Abstract
Methylobacterium species, the representative bacteria distributed in phyllosphere region of plants, often synthesize carotenoids to resist harmful UV radiations. Methylobacterium extorquens is known to produce a carotenoid pigment and recent research revealed that this carotenoid has a C30 backbone. However, its exact structure remains unknown. In the present study, the carotenoid produced by M. extorquens AM1 was isolated and its structure was determined as 4-[2-O-11Z-octadecenoyl-β-glucopyranosyl]-4,4′-diapolycopenedioc acid (1), a glycosylated C30 carotenoid. Furthermore, the genes related to the C30 carotenoid synthesis were investigated. Squalene, the precursor of the C30 carotenoid, is synthesized by the co-occurrence of META1p1815, META1p1816 and META1p1817. Further overexpression of the genes related to squalene synthesis improved the titer of carotenoid 1. By using gene deletion and gene complementation experiments, the glycosyltransferase META1p3663 and acyltransferase META1p3664 were firstly confirmed to catalyze the tailoring steps from 4,4′-diapolycopene-4,4′-dioic acid to carotenoid 1. In conclusion, the structure and biosynthetic genes of carotenoid 1 produced by M. extorquens AM1 were firstly characterized in this work, which shed lights on engineering M. extorquens AM1 for producing carotenoid 1 in high yield.
Keywords: C30 carotenoid, Methylobacterium extorquens, Biosynthetic gene cluster, Glycosyltransferase, Acyltransferase
1. Introduction
The lipophilic natural carotenoids belong to a class of isoprenoid derivatives. So far, more than 1100 carotenoids have been isolated from various plants and microorganisms [1]. The carotenoids have multiple conjugated double bonds, which enable two essential features of carotenoids: the light-harvesting capability and powerful anti-oxidant effect by quenching of free radicals, singlet oxygen and reactive oxygen species [1]. Carotenoids are widely used as colorants and additives in food industry [2]. Additionally, for human beings, carotenoids are shown to inhibit cancer cells, serve as antioxidants, and enhance the immune response to decrease the risk of multiple diseases, especially eye diseases [[3], [4], [5]]. Structurally, carotenes such as lycopene, β-carotene, and α-carotene are hydrocarbons that can be linear or cyclized, while xanthophylls like lutein and astaxanthin are oxygenated derivatives of carotenes with hydroxyl, keto, or epoxy groups [[6], [7], [8]]. Most of carotenoids are typical C40 based derivatives, which are produced by photosynthetic bacteria and plants. Whereas for several non-photosynthetic bacteria species such as Rubritalea squalenifaciens [9], Staphylococcus aureus [10], Bacillus firmus [11], Streptococcus faecium [12], Methylomonas sp. strain 16a [13], Methylobacterium spp. strains [14], and Planococcus spp. strains [[15], [16], [17], [18]], the unique C30 based carotenoids were identified.
The biosynthetic pathway of C30 carotenoid staphyloxanthin, a virulence factor generated by the conditional pathogen S. aureus, was identified by employing a combination of gene deletion and heterologous expression experiments, which includes a cluster of five genes crtMNPQO and an individual gene aldH [19,20]. In addition, the genes involved in synthesis of the C30 based carotenoids in Bacillus firmus [21], Methylomonas sp. strain 16a [13], Planococcus maritimus strain iso-3 [15], Planococcus faecalis AJ003T [22], and P. limnophila were also identified [17]. Notably, the genes responsible for synthesis of C30 based carotenoids are not always rigidly restricted in one locus [13,20,22]. Two different enzymes are characterized to catalyze the formation of the first intermediate with C30 backbone. The 4,4′-diapophytoene synthase CrtM and its analogues catalyze the condensation of two molecules of farnesyl diphosphate (FPP) to afford C30 based backbone 4,4′-diapophytoene (dehydrosqualene) or 15-cis-4,4′-diapophytoene [15,19]. Whereas for synthesis of C30 carotenoids in P. limnophila and Methylobacterium extorquens PA1, the squalene synthesized by three co-occurrence of enzymes HpnCDE is used as the first C30 precursor [17,23]. Next, the 4,4′-diapophytoene desaturase CrtN, 4,4′-diaponeurosporene oxidase CrtP, aldehyde dehydrogenase AldH, glycosyltransferase CrtQ, and acyltransferase CrtO are employed to introduce further tailoring modifications to diversify C30 carotenoids.
Methylobacterium extorquens AM1, one representative of methylotrophs, is a facultative methylotroph α-proteobacterium that is capable of growing in the medium with one-carbon compound as the sole carbon and energy source [24,25]. M. extorquens AM1 produces a carotenoid pink pigment, which is proposed to be C30 carotenoid rather than C40 carotenoid based on the following two reasons. Firstly, the pink pigment produced in M. extorquens PA1, the strain with closest genetic background to M. extorquens AM1, was shown to be C30 carotenoid, which is derived from squalene [23]. Secondly, phytoene synthase gene crtB involved in synthesis of C40 carotenoid in M. extorquens AM1 had no influence on synthesis of the pink pigment [26]. Furthermore, two desaturases META1p3665 and META1p3670 involved in synthesis of this pink pigment were identified [26,27]. Although M. extorquens AM1 is known to produce carotenoid for a long time, the exact structure of this carotenoid remains unknown. Herein, we report the structure of the carotenoid, as well as identification of its biosynthetic genes in M. extorquens AM1 by using gene deletion and gene complementation experiments.
2. Materials and methods
2.1. Strains, media and culture conditions
The plasmids and strains used and generated in this study are listed in Table 1. All E. coli strains were cultivated in Luria-Bertani (LB) agar or liquid medium at 37 °C supplemented with appropriate antibiotics. M. extorquens AM1 and its derivative strains were routinely cultivated in a minimal medium at 30 °C as described previously [31]. The final concentrations of antibiotics used in this study are 20 μg/mL tetracycline (Tet) and 25 μg/mL kanamycin (Km). All chemicals used for media were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
Table 1.
Strains and plasmids used in this study.
