Abstract
OBJECTIVE:
Retained placenta complicates 2-3% of vaginal deliveries and is a known cause of postpartum hemorrhage. Treatment includes manual or operative placental extraction, potentially increasing risks of hemorrhage, infections, and prolonged hospital stays. We sought to evaluate risk factors for retained placenta, defined as more than 30 minutes between the delivery of the fetus and placenta, in a large US obstetrical cohort.
STUDY DESIGN:
We included singleton, vaginal deliveries ≥24 weeks (n = 91,291) from the Consortium of Safe Labor from 12 US institutions (2002–2008). Multivariable logistic regression analyses estimated the adjusted odds ratios (OR) and 95% confidence intervals (CI) for potential risk factors for retained placenta stratified by parity, adjusting for relevant confounding factors. Characteristics such as stillbirth, maternal age, race, and admission body mass index were examined.
RESULTS:
Retained placenta complicated 1047 vaginal deliveries (1.12%). Regardless of parity, significant predictors of retained placenta included stillbirth (nulliparous adjusted OR, 5.67; 95% CI, 3.10–10.37; multiparous adjusted OR, 4.56; 95% CI, 2.08–9.94), maternal age ≥30 years, delivery at 24 0/7 to 27 6/7 compared with 34 weeks or later and delivery in a teaching hospital. In nulliparous women, additional risk factors were identified: longer first- or second-stage labor duration, whereas non-Hispanic black compared with non-Hispanic white race was found to be protective. Body mass index was not associated with an increased risk.
CONCLUSION:
Multiple risk factors for retained placenta were identified, particularly the strong association with stillbirth. It is plausible that there could be something intrinsic about stillbirth that causes a retained placenta, or perhaps there are shared pathways of certain etiologies of stillbirth and a risk of retained placenta.
Keywords: postpartum hemorrhage, preterm birth, retained placenta, stillbirth
Postpartum hemorrhage is the primary cause of maternal mortality in developing countries and reported by the World Health Organization to be responsible for 25% of all maternal fatalities.1 Postpartum hemorrhage complicates approximately 2–3% of vaginal deliveries.2 Although uterine atony is the most common etiology of postpartum hemorrhage, other etiologies include cervical or vaginal lacerations, coaglopathies, and a retained placenta.
Although there is no universal consensus for the length of time allotted for the placenta to deliver before it is diagnosed as retained, intrapartum guidelines from the National Institute for Health and Clinical Excellence in London and the World Health Organization suggest using 30 minutes following delivery of the neonate as the length of time after which some type of intervention is advised, especially in the presence of bleeding.3,4 Treatment may involve manual or operative extraction of the placenta, potentially increasing the risks of hemorrhage, postpartum infections, and prolonged hospital stays.5,6
Several reports since the early 1990s have identified risk factors for retained placenta to be induction of labor, high parity (one study citing parity of ≥5), history of retained placenta, previous dilatation and curettage, preterm delivery, and small placental weight.7-11 The study by Endler et al9 in 2014 was the first to suggest an association between term stillbirth and retained placenta in a Swedish population. However, no studies have examined the US population.
It also remains unknown whether there are differences in retained placenta among maternal races or an association with increasing body mass index (BMI), both factors that may differ from non-US populations. The goal of this study was to identify underlying factors for retained placenta, specifically focusing on potential racial differences and increasing maternal BMI by using the Consortium on Safe Labor database.
Materials and Methods
We performed a secondary analysis of deidentified data collected from the Consortium on Safe Labor database, a retrospective cohort study of 228,562 deliveries from 12 US clinical centers between 2002 and 2008.12 Data were collected from obstetric, labor progression, and newborn electronic medical records linked to hospital discharge codes. This original study received institutional review board approval from all participating institutions, and the current analysis was deemed exempt by the MedStar Washington Hospital Center’s Institutional Review Board on Oct. 17, 2013.
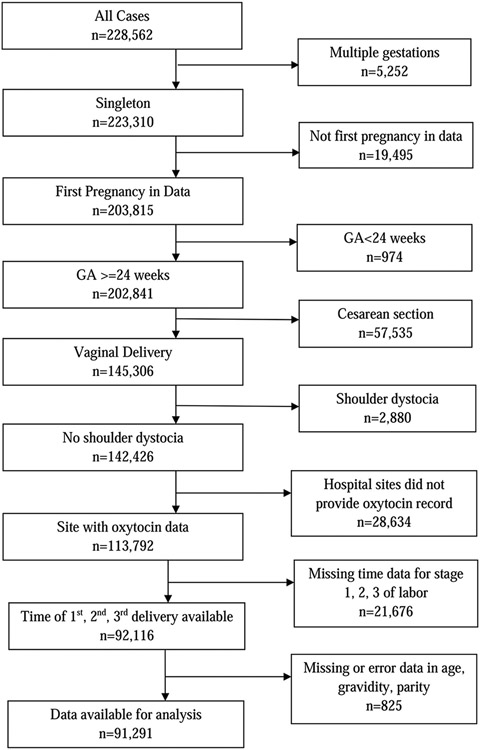
For the present analysis, we included women with singleton gestations, delivery gestational age ≥ 24 weeks, and vaginal deliveries. Only the patient’s first documented pregnancy in the Consortium on Safe Labor database was used for analysis. Cases with shoulder dystocia or hospitals without adequate documentation of pertinent variables were excluded (Figure 1). The total number of deliveries available for analysis was 91,291.
