Abstract
Bone graft in trauma surgery is commonly used in managing bone defects, non-union, fracture related infections, arthrodesis or to provide structural support in fractures. A variety of bone grafts are made available to the treating physician, which includes autograft, allograft and bone graft substitutes. The future of bone grafting in trauma surgery is exciting with the incorporation of technological advancement such as gene therapy, 3D-printing and tissue engineering. Regardless, there are still limitations to what we understand regarding current bone grafting techniques with conflicting literature on their clinical utility and indication. The aim of this review article therefore is to take a step back and critically evaluate the current concepts of bone grafting in trauma surgery, with special emphasis made on reviewing the types of bone graft, biology of bone graft incorporation and indication for its use in various clinical scenarios.
Keywords: Bone graft, Autograft, Allograft, Bone graft substitutes, Trauma surgery
1. Introduction
Bone grafting is used in trauma surgery to manage bone defects, non-union, fracture-related infections (FRI), arthrodesis and to provide structural support in fractures.1 The field of bone grafting has expanded in terms of clinical utility and types of bone graft and bone graft substitutes (BGS) used. Studies evaluating tissue engineering, 3D-printing and gene therapy are also underway to enhance bone graft use.2,3 Nonetheless, there is limitation to what we understand and can achieve with present bone grafting techniques. This review article aims to critically evaluate the current concepts of bone grafting in trauma surgery by reviewing the types of bone graft, biology of bone graft incorporation, and their clinical indication.
2. Types of bone grafts
2.1. Autografts
Autologous grafts confer the lowest immunological rejection risk with high osteogenic, osteoinductive and osteoconductive properties, and remain the gold standard for managing bone defects.4 Cancellous autografts may be harvested from the iliac crest, femur, proximal tibia, calcaneum, olecranon and distal radius. It is useful in filling bone defects and provides a scaffold for new bone formation, containing osteogenic cells, mesenchymal stem cells (MSCs), bone morphogenic proteins (BMPs) and other growth factors (GFs).1,5 However, it is unable to provide structural support (Fig. 1).
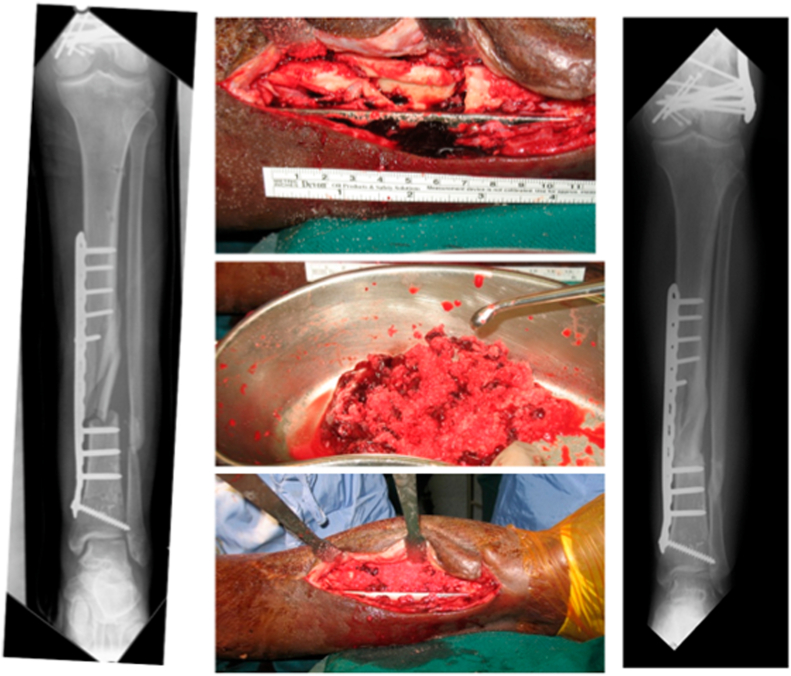
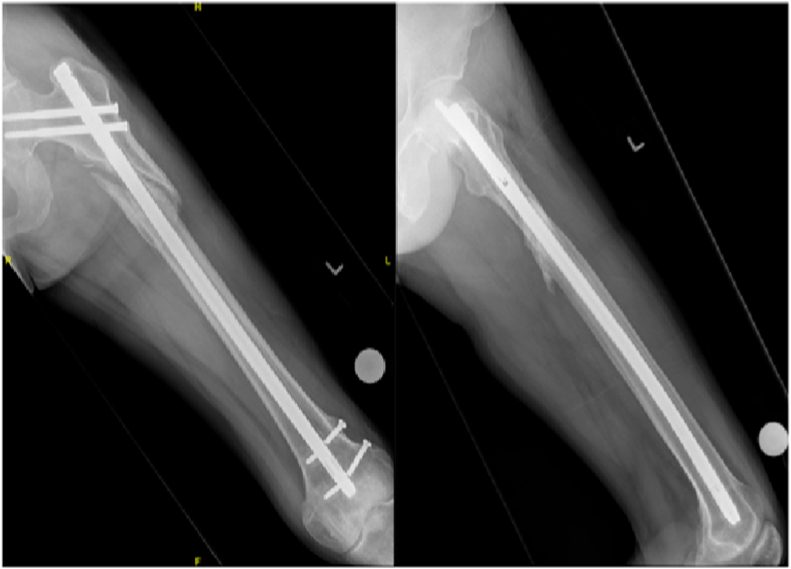
Fig. 1.
Management of tibial shaft non-union using iliac crest bone graft, demonstrating consolidation at 1 year post-operatively.
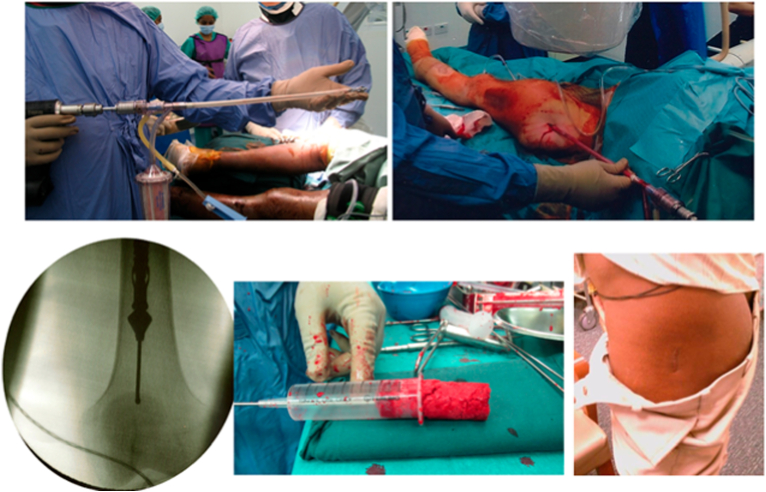
The reamer-irrigator-aspirator (RIA) technique allows one to obtain greater volume of cancellous graft compared to anterior iliac crest bone graft (ICBG) harvesting (Fig. 2). It has reduced risks of post-operative donor site pain and infection.6 The reamer is a single pass device, while the irrigator and aspirator reduce heat generation, intramedullary pressure and fat embolism rates.1 The graft has equivalent union rates as compared to ICBG use. The aforementioned advantages make it an attractive option for dealing with large bone defects. The risks of this technique include blood loss, fractures and pulmonary embolism, although the overall prevalence is low.6
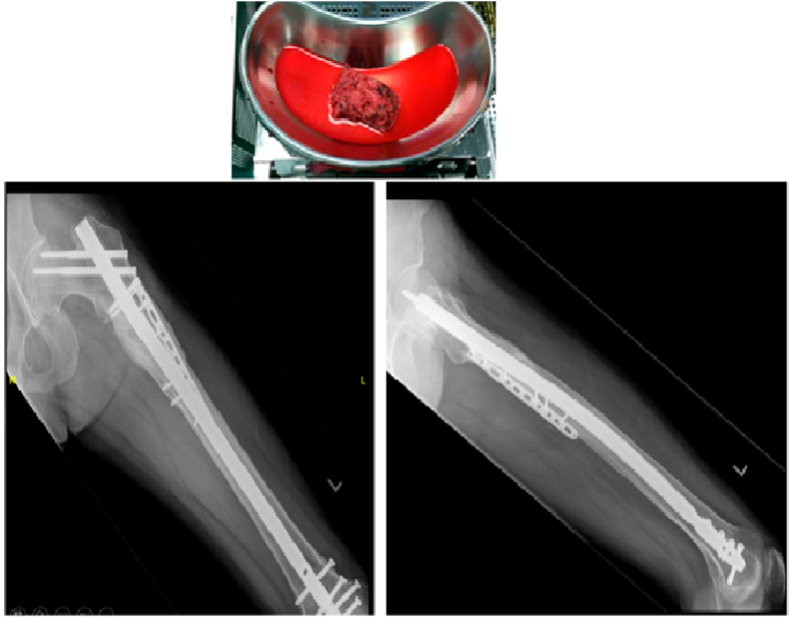
Fig. 2.
