Summary
Mesenchymal stem cells (MSCs), also referred to as “medicinal signaling cells,” have gained prominence as candidates for cell-based therapy and in clinical trials owing to their regenerative and therapeutic properties. Here, we present a protocol for isolating MSCs from the decidua basalis layer of human placenta using an explant culture approach. We describe steps for collecting, disinfecting, and plating placental tissue. We then detail procedures for characterizing the isolated MSCs through flow cytometry and in vitro differentiation.
Subject areas: Cell Biology, Cell Culture, Cell Isolation, Flow Cytometry/Mass Cytometry, Stem Cells, Cell Differentiation
Graphical abstract

Highlights
-
•
Isolation of MSCs from human placenta using explant culture method
-
•
Expansion in culture, cryopreservation, and revival of the placental MSCs
-
•
Basic characterization by flow cytometry and in vitro differentiation
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Mesenchymal stem cells (MSCs), also referred to as “medicinal signaling cells,” have gained prominence as candidates for cell-based therapy and in clinical trials owing to their regenerative and therapeutic properties. Here, we present a protocol for isolating MSCs from the decidua basalis layer of human placenta using an explant culture approach. We describe steps for collecting, disinfecting, and plating placental tissue. We then detail procedures for characterizing the isolated MSCs through flow cytometry and in vitro differentiation.
Before you begin
MSCs provide ease of accessibility for isolation and they can be derived from numerous sources like bone marrow, adipose tissue, umbilical cord etc. An alternative source to give a higher yield of MSCs, as compared to other well-known sources of adult tissues, is the human placenta.1 Placenta is usually abundantly available and discarded after birth, hence, avoiding the use of any invasive procedures for collection, making it a convenient source. The decidua basalis layer forms a part of the maternal side of the human placenta.2 This protocol outlines a simple step-wise procedure by which MSCs of “maternal” origin can be isolated from the decidua basalis layer of human placenta by explant culture method without the use of any enzymatic digestion or centrifugation steps.
The isolated placental MSCs were analyzed for the expression of MSC-specific surface antigen markers and were found to be positive for CD73, CD90, CD105 and CD166; while being negative for CD34 and HLA-DR. The isolated placental MSCs also demonstrated successful differentiation towards the osteogenic and adipogenic lineages, which demonstrated their multipotent differentiation potential.
A similar explant culture protocol had been standardized in our lab for the isolation of MSCs from Wharton’s jelly of the human umbilical cord.3
Institutional permissions
Immediately after full-term births by cesarean delivery, collection of human placenta samples (n = 5) was done with informed consent from the donors. The experimental protocol was approved, following the guidelines laid down by the Institutional Ethics Committee (IEC) and Institutional Committee for Stem Cell Research and Therapy (IC-SCRT) at IISER Kolkata, India. All methods were performed in accordance with the guidelines and regulations of the Declaration of Helsinki.
Preparation of items for collection of placental tissue
Timing: 10 min
-
1.
Prepare a set of sterile surgical instruments (a pair of sharp scissors and forceps), for cutting the decidua basalis layer of the placenta.
-
2.
Prepare 20 mL of normal saline containing 1x Antibiotic-Antimycotic solution (100 U/ml of penicillin, 100 μg/mL of streptomycin and 250 ng/mL of amphotericin B), with additional 6.25 μg/mL amphotericin B, in a 50 mL sterile centrifuge tube, for collecting the placental tissue pieces.
Note: Total anti-fungal concentration of prepared solution at this point is maintained at 6.5 μg/mL.
CRITICAL: Use sterile centrifuge tubes and surgical instruments.
Preparation of items for washing and disinfection of placental tissue
Timing: 30 min
-
3.
Prepare 30 mL of normal saline containing 1x Antibiotic-Antimycotic solution (100 U/ml of penicillin, 100 μg/mL of streptomycin and 250 ng/mL of amphotericin B), with additional 12.5 μg/mL amphotericin B, in a 50 mL sterile centrifuge tube, for storing the placental tissue pieces, after washing with saline.
Note: Total anti-fungal concentration of prepared solution at this point is maintained at 12.75 μg/mL.
-
4.For the next steps of disinfection, inside the laminar flow hood, prepare a total of seven 15 mL sterile centrifuge tubes and label them in the following order and add the respective components into the tubes.
-
a.Sterile phosphate-buffered saline (PBS).
-
b.Sterile PBS.
-
c.70% Ethanol.
-
d.Sterile PBS.
-
e.Sterile PBS.
-
f.Sterile PBS.
-
g.Sterile PBS.
-
a.
CRITICAL: Use sterile centrifuge tubes and PBS for washing.
Preparation of MSC isolation and growth medium
Timing: 20 min
-
5.
Prepare 50 mL of MSC isolation or growth medium (see materials and equipment for recipes).
-
6.
Sterile filter the prepared medium into fresh 50 mL centrifuge tubes using 0.22 μm syringe filters.
-
7.
Aliquot the desired amount of medium and pre-warm it at 37°C before use.
Preparation of MSC freezing mixture
Timing: 10 min
-
8.
Prepare 5 mL of MSC freezing mixture (see materials and equipment for recipe).
-
9.
Sterile filter the prepared mixture into a fresh 15 mL centrifuge tube using a 0.22 μm syringe filter.
-
10.
Store the freezing mixture at 4°C.
Preparation of adipogenic differentiation medium
Timing: 10 min
-
11.
Prepare 10 mL of adipogenic differentiation medium (see materials and equipment for recipe).
-
12.
Sterile filter the prepared medium into a fresh 15 mL centrifuge tube using a 0.22 μm syringe filter.
-
13.
Pre-warm it at 37°C before use.
Preparation of osteogenic differentiation medium
Timing: 10 min
-
14.
Prepare 10 mL of osteogenic differentiation medium (see materials and equipment for recipe).
-
15.
Sterile filter the prepared medium into a fresh 15 mL centrifuge tube using a 0.22 μm syringe filter.
-
16.
Pre-warm it at 37°C before use.
OR
-
17.
Prepare the osteogenic differentiation medium using the StemProTM Osteogenesis Differentiation Kit as per manufacturer’s protocol (see https://tools.thermofisher.com/content/sfs/manuals/StemProOsteoDiff.pdf ).
-
18.
Pre-warm it at 37°C before use.
Preparation of Alizarin Red S stain
Timing: 20 min
-
19.
Make 1% Alizarin Red S solution in 10 mL of ultrapure distilled water.
-
20.
Adjust the pH to 4.2 using 0.05 M HCl.
-
21.
Filter through a 0.22 μm syringe filter to remove any undissolved stain particles.