| Plasmids/strains | Description | Sources |
|---|---|---|
| Plasmids | ||
| pCM80 | vector used for gene expression in M. extorquens; promoter, PmxaF; antibiotics, TetR, KmR | [28] |
| pCM433 | sacB-based allelic exchange vector; antibiotics, ApR, CmR, TetR | [29] |
| pAIO | pCM80 derivative with dcas9 and sgRNA expression cassette; dcas9 and sgRNA under the control of inducible promoter PR/tetO and PmxaF-g, respectively | [26] |
| pAI1815 | pAIO derivative plasmid carrying the sgRNA targeting META1p1815 | This study |
| pAI1816 | pAIO derivative plasmid carrying the sgRNA targeting META1p1816 | This study |
| pCM3663 | pCM80 derivative harboring META1p3663 under the downstream of PmxaF, TetR | This study |
| pCM3664 | pCM80 derivative harboring META1p3664 under the downstream of PmxaF, TetR | This study |
| pCM1815 | pCM80 derivative harboring META1p1815 under the downstream of PmxaF, TetR | This study |
| pCM1816 | pCM80 derivative harboring META1p1816 under the downstream of PmxaF, TetR | This study |
| pCM1815-16 | pCM80 derivative harboring the operon of META1p1815 and META1p1816 under the downstream of PmxaF, TetR | This study |
| pCM3665-70 | pCM80 derivative harboring the operon of META1p3665 and META1p3670 under the downstream of PmxaF, TetR | This study |
| pCM1815-3665-70 | pCM80 derivative harboring the operon of META1p1815, META1p3665 and META1p3670 under the downstream of PmxaF, TetR | This study |
| pCM-△3663 | pCM433 with about 1 kb upstream and 1 kb downstream fragments of META1p3663; ApR, CmR, TetR | This study |
| pCM-△3664 | pCM433 with about 1 kb upstream and 1 kb downstream fragments of META1p3664; ApR, CmR, TetR | This study |
| pCM-△3652 | pCM433 with about 1 kb upstream and 1 kb downstream fragments of META1p3652; ApR, CmR, TetR | This study |
| pCM-△4598 | pCM433 with about 1 kb upstream and 1 kb downstream fragments of META1p4598; ApR, CmR, TetR | This study |
| Strains | ||
| E. coli DH5αα | Host strain for general clone | Lab storage |
| M. extorquens AM1 | Wild-type, pink color, rifamycin-resistant strain | [30] |
| YA | M. extorquens AM1::pCM80 | [26] |
| YAIO | M. extorquens AM1 containing the plasmid pAIO | [26] |
| YA3663 | The gene META1p3663 deleted from M. extorquens AM1 | This study |
| YA3664 | The gene META1p3664 deleted from M. extorquens AM1 | This study |
| YA3652 | The gene META1p3652 deleted from M. extorquens AM1 | This study |
| YA4598 | The gene META1p4598 deleted from M. extorquens AM1 | This study |
| YCM3663 | The strain YA3663::pCM-exp3663, TetR | This study |
| YCM3664 | The strain YA3664::pCM-exp3664, TetR | This study |
| YAMZ1815 | M. extorquens AM1 harboring the CRISPRi plasmid pAI1815 | This study |
| YAMZ1816 | M. extorquens AM1 harboring the CRISPRi plasmid pAI1816 | This study |
| YCM1815 | M. extorquens AM1::pCM-exp1815, TetR | This study |
| YCM1816 | M. extorquens AM1::pCM-exp1816, TetR | This study |
| YCM1815-16 | M. extorquens AM1::pCM-exp1815-16, TetR | This study |
| YCM3665-70 | M. extorquens AM1::pCM-exp3665-70, TetR | This study |
| YCM1816-3665-70 | M. extorquens AM1:: pCM-exp1815-3665-70, TetR | This study |
2.2. Extraction and analysis of carotenoid produced by M. extorquens AM1 and its derivative strains
The carotenoids were extracted from M. extorquens AM1 as described previously [26,32]. The extract from 100 mL of cell culture was dissolved in 1 mL of CH3OH, and 30 μL was subject to HPLC analysis by using Waters HPLC 1260 (Waters, MA, USA) equipped with a Waters Spherisorb 5.0 μm ODS2 (4.6 mm × 250 mm, 5 μm) column [27]. The mobile phases contained solvent A (acetonitrile-water 9:1, V/V) and solvent B (methanol-isopropanol, 3:2, V/V). The elution program was set as follow: 100% A to 5% A in 0–10 min, 5% A retained from 10 to 20 min, and 5% A to 100% A in 20–25 min. The flow rate was 1.0 mL/min and UV absorbance of the peaks were detected by using a photodiode array detector.
2.3. Extraction and purification of carotenoid from M. extorquens AM1
M. extorquens AM1 was cultivated by using 30 L media for 5 days. Subsequently, the broth was centrifuged (8000 g for 3 min) and the pellets were extracted by using 600 mL of solvents (CH3OH: CHCl3: H2O = 10:3:4) until all visible pigments were removed. Next, all the bottom organic phases were combined and equal volume of acetone was added, which were incubated at 4 °C for 12 h. The insoluble pellets were removed by centrifuge and the supernatants were concentrated under reduced pressure to give the organic extract. The organic extract was fractionated by a silica gel column using gradient elution with petroleum ether-CHCl3-MeOH to give seven fractions (fractions 1–7). Fractions 3 and 4 eluted respectively with 98:2 petroleum ether-CHCl3-MeOH and 96:4 petroleum ether-CHCl3-MeOH were combined and separated by semi-preparative HPLC to obtain the target pigment. The elution program was identical to that used in the analysis program and the flow rate was 3.0 mL/min.
2.4. Gene deletion and complementation
The primers used in this study are listed in Table 2. Allelic replacement of the genes META1p3663, META1p3664, META1p3652 and META1p4598 in M. extorquens AM1 were performed by using the method described previously [26]. Briefly, about 1000 bp to 1200 bp DNA fragments located in the upstream and downstream of META1p3663, META1p3664, META1p3652 and META1p4598 were amplified from M. extorquens AM1 by PCR, respectively. Then, the two fragments of the corresponding gene were fused by overlapping PCR. The PCR products with correct sizes were purified and ligated into linear pCM433 vector digested by Bgl II and Sac I through recombinant clone strategy, respectively. The transformants were screened by PCR and the positive clones were verified by sequencing, the correct plasmids were termed as pCM-△3663, pCM-△3664, pCM-△3652 and pCM-△4598. Subsequently, the plasmids pCM-△3663, pCM-△3664, pCM-△3652 and pCM-△4598 were electroporated into M. extorquens AM1, respectively. M. extorquens AM1 bearing the corresponding plasmid was firstly selected by using tetracycline, then, the double-crossover mutants occurred by growing on the plates containing 5% sucrose (w/v). The genotype of mutant strains with successful allele swapping were firstly screened by PCR, followed by sequencing of the PCR fragments with expected size. The correct mutant strains were termed as YA3663, YA3664, YA3652 and YA4598.
Table 2.