Cancellous autograft harvested from the left femur using the Reamer-Irrigator-Aspirator system.
Cortical autografts provide excellent mechanical stability and may be harvested with or without their vascular supply.1,4 Non-vascularized grafts may be obtained from the iliac crest, fibula or distal radius, whereas vascularized grafts may be harvested from the iliac crest, fibula, distal radius or ribs (Fig. 3). Non-vascularized cortical autografts are useful when dealing with bone defects measuring more than 6 cm, but for those greater than 12 cm, vascularized cortical grafts are recommended, demonstrating lower failure rates.1 Donor site morbidity such as pain, infection, sensory deficits and fractures are issues with autologous bone graft harvesting.4 There is also a limited volume of cancellous autograft that can be obtained at each site (Table 1).
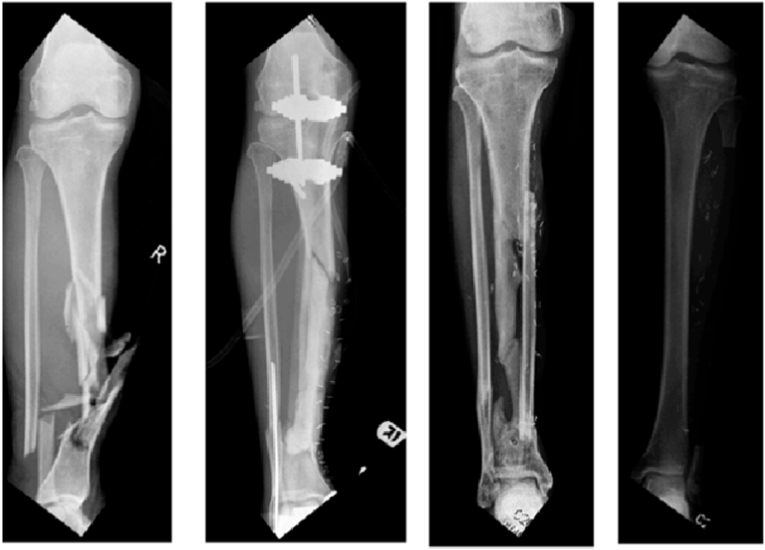
Fig. 3.
Vascularized fibula autograft harvested from the contralateral leg to manage tibial bone loss in a patient presenting with open fracture of the right tibia initially managed with debridement, external fixation and cement spacer.
Table 1.
Average volume of cancellous autograft obtained from different anatomical sites.
| Site | Average volume (cm3) |
|---|---|
| Anterior iliac crest bone graft | 13 |
| Posterior iliac crest bone graft | 30 |
| Reamer-Irrigator-Aspirator system | 30–90 |
| Proximal tibia | 25 |
| Distal radius | 3 |
| Distal tibia | 3 |
| Greater trochanter | 5–10 |
2.2. Bone marrow aspirate concentrate
Bone marrow aspirate concentrate (BMAC) demonstrates osteogenic and osteoinductive properties, containing GFs, MSCs and cytokines.7 BMAC can be obtained from aspirating the posterior ilium.1 The aspirate undergoes centrifugation, leaving a concentrate of stem cells and GFs.8 Hernigou et al. evaluated the outcome of injecting 20 cm3 of BMAC obtained from the iliac crest in tibial non-union, demonstrating close to 90% union rate at 4 months post-injection.9 The modified Hernigou technique was subsequently described, where BMAC was mixed with either demineralized bone matrix (DBM) or BMP-2. Desai et al. utilised this technique to manage tibial non-union, demonstrating 80% union rates at 5 months post-intervention.8 Risks of BMAC are divided to harvesting risks (sciatic nerve or gluteal vessel injury, infection, chronic pain) and administration risks (infection, fat embolism).7,10
2.3. Allograft
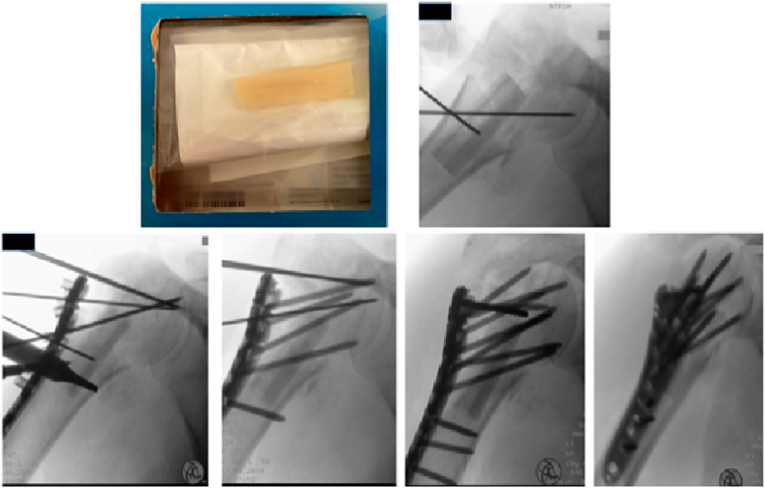
Allografts can be harvested from the pelvis, ribs, fibula or femur and may be structural or particulate in nature.1 Cortical strut allograft is used in proximal humerus, tibial plateau and periprosthetic fractures11, 12, 13, 14 (Fig. 4a, Fig. 4b, Fig. 5). They provide structural stability, aid in fracture reduction and support poor host bone stock. However, there is no Level 1 evidence demonstrating its superiority over locking plates (LP) in the management of these fractures.11,15 Particulate allografts have been used to fill metaphyseal defects and manage non-unions, but again, the evidence for their use is limited.16
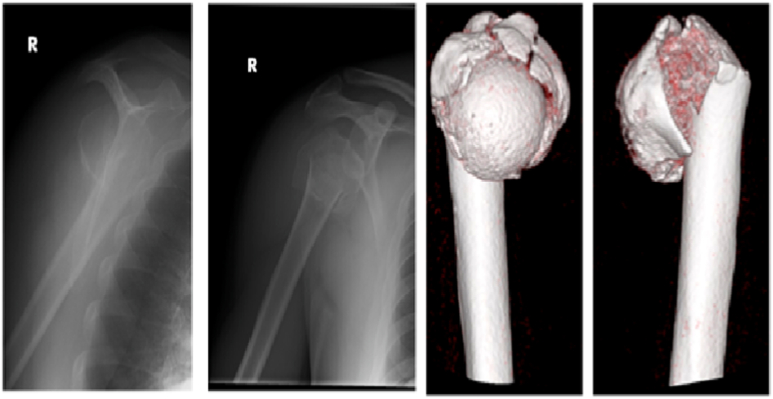
Fig. 4a.
Patient presenting with an extra-articular, comminuted, proximal humerus fracture.
Fig. 4b.
Patient undergoing surgical fixation of right proximal humerus fracture with a locking plate construct supplemented with a fibular cortical strut graft aiding with reduction of varus deformity.
Fig. 5.
Patient presenting with a right periprosthetic femoral fracture with previous cemented unipolar hemiarthroplasty, treated with surgical fixation using a locking plate construct supplemented with strut allograft.
The use of allografts is nonetheless appealing, avoiding risks of donor site morbidity and limited graft volume. The risk of infection and disease transmission is present but low with strict protocols in terms of patient selection and sterilization methods.17 Other risks include inflammatory and antigen-dependent immunological reactions.
