Abstract
Introduction:
Septic shock is often characterized by tachycardia and a hyperdynamic hemodynamic profile. Use of the beta antagonist esmolol has been proposed as a therapy to lower heart rate, thereby improving diastolic filling time and improving cardiac output, resulting in a reduction in vasopressor support.
Methods:
We conducted a two-center, open-label, randomized, Phase II trial comparing esmolol to placebo in septic shock patients with tachycardia. The primary endpoint was improvement in hemodynamics as measured by the difference in norepinephrine equivalent dose (NED) between groups at 6 hours after initiation of study drug. Secondary outcomes included assessing differences in inflammatory biomarkers and oxygen consumption (VO2).
Results:
A total of 1,122 patients were assessed for eligibility and met inclusion criteria; 42 underwent randomization, and 40 received study interventions (18 in the esmolol arm and 22 in the usual care arm). The mean NED at 6 h was 0.30 ± 0.17 mcg/kg/min in the esmolol arm compared to 0.21 ± 0.19 in the standard care arm (P= 0.15). There was no difference in number of shock free days between the esmolol (2, IQR 0, 5) and control groups (2.5, IQR 0, 6) (P= 0.32). There were lower levels of C-reactive protein at 12 and 24 h in the esmolol arm, as well as a statistically significant difference in trend over time between groups. There were no differences in terms of IL-4, IL-6, IL-10, and TNFα. Among a subset who underwent VO2 monitoring, there was decreased oxygen consumption in the esmolol patients; the mean difference between groups at 24 h was −2.07 mL/kg/min (95% CI −3.82, −0.31) (P= 0.02), with a significant difference for the trend over time (P< 0.01).
Conclusion:
Among patients with septic shock, infusion of esmolol did not improve vasopressor requirements or time to shock reversal. Esmolol was associated with decreased levels of C-reactive protein over 24 h.
Keywords: Esmolol, septic shock, tachycardia
INTRODUCTION
Septic shock is a leading cause of death around the world, with a mortality that often ranges 30% to 50% but in some locations may be even higher (1). Despite advances in critical care medicine over the last several decades, few therapeutic interventions have demonstrated a mortality benefit in this population besides antimicrobial medications, intravenous fluids, and controlling the source of the infection.
After resuscitation for hypovolemia and in the absence of myocardial suppression, septic shock is traditionally characterized by a hyperdynamic hemodynamic profile with a high cardiac output (CO) and low systemic vascular resistance (SVR) in association with excessive catecholamine stimulation. Tachycardia is a common finding as an early compensatory mechanism to increase cardiac output in the setting of low SVR. Often tachycardia persists beyond the initial stages of septic shock, and has been associated with restricted diastolic ventricular filling, increased oxygen requirements, and tachycardia-induced cardiomyopathy, as well as myocardial depression and direct myocyte toxicity via calcium overload (2–5). Generally, clinical practice has been to avoid trying to control the tachycardic response for fear of worsening cardiac output and causing cardiovascular collapse. However, a previous single center randomized trial (6) of the intravenous beta-1 adrenoreceptor antagonist esmolol demonstrated that control of heart rate to a more “normal” range was safe, well-tolerated, and appeared beneficial, with a 30% reduction in mortality found in that trial. In previous human and animal studies, esmolol has been associated with decreased inflammation as represented by attenuation of inflammatory cytokines and biomarkers (7–10).
In this trial, we evaluated the use of beta-1 blockade infusion in a septic shock cohort. We hypothesized that the administration of esmolol to patients with vasopressor-dependent septic shock would lower the heart rate, thereby improving diastolic filling time and improving cardiac output, resulting in a reduction in need for vasopressor support. We also sought to explore whether esmolol infusion would have an effect on the inflammatory cascade or on oxygen consumption.
METHODS
Design and setting
This was a two-center, open-label, randomized, Phase II trial comparing esmolol to placebo in septic shock patients with tachycardia. The study was conducted at Beth Israel Deaconess Medical Center (coordinating site) and Lahey Hospital and Medical Center (LHMC), both tertiary care centers in Massachusetts. The study was approved by both Institutional Review Boards, and patients or legally authorized surrogates provided written informed consent. The trial was registered at clinicaltrials.gov (NCT02369900). A Data and Safety Monitoring Board evaluated and monitored for safety.
Study population
The hospitals’ emergency departments and intensive care units (ICU) were screened between January 2015 and December 2019. Inclusion criteria were: age ≥18years, sepsis (presence of two or more systemic inflammatory response syndrome [SIRS] criteria with documented or suspected infection), norepinephrine (minimum 0.1 mcg/kg/min) support to maintain a mean arterial pressure ≥ 65 mm Hg despite appropriate volume resuscitation (as defined by the clinical team, however at least 30mL/kg intravenous fluid), heart rate ≥ 95 per minute for at least 2 h prior to enrollment. If patients were on additional vasopressors, those doses were converted to norepinephrine equivalent doses (NED) to provide a total combined vasopressor dose (11).
Patients were excluded from the trial if they had received intravenous beta-blocker therapy or other antiarrhythmic medication prior to randomization, had evidence of cardiac dysfunction (i.e., cardiac index [CI] ≤ 2.2 L/min/m2), had a history of significant (more than “mild” by previous echocardiography) valvular heart disease, had a known allergy/sensitivity to esmolol or history of asthma/COPD, were designated “Do-not-resuscitate (DNR)” or “do-not-intubate (DNI)” or “comfort measures only (CMO),” were receiving an infusion of epinephrine, dopamine, dobutamine, or milrinone at time of enrollment, or the patient/surrogate or clinical team refused. In some cases, subjects were excluded when there were active discussions about goals of care but the patient had not officially transitioned to DNR/CMO, or the patient was moribund on maximal vasopressor therapy. Additional detail is provided in Figure 1.
FIG. 1.
