Summary
Memory generalization is the ability to abstract knowledge from prior experiences and is critical for flexible behavior in novel situations. Here, we describe a protocol for simultaneous recording of hippocampal (area CA1)-prefrontal cortical neural ensembles in Long-Evans rats during task generalization across two distinct environments. We describe steps for building and assembling experimental apparatuses, animal preparation and surgery, and performing experiments. We then detail procedures for histology, data processing, and assessing population geometry using Uniform Manifold Approximation and Projection.
For complete details on the use and execution of this protocol, please refer to Tang et al. (2023).1
Subject areas: Neuroscience, Cognitive Neuroscience, Behavior
Graphical abstract

Highlights
-
•
Build behavioral apparatus to perform linear and W-Track experiments
-
•
Fabrication of 32-tetrode and 64-tetrode microdrive arrays
-
•
Microdrive implant surgery, electrode adjustment, and experimental design
-
•
Assessment of CA1-PFC latent space representations using UMAP
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Memory generalization is the ability to abstract knowledge from prior experiences and is critical for flexible behavior in novel situations. Here, we describe a protocol for simultaneous recording of hippocampal (area CA1)-prefrontal cortical neural ensembles in Long-Evans rats during task generalization across two distinct environments. We describe steps for building and assembling experimental apparatuses, animal preparation and surgery, and performing experiments. We then detail procedures for histology, data processing, and assessing population geometry using Uniform Manifold Approximation and Projection.
Before you begin
In this protocol, we lay out methods for multi region in vivo tetrode recordings to investigate geometric representations in the hippocampal-prefrontal circuit during exposure to learning of a spatial working memory task in novel and familiar environments.1 Furthermore, the behavioral protocol for single-day W-Track learning is described2 as training step prior to novel environment exposure. Successful execution of this protocol requires access to a fully functional experimental rig room with electrophysiological and behavior recording capabilities as well as an approved surgical suite. In this protocol, we provide detailed step-by-step instructions on how to train animals, build microdrive arrays, perform surgeries, conduct the experiments mentioned above, and perform the analysis required for visualization of CA1 and PFC population manifolds across different environments.
Institutional permissions
All procedures were approved by the Institutional Animal Care and Use Committee at the Brandeis University and conformed to US National Institutes of Health guidelines.
Preparation of experimental rig rooms
Timing: 2–3 weeks
-
1.Setup recording systems in two separate rooms.Note: Main components for setting up a SpikeGadgets recording system are listed below.
-
a.Environmental control unit (ECU).
-
b.Main control unit (MCU);
-
c.128 or 256 channel headstages;
-
d.General purpose commutator.
-
e.Active HDMI cables (MCU to commutator).
-
f.HDMI cables (MCU to ECU);
-
g.HDMI to micro-HDMI cables (commutator to headstage).
-
h.Overhead cameras.
-
i.Ethernet cables.
-
j.PC running Linux.Note: Details for setup can be found on the SpikeGadgets website (https://spikegadgets.com/documentation/).
 CRITICAL: Two experimental rooms are required to perform the novel-familiar experiment for distinct environmental contexts.
CRITICAL: Two experimental rooms are required to perform the novel-familiar experiment for distinct environmental contexts.
-
a.
-
2.
Build all required behavioral components as instructed in the section “construct W-mazes, linear track, sleep box, and reward wells.”
-
3.
Prepare behavioral scripts to run linear and W-Track behaviors (example code can be found on our lab GitHub: http://github.com/JadhavLab/GeometricTransformationProtocol).
Preparation of animals for pretraining
Timing: 1–2 weeks
-
4.
Obtain animals (rats aged 3–4 months upon receipt) and habituate to the new vivarium for 2–3 days before beginning handling.
Note: Habituation to the new vivarium can be extended (∼1 week) to ensure minimal stress before handling.
-
5.Handle and habituate animals to human interaction daily for 1–2 weeks using the following protocol as a guide.
-
a.Day 1.
-
i.Allow the animal to freely move around the cage in the handling environment for a few minutes.
-
ii.Periodically interact with the animal by petting its back.
-
iii.If the animal is not exhibiting any anxious or fearful behavior such as freezing, gently lift the rat by the base of the tail and transfer to a separate, confined table.
-
iv.Let the animal explore and return to the cage after a few minutes.
-
i.
-
b.Day 2.
-
i.Repeat the steps from day 1 and constantly monitor for anxious behavior.
-
ii.Pick up the animal by the base of the tail and cradle it between your opposite forearm and chest.
-
iii.Stay close to the animals’ home cage or handling table in case they get ‘jumpy’ or want to escape.
-
i.
-
c.Day 3.
-
i.Repeat the steps from day 2 until the animal is comfortable being held for minutes at a time.
-
ii.While in your arms, begin gently grabbing the animal from under the armpits and monitor for anxious behavior.
-
i.
-
d.Subsequent days.
-
i.Assess comfort level by gently grabbing the animal from the home cage using the ‘underarm’ method.
-
ii.If the animal exhibits anxious behavior, return to the cage and repeat steps from day 2.
-
iii.Animals should be sufficiently handled in 1–2 weeks.
-
i.
-
a.
Note: Animals should be comfortable with being picked up directly from the home cage using the ‘underarm’ method and held for minutes at a time without showing any signs of stress or discomfort.
-
6.
Once animals reach a protocol approved weight, section “animal pre-training” can begin.
Preparation for implant surgery
Timing: 1–2 h
-
7.
Autoclave all necessary tools for surgery.
-
8.Prepare injectables.
 CRITICAL: Be sure to refer to the lab’s approved protocol to ensure that proper amounts and concentrations of each drug are used. Do not utilize any of the following substances unless previously approved.
CRITICAL: Be sure to refer to the lab’s approved protocol to ensure that proper amounts and concentrations of each drug are used. Do not utilize any of the following substances unless previously approved.-
a.Ketamine, xylazine, atropine (KXA) solution.
-
b.Dexamethasone.
-
c.Ringer’s solution.
-
d.Meloxicam.
-
e.Lidocaine.
-
f.Buprenorphine-ER.
-
a.
-
9.
Prepare all necessary tools/materials for surgery (Figure 3A).
-
10.
Fill vaporizer with isoflurane.
-
11.
Check weight of isoflurane scavengers and replace if full.
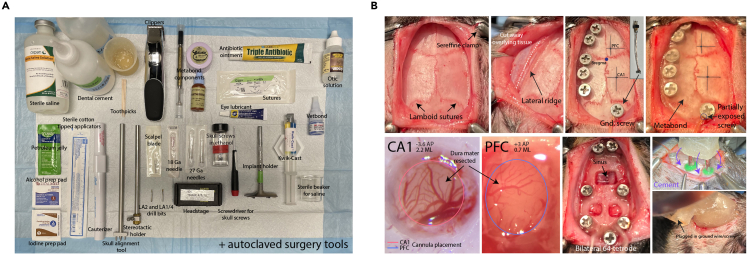
Figure 3.
Surgery preparation and microdrive implantation
(A) Surgical materials to prepare for implantation of microdrive array.
(B) Surgical procedures for microdrive implantation.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Data underlying the results | DANDI | https://doi.org/10.48324/dandi.000447/0.230316.2133 |
| Code underlying the results | GitHub | https://github.com/JadhavLab/GeometricTransformation |
| Experimental models: Organisms/strains | ||
| Wild-type male Long-Evans rats (Rattus norvegicus, 3–4 months old) | Charles River Laboratories | Cat#Crl:LE 006; RRID: RGD_2308852 |
| Software and algorithms | ||
| Matclust v4 | Mattias Karlsson | https://www.mathworks.com/matlabcentral/fileexchange/39663-matclust |
| Software for analysis and processing | MATLAB | https://www.mathworks.com |
| Other | ||
| 1/8″ aluminum sheets for track components | Grainger | Cat#786CV3 |
| 1/4″ black delrin sheets | McMaster-Carr | Cat#8578K512 |
| Corner brace | Grainger | Cat#4PE75 |
| Fixed eye hasp | Grainger | Cat#4PE39 |
| Hinges | Home Depot | Cat#030699135929 |
| Wingnuts | Home Depot | Cat#204775371 |
| Machine screws for wingnuts | Home Depot | Cat#204274614 |
| Black spray paint | Home Depot | Cat#307244849 |
| Black kraft paper | ULINE | Cat#S-13633 |
| Reward pumps | New Era Pump Systems Inc | Cat#NE-500 |
| Polyurethane tubing | Clippard Instrument Lab Inc | Cat#URH1-0804-CLT-500 |
| 60 mL syringes | Fisher Scientific | Cat#14955461 |
| Reward wells | http://github.com/JadhavLab/GeometricTransformationProtocol | N/A |
| Reward well PCB | http://github.com/JadhavLab/GeometricTransformationProtocol | N/A |
| Liquid electrical tape | Amazon | Cat#B000FPAN2K |
| Evaporated milk | Amazon | Cat#B003G2JMVW |
| IR emitter and receiver | Amazon | Cat#EK8443 |
| Surface mount green LEDs | Mouser Electronics | Cat#604-APT3216LZGCK |
| Surface mount red LEDs | Mouser Electronics | Cat#604-APT3216LSECKJ3RV |
| Surface mount resistors (220 Ω, 10 kΩ, 0 Ω) | Amazon | Cat#B00BXXJBSA |
| RJ45 Ethernet connector | Mouser Electronics | Cat#538-44620-0002 |
| Mill-Max connector (male) | DigiKey | Cat#ED83100-ND |
| Mill-Max connector (female) | DigiKey | Cat#ED90267-ND |
| Flux | Amazon | Cat#B004RI6G5E |
| Solder | Grainger | Cat#1UYH4 |
| Electroplater | White Matter | NanoZ |
| Impedance tester | Bak Electronics | Cat#IMP-2A |
| Gold solution | Sifco | Cat#80535500 |
| Parafilm | Fisher Scientific | Cat#50-998-943 |
| Tetrode assembly station | Neuralynx | Tetrode assembly station |
| Current generator | World Precision Instruments | Cat#A365RC |
| UV sterilization box | Grainger | Cat#18AX46 |