Preparation of Oil Red O stain
Timing: 1 h
-
22.
Dissolve 0.05 g of Oil Red O in 10 mL of isopropanol (stock). For dissolution, keep in a warm water bath for 30 min.
-
23.
To prepare the working solution, add 3 mL of the prepared stock solution to 2 mL of ultrapure distilled water. Filter twice using Whatman filter paper, to remove any undissolved stain particles.
Preparation of Toluidine Blue stain
Timing: 30 min
-
24.
Prepare 0.1% Toluidine Blue solution in 1% paraformaldehyde (PFA) in PBS.
-
25.
Filter twice using Whatman filter paper, to remove any undissolved stain particles.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE Mouse Anti-Human CD73 (1:33) | BD Pharmingen | Catalog No 550257 |
| PE Mouse Anti-Human CD90 (1:33) | BD Pharmingen | Catalog No 555596 |
| CD105 PE-conjugated Antibody (1:50) | R&D Systems | Catalog No FAB10971P |
| PE Mouse Anti-Human CD34 (1:50) | BD Pharmingen | Catalog No 550761 |
| PE Mouse Anti-Human CD166 (1:50) | BD Pharmingen | Catalog No 559263 |
| FITC Mouse Anti-Human HLA-DR (1:33) | BD Pharmingen | Catalog No 555811 |
| PE Mouse IgG, κ Isotype Control (1:33 as isotype for CD90, 1:800 as isotype for CD34/CD73/CD166) | BD Pharmingen | Catalog No 550617 |
| Mouse IgG1 PE-conjugated Antibody (1:50) | R&D Systems | Catalog No IC002P |
| FITC Mouse IgG2a, κ Isotype Control (1:33) | BD Pharmingen | Catalog No 555573 |
| Vimentin (D21H3) XP Rabbit mAb Antibody (1:150) |
Cell Signaling Technology | Catalog No 5741S |
| Human STRO-1 antibody (1:100) | R&D Systems | Catalog No MAB1038 |
| Oct-4 (D705Z) Mouse mAb (1:400) | Cell Signaling Technology | Catalog No 75463S |
| Alexa Fluor 488 goat anti-mouse (1:500) | Invitrogen | Catalog No A11001 |
| Alexa Fluor 568 goat anti-rabbit (1:500) | Invitrogen | Catalog No A11011 |
| Alexa Fluor 488 anti-rabbit (1:500) | Cell Signaling Technology | Catalog No 4412S |
| Biological samples | ||
| Human placenta | N/A | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| 0.9% w/v Saline | Kunal Remedies Pvt. Ltd. | Catalog No 01/LVP/SC/P |
| Antibiotic-antimycotic (100X) | Thermo Fisher Scientific, Gibco | Catalog No 15240096 |
| Isopropanol | Merck | Catalog No 1.07022.0521 |
| Autoclaved distilled water | N/A | N/A |
| Ultrapure distilled water | N/A | N/A |
| Hydrochloric acid (HCl) | Merck | Catalog No 1.93401.0521 |
| Absolute ethanol | Merck | Catalog No 1.00983.0511 |
| Sodium hypochlorite solution | Merck | Catalog No 1.93607.1021 |
| Dulbecco’s phosphate-buffered saline (DPBS), no calcium, no magnesium | Thermo Fisher Scientific, Gibco | Catalog No 14190144 |
| TrypLE Express Enzyme (1x), no phenol red | Thermo Fisher Scientific, Gibco | Catalog No 12604013 |
| KnockOut DMEM (DMEM-KO) | Thermo Fisher Scientific, Gibco | Catalog No 10829018 |
| L-Glutamine (200 mM) | Thermo Fisher Scientific, Gibco | Catalog No 25030149 |
| Fetal bovine serum (FBS) mesenchymal stem cell-qualified | Thermo Fisher Scientific, Gibco | Catalog No 12662029 |
| Penicillin-streptomycin (10,000 U/mL) | Thermo Fisher Scientific, Gibco | Catalog No 15140148 |
| Amphotericin B (AMT) solution (250 ug/mL) for cell culture, endotoxin (BET) 1 EU/m | Sisco Research Laboratories Pvt. Ltd. | Catalog No 15875 |
| Phosphate-buffered saline | Sigma-Aldrich | Catalog No P4417 |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | Catalog No D2650 |
| Sheath fluid | BD Biosciences | Catalog No 342003 |
| Paraformaldehyde | Sigma-Aldrich | Catalog No P6148 |
| Triton X-100 | Sigma-Aldrich | Catalog No T8787 |
| Bovine serum albumin (BSA) | Sigma-Aldrich | Catalog No A7906 |
| 4′,6-Diamidino-2-phenylindole dihydrochloride, 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) | Sigma-Aldrich | Catalog No D9542 |
| Vectashield Antifade Mounting Medium | Vector Laboratories | Catalog No H-1000 |
| Dexamethasone | Sigma-Aldrich | Catalog No D4902 |
| Insulin solution human | Sigma-Aldrich | Catalog No I9278 |
| ꞵ-Glycerophosphate disodium salt hydrate | Sigma-Aldrich | Catalog No G9422 |
| L-ascorbic acid | Sigma-Aldrich | Catalog No A5960 |
| Indomethacin | Sigma-Aldrich | Catalog No I8280 |
| Butylated hydroxyanisole | Sigma-Aldrich | Catalog No B1253 |
| Toluidine blue | Loba Chemie | Catalog No 52040 |
| Alizarin red S | Sigma-Aldrich | Catalog No A5533 |
| Oil Red O | Sigma-Aldrich | Catalog No 00625 |
| StemPro Osteogenesis Differentiation Kit | Thermo Fisher Scientific, Gibco | Catalog No A1007201 |
| RNAiso Plus | Takara | Catalog No 9108 |
| Chloroform | Sigma-Aldrich | Catalog No C2432-25ML |
| 2-Propanol | Sigma-Aldrich | Catalog No I9516-25ML |
| UltraPure DNase/RNase-Free Distilled Water | Invitrogen | Catalog No 10977015 |
| Verso cDNA Synthesis Kit | Thermo Fisher Scientific | Catalog No AB1453A |
| PowerUp SYBR Green Master Mix | Applied Biosystems | Catalog No A25742 |
| TaqMan Gene Expression Assay (FAM) | Applied Biosystems | Catalog No 4331182 |
| TaqMan Fast Universal Master Mix | Applied Biosystems | Catalog No 4352042 |
| Oligonucleotides | ||
| Vimentin (VIM) forward and reverse primers | N/A | N/A |
| Oct-4 (POU5F1) (Hs00742896_s1) | Applied Biosystems | Catalog No 4331182 |