2.4. Demineralized bone matrix
DBM is produced through acid extraction of allograft bone, containing collagen, proteins and GFs but lacks mechanical stability.1,18 Its osteoinductive properties are dependent on the amount of BMP-2 and BMP-7. There is a low risk of disease transmission with DBM use. Uncommon adverse effects in animal studies include allergic reactions from antibiotics used during the disinfection process and immunological reaction.19
There are only a few studies evaluating DBM use in managing metaphyseal or long bone defects and non-union.20 These studies, however, are of low quality, with systematic reviews not recommending it's routine use in trauma surgery for now.21 Cost is another barrier to utilizing allografts and DBM, where the average cost ranges from US $8.7 to US $55.86 per cm3 and US $726 to US $1225 per cm3 respectively.20
2.5. Synthetic bone graft substitutes
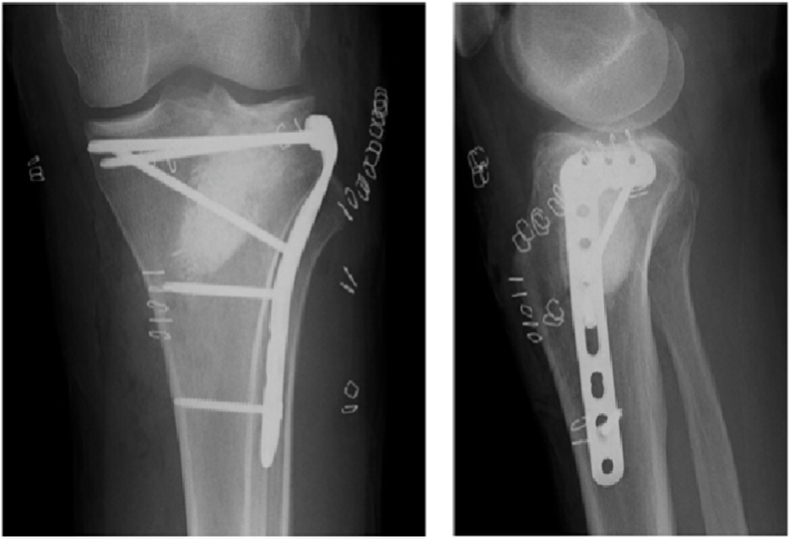
Various commercially available BGS such as mineral ceramics, BMPs and bioactive glass are available. These demonstrate osteoconductive and osteoinductive properties.22 Examples of mineral ceramics include calcium sulfate, hydroxyapatite and tricalcium phosphate (TCP). TCP provides structural stability and is usually mixed with hydroxyapatite as it is less porous.5,23 TCP use is contraindicated in active infection, areas of poor vascularity or regions of bone subject to major compressive forces such as that in the femur.22,24 Calcium sulfate may be used as a block, pellets or injectable for metaphyseal or subchondral bone defects, gradually being absorbed and remodels forming new bone22 (Fig. 6).
Fig. 6.
Use of an injectable calcium sulfate cement to fill metaphyseal bone defect in a depressed lateral tibial plateau fracture.
BMP is extracted from allograft bone and is part of the transforming GF superfamily involved in osteogenesis.1 Molecular cloning technology allows for generating large quantities of recombinant human BMPs (rhBMP). Currently, only rhBMP-2 and rhBMP-7 are approved by the US Food and Drug Administration for intramedullary nailing (IMN) of open tibial shaft fractures within 14 days of injury, and tibia shaft non-union and traumatic bone defect respectively.25 The cost-effectiveness of BMP-2 use is debatable, with some demonstrating savings close to USD $3000 when evaluating time to fracture healing, revision and infection rates in the management of Gustilo IIIB tibial fractures.26 Contraindications to rhBMP use include pediatric patients, pregnant or those planning to be pregnant within a year, and cancer patients.1 Risks include seroma and ectopic bone formation, wound dehiscence, surgical site infection and renal/hepatic failure.27
Bioactive glass is an amorphous structure consisting of calcium, phosphorus and silicon. It is biodegradable, osteoinductive and osteoconductive, and is able to induce the formation of hydroxyapatite by releasing calcium ions.28 It is useful in filling bone voids in infection as it has inherent antimicrobial properties against common organisms causing osteomyelitis. It also has angiogenic properties and resorbs with time.29
3. Biology of bone graft incorporation
Successful factors for bone healing were encapsulated by Giannoudis PV et al. as the “diamond concept” which is broken down into mechanical stability, vascularity, osteogenesis, osteoconduction, osteoinduction and host factors5,30 (Table 2). An ideal bone graft should possess the above properties, while also being biocompatible with no risk of disease transmission.31,32 Table 3 demonstrates the different types of bone grafts and the extent of these properties. Once the above factors are met, graft incorporation may then take place (Table 4).
Table 2.
Definition of bone graft properties.
| Property | Definition |
|---|---|
| Osteogenesis | Generation of new bone by cells derived from either the graft or hosta |
| Osteoinductive | Ability of a graft to provide a scaffold for bone growth to occur on its surface, pores or channelsb |
| Osteoconductive | Ability to recruit mesenchymal stem cells and stimulating them to differentiate into chondroblasts and osteoblastsc |
Table 3.
Properties and cost of different types of bone grafts and bone graft substitutes.
| Graft | Mechanical Properties | Vascularity | Osteogenesis | Osteoconduction | Osteoinduction | Cost (USD $)31,32 |
|---|---|---|---|---|---|---|
| Autograft | ||||||
| 1. Cortical | ✓✓ | ✕ | ✓ | ✓ | ✓ | |
| 2. Cancellous | ✓ | ✕ | ✓✓ | ✓✓ | ✓ | |
| 3. Vascularized | ✓✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | |
| 4. Bone Marrow | ✕ | ✕ | ✓✓ | ✓ | ✓ | |
| Allograft | ||||||
| 1. Cortical | ✓✓ | ✕ | ✕ | ✓ | ✓ | 530-1681/3–20 cm |
| 2. Cancellous | ✓ | ✕ | ✕ | ✓✓ | ✓ | 376/30 cc |
| 3. Demineralized | ✕ | ✕ | ✕ | ✓✓ | ✓✓✓ | 726-1225/10 ml |
| Bone Graft Substitutes | ||||||
| 1. Calcium phosphate | ✓∗ | ✕ | ✕ | ✓✓ | ✕ | 1520/10 ml |
| 2. Calcium sulfate | ✕ | ✕ | ✕ | ✓✓ | ✕ | 655/10 ml |
| 3. Tricalcium phosphate | ✓✓ | ✕ | ✕ | ✓ | ✕ | 875/10 ml |
| 4. Bone morphogenic proteins | ✕ | ✕ | ✕ | ✕ | ✓✓ | 3500–5000 |
✕, ✓, ✓✓, ✓✓✓: extent of activity; ✕: absent activity, ✓✓✓: greatest activity∗: for tri-calcium phosphate.
Table 4.
Stages of bone graft incorporation.
| Stage | Events | ||
|---|---|---|---|
| Inflammation & Revascularization | Week 1: Haematoma formation with presence of lymphocytes, plasma cells, osteoclasts, mononuclear and polynuclear cells | ||
| Week 2: Increased osteoclastic activity with formation of fibrous granulation tissue. Macrophages remove necrotic debris and release intra-cellular substances that attracts mesenchymal stem cells (MSCs) | |||
| Revascularization allows for MSCs from donor and recipient to reach the marrow spaces | |||
| Greater inflammatory response in allograft incorporation | |||
| Osteogenesis | MSCs differentiate into bone forming cells in the marrow spaces | ||
| Osteoinduction | Both cortical and cancellous autologous bone graft allows for bone growth to occur on its surface, pores or channels | ||
| Osteoconduction | Cortical Grafts | Cancellous Graft | Allografts |
| Initial resorbed by osteoclasts before new osteoid are laid down by osteoblasts (“cutting cones”), thus leading to initial reduction in mechanical strength | Creeping substitution occurs where new bone is laid down by osteoblasts on a necrotic bed that is simultaneously resorbed by osteoclasts. This leads to an initial increase in mechanical strength | Intra-membranous and endochondral ossification occurs on the surface, forming a bridging external callus. Creeping substitution of cortical bone occurs. |
|
| Remodelling | Incomplete incorporation with no remodelling phase | Occurs along lines of force with complete incorporation of bone graft | Fusion occurs only at the bone-graft interface with deeper layers of the grafted bone containing dead trabeculae |
To increase the success of bone graft incorporation, studies are evaluating the effect of the immune system and endogenous GFs.33 Processes involved in regulating bone formation and resorption can be divided into systemic regulation and local regulation by GFs and immunomodulatory cytokines e.g., interleukin-1 and interleukin-6 (Table 5).34 These factors may be isolated via recombinant technology and utilised for their osteoinductive and osteoconductive properties to promote bone healing. rhBMP-2 and rhBMP-7 in particular have been widely studied in the management of upper and lower limb non-union.35 There is however no evidence that BMP use alone is more or less effective than bone graft in such cases, with its use reserved for treatment failures with bone graft.36
Table 5.