| 30-gauge stainless steel tubing | Component Supply Co. | Cat#HTX-30R-60 |
| 23-gauge stainless steel tubing | Component Supply Co. | Cat#HTX-23R-60 |
| 14-gauge stainless steel tubing | Component Supply Co. | Cat#HTX-14T-30 |
| 0.0055″ medical mandrel wire | Component Supply Co. | Cat#GWX-0055-72 |
| 18-gauge needles | Fisher Scientific | Cat#148265G |
| 23-gauge needles | Fisher Scientific | Cat#14826A |
| 27-gauge needles | Fisher Scientific | Cat#1482648 |
| Polyimide inners | MicroLumen Inc | Cat#039-I |
| 0-80 threaded rods | Amazon | Cat#B000FMWBNC |
| 0-80 tap | Amazon | Cat#B007BTRKZQ |
| Custom brass nut piece | http://github.com/JadhavLab/GeometricTransformationProtocol | N/A |
| Custom brass nut piece mold | http://github.com/JadhavLab/GeometricTransformationProtocol | N/A |
| Custom M1.2×0.25 screws | http://github.com/JadhavLab/GeometricTransformationProtocol | N/A |
| M1.2×0.25 tap | Amazon | Cat#B07WGRWTQ8 |
| Scotch tape | Amazon | Cat#B0000DH8HQ |
| Pin vise | Amazon | Cat#B00J9NX8N0 |
| Vise | Amazon | Cat#B0002BC1AY |
| Student anatomical standard pattern forceps | Fine Science Tools | Cat#91100-12 |
| Benchtop microscope | TEquipment | Cat#EMZ-13TR |
| Microscope stand | TEquipment | Cat#KBL |
| Microscope eyepieces | TEquipment | Cat#MA504 |
| 3D printer | Formlabs | Form 3+ |
| Dremel | Amazon | Cat#B00COSTSTA |
| Diamond cutting wheel (thin) | Stoelting Co. | Cat#58605 |
| Diamond cutting wheel (thick) | Home Depot | Cat#100346850 |
| Diamond cutting wheel (thick - alternative) | Dremel | Cat#409 |
| Sanding wheel | Amazon | Cat#B00004UDH3 |
| Soldering iron | Amazon | Cat#B00NRZ6I1A |
| Insulated copper wire | Cooner Wire | Cat#CZ1187 |
| Tetrode cutting scissors | Fine Science Tools | Cat#14568-09 |
| Super glue | Amazon | Cat#B00VG22QAI/ B01FIL87AK |
| 32-tetrode drive body | http://github.com/JadhavLab/GeometricTransformationProtocol | N/A |
| 32-tetrode drive cone and cap | http://github.com/JadhavLab/GeometricTransformationProtocol | N/A |
| 64-tetrode drive body | http://github.com/JadhavLab/GeometricTransformationProtocol | N/A |
| Ceramic tipped forceps | Fisher Scientific | Cat#501927539 |
| Ketamine | Patterson Veterinary | Cat#07-803-6637 |
| Xylazine | Patterson Veterinary | Cat#07-893-8424 |
| Atropine | Patterson Veterinary | Cat#07-869-6061 |
| Bacteriostatic saline | Mountainside Medical Equipment | Cat#63323-0924-10 |
| Meloxicam | Patterson Veterinary | Cat#07-845-6986 |
| Dexamethasone | Patterson Veterinary | Cat#07-808-8194 |
| Lidocaine | Patterson Veterinary | Cat#07-889-0316 |
| Buprenorphine ER | ZooPharm | Cat#1Z-73000 |
| Beuthanasia-D | Patterson Veterinary | Cat#:07-807-3963 |
| Kwik-Cast | World Precision Instruments | Kwik-Cast |
| Vetbond | Patterson Veterinary | Cat#07-805-5031 |
| Petroleum jelly | Amazon | Cat#B00CPPR9UC |
| Cort/astrin solution (otic) | Patterson Veterinary | Cat#07-869-0640 |
| Eye ointment | Patterson Veterinary | Cat#07-888-2572 |
| Sutures | eSutures | Cat#A1667N |
| Antibiotic ointment | Amazon | Cat#B08L34KLXX |
| Alcohol prep pads | Fisher Scientific | Cat#23501711 |
| Surgical heating pad | Physitemp Instruments | Cat#HP-1M |
| SpO2 monitor | Starr Life Sciences | MouseOx Plus |
| Clippers | Amazon | Cat#B00MNMDO3S |
| Timer | Amazon | Cat#B09GY5ZS78 |
| Dental drill | KaVo | Cat#1.007.5540 |
| Dental unit | KaVo | Cat#1.012.8541 |
| LA2 burs | Net32 | Cat#05-61148 |
| LA1/4 burs | Net32 | Cat#05-60148 |
| Freer elevator | Roboz | Cat#RS-8820 |
| Surgical scissors | Fine Science Tools | Cat#14568-09 |
| Fine-tipped forceps | Fine Science Tools | Cat#11251-35 |
| Bulldog serrefines | Fine Science Tools | Cat#18050-35 |
| Scalpel handle | Fine Science Tools | Cat#10003-12 |
| Scalpel blades | Fine Science Tools | Cat#10011-00 |
| Cauterizer | Fine Science Tools | Cat#18010-00 |
| Rat skull alignment tool | Kopf Instruments | Model 944 |
| Stereotaxic holder | Kopf Instruments | Model 1770 |
| Ear bars | Kopf Instruments | Model 955 |
| Stereotax | Kopf Instruments | Model 942 |
| Nose cone | Kopf Instruments | Model 906 |
| Ringers solution | Patterson Veterinary | Cat#07-869-6319 |
| Anesthesia system | Colonial Medical Supply | Cat#901822 |
| Isoflurane gas scavenger | Colonial Medical Supply | Cat#931401 |
| Oxygen concentrator | Supera Anesthesia Innovations | Cat#M6100 |
| Induction box (7L) | Colonial Medical Supply | Cat#941448 |
| Isoflurane | Patterson Veterinary | Cat#07-893-1389 |
| Kimwipes | Fisher Scientific | Cat#06666A |
| Cotton-tipped applicators | Fisher Scientific | Cat#22363172 |
| Metabond | Net32 | Cat#S380 |
| Dental acrylic powder | Amazon | Cat#B008OLBEC0 |
| Dental acrylic liquid | Amazon | Cat#B01I3ZIV2M |
| Skull screws | Amazon | Cat#B07CBFX8SH |
| Rongeur | Fine Science Tools | Cat#16000-14 |
| Metrizyme | VWR | Cat#89132-869 |
| 37% Formaldehye | Fisher Scientific | Cat#RSOF0010500 |
| Sterile saline | Patterson Veterinary | Cat#07-869-6640 |
| Microtome | Leica | Cat#SM2010 R |
| Fluorescence microscope | Keyence | BZ-X series |
| Sucrose | Sigma-Aldrich | Cat#S8501-5KG |
| OCT | Fisher Scientific | Cat#50363579 |
| 10× PBS | Fisher Scientific | Cat#70011044 |
| 48-well plates | Fisher Scientific | Cat#087721C |
| Parafilm | Fisher Scientific | Cat#1337410 |
| 50% ethanol | Fisher Scientific | Cat#LC222204 |
| 70% ethanol | Fisher Scientific | Cat#LC222102 |
| 95% ethanol | Fisher Scientific | Cat#MAX04413 |
| Absolute ethanol | Fisher Scientific | Cat#BP2818500 |
| Glacial acetic acid | Fisher Scientific | Cat#18-602-917 |
| Xylene | Fisher Scientific | Cat#AC422680040 |
| Cresyl violet | Fisher Scientific | Cat#AC229630050 |
| Sodium acetate trihydrate | Fisher Scientific | Cat#S220-1 |
| Gelatin-coated slides | Fisher Scientific | Cat#OBSLD01CS |
| Permount | Fisher Scientific | Cat#SP15-100 |
| DPX | Fisher Scientific | Cat#50-980-370 |
| Coverslips | Fisher Scientific | Cat#12-541-055 |
| NiCr wire | Amazon | Cat#B0BN74Q925 |
| Gold pins | Neuralynx | Small EIB pins |
| Overhead color camera | Edmund Optics | Cat#88-468 |
| Varifocal lens | 123Security | Cat#12VM412ASIR |
| Studio monitor | Amazon | Cat#B000Z75616 |
| Active HDMI cable | Amazon | Cat#B0186H3TH4 |
| SpikeGadgets main control unit | SpikeGadgets | MCU |
| SpikeGadgets environmental control unit | SpikeGadgets | ECU |
| SpikeGadgets commutator | SpikeGadgets | General purpose commutator |
| SpikeGadgets 128-channel headstage | SpikeGadgets | HH128 |
| SpikeGadgets 256-channel headstage | SpikeGadgets | HH256 |
| SpikeGadgets 128-channel electrode interface boards | SpikeGadgets | EIB128 |
| SpikeGadgets 256-channel electrode interface boards | SpikeGadgets | EIB256 |
| SpikeGadgets NanoZ adapter | SpikeGadgets | NanoZ adapter |
Step-by-step method details
Construct W-mazes, linear track, sleep box, and reward wells
Timing: 1–2 weeks
These steps describe how to fabricate necessary behavioral apparatuses for the experiments outlined in this protocol. There are multiple ways to approach this step – apparatuses may be fabricated in-house or can be outsourced to external companies. Design files can be found on our lab GitHub (http://github.com/JadhavLab/GeometricTransformationProtocol).
-
1.Sleep box fabrication.
-
a.Using the appropriate number of sheets of 0.65 cm thick black Delrin, cut four pieces with the dimensions 30 × 40 cm (sides) and one piece with the dimensions 34 × 34 cm (bottom) (Figure 1A).
-
b.Cut 0.65 cm width shallow grooves around the perimeter of the bottom piece, approximately 1 cm inward from the edges. These grooves will hold the side pieces in place, with exception of the front panel, which will be movable.
-
c.Align the three side panels with the grooves in the bottom panel and attach panels using 3 screws per side panel from the bottom.
-
i.To further stabilize side panels, attach corner braces at four points to the side and back panels.
-
i.
-
d.Attach the front panel using 5 cm hinges to one of the side panels.
-
e.Attach the hinged metal strap to the opposite side panel and the looped staple to the front panel.
-
f.Smooth out any sharp edges to prevent injury.
-
a.
Note: Be sure to use correct length screws that do not protrude in any location.
-
2.Linear and W-Track fabrication.
-
a.For each track component, cut 0.3 cm thick aluminum sheets with the track dimensions illustrated in Figure 1B.Note: You will need materials for two W-Tracks, one each for the novel and familiar components of the experiment.
-
b.Drill two appropriately sized and spaced holes at the end of each track arm to secure reward wells.
-
c.In a well-ventilated area, spray paint the track components matte black to reduce light reflection, which will help with downstream position tracking.Note: Due to the heavy machinery required to fabricate these maze parts from 0.3 cm thick aluminum, using an external company or campus machine shop that specializes in machining may be required. Xometry (www.xometry.com) has been previously used to manufacture custom maze parts for adaptable mazes that are currently used in the laboratory.3Note: Instructions for the fabrication of track bases are not covered in this protocol, as appropriate track height may differ between laboratory setups based on the height of various recording equipment.
-
a.
-
3.Reward well fabrication and assembly.
-
a.3D print 6 reward wells using the Form 3 printer or an external company (e.g., Stratasys, Shapeways, etc.). The reward well pictured was printed using the Form 3 (Material, Grey Pro).
-
b.Get printed circuit boards (PCBs) fabricated.