| Software and algorithms | ||
| BD FACSDiva | BD Biosciences | https://www.bdbiosciences.com/en-in/products/software/instrument-software/bd-facsdiva-software |
| ZEN | Zeiss | https://www.zeiss.com/microscopy/en/products/software/zeiss-zen-lite.html |
| NanoDrop 2000 | Thermo Fisher Scientific | https://www.thermofisher.com/in/en/home/industrial/spectroscopy-elemental-isotope-analysis/molecular-spectroscopy/uv-vis-spectrophotometry/instruments/nanodrop/resources.html |
| CFX Manager | Bio-Rad | https://www.bio-rad.com/en-in/sku/1845000-cfx-manager-software?ID=1845000 |
| GraphPad Prism 8 | GraphPad | https://www.graphpad.com/dl/1057948/D599DCF7/ |
| Other | ||
| 15 mL Centrifuge tubes | Tarsons | Catalog No 500031 |
| 50 mL Centrifuge tubes | Tarsons | Catalog No 500041 |
| 1.5 mL Microcentrifuge tubes | Corning, Axygen | Catalog No MCT-150-C |
| 90 mm Petri dish sterile | Tarsons | Catalog No 460090 |
| 35 mm Cell culture dish | Corning, Falcon | Catalog No 353001 |
| 1.2 mL External threaded polypropylene cryogenic vial, self-standing with conical bottom | Corning | Catalog No 430658 |
| FACS tubes | Corning, Falcon | Catalog No 352054 |
| Millex-GP Syringe Filter Unit, 0.22 μm, polyether sulfone, 33 mm, gamma sterilized | Merck | Catalog No 2SLGP033RS |
| 20 and 5 mL Syringes | Dispo Van | N/A |
| Sterile scalpel blade no. 20 | HiMedia | Catalog No LA771 |
| 0.5–10 μL Microtips | Corning, Axygen | Catalog No T-300 |
| 20–200 μL pipette tips | Corning, Axygen | Catalog No T-200-C |
| 100–1000 μL pipette tips | Corning, Axygen | Catalog No T-1000-C |
| Laminar flow hood | Thermo Fisher Scientific | Catalog No 51025411 |
| Surgical tools including scalpel holder, forceps (large and small), pointed scissors | HiMedia | Catalog No LA830, LA824-2NO, LA710, |
| Incubator | BINDER | Model: C 150 CO2 Incubator; Catalog No 9040-0078 |
| Centrifuge 5702 | Eppendorf | Catalog No 0015761 |
| Hemocytometer depth 0.1 mm | Rohem Instruments Pvt. Ltd. | Catalog No B.S. 748 |
| Mr. Frosty Freezing Container | Thermo Fisher Scientific | Catalog No 5100-0001 |
| −20°C Freezer | Celfrost | Model: BSF 150 |
| −86°C Upright ultra-low temperature freezer | Thermo Fisher Scientific | Model: ULT-1386-3 |
| Liquid nitrogen tank | Thermo Fisher Scientific | Model: Model 8031 |
| Flow cytometer | BD Biosciences | Model: BD LSRFortessa Cell Analyzer; Catalog No 647177I1 |
| Inverted microscope | Nikon | Model: Eclipse TS100 |
| Bright field microscope | Olympus | Model: BX51 |
| Fluorescence microscope | Zeiss | Model: Axio Observer Z1 |
| NanoDrop spectrophotometer | Thermo Fisher Scientific | Model: 2000 |
| Real-Time PCR Detection System | Bio-Rad | Model: CFX96; Catalog No 3600037 |
| Refrigerator | Samsung | Model: RT31 |
| 2–20 μL single channel variable pipette | Eppendorf | Model: Research plus; Catalog No. 3120000038 |
| 20–200 μL single channel variable pipette | Eppendorf | Model: Research plus; Catalog No 3120000054 |
| 100–1,000 μL single channel variable pipette | Eppendorf | Model: Research plus; Catalog No 3120000062 |
| Autoclave | Tuttnauer | Model: 3850 ML |
| Plastic beakers | Tarsons | Catalog No 421020 |
| Biohazards disposable waste bags | N/A | N/A |
| Microscope slides | Riviera | Catalog No 72910 135 |
| Glass funnels | Borosil | Catalog No 6140077 |
| Filter papers | Whatman | Catalog No 1001110 |
| Purple nitrile exam gloves | Kimtech | Catalog No 55081 |
Materials and equipment
MSC Isolation Medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM-KO | 44 mL | |
| MSC-Qualified FBS | 10% | 5 mL |
| L-Glutamine | 2 mM | 500 μL |
| Antibiotic-Antimycotic | 100 U/ml penicillin, 100 μg/mL streptomycin and 250 ng/mL amphotericin B | 500 μL |
| Amphotericin B | Variable | Variable |
| Total | 50 mL |
Store at 4°C, use within 2 weeks.
MSC Growth Medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM-KO | 44 mL | |
| MSC-Qualified FBS | 10% | 5 mL |
| L-Glutamine | 2 mM | 500 μL |
| Penicillin-Streptomycin | 100 U/ml penicillin and 100 μg/mL streptomycin | 500 μL |
| Total | 50 mL |
Store at 4°C, use within 2 weeks.
MSC Freezing Mixture
| Reagent | Final concentration | Amount |
|---|---|---|
| MSC-Qualified FBS | 90% | 4.5 mL |
| DMSO | 10% | 500 μL |
| Total | 5 mL |
Store at 4°C, use within 2 weeks.
Adipogenic Differentiation Medium
| Reagent | Final concentration | Amount |
|---|---|---|
| MSC growth medium | 9.8 mL | |
| Dexamethasone (Stock- 200 μM in ethanol) | 1 μM | 50 μL |
| Indomethacin (Stock- 70 mM in ethanol) | 0.5 mM | 77.14 μL |
| Butylated-hydroxy anisole (Stock- 45 mM in ethanol) | 60 μM | 13.33 μL |
| Insulin (Stock- 10 mg/mL) | 10 μg/mL | 10 μL |
| Total | 10 mL |
Store at 4°C, use within 2 weeks.
Osteogenic Differentiation Medium
| Reagent | Final concentration | Amount |
|---|---|---|
| MSC growth medium | 9.8 mL | |
| Dexamethasone (Stock- 200 μM in ethanol) | 0.1 μM | 5 μL |
| Ascorbic acid (Stock- 50 mM in water) | 0.2 mM | 40 μL |
| ꞵ-glycerophosphate disodium salt hydrate (Stock- 0.5 M in water) | 10 mM | 200 μL |
| Total | 10 mL |
OR
StemPro Osteogenesis Differentiation Kit.