Systemic and local regulation pathways and their effect on bone.
| Bone Resorption | Bone Formation | |
|---|---|---|
| Calcium-Regulating Hormones | ||
| 1. Parathyroid hormone | ↑ | ↓ |
| 2. 1,25-dihydroxyvitamin D | ↑ | ↓ |
| 3. Calcitonin | ↓ | – |
| Systemic Hormones | ||
| 1. Glucocorticoids | ↓ | ↓ |
| 2. Insulin | – | ↑ |
| 3. Thyroxine | ↑ | ↑ |
| 4. Sex hormones | ↓ | ↓↑a |
| 5. Growth hormones | – | ↑ |
| Growth Factors | ||
| 1. Insulin-like growth factors | – | ↑ |
| 2. Fibroblast growth factor | ↑ | ↓ |
| 3. Platelet-derived growth factor | ↑ | ↑ |
| 4. Transforming growth factor-β | ↑↓ | ↑↓ |
| Local Factors | ||
| 1. Prostaglandin E | ↑ | ↑↓b |
| 2. Interleukin-1 | ↑ | ↑↓b |
| 3. Tumour necrosis factor | ↑ | ↑↓ |
| 4. Bone morphogenic protein | – | ↑↓ |
Effects dependent on age and concentration.
Effects dependent on presence and concentration of glucocorticoids.
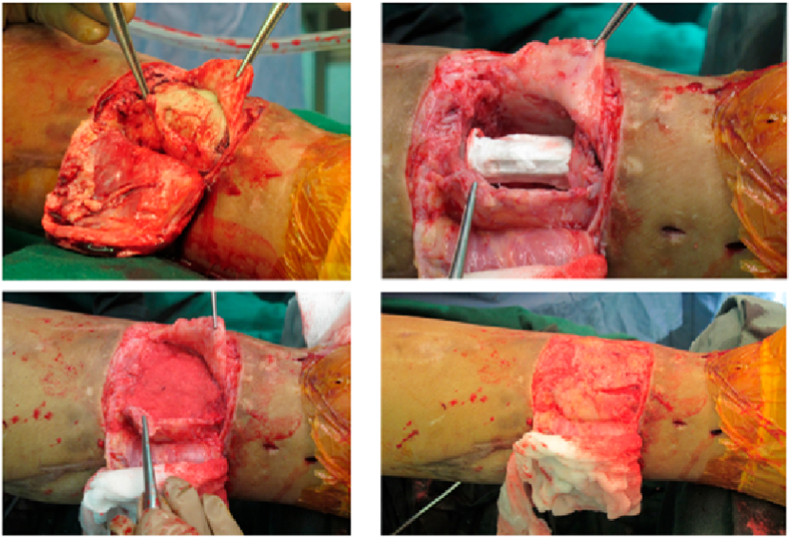
BMAC is used in managing delayed or non-union, bone defects and distraction osteogenesis.7 The exact mechanism of action is unclear but is believed to provide a direct source of GFs and cytokines to promote bone healing.37 The Masquelet technique is also useful as the induced membrane secretes GFs, vascular and osteoconductive factors and provides physical containment for subsequent bone grafting (Fig. 7). It has been shown to be more cost-effective in the management of tibial bone defects when compared to distraction osteogenesis techniques.38,39 It is imperative however to not only understand the factors that promote bone graft incorporation, but also those that impair it (Table 6).
Fig. 7.
Use of the Masquelet technique in the management of tibial bone defect.
Table 6.
Factors that affect bone graft incorporation.
| Factors | Promotes | Impairs |
|---|---|---|
| 1. Local | Vascularity | Infection |
| Mechanical Stability | Radiation | |
| Growth factors | Tumour | |
| Innervation | ||
| 2. Systemic | Growth hormones | Steroids |
| Parathyroid hormones | Non-steroidal anti-inflammatory drugs | |
| Insulin-like growth factors | Smoking | |
| Somatomedins | Malnutrition | |
| Thyroid hormones | Diabetes | |
| Vitamins A & D | Chemotherapy | |
| Metabolic bone disease |
4. Indications for bone graft use in trauma
4.1. Atypical femoral fractures
Atypical femoral fractures (AFF) are at increased risk of delayed or non-union, leading to implant failure requiring re-operations.40 AFF are considered pathological due to chronic osteoclast inhibition leading to defective bone remodelling. These fractures are managed with IMN or plate osteosynthesis. With regards to plating, there is no consensus on whether an absolute or relative stability construct is better. Some utilise compression plating with angled blade plates, while others perform bridge plating with a medial strut allograft to promote callus formation at the fracture site.41
There is little evidence for using bone graft or BGS to enhance healing potential at the index surgery.41 Theoretically, the use of autologous bone graft or BMAC may promote healing by introducing osteogenic precursors at the fracture site. It is however unclear if the systemic suppression of osteoclasts has a negative effect on all autologous bone. There are also contrasting studies regarding the population of osteoblast and osteoclasts, and healing potential in iliac crest biopsies of AFF patients.42
Kulachote et al. in his case-control study utilised DBM together with IMN and found that nine patients treated with both healed at an average of 28.1 weeks, whereas those managed with IMN alone healed at an average of 57.9 weeks (p = 0.04).43 Use of BMP in AFF has not been evaluated. Despite its positive effect on osteoblast differentiation, it also upregulates osteoclast activity as part of the bone remodelling process. Robust studies are required to determine if such fractures require primary augmentation.
The use of bone graft and/or BGS to manage non-union of surgically treated AFF is more common. Nagy MT et al. managed 10 patients with failed IMN using compression plating and autologous iliac crest bone graft, achieving union in all patients at a mean of 16 months.44 A case report of revision IMN with autologous iliac crest bone graft and BMP was described by Lu J et al., achieving union at 9 months.45 The use of bone graft augmentation for non-union in AFF is further supported by Shin WC et al., who found that patients who underwent revision exchange IMN alone were at higher risk of treatment failure compared to those with both IMN and iliac crest bone grafting.46 The authors thus recommended open reduction and autogenous bone grafting for AFF patients with non-union.