-
c.Solder surface mount resistors (Figure 1C, R1-220 Ω, R2-10 kΩ, R3-0 Ω (jumper)), an IR emitter and receiver, and an RJ45 Ethernet port onto designated regions of the PCB. Note that the IR emitter/receiver and RJ45 plug are soldered on the opposite side of the resistors (Figure 1C).Note: Make sure the legs of the emitter/receiver extend far enough out from the PCB to allow the lens of the LEDs to fit into the sides of the reward well when bent inward.
-
d.Cover all soldered components with liquid electric tape.
 CRITICAL: Not thoroughly covering electrical components at this step may lead to downstream noise issues, such as amplified lick artifacts.
CRITICAL: Not thoroughly covering electrical components at this step may lead to downstream noise issues, such as amplified lick artifacts. -
e.Insert the PCB into the back of the reward well, slide on the 3D printed back cover, and secure the cover by inserting screws into the side holes.
-
a.
-
4.
Once all components have been constructed, the mazes can be set up for automated reward delivery via pumps, tubing, and an environmental control unit (ECU, SpikeGadgets).
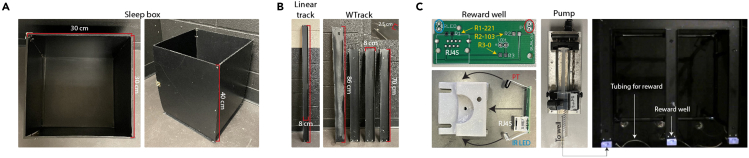
Figure 1.
Fabrication of behavioral apparatuses
(A) Dimensions of the sleep box.
(B) Dimensions of the linear and W-Track.
(C) Instructions for assembly of 3D printed reward well. Reward wells are connected to 60 mL syringes via polyurethane tubing, and precise reward amounts are delivered through programmed pumps. Connections between components can be made with appropriate Luer lock fittings.
Drive fabrication
Timing: 5–10 days
These steps describe the protocols for fabrication of 32 and 64-tetrode microdrive arrays for use in chronic in vivo electrophysiology experiments. Although the instructions here will focus on targeting the prefrontal cortex and the dorsal CA1 layer of the hippocampus in the rat, these methods can be extended to record from many other regions in the brain. The drive weight should ideally be <10% of body weight of animals; therefore, larger rats with weights >=400 g at the time of implant are usually required.
-
5.Fabrication of custom drive components.
-
a.Drive bodies can be 3D printed using Form 3 or can be outsourced. The drive body pictured in Figure 2A was printed using PolyJet technology through Stratasys (Material, VeroBlue).
-
b.Custom metal components (brass nut pieces, molds, and M1.2 screws) were ordered through eMachineShop.
-
a.
Note: The 32 tetrode microdrive body only has 30 shuttle lanes. To maximize the number of tetrodes, some shuttles can be double loaded.
Note: The length of the custom M1.2 screws for the 64-tetrode drive will determine how far electrodes can penetrate the brain. If targeting deeper structures, it may be necessary to lengthen the custom screws.
-
6.Preparation of materials and drive body.
-
a.Using a 23 Ga needle, ream out excess 3D printing material from the drive body.Note: Blockage of these holes will result in a failure to mobilize shuttles.
-
i.For the 32-tetrode drive, ream out the inner and outermost holes.
-
ii.For the 64-tetrode drive, ream out the inner hole.Note: When reaming the inner hole, stop a few millimeters before penetrating the drive through the bottom. This will simplify the future step of loading 30 Ga tubes into the drive body.
-
i.
-
b.Tap each larger hole with the appropriately sized tap (0–80 or M1.2 tap for 32 or 64 tetrode drive, respectively).
 CRITICAL: While tapping holes, make sure not to apply excess lateral pressure. This will cause the tap to break off in the drive body, effectively rendering that shuttle lane inoperable.
CRITICAL: While tapping holes, make sure not to apply excess lateral pressure. This will cause the tap to break off in the drive body, effectively rendering that shuttle lane inoperable.
-
a.
-
7.Preparation of microdrive components.
-
a.With a diamond cutting wheel, cut appropriate number of 30 Ga steel tubing and 0.0055″ medical mandrel wire (5.5 m wire from now on) measured out to 6 and 8 cm, respectively.
-
i.Sharpen 5.5 m wires by briefly grinding the ends on the face of the diamond cutting wheel at around 45 degrees. Multiple wires can be sharpened simultaneously.
-
ii.Briefly grind down the ends of the 30 Ga tubes to clear off any debris or sharp points from cutting.
-
i.
-
b.Ream a 5.5 m wire through each 30 Ga tube multiple times until the wire passes through smoothly.Note: If it is difficult to pass a 5.5 m wire through, an end of the 30 Ga tube may be welded shut. Remember to only briefly grind down the ends.
-
c.Insert the reamed 30 Ga tubes into the inner holes of the drive body.
-
d.Cut 2 lengths of 14 Ga tubing (cannulae) measuring ∼1.5–1.75 cm and scuff the sides of one end.Note: Scuffing the ends will help dental cement adhere to the tubes.
-
e.Make a targeting block.
-
i.Cut a piece of 2 cm thick Delrin into a 4 cm × 4 cm square.
-
ii.Mark out the coordinates dorsal CA1 (−3.6 AP, 2.2 ML) and PFC (3 AP, 0.7–1.0 ML).
-
iii.Drill 1 cm deep holes with an appropriately sized drill bit at the marked sites.Note: Depending on the number of tetrodes per region, the size of the guide cannula will have to change (i.e., 14 Ga to 15 Ga for fewer tetrodes).
-
i.
-
f.Insert the 14 Ga cannulae into the targeting block, scuffed side up.
-
g.Carefully start feeding in the appropriate number of 30 Ga tubes into each 14 Ga cannula and make sure each 30 Ga tube contacts the bottom of the targeting block.
-
h.After inserting all tubes, recheck that all 30 Ga tubes are at their bottom most position in the block.
 CRITICAL: If tubes are not at the bottom, some may be recessed after dental cementing, which may cause tetrode instability due to insufficient contact with the brain.
CRITICAL: If tubes are not at the bottom, some may be recessed after dental cementing, which may cause tetrode instability due to insufficient contact with the brain. -
i.Gently push the drive body downward to reduce the height of the drive but take care not to kink any of the 30 Ga tubes.Note: Test whether the 5.5 m wires within the 30 Ga tubes are still mobile to determine if the bend is too extreme.
-
j.Apply a thin layer of mineral oil on the surface of the Delrin block with a cotton-tipped applicator.
-
k.Dental cement the drive body, 30 Ga tubes, and 14 Ga cannulae together.
-
l.Let fully harden then remove from block.Note: All 30 Ga tubes should be flush with the bottom of the 14 Ga cannulae (or slightly protruding).
-
m.Extend all 5.5 m wires out ∼2 cm out from the bottom.
-
n.With the sharp diamond cutting wheel, cut each 30 Ga tube at the top of the drive body such that ∼2 mm protrudes.
-
o.Using pliers or serrated forceps (Fine Science Tools, Cat#91100-12), push each 5.5 m wire from the bottom through the freshly cut 30 Ga tubes at the top.Note: There will be some resistance so take care not to bend any 5.5 m wires.
-
p.With all the 5.5 m wires now protruding out the top of the drive, cut the two 14 Ga cannulae to a desired length (typically 0.5–0.75 cm) with a thick diamond wheel.Note: Using the thin diamond wheel can cause warping of the wheel.
-
q.Using pliers, push each 5.5 m wire from the top through the freshly cut 30 Ga tubes at the bottom, and ensure the 5.5 m wire passes smoothly through each 30 Ga tube.
-
r.Remove 5.5 m wires and sand the excess dental cement around the cannulae into a rectangular shape.Note: The dental cemented portion should clamp securely in a Panavise.
-
s.Take an image of the bottom of the cannulae and map out the location of each 30 Ga tube from the top of the drive using a 5.5 m wire.
-
t.Attach a 128 or 256 channel electrode interface board (EIB) to the drive body using screws.
-
u.Solder a ground wire to the ground pin hole on the EIB and route the wire to the back of the drive body.
-
v.Solder a female 4 pin Mill-Max connector to the wire.
-
w.Dental cement the ground wire to the drive body at multiple points, taking care not to get cement into the holes.
-
a.
-
8.Assembling turn tools (Figure 2B).
-
a.32 tetrode drive turn tool for threaded rods (Tool A) – Using a pin vice, a 7.5 cm piece of 14 Ga stainless steel tubing, and a 5 cm piece of 0–80 threaded rod, assemble a turn tool.
-
i.At one end of the threaded rod, cut a 0.5 cm long half-moon, similar to the top of the custom M1.2 screw.
-
ii.Secure the 14 Ga tube in the pin vice and dental cement to lock in place.
-
iii.Insert the threaded rod into the 14 Ga tube until the half-moon end is flush with the end of the 14 Ga tube.
-
iv.Using pliers, clamp the 14 Ga tube to the inserted threaded rod.
-
i.
-
b.32 tetrode drive turn tool for nut pieces (Tool B) – Using a pin vice, and a 7.5 cm piece of 14 Ga stainless steel tubing, assemble a turn tool.
-
i.Secure the 14 Ga tube in the pin vice and dental cement to lock in place.
-
ii.Using a Dremel, shave down opposite sides of the 14 Ga tip to securely mate with the grooves in the custom brass nut piece.
-
i.
-
c.64 tetrode drive turn tool – Using a pin vice, a 7.5 cm piece of 16 Ga stainless steel tubing, and a custom M1.2 screw, assemble a turn tool.
-
i.Secure the 16 Ga tube in the pin vice with dental cement, insert the M1.2 screw into the top, and clamp (similar to Tool A above).Note: The 32-tetrode drive requires two tools, one to screw the threaded rods into the drive body (Tool A), and the other to move shuttles up and down the threaded rod (Tool B).
-
i.
-
a.
-
9.Assembling shuttles for the 32-tetrode drive (Figure 2B).
-
a.Insert 23 Ga tubes into the base of the shuttle mold.
-
b.Thread custom nut pieces onto cut pieces of 0–80 threaded rod and screw into the mold until the bottom of the nut piece comes in contact with the mold.
-
c.Lightly coat the surface of the mold, nut pieces, and 23 Ga tubes with mineral oil.
-
d.Wrap a piece of Scotch tape around the perimeter of the mold.
-
e.Pour dental cement into the mold, making sure not to fill the notches of the brass nut pieces.Note: The cement should sit right below the notches of the nut pieces.
-
f.As the dental cement begins to harden, turn the nut pieces counterclockwise ¼ of a turn and then clockwise ¼ of a turn. This will allow the nut piece to move freely within the dental cement.Note: Do not turn counterclockwise too far or the nut piece will wiggle in the dental cement after hardening.
-
g.Repeat step f above until the dental cement has fully hardened.
-
h.Remove the tape, 23 Ga tubes, and the threaded rods.
-
i.Snap off the dental cement from the mold and shave down the shuttles with a Dremel.Note: The shuttles should be shaved down as thin as possible without compromising the strength of the dental cement. Thick shuttles will clash when loaded into the microdrive.
