however, previous studies indicate that proinflammatory cytokines released by infiltrating lymphocytes lead to secretory dysfunction by damaging salivary gland tight junctions,50,79–84 a finding that we will seek to expand on in our future investigation. Moreover, lymphocyte receptors may prove unresponsive to AT-RvD1, thereby allowing for the continuation of inflammatory cytokine secretion and leading to tight junction disruption.50,79–84 Such an effect could result in relapse among the AT-RvD1-treated cohort, and future studies are warranted to determine if this is the case. Finally, adverse effects of DEX are well-known, and lower doses will predictably reduce adverse effects85–88; however, we have yet to determine the lowest DEX concentration at which point treatment effects would be lost. As such, future studies will conduct dose-response experiments to determine the appropriate minimum dosage of DEX with AT-RvD1. Having thus determined the lowest permissible DEX dosage, later studies may be extended to establish the sustainability of treatment gains.
Conclusions
This study showed that a combination of low-dose DEX with AT-RvD1 reduced the severity of SS-like features and prevented the development of advanced and potentially irreversible damage, all in a form that can be administered indefinitely without the need to cease treatment because of secondary effects. Finally, it is worth noting that the cohort in our study was composed entirely of female mice in light of the heavy predominance of females affected by SS, with a ratio of 9:1 compared with males.57 That said, once the management of the primary treatment group has been established, further studies would seek to apply these techniques to the remaining cases appearing among males while also extending these findings to the potential reversal of late-stage SS damage.
Why Is This Important?
Treatments for Sjögren syndrome are ineffective in that they either address only part of the problem, do so at a considerable cost in terms of secondary effects when used indefinitely, or both. This study aimed to combine 2 promising treatments for Sjögren syndrome (ie, dexamethasone and aspirin-triggered resolvin D1) to retain their benefits when given in isolation while reducing their respective deficits.
Acknowledgments
This study was supported by grants R01DE022971 (O.B.J.), R01DE027884 (O.J.B.), R01DE007389 (G.A.W.), and R01DE029833 (G.A.W.) by the National Institute of Dental and Craniofacial Research, National Institutes of Health.
Footnotes
Disclaimer. Dr Baker serves as an Editorial Board member for JADA Foundational Science. She was not involved in decisions about her article, and peer review was handled independently.
Disclosure. None of the authors reported any disclosures.
References
- 1.Jonsson R. Disease mechanisms in Sjögren’s syndrome: what do we know? Scand J Immunol. 2022;95(3):e13145. 10.1111/sji.13145 [DOI] [PubMed] [Google Scholar]
- 2.Baldini C, Talarico R, Tzioufas AG, Bombardieri S. Classification criteria for Sjogren’s syndrome: a critical review. J Autoimmun. 2012;39(1–2):9–14. 10.1016/j.jaut.2011.12.006 [DOI] [PubMed] [Google Scholar]
- 3.Fox RI, Stern M. Sjögren’s syndrome: mechanisms of pathogenesis involve interaction of immune and neurosecretory systems. Scand J Rheumatol Suppl. 2002;116(2):3–13. 10.1080/030097402317474874 [DOI] [PubMed] [Google Scholar]
- 4.Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjögren’s syndrome. Nat Rev Rheumatol. 2013;9(9):544–556. 10.1038/nrrheum.2013.110 [DOI] [PubMed] [Google Scholar]
- 5.Seror R, Nocturne G, Mariette X. Current and future therapies for primary Sjögren syndrome. Nat Rev Rheumatol. 2021;17(8):475–486. 10.1038/s41584-021-00634-x [DOI] [PubMed] [Google Scholar]
- 6.Argyropoulou OD, Tzioufas AG. Update on Sjögren’s syndrome 2018. Mediterr J Rheumatol. 2018;29(4):193–198. 10.31138/mjr.29.4.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldini C, Cecchettini A, Gallo A, Bombardieri S. Updates on Sjögren’s syndrome: from proteomics to protein biomarkers. Expert Rev Proteomics. 2017;14(6):491–498. 10.1080/14789450.2017.1333904 [DOI] [PubMed] [Google Scholar]