4.2. Subtrochanteric fractures
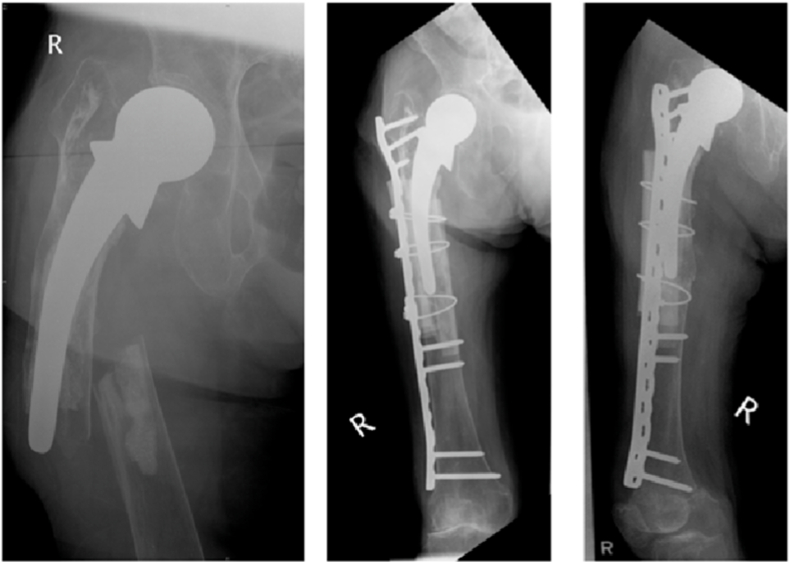
Subtrochanteric fractures are at risk of non-union due to increased stress loading at the fracture site, with shear and bending forces acting at the cancellous and cortical bone respectively47 (Fig. 8a, Fig. 8b). Risk factors for non-union in surgically managed subtrochanteric fractures include varus malreduction, residual displacement after reduction and lack of medial cortical support.48 Malkawi H in 1982 first described the use of plate fixation and autologous cancellous bone graft in subtrochanteric fractures with medial cortex comminution.49
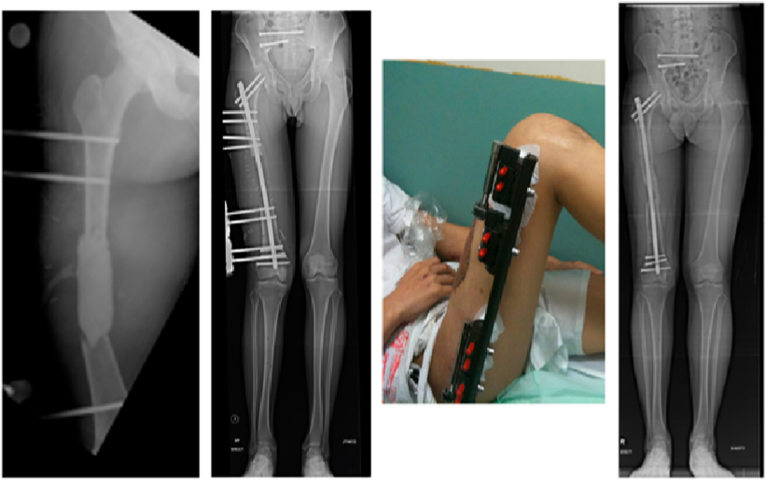
Fig. 8a.
Non-union of a left subtrochanteric fracture previously managed with an intra-medullary nail.
Fig. 8b.
Successful union after treatment with bone graft obtained using the Reamer-Irrigator-Aspirator system and supplemental plating.
The use of bone graft in subtrochanteric fractures presently is described for non-union cases as IMN for primary fixation is preferred with preservation of biology at the fracture site. However, recent literature has questioned if bone grafting is even required for non-union cases. Shin WC et al. found that exchange nailing with emphasis on proper reduction can successfully treat non-union in subtrochanteric fractures.46 Interestingly, Balasubramanian N et al. in their prospective study of 13 patients with subtrochanteric fractures non-union managed with an anatomical proximal femur locked compression plate, found that 2 patients treated with primary bone grafting had delayed union, presumably due to disruption of biology at fracture site.50 Clinicians should thus focus on proper reduction and preserving biology at the fracture site rather than depending on bone grafting, even for non-union cases.
4.3. Tibial plateau fractures
Comparative studies have been performed evaluating the use of bone graft and/or BGS in the management of depressed tibial plateau fractures.51,52 A study by Hoffmann et al. compared autologous ICBG with biphasic hydroxyapatite and calcium sulfate cement (cerament bone void filler (CBVF)) in the management of depressed and split-depressed tibial plateau fractures.51 They found no difference in terms of fracture healing rates, defect remodelling or degree of articular subsidence between the two groups. There was however a significant reduction of blood loss and pain levels at post-operative day 1 in the CBVF group.
A prospective, randomized controlled trial (RCT) with 11-year clinical follow-up conducted by Pernaa K et al. also found no difference in the degree of articular surface depression or deviation of mechanical axes when either bioactive glass S53P4 or autologous ICBG was used.53 Another RCT comparing bioactive glass S53P4 and autologous ICBG by Heikkilä JT et al. also found no difference in patient reported outcome measures (PROMS) or degree of articular depression.54
Russell TA et al. in their RCT comparing autogenous ICBG against calcium phosphate cement however found a statistically significant difference in terms of articular subsidence levels favoring the cement group.52 However, there was no PROMS data with incomplete clinical evaluation in their multicenter study. The use of porous titanium granules was also compared against autologous ICBG by Jónsson BY et al. who, despite identifying a significant difference in terms of articular subsidence rate favoring granules use, found no difference in functional outcome at 1 year post-operatively.55 Most of these studies justified the use of BGS to avoid donor site morbidity associated with autologous ICBG harvesting.
Although trials comparing use of bone graft/BGS against no bone graft are lacking, a most recent study by Hartwich M et al. seems to suggest that there is no functional outcome difference between the two.56 Further studies are required to determine if bone graft/BGS are required for depressed tibial plateau fractures.
4.4. Tibial pilon fracture
There is a dearth of literature regarding the use of bone grafts in pilon fractures. A case report by Kim WY et al. utilised a periarticular distal tibia LP and fibula strut graft to provide stability in bone defect >7 cm.57 Other smaller studies looked at using autologous osteochondral grafts from the non-weightbearing portion of the femoral condyle to manage comminuted pilon fractures or post-traumatic ankle arthritis, demonstrating positive PROMS.58,59
4.5. Proximal humerus fractures
Biomechanical studies have demonstrated significantly improved maximum load to failure and initial construct stiffness in LP construct with intramedullary fibular strut graft compared to LP use alone for proximal humerus fractures.60 This has been translated to clinical practice, with better clinical and radiological outcome seen in elderly patients managed with LP and fibular strut graft.12 Contrasting results however were demonstrated by Wang Q et al. in their RCT comparing use of fibular strut allograft for medial column comminuted proximal humerus fractures.11 Even though they found no statistical difference between the two groups in terms of functional and radiological outcome scores, their protocol allowed surgeons to use cancellous bone grafts in the LP group which might confer some stability. Also, even though subgroup analysis did not demonstrate clinical benefit in the young or elderly, this was underpowered.11
Autologous ICBG has also been used in comminuted proximal humerus fractures, demonstrating improved range of motion, functional and radiological outcome measures.61,62 There is however to date no comparative study between autologous ICBG and fibular cortical strut graft.
4.6. Fractures with non-union/avascular necrosis risk
Talar neck, scaphoid, proximal humerus and femoral neck fractures are at risk non-union and avascular necrosis (AVN). Bone grafts have been used primarily or secondarily to prevent or manage established delayed or non-union. Results, however, have not always been encouraging. McMurtie JT et al. attempted to use autologous tibial bone graft for talar neck fractures with “substantial bone defect” defined as a gap of more than 5 mm in the sagittal plane and greater than 1/3 of the talar neck's width in the coronal plane.63 Despite achieving excellent union rates, most continued to develop AVN and post-traumatic arthritis of the ankle and subtalar joint with poor PROMS. Tang H et al. utilised a vascularized cuboid pedicle bone graft for the management of Hawkin's II talar neck fracture combined with both internal and external fixation. No patients developed AVN at 8 weeks post-operatively with good functional outcome. However, the number of patients was small with no long-term follow-up evaluation.64
No studies have evaluated primary bone grafting in scaphoid fractures, but plenty have looked at bone graft use in scaphoid non-union with AVN.65 There is controversy about the type of bone graft required, although most support the use of non-vascularized over vascularized bone graft in such cases.65 In cases of non-union without AVN, again there is divide as to whether bone grafting is actually required. A systematic review by Elgayar L et al. concluded that for stable, well-aligned scaphoid fractures complicated by non-union, rigid screw fixation achieving compression alone is sufficient.66 Some authors have also managed scaphoid non-union using BMP together with bone graft and screw and/or wires. In a review article by Polmear MM et al., 90.5% of patients achieved union, although heterotopic ossification was a common complication.67
Use of fibula strut grafts have been described for managing fresh femoral neck fractures, particularly those with posterior comminution.68,69 However, an RCT by Kumar S et al. comparing cancellous screw fixation with and without fibular strut graft in young adults found no statistical difference in terms of time to weight-bearing and union rates.70 Yin J et al. evaluated patients with an average age of 35.6 years who developed femoral neck non-union after their index surgery.71 Revision surgery with use of vascularized fibular graft and locking plates or cannulated screws achieved 77% success rate. Others have also used muscle-pedicle bone grafting with tensor fascia lata.72 These procedures, however, require specialized expertise which might not be available in every institution.