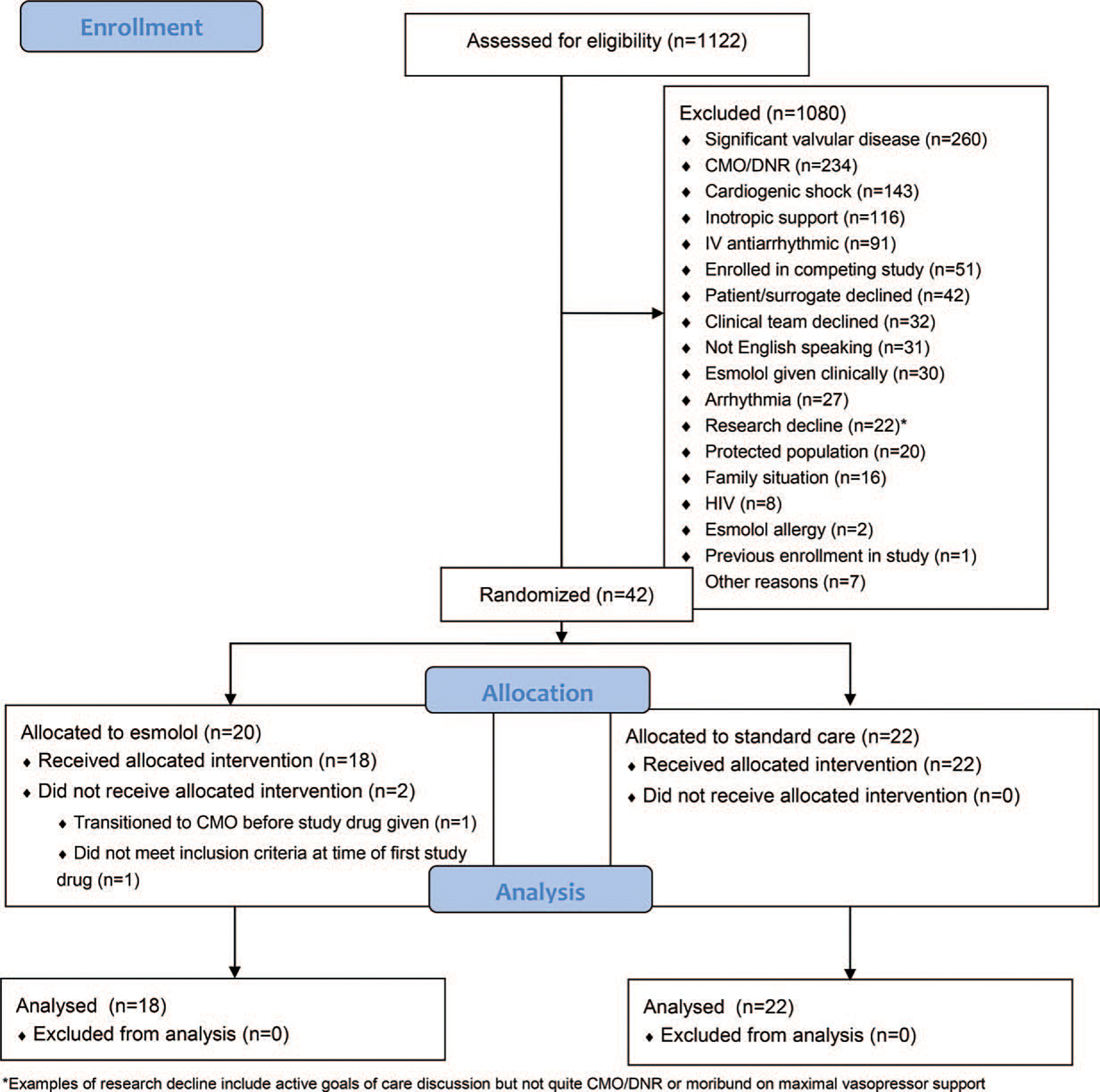
CONSORT diagram.
Randomization, study drug, and blinding
Patients were randomized to esmolol infusion or standard care in a 1:1 ratio stratified by site. A saline placebo was added as a time stamp for the beginning of study interventions after the seventh enrollment; prior to that, we used the first blood draw after randomization as the time stamp for enrollment. Patients, irrespective of treatment group, were managed at the discretion of the clinical teams. Both enrollment sites have internal guidelines and processes for the management of septic shock which reflect the 2012 Surviving Sepsis Campaign guidelines and are incorporated into the care of patients with septic shock in the ICUs (12). Hemodynamic monitoring was not standardized beyond what was used by the ICU teams for clinical purposes. Esmolol was titrated to a heart rate of 80 to 94 per minute, starting at 50mcg/kg/min and subsequently increasing every 20 min in increments of 50mcg/kg/min (or slower at the discretion of the clinical team) until target was achieved. The maximum allowed dose was 300mcg/kg/min. Esmolol was continued for 24 h, unless held or discontinued by the clinical team at their discretion, or if any of the stopping parameters were reached. The cardiac index was monitored at baseline and throughout infusion of esmolol with the use of the Noninvasive Cardiac Output Monitor (NICOM) (Cheetah Medical, Oregon), a validated tool for hemodynamic measurements in the critically ill (13). Esmolol infusion was held if the CI dropped below 2.2 L/min/m2. In the event of a sustained decrease in heart rate below 50, the infusion was paused or discontinued, and restarted later at the discretion of the clinical team; if the heart rate remained below target, the infusion was not restarted.
Outcomes
The primary endpoint was improvement in the hemodynamic profile as measured by a decrease in the need for norepinephrine support. This was defined as the difference in norepinephrine dose equivalents between groups at 6 h after initiation of study drug. The difference in vasopressor dose between groups at 12 h and over time, and the differences in heart rate at the 6 and 12 h time points and over time were also assessed. Other secondary hemodynamic endpoints included number of shock-free days (cessation of all vasopressors), time to shock reversal (defined as cessation of all vasopressor use for 24 h), and change in lactate over time (12, 24 h time points). Differences between groups in five inflammatory biomarkers (IL-4, IL-6, IL-10, TNF-α, and CRP) at 12 h, 24 h, and over time were also assessed. Additional outcomes assessed included days of mechanical ventilation, need for dialysis, length of ICU stay, and hospital length of stay. Change in VO2 was assessed between the two groups among a subset of patients who were mechanically ventilated and had VO2 monitoring available.
Blood samples and data collection
Trained research personnel collected all data according to a detailed, predefined data dictionary, and a physician verified all outcome variables. We entered data into a Research Electronic Data Capture (REDCap) database, a secure, web-based database tool (14).
Blood was collected at enrollment before (< 10 min) administration of the study drug (time 0) and at 6, 12 and 24 h thereafter (±1 h). Blood for lactate measurement was sent for immediate analysis at the hospitals’ clinical laboratories; this was measured using whole blood collected in a standard heparinized blood gas syringe by the clinical nursing team with our research staff at the bedside. Research blood samples were centrifuged, aliquoted into light-protected cryotubes, and frozen at −80°C. Plasma IL-4, IL-6, IL-10, and TNF-α levels were measured using V-PLEX Proinflammatory Panel 1 (human) kits from Meso Scale Discovery (MSD, Rockville, MD). Plasma C-reactive protein (CRP) levels were tested using V-PLEX Vascular Injury Panel 2 (human) kits from MSD. All above assays were done by the Metabolism and Mitochondrial Research Core (Beth Israel Deaconess Medical Center, Boston, MA) following manufacturer’s instructions.