- 8.Segal B, Bowman SJ, Fox PC, et al. Primary Sjögren’s syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes. 2009;7(1):746. 10.1186/1477-7525-7-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Easley JT, Nelson JW, Mellas RE, et al. Aspirin-triggered resolvin D1 versus dexamethasone in the treatment of Sjögren’s syndrome-like NOD/ShiLtJ mice: a pilot study. J Rheum Dis Treat. 2015;1(4):027. 10.23937/2469-5726/1510027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CS, Maruyama CL, Easley JT, Trump BG, Baker OJ. AT-RvD1 promotes resolution of inflammation in NOD/ShiLtJ mice. Sci Rep. 2017;7(1):45525. 10.1038/srep45525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prezant DJ, Karwa ML, Richner B, et al. Short-term vs long-term dexamethasone treatment: effects on rat diaphragm structure and function. Lung. 1998;176(4):267–280. 10.1007/pl00007609 [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. 10.1186/1710-1492-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusuda Y, Kondo Y, Miyagi Y, et al. Long-term dexamethasone treatment diminishes store-operated Ca2+ entry in salivary acinar cells. Int J Oral Sci. 2019;11(1):1. 10.1038/s41368-018-0031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bighetti BB, d Assis GF, Vieira DC, et al. Long-term dexamethasone treatment alters the histomorphology of acinar cells in rat parotid and submandibular glands. Int J Exp Pathol. 2014;95(5):351–363. 10.1111/iep.12092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017;58:114–129. 10.1016/j.mam.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recchiuti A, Codagnone M, Pierdomenico AM, et al. Immunoresolving actions of oral resolvin D1 include selective regulation of the transcription machinery in resolution-phase mouse macrophages. FASEB J. 2014;28(7):3090–3102. 10.1096/fj.13-248393 [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN. Systems approach with inflammatory exudates uncovers novel anti-inflammatory and pro-resolving mediators. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):157–163. 10.1016/j.plefa.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. 10.1038/nature13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med. 2017;58:1–11. 10.1016/j.mam.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31(4):1273–1288. 10.1096/fj.201601222R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(suppl 1):S200–S215. 10.1038/sj.bjp.0707489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyall SC, Balas L, Bazan NG, et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: recent advances in the understanding of their biosynthesis, structures, and functions. Prog Lipid Res. 2022;86:101165. 10.1016/j.plipres.2022.101165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun YP, Oh SF, Uddin J, et al. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282(13):9323–9334. 10.1074/jbc.M609212200 [DOI] [PubMed] [Google Scholar]
- 24.Keinan D, Leigh NJ, Nelson JW, De Oleo L, Baker OJ. Understanding resolvin signaling pathways to improve oral health. Int J Mol Sci. 2013;14(3):5501–5518. 10.3390/ijms14035501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Wang J, Nie R, Zhou S. Endogenous pro-resolving and anti-inflammatory lipid mediators: the new hope of atherosclerotic diseases. Med Hypotheses. 2008;71(2):237–240. 10.1016/j.mehy.2008.03.026 [DOI] [PubMed] [Google Scholar]
- 26.Fredman G, Serhan CN. Specialized pro-resolving mediators: wiring the circuitry of effector immune and tissue homeostasis. Endod Topics. 2011;24(1):39–58. 10.1111/etp.12010 [DOI] [Google Scholar]
- 27.Haas-Stapleton EJ, Lu Y, Hong S, et al. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One. 2007;2(12):e1316. 10.1371/journal.pone.0001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y, Arita M, Zhang Q, et al. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci. 2009;50(10):4743–4752. 10.1167/iovs.08-2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norris PC, Arnardottir H, Sanger JM, Fichter D, Keyes GS, Serhan CN. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins Leukot Essent Fatty Acids. 2016;138:81–89. 10.1016/j.plefa.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol. 2012;189(2):1036–1042. 10.4049/jimmunol.1103483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benabdoune H, Rondon EP, Shi Q, et al. The role of resolvin D1 in the regulation of inflammatory and catabolic mediators in osteoarthritis. Inflamm Res. 2016;65(8):635–645. 10.1007/s00011-016-0946-x [DOI] [PubMed] [Google Scholar]