-
j.Cut 40 pieces of 0–80 threaded rod (2.5 cm long).
-
i.Cut a half-moon shape on one end and bevel the other end with a Dremel.Note: The beveling makes threading into the nut piece easier.
-
k.Cut 40 pieces of 23 Ga tubing (2.5 cm long). These will be supporting pieces.
-
l.Cut 40 pieces of 23 Ga tubing (1.5 cm long). These will mate with the 30 Ga tubes in the drive body and house the tetrodes.
-
m.Cut 40 pieces of polyimide tubing (2 cm long).
-
n.Assemble the 32 tetrode drive shuttles.
-
i.Thread the 0–80 rods into the nut pieces.
-
ii.Insert the support 23 Ga tubes.
-
iii.Insert the shorter 23 Ga tubes halfway into the shuttle.
-
iv.Lightly coat the top half of the short 23 Ga tubes with super glue and advance into the shuttle such that ∼2 mm sticks out from the top.
-
v.Apply a small amount of super glue to the 23 Ga and dental cement to secure.
 CRITICAL: Do not get super glue into the 23 Ga tubes or in between the dental cement and the nut pieces.
CRITICAL: Do not get super glue into the 23 Ga tubes or in between the dental cement and the nut pieces. -
vi.Insert the polyimide tubes and secure to the top of the 23 Ga tubes with super glue. 1–2 mm of polyimide should be protruding from the top.Note: Once the polyimide tubes are in, super glue can enter the 23 Ga tubes.Note: If double loading the shuttles to maximize recording channel count for the 32-tetrode drive (design provided only has 30 lanes), do not load polyimide tubes in two shuttles. These will be double loaded.
-
vii.Cut the excess polyimide from the bottom so 1–2 mm protrudes.
 CRITICAL: Do not crimp the polyimide at any stage or it will have to be replaced.
CRITICAL: Do not crimp the polyimide at any stage or it will have to be replaced. CRITICAL: Do not use too much super glue or it will flow to the bottom of the tubes, which will impede loading of the shuttles into the drive body.
CRITICAL: Do not use too much super glue or it will flow to the bottom of the tubes, which will impede loading of the shuttles into the drive body.
-
i.
-
o.Assemble the 64 tetrode drive shuttles (Figure 2B).
-
i.3D print the components for the 64 tetrode drive shuttle (80 each).Note: These 3D printed pieces are prone to breakage, so printing an ample supply is advantageous.
-
ii.Cut 70 pieces of 23 Ga tubing (1.5 cm long).
-
iii.Cut 70 pieces of polyimide tubing (2 cm long).
-
iv.Mate the U-shaped piece with the groove on the M1.2 screws and slide in the second piece.
-
v.Apply liquid super glue to the seams on both sides where the two pieces meet. It should flow between the two pieces.
-
vi.Make a batch of Metabond in a chilled ceramic mixing container and keep on ice to increase working time.
-
vii.Apply a thin layer of Metabond around the entire shuttle. This significantly strengthens the shuttles and decreases attrition.
-
viii.Insert the polyimide tubes and secure to the top of the 23 Ga tubes with super glue. 1–2 mm of polyimide should be protruding from the top.
-
ix.Cut the excess polyimide from the bottom so 1–2 mm protrudes.
-
i.
-
a.
-
10.Loading shuttles.
-
a.Under a microscope, carefully load each shuttle into the drive body.
-
i.Align the polyimide tube in the shuttle with the 30 Ga tube in the drive body and insert it until the 23 Ga tube mates with the 30 Ga tube.
-
ii.With forceps or pliers, grab the bottom of the 0–80 threaded rod and advance to the surface of the drive body.
-
iii.Screw in the threaded rod with Tool A until secure.
-
iv.Guide the support 23 Ga tube into the support hole.
-
v.Using Tool B, advance the shuttle to the bottom position (∼4 mm from the drive body).
-
i.
-
a.
Note: Steps are the same for the 64 tetrode drive shuttles except that there are no support pieces.
Note: The shuttles in the 64-tetrode drive are moved by direct movement of the M1.2 screw in the drive body.
-
11.Tetrode fabrication.
-
a.Measure out 70 cm of NiCr wire (Alleima, 0.0127 mm, polyimide coated).Note: If longer tetrodes are required, 80–90 cm of wire can be used. Note that the number of turns must be adjusted accordingly. Consult the Neuralynx tetrode assembly station user manual for detailed instructions (https://neuralynx.com/documents/TAS_Manual.pdf).
-
b.Loop the wire and drape over the bar of the tetrode assembly station (Neuralynx).
-
c.Clamp the 4 wires at the bottom with an alligator clip and position the clip in the spinning unit.Note: Make sure the alligator clip is wrapped with heat shrink tubing. In addition to the heat shrink, wrapping the clips with Parafilm can improve gripping of tetrode wires prior to spinning.Note: Do not let the clip contact the magnet in the spinning unit. This can cause the electrodes to snap.
-
d.Spin the tetrode 90× clockwise and 30× counterclockwise.
-
e.Using a heat gun, anneal the tetrode wire evenly from three sides approximately 2 cm away. It should take ∼5 s from top to bottom.Note: To avoid misshapen tetrodes and electrode bridges, do not heat excessively. For polyimide coated wire, the annealing temperature should be ∼500°C.
-
f.Lift the bar, releasing the alligator clip from the spinning unit and allow it to spin until it stops.Note: Skipping this step can result in tangled leads at the top due to build up tension.
-
g.Anneal the newly spun portion from three sides using the heat gun.
-
h.Lift the alligator clip to relieve tension and cut ∼1 cm above the clip.
-
i.Remove the tetrode and cut the two loops formed at the top.
-
j.Store in a dust free container until use.
-
a.
-
12.Loading tetrodes.
-
a.Grab a tetrode with clean fingers or ceramic tipped forceps and, under a stereoscope, carefully load it into polyimide tube (MicroLumen, Cat#039-I, inner diameter: 0.0039″) until slight resistance is met.
-
b.Using ceramic tipped forceps (Fisher Scientific, Cat#501927539, tip width: 0.4 mm), gently grab the tetrode near the point of entry and advance the tetrode into the drive gently until 1–2 cm of the annealed portion of the tetrode is left at the top.
-
c.Thread each electrode lead into the pin holes in the EIB and adjust the slack such that the tetrode bends away from the shuttle.
-
d.Pin the electrodes to the EIB with gold pins.
-
e.Note the electrode group on the EIB the tetrode is pinned to.Note: Using the electrode group ID along with the microdrive mapping information will allow for matching of neural signals to tetrode spatial location, which is useful if precise targeting is required.
-
f.After all shuttles have been loaded, cut the tetrodes protruding from the bottom on the cannulae to an even length, keeping them much longer than what is needed to reach the brain areas of interest.
-
g.Test connectivity of each electrode using a saline bath the NanoZ (detailed instructions in Gold Plating section).
-
h.Replace any tetrodes with bad connectivity.
-
i.After connectivity is confirmed, super glue each tetrode to the polyimide tube at the top of the shuttle.Note: Gel super glue works best for this step. If liquid super glue is used, it is critical to only apply a small amount to prevent glue from invading the polyimide tube.
 Pause point: Loaded drives can be stored at this point for later use.
Pause point: Loaded drives can be stored at this point for later use.
-
a.
-
13.Gold plating and final steps (Figure 2D).
-
a.Place the drive in a UV box (Grainger, Cat#18AX46, output: 52.5 W UVC radiation) and sterilize for 15 min.
-
b.Under a stereoscope, cut the tetrodes to their final length using sharp surgical scissors.
 CRITICAL: Do not UV sterilize after the final cut. We’ve experienced inconsistent impedance values if sterilization takes place after the final cuts.
CRITICAL: Do not UV sterilize after the final cut. We’ve experienced inconsistent impedance values if sterilization takes place after the final cuts. -
c.Connect the drive to the NanoZ via an adapter (custom or SpikeGadgets).
-
d.Fill one plating well with gold solution and connect to the NanoZ via a wire.
-
e.Fill the other well with distilled water.
-
f.Rinse tetrodes in distilled water for 1–2 min.
-
g.Move tetrodes to the gold solution.
 CRITICAL: Only the tetrode tips should be in the gold solution. Do not allow solution to enter the metal cannulae. This will cause crystallization in the tubes and impede tetrode movement.
CRITICAL: Only the tetrode tips should be in the gold solution. Do not allow solution to enter the metal cannulae. This will cause crystallization in the tubes and impede tetrode movement. -
h.Test connectivity and impedance.
-
i.Setting – Test Impedances.Note: Baseline impedance for un-plated electrodes should be around 5–6 MOhms. Much higher impedances may indicate faulty channels and/or connections.
-
i.
-
i.Burn the electrode tips.
-
i.Setting – DC electroplate.
-
ii.Mode set to fixed plating time.
-
iii.Plating current set to +1 μA.
-
iv.Interval set to 2 s.
-
v.Autoplate.Note: Impedances should drop down to around 2–3 MOhms
-
i.
-
j.Gold plate the electrodes.
-
i.Set interval to 2 s and number of runs to 5.
-
ii.Round 1 – Current set to −0.5 μA; target set to 500 kOhms.
-
iii.Round 2 – Current set to −0.3 μA; target set to 400 kOhms.
-
iv.Round 3 – Current set to −0.1 μA; target set to 300 kOhms.
-
v.Round 4 – Current set to −0.05 μA; target set to 250 kOhms.
-
vi.After each round, rinse in distilled water for 5 min.
 CRITICAL: Over plating will cause bridges (shorts) between channels. The above settings are provided as a general guide. Adjust current to lower values for each round if necessary.Note: Gold plating can take place over the course of a few days.Note: Electrode impedances should be ∼250 kOhms at the end of gold plating.
CRITICAL: Over plating will cause bridges (shorts) between channels. The above settings are provided as a general guide. Adjust current to lower values for each round if necessary.Note: Gold plating can take place over the course of a few days.Note: Electrode impedances should be ∼250 kOhms at the end of gold plating.
-
i.
-
k.Under a stereoscope, retract all tetrodes slightly past the end of the 30 Ga tubes at the bottom of the drive.Note: Record the number of turns required to retract each tetrode to approximate the maximum travel distance.
-
l.Secure the 3D printed cone to the drive with dental cement.
-
a.
-
14.Ground screw fabrication (Figure 2E).
-
a.Cut 2–3 pieces of insulated copper wire (∼5 cm long).
-
b.Strip 0.5 cm of insulation off both ends.
-
c.Twist the wires together and solder one end to a 4 pin male Mill-Max connector.
-
d.Wrap the opposite stripped end around a skull screw at the base of the head.
-
e.Apply a small amount of flux and solder.
 CRITICAL: Do not apply too much solder. If excess solder invades the threads of the ground screw, it will not properly secure within the skull.
CRITICAL: Do not apply too much solder. If excess solder invades the threads of the ground screw, it will not properly secure within the skull. -
f.Test connectivity between the base of the screw and the pins of the Mill-Max connector with a multimeter.