For patients who were mechanically ventilated at the coordinating center site, oxygen consumption (VO2) was measured using the Compact Anesthesia Monitor by General Electric. This device, which connects to the ventilator circuit, has a gas exchange module which measures the volume of inspired gas using a pneumotachograph and the partial pressure of oxygen with inspiration and expiration using a rapid paramagnetic analyzer. Measurements were taken by a sensor placed in the ventilator tubing and connected to the gas analyzing module (15), providing continuous readings with each patient breath. The Compact Anesthesia Monitor has been approved for the measurement of VO2 in mechanically ventilated patients. The technology has been validated against indirect calorimetry, and is perhaps even more accurate at higher FiO2 (15, 16). The VO2 monitor was only available for use at the coordinating center.
We collected data elements including laboratory values, information on mechanical ventilation duration and length of stay, and other clinical variables.
Statistical analyses
Preliminary observational data on a cohort of septic shock patients indicated a mean norepinephrine equivalent dose was 0.15 ± 0.09 mcg/kg/min at the 6 h time point. We anticipated a 33% reduction in mean norepinephrine dose to 0.10 ± 0.09 mcg/kg/min after 6 h of continuous treatment with esmolol. Based on these estimates, for a two-sample t test with a power of 80% and a type I error of 0.05, we estimated that a total of 104 patients would be required to show a statistically significant difference between treatment and control groups.
Descriptive statistics are provided for baseline and outcome variables. Continuous variables are presented as means with standard deviations or medians with interquartile ranges (IQR), based on the distribution of the data. Categorical variables are presented as counts with percentages. In univariate analysis, continuous variables were analyzed using a Student’s t test if normally distributed and a Wilcoxon ranked sum test if non-normal. Categorical variables were analyzed using a Fisher’s exact test.
The association between continuous infusion of esmolol and need for vasopressor support was assessed by comparing the median 6 h norepinephrine equivalent dose (NED) between groups using a Wilcoxon rank-sum test. No patients expired prior to 6 h after enrollment. To analyze the difference in vasopressor dose between groups at 12 h and over time, we compared NED (recorded hourly for 12 h) over time between groups using linear mixed effect models accounting for repeated measures and adjusting for preintervention NED. The appropriate variance-covariance structure was selected based on Akaike information criterion. Using this model, we tested whether NED was different at 12 h and for differences in the overall trend over time. A similar analysis was used to assess the difference in heart rate between groups over the first 12 h and lactate between groups over the first 24 h; however, in the heart rate analysis, we tested for mean differences at 6 h, 12 h and for differences in the overall trend over time, and in the lactate analysis, we used a log-transformed lactate value to test for mean differences at 12 and 24 h and for differences in the overall trend over time. We used a Wilcoxon-rank sum to compare the median number of shock free days, defined as cessation of all vasopressors during the first 7days, between the two groups.
To characterize the effects of esmolol infusion on VO2, we limited the analysis to patients who were mechanically ventilated during the study period. The VO2 values were cleaned based on a custom algorithm that eliminated points that met any of the following four criteria:
VO2 value corresponding to a Fraction of Inspired Oxygen (FiO2) value above 61%.
VO2 value within 10 min of a 10-unit change in FiO2.
VO2 value above 800mL/kg/min or below 80mL/kg/min that is not sustained for at least 30 consecutive minutes.
VO2 value that is 15% higher or lower than the average of the VO2 values 5 min before and after the point in question.
To analyze the difference in oxygen consumption between groups at 12 h, 24 h and over time for patients who were on mechanical ventilation at enrollment, we compared VO2 measurements (standardized by bodyweight in kilograms) over time (recorded every minute from the time of study drug administration over a period of at least 24 h) between groups using mixed linear model accounting for repeated measures (n = 15). We did not adjust for preintervention VO2 measurements. Using this model, we tested for mean differences at 12 h, 24 h and for differences in the overall trend over time. The appropriate variance-covariance structure was selected based on Akaike information criterion. Additionally, a figure was created by first computing the mean of all the VO2 observations for a patient by timepoint, and then computing the mean of the means for each timepoint by treatment group.
To characterize effects of esmolol on inflammatory markers in patients with vasopressor-dependent septic shock, we compared log-transformed values of biomarkers at 12 and 24 h and over time between groups using mixed linear model accounting for repeated measures and adjusting for preintervention biomarkers. The appropriate variance-covariance structure was selected based on Akaike information criterion.
In-hospital mortality was compared between groups using the Fisher’s Exact test. Baseline covariates appeared to be balanced, so we did not adjust for any additional covariates. Length of hospital and ICU stay was compared between groups using a Wilcoxon ranked sum test and stratified based on hospital survival. To characterize effects of esmolol on SOFA score over the first 24 h, we compared SOFA at 6, 12, and 24 h and over time between groups using a mixed linear model accounting for repeated measures and adjusting for preintervention SOFA.
All analyses were conducted using Stata 14.2 (College Station, TX) and a P-value of <0.05 was considered significant.
RESULTS
A total of 1,122 patients were assessed for eligibility and met inclusion criteria; 42 underwent randomization, and 40 received study interventions (18 in the esmolol arm and 22 in the usual care arm). After randomization, two patients assigned to the esmolol arm did not receive any study interventions and thus were excluded from the analysis; one was transitioned to comfort measures only by family and the other no longer met inclusion criteria before drug could be initiated. The trial was stopped before the target enrollment number of 104 due to low enrollments and the end of funding. Figure 1 shows the flow of patients through the trial. Five subjects were enrolled at LHMC; the remainder were enrolled at the coordinator center.
Baseline characteristics are presented in Table 1. There was no statistically significant difference between esmolol and placebo patients at baseline in terms of mean vasopressor dose, median lactate, median heart rate, mean SOFA scores, or patient characteristics.
TABLE 1.