Primers and sgRNAs used in this study.
| Primers | Sequences (5′-3′) | Usage |
|---|---|---|
| 3663Up-F61 | GCCACCTGACGTCTAGATCTCGAGCAGATCCTCGATCGCG | Construction of pCM-△3663 and PCR verification of YA3663 |
| 3663Up-R58 | TCCGAGGAGACGCGGACCCGGGGACTTTTCGCGAAACAATCC | Construction of pCM-△3663 |
| 3663Down-F63 | ATTGTTTCGCGAAAAGTCCCCGGGTCCGCGTCTCCTCG | Construction of pCM-△3663 |
| 3663Down-R61 | CTGGATCCTCTAGTGAGCTCCGCGATCTACGGGCTGGC | Construction of pCM-△3663 and PCR verification of YA3663 |
| 3664Up-F59 | GCCACCTGACGTCTAGATCTGAGAATCGTCGCGAACGGC | Construction of pCM-△3664 and PCR verification of YA3664 |
| 3664Up-R60 | ACCGCCTGAGTTGATTCGACCACTCACCCTCCTCGTCCTC | Construction of pCM-△3664 |
| 3664Down-F58 | GAGGACGAGGAGGGTGAGTGGTCGAATCAACTCAGGCGGT | Construction of pCM-△3664 |
| 3664Down-R60 | CGCTCGAGCTGCAGCATATGGGCAAGGTCAAGGATGCGC | Construction of pCM-△3664 and PCR verification of YA3664 |
| 3652up-F58 | GCCACCTGACGTCTAGATCTCCGGCTTCCTCTCGATCAAC | Construction of pCM-△3652 |
| 3652up-R62 | GTCTCGAACTCTCGAAGGTCCGCGTTTCCTCCATTGCGCT | Construction of pCM-△3652 |
| 3652down-F55 | AGCGCAATGGAGGAAACGCGGACCTTCGAGAGTTCGAGAC | Construction of pCM-△3652 |
| 3652down-R57 | CTGGATCCTCTAGTGAGCTCTGCTCACCTCGAAGTTCAGA | Construction of pCM-△3652 |
| 3652-tF | AGATCCGCTTCACCGCCGA | PCR verification of strain YA3652 |
| 3652-tR | TGACAGGGACGGGCTGAAGC | PCR verification of strain YA3652 |
| 4598-Up-F | AAAGTGCCACCTGACGTCTAGATCTACGAGATGGTCGAACATCGCGT | Construction of pCM-△4598 |
| 4598-Up-R | TGCCTATATGAAGGCCATCCCTCGTATCACCGGTCAAGCGTGTCTCGAA | Construction of pCM-△4598 |
| 4598-Down-F | TTCTTCGAGACACGCTTGACCGGTGATACGAGGGATGGCCTTCATATA | Construction of pCM-△4598 |
| 4598-Down-R | TCGGCTGGATCCTCTAGTGAGCTCTCGATACCGCCTCGACCTATT | Construction of pCM-△4598 |
| 4598-tF | GGGATTGAACGGGTTTTCGC | PCR verification of YA4598 |
| 4598-tR | GGTGCCCGTGATGTGCCTGA | PCR verification of YA4598 |
| Com-R | CTATATTTTCTAGGCTTTGATTG | Construction of plasmid pAI1815 and pAI1816 |
| 1815-NT766-F | TCAAAGCCTAGAAAATATAGTGATGGACTTTCTCGCTGAGGTTTTAGAGCTAGAAATAG | Construction of plasmid pAI1815 |
| 1816-NT802-F | TCAAAGCCTAGAAAATATAGAGGAGAAGGGGGAATTTGCCGTTTTAGAGCTAGAAATAG | Construction of plasmid pAI1816 |
| 80-15F63 | ACCATGATTACGCCAAGCTTATGAGCGCCGCGCTTCAAAC | Construction of pCM-exp1815 and pCM-exp1815-1816 |
| 80-15R70 | ACGGGATTCTGTGAGGATCCTCATCGGCCGGCTCCGGCGG | Construction of pCM-exp1815 |
| 80-16F69 | ACCATGATTACGCCAAGCTTATGAGCGCCACCGCCACCCC | Construction of pCM-exp1816 |
| 80-16R63 | AGCTCGGTACCCGGGGATCCTCACAGAATCCCGTGGCGCA | Construction of pCM-exp1816 and pCM-exp1815-1816 |
| 80-3663F-57 | ACCATGATTACGCCAAGCTTATGACACTCACCCTCCTCG | Construction of pCM-exp3663 |
| 80-3663R-58 | AGCTCGGTACCCGGGGATCCTCAACCCGGTTCCTGCC | Construction of pCM-exp3663 |
| 80-3664-F60 | ACCATGATTACGCCAAGCTTATGGCGCGGGGCAAAC | Construction of pCM-exp3664 |
| 80-3664-R60 | AGCTCGGTACCCGGGGATCCTCATCGGGTCCGCGTCTC | Construction of pCM-exp3664 |
For the mutant strains YA3663 and YA3664, the gene complementation experiments were performed as described previously [26]. The genes META1p3663 and META1p3664 were amplified from M. extorquens AM1 by using PCR, and then cloned into vector pCM80 under downstream of promoter PmxaF to afford the plasmids pCM-exp3663 and pCM-exp3664, respectively. After confirming the inserted fragments by sequencing, pCM-exp3663 and pCM-exp3664 were introduced into corresponding mutant strains by using electroporation to afford the strains YCM3663 and YCM3664, respectively.
2.5. CRISPR interfering of META1p1815 and META1p1816
By using reverse PCR technology with plasmid pAIO as template [26], two plasmids pAI1815 (targeting METAp1815) and pAI1816 (targeting METAp1816) were generated, which were introduced into M. extorquens AM1 to afford the strains YAMZ1815 and YAMZ1816, respectively.
2.6. Gene overexpression in M. extorquens AM1
The gene overexpression strains YCM1815, YCM1816 and YCM1815-16 were generated as below. The individual genes META1p1815, META1p1816 and the operon META1p1815-META1p1816 were amplified from M. extorquens AM1 by using PCR, respectively. Then, the PCR fragments with expected size were purified and then ligated into vector pCM80 under downstream of promoter PmaxF, generating plasmids pCM1815, pCM1816 and pCM1815-16, respectively. After verifying by sequencing, pCM1815, pCM1816 and pCM1815-16 were introduced into M. extorquens AM1 by electroporation to afford YCM1815, YCM1816 and YCM1815-16, respectively.
The strain YCM3665-70 was generated as below. The individual genes META1p3665 and META1p3670 were amplified by using PCR. Then, the two fragments with correct size were purified, which then were fused by overlapping PCR. Next, the fused META1p3665 and META1p3670 fragment was cloned into pCM80 vector to afford plasmid pCM3665-70. After confirming by sequencing, the plasmid pCM3665-70 was electroporated into M. extorquens AM1 to generate YCM3665-70.
The construction of YCM1815-3665-70 was described as below. Briefly, the fragments of META1p1815 and META1p3665-META1p3670 were fused by overlapping PCR to generate META1p1815-META1p3665-META1p3670 cassette, which was then cloned into pCM80 vector to afford the plasmid pCM1815-3665-70. After confirming by sequencing, pCM1815-3665-70 was introduced into M. extorquens AM1 by using electroporation, leading to generate YCM1815-3665-70.
2.7. Spectroscopic analyses of carotenoids from M. extorquens AM1 and its derivative strains
1H NMR spectrum was recorded at 25 °C on Bruker AV 500 instruments. LC-HR-MS data were acquired on a Thermo MAT95XP high-resolution mass spectrometer or a Waters micro MS Q-Tof spectrometer.