Store at 4°C, use within 2 weeks.
1x Sterile PBS, pH 7.4
Dissolve two tablets of PBS in 400 mL of distilled water. Sterilize by autoclaving. Store at 4°C. Use within 1 month.
Alizarin Red S solution, pH 4.2
Prepare freshly before use.
Oil Red O solution
Prepare freshly before use.
Toluidine Blue Solution
Prepare freshly before use.
Step-by-step method details
Collection of placental tissue
Timing: 15 min
This will demonstrate how the decidua basalis layer of the placenta is identified, cut into small pieces and carried to the laboratory for further processing.
-
1.
Collect the placenta, along with the umbilical cord, in an aseptic tray, after cesarean delivery.
-
2.
Flip the placenta over such that the maternal surface (decidua basalis) is facing upwards.
CRITICAL: Make sure that the fetal side with the umbilical cord is facing downwards.
-
3.
Cut pieces of approximately 0.5 cm thickness by using a sterile, sharp pair of scissors; from the decidua basalis tissue layer, as depicted in Figure 1A and Methods video S1.
-
4.
Transfer the pieces to a 50 mL sterile centrifuge tube containing 20 mL of normal saline supplemented with 1X Antibiotic-Antimycotic solution (100 U/ml of penicillin, 100 μg/mL of streptomycin and 250 ng/mL of amphotericin B) and additional 6.25 μg/mL of amphotericin B.
Note: Total anti-fungal concentration of the prepared solution at this point is maintained at 6.5 μg/mL.
-
5.
Transport the tube back to the laboratory on ice for further processing.
Figure 1.
Isolation of PL-MSCs by explant culture method
(A–F) (A) Collection of the decidua basalis layer of the human placenta (B) Wash to remove excess blood (C) Disinfection and washing (D) Mincing of tissue explants (E) Plating of tissue explants (F) Emerging of MSCs. Scale bar: 250 μm.
Washing and disinfection of placental tissue
Timing: 2.5 h
This will demonstrate how the placental tissue pieces are thoroughly washed to remove all traces of blood sticking to them, and meticulously disinfected prior to further processing.
-
6.
Take the tissue pieces out, one by one, onto a 90 mm sterile petri-plate, and rinse them with normal saline, till all traces of blood and blood clots sticking to it are completely removed, as depicted in Figure 1B and Methods video S2.
Note: To avoid spillage of human blood and contaminated liquid, we carried out this step outside the laminar flow hood. However, it is advisable to carry out this procedure inside a laminar flow hood, if possible, to evade contamination issues.
-
7.
Transfer the tissue pieces to another sterile 50 mL centrifuge tube containing 30 mL of normal saline supplemented with 1x Antibiotic-Antimycotic solution (100 U/ml of penicillin, 100 μg/mL of streptomycin and 250 ng/mL of amphotericin B) with an additional 12.5 μg/mL of amphotericin B.
Note: Total anti-fungal concentration of the prepared solution at this point is maintained at 12.75 μg/mL.
-
8.
Keep the tube at 4°C for 1.5–2 h.
-
9.After 1.5–2 h, transfer the tissue pieces to the laminar flow hood for further disinfection. Perform the following steps as depicted in Methods video S3.
-
a.Take out a tissue piece on a fresh 90 mm sterile petri-plate.
-
b.Dip it 3 to 4 times in a 15 mL centrifuge tube containing sterile PBS to give a wash.
-
c.Again, transfer it to another 15 mL centrifuge tube containing sterile PBS, and dip it 3 to 4 times.
-
d.Next, dip the tissue piece in a tube containing 70% ethanol for exactly 15 s.
-
e.Immediately transfer to another tube containing sterile PBS and dip 3 to 4 times, to remove any traces of ethanol.
-
f.Repeat step (e) 3 more times (Figure 1C).
-
a.
Mincing and plating of placental tissue
Timing: 15 min
-
10.Place a washed tissue piece from step 9f onto a 90 mm sterile petri-plate.
-
a.Store the tube containing the other pieces on ice.
-
b.Using forceps and a scalpel fixed to a scalpel holder, cut it into small explants of 3–5 mm (Figure 1D).
-
c.Carefully plate about 10–15 explants onto a 35 mm sterile tissue culture dish using fine forceps.
-
d.Allow them to air-dry for 5–7 min.
-
a.
Note::This would facilitate the attachment of the explants to the surface of the dish.
-
11.
Next, add 2 mL of warm fresh MSC isolation medium drop-wise, very gently, taking care not to dislodge the explants as depicted in Figure 1E and Methods video S4.
Note: At this stage, the MSC isolation medium contains 6 μL per ml of additional amphotericin B (250 μg/mL) to maintain the total anti-fungal component concentration at 1.75 μg/mL.
-
12.
Transfer the tissue culture dishes containing the placental explants to a CO2 incubator maintaining 37°C and 5% CO2.
CRITICAL: Discard the leftover minced explants in a biohazard waste bag. All the liquid waste should be collected in a beaker containing sodium hypochlorite solution and discarded appropriately.
-
13.
After 48 h, give the first medium change with 2 mL of warm MSC isolation medium. After this, give consecutive media changes every 72 h.
Note: Progressively lower the amphotericin B component from the 8th day of plating of the explants. On the 8th day, reduce the total anti-fungal concentration to 1.5 μg/mL and further reduce it to 0.75 μg/mL on the 11th day.
-
14.
After approximately 10–11 days, the MSCs are expected to come out of the explants.
-
15.
On ∼ 14th day, after enough cells have come out of the explants, as depicted in Figure 1F, remove them using 1 mL sterile blunt-end tips and add 2 mL of warm fresh MSC isolation medium (without any additional amphotericin B added) to the dishes.
Note: It is critical to remove the explants once sufficient cells have emerged, to avoid the presence of necrotic tissue.
Establishment of MSC culture
Timing: Variable
-
16.
After 24–48 h of explant removal, check the confluency of the cell patches on the dishes, aspirate the medium, and wash the cells twice with DPBS.
CRITICAL: Ensure that the patches of cells on the dishes are around 50–80% confluent.
-
17.
Add 250 μL of TrypLE to each 35 mm tissue culture dish and allow the cells to dissociate from the plastic surface for 4–6 min at 37°C.
Note: Trypsin can also be used in place of TrypLE. TrypLE is an animal origin-free recombinant enzyme that is gentle on stem cells and preserves their surface proteins.
-
18.Add 1 mL of warm fresh MSC growth medium to the dish.