Baseline characteristics
| All (n = 40) | Esmolol (n = 18) | Control (n = 22) | P | |
|---|---|---|---|---|
|
| ||||
| Age (years, median, IQR) | 63 (58, 68) | 62 (53, 67) | 64 (59, 71) | 0.26 |
| Female sex (n, %) | 17 (43) | 8 (44) | 9 (41) | >0.82 |
| Enrollment location (n, %) | >0.99 | |||
| ED | 6 (15) | 3 (17) | 3 (14) | |
| ICU | 34 (85) | 15 (83) | 19 (86) | |
| BMI (kg/m2, median, IQR) | 26.3 (23.8, 30.2) | 25.7 (22.6, 27.2) | 26.9 (24.2, 30.6) | 0.45 |
| Race (n, %) | 0.92 | |||
| White | 31 (78) | 14 (78) | 17 (77) | |
| Black | 3 (8) | 1 (6) | 2 (9) | |
| Other | 1 (3) | 1 (6) | 0 (0) | |
| Unknown/not reported | 5 (13) | 2 (11) | 3 (14) | 0.56 |
| Ethnicity (n, %) | ||||
| Hispanic | 1 (3) | 1 (6) | 0 (0) | |
| Not Hispanic | 31 (78) | 13 (72) | 18 (82) | |
| Unknown | 8 (20) | 4 (22) | 4 (18) | |
| Clinical characteristics | ||||
| Mechanical ventilation during study (n, %) | 28 (70) | 12 (67) | 16 (73) | 0.68 |
| Intubated prior to enrollment (n, %) | 25 (89) | 11 (92) | 14 (88) | >0.99 |
| Fluids in the 12 hours prior to enrollment (mL, median IQR) | 2666 (1328, 4703) | 2930 (1412, 5554) | 2621 (1100, 4272) | 0.71 |
| Source of sepsis (n, %)* | ||||
| Pulmonary | 13 (33) | 6 (33) | 7 (32) | >0.99 |
| Urinary | 8 (20) | 6 (33) | 2 (9) | 0.11 |
| Intra-abdominal/biliary | 12 (30) | 5 (28) | 7 (32) | >0.99 |
| Skin/soft tissue | 4 (10) | 3 (17) | 1 (5) | 0.31 |
| Other (includes retropharyngeal abscess, bacteremia, tick-borne illness, meningitis) | 8 (20) | 2 (11) | 6 (27) | 0.26 |
| Past medical history | ||||
| CAD (n, %) | 4 (10) | 2 (11) | 2 (9) | >0.99 |
| Cancer (n, %) | 9 (23) | 3 (17) | 6 (27) | 0.48 |
| CHF (n, %) | 3 (8) | 2 (11) | 1 (5) | 0.58 |
| COPD (n, %) | 10 (25) | 5 (28) | 5 (23) | 0.73 |
| Dementia (n, %) | 1 (3) | 0 (0) | 1 (5) | >0.99 |
| Diabetes mellitus (n, %) | 12 (30) | 7 (38) | 5 (23) | 0.32 |
| Type 1 | 6 (15) | 2 (11) | 4 (18) | |
| Type 2 | 6 (15) | 5 (28) | 1 (5) | |
| Alcohol abuse (n, %) | 12 (30) | 6 (33) | 6 (27) | 0.74 |
| HIV (n, %) | 2 (5) | 0 (0) | 2 (9) | 0.49 |
| HTN (n, %) | 15 (38) | 7 (39) | 8 (36) | >0.99 |
| Hyperlipidemia (n, %) | 12 (30) | 8 (44) | 4 (18) | 0.09 |
| Liver disease (n, %) | 12 (30) | 5 (28) | 7 (32) | >0.99 |
| Noncirrhotic | 1 (3) | 1 (6) | 0 (0) | |
| Cirrhotic | 11 (28) | 4 (22) | 7 (32) | |
| Renal disease (n, %) | 7 (18) | 3 (17) | 4 (18) | >0.99 |
| Chronic renal insufficiency | 5 (13) | 2 (11) | 3 (14) | |
| ESRD | 1 (3) | 1 (6) | 0 (0) | |
| Unknown/other | 1 (3) | 0 (0) | 1 (5) | |
| Stroke (n, %) | 2 (5) | 2 (11) | 0 (0) | 0.20 |
| Thyroid disease (n, %) | 0 (0) | 0 (0) | 0 (0) | N/A |
| Tobacco use (n, %) | 6 (15) | 2 (11) | 4 (18) | 0.67 |
| Transplant (n, %) | 0 (0) | 0 (0) | 0 (0) | N/A |
| Baseline values (prestudy drug) | ||||
| HR (beats per minute, mean ± SD) | 106 ± 11 | 107 ± 13 | 105 ± 10 | 0.55 |
| Vasopressor dose (norepinephrine equivalent units, mean ± SD) | 0.25 ± 0.18 | 0.28 ± 0.17 | 0.22 ± 0.19 | 0.33 |
| Baseline lactate (mmol/L, median, IQR) | 2.8 (1.8, 4.5) | 2.9 (1.8, 4.5) | 2.8 (1.6, 3.8) | 0.55 |
| Baseline SOFA (mean ± SD) | 10.5 ± 3.6 (n = 37) | 10.0 ± 3.6 (n = 16) | 11.0 ± 3.6 (n = 21) | 0.43 |
Some patients could have multiple sources identified.
Six patients (15%) were enrolled in the ED (three in the esmolol arm and three in the control arm). All were admitted to the ICU on the same day as enrollment. There were no significant differences in median enrollment systolic or diastolic blood pressure between the groups when comparing ED (116 mm Hg, IQR: 100, 132; 60 mm Hg, IQR: 52, 74) or ICU (103 mm Hg, IQR: 96,118; 58 mm Hg, IQR: 51, 65) enrollment location (P=0.34 and P=0.43, respectively). There was also no significant difference in enrollment hour systolic or diastolic blood pressures between the three esmolol patients and the three control patients who were enrolled in the ED (P=0.20 and P>0.99, respectively). The median time to from ICU admission to study intervention was 17.7 (IQR: 11.2, 28.5) hours in the esmolol group and 14.2 (IQR: 5.7, 27.3) hours in the standard care arm (P=0.69). The median esmolol dose received by patients in the esmolol group during the 24 h study period was 1208.9 mg (IQR: 520.7, 2189.2). Figure 2 reports the hourly median doses of esmolol received by patients in the interventional arm during the trial period. The median duration of the esmolol infusion (the number of hours over the first 24 h in which a patient received esmolol) was 15 (IQR: 10, 21) hours.
FIG. 2.