3. Results
3.1. Isolation and structural determination of the C30 carotenoid pigment
The carotenoid pigments extracted from M. extorquens AM1 were analyzed by HPLC. The major peak 1 at 11.6 min with characteristic absorption at 490 nm as well as several trace congers were detected (Fig. 1A). Next, LC-MS analysis revealed that peak 1 has a molecular mass of 886.5521 (detected as [M − H]- = 885.5521, Fig. 1B), implying that its molecular formula is C54H78O10 (calculated [M − H]- = 885.5517). By searching the carotenoids produced by Methylorubrum species, we found that compound 1 has the same molecular mass and molecular formula to 4-[2-O-11Z-octadecenoyl-β-glucopyranosyl]-4,4′-diapolycopenedioc acid, a glycosylated carotenoid produced by M. populi BJ001 [14], suggesting they shared the same chemical structure. Further analysis of 1H NMR data proved our speculation, as their hydrogen signals were almost identical (Fig. S1). Therefore, the compound 1 was proposed to be 4-[2-O-11Z-octadecenoyl-β-glucopyranosyl]-4,4′-diapolycopenedioc acid.
Fig. 1.
HPLC analysis (A), mass spectrophotometry (MS) analysis (B), and structure (C) of the carotenoids extracted from M. extorquens AM1. (A) The HPLC profile of the metabolites produced by M. extorquens AM1. The compound 1 with retention time at 11.6 min was isolated for further NMR analyses; (B) The mass of compound 1, [M − H]- = 885.5521 (observed), [M − H]- = 885.5517 (calculated); (C) the structure of compound 1.
3.2. Three enzymes META1p1815, META1p1816 and META1p1817 participate in synthesis of precursor squalene
By using diapophytoene synthase CrtM as a probe, two analogous proteins, presqualene diphosphate synthase META1p1816 (HpnD, 28.5% identity to CrtM) and META1p3220 (CrtB, 27.7% identity to CrtM) were identified. Previous studies demonstrated that the phytoene synthase CrtB is not related to synthesize carotenoids in M. extorquens AM1 and M. extorquens PA1 [23,27], which suggest META1p1816 is probably the candidate protein. Further analyses of M. extorquens AM1 genome revealed that four genes encoding META1p1815 (HpnC), META1p1816 (HpnD), META1p1817 (HpnE), and squalene-hopene cyclase META1p1818 (HpnF/SchC) were clustered in one locus. The three co-occurrence of enzymes HpnCDE were confirmed to synthesize precursor squalene in C30 carotenoid pathway in M. extorquens PA1 and P. limnophila [17,23]. Given META1p1815, META1p1816, META1p1817 show nearly 100% identities to their counterpart proteins HpnCDE presented in M. extorquens PA1, we therefore propose that META1p1815, META1p1816, META1p1817 are used to synthesize C30 precursor squalene in compound 1 biosynthetic pathway in M. extorquens AM1.
The pink carotenoid pigment and hopanoid share the same biosynthetic intermediate in M. extorquens AM1 [23,26]. Hopanoid plays essential roles in physiological processes such as membrane fluidity and lipid packing in M. extorquens, thus, deletion of gene shc encoding squalene-hopene cyclase led the mutant strain has a bad growth [23,32]. To avoid impairment on the growth of M. extorquens AM1 caused by deleting genes related to synthesis of precursor squalene, we used CRISPR interfering technology to decrease the expression of META1p1815 and META1p1816. No matter of interfering META1p1815 and META1p1816, the growth of M. extorquens AM1 was significantly attenuated (Table S1).
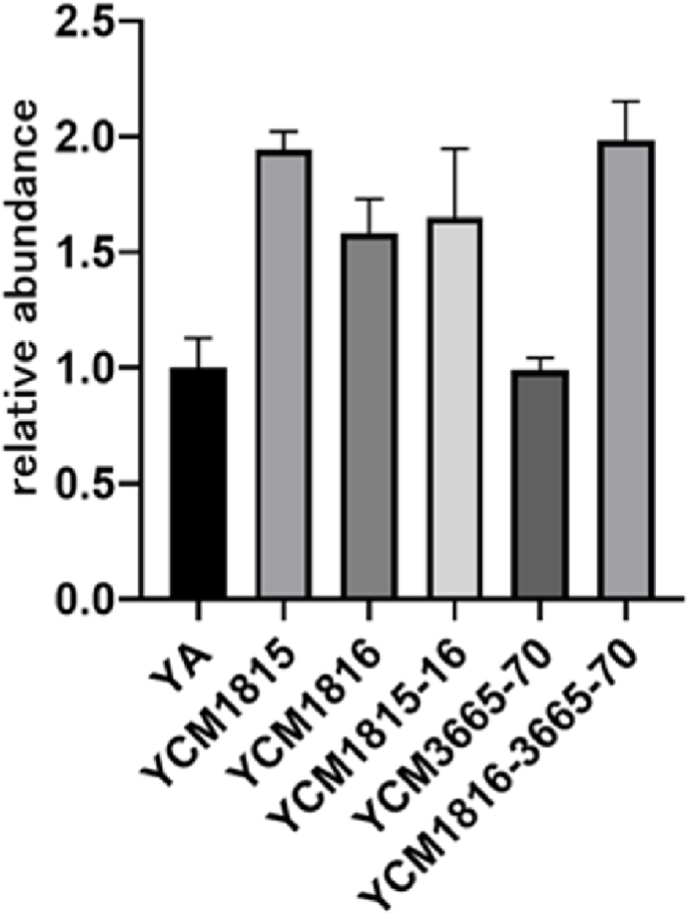
Next, the involvement of META1p1815 (HpnC) and META1p1816 (HpnD) in biosynthetic pathway of compound 1 was investigated by gene overexpression experiments. Compared to M. extorquens YA harboring empty pCM80, the titer of 1 in YCM1815 (overexpression of META1p1815), YCM1816 (overexpression of META1p1816), and YCM1815-16 (overexpression of META1p1815 and META1p1816 cassette) increased by 94.4%, 58.2%, and 65.1%, respectively (Fig. 2), suggesting that increasing the precursor supply improves the titer of compound 1. As revealed from the titer of 1 in YCM3665-70, overexpression of genes encoding enzymes related to modify precursor squalene cannot improve the titer of 1 (Fig. 2). However, co-expression of genes involved in precursor squalene synthesis and modification can significantly improve the titer of 1, as revealed from the titer of 1 in YCM1816-3665-70 (Fig. 2). These results suggested that the supply of the precursor squalene maybe the bottleneck of the titer of carotenoid 1.
Fig. 2.
The relative titer of 1 in M. extorquens AM1 derivative strains. YA is M. extorquens AM1 harboring empty pCM80, which is used as control strain. YCM1815, YCM1816, and YCM1815-16 are M. extorquens AM1 derivative strains which carry plasmids for overexpressing the genes META1p1815, META1p1816, META1p1815 and META1p1816, respectively. YCM3665-70 is M. extorquens AM1 derivative strain which carries plasmid for overexpressing genes META1p3665 and META1p3670. YCM1816-3665-70 is M. extorquens AM1 derivative strain which carries the plasmid for overexpressing the cassette of META1p1816, META1p3665 and META1p3670.