-
a.
- 15.
Note: Two LEDs are used for tracking to extract head direction during behavior.
Figure 2.
Microdrive fabrication
(A) Consumables required for microdrive fabrication.
(B) Instructions for assembly of shuttles and turn tools.
(C) Steps for microdrive assembly.
(D) Gold plating with the NanoZ electroplater.
(E) Fabrication of the ground screw.
(F) Circuit diagram for a SpikeGadgets compatible LED array.
Animal pre-training
Timing: 2–5 days
These pre-training steps are performed to habituate the animal to running on elevated mazes for reward.
Note: All behavioral experiments were performed between ZT0 (zeitgeber time) and ZT12.
-
16.
Once an animal has been sufficiently handled and has reached a protocol approved weight (e.g., 300–400 g), food restriction can begin.
Note: The growth chart of the animal should be taken into consideration before beginning food deprivation. For example, if a male rat weighs ∼350 g at 11 week (typical) and food deprivation begins, the weight of the animal should not cross 85% (or approved food restriction limit) of the projected weight at the corresponding age. Food deprivation should be monitored carefully to adhere to this. Check your supplier’s animal documentation for more information, and be sure to check your lab’s protocol for instructions on proper food restriction/deprivation.
-
17.
Start habituating the animal to the reward that will be used during experiments. In our case, evaporated milk was used.
Note: Evaporated milk reward can also be mixed with sugar (20 g sugar per 350 mL of milk) or blended with Reese’s Pieces (20 g per 350 mL). If blending with Reese’s Pieces, use a strainer to get rid of chunks.
-
18.
Habituate animal to the sleep box for ∼20 min per day for 3 days.
-
19.When the animal has been sufficiently food restricted (e.g., reaches 85% of baseline weight), begin linear track pretraining.
-
a.Run 2–3 15–20-min linear track sessions daily until good performance has been reached.
-
b.Animals should be able to reach a reward receipt rate of at least 4 per minute for 2 consecutive days before proceeding to drive implantation.
-
c.If acceptable performance is not reached within a week, do not use animal for downstream experiments.
-
i.Alternatively, it may be helpful to change the reward type to get the animal to an acceptable performance level.Note: Due to the rigorous behavioral protocol of the single day experiment, if animals did not reach criterion (∼60–80 rewards in 15–20 min for two consecutive days), they did not proceed to subsequent steps.Note: It’s advantageous to run a few animals in parallel, in case of issues with behavioral performance, etc.
-
i.
-
d.After training, take the animal off food restriction and let the animal reach an appropriate weight before proceeding to drive implantation.
-
a.
Implant surgery
Timing: 4–6 h
This section provides detailed, step-by-step instructions for drive implantation.
-
20.Preparing the animal for implantation (Figure 3B).
-
a.Place a 7 L induction box on a heating pad to keep the floor of the box warm.
-
b.Place the animal in the induction box and flow in 5% isoflurane at an airflow rate of 2 L/min (Anesthesia system, Colonial Medical Supply, Cat#901822).
 CRITICAL: Limit the animals’ exposure to 5% isoflurane to <5 min. Prolonged exposure to high isoflurane concentrations will harm the animal.
CRITICAL: Limit the animals’ exposure to 5% isoflurane to <5 min. Prolonged exposure to high isoflurane concentrations will harm the animal. -
c.Once the breath rate of the animal has reached 60 breaths/min, set the isoflurane to 0%, purge the chamber with oxygen, and quickly remove the animal from the chamber.
-
d.Inject a solution of ketamine (50 mg/kg), xylazine (6 mg/kg), and atropine (0.1 mg/kg) intraperitonially, and return to the induction box for at least 5 min at 1% isoflurane.
-
e.Once the animal is sufficiently anesthetized, purge the chamber, remove the animal, and shave the animal’s head using clippers (WAHL, Cat#09861-900).Note: Keep the animal on a heating pad while shaving. Shaving should take ∼30 s.
-
f.Switch the airflow from the induction box to the stereotaxic nose cone and set the isoflurane to an initial concentration of 0.25%–0.5%.
 CRITICAL: At the beginning of surgery, it is important to find the proper anesthetic depth by adjusting the isoflurane concentration. Too much isoflurane in combination with initial KXA induction may lead to complications, such as very low breath rate or loss of the animal.
CRITICAL: At the beginning of surgery, it is important to find the proper anesthetic depth by adjusting the isoflurane concentration. Too much isoflurane in combination with initial KXA induction may lead to complications, such as very low breath rate or loss of the animal. -
g.Place the animal in the stereotax (Kopf, Model 942) on top of the heating pad.
-
h.Connect the foot clip of the MouseOx (Starr Life Sciences, MouseOx Plus) to the animal’s hind paw, and make sure accurate vital readings are obtained. Please refer to the MouseOx user manual for proper positioning of the sensor.
-
i.Using petroleum jelly, coat the rectal temperature probe, insert, and tape to the base of the tail. Check that there is no obstruction prior to insertion.
-
j.Coat a sterile cotton-tipped applicator with eye lubrication and apply generously to eyes.Note: During this step, check for a blink reflex. Wait for the reflex to diminish before proceeding to the next step.
 CRITICAL: It is imperative to administer ringers’ solution every hour (at least ∼5 mL/hr) to prevent dehydration during long surgeries.
CRITICAL: It is imperative to administer ringers’ solution every hour (at least ∼5 mL/hr) to prevent dehydration during long surgeries. -
k.Stabilize the animal’s head using non-rupturing ear bars (Kopf, Model 955) and align with the center of the nose cone.
-
l.Inject dexamethasone (1–2 mg/kg) and meloxicam (2 mg/kg) subcutaneously at a site in the back.
-
m.Clean the scalp with an alcohol prep pad followed by an iodine pad. Start at the center and move outwards in circular motion on the scalp with q tips.
-
n.Inject the incision site with 0.2 mL of undiluted 2% lidocaine HCl at multiple points along the midline.
-
o.Using a scalpel, make a 2.5–3 cm incision in the scalp, starting from the midpoint between the eyes.
-
p.Using bulldog sereffine clamps (Fine Science Tools, Cat#18050-35), clip the subcutaneous tissue at four points to expose the skull.
-
q.Scrape away underlying periosteum completely using a freer elevator (Roboz, Cat#RS-8820) while wiping away blood with cotton-tipped applicators. Make sure to detach the tissue at the lateral ends of the lamboid sutures on the skull.Note: Completely detaching this connection reduces the chances that tissue will regrow under the implant.
-
r.Stop any excessive bleeding from the skull and/or tissue with the cauterizer.Note: Minor bleeding can be stopped by positioning the cautery slightly above (not touching) the tissue or skull. The heat given off the tip often stops bleeding without causing excessive damage.
-
s.Using the freer elevator, push the tissue overlying the lateral ridge upward and cut along the ridge from posterior to anterior on both sides with surgical scissors (Figure 3B, white dotted line).Note: Initial displacement of the overlying tissue may take moderate pressure. Removing this tissue will prevent sharp edges from forming during later steps where dental cement is applied to the skull.
-
t.Make shallow cuts in the skull in a crosshatch pattern with a scalpel to allow robust adhesion of the implant.
-
u.After sufficiently cleaning the skull, level the head with the rat skull alignment tool.
-
v.Slide an 18 Ga needle into the stereotactic holder to target bregma.
-
w.Find bregma and mark the locations of the craniotomies with crosshairs.
-
x.Using an LA2 drill bit, drill holes for stabilizing screws on the contralateral side of the craniotomies.Note: Skull screw placement for bilateral implants will need to be more carefully planned out to ensure the screws do not occlude the craniotomy sites.
-
y.Drill a hole above the cerebellum on the ipsilateral side of the craniotomies for the ground screw.
 CRITICAL: Avoid drilling at sites above the sinus and take care to not break through the skull aggressively for any of the skull screws, as this may cause brain damage and excessive bleeding.Note: If desired, an additional ground screw can be used as a backup.
CRITICAL: Avoid drilling at sites above the sinus and take care to not break through the skull aggressively for any of the skull screws, as this may cause brain damage and excessive bleeding.Note: If desired, an additional ground screw can be used as a backup. -
z.Using a small Phillips screwdriver, insert the skull screws about halfway.Note: The pitch (distance between threads) of the 0–80 skull screws used here is ∼0.3 mm. To avoid pressing down on the brain, the skull screws should be adjusted ∼0.75 mm into the skull (2–3 turns).
-
i.For the ground screw, make sure the wire is facing toward the back of the animal.
-
i.
-
aa.Make a batch of Metabond and lay down a base layer over the skull screws.
 CRITICAL: It is imperative that the skull is completely dry before applying Metabond.
CRITICAL: It is imperative that the skull is completely dry before applying Metabond.-
i.The Metabond should not cross over to the sites of the craniotomies or be applied to the tissue overlying the ridge.Note: Applying Metabond often causes minor bleeding from surrounding tissue. Wick away any blood during this process to ensure no moisture gets under the base layer of cement.
-
ii.Leave the ground screw partially exposed to check ground connection after drive implantation.
 CRITICAL: Let the Metabond set in place undisturbed. If cement is accidentally applied to tissue, let dry and drill the excess away. Disrupting the setting process of the cement may cause drive instability.
CRITICAL: Let the Metabond set in place undisturbed. If cement is accidentally applied to tissue, let dry and drill the excess away. Disrupting the setting process of the cement may cause drive instability.
-
i.
-
bb.After Metabond has set sufficiently, drill the craniotomies.
-
i.Start by outlining the perimeter of the craniotomies with an LA2 drill bit.
 CRITICAL: The craniotomies should be significantly larger than the diameter of the drive cannula to ensure residual bone and dura at the edges do not occlude the tetrodes from entering the brain.
CRITICAL: The craniotomies should be significantly larger than the diameter of the drive cannula to ensure residual bone and dura at the edges do not occlude the tetrodes from entering the brain. -
ii.Drill evenly around the perimeter until ¾ of the way through the skull.
-
iii.Switch to an LA1/4 drill bit and continue drilling around the outer edge of the craniotomy until the skull piece is loose enough to pluck off with a pair of forceps.Note: After switching to the LA1/4 drill bit, keep the craniotomy well irrigated with cold, sterile saline. Repeated wicking away of moisture may cause excess drying if there is premature breakthrough/rupture of the underlying dura. This may cause dura to adhere to the brain, making resection difficult.
 CRITICAL: Take care not to tear the underlying dura or damage the brain while drilling. This will cause bleeding and subsequent clotting, which could impede visibility and the removal of dura.
CRITICAL: Take care not to tear the underlying dura or damage the brain while drilling. This will cause bleeding and subsequent clotting, which could impede visibility and the removal of dura. -
iv.Cover the craniotomy site with a small, saline moistened piece of cotton from the tip of a cotton-tipped applicator.
-
v.Repeat the above steps for all implant sites.
-
i.