-
a.Collect the cells and transfer them to a 15 mL sterile centrifuge tube
-
b.Centrifuge at 24°C–26°C for 2 min at 500 × g.
-
a.
-
19.After centrifugation, aspirate out the medium supernatant.
-
a.Add 500 μL of warm fresh MSC growth medium to the cell pellet to suspend it.
-
b.Take out a 10 μL aliquot of the cell suspension to count the cells using a hemocytometer.
-
a.
-
20.
As a next step, seed the placental MSCs (PL-MSCs) at a density of 6000 cells/cm2 in 2 mL of warm fresh MSC growth medium in a 35 mm tissue culture dish.
Note: From passage 1 onwards, do not add any anti-fungal component (Antibiotic-Antimycotic or amphotericin B) to the cultures.
-
21.
Culture them for 72 h till cells are 70–80% confluent.
Note: No medium change is required in between.
-
22.
Detach the cells with TrypLE, count them, and seed them for the next passage, repeating Steps 17–22.
Note: Warm TrypLE, MSC growth medium and DPBS to 37°C prior to starting the detachment of cells.
Freezing and reviving of PL-MSCs
Timing: Variable
-
23.
Once the PL-MSCs are 70–80% confluent, detach them with 250 μL TrypLE.
-
24.Add 1 mL of warm fresh MSC growth medium to the cells.
-
a.Transfer them to a 15 mL centrifuge tube.
-
b.Take an aliquot for counting
-
c.Centrifuge the remaining cells at 500 × g for 2 min at 24°C–26°C.
-
a.
Note: Till passage 4, freeze the excess cells obtained during cell culture.
-
25.
Aspirate the supernatant and suspend the cells directly in freezing medium at ∼2 × 106 cells/mL.
-
26.
Put the cell suspension into cryogenic vials and place them in a Mr. Frosty freezing container containing isopropanol.
-
27.
Keep the Mr. Frosty freezing container in −80°C freezer for ∼24 h before transferring the cryogenic vials to a liquid nitrogen tank for long-term storage.
-
28.
For cell revival, take out a cryogenic vial with frozen PL-MSCs from the liquid nitrogen tank and immediately transfer it to a beaker containing water at 37°C for gentle thawing.
-
29.
Once the cell suspension has thawed, add 1 mL of warm fresh MSC growth medium to the cryogenic vial, mix, and transfer the contents to a 15 mL centrifuge tube containing ∼ 3 mL of additional warm fresh MSC growth medium.
-
30.
Centrifuge the cells at 500 × g for 2 min at 24°C–26°C to wash off the freezing medium.
-
31.Aspirate the supernatant and resuspend the cell pellet in 500 μL of warm, fresh MSC growth medium.
-
a.Add 2 mL of warm, fresh MSC growth medium to a 35 mm tissue culture dish
-
b.Add the cells and gently swirl to distribute them evenly.
-
a.
Note: During thawing and revival of MSCs, plate the cells at a higher density to maximize recovery.
-
32.
Transfer the dish to a CO2 incubator and replace the medium with 2 mL of warm, fresh MSC growth medium the next day.
-
33.
After 48 h of reviving and plating, detach the cells with TrypLE, count and seed an appropriate number of cells for the next passage.
Characterization of PL-MSCs
Immunophenotyping by flow cytometry
Timing: 2 h
-
34.
At passages 4–5, detach the PL-MSCs with TrypLE and calculate the cell number as per Step 19.
-
35.
Wash the harvested cells 1–2 times with ice-cold PBS by centrifuging at 500 × g for 2 min.
-
36.
Discard the supernatant and suspend the cells in PBS at 2 × 106 cells/ml.
-
37.
Aliquot 1 × 105 cells or 50 μL of cell suspension in each of the pre-labeled 5 mL FACS tubes.
-
38.
Add the respective antibodies to the tubes and incubate in ice for 1 h. Mouse isotype antibodies serve as controls.
-
39.
Acquire at least 10,000 events on BD LSRFortessa Cell Analyzer flow cytometer and analyze the results using BD FACSDiva software.
-
40.
Representative data from a PL-MSC culture at passage 4 is presented in Figures 2A–2F.
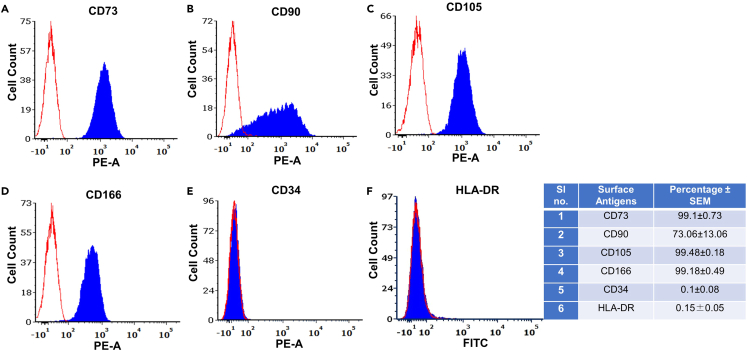
Figure 2.
Immunophenotypic characterization of isolated PL-MSCs
The antibodies used were (A) human CD73, (B) human CD90 (C) human CD105 (D) human CD166 (E) human CD34 (F) human HLA-DR and their respective isotype controls. Open histogram indicates background signal, while, shaded histogram represents positive reactivity with the indicated antibodies. Table shows percentage of cells expressing the respective surface markers (n = 3) in mean ± SEM.
In vitro differentiation
Timing: ∼25 days
-
41.Use PL-MSCs at passages 4–5 to perform the in vitro differentiation studies.
-
a.Seed the isolated PL-MSCs at a density of 6000 cells/cm2 on a 35 mm tissue culture dish in 2 mL of warm, fresh MSC growth medium.
-
b.Incubate the dishes at 37°C and 5% CO2 until they reach 85–90% confluency.
-
a.
-
42.
Next, remove the growth medium, and initiate in vitro differentiation by adding 2 mL of pre-warmed differentiation medium.
-
43.
Continue adipogenic differentiation for a total of ∼20 days (or till matured oil drops become visible within the cells)4 and osteogenic differentiation for 21–22 days, with media changes given every third day.
Note: Plate the uninduced control cultures at an identical density and maintain it in MSC growth medium for a similar duration.
-
44.After completion of differentiation, stain the adipogenic differentiated dish with Oil Red O and osteogenic differentiated dish with Alizarin Red S, respectively, and observe under bright field microscope.Note: Stain the corresponding uninduced control cultures as well.
Staining –
Oil Red O Staining –-
a.Aspirate the medium from the adipogenic differentiation dish and give a PBS wash at 24°C–26°C.