- 32.Eickmeier O, Seki H, Haworth O, et al. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6(2):256–266. 10.1038/mi.2012.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie W, Wang H, Wang L, Yao C, Yuan R, Wu Q. Resolvin D1 reduces deterioration of tight junction proteins by upregulating HO-1 in LPS-induced mice. Lab Invest. 2013;93(9):991–1000. 10.1038/labinvest.2013.80 [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Wang T, Gui P, et al. Resolvin D1 reverts lipopolysaccharide-induced TJ proteins disruption and the increase of cellular permeability by regulating IκBα signaling in human vascular endothelial cells. Oxid Med Cell Longev. 2013;2013:185715. 10.1155/2013/185715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.English JT, Norris PC, Hodges RR, Dartt DA, Serhan CN. Identification and profiling of specialized pro-resolving mediators in human tears by lipid mediator metabolomics. Prostaglandins Leukot Essent Fatty Acids. 2017;117:17–27. 10.1016/j.plefa.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34(11):599–609. 10.1016/j.tins.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leigh NJ, Nelson JW, Mellas RE, Aguirre A, Baker OJ. Expression of resolvin D1 biosynthetic pathways in salivary epithelium. J Dent Res. 2014;93(3):300–305. 10.1177/0022034513519108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. 10.1146/annurev.pathmechdis.3.121806.151409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werz O, Gerstmeier J, Libreros S, et al. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun. 2018;9(1):59. 10.1038/s41467-017-02538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnardottir H, Orr SK, Dalli J, Serhan CN. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016;9(3):757–766. 10.1038/mi.2015.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sommakia S, Baker OJ. Regulation of inflammation by lipid mediators in oral diseases. Oral Dis. 2017;23(5):576–597. 10.1111/odi.12544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duvall MG, Levy BD. DHA- and EPA-derived resolvins, protections, and maresins in airway inflammation. Eur J Pharmacol. 2016;785:144–155. 10.1016/j.ejphar.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016;30(8):2792–2801. 10.1096/fj.201500155R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen TV, Dalli J, Serhan CN. The novel lipid mediator PD1n-3 DPA: an overview of the structural elucidation, synthesis, biosynthesis and bioactions. Prostaglandins Other Lipid Mediat. 2017;133:103–110. 10.1016/j.prostaglandins.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orr SK, Butler KL, Hayden D, Tompkins RG, Serhan CN, Irimia D. Gene expression of proresolving lipid mediator pathways is associated with clinical outcomes in trauma patients. Crit Care Med. 2015;43(12):2642–2650. 10.1097/CCM.0000000000001312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serhan CN. Controlling the resolution of acute inflammation: a new genus of dual anti-inflammatory and proresolving mediators. J Periodontol. 2008;79(8 suppl):1520–1526. 10.1902/jop.2008.080231 [DOI] [PubMed] [Google Scholar]
- 47.Parashar K, Schulte F, Hardt M, Baker OJ. Sex-mediated elevation of the specialized pro-resolving lipid mediator levels in a Sjögren’s syndrome mouse model. FASEB J. 2020;34(6):7733–7744. 10.1096/fj.201902196R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dos Santos HT, Nam K, Maslow F, Trump B, Baker OJ. Specialized pro-resolving receptors are expressed in salivary glands with Sjögren’s syndrome. Ann Diagn Pathol. 2022;56:151865. 10.1016/j.anndiagpath.2021.151865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson JW, Leigh NJ, Mellas RE, McCall AD, Aguirre A, Baker OJ. ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am J Physiol Cell Physiol. 2014;306(2):C178–C185. 10.1152/ajpcell.00284.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-α-mediated disruption of salivary epithelial formation. Am J Physiol Cell Physiol. 2012;302(9):C1331–C1345. 10.1152/ajpcell.00207.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CS, Wee Y, Yang CH, Melvin JE, Baker OJ. ALX/FPR2 modulates anti-inflammatory responses in mouse submandibular gland. Sci Rep. 2016;6(1):24244. 10.1038/srep24244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Easley JT, Maruyama CL, Wang CS, Baker OJ. AT-RvD1 combined with DEX is highly effective in treating TNF-α-mediated disruption of the salivary gland epithelium. Physiol Rep. 2016;4(19):e12990. 10.14814/phy2.12990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang CS, Baker OJ. The G-protein-coupled receptor ALX/Fpr2 regulates adaptive immune responses in mouse submandibular glands. Am J Pathol. 2018;188(7):1555–1562. 10.1016/j.ajpath.2018.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barone F, Campos J, Bowman S, Fisher BA. The value of histopathological examination of salivary gland biopsies in diagnosis, prognosis and treatment of Sjögren’s syndrome. Swiss Med Wkly. 2015;145: w14168. 10.4414/smw.2015.14168 [DOI] [PubMed] [Google Scholar]