3.3. Mining the oxidases in synthesis of compound 1
After formation of squalene, multiple oxidative modifications are required to afford the intermediate 4,4′-diapolycopene-4,4′-dioic acid [20]. META1p3670 and META1p3665 were confirmed in synthesizing the C30 carotenoid in M. extorquens AM1 [26,27], however, the exact roles of META1p3670 and META1p3665 are still not well characterized. META1p3670 shows 30.4% identity to CrtN involved in staphyloxanthin biosynthetic pathway. Since its counterpart WP_012254689.1 (Mext_3436) presented in M. extorquens PA1 (100% identity to META1p3670) is classified as CrtN group protein by using phylogenetically analysis [23], therefore, META1p3670 is proposed to act as a desaturase to catalyze multiple desaturation steps. META1p3665 shows 32.5% identity to CrtP involved in staphyloxanthin biosynthetic pathway, and its counterpart protein WP_003603441.1 (100% identity to META1p3665) in M. extorquens PA1 acts as a CrtP-type oxidase [23], therefore, META1p3665 is proposed to act as an oxidase to catalyze the formation of terminal aldehyde groups.
AldH was reported to catalyze the formation of terminal carboxylic acid in staphyloxanthin biosynthetic pathway [20]. Then, by using AldH as a probe, three homologous proteins, the aldehyde dehydrogenase META1p3652 (29.6% identity to AldH), the succinate-semialdehyde dehydrogenase I META1p4598 (25.8% identity to AldH), and the proline dehydrogenase META1p0211 (27.2% identity to AldH) were found in M. extorquens AM1 genome. META1p3652 (507 amino acids, a.a.) and META1p4598 (477 a.a.) have the similar amino acid numbers to AldH (459 a.a.), whereas the protein length of META1p0211 (1035 a.a.) is much longer than that of META1p3652 and META1p4598. Thus, the candidates of AldH in M. extorquens AM1 are more likely to be META1p3652 and META1p4598.
Subsequently, the roles of META1p3652 and META1p4598 in compound 1 biosynthetic pathway were investigated by using gene deletion experiments. Unfortunately, both mutant strains YA3652 (ΔMETA1p3652) and YA4598 (ΔMETA1p3652) still produce carotenoid 1 (Fig. S2), suggesting both META1p3652 and META1p4598 are not involved in synthesis of 1.
3.4. META1p3663 acts as a glycosyltransferase in compound 1 biosynthetic pathway
The subsequent modification of 4,4′-diapolycopene-4,4′-dioic acid is glycosylation. In M. extorquens AM1, the glycosyltransferase META1p3663, displaying 27.32% identity to CrtQ, is found to be candidate to catalyze the glycosylation reaction, leading to afford glucosyl-4,4′-diapolycopene-4,4′-dioic acid. To verify the role of glycosyltransferase META1p3663 in compound 1 biosynthetic pathway, META1p3663 was deleted from M. extorquens AM1 genome, generating the mutant strain YA3663 (Fig. 3). The strain YA3663 still shows light pink color, implying that the carotenoid pigment is still produced in this strain. The light pink pigment extracted from YA3663 was analyzed by HPLC. Compared to M. extorquens AM1 wild type, YA3663 abolished the production of compound 1, instead, two new peaks (the main product 2 at 6.02 min and one minor peak at 9.5 min) with the characteristic ultraviolet absorption of carotenoid were detected. Then, molecular formula of the compound 2 was determined as C30H36O4 based on LC-HR-MS data (observed mass [M − H]- = 459.2536, [2M − H]- = 919.5324, calculated mass [M − H]- = 459.2535), which was identical to the expected intermediate 4,4′-diapolycopene-4,4′-dioic acid (Fig. 3). Furthermore, to evaluate whether the production of 4,4′-diapolycopene-4,4′-dioic acid was caused by deletion of META1p3663, the gene META1p3663 was re-introduced into YA3663 to afford strain YCM3663. HPLC analyses results revealed that YCM3663 strain restores the production of compound 1, suggesting the glycosyltransferase META1p3663 is responsible for the glycosylation process.
Fig. 3.
Generating META1p3663 deletion mutant strain YA3663 and analyzing its metabolites. (A) Verification of genotype of YA3663 by using PCR, lane M: DNA ladder marker, lane 1: PCR fragments (2106 bp) from M. extorquens AM1 wild type, lane 2: PCR fragments (3267 bp) from mutant strain YA3663. (B) HPLC analyses of metabolites from YA3663, M. extorquens AM1 wild type, and YCM3663. (C) LC-HR-MS analyses of compound 2 generated by strain YA3663. The observed mass of compound 2 is [M − H]- = 459.2541, [2M − H]- = 919.5324, the calculated mass is [M − H]- = 459.2535. (D) The proposed structure of compound 2 based on mass and biosynthetic pathway.
3.5. META1p3664 acts as an acyltransferase in compound 1 biosynthetic pathway
The last tailoring step in compound 1 biosynthetic pathway is acylation of glucosyl-4,4′-diapolycopene-4,4′-dioic acid. When the acyltransferase CrtO was used as a probe, no homologous proteins were found in M. extorquens AM1 genome. Further bioinformatics analyses demonstrated that a lysophospholipid acyltransferase META1p3664 located next to the glycosyltransferase META1p3663. Because of the co-occurrence of META1p3664 and META1p3663, META1p3664 is probably the candidate enzyme to catalyze the last step in compound 1 biosynthetic pathway.
To check the role of META1p3664 in synthesis of compound 1, META1p3664 was deleted from the genome to afford the strain YA3664 (Fig. 4). The strain YA3664 still shows light pink color. By comparison of the wild type M. extorquens AM1, YA3664 lost the capacity to produce carotenoid 1, instead, several peaks with characteristic ultraviolet absorption of carotenoid at 490 nm were detected (Fig. 4). To check whether the production of these carotenoid derivatives in YA3664 was caused by deletion of META1p3664, the gene META1p3664 was re-introduced into YA3664. The strain YCM3664 harboring META1p3664 driven by promoter pmxaF in pCM80 plasmid can restore the production of compound 1, thus, META1p3664 was confirmed to be involved in synthesis of compound 1. Then, the metabolites produced in YA3664 were analyzed by LC-HR-MS. The compound 3 with target molecular mass was observed ([M − H]- = 621.3110), which is in accordance with the predicted intermediate glucosyl-4,4′-diapolycopene-4,4′-dioic acid (calculated mass [M − H]- = 621.3064) (Fig. 4). Based on these results, META1p3664 was confirmed to catalyze the last acylation reaction to afford compound 1.
Fig. 4.
Generating META1p3664 deletion mutant strain YA3664 and analyzing its metabolites. (A) Verification of the genotype of the mutant strain YA3664 by using PCR, lane M: DNA ladder marker, lane 1: PCR fragments (2830 bp) from M. extorquens AM1 wild type, lane 2: PCR fragments (2014 bp) from mutant strain YA3664. (B) HPLC analyses of the metabolites from YA3664, M. extorquens AM1 wild type, and YCM3664 (YA3664 complemented with META1p3664). (C) LC-HR-MS analyses of compound 3 generated by mutant strain YA3664. The observed mass of compound 3 is [M − H]- = 621.3110, the calculated mass is [M − H]- = 621.3064. (D) The proposed structure of compound 3 based on mass and biosynthetic pathway analyses.