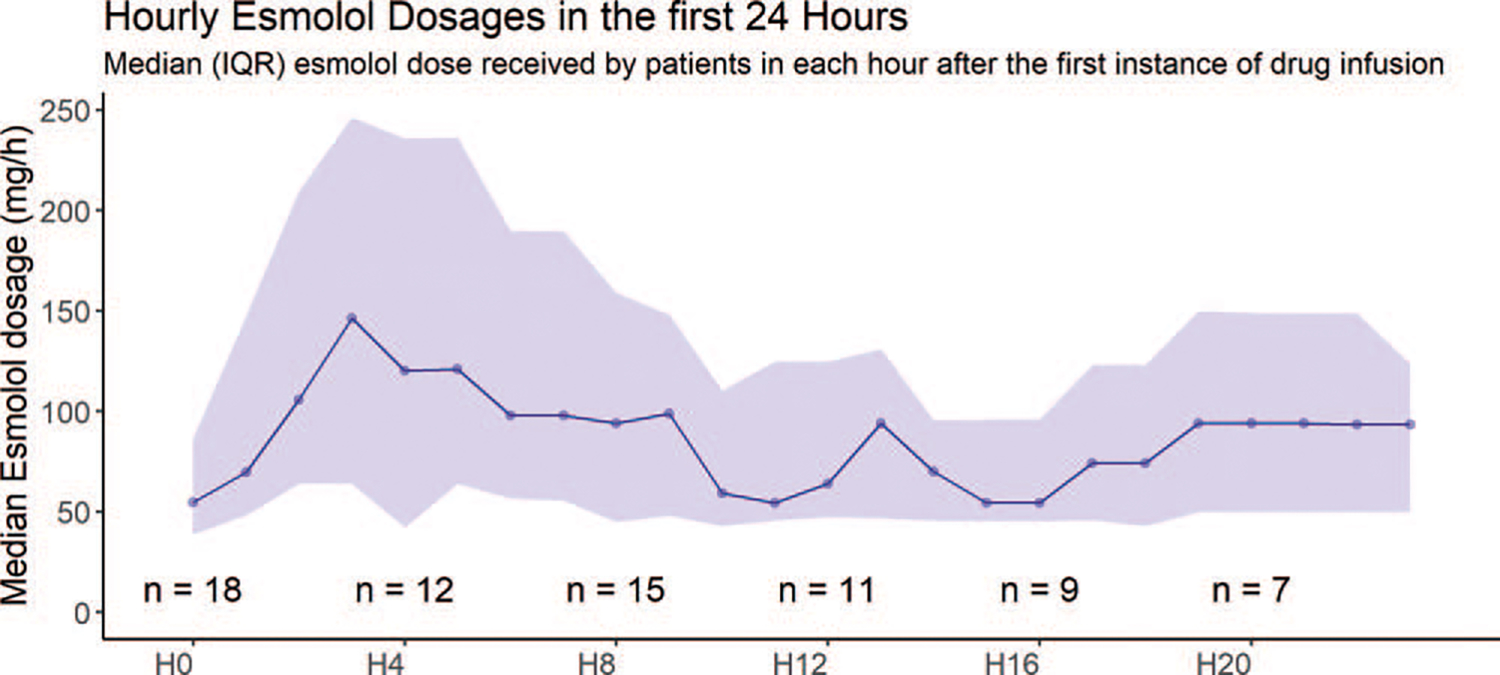
Hourly esmolol dose during the duration of the study. H0 indicates the first hour after initial drug infusion, that is, hour 0 to 1. Ticks on X axis indicate every 4th hour after drug infusion. The number of patients who contributed to the calculation of the median dose at each time point are indicated with the “n=“ annotations. The shaded purple area represents the inter-quartile range, with the lower and upper edges of the shaded area representing the 1st and 3rd quartiles of the esmolol dose, respectively. The central point within the shaded area represents the median esmolol dose.
Primary and Secondary Outcomes
The mean norepinephrine equivalent dose at 6 h was 0.30 ± 0.17mcg/kg/min in the esmolol arm compared to 0.2 ± 10.19mcg/kg/min in the control arm (P=0.15) (Table 2). In a mixed model comparing norepinephrine equivalent doses of vasopressors over 12 h between groups, controlling for baseline vasopressor dose, the mean difference between esmolol and control arms at 12 h was statistically significantly different with higher norepinephrine equivalent dose values in the esmolol arm (mean difference 0.09 [95% CI: 0.03–0.15], P<0.01) and there was a statistically significant trend over time (P<0.05). In a mixed model of heart rate over 12 h controlling for baseline heart rate, the mean difference between esmolol and control arms was not different (mean difference −2.9 [95% CI: −10.9 to 5.14] at 6 h, P=0.48; −3.6 [95% CI: −11.6 to 4.4] at 12 h, P=0.38; and there was no difference between the groups for the heart rate trend over time (P=0.81). In a mixed model comparing lactate (log) over 24 h between groups, and controlling for baseline values, there was no difference (mean difference between esmolol and control arms at 12 h −0.06 [95% CI: −0.27 to 0.15], P=0.57; −0.17 [95% CI: −0.38 to 0.04] at 24 h, P=0.11; and no difference for the trend over time (P=0.45).
TABLE 2.
Univariate outcomes
| Overall (n = 40) | Esmolol (n = 18) | Control (n = 22) | P | |
|---|---|---|---|---|
|
| ||||
| Mean norepinephrine equivalent at 6 h (mean ± SD) | 0.25 ± 0.19 | 0.30 ± 0.17 | 0.21 ± 0.19 | 0.15 |
| Mean norepinephrine equivalent at 12 h (mean ± SD) | 0.23 ± 0.19 | 0.31 ± 0.19 | 0.17 ± 0.17 | 0.026 |
| Mean norepinephrine equivalent at 24 h (mean ± SD) | 0.20 ± 0.18 | 0.24 ± 0.19 | 0.16 ± 0.17 | 0.136 |
| Median heart rate at 12 h (beats per minute, median, IQR) | 92 (82, 105) | 89 (79, 105) | 96 (85, 105) | |
| Median lactate at 12 h (mmol/L, median, IQR)* | 2.1 (1.5, 3.3) | 2.1 (1.6, 3.1) | 2.1 (1.4, 3.3) | |
| Median lactate at 24 h (mmol/L, median, IQR)† | 1.8 (1.4, 2.6) | 1.8 (1.4, 2.6) | 1.8 (1.5, 3.3) | |
1 patient in the control group did not have a lactate value at 12 h.
2 patients in the control group did not have a lactate value at 24 h.