- 55.Giovelli RA, Santos MCS, Serrano ÉV, Valim V. Clinical characteristics and biopsy accuracy in suspected cases of Sjögren’s syndrome referred to labial salivary gland biopsy. BMC Musculoskelet Disord. 2015;16(1):30. 10.1186/s12891-015-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen W, Cao H, Lin J, Olsen N, Zheng SG. Biomarkers for primary Sjögren’s syndrome. Genomics Proteomics Bioinformatics. 2015;13(4): 219–223. 10.1016/j.gpb.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parisis D, Chivasso C, Perret J, Soyfoo MS, Delporte C. Current state of knowledge on primary Sjögren’s syndrome, an autoimmune exocrinopathy. J Clin Med. 2020;9(7):2299. 10.3390/jcm9072299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciháková D, Talor MV, Barin JG, et al. Sex differences in a murine model of Sjögren’s syndrome. Ann N Y Acad Sci. 2009;1173(1):378–383. 10.1111/j.1749-6632.2009.04760.x [DOI] [PubMed] [Google Scholar]
- 59.Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18(7):e3000410. 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaieda S, Fujimoto K, Todoroki K, et al. Mast cells can produce transforming growth factor β1 and promote tissue fibrosis during the development of Sjögren’s syndrome-related sialadenitis. Mod Rheumatol. 2022;32(4):761–769. 10.1093/mr/roab051 [DOI] [PubMed] [Google Scholar]
- 61.Conti P, Stellin L, Caraffa A, et al. Advances in mast cell activation by IL-1 and IL-33 in Sjögren’s syndrome: promising inhibitory effect of IL-37. Int J Mol Sci. 2020;21(12):4297. 10.3390/ijms21124297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Konttinen YT, Hietanen J, Virtanen I, et al. Mast cell derangement in salivary glands in patients with Sjögren’s syndrome. Rheumatol Int. 2000;19(4):141–147. 10.1007/s002960050118 [DOI] [PubMed] [Google Scholar]
- 63.Konttinen YT, Tuominen S, Segerberg-Konttinen M, et al. Mast cells in the labial salivary glands of patients with Sjögren’s syndrome: a histochemical, immunohistochemical, and electron microscopical study. Ann Rheum Dis. 1990;49(9):685–689. 10.1136/ard.49.9.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuzaki T, Susa T, Shimizu K, et al. Function of the membrane water channel aquaporin-5 in the salivary gland. Acta Histochem Cytochem. 2012;45(5):251–259. 10.1267/ahc.12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delporte C, Steinfeld S. Distribution and roles of aquaporins in salivary glands. Biochim Biophys Acta. 2006;1758(8):1061–1070. 10.1016/j.bbamem.2006.01.022 [DOI] [PubMed] [Google Scholar]
- 66.Rajasekaran SA, Beyenbach KW, Rajasekaran AK. Interactions of tight junctions with membrane channels and transporters. Biochim Biophys Acta. 2008;1778(3):757–769. 10.1016/j.bbamem.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 67.Wu BJ, Zhu J, Tan WP, et al. Effect of dexamethasone on the expression of aquaporin-5 in the lungs of mice with acute allergic asthma. Article in Chinese. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28(9):1670–1673. [PubMed] [Google Scholar]
- 68.Miyawaki S, Nishiyama S, Matoba K. Efficacy of low-dose prednisolone maintenance for saliva production and serological abnormalities in patients with primary Sjögren’s syndrome. Intern Med. 1999;38(12):938–943. 10.2169/internalmedicine.38.938 [DOI] [PubMed] [Google Scholar]
- 69.Zandbelt MM, van den Hoogen FH, de Wilde PC, van den Berg PJ, Schneider HG, van de Putte LB. Reversibility of histological and immunohistological abnormalities in sublabial salivary gland biopsy specimens following treatment with corticosteroids in Sjögren’s syndrome. Ann Rheum Dis. 2001;60(5):511–513. 10.1136/ard.60.5.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson DA, Alvares OF, Etzel KR, Kalu DN. Regulation of salivary proteins. J Dent Res. 1987;66(2):576–582. 10.1177/00220345870660023201 [DOI] [PubMed] [Google Scholar]