4. Discussion and conclusion
Although M. extorquens AM1 is known to produce pigment for a long time, the structure of the carotenoid remains unknown. Herein, we firstly purified the carotenoid and determined its structure as 4-[2-O-11Z-octadecenoyl-β-glucopyranosyl]-4,4′-diapolycopenedioc acid, a glycosylated carotenoid with C30 backbone. Furthermore, two enzymes, the glycosyltransferase META1p3663 and lysophospholipid acyltransferase META1p3664, were identified to participate in synthesis of the C30 carotenoid.
Methylobacterium species, a representative group of methylotroph strains widely distributed in phyllosphere region of plants, are found to be good candidates as plant growth-promoting bacteria because they can provide nutrients to plants, modulate phytohormone levels, and protect plants against pathogens [33,34]. To resist harmful UV radiations during phyllosphere colonization and/or used for anoxygenic photosynthesis, Methylobacterium species often synthesize carotenoids to provide natural antioxidant activity [14,33,35]. Up to now, only C30 carotenoids were reported from Methylobacterium species such as M. populi BJ001, M. radiotolerans JCM2831 and M. rhodinum ATCC 14821 [14,36]. Genome analyses revealed that the key enzyme CrtM in C40 carotenoid synthesis via CrtB-CrtI-CrtD pathway is absent in alpha-proteobacteria [23], therefore, the Methylobacterium species belonging to alpha-proteobacteria cannot produce C40 carotenoid. Some C30 carotenoids possess better antioxidant activity in both the physical and chemical quenching of reactive oxygen species [37]. The compound 1 isolated here was reported to show better antioxidative activity than C40 carotenoid astaxanthin by using singlet oxygen quenching model experiment [14]. Some other C30 carotenoids such as 4,4′-diapolycopene-4,4′-dial, methyl glucosyl-3,4-dehydroapo-8′-lycopenoate also displayed better antioxidant activity than C40 carotenoids [14,38,39]. These C30 carotenoids are proposed to protect Methylobacterium species against photosensitization reactions because they grow on plant leaves where they are exposed to strong sunlight [14]. It was shown that disruption of C30 carotenoid synthesis led slightly increasing sensitivity of M. extorquens to oxidative stress [23].
As for the first C30 intermediate in C30 carotenoid biosynthetic pathway, two different routes were characterized. The first route is diapophytoene synthase CrtM catalyzes the condensation of two molecules of FPP to afford the C30 intermediate 4,4′-diapophytoene (staphyloxanthin biosynthetic pathway in Staphylococcus aureus) or 15-cis-4,4′-diapophytoene (methyl 5-glucosyl-5,6-dihydro-4,4′-diapolycopenoate biosynthetic pathway in marine bacterium Planococcus maritimus strain iso-3) [15,20]. The secondary route is that squalene synthesized by squalene synthase or three co-occurrence of enzymes HpnCDE is used as precursor [17,23]. Bioinformatics analyses demonstrated that squalene used as precursor in C30 carotenoid biosynthesis via HpnCDE pathway is widespread in prokaryotes [17,23]. In M. extorquens PA1 and P. limnophila, this squalene route to C30 carotenoids is confirmed by gene knockout or gene heterologous expression experiments [17,23]. In this study, the three enzymes META1p1815 (HpnC), META1p1816 (HpnD) and META1p1817 (HpnE) were assumed to synthesize the precursor squalene (Fig. 5), further gene overexpression experiments also confirmed their participation in synthesis of the C30 carotenoid.
Fig. 5.
The gene organization and biosynthetic pathway of compound 1. The arrows in pink represent the genes involved in biosynthesis of compound 1.
The tailoring steps from squalene to 4,4′-diapolycopene-4,4′-dioic acid in M. extorquens AM1 were not well characterized. Based on phylogenetically analyses, META1p3670 and META1p3665 were classified as desaturase (CrtN) and oxidase (CrtP), respectively. However, the exact tailoring timing of META1p3670 and META1p3665 remains elusive. Previous studies revealed that ΔMETA1p3665 and ΔMETA1p3670 strains are colorless [26,27]. Likewise, deletion of WP_012254689.1 (crtN) or WP_003603441.1 (crtP) also lead to abolish the production of carotenoid in M. extorquens PA1, moreover, the precursors for carotenoid synthesis were not detectable in both mutant strains [23]. The colorless phenotype of the mutant strains (ΔMETA1p3665 and ΔWP_003603441.1) demonstrated that the native substrate of META1p3665 or WP_003603441.1 is not 4,4′-diapolycopene, otherwise, the mutant strains can still present pink color for the accumulation of 4,4′-diapolycopene. These results give us a hint that the substrate of META1p3665 should be an intermediate in biosynthesis of 4,4′-diapolycopene rather than 4,4′-diapolycopene. As for the exact timing of terminal aldehyde group catalyzed by META1p3665, it remains unknown (Fig. 5). The aldehyde dehydrogenases such as AldH catalyzing the transformation from 4,4′-diapolycopene-dial to 4,4′-diapolycopene-4,4′-dioic acid are often not clustered with other biosynthetic genes [13,20]. In this study, two AldH analogues META1p3652 and META1p4859, albeit the identities to AldH are low, showed no influence on production of carotenoid 1. This result suggests that the missing aldehyde dehydrogenase in M. extorquens AM1 maybe different from AldH and its analogues, which requires further investigations. After formation of 4,4′-diapolycopene-4,4′-dioic acid, META1p3663 and META1p3664 catalyze the glycosylation and acylation modification of 4,4′-diapolycopene-4,4′-dioic acid to afford compound 1 (Fig. 5).
Some new peaks harboring almost identical UV–visible spectra to the C30 carotenoid were detected in mutant strains YA3664 and YA3663, but further LC-HR-MS analyses revealed that they were not related to the intermediates in compound 1 biosynthetic pathway. Given the terminal carboxylic acid is prone to be esterified, we hypothesize that these peaks are esterified derivatives, and this phenomenon was also observed in Planctopirus limnophila and M. extorquens PA1 [[17], [23], [40], [41]]. Bioinformatics analyses revealed that the three genes cassette containing META1p3663, META1p3664 and META1p3665 located in the genomes of Methylobacterium strains M. extorquens PA1, M. populi BJ001, M. radiotolerans JCM2831 and M. rhodinum ATCC 14821 (Fig. 6). M. populi BJ001, M. radiotolerans JCM2831 and M. rhodinum ATCC 14821 were found to produce glycosylated C30 carotenoid, which were consistent with the biosynthetic genes in these strains. Notably, the C30 carotenoids produced by M. extorquens PA1 are different from M. extorquens AM1 [23]. Based on the gene structure analyses, Mext_3434, Mext_3435 and Mext_3436 (crtP) probably are transcribed as one operon, in which Mext_3436 is proven to be active [23]. However, Mext_3434 and Mext_3435 show no modification toward 4,4′-diapolycopene-4,4′-dioic acid. One possible reason is that an unknown methyltransferase in M. extorquens PA1 is more active than the glycosyltransferase Mext_3434, which can easily methylate the terminal carboxylic acid at 4,4′-diapolycopene-4,4′-dioic acid to form ester bond, therefore, further glycosylation cannot proceed.