-
b.Fix the cells with 4% PFA in PBS and incubate for 20 min at 24°C–26°C.
-
c.After this, give a PBS wash, and next, add 60% isopropanol to the dish and incubate for 3 min at 24°C–26°C.
-
d.Remove the isopropanol and add 1–2 mL of Oil Red O stain solution. Incubate the dish in dark for 60 min at 24°C–26°C.
-
e.Following the incubation, give three washes with double distilled water to remove excess stain.
-
f.Add a small amount of double distilled water to the dish to prevent the cells from drying and then visualize under bright field microscope.
-
g.Upon Oil Red O staining, the oil droplets formed in the adipogenic differentiated culture appear red in color, whereas the uninduced control culture remains unstained.
-
h.Representative images of the same are presented in Figure 3A.Alizarin Red S Staining –
-
i.Aspirate the medium from the osteogenic differentiation dish, and give a PBS wash at 24°C–26°C.
-
j.Fix the cells with 4% PFA in PBS and incubate for 20 min at 24°C–26°C.
-
k.Give two washes with double distilled water, and add 2 mL of 1% Alizarin Red S solution. Incubate the dishes in the dark for 1 h at 24°C–26°C.
-
l.After this, again give two washes with double distilled water to remove excess stain.
-
m.Add a small amount of double distilled water to the dish to prevent the cells from drying and then visualize under bright field microscope.
-
n.Upon Alizarin Red S staining, the calcium phosphate deposits in the osteogenic differentiated culture appear red in color, whereas the uninduced control culture remains unstained.
-
o.Representative images of the same are presented in Figure 3B.
-
a.
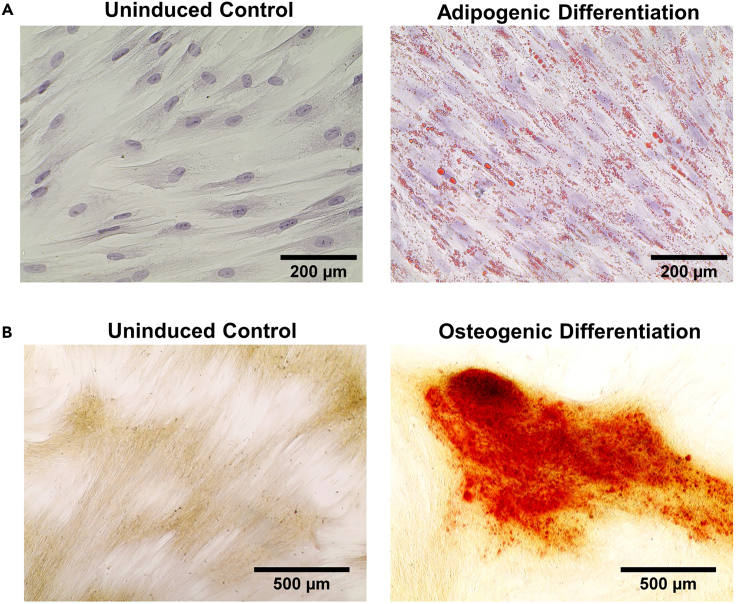
Figure 3.
Differentiation potential of PL-MSCs
(A) Oil Red O staining to demonstrate adipogenic differentiation (40× magnification), Scale bar: 200 μm.
(B) Alizarin Red S staining to demonstrate osteogenic differentiation (10× magnification) (n = 3). The corresponding uninduced control cultures did not show any staining (40× magnification), Scale bar: 500 μm.
CFU-F assay (colony forming unit-fibroblast assay)
Timing: ∼ 10 days
-
45.
Perform the CFU-F assay with PL-MSCs at passage 2–3. For this, seed the PL-MSCs at a density of 10 cells/cm2 in a 35 mm tissue culture dish.
-
46.
An outgrowth of around 50 cells can be referred to as a colony. After 9–10 days, the plated cells are seen to give rise to colonies when visualized under an Eclipse TS100 inverted phase-contrast microscope.
-
47.
Then, aspirate the medium out from the dish and give it a wash with DPBS.
-
48.
After this, fix the cells with 1% PFA in PBS for 20 min.
-
49.
Remove the PFA, give a wash with PBS, and add 0.1% Toluidine blue stain (in 1% PFA) to the dish for staining.
-
50.
After 1 h, remove the stain from the dish and give two consecutive washes with double distilled water.
-
51.
Manually count the stained colonies and report the data as a total number of colonies per 100 cells plated.
-
52.
Representative image of the CFU-F assay has been presented in Figure 4A.
Figure 4.
CFU-F assay, growth kinetics, immunofluorescence and qRT-PCR analysis of isolated PL-MSCs
(A) CFU-F assay was performed in triplicates from PL-MSCs (n = 3).
(B and C) Growth kinetics of PL-MSCs represented in terms of (B) population doublings and (C) population doubling time obtained for P1 to P6. Passage numbers are denoted as P1, P2, P3, P4, P5, and P6 (n = 4). Each bar represents mean ± SEM.
(D–F) Representative images of PL-MSCs immunostained with (D) vimentin (E) Stro-1 and (F) Oct-4. DAPI (blue) was used to stain the nuclei (n = 3), Scale bar: 50 μm.
(G) Average Ct ± SEM values of VIM and POU5F1 (n = 3).
Growth kinetics
Timing: 15 min
-
53.
As described in step 19, during passaging of cells count the cell numbers using a hemocytometer.
-
54.
Calculate the population doublings and population doubling time for passage 1 to passage 6 using the formula below:
| Population doublings = {log 10 (Nh ) – log 10 (Ni )}/{log 10 2} |
| Population doubling time = {Time in culture (in hrs)}/{Population doublings} |
where, Ni = inoculum cell number and Nh is the harvest cell number.
-
55.
The data obtained has been presented as population doublings and population doubling time across increasing passages in Figures 4B and 4C.
Immunofluorescence
Timing: 2 days
-
56.Perform the immunofluorescence studies with PL-MSCs at passage 4–6.
-
a.Plate PL-MSCs at a density of 6000 cells/cm2 on glass cover-slips
-
b.Incubate at 37°C under standard culture conditions for 72 h.
-
a.
-
57.
After 72 h, aspirate the medium and transfer the cover-slips to clean 35 mm tissue culture dishes for further processing.
-
58.
Give two consecutive washes with PBS at 24°C–26°C.
-
59.
Add ∼1.5 mL of 4% PFA in PBS to each dish with the cover-slips for fixation.
-
60.
Fix the cells on the cover-slips for 20 min at 24°C–26°C.