Table 3 reports secondary outcomes for the trial. There was no difference in median number of shock free days between the esmolol group (2, IQR: 0, 5) and the control group (2.5, IQR: 0, 6) (P=0.32) or the median time to shock reversal (3.9 [IQR: 1.9, 6.5] in the esmolol group versus 2.5 [IQR: 1.5, 6.1] in the control group, P=0.20). Need for renal replacement therapy and need for mechanical ventilation were similar in both groups. Among those ventilated patients who survived, the median duration of mechanical ventilation was 7days (IQR: 3, 16) in the esmolol group compared with 6.5days (IQR: 1, 13) in the control arm (P=0.56). Total intravenous fluid volumes during the study period were similar between groups. There was no difference in urine output between the groups during the study period.
TABLE 3.
Secondary clinical outcomes
| All (n = 40) | Esmolol (n = 18) | Control (n = 22) | P | |
|---|---|---|---|---|
|
| ||||
| Median shock free days (IQR) | 2 (0, 5) | 2 (0, 5) | 2.5 (0, 6) | 0.32 |
| Time to shock reversal (days) | 3.1 (1.7, 6.2) | 3.9 (1.9, 6.5) | 2.5 (1.5, 6.1) | 0.20 |
| Intubated after enrollment (n, %) | 4 (10) | 2 (11) | 2 (9) | >0.99 |
| Hospital mortality (n, %) | 14 (35) | 6 (33) | 8 (36) | >0.99 |
| Dialysis (n, %) | 10 (25) | 3 (17) | 7 (32) | 0.46 |
| Median Hospital LOS in all patients (IQR) | 12 (6, 28) | 10 (6, 25) | 13 (6, 30) | 0.82 |
| Median Hospital LOS in survivors (IQR) | 15 (7, 30) | 13 (7, 29) | 15 (7, 35) | 0.86 |
| Median ICU LOS in all patients (IQR) | 6 (3, 13) | 8 (3, 14) | 6 (3, 12) | 0.44 |
| Median ICU LOS in survivors (IQR) | 5 (3, 11) | 8 (3, 13) | 4 (2, 11) | 0.41 |
| Median Ventilator days in all ventilated patients (IQR) | 6 (3, 12) | 7 (4, 13) | 6 (2, 12) | 0.56 |
| Median Ventilator days in ventilated patients who survived (IQR) | 7 (2, 16) | 7 (3, 16) | 6.5 (1, 13) | 0.56 |
| Fluids during the study (mL, median IQR) | 4916 (2507, 7375) | 6089 (3077, 8715) | 3990 (2027, 7145) | 0.14 |
| Urine output between 0 and 12 h after study start | 488 (267, 1099) | 572 (214, 1300) | 488 (280, 1007) | 0.86 |
| Urine output between 12 and 24 h after study start* | 491 (310, 895) | 508 (310, 1075) | 491 (310, 800) | 0.61 |
1 value missing in placebo group.
VO2 analyses
There were 15 patients (6 esmolol patients and 9 control patients) who had VO2 data available for analysis (see Table S-1,http://links.lww.com/SHK/B397 for additional patient detail). In a mixed model of VO2 over 24 h, there was a mean difference of −0.17mL/kg/min (95% CI: −1.92, 1.59) between the esmolol arm and the control arm at 12 h (P=0.85). The mean difference between groups at 24 h was −2.07mL/kg/min (95% CI: −3.82, −0.31) (P=0.02), with a significant difference for the trend over time (P<0.01). Figure 3 describes the VO2 over time at hourly intervals.
FIG. 3.

Plot of mean VO2/kg over time at hourly intervals after study drug administration. On the X axis, 1 indicates the 1st hour after drug administration(all data between hour 0 [time of drug administration] and hour 1), 2 indicates the 2nd hour (data between hour 1 and hour 2), and soon. The number of patients contributing to means at certain select timepoints is specified in the annotations (annotating text is colored red for the placebo group and blue for the esmolol group). Over all timepoints, a total of 15 patients (6 patients from esmolol group and 9 from control group) contributed VO2 data.
Biomarker analyses
The median inflammatory marker values at baseline, 6, 12, and 24 h are reported in a supplemental table (Table S-2, http://links.lww.com/SHK/B398). Table 4 reports the log-transformed values in a mixed model comparing inflammatory markers over 24 h between groups, controlling for baseline inflammatory marker level. There were statistically significantly lower levels of CRP at 12 and 24 h in the esmolol arm compared with the control arm, and there was a statistically significantly different trend over time. There were no differences between groups with regard to IL-4, IL-6, IL-10, or TNFα. Figure 4 shows the median percent change from baseline for each inflammatory marker during the study period.
TABLE 4.
Mixed model comparing inflammatory markers over 24 h between groups, controlling for baseline inflammatory marker level
| Mean difference between esmolol and control at 12 h | P at 12 h | Mean difference between esmolol and group at 24 h | P at 24 h | P for trend over time | |
|---|---|---|---|---|---|
|
| |||||
| Log IL-4 in the first 24 h | −0.02 (−0.89 to 0.84) | 0.96 | 0.39 (−0.45 to 1.24) | 0.36 | 0.85 |
| Log IL-6 in the first 24 h | 0.32 (−0.24 to 0.89) | 0.26 | 0.49 (−0.06 to 1.06) | 0.08 | 0.30 |
| Log IL-10 in the first 24 h | 0.28 (−0.36 to 0.93) | 0.39 | 0.53 (−0.11 to 1.16]) | 0.11 | 0.19 |
| Log TNFα in the first 24 h | 0.05 (−0.26 to 0.36) | 0.76 | 0.02 (−0.28 to 0.33) | 0.89 | 0.65 |
| Log CRP in the first 24 h | −0.35 (−0.07 to 0.50) | 0.01 | −0.50 (−0.77 to [−0.23]) | <0.01 | <0.01 |
FIG. 4.

Inflammatory markers by treatment group.
DISCUSSION
In this two-center trial of esmolol in septic shock, there was no difference in vasopressor requirement at 6 h in the esmolol group compared with the usual care arm. Vasopressor requirements were higher at 12 h and over the study period in patients who received esmolol, however there was no significant difference in terms of time to shock reversal, nor number of shock free days, between the groups. There was evidence of lower CRP in patients who received esmolol, however there were no other differences among other biomarkers of inflammation.
Despite advances in critical care and increased awareness of the need for early identification and aggressive treatment of sepsis, morbidity and mortality for septic shock remains high. The downstream cardiovascular effects of septic shock, beyond the shock period itself, are not trivial: myocardial depression, tachycardia-induced cardiomyopathy, and direct myocyte toxicity (2–5). Novel therapies for septic shock have repeatedly failed to demonstrate improved outcomes, and in some cases, have actually conferred harm (17–22). Beyond the use of vasopressors and inotropes to support failing hemodynamics, there is currently no specific therapy targeted at the cardiovascular effects of septic shock.