-
cc.Resect the dura mater.
-
i.Bend the 1–2 mm of the tip of a sterile 27 Ga needle into a 45- or 90-degree angle and gently make an incision in the dura at a location close to the edge of the craniotomy. Avoid large vessels.Note: Further bending the angled needle tip into a tiny hook can help with gripping and incising the dura.Note: Alternatively, a von Graefe knife can be used to make the initial incision.
-
ii.Insert the tip of the needle under the dura flap, gently lift, and start cutting toward the opposite side of the initial incision.Note: A pair of sharp forceps can also be used to cut dura.
-
iii.Repeat this cutting procedure orthogonal to the first incision and push dura toward the edges of the craniotomy.
-
iv.Excess dura can be resected using sharp forceps.
-
v.Ensure that the area of the exposed brain will be able to accommodate the size of your implant cannula.
-
vi.After dura has been resected from all craniotomies, gently rinse the skull with saline to get rid of any debris.
-
vii.Dry the exposed skull completely but keep the surface of the brain hydrated with sterile saline until implant.
-
i.
-
a.
-
21.Drive implantation (Figure 3B).
-
a.Mount the drive onto a stereotaxic holder with an EIB connector.
-
b.Position the drive such that the targeting cannulae are above the implant sites.
-
c.Position the microscope so the cannulae can be visualized contacting the brain surface.
-
d.Using a rolled piece of a Kimwipe, wick away excess moisture in the craniotomies.
-
e.Slowly lower the drive until contact with the surface of the brain and advance until the brain slightly dimples at all implant locations.
-
f.Fill the craniotomies with a small amount of Kwik-Cast and let set.
 CRITICAL: Do not allow any Kwik-Cast flow under the implant cannulae.
CRITICAL: Do not allow any Kwik-Cast flow under the implant cannulae. -
g.Mix a batch of Metabond, and when it is moderately thick, apply it to the exposed skull. This ensures a strong bond to the skull on the ipsilateral side of the implant.
-
h.Using regular dental cement, bond the drive to the base Metabond layer. This will most likely take a few batches of cement, as the cement tends to dry quickly.
-
i.Shape the dental cement to match the margins of the incision as accurately as possible.
-
j.Drill away any sharp cement edges with the dental drill to prevent irritation.
-
k.Once the dental cement has set, plug in the ground screw wire to the drive and test connectivity between the exposed ground screw and the ground pin on the EIB with a multimeter.
-
l.Confirm connectivity and thoroughly dental cement the ground wire and connector in place.
-
a.
-
22.Finishing steps.
-
a.After the drive has been implanted, remove the bulldog sereffines and carefully cut off any dead tissue caused by the clamps.
-
b.Rinse the tissue around the drive with sterile saline to clear out any debris.
-
c.Inject 0.2 mL of lidocaine at multiple sites in the tissue surrounding the implant.
-
d.Generously apply antibiotic ointment to exposed tissue.
-
e.If necessary, suture up the skin both anterior and posterior to the implant.Note: Make sure the skin is not too tight around the implant after suturing. This may cause irritation. The skin should sit loosely around the implant but should be snug enough, so no underlying tissue is exposed (Figure 3B).
-
f.Apply a small amount of vetbond to the sutured areas.Note: Vetbond has a low viscosity so it may flow out of the tube quickly. Take care not to apply too much to prevent irritation.
-
g.Initial tetrode adjustments.
-
i.Using the appropriate turn tool, advance each tetrode down ∼1 mm into the brain while the animal is still under isoflurane.
Note: If surgery takes over 6 h, it may be safer to perform initial adjustments after the animal has woken up from anesthesia. Extended periods on isoflurane may lead to negative post-surgery outcomes. -
i.
-
h.Attach the headstage (optional) and cap for the drive cone and remove animal from the stereotax.
-
i.Add a couple drops of otic solution (Patterson Veterinary, Cat# 07-869-0640) to each ear to help with inflammation from the ear bars.
-
j.When the animal starts waking up, give a subcutaneous injection of buprenorphine ER (1.2 mg/kg) for analgesia and monitor for a couple hours.
-
k.Follow any approved post-operative procedures.Note: If approved, post-operative medication (e.g., Baytril, penicillin, topical lidocaine, etc.) may be helpful during recovery.
-
a.
Animal recovery, tetrode adjustments, and re-training
Timing: 2–3 weeks
These post-surgery steps describe the procedures for animal monitoring during recovery, tetrode advancement to regions of interest, and re-training prior to experiments.
-
23.Monitor the animal’s health after surgery.Note: The first few days of recovery post-surgery are the most important.
-
a.Closely monitor the amount of water consumed on the days following surgery. The implant may impede the animal’s ability to access water, so adjustment to the location of the water spout may have to be made.Note: Animals will likely have difficulty lifting their head after surgery, so it may be necessary to drill holes in the cage to provide access to a lower, angled water spout.
-
i.If the animal is showing signs of dehydration, (i.e., blood in the urine and decrease in skin turgor), give 5–10 mL of ringers solution subcutaneously as needed.
-
i.
-
b.Monitor the animal’s weight and food intake daily.Note: It is expected that the animal will lose weight after any major surgical procedure. To encourage the animals to eat, it may be necessary to break up food pellets and/or treats into more manageable pieces. The animal’s weight should stabilize 7–10 days post-surgery.
-
a.
-
24.Tetrode adjustments (Figure 4).
-
a.Tetrode adjustments should be made every day to keep tetrodes mobile, but in our experience, electrodes can be advanced every other day, if desired, without issue.
-
b.PFC adjustments.
-
i.PFC is targeted primarily based on depth (∼3.5–4.5 mm from brain surface).Note: To our knowledge, there are no audiovisual detectable electrophysiological markers that delineate regions of PFC (Figures 4B and 4C, blue traces).
-
ii.Daily adjustments can range from 50–500 μM, depending on distance from desired recoding location and cell quality.
-
iii.Pick a tetrode to use as a reference and pull it back ∼500 μM relative to the others.
-
i.
-
c.Dorsal CA1 adjustments.
-
i.During the first week post-surgery, adjust tetrodes down ∼300 μM every other day (or ∼150 μM daily).Note: Depending on the quality of the implant site (i.e., bleeding, swelling, etc.), there may be residual swelling that could cause significant electrode drift during the first week post-surgery. Do not advance tetrodes too aggressively.
-
ii.At the beginning of week 2, slowly take down a tetrode that will be used as a reference to gauge the depth of the CA1 layer.
-
iii.Once the layer has been reached, record the depth, and pull the tetrode back ∼500 μM into corpus callosum to use as a reference electrode.Note: Ripple magnitude and theta modulation can be estimated using a studio monitor.Note: Referencing other electrodes to the electrode in corpus callosum will exclude high frequency noise due to animal movements, which allows for more accurate estimation of ripple magnitude while advancing tetrodes.
-
iv.Leading up to the experiment, slowly (∼50–100 μM/day) advance tetrodes and listen for the physiological markers mentioned above (Figures 4B and 4C).
-
v.When large amplitude ripples and near spiking activity is observed on your tetrodes, micro-adjust tetrodes (∼10–50 μM/day) until clusters can be resolved.
-
i.
-
d.When the regions of interest have been reached and recordings are deemed acceptable, the experiment can begin.Note: Ideally, the majority of CA1 tetrodes will land in the pyramidal cell layer (SP, Figure 4A).
-
a.
-
25.Linear track re-training.
-
a.Linear track re-training is a necessary step prior to the single day W-Track and novel-familiar experiments and should be started ∼2 weeks post-surgery.Note: Linear track re-training should take place concurrently with tetrode adjustments.
-
b.After the animal’s weight has stabilized, food restrict the animal according to an approved protocol.
-
c.After ∼1 week of food restriction, reintroduce the animal to the linear track in the same room as initial linear track training. This room will be used as the familiar environment for downstream experiments.
-
d.Train the animal (20-min sessions) until a reward receipt rate of 3 rewards per minute is reached.Note: Retraining may take a few days as the animal adjusts to running with the implanted microdrive. If retraining exceeds one week, it may be necessary to begin the experiments due to eventual deterioration of recording quality and implant stability.Note: Performing tetrode adjustments after daily re-training sessions may be advantageous, since the animal is not as active after running.
-
e.Once acceptable performance has been reached, the following experiments can begin.Note: At this point, both recording quality and behavior should be satisfactory.
-
a.
Figure 4.
Electrophysiological signatures of dorsal CA1
(A) Example coronal brain slice illustrating the regions of dorsal CA1. Note that the overlying “cortex” label is not the recording location for the PFC traces shown in B and C (PFC recording location not shown. See Figure 6B for example recording locations).
(B) Example LFP traces during immobility showing the range of ripple magnitudes as well as differences in the sharp-wave polarity across dorsal CA1 layers for an example ripple event (CC – corpus callosum, SO – stratum oriens, SP – stratum pyramidale, SR – stratum radiatum).
(C) Example LFP traces during active behavior. Note the prominent theta band activity (8–12 Hz fluctuations) on the CA1 electrodes.
Single day W-Track learning
Timing: 6–8 h
This step explains the procedures for performing the single day W-Track learning experiment, allowing for longitudinal recording of CA1 and PFC ensembles over the course of learning a novel spatial learning task.2
-
26.
Check the quality of the recordings (i.e., unit yield) to determine whether to begin experiment.
Note: If additional adjustments must be made, wait ∼6 h for tetrodes to stabilize before starting the single day experiment. Alternatively, the experiment can be postponed to a subsequent day.
-
27.
Setup the W-Track in the familiar room where linear track pre-training took place.
-
28.
Run eight W-Track sessions, interleaved with 9 sleep sessions (Figure 5A).
Note: Sleep sessions may be extended if the animals’ behavior on the track deteriorates (i.e., immobile for minutes at a time). Generally, the session duration can be doubled, but avoid sessions >60 min. Keep in mind that longer sessions may increase the magnitude of electrode drift, which will affect the ability to track units over behavioral sessions.
CRITICAL: Do not change reward type or amount in the middle of the experiment. Changes in reward alters neural activity, which may affect interpretation of downstream results.5
Figure 5.
Single day and novel-familiar experimental setup
(A) Behavioral paradigm for the single day W-Track experiment (de novo learning).
(B) Behavioral paradigm for the novel-familiar experiment.
(C) Room layout for novel and familiar environments. Note the visible cue cards on the walls to maximally differentiate the novel and familiar environments.
Novel-familiar-novel experiment
Timing: 1–2 days
This step explains the procedures for performing the novel-familiar experiment, allowing for characterization of CA1 and PFC ensembles across separate experiences.1
-
29.
Check recordings and make tetrode adjustments if necessary.
-
30.
Setup the second W-Track in a different experimental room (novel, Figure 5C).
Note: The sleep box and reward wells were kept consistent throughout both the single day and novel-familiar experiments.
-
31.
Cover any empty wall space with black kraft paper and decorate with salient distal cues (i.e., shapes made with colored tape, etc.).