Fig. 6.
The gene cassettes containing META1p3663 (encoding glycosyltransferase), META1p3664 (acyl transferase) and META1p3665 (crtP) were identified from Methylobacterium strains which produce C30 carotenoids with characterized structures.
In conclusion, the C30 carotenoid 4-[2-O-11Z-octadecenoyl-β-glucopyranosyl]-4,4′-diapolycopenedioc acid was firstly identified from M. extorquens AM1. By combination of bioinformatics analyses and gene deletion experiments, the genes involved in synthesis of compound 1 were identified. The genes related to synthesizing C30 carotenoid are not rigidly clustered in one locus of M. extorquens AM1 genome. At last, the function of two new enzymes glycosyltransferase META1p3663 and lysophospholipid acyltransferase META1p3664 in C30 carotenoid biosynthetic pathway were characterized by using gene deletion and gene complementation experiments.
CRediT authorship contribution statement
Xu-Hua Mo: Designed the project, Formal analysis, analyzed the data, Writing – original draft, wrote original draft. Yu-Man Sun: Carried out the experiments, Formal analysis, analyzing the data. Yu-Xing Bi: Carried out the experiments, Formal analysis, analyzed the data. Yan Zhao: Carried out the experiments. Gui-Hong Yu: Analyzed the data. Ling-ling Tan: Supervision, Designed and supervised the project, Revised the manuscript. Song Yang: Supervision, Designed and supervised the project, Revised the manuscript.
Declaration of competing interest
The paper entitled “Characterization of C30 carotenoid and identification of its biosynthetic gene cluster in Methylobacterium extorquens AM1” was submitted to Synthetic and Systems Biotechnology. All authors declare that they do not have any financial or commercial conflict of interest in connection with the work submitted.
Acknowledgments
This work was supported by the National Key R&D Program of China (grant No. 2021YFC2103500); National Natural Science Foundation of China (grant No. 22078169); Natural Science Foundation of Shandong Province, China (ZR2021MC074; ZR2020MC008); and Shandong Provincial Key Research and Development Plan (2021ZDSYS28).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2023.08.002.
Contributor Information
Ling-ling Tan, Email: tanlingling80@163.com.
Song Yang, Email: yangsong1209@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Liu C., Hu B., Cheng Y., Guo Y., Yao W., Qian H. Carotenoids from fungi and microalgae: a review on their recent production, extraction, and developments. Bioresour Technol. 2021;337 doi: 10.1016/j.biortech.2021.125398. [DOI] [PubMed] [Google Scholar]
- 2.Santos P.D.F., Rubio F.T.V., da Silva M.P., Pinho L.S., Favaro-Trindade C.S. Microencapsulation of carotenoid-rich materials: a review. Food Res Int. 2021;147 doi: 10.1016/j.foodres.2021.110571. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Z., Chen J., Ci F., Pang H., Cheng N., Xing A. α-Carotene: a valuable carotenoid in biological and medical research. J Sci Food Agric. 2022;102:5606–5617. doi: 10.1002/jsfa.11966. [DOI] [PubMed] [Google Scholar]
- 4.Hussain Y., Abdullah, Alsharif K.F., Aschner M., Theyab A., Khan F., et al. Therapeutic role of carotenoids in blood cancer: mechanistic insights and therapeutic potential. Nutrients. 2022;14:1949. doi: 10.3390/nu14091949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donoso A., González-Durán J., Muñoz A.A., González P.A., Agurto-Muñoz C. Therapeutic uses of natural astaxanthin: an evidence-based review focused on human clinical trials. Pharmacol Res. 2021;166 doi: 10.1016/j.phrs.2021.105479. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S., Kumar R., Kumari A., Panwar A. Astaxanthin: a super antioxidant from microalgae and its therapeutic potential. J Basic Microbiol. 2022;62:1064–1082. doi: 10.1002/jobm.202100391. [DOI] [PubMed] [Google Scholar]
- 7.Ashokkumar V., Flora G., Sevanan M., Sripriya R., Chen W.H., Park J.H., et al. Technological advances in the production of carotenoids and their applications- A critical review. Bioresour Technol. 2023;367 doi: 10.1016/j.biortech.2022.128215. [DOI] [PubMed] [Google Scholar]
- 8.Morrison E.S., Badyaev A.V. Structure versus time in the evolutionary diversification of avian carotenoid metabolic networks. J Evol Biol. 2018;31:764–772. doi: 10.1111/jeb.13257. [DOI] [PubMed] [Google Scholar]
- 9.Shindo K., Asagi E., Sano A., Hotta E., Minemura N., Mikami K., et al. Diapolycopenedioic acid xylosyl esters A, B, and C, novel antioxidative glyco-C30-carotenoic acids produced by a new marine bacterium Rubritalea squalenifaciens. J Antibiot. 2008;61:185–191. doi: 10.1038/ja.2008.28. [DOI] [PubMed] [Google Scholar]
- 10.Marshall J.H., Wilmoth G.J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriol. 1981;147:900–1013. doi: 10.1128/jb.147.3.900-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osawa A., Iki K., Sandmann G., Shindo K. Isolation and identification of 4, 4'-diapolycopene-4,4'-dioic acid produced by Bacillus firmus GB1 and its singlet oxygen quenching activity. J Oleo Sci. 2013;62:955–960. doi: 10.5650/jos.62.955. [DOI] [PubMed] [Google Scholar]
- 12.Taylor R.F., Davies B.H. Triterpenoid carotenoids and related lipids. The triterpenoid carotenes of Streptococcus faecium UNH 564P. Biochem J. 1974;139:751–760. doi: 10.1042/bj1390751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao L., Schenzle A., Odom J.M., Cheng Q. Novel carotenoid oxidase involved in biosynthesis of 4,4'-diapolycopene dialdehyde. Appl Environ Microbiol. 2005;71:3294–3301. doi: 10.1128/AEM.71.6.3294-3301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osawa A., Kaseya Y., Koue N., Schrader J., Knief C., Vorholt J.A., et al. 4-[2-O-11Z-Octadecenoyl-β-glucopyranosyl]-4,4'-diapolycopene-4,4-dioic acid and 4-[2-O-9Z-hexadecenoyl-b-glucopyranosyl]-4,4'-diapolycopene-4,4'-dioic acid: new C30-carotenoids produced by Methylobacterium. Tetrahedron Lett. 2015;56:2791–2794. [Google Scholar]