4.7. Critical bone defects, open fractures and fracture-related infections
Currently, there is no agreed definition of a “critical bone defect”. Examples include a bone defect which is not expected to heal without a secondary surgical intervention, or bone loss involving greater than 2 times the diameter of the long bone diaphysis.73 The Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures (SPRINT) study described it as a defect involving 50% of the cortical diameter with a minimum length of 1 cm74. Haines et al. introduced the concept of average radiographic apparent bone gap (RABG) which measures bone loss across 4 cortices and found that a RABG of <25 mm had a union rate of 54% whereas that which is > 25 mm had a union rate of 0%.75
Treatment of bone defects associated with open fractures is uncommon. In a ten-year prospective audit of patients admitted to the Edinburgh Orthopaedic Unit, 0.4% of all fractures were associated with bone loss. Of this, bone loss was observed in only 11.4% of all open fractures, most being Gustilo IIB or IIIC tibial fractures.76 This was echoed in the SPRINT study which looked at the surgical treatment of open tibial fractures. Only 3% of their population had a “critical” bone defect as defined above, with 47% of these patients achieving union without any secondary procedures. Degree of bone defect alone does not determine union rates, with anatomical, biomechanical and biological factors playing a role as well.74,77 Surgical options at the index surgery include the Masquelet technique or acute limb shortening with or without subsequent distraction osteogenesis (Fig. 9). Bone grafts are typically used in secondary procedures. However, there is data demonstrating successful management of open fractures of the hand with internal fixation and bone graft at the primary setting.78 Early bone grafting in these injuries allowed for faster union rates and lesser soft tissue contracture, leading to better hand function with low infection rates.78,79
Fig. 9.
Successful treatment of a previous open right femoral shaft fracture with distraction osteogenesis technique over a cephalomedullary nail.
The management of critical bone defects in FRI is highly variable and when evaluated in systematic reviews, demonstrates significant heterogeneity. Common options include use of autologous bone graft and/or BGS for defects measuring up to 2 cm, or distraction osteogenesis and Masquelet technique for defects measuring more than 5 cm, with some reporting success in defects measuring 20–30 cm even.80, 81, 82, 83 There is no one superior technique over the other, and treatment has to be tailored to host factors as well as local soft tissue and defect factors.84
5. Conclusion
The future of bone grafting in Trauma appears promising, with studies evaluating use of GFs and MSCs to augment bony healing, nanotechnology, bio-scaffolds and 3D-printed bone models infused with osteogenic cells. There is nonetheless much to learn about existing techniques and to optimise what we currently understand to maximize clinical outcomes.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author contribution
Nazrul NASHI; performed literature review, wrote the paper.
Fareed HY, KAGDA; contributed figures, reviewed the paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Baldwin P., Li D.J., Auston D.A., Mir H.S., Yoon R.S., Koval K.J. Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J Orthop Trauma. 2019;33(4):203–213. doi: 10.1097/BOT.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 2.Meinel L., Karageorgiou V., Fajardo R., et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112–122. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi R.T., Yang S., Rutherford R.B., Krebsbach P.H., Zhao M., Wang D. Gene therapy approaches for bone regeneration. Cells Tissues Organs. 2004;176:95–108. doi: 10.1159/000075031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt A.H. Autologous bone graft: is it still the gold standard? Injury. 2021;52(Suppl 2):S18–S22. doi: 10.1016/j.injury.2021.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Sohn H.S., Oh J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res. 2019;23(4):9. doi: 10.1186/s40824-019-0157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox G., Jones E., McGonagle D., Giannoudis P.V. Reamer-irrigator-aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951–956. doi: 10.1007/s00264-010-1189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imam M.A., Holton J., Ernstbrunner L., et al. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int Orthop. 2017;41(11):2213–2220. doi: 10.1007/s00264-017-3597-9. [DOI] [PubMed] [Google Scholar]
- 8.Desai P., Hasan S.M., Zambrana L., et al. Bone mesenchymal stem cells with growth factors successfully treat nonunions and delayed unions. HSS J. 2015;11(2):104–111. doi: 10.1007/s11420-015-9432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernigou P., Poignard A., Beaujean F., Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 10.Hernigou J., Picard L., Alves A., Silvera J., Homma Y., Hernigou P. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop. 2014;38(11):2377–2384. doi: 10.1007/s00264-014-2343-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q., Sheng N., Huang J.T., et al. Effect of fibular allograft augmentation in medial column comminuted proximal humeral fractures: a randomized controlled trial. J Bone Joint Surg Am. 2023;105(4):302–311. doi: 10.2106/JBJS.22.00746. [DOI] [PubMed] [Google Scholar]
- 12.Dasari S.P., Kerzner B., Fortier L.M., et al. Improved outcomes for proximal humerus fracture open reduction internal fixation augmented with a fibular allograft in elderly patients: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2022;31(4):884–894. doi: 10.1016/j.jse.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Berkes M.B., Little M.T., Schottel P.C., et al. Outcomes of Schatzker II tibial plateau fracture open reduction internal fixation using structural bone allograft. J Orthop Trauma. 2014;28:97–102. doi: 10.1097/BOT.0b013e31829aaee1. [DOI] [PubMed] [Google Scholar]
- 14.Haddad F.S., Duncan C.P., Berry D.J., Lewallen D.G., Gross A.E., Chandler H.P. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A:945–950. [PubMed] [Google Scholar]
- 15.Dehghan N., McKee M.D., Nauth A., Ristevski B., Schemitsch E.H. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28:721–727. doi: 10.1097/BOT.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 16.Flierl M.A., Smith W.R., Mauffrey C., et al. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: a retrospective cohort study in 182 patients. J Orthop Surg Res. 2013;8:33. doi: 10.1186/1749-799X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archunan M.W., Petronis S. Bone grafts in trauma and orthopaedics. Cureus. 2021;13(9) doi: 10.7759/cureus.17705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson B., Whang P.G., Iglesias R., Wang J.C., Lieberman J.R. Osteoinductivity of commercially available demineralized bone matrix. Preparations in a spine fusion model. J Bone Joint Surg Am. 2004;86-A:2243–2250. doi: 10.2106/00004623-200410000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Dinopoulos H.T.H., Giannoudis P. Safety and efficacy of use of demineralised bone matrix in orthopaedic and trauma surgery. Expet Opin Drug Saf. 2006;5:847–866. doi: 10.1517/14740338.5.6.847. [DOI] [PubMed] [Google Scholar]