-
61.
Again, give two consecutive washes with PBS at room temperature to remove all traces of PFA.
Pause point: After fixation, the cells can be stored at 4°C for up to one week.
-
62.
Perform permeabilization with 0.1% Triton X 100 in PBS. Add it to the cover-slips and keep at 24°C–26°C for 10 min.
-
63.
To remove the Triton X 100, give one wash with PBS.
-
64.
Add ∼1.5 mL (or sufficient volume to cover) of 3% BSA in PBS to each dish with the cover-slips for blocking, and keep the dishes for 60 min at 24°C–26°C.
-
65.
Following this, add the primary antibodies, vimentin, Stro-1, and Oct-4 at 1:150, 1:100, and 1:400 dilutions (or an optimized dilution as per manufacturer’s instruction) in 3% BSA, respectively.
Note: For each antibody, plate one cover-slip of cells for staining. Keep a cover-slip, which would serve as a negative control. In the negative control, add the secondary antibody to the fixed cells, but eliminate the addition of primary antibody. This can detect any false positives expressed due to non-specific binding of secondary antibody.
Note: Here, we have optimized a new protocol for the isolation of MSCs from the placenta, which has not been previously studied by our group. Hence, to test if the isolated PL-MSCs exhibit any pluripotency, we checked for the expression of Oct-4, a known pluripotency marker.
-
66.
Keep the cover-slips at 4°C for 12–16 h. To ensure that the antibody solution does not dry up, place in a humidified chamber or box.
-
67.
Next day, remove the primary antibody and give three consecutive washes with PBS (each wash of 5 min) at 24°C–26°C.
-
68.
Add the respective secondary antibodies to the cover-slips, in 1:500 dilution (or an optimized dilution as per manufacturer’s instruction) in 1.5% BSA in PBS. Incubate in dark for 1 h.
-
69.
To remove the secondary antibody, again, give three consecutive washes with PBS (each wash of 5 min) at 24°C–26°C.
-
70.
Add DAPI (1:5000 dilution in PBS or an optimized dilution as per manufacturer’s instruction) to each dish containing the cover-slips, and incubate them for 10 min in the dark.
-
71.
Again, give three consecutive washes with PBS (each wash of 5 min) at 24°C–26°C to remove DAPI.
-
72.
Mount the cover-slips using VECTASHIELD as anti-fade mounting medium. Capture the cell images at 24°C–26°C at 40× magnification using fluorescence microscope.
-
73.
Representative images of PL-MSCs stained with vimentin, Stro-1 and Oct-4 are presented in Figures 4D–4F.
Gene expression analysis
RNA isolation and cDNA synthesis
Timing: 2.5 h
-
74.
Seed the isolated PL-MSCs at a density of 6000 cells/cm2 on a 35 mm tissue culture dish in 2 mL of warm, fresh MSC growth medium at passage 4–6. Incubate the dishes at 37°C and 5% CO2 for 72 h.
-
75.Aspirate the medium from the dish.
-
a.Give a wash with PBS at 24°C–26°C
-
b.Add 500 μL of RNAiso Plus to the dish to isolate total RNA as per manufacturer’s protocol (see https://www.takarabio.com/assets/a/112212).
-
a.
-
76.
Determine the concentration of RNA isolated using a NanoDrop spectrophotometer.
Pause point: The isolated RNA can be stored at −80°C before cDNA synthesis.
-
77.
Prepare cDNA from the RNA using Verso cDNA synthesis kit, as per the manufacturer’s protocol (see https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0012822_Verso_cDNA_Synthesis_AB1453A_UG.pdf).
Pause point: The cDNA can be stored at −20 or −80°C before qRT-PCR.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Timing: 2 h
-
78.
Use PowerUp SYBR Green Master Mix to detect and quantify the mRNA expression level for vimentin (VIM) .
PowerUp SYBR Green PCR Reaction Mix
| Reagent | Amount |
|---|---|
| PowerUp SYBR Green Master Mix | 5 μL |
| UltraPure DNase/RNase-Free Distilled Water | 3.5 μL |
| Forward primer | 0.5 μL |
| Reverse primer | 0.5 μL |
| cDNA | 0.5 μL |
| Total | 10 μL |
PCR Cycling Conditions
| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Activation 1 | 50°C | 2 min | 1 |
| Activation 2 | 95°C | 2 min | 1 |
| Denaturation | 95°C | 15 s | 40 |
| Annealing | 60°C | 15 s | |
| Extension | 72°C | 1 min | |
| Hold | 4°C | Forever | N/A |
Primer sequences
| Gene | Forward primer (5'->3′) | Reverse primer (5'->3′) |
|---|---|---|
| VIM | GACAATGCGTCTCTGGCACGTCTTGACCTTGAACGC | GCATCTGGCGTTCCAGGGACTCATTGGTTC |
-
79.
Use TaqMan Gene Expression Assay (FAM) and TaqMan Fast Universal Master Mix to detect and quantify the mRNA expression level for Oct-4 (POU5F1).
TaqMan PCR Reaction Mix
| Reagent | Amount |
|---|---|
| TaqMan Fast Universal Master Mix | 5 μL |
| UltraPure DNase/RNase-Free Distilled Water | 4 μL |
| TaqMan Gene Expression Assay (FAM) | 0.5 μL |
| cDNA | 0.5 μL |
| Total | 10 μL |
PCR Cycling Conditions
| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Activation | 95°C | 10 min | 1 |
| Denaturation | 95°C | 15 s | 40 |
| Annealing/Extension | 60°C | 1 min | |
| Hold | 4°C | Forever | N/A |
-
80.
The Ct values (mean ± SEM) obtained for VIM and POU5F1 are presented in Figure 4G.
Expected outcomes
This protocol outlines a simple method to isolate MSCs from the decidua basalis layer of the human placenta by the explant culture method. Though this is an elaborate process, and it takes around 14 days for the MSCs to emerge from the tissue explants, it successfully evades the need for harsh enzymatic digestions and centrifugation steps, thus leading to increased viability and better yield of cells.5,6,7
The primary yield per gram of placental tissue was found to be ∼105 cells. On evaluating the growth kinetics, the mean population doubling times for PL-MSCs, obtained from four independent biological samples, were found to be 37.82 ± 7.40 h, 38.24 ± 1.16 h, 39.94 ± 4.57 h and 42.07 ± 2.33 h at passages 2, 3, 4 and 5 respectively. The isolated PL-MSCs were characterized by flow cytometry for the presence of surface antigens. The PL-MSCs were seen to be strongly positive for the expression of CD73 and CD105 and negative for the expression of CD34 and HLA-DR. Interestingly, PL-MSCs were found to be exhibiting lesser percentage of CD90 positive cells, an MSC representative marker as per the guidelines of the International Society for Cell and Gene Therapy (ISCT).8 However, our flow cytometry data depicted that PL-MSCs were strongly positive for CD166, another MSC-specific marker, which successfully differentiated MSCs from fibroblast cells.9
MSCs have multipotent differentiation potential. Hence, they can differentiate into osteocytes, adipocytes and chondrocytes when proper in-vitro stimuli are provided. We attempted and successfully differentiated the isolated PL-MSCs towards adipogenic and osteogenic lineages.