- 15.Takemura M., Takagi C., Aikawa M., Araki K., Choi S.K., Itaya M., et al. Heterologous production of novel and rare C30-carotenoids using Planococcus carotenoid biosynthesis genes. Microb Cell Factories. 2021;20:194. doi: 10.1186/s12934-021-01683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J.W., Choi B.H., Kim J.H., Kang H.J., Ryu H., Lee P.C. Complete genome sequence of Planococcus faecalis AJ003T, the type species of the genus Planococcus and a microbial C30 carotenoid producer. J Biotechnol. 2018;266:72–76. doi: 10.1016/j.jbiotec.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Santana-Molina C., Henriques V., Hornero-Méndez D., Devos D.P., Rivas-Marin E. The squalene route to C30 carotenoid biosynthesis and the origins of carotenoid biosynthetic pathways. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2210081119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moyo A.C., Dufossé L., Giuffrida D., van Zyl L.J., Trindade M. Structure and biosynthesis of carotenoids produced by a novel Planococcus sp. isolated from South Africa. Microb Cell Factories. 2022;21:43. doi: 10.1186/s12934-022-01752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelz A., Wieland K.P., Putzbach K., Hentschel P., Albert K., Götz F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem. 2005;280:32493–32498. doi: 10.1074/jbc.M505070200. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.H., Lee P.C. Functional expression and extension of staphylococcal staphyloxanthin biosynthetic pathway in Escherichia coli. J Biol Chem. 2012;287:21575–21583. doi: 10.1074/jbc.M112.343020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiger S., Perez-Fons L., Cutting S.M., Fraser P.D., Sandmann G.J.M. Annotation and functional assignment of the genes for the C30 carotenoid pathways from the genomes of two bacteria: Bacillus indicus and Bacillus firmus. Microbiology. 2015;161:194–202. doi: 10.1099/mic.0.083519-0. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.H., Kim J.W., Lee P.C. Genome mining reveals two missing crtp and aldh enzymes in the C30 carotenoid biosynthesis pathway in Planococcus faecalis AJ003T. Molecules. 2020;25:5892. doi: 10.3390/molecules25245892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizk S., Henke P., Santana-Molina C., Martens G., Gnädig M., Nguyen N.A., et al. Functional diversity of isoprenoid lipids in Methylobacterium extorquens PA1. Mol Microbiol. 2021;116:1064–1078. doi: 10.1111/mmi.14794. [DOI] [PubMed] [Google Scholar]
- 24.Peel D., Quayle J.R. Microbial growth on C1 compounds. 1. Isolation and characterization of Pseudomonas AM1. Biochem J. 1961;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan X.J., Chen W.J., Ma Z.X., Yuan Q.Q., Zhang M., He L. Rewiring the native methanol assimilation metabolism by incorporating the heterologous ribulose monophosphate cycle into Methylorubrum extorquens. Metab Eng. 2021;64:95–110. doi: 10.1016/j.ymben.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Mo X.H., Zhang H., Wang T.M., Zhang C., Zhang C., Xing X.H., et al. Establishment of CRISPR interference in Methylorubrum extorquens and application of rapidly mining a new phytoene desaturase involved in carotenoid biosynthesis. Appl Microbiol Biotechnol. 2020;104:4515–4532. doi: 10.1007/s00253-020-10543-w. [DOI] [PubMed] [Google Scholar]
- 27.Van Dien S.J., Marx C.J., O'Brien B.N., Lidstrom M.E. Genetic characterization of the carotenoid biosynthetic pathway in Methylobacterium extorquens AM1 and isolation of a colorless mutant. Appl Environ Microbiol. 2003;69:7563–7566. doi: 10.1128/AEM.69.12.7563-7566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marx C.J., Lidstrom M.E. Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology. 2001;147:2065–2075. doi: 10.1099/00221287-147-8-2065. [DOI] [PubMed] [Google Scholar]
- 29.Marx C.J. Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res Notes. 2008;1:1. doi: 10.1186/1756-0500-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunn D.N., Lidstrom M.E. Isolation and complementation analysis of 10 methanol oxidation mutant classes and identification of the methanol dehydrogenase structural gene of Methylobacterium sp. strain AM1. J Bacteriol. 1986;166:581–590. doi: 10.1128/jb.166.2.581-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y.M., Chen W.J., Yang J., Zhou Y.M., Hu B., Zhang M., et al. Production of 3-hydroxypropionic acid in engineered Methylobacterium extorquens AM1 and its reassimilation through a reductive route. Microb Cell Factories. 2017;16:179. doi: 10.1186/s12934-017-0798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley AS, Swanson PK, Muller EE, Bringel F, Caroll SM, Pearson A, et al. Hopanoid-free Methylobacterium extorquens DM4 overproduces carotenoids and has widespread growth impairment. PLoS One;12:e0173323. [DOI] [PMC free article] [PubMed]
- 33.Bajpai A., Mahawar H., Dubey G., Atoliya N., Parmar R., Devi M.H., et al. Prospect of pink pigmented facultative methylotrophs in mitigating abiotic stress and climate change. J Basic Microbiol. 2022;62:889–899. doi: 10.1002/jobm.202200087. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C., Wang M.Y., Khan N., Tan L.L., Yang S. Potentials, utilization, and bioengineering of plant growth-promoting Methylobacterium for sustainable agriculture. Sustainability. 2021;13:3941. [Google Scholar]
- 35.Stiefel P., Zambelli T., Vorholt J.A. Isolation of optically targeted single bacteria by application of fluidic force microscopy to aerobic anoxygenic phototrophs from the phyllosphere. Appl Environ Microbiol. 2013;79:4895–4905. doi: 10.1128/AEM.01087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinig H., Schmitt R., Meister W., Englert G., Thommen H. New C30-carotenoic acid glucosyl esters from Pseudomonas rhodos. Z Naturforsch. 1979;34c:181–185. [Google Scholar]
- 37.Siziya I.N., Hwang C.Y., Seo M.J. Antioxidant potential and capacity of microorganism-sourced C30 carotenoids-A Review. Antioxidants. 2022;11:1963. doi: 10.3390/antiox11101963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S.H., Kim M.S., Lee B.Y., Lee P.C. Generation of structurally novel short carotenoids and study of their biological activity. Sci Rep. 2016;6 doi: 10.1038/srep21987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shindo K., Endo M., Miyake Y., Wakasugi K., Morritt D., Bramley P.M., et al. Methyl 5-glucosyl-5,6-dihydro-apo-4,4'-lycopenoate, a novel antioxidative glyco-C(30)-carotenoic acid produced by a marine bacterium Planococcus maritimus. J Antibiot. 2008;61:729–735. doi: 10.1038/ja.2008.86. [DOI] [PubMed] [Google Scholar]
- 40.Furubayashi M., Li L., Katabami A., Saito K., Umeno D. Construction of carotenoid biosynthetic pathways using squalene synthase. FEBS Lett. 2014;588:436–442. doi: 10.1016/j.febslet.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Steiger S., Perez-Fons L., Fraser P.D., Sandmann G. Biosynthesis of a novel C30 carotenoid in Bacillus firmus isolates. J Appl Microbiol. 2012;113:888–895. doi: 10.1111/j.1365-2672.2012.05377.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.