Esmolol, as a beta-antagonist used for heart rate control but not typically in the setting of shock, has been queried as a potential therapy in septic shock. In a single center trial of 10 septic shock patients treated with esmolol infusion, researchers demonstrated efficacy in terms of heart rate reduction as well as the safety of this intervention (no change in norepinephrine infusion dose, nonsignificant changes in stroke volume, oxygen delivery, VO2, or lactate) (23). In the Morelli et al. open-label Phase II trial, patients with septic shock who had a heart rate >95/min and were receiving high dose norepinephrine to maintain a mean arterial pressure (MAP) >65mmHg, were randomized to esmolol infusion or standard care. In this trial, powered to a primary outcome of reduction in heart rate, the authors found that they were able to achieve heart rate control in all patients in the esmolol group without adverse effects, and reported a 28day mortality of 49.4% in the esmolol group vs. 80.5% in the control group (hazard ratio, 0.39; 95% CI, 0.26–0.59; P<0.01). Overall, the mortality rates in these two studies were higher than typically reported in U.S. septic shock populations (generally 25%–50% in most U.S. ICUs) (24, 25) raising considerations about generalizability.
More recently, Levy et al. reported the results of the Esmosepsis study (26), which administered esmolol for 6 h to septic shock patients with the aim of a 20% reduction in heart rate and a primary outcome of change in cardiac index (CI). The investigators also monitored other hemodynamic parameters. They reported that heart rate reduction using esmolol titration was associated with an increased risk of hypotension and decreased cardiac index; this study was terminated early due to low enrollments.
In the current trial, we found that esmolol did not have a favorable impact on hemodynamic parameters, with no significant change in mean norepinephrine equivalent dose at 6 h, our primary endpoint for the study. Although vasopressor requirements were higher at 12 h and over the study period in patients in the esmolol group, there was no significant differences in time to shock reversal or number of shock free days. While our protocol did have holding and stopping parameters for esmolol, ultimately the clinical team had authority to hold or discontinue the infusion if they had concern about hemodynamics. Intravenous fluid management was at the discretion of the clinical team; there was no difference in the amount of intravascular volume received during the study period (Table 2). There was no difference in lactate between the groups.
The metabolic rate increases in sepsis as the body’s demand for oxygen increases due to fever, hypotension, tachycardia, and catecholamine surge. In contrast, the ability of cells to utilize oxygen by aerobic metabolism is often compromised, in a phenomenon referred to as cytopathic hypoxia (27). Therefore, interventions that can either support aerobic metabolism, enabling cells to utilize oxygen more effectively, or interventions to decrease oxygen demand, may be beneficial. Morelli et al. reported lower oxygen consumption (VO2) in the esmolol group vs the control group at every time point measured (P=0.001). In that study, VO2 was measured indirectly via pulmonary artery catheter measurements. Measuring total body VO2 can be achieved by use of the GE Compact Anesthesia monitor, with a gas exchange module that allows for continuous measurement of VO2. We hypothesized that esmolol infusion, by decreasing the workload of cardiac myocytes, would decrease oxygen demand and thus in part lead to lower total body oxygen consumption. While our analysis of VO2 was limited by the number of patients receiving mechanical ventilation and restricted to one site, the results do appear consistent with decreased oxygen consumption among patients treated with esmolol compared to those who were not treated with esmolol. Given the small numbers, no definitive assessments can be made about how that might translate into meaningful clinical outcomes, but further investigation is warranted.
Esmolol may have an effect on inflammation and immune response. In our trial, we found that CRP levels were reduced at 12 and 24 h in the esmolol group compared with standard care; no differences were seen in IL-4, IL-6, IL-10, or TNF-alpha levels. In an animal model of septic shock, mice were randomized to either esmolol infusion or normal saline infusion; there was significantly higher survival at 120h in the esmolol group (34.9%) vs. the saline group (11.6%), P=0.01. Additionally, the investigators found that there was increased gene expression in immunologic and apoptotic pathways which may have a role in the survival difference (8). Kim et al. (7) performed a trial of esmolol infusion in patients undergoing laparoscopic gastrectomy; patients were randomized to either saline, a clinical dose of esmolol, or a subclinical dose of esmolol. They measured levels of IL-6, IL-4, IL-10, CRP, and found that postoperative levels of IL-4 were significantly decreased in the clinical dose group compared to the saline group (2.14 vs. 21.91pg/mL, P=0.022), as well as lower CRP levels on postoperative day 1 in the esmolol-treated groups in a dose-dependent manner. In another animal study of abdominal sepsis, investigators found that mean survival time in the esmolol group was significantly longer compared with the control group, intraperitoneal fluid TNF-alpha levels were elevated in the control group but significantly depressed in the esmolol group, and the gut mucosal injury score was elevated suggesting that the esmolol infusion may have modified gut barrier function in this animal model of intra-abdominal sepsis (9). While we detected a difference in CRP levels between groups in our trial, suggesting less inflammation in the patients exposed to esmolol, we did not detect differences in terms of other inflammatory markers; the clinical relevance of these findings is uncertain.
Limitations
There are several limitations of this clinical trial which should be acknowledged. First, the trial ended early due to slow enrollments and ending of funding before the target enrollment was reached, limiting our statistical power; conclusions about the true impact of esmolol on our primary outcome measure are inherently limited. Whether the results would have been different if we had achieved our target enrollment based on our original power calculation is unknown. Many patients with septic shock were excluded because of the absence of tachycardia, or due to the presence of myocardial dysfunction, which limited our ability to enroll adequate numbers of subjects. Among those we did enroll, the relatively lower baseline heart rates in this trial compared with those in the Morelli et al trial may have limited the effectiveness of esmolol as a rate-control agent; it is possible that with more pronounced tachycardia, the benefit of slowing the heart rate and allowing improved diastolic filling would be more pronounced. In our trial, esmolol was administered for up to 24 h; patients in the esmolol arm had differing exposures to esmolol, based on variable dosing and duration due to individual patient characteristics (heart rate response) as well as clinical teams’ decision-making around holding the infusion depending on blood pressure or other clinical parameters. It is unknown whether a longer duration of exposure to esmolol would have impacts on hemodynamics, inflammatory markers, or clinical outcomes.