- 71.Dean S, Wang CS, Nam K, Maruyama CL, Trump BG, Baker OJ. Aspirin triggered resolvin D1 reduces inflammation and restores saliva secretion in a Sjögren’s syndrome mouse model. Rheumatology (Oxford). 2019;58(7):1285–1292. 10.1093/rheumatology/kez072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang SH, Woo JS, Moon J, et al. IL-17 and CCR9+α4β7− Th17 cells promote salivary gland inflammation, dysfunction, and cell death in Sjögren’s syndrome. Front Immunol. 2021;12:721453. 10.3389/fimmu.2021.721453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verstappen GM, Gao L, Pringle S, et al. The transcriptome of paired major and minor salivary gland tissue in patients with primary Sjögren’s syndrome. Front Immunol. 2021;12:681941. 10.3389/fimmu.2021.681941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin L, Yu D, Li X, et al. CD4+CXCR5+ follicular helper T cells in salivary gland promote B cells maturation in patients with primary Sjogren’s syndrome. Int J Clin Exp Pathol. 2014;7(5):1988–1996. [PMC free article] [PubMed] [Google Scholar]
- 75.Blokland SLM, Kislat A, Homey B, et al. Decreased circulating CXCR3 + CCR9+T helper cells are associated with elevated levels of their ligands CXCL10 and CCL25 in the salivary gland of patients with Sjögren’s syndrome to facilitate their concerted migration. Scand J Immunol. 2020;91(3):e12852. 10.1111/sji.12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varin MM, Guerrier T, Devauchelle-Pensec V, Jamin C, Youinou P, Pers JO. In Sjögren’s syndrome, B lymphocytes induce epithelial cells of salivary glands into apoptosis through protein kinase C delta activation. Autoimmun Rev. 2012;11(4):252–258. 10.1016/j.autrev.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 77.Cornec D, Devauchelle-Pensec V, Tobón GJ, Pers JO, Jousse-Joulin S, Saraux A. B cells in Sjögren’s syndrome: from pathophysiology to diagnosis and treatment. J Autoimmun. 2012;39(3):161–167. 10.1016/j.jaut.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 78.Barr JY, Wang X, Kreiger PA, Lieberman SM. Salivary-gland-protective regulatory T-cell dysfunction underlies female-specific sialadenitis in the non-obese diabetic mouse model of Sjögren syndrome. Immunology. 2018;155(2):225–237. 10.1111/imm.12948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baker OJ. Current trends in salivary gland tight junctions. Tissue Barriers. 2016;4(3):e1162348. 10.1080/21688370.2016.1162348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baker OJ, Camden JM, Redman RS, et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol. 2008;295(5):C1191–C1201. 10.1152/ajpcell.00144.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baker OJ, Schulz DJ, Camden JM, et al. Rat parotid gland cell differentiation in three-dimensional culture. Tissue Eng Part C Methods. 2010;16(5):1135–1144. 10.1089/ten.TEC.2009.0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ewert P, Aguilera S, Alliende C, et al. Disruption of tight junction structure in salivary glands from Sjögren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum. 2010;62(5): 1280–1289. 10.1002/art.27362 [DOI] [PubMed] [Google Scholar]
- 83.Zhang LW, Cong X, Zhang Y, et al. Interleukin-17 impairs salivary tight junction integrity in Sjögren’s syndrome. J Dent Res. 2016;95(7):784–792. 10.1177/0022034516634647 [DOI] [PubMed] [Google Scholar]
- 84.Verstappen GM, Pringle S, Bootsma H, Kroese FGM. Epithelial–immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis. Nat Rev Rheumatol. 2021;17(6):333–348. 10.1038/s41584-021-00605-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Shi G, Zhang H, et al. Dexamethasone enhances the lung metastasis of breast cancer via a PI3K-SGK1-CTGF pathway. Oncogene. 2021;40(35):5367–5378. 10.1038/s41388-021-01944-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Westhoff PG, de Graeff A, Geerling JI, Reyners AK, van der Linden YM. Dexamethasone for the prevention of a pain flare after palliative radiotherapy for painful bone metastases: a multicenter double-blind placebo-controlled randomized trial. BMC Cancer. 2014;14:347. 10.1186/1471-2407-14-347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kizawa Y, Furuya M, Saito K, Masuko T, Kusama T. Effects of dexamethasone and aminophylline on survival of Jurkat and HL-60 cells. Biol Pharm Bull. 2006;29(2):281–285. 10.1248/bpb.29.281 [DOI] [PubMed] [Google Scholar]
- 88.Kusuda Y, Kondo Y, Miyagi Y, et al. Long-term dexamethasone treatment diminishes store-operated Ca2+ entry in salivary acinar cells. Int J Oral Sci. 2019;11(1):1. 10.1038/s41368-018-0031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