-
32.
Perform the novel-familiar experiment as laid out in Figure 5B.
Note: Quickly transfer the animal between rooms to preserve recording continuity.
Drive recovery and histology
Timing: 1 week
This section explains the procedures for drive recovery and histological verification of electrode placement after all experiments have been completed.
-
33.Electrolytic lesions and perfusion.
-
a.Put the animal in a warmed 7 L induction chamber and flow in 5% isoflurane at 2 L/min.
-
b.After a breath rate of 60 bpm is reached, transfer the animal to the stereotactic nose cone and set the isoflurane to 2%.
-
c.Connect the black terminal of the current generator to the Gnd pin on the EIB.
-
d.Connect the red terminal to a wire with a pin soldered at the end.
-
e.Set the output on the current generator to red.
-
f.Set the current to 30 μA.
-
g.Touch each gold pin on the EIB for ∼1–2 s to lesion each site.
 CRITICAL: Do not send too much current down any electrode. This may cause overlapping of lesions, which can be problematic if precise reconstruction of electrode tip locations is required.
CRITICAL: Do not send too much current down any electrode. This may cause overlapping of lesions, which can be problematic if precise reconstruction of electrode tip locations is required. -
h.After ∼24 h, place the animal in a 7 L induction box at 5% isoflurane.
-
i.When a breath rate of 60 bpm is reached, inject an appropriate dose of Beuthanasia-D (Patterson Veterinary, Cat#:07-807-3963) intraperitonially.
-
j.Once deeply anesthetized, intracardially perfuse with 0.9% saline followed by 3.7% formaldehyde.
-
k.Remove the head with the drive still attached and strip away all tissue, leaving only the skull.
-
l.Submerge the entire skull in 3.7% formaldehyde overnight.Note: Letting the skull sit in formaldehyde overnight helps with downstream tetrode track visualization.
-
m.In a hood, dry off the skull and retract all tetrodes to their top positions.
-
n.Extract the brain from the skull (Figure 6A) and store in a 30% sucrose and 3.7% formaldehyde solution in the fridge.
-
o.Submerge the recovered drive in a Metrizyme solution (VWR, Cat#89132-869) for 24–48 h to clean.
 CRITICAL: Submerging immediately in a Metrizyme solution prevents clogging of 30 Ga tubes and makes clearing debris out much easier.
CRITICAL: Submerging immediately in a Metrizyme solution prevents clogging of 30 Ga tubes and makes clearing debris out much easier. -
p.Recover shuttles and clean 23 Ga tubes and threaded rods in acetone for 24 h.Note: Shuttles for the 32-tetrode drive can be reused if the dental cement is intact.Note: Carefully unscrew the threaded rods from the drive to retain the threads in the body.
-
q.Ream out 30 Ga tubes in the drive body with 5.5 m wire.
-
r.Remove the residual skull, EIB, and ground wire.
-
s.Shave down excess dental cement.Note: As long as the dental cement is intact and the 30 Ga tubes are not bent or clogged, drive bodies can be expected to be reused ∼5–6 times.Note: The drive body can now be reloaded with shuttles and tetrodes for a subsequent experiment.Note: The shuttles of the 64-tetrode drive will have to be remade due to their fragility.
-
a.
-
34.Brain slicing and Nissl staining.
-
a.Using a microtome, collect 50 μM thick slices around the regions of interest and store in 1× PBS filled 48 well plates.Note: If using a freezing microtome, cover brain in OCT and let it freeze sufficiently before slicing.
-
b.Mount slices on gelatin coated slides and thoroughly dry in a hood for 2–3 days.
 CRITICAL: Insufficient drying may cause slices to fall off the slides during staining.
CRITICAL: Insufficient drying may cause slices to fall off the slides during staining. -
c.Nissl stain the slices in a hood using the following protocol:
-
i.Xylene – 5 min.
-
ii.100% ethanol – 3 min.
-
iii.70% ethanol – 3 min.
-
iv.50% ethanol – 3 min.
-
v.d2H2O – 1 min.
-
vi.Cresyl violet (CV6) – 3.5 min.Note: Recipe for cresyl violet solution can be found in reference above.
-
vii.d2H2O – Quick rinse.
-
viii.CV differentiator – 45 s.Note: CV differentiator can be made by mixing 5 mL of glacial acetic acid in 500 mL of 70% ethanol.
-
ix.d2H2O – Quick rinse followed by 1 min.
-
x.50% ethanol – 1 min.
-
xi.70% ethanol – 2 min.
-
xii.95% ethanol – 1 min.
-
xiii.100% ethanol – 1 min.
-
xiv.Xylene – 5 min.Note: Keep in mind that slices will continue to lighten during the dehydration steps (x–xiv) after the CV differentiator wash.
-
i.
-
d.Mount a coverslip with Permount or DPX mounting media directly from the xylene wash.
 CRITICAL: Do not allow slices to dry out in between the xylene wash and mounting. This will cause significant cracking and result in poor visualization of electrolytic sites.
CRITICAL: Do not allow slices to dry out in between the xylene wash and mounting. This will cause significant cracking and result in poor visualization of electrolytic sites. -
e.After mounting media sufficiently dries, seal the edges of the coverslip with nail polish to prevent bubbles from forming.
-
f.Image slices using a brightfield microscope at 4 or 10× and stitch together to generate a composite for each slice.
-
g.Reconstruct electrode positions using composite images (Figure 6B).
-
a.
Figure 6.
Drive recovery and histological verification of electrode tips
(A) Rat skull attached to a microdrive after tetrode retraction, brain extraction, and cleaning in Metrizyme.
(B) Examples of Nissl stained CA1 and PFC slices. Note the lesions indicated by the red arrows.
Data processing, spike clustering, and single-trial linearized firing rates
Timing: 1–2 weeks
This section provides a general overview of the steps required to pre-process the acquired data in preparation for manifold visualization.
-
35.Position tracking the animals’ behavior.Note: Here, position was semi-manually tracked using the cameraModule software (SpikeGadgets).
-
a.After position tracking in 2D, generate linearized position for each of the 4 trajectories (Figure 7C).Note: Track linearization can be accomplished using publicly available code. (https://github.com/LorenFrankLab/track_linearization).
-
a.
-
36.Spike sorting.Note: Here, all single units were manually clustered using matclust (Figure 7A). (https://bitbucket.org/mkarlsso/matclust/src/master/).
-
a.For both experimental paradigms, track as many cells throughout the entire duration of the experiment.Note: Tracking across the single day experiment allows for characterization of defined hippocampal-prefrontal populations over the course of learning.
-
a.
-
37.Generate linearized place fields (Figure 7D).
-
a.For each cell, generate occupancy normalized firing fields on a trial-by-trial basis for each trajectory at 1 cm spatial resolution.
-
a.
-
38.
Compile CA1 and PFC pseudo-population matrices for all trajectories and epochs as well as trajectory labels at each position (Figure 7E).
Note: Example code for steps 37 and 38 can be found at http://github.com/JadhavLab/GeometricTransformationProtocol in the “ExampleCode” folder.
Note: The trajectory label example in Figure 7E is a schematic and only displays labels for one trajectory.
Note: The length of the trajectory label column vector should match the number of rows in the pseudo-population spike matrix.
Figure 7.
Spike sorting, position linearization, and pseudo-population matrices
(A) Example spike clusters in CA1 across novel and familiar environments. Note the purple cell that is only active in the familiar environment.
(B) Same is in (A) but for PFC.
(C) Linearization of animals’ position into 4 separate trajectories.
(D) Example linearized firing fields for CA1 (top) and PFC (bottom).
(E) Schematic showing the construction of the n-dimensional pseudo-population matrix and trajectory labels. Note that the example compiled matrix only shows three trials of one trajectory type.
Geometric transformation of cognitive maps
Timing: 1–2 h
This step takes the pseudo-population activity matrices generated above and plots the low dimensional embeddings for each trajectory using UMAP.11
-
39.
Download the MATLAB UMAP toolbox and add to path. (https://www.mathworks.com/matlabcentral/fileexchange/71902-uniform-manifold-approximation-and-projection-umap).
-
40.
Run the following code snippet to obtain low dimensional embeddings of either CA1 or PFC populations for all trajectories from a novel epoch. This code will also save a “template file” that is used to project population activity from the familiar environment using the novel environment embeddings (Figure 8, left).
>spike_umap = run_umap(spike_matrix_novel, 'min_dist', 0.6, 'n_neighbors', 50, 'metric', 'cosine', 'n_components', 3, 'save_template_file', 'all_umap_trials_novel.mat')
Note: The ‘spike_umap’ output file will have the same number of rows as the input ‘spike_matrix’ file.
Note: The hyperparameters used here are similar to previous studies.12,13
-
41.
Using the saved “template file”, which contains the novel UMAP object, project familiar environment population activity to visualize transformation of CA1 or PFC maps across the novel and familiar experiences (Figure 8, right).
>spike_umap = run_umap(spike_matrix_familiar, 'min_dist', 0.6, 'n_neighbors', 50, 'metric', 'cosine', 'n_components', 3, 'template_file', 'all_umap_trials_novel.mat')
-
42.
To plot, assign colormaps to the different trajectories and align trajectories from start to end.
>traj1 = inferno(length(pos_interp))
>traj2 = parula(length(pos_interp))
>traj3 = viridis(length(pos_interp))
>traj4 = plasma(length(pos_interp))
>traj_colormap = [traj1;traj2(end:-1:1,:);traj3;traj4(end:-1:1,:)]
-
43.
Plot the data in ‘spike_umap’ in 3D and assign temporary colors using the trajectory label information in ‘spike_matrix_label’.
-
44.
Lastly, color data points according to trajectory and position using the compiled colormap.
>scatter3(spike_umap(:,1),spike_umap(:,2),spike_umap(:,3),30∗ones(size(spike_matrix_label)),spike_matrix_label,'filled')
>colormap(traj_colormap)
Note: Detailed code for additional analyses can be found on GitHub (http://github.com/JadhavLab/GeometricTransformation).
Figure 8.
Geometric transformation of cognitive maps
(A) Low dimensional representation of CA1 activity during the second exposure to the novel environment (left). Familiar environment activity projected into the latent space constructed based on novel environment activity (right).
(B) Same as in (A) but for PFC.
Expected outcomes
Upon successful completion of the experimental protocol, hippocampal tetrodes in the pyramidal cell layer can detect upwards of ∼15–20 units. Conservatively, if 1/4th–1/3rd of the tetrodes reaches the CA1 layer, one can expect a yield of ∼30–60 place cells. The prelimbic region of PFC, on the other hand, yields sparse recordings per tetrode, and a yield of ∼30–40 units can be expected using the 32 tetrode microdrive array (16 tetrodes for each area). These values will scale with the total number of tetrodes targeting these regions, so using the 64-tetrode drive design will yield significantly larger ensembles. Regardless of what design is used, the unit yield should be sufficient for several common downstream analytical methods, such as position decoding and replay detection. For the novel-familiar experiment, remapping in CA1 across environments may decrease the number of consistent place cells recorded across exposures, so it may be advantageous to target a larger proportion of tetrodes to CA1. PFC cells can be expected to have relatively consistent firing across experiences in this paradigm, so longitudinal tracking of single units is not as much of a concern.