We did not collect echocardiographic data on these patients during the study period, which may have added valuable information to our comparisons. While we did not collect detailed measurements of intravascular volume status, as we relied on the clinical team’s assessment that the patient was adequately volume resuscitated, there were similar amounts of intravenous fluids administered both pre-enrollment and during the study period between the two groups. We did not collect patient data beyond the 24-h study period, other than clinical outcomes such as length of stay or mortality; it is unknown whether additional differences between the control and intervention cohorts would have been identified in inflammatory markers or other variables. Finally, we did not control for enrolling site in our analysis, given the small number of patients (5 of 40) enrolled at Lahey Hospital and Medical Center, however a comparison of the primary outcome for patients enrolled at BIDMC (n=35) showed similar results to those of the entire combined cohort. This finding is reassuring that there was not a meaningful difference attributable to the contribution of subjects from the LHMC site.
CONCLUSION
Among patients with septic shock, infusion of esmolol did not improve vasopressor requirements or time to shock reversal. Esmolol was associated with decreased CRP levels over 24 h.
Supplementary Material
ACKNOWLEDGMENTS
We thank Francesca Montillo for her administrative support and preparation of the manuscript. We thank Robb Kociol, MD, Marwa Sabe, MD, Peter Clardy, MD, Myles Dustin Boone, MD, and Tenzin Dechen, MPH, for their participation on the Data Safety and Monitoring Board. Finally, we thank all the research staff, pharmacy staff, and clinical staff who made this trial possible. The author group would like to specifically acknowledge our co-investigator, Dr James Dargin, MD, who passed away suddenly in October 2021; he is fondly remembered by his colleagues and collaborators.
This trial was funded by a Scientist Development Grant from the American Heart Association (15SDG22420010).
Footnotes
The authors report no conflict of interests.
Ethical approval and consent to participate: The study was conducted at Beth Israel Deaconess Medical Center (coordinating site) and Lahey Hospital and Medical Center, both tertiary care medical centers in Massachusetts, USA. The study was approved by both Institutional Review Boards, and patients or legally authorized surrogates provided written informed consent. The trial was registered at clinicaltrials.gov (NCT02369900). A Data and Safety Monitoring Board evaluated and monitored for safety.
Availability of supporting data: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.shockjournal.com).
Trial Registration: www.clinicaltrials.gov. Registered February 24, 2015, https://clinical-trials.gov/ct2/show/NCT02369900
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29(7):1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Rudiger A, Singer M: Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35(6):1599–1608, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Schmittinger CA, Dunser MW, Torgersen C, Luckner G, Lorenz I, Schmid S, Joannidis M, Moser P, Hasibeder WR, Halabi M, et al. : Histologic pathologies of the myocardium in septic shock: a prospective observational study. Shock 39(4):329–335, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ: Sepsis-induced cardiomyopathy. Curr Cardiol Rev 7(3):163–183, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunser MW, Hasibeder WR: Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med 24(5): 293–316, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, Orecchioni A, D’Egidio A, D’Ippoliti F, Raffone C, et al. : Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 310(16):1683–1691, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Hwang W, Cho ML, Her YM, Ahn S, Lee J: The effects of intraoperative esmolol administration on perioperative inflammatory responses in patients undergoing laparoscopic gastrectomy: a dose-response study. Surg Innov 22(2):177–182, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim-Zada I, Rhee P, Gomez CT, Weller J, Friese RS: Inhibition of sepsis-induced inflammatory response by beta1-adrenergic antagonists. J Trauma Acute Care Surg 76:320–327, 2014. discussion 327–8. [DOI] [PubMed] [Google Scholar]
- 9.Mori K, Morisaki H, Yajima S, Suzuki T, Ishikawa A, Nakamura N, Innami Y, Takeda J: Beta-1 blocker improves survival of septic rats through preservation of gut barrier function. Intensive Care Med 37(11):1849–1856, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz D, Wilsenack K, Lendemanns S: Schedlowski M and Oberbeck R. beta-Adrenergic blockade during systemic inflammation: impact on cellular immune functions and survival in a murine model of sepsis. Resuscitation 72(2):286–294, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, Busse LW, Altaweel L, Albertson TE, Mackey C, et al. : Angiotensin II for the treatment of vasodilatory shock. N Engl J Med 377:419–430, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. : Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Squara P, Denjean D, Estagnasie P, Brusset A, Dib JC, Dubois C: Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med 33(7):1191–1194, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson L, Dodds S, Walsh TS: Clinical evaluation of a continuous oxygen consumption monitor in mechanically ventilated patients. Anaesthesia 58(5):455–460, 2003. [DOI] [PubMed] [Google Scholar]
- 16.McLellan S, Walsh T, Burdess A, Lee A: Comparison between the Datex-Ohmeda M-COVX metabolic monitor and the Deltatrac II in mechanically ventilated patients. Intensive Care Med 28(7):870–876, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Marti-Carvajal AJ, Sola I, Gluud C, Lathyris D, Cardona AF: Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev 12:CD004388, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schedel I, Dreikhausen U, Nentwig B, Hockenschnieder M, Rauthmann D, Balikcioglu S, Coldewey R, Deicher H: Treatment of gram-negative septic shock with an immunoglobulin preparation: a prospective, randomized clinical trial. Crit Care Med 19(9):1104–1113, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, DalNogare A, Nasraway S, Berman S, Cooney R, et al. : Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet 351(9107):929–933, 1998. [PubMed] [Google Scholar]
- 20.Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R, et al. : Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA 273(12):934–941, 1995. [PubMed] [Google Scholar]
- 21.Deans KJ, Haley M, Natanson C, Eichacker PQ, Minneci PC: Novel therapies for sepsis: a review. J Trauma 58(4):867–874, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, Gerstenberger EP, Fitz Y, Danner RL, Natanson C: Risk and the efficacy of anti-inflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med 166(9):1197–1205, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Balik M, Rulisek J, Leden P, Zakharchenko M, Otahal M, Bartakova H, KorinekJ: Concomitant use of beta-1 adrenoreceptor blocker and norepinephrine in patients with septic shock. Wiener klinische Wochenschrift 124(15–16):552–556, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Linde-Zwirble WT, Angus DC: Severe sepsis epidemiology: sampling, selection, and society. Crit Care 8(4):222–226, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin GS, Mannino DM, Eaton S, Moss M: The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Levy B, Fritz C, Piona C, Duarte K, Morelli A, Guerci P, Kimmoun A, Girerd N: Hemodynamic and anti-inflammatory effects of early esmolol use in hyperkinetic septic shock: a pilot study. Crit Care 25(1):21, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink MP: Bench-to-bedside review: cytopathic hypoxia. Crit Care 6(6):491–499, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