- 20.Brink O. The choice between allograft or demineralized bone matrix is not unambiguous in trauma surgery. Injury. 2020;202(52 Suppl 2):S23–S28. doi: 10.1016/j.injury.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 21.van der Stok J., Hartholt K.A., Schoenmakers D.A.L., Arts J.J.C. The available evidence on demineralised bone matrix in trauma and orthopaedic surgery: a systematic review. Bone Joint Res. 2017;6(7):423–432. doi: 10.1302/2046-3758.67.BJR-2017-0027.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobb D.C., DeGeorge B.R., Jr., Chhabra A.B. Bone graft substitutes: current concepts and future expectations. J Hand Surg Am. 2019;44(6):497–505.e2. doi: 10.1016/j.jhsa.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 23.Moore D., Chapman M., Manske D. The evaluation of a biphasic calcium phosphate ceramic for use in grafting long-bone diaphyseal defect. J Orthop Res. 1987;5:356–365. doi: 10.1002/jor.1100050307. [DOI] [PubMed] [Google Scholar]
- 24.Beuerlein M.J., McKee M.D. Calcium sulfates: what is the evidence? J Orthop Trauma. 2010;24(suppl 1):S46–S51. doi: 10.1097/BOT.0b013e3181cec48e. [DOI] [PubMed] [Google Scholar]
- 25.Govender S., Csimma C., Genant H.K., et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Hagen A., Gorenoi V., Schönermark M.P. Bone graft substitutes for the treatment of traumatic fractures of the extremities. GMS Health Technol Assess. 2012;8:Doc04. doi: 10.3205/hta000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James A.W., LaChaud G., Shen J., et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B. 2016;22(4):284–297. doi: 10.1089/ten.teb.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tetzel L., Guyard M. Saving the lower limb with GlassBONE™ - successful surgical revision of pseudarthrosis after infected open proximal tibia fracture type IIIC with bioactive glass grafting - a case report. Trauma Case Rep. 2020;13:31. doi: 10.1016/j.tcr.2020.100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drago L., Romanò D., De Vecchi E., et al. Bioactive glass bag-S53P4 for the adjunctive treatment of chronic osteomyelitis of the long bones: an in vitro and prospective clinical study. BMC Infect Dis. 2013;13(1):584. doi: 10.1186/1471-2334-13-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannoudis P.V., Einhorn T.A., Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl 4):S3–S6. doi: 10.1016/s0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 31.Bostrom M.P., Seigerman D.A. The clinical use of allografts, demineralized bone matrices, synthetic bone graft substitutes and osteoinductive growth factors: a survey study. HSS J. 2005;1(1):9–18. doi: 10.1007/s11420-005-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts T.T., Rosenbaum A.J. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8(4):114–124. doi: 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi D., Tank P., Mahida H., Dhami M., Vedpathak H., Karle A. Bone grafting: an overview. Vet World. 2010;3(4):198–200. [Google Scholar]
- 34.Copelan E.A. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 35.Haubruck P., Tanner M.C., Vlachopoulos W., et al. Comparison of the clinical effectiveness of Bone Morphogenic Protein (BMP) -2 and -7 in the adjunct treatment of lower limb nonunions. Orthop Traumatol Surg Res. 2018;104(8):1241–1248. doi: 10.1016/j.otsr.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Garrison K.R., Donell S., Ryder J., et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess. 2007;11(30):1–150. doi: 10.3310/hta11300. [DOI] [PubMed] [Google Scholar]
- 37.Lee D.H., Ryu K.J., Kim J.W., Kang K.C., Choi Y.R. Bone marrow aspirate concentrate and platelet-rich plasma enhanced bone healing in distraction osteogenesis of the tibia. Clin Orthop Relat Res. 2014;472(12):3789–3797. doi: 10.1007/s11999-014-3548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelissier P., Masquelet A.C., Bareille R., Pelissier S.M., Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22(1):73–79. doi: 10.1016/S0736-0266(03)00165-7. [DOI] [PubMed] [Google Scholar]
- 39.Kanakaris N.K., Harwood P.J., Mujica-Mota R., Mohrir G., Chloros G., Giannoudis P.V. Treatment of tibial bone defects: pilot analysis of direct medical costs between distraction osteogenesis with an Ilizarov frame and the Masquelet technique. Eur J Trauma Emerg Surg. 2022;49(2):951–964. doi: 10.1007/s00068-022-02162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teo B.J., Koh J.S., Goh S.K., Png M.A., Chua D.T., Howe T.S. Post-operative outcomes of atypical femoral subtrochanteric fracture in patients on bisphosphonate therapy. Bone Joint Lett J. 2014;96-B(5):658–664. doi: 10.1302/0301-620X.96B5.32887. [DOI] [PubMed] [Google Scholar]
- 41.Githens M., Garner M.R., Firoozabadi R. Surgical management of atypical femur fractures associated with bisphosphonate therapy. J Am Acad Orthop Surg. 2018;26(24):864–871. doi: 10.5435/JAAOS-D-16-00717. [DOI] [PubMed] [Google Scholar]
- 42.Shane E., Burr D., Abrahamsen B., et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 43.Kulachote N., Sa-ngasoongsong P., Sirisreetreerux N., et al. Demineralized bone matrix add-on for acceleration of bone healing in atypical subtrochanteric femoral fracture: a consecutive case-control study. BioMed Res Int. 2016 doi: 10.1155/2016/4061539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy M.T., Pydisetty G., Kwaees T.A., Saldanha K. Outcome of revision surgery for bisphosphonate related subtrochanteric fracture non-union following failed intramedullary nailing. Injury. 2021;52(3):582–588. doi: 10.1016/j.injury.2020.09.051. [DOI] [PubMed] [Google Scholar]
- 45.Lu J., Maruo Holledge M. Surgical management of an atypical femoral non-union fracture with bone morphogenic protein supplementation. Trauma Cases Rev. 2017;3:48. [Google Scholar]
- 46.Shin W.C., Jang J.H., Moon N.H., Jun S.B. Is open bone graft always necessary when treating aseptic subtrochanteric nonunion with a reamed intramedullary nail? BMC Muscoskel Disord. 2021;22(1):145. doi: 10.1186/s12891-021-04016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gdoutos E.E., Raftopoulos D.D., Baril J.D. A critical review of the biomechanical stress analysis of the human femur. Biomaterials. 1982;3:2–8. doi: 10.1016/0142-9612(82)90053-9. [DOI] [PubMed] [Google Scholar]
- 48.Park S.H., Kong G.M., Ha B.H., Park J.H., Kim K.H. Nonunion of subtrochanteric fractures: comminution or malreduction. Pakistan J Med Sci. 2016;32:591–594. doi: 10.12669/pjms.323.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malkawi H. Bone grafting in subtrochanteric fractures. Clin Orthop Relat Res. 1982;168:69–72. [PubMed] [Google Scholar]
- 50.Balasubramanian N., Babu G., Prakasam S. Treatment of non-unions of subtrochanteric fractures using an anatomical proximal femur locked compression plate - a prospective study of 13 patients. J Orthop Case Rep. 2016;6(1):65–68. doi: 10.13107/jocr.2250-0685.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofmann A., Gorbulev S., Guehring T., et al. CERTiFy Study Group Autologous iliac bone graft compared with biphasic hydroxyapatite and calcium sulfate cement for the treatment of bone defects in tibial plateau fractures: a prospective, randomized, open-label, multicenter study. J Bone Joint Surg Am. 2020;102(3):179–193. doi: 10.2106/JBJS.19.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell T.A., Leighton R.K., Alpha-BSM Tibial Plateau Fracture Study Group Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Joint Surg Am. 2008;90(10):2057–2061. doi: 10.2106/JBJS.G.01191. [DOI] [PubMed] [Google Scholar]
- 53.Pernaa K., Koski I., Mattila K., et al. Bio active glass S53P4 and autograft bone in treatment of depressed tibial plateau fractures - a prospective randomized 11-year follow-up. J Long Term Eff Med Implants. 2011;21(2):139–148. doi: 10.1615/jlongtermeffmedimplants.v21.i2.40. [DOI] [PubMed] [Google Scholar]
- 54.Heikkilä J.T., Kukkonen J., Aho A.J., Moisander S., Kyyrönen T., Mattila K. Bioactive glass granules: a suitable bone substitute material in the operative treatment of depressed lateral tibial plateau fractures: a prospective, randomized 1 year follow-up study. J Mater Sci Mater Med. 2011;22(4):1073–1080. doi: 10.1007/s10856-011-4272-0. [DOI] [PubMed] [Google Scholar]