The CFU-F assay demonstrated the clonal expansion ability of the PL-MSCs as they could give rise to individual colonies.
The isolated PL-MSCs were noted to be positive for the expression of the mesenchymal markers vimentin and Stro-1; while they did not express the pluripotency marker Oct-4, as demonstrated by immunofluorescence staining. These results were also validated at the molecular level by qRT-PCR. The Ct values obtained for VIM and POU5F1 were 18.84 ± 0.64 and 30.37 ± 0.93, respectively. This indicated that the PL-MSCs exhibited a strong expression for VIM and a weak expression for POU5F1.
The PL-MSCs could be successfully passaged till ∼ passage 15. Moreover, we noted that the excess cells obtained underwent cryopreservation and revival. Even though this protocol involved the usage of FBS for the isolation and culture of PL-MSCs, it minimized the use of other xenogeneic components by omitting the use of trypsin and enzymes for tissue digestion.
Quantification and statistical analysis
All experiments have been performed with at least three independent biological samples. All data has been documented as mean ± standard error of the mean (SEM). Graphical representations were plotted using GraphPad Prism 8 software. Statistical comparisons were assessed using one-way ANOVA followed by Bonferroni’s multiple comparison test. Significance was accepted at p ≤ 0.05.
Limitations
The explant culture method of isolation is a time-consuming process, and the cells take longer to emerge from the tissue explants as compared to conventional enzymatic digestion methods. For the characterization of PL-MSCs, the expression of only two negative markers was tested. Anti-fungal component has been used during the PL-MSC isolation steps, till passage 0.
Troubleshooting
Problem 1
Yeast infection is usually a challenge with birth-associated tissues. Hence, the placenta may harbor yeast contamination and the placental explants may get contaminated during the initial days of culture.
Potential solution
Use an additional amount of anti-fungal components like amphotericin B and modulate/optimize its concentration at different steps according to the necessity to evade contamination issues. Once the MSC cultures have been established from these placental explants, the anti-fungal treatment should be stopped.
Problem 2
Excessive 70% ethanol treatment during the washes can dehydrate the tissue and prevent cells from coming out of the explant pieces (step 9d).
Potential solution
Ensure that the tissue pieces are dipped in ethanol for not more than 15 s. This step has been optimized with respect to a similar protocol previously standardized by our group (Goyal et al., 20183).
Problem 3
No MSCs emerging from the tissue explants (step 15).
Potential solution
Handle the tissue culture dishes containing the tissue explants with care during the initial period after plating so that the explants do not get dislodged. The explants need to stick to the tissue culture dish surface for the MSCs to migrate out.
The explant pieces should be 3–5 mm in size, as too small explant size might dislodge easily and start to float.
The air-drying step for 5–7 min after adding the tissue explants helps the pieces to adhere better to the tissue culture dish surface.
Problem 4
Sometimes, the PL-MSCs might demonstrate an enlarged and flattened morphology as compared to their conventional spindle-shaped appearance due to cellular senescence. (step 16–22).
Potential solution
Passage the cells routinely when they are 70–80% confluent and plate at not less than 6000 cells/cm2 seeding density. Also, give a medium change to the culture every 72 h. Plan experiments using PL-MSCs within passages 4–7.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Malancha Ta, malancha.ta@iiserkol.ac.in.
Materials availability
This study did not generate any unique reagent.
Acknowledgments
The work was funded by IISER, Kolkata. We thank UGC, India, for the fellowship of Ms Srishti Dutta Gupta. We are grateful to Dr Jayanta Chatterjee, Astha, Kalyani for providing placenta samples. We thank Mr Ritabrata Ghosh and Mr Tamal Ghosh for technical assistance with microscopy and flow cytometry.
Author contributions
M.T. conceptualized the study and supervised the project. M.T., S.D.G., and A.S. developed the methodology. S.D.G., P.P., and A.S. performed the experiments and analyzed the data. S.D.G. wrote the manuscript with comments from all authors.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2023.102498.
Data and code availability
The data is available from the corresponding author on request.
References
- 1.Pelekanos R.A., Sardesai V.S., Futrega K., Lott W.B., Kuhn M., Doran M.R. Isolation and Expansion of Mesenchymal Stem/Stromal Cells Derived from Human Placenta Tissue. J. Vis. Exp. 2016;112 doi: 10.3791/54204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang Y.C., Yang Z.M., Chen X.H., Tan M.Y., Wang J., Li X.Q., Xie H.Q., Deng L. Isolation of Mesenchymal Stem Cells from Human Placental Decidua Basalis and Resistance to Hypoxia and Serum Deprivation. Stem Cell Rev. Rep. 2009;5:247–255. doi: 10.1007/s12015-009-9069-x. [DOI] [PubMed] [Google Scholar]
- 3.Goyal U., Jaiswal C., Ta M. Isolation and Establishment of Mesenchymal Stem Cells from Wharton's Jelly of Human Umbilical Cord. Bio. Protoc. 2018;8 doi: 10.21769/BioProtoc.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y.H.K., Ogando C.R., Wang See C., Chang T.Y., Barabino G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018;9:131. doi: 10.1186/s13287-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon J.H., Roh E.Y., Shin S., Jung N.H., Song E.Y., Chang J.Y., Kim B.J., Jeon H.W. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton's jelly. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/428726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendijani F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017;50 doi: 10.1111/cpr.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman L.S., Condé-Green A., Naaldijk Y., Lee E.S., Rameshwar P. An Enzyme-free Method for Isolation and Expansion of Human Adipose-derived Mesenchymal Stem Cells. J. Vis. Exp. 2019;154:e59419. doi: 10.3791/59419. [DOI] [PubMed] [Google Scholar]
- 8.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D.J., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9.Brinkhof B., Zhang B., Cui Z., Ye H., Wang H. ALCAM (CD166) as a gene expression marker for human mesenchymal stromal cell characterization. Gene. 2020;763S doi: 10.1016/j.gene.2020.100031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is available from the corresponding author on request.