As for behavior, ∼80%–90% of animals will learn the single day W-Track task, given that the animals exhibit consistent behavior over the entirely of the experiment and do not lose the motivation to run. However, successful experiments necessitate sufficient unit yields as well as good behavioral performance, which increases the difficulty of these experiments. Taking all factors into consideration, this lowers the success rate for the single day paradigm to ∼75%. If animals do not learn the task in a single day, subsequent days should be used to train the animal in the familiar environment until learning has been established. Only then can the novel-familiar experiment begin. If unit yields are sufficient, novel-familiar experiments can be expected to be successful ∼90% of the time.
Limitations
Although tetrode recordings allow for precise targeting, there are several limitations that come with their use. Given the propensity for tubes to get clogged with biological material, tetrodes need to be adjusted regularly, increasing the time tending to implanted animals. Also, electrode drift is a common factor that hinders consistent recordings over the course of days. Given this, it is difficult to maintain good recordings without performing small adjustments every 1–2 days. On the topic of unit yields, although tetrodes are well suited for recording from CA1, they may not be optimal for structures such as PFC. A dual recording approach combining tetrode and probe recordings may optimize these experiments.14 Lastly, as with most in vivo experimental paradigms, the learning curve for performing successful experiments from beginning to end can be steep.
In relation to the dimensionality reduction method used here, there are a few factors that need to be taken into consideration. As the effective dimensionality of CA1 and PFC is still unknown, it is difficult to determine the number of units required for optimal dimensionality reduction in each region. However, we suggest that, at a minimum, the number of units to include be estimated by calculating the spatial coverage of the environment afforded by the recorded population. For example, if a requirement is set that each positional bin have at least 5 neurons active, the smaller the population, the greater the proportion of excluded bins. We advise that no more than 5% of the bins be excluded at any time. As a guide, the minimum numbers of CA1 and PFC neurons needed to fulfill this requirement for the W-Track are shown in Figure 9. Note that this value will change depending on the nature of the experiment being performed (i.e., brain region recorded from, size of the environment, etc.).
Figure 9.
Minimum number of units to include for dimensionality reduction
(A) The minimum number of units to use for dimensionality reduction can be estimated by the proportion of positional bins excluded given a certain cell inclusion criterion. <5% of bins should be excluded. Note that this is a general recommendation and has not been empirically validated since the effective dimensionality of brain regions is difficult to determine. Data are represented as mean ± S.E.M.
Troubleshooting
Problem 1
Animal does not recover from surgery (Implant surgery problem).
Potential solution
-
•
Perform the surgery as quickly as possible without compromising good surgical technique. This will take some time to master. Longer surgeries may necessitate the use of higher isoflurane concentrations to maintain anesthetic depth, which is detrimental to post-surgical recovery.
-
•
Try not to exceed an anesthesia maintenance isoflurane level of ∼2% through the nose cone.
Problem 2
Drive falls off after surgery (General problem).
Potential solution
-
•
During surgery, ensure all tissue is removed from the skull.
-
•
Completely dry off the skull prior to adding the base layer of Metabond to the skull/screws.
-
•
Do not disturb the base layer of Metabond.
-
•
Keep a sterile environment during surgery to reduce the risk of infection.
-
•
Antibiotics, such as penicillin, can be administered for a few days post-surgery if approved.
Problem 3
Electrode impedance does not drop to ∼250 kOhms (Drive fabrication problem).
Potential solution
-
•
Replace gold solution if it starts to get cloudy.
-
•
Recut the affected tetrodes with sharp scissors. Bad cuts can prevent effective gold plating.
Problem 4
Recordings are noisy (Recording problem).
Potential solution
-
•
If the ground screw in the skull has lost connection to the ground pin in the EIB, there will be excessive noise that occludes any real electrophysiological signal.
-
•
Attempt to reestablish a connection, which may involve anesthetizing the animal with isoflurane and soldering any broken connection.
-
•
For future surgeries, double check connectivity with a multimeter and sufficiently cover all components of the ground wire, 4 pin connectors, and screw with dental cement.
-
•
Ambient electrical noise is also a common problem. This may be remedied by coating the interior of the drive cone with copper tape and grounding to the ground pin of the EIB.
-
•
Ensure that all electrical components are grounded according to the manufacturer’s instructions.
Problem 5
Recordings are unstable (Recording problem).
Potential solution
-
•
Do not use excess force when plugging or unplugging the animal from the recording system.
-
•
Ensure that the animal has enough slack to move freely in the sleep box and on the linear and W-Tracks.
-
•
Recording instability may also be due to poor adherence of the drive to the skull. Follow instructions for the above problem “Drive falls off after surgery.”
Problem 6
No change in recordings is observed after moving tetrodes (Recording problem).
Potential solution
-
•
The 23 Ga tubing may have broken loose from the shuttle when adjusting down. Use a new tube of super glue when assembling drive components to reduce the chance that bad glue is the cause of this problem.
-
•
Sufficiently ream out the drive body with a 23 Ga needle prior to loading shuttles to ensure that 23 Ga tubes slide smoothly into the drive body. Excess resistance may cause 23 Ga tubes to break away from the shuttles.
-
•
For targeting PFC, the cannula should be as medial as possible without disturbing the sinus. If the cannula is too lateral, tetrodes will enter the white matter, which may not result in robust electrophysiological recordings.
-
•
Refine surgical skills. Excess swelling and cell death caused by incorrect surgical technique will result in a quiet zone as tetrodes are advanced.
-
•
Excess bleeding in the craniotomy may cause blood to clot within the 30 Ga tubes, preventing advancement of tetrodes. Avoid larger vessels when implanting the drive.
Problem 7
Animals do not learn the W-Track task in a single day (Behavior problem).
Potential solution
-
•
Change linear track performance criterion to 5 rewards/min (pre-surgery) or 4 rewards/min (post-surgery).
-
•
Make sure that no loud work will be performed on the day of the experiment. Loud noises may cause animals to pause for extended periods of time.
-
•
Have the animal reach the approved food deprivation limit before starting the experiment.
Problem 8
Brain slices fall off slides during Nissl staining (Histology problem).
Potential solution
-
•
Dry the slices for an additional 1–2 days.
-
•
During mounting, wick away excess moisture from the edges of the slices using Kimwipes to prevent edges from curling upward while drying.
-
•
Make fresh Nissl staining solutions. While staining solutions can be reused, dilution of some of the components may affect staining quality.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Shantanu Jadhav (shantanu@brandeis.edu).
Materials availability
No new materials were generated in this study.
Acknowledgments
Figures in the graphical abstract were created with BioRender.com. The authors thank the Frank and Foster laboratories for providing initial 3D printing files and Blake Porter for providing example LFP traces. This work was supported by NIH grant R01MH112661 to S.P.J.
Author contributions
Visualization, J.D.S. and W.T.; writing – original draft, J.D.S.; writing – review and editing, J.D.S. and S.P.J.; funding acquisition, S.P.J.; supervision, S.P.J.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Justin D. Shin, Email: jdshin@brandeis.edu.
Shantanu P. Jadhav, Email: shantanu@brandeis.edu.
Data and code availability
Data underlying these results have been uploaded in the NWB (Neurodata Without Borders) format15 to DANDI: ID#000447 (https://doi.org/10.48324/dandi.000447/0.230316.2133). Code to replicate these results are available on our lab GitHub (http://github.com/JadhavLab/GeometricTransformation).
References
- 1.Tang W., Shin J.D., Jadhav S.P. Geometric transformation of cognitive maps for generalization across hippocampal-prefrontal circuits. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin J.D., Tang W., Jadhav S.P. Dynamics of Awake Hippocampal-Prefrontal Replay for Spatial Learning and Memory-Guided Decision Making. Neuron. 2019;104:1110–1125.e7. doi: 10.1016/j.neuron.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson J.M., Leppla C.A., Bladon J.H., Jadhav S.P. Adaptable and automated rodent behavior maze system. bioRxiv. 2021 doi: 10.1101/2021.06.05.447225. Preprint at: [DOI] [Google Scholar]
- 4.Buzsáki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25:1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrose R.E., Pfeiffer B.E., Foster D.J. Reverse Replay of Hippocampal Place Cells Is Uniquely Modulated by Changing Reward. Neuron. 2016;91:1124–1136. doi: 10.1016/j.neuron.2016.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cresyl violet acetate stain. Cold Spring Harbor Protocols. 2008;2008 doi: 10.1101/pdb.rec11201. pdb.rec11201. [DOI] [Google Scholar]
- 7.Tang W., Shin J.D., Jadhav S.P. Multiple time-scales of decision-making in the hippocampus and prefrontal cortex. Elife. 2021;10 doi: 10.7554/eLife.66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller R.U., Kubie J.L. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leutgeb S., Leutgeb J.K., Treves A., Moser M.B., Moser E.I. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- 10.Alme C.B., Miao C., Jezek K., Treves A., Moser E.I., Moser M.B. Place cells in the hippocampus: eleven maps for eleven rooms. Proc. Natl. Acad. Sci. USA. 2014;111:18428–18435. doi: 10.1073/pnas.1421056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McInnes L., Healy J., Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv. 2018 doi: 10.48550/arXiv.1802.03426. Preprint at. [DOI] [Google Scholar]
- 12.Campbell M.G., Attinger A., Ocko S.A., Ganguli S., Giocomo L.M. Distance-tuned neurons drive specialized path integration calculations in medial entorhinal cortex. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner R.J., Hermansen E., Pachitariu M., Burak Y., Baas N.A., Dunn B.A., Moser M.B., Moser E.I. Toroidal topology of population activity in grid cells. Nature. 2022;602:123–128. doi: 10.1038/s41586-021-04268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guardamagna M., Eichler R., Pedrosa R., Aarts A., Meyer A.F., Battaglia F.P. The Hybrid Drive: a chronic implant device combining tetrode arrays with silicon probes for layer-resolved ensemble electrophysiology in freely moving mice. J. Neural. Eng. 2022;19:e036030. doi: 10.1088/1741-2552/ac6771. [DOI] [PubMed] [Google Scholar]
- 15.Rübel O., Tritt A., Ly R., Dichter B.K., Ghosh S., Niu L., Baker P., Soltesz I., Ng L., Svoboda K., et al. The Neurodata Without Borders ecosystem for neurophysiological data science. Elife. 2022;11 doi: 10.7554/eLife.78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying these results have been uploaded in the NWB (Neurodata Without Borders) format15 to DANDI: ID#000447 (https://doi.org/10.48324/dandi.000447/0.230316.2133). Code to replicate these results are available on our lab GitHub (http://github.com/JadhavLab/GeometricTransformation).