- 55.Jónsson B.Y., Mjöberg B. Porous titanium granules are better than autograft bone as a bone void filler in lateral tibial plateau fractures: a randomised trial. Bone Joint Lett J. 2015;97-B(6):836–841. doi: 10.1302/0301-620X.97B6.34552. [DOI] [PubMed] [Google Scholar]
- 56.Hartwich M., Lans J., Jupiter J.B., Babst R., Regazzoni P., Dell'Oca A.F. Joint depression in tibial plateau fractures: to bone graft or not to bone graft? Injury. 2023;2(23):S0020–S1383. doi: 10.1016/j.injury.2023.02.050. 00184-5. [DOI] [PubMed] [Google Scholar]
- 57.Kim W.Y., Ji J.H., Park S.E., Kim Y.Y., Jeong J.J., Kang H.T. Surgical management of pilon fractures with large segmental bone defects using fibular strut allografts: a report of two cases. Eur J Orthop Surg Traumatol. 2011;21(6):439–444. doi: 10.1007/s00590-010-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X., Zhang H., Li Y., et al. Osteochondral autograft transplantation in the treatment of AO/OTA type C3 tibial plafond fractures with irreducibly comminuted area and/or cartilage delamination in the distal tibial facet. Injury. 2022;53(4):1523–1531. doi: 10.1016/j.injury.2022.01.040. [DOI] [PubMed] [Google Scholar]
- 59.Liu X., An J., Zhang H., Li Y., Chen Y., Zhang W. Autologous osteochondral graft for early posttraumatic arthritis of tibiotalar joints after comminuted pilon fractures in young patients. Foot Ankle Int. 2020;41(1):69–78. doi: 10.1177/1071100719875728. [DOI] [PubMed] [Google Scholar]
- 60.Jang Y., Kim D. Biomechanical study of Proximal humeral fracture fixation: locking plate with medial support screw vs. locking plate with intramedullary fibular graft. Clin Biomech. 2021;90 doi: 10.1016/j.clinbiomech.2021.105510. [DOI] [PubMed] [Google Scholar]
- 61.Kim S.H., Lee Y.H., Chung S.W., et al. Outcomes for four-part proximal humerus fractures treated with a locking compression plate and an autologous iliac bone impaction graft. Injury. 2012;43(10):1724–1731. doi: 10.1016/j.injury.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 62.Zhu L., Liu Y., Yang Z., et al. Locking plate fixation combined with iliac crest bone autologous graft for proximal humerus comminuted fracture. Chin Med J. 2014;127(9):1672–1676. [PubMed] [Google Scholar]
- 63.McMurtrie J.T., Patch D.A., Frazier M.B., et al. Union rates of talar neck fractures with substantial bone defects treated with autograft. Foot Ankle Int. 2022;43(3):343–352. doi: 10.1177/10711007211050032. [DOI] [PubMed] [Google Scholar]
- 64.Tang H., Han K., Li M., et al. Treatment of Hawkins type II fractures of talar neck by a vascularized cuboid pedicle bone graft and combined internal and external fixation: a preliminary report on nine cases. J Trauma. 2010;69(4):E1–E5. doi: 10.1097/TA.0b013e3181cda6ad. [DOI] [PubMed] [Google Scholar]
- 65.Duncumb J.W., Robinson P.G., Williamson T.R., et al. Bone grafting for scaphoid non-union surgery: a systematic review and meta-analysis. Bone Joint Lett J. 2022;104-B(5):549–558. doi: 10.1302/0301-620X.104B5.BJJ-2021-1114.R1. [DOI] [PubMed] [Google Scholar]
- 66.Elgayar L., Elmajee M., Aljawadi A., Abdelaal A., Khan S., Pillai A. A systematic review of mechanical stabilization by screw fixation without bone grafting in the management of stable scaphoid non-union. J Clin Orthop Trauma. 2021;17:112–117. doi: 10.1016/j.jcot.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Polmear M.M., Anderson A.B., Lanier P.J., Orr J.D., Nesti L.J., Dunn J.C. Bone morphogenetic protein in scaphoid non-union: a systematic review. J Wrist Surg. 2021;10(3):184–189. doi: 10.1055/s-0040-1722332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elgeidi A., El Negery A., Abdellatif M.S., El Moghazy N. Dynamic hip screw and fibular strut graft for fixation of fresh femoral neck fracture with posterior comminution. Arch Orthop Trauma Surg. 2017;137(10):1363–1369. doi: 10.1007/s00402-017-2758-z. [DOI] [PubMed] [Google Scholar]
- 69.Mehraj M., Khurana S., Kumar B., Chahal J.S. Fixation of fresh femoral neck fractures using fibular strut graft along with cannulated screws. Ortop Traumatol Rehabil. 2022;24(5):319–323. doi: 10.5604/01.3001.0016.1364. [DOI] [PubMed] [Google Scholar]
- 70.Kumar S., Bharti A., Rawat A., Kumar V., Avasthi S. Comparative study of fresh femoral neck fractures managed by multiple cancellous screws with and without fibular graft in young adults. J Clin Orthop Trauma. 2015;6(1):6–11. doi: 10.1016/j.jcot.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yin J., Zhu H., Gao Y., Zhang C. Vascularized fibular grafting in treatment of femoral neck non-union: a prognostic study based on long-term outcomes. J Bone Joint Surg Am. 2019;101(14):1294–1300. doi: 10.2106/JBJS.18.01132. [DOI] [PubMed] [Google Scholar]
- 72.Biswas S.K., Salgotra K.R., Lahree P.K. Tensor fascia lata/gluteus medius muscle pedicle bone graft for non-union of femoral neck fractures. Med J Armed Forces India. 1997;53(1):19–23. doi: 10.1016/S0377-1237(17)30638-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McBride J.C.M., Banks R.E., Taylor D., Ryan J. Healing of segmental bone defects in goat tibia. J Invest Surg. 1993;6:369. [Google Scholar]
- 74.Sanders D.W., Bhandari M., Guyatt G., et al. Critical-sized defect in the tibia: is it critical? Results from the SPRINT trial. J Orthop Trauma. 2014;28:632–635. doi: 10.1097/BOT.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 75.Haines N.M., Lack W.D., Seymour R.B., Bosse M.J. Defining the lower limit of a "critical bone defect" in open diaphyseal tibial fractures. J Orthop Trauma. 2016;30(5):e158–e163. doi: 10.1097/BOT.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 76.Keating J.F., Simpson A.H., Robinson C.M. The management of fractures with bone loss. J Bone Jt Surg Br. 2005;87:142–150. doi: 10.1302/0301-620x.87b2.15874. [DOI] [PubMed] [Google Scholar]
- 77.Adamczyk A., Meulenkamp B., Wilken G., Papp S. Managing bone loss in open fractures. OTA Int. 2020;3(1):e059. doi: 10.1097/OI9.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saint-Cyr M., Gupta A. Primary internal fixation and bone grafting for open fractures of the hand. Hand Clin. 2006;22(3):317–327. doi: 10.1016/j.hcl.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 79.Stahl S., Lerner A., Kaufman T. Immediate autografting of bone in open fractures with bone loss of the hand: a preliminary study. Scand J Plast Reconstr Hand Surg. 1999;33:117–122. doi: 10.1080/02844319950159721. [DOI] [PubMed] [Google Scholar]
- 80.Rao N., Ziran B.H. Treating osteomyelitis: antibiotics and surgery. Plast Reconstr Surg. 2011;127(Suppl 1):177S–187S. doi: 10.1097/PRS.0b013e3182001f0f. [DOI] [PubMed] [Google Scholar]
- 81.El-Gammal T.A., Shiha A.E., El-Deen M.A., et al. Management of traumatic tibial defects using free vascularized fibula or Ilizarov bone transport: a comparative study. Microsurgery. 2008;28:339–346. doi: 10.1002/micr.20501. [DOI] [PubMed] [Google Scholar]
- 82.Marais L.C. Bone transport through an induced membrane in the management of tibial bone defects resulting from chronic osteomyelitis. Strategies Trauma Limb Reconstr. 2015;10:27–33. doi: 10.1007/s11751-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kubes K., Friedman A., Pyle C., Diaz G., Hargett D. Management of twenty centimeter segmental bone defect of femoral shaft secondary to infected non-union of fracture using masquelet technique: a case report. Int J Surg Case Rep. 2021;84 doi: 10.1016/j.ijscr.2021.106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bezstarosti H., Metsemakers W.J., van Lieshout E.M.M., et al. Management of critical-sized bone defects in the treatment of fracture-related infection: a systematic review and pooled analysis. Arch Orthop Trauma Surg. 2021;141(7):1215–1230. doi: 10.1007/s00402-020-03525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]