FIGURE 1. Case selection diagram.
GA, gestational age.
The third stage of labor was calculated from the time of neonate delivery to the time of placental delivery as recorded in the electronic medical record. Retained placenta was defined as longer than 30 minutes.3,4 Bivariate analyses were performed to assess the relationship between a retained placenta and maternal demographic or clinical characteristics with a χ2 test, Fisher exact test, Student t test, or Wilcoxon rank sum test, if applicable. Multivariable logistic regression analyses estimated the adjusted odds ratios and 95% confidence intervals for potential risk factors for retained placenta stratified by parity.
Risk factors for retained placenta were identified from the medical record or International Classification of Disease, 9th revision (ICD-9), codes and included parity, maternal age, gestational age, admission BMI, race, history of abortion (ICD-9), history of cesarean delivery, large for gestational age (ICD-9), intrauterine growth restriction (IUGR; ICD-9), duration of first and second stages of labor, duration of rupture of membranes to delivery, labor induction agent (misoprostol, dinoprostone, artificial rupture of membranes, or oxytocin), group B streptococcal status, chorioamnionitis, use of an epidural, episiotomy, stillbirth, hospital type, and duration of exposure to oxytocin were examined and adjusted for in the analysis.
A value of P < .05 was determined significant. Forest plots were developed to compare the odds ratios between categories of gestational age groups, based on the result of multivariable logistic regression models. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC).
Results
The incidence of retained placenta in the study population was 1.12% (1047 deliveries). The demographics of the study population are described in Table 1. Women with retained placenta compared with women without retained placenta were more likely to be older (27.5 years vs 26.6 years; P < .001), multiparous, and non-Hispanic black or Asian, but there was no difference in maternal BMI (P < .18).
TABLE 1.
Demographic and obstetrical risk factors for retained placenta
| Variablea | Retained placenta no (n = 89,765) |
Retained placenta yes (n = 1047,1.12%) |
P value |
|---|---|---|---|
| Maternal age, yb | 26.64(5.81) | 27.53 (6.43) | < .001 |
| Gravidityc | 2 (1,5) | 2 (1,5) | .700 |
| Parityc | 1 (0,3) | 1 (0,3) | .008 |
| Nulliparous | .002 | ||
| No | 50,634 (56.41%) | 541 (51.67%) | |
| Yes | 39,131 (43.59%) | 506 (48.33%) | |
| Gestational age group | < .001 | ||
| 24 wks 0 d-27 wks 6 d | 239 (0.27%) | 23 (2.20%) | |
| 28 wks 0 d-31 wks 6 d | 500 (0.56%) | 16 (1.53%) | |
| 32 wks 0 d-33 wks 6 d | 855 (0.95%) | 18 (1.72%) | |
| 34 wks 0 d-36 wks 6 d | 6190 (6.90%) | 87 (8.31%) | |
| 37 wks 0 d-38 wks 6 d | 26,702 (29.75%) | 306 (29.23%) | |
| 39 wks 0 d-40 wks 6 d | 48,503 (54.03%) | 497 (47.47%) | |
| 41 wks 0 d-41 wks 6 d | 6212 (6.92%) | 95 (9.07%) | |
| 42 wks 0 d and above | 564 (0.63%) | 5 (0.48%) | |
| BMI at admission, kg/m2 | .184 | ||
| <18.5 | 55 (0.06%) | 1 (0.10%) | |
| 18.5–25 | 12,323 (13.74%) | 138 (13.18%) | |
| 25–30 | 30,597 (34.09%) | 357 (34.10%) | |
| 30–35 | 19,428 (21.64%) | 227 (21.68%) | |
| 35–40 | 7794 (8.68%) | 94 (8.98%) | |
| ≥40 | 4325 (4.82%) | 69 (6.59%) | |
| Missing | 15,243 (16.98%) | 161 (15.38%) | |
| Race | .039 | ||
| Non-Hispanic white | 48,848 (54.42%) | 552 (52.72%) | |
| Non-Hispanic black | 16,087 (17.92%) | 193 (18.43%) | |
| Hispanic | 14,736 (16.42%) | 204 (19.48%) | |
| Asian/Pacific Islander | 3203 (3.57%) | 25 (2.39%) | |
| Others | 1854 (2.07%) | 22 (2.10%) | |
| Unknown/declined | 5037 (5.61%) | 51 (4.87%) | |
| Stillbirth | < .0011 | ||
| No | 89,511 (99.72%) | 1016 (97.04%) | |
| Yes | 254 (0.28%) | 31 (2.96%) | |
| Stillbirth, GA 24 wks 0 d to 27 wks 6 d | .0031 | ||
| No | 199 (83.26%) | 11 (47.83%) | |
| Yes | 40 (16.74%) | 12 (52.17%) | |
| Stillbirth, GA 28 wks 0 d to 31 wks 6 d | .011 | ||
| No | 458 (91.60%) | 11 (68.75%) | |
| Yes | 42 (8.40%) | 5 (31.25%) | |
| Stillbirth, GA 32 wks 0 d to 33 wks 0 d | .0421 | ||
| No | 819 (95.79%) | 15 (83.33%) | |
| Yes | 36 (4.21%) | 3 (16.67%) | |
| Stillbirth, GA 34 wks 0 d to 36 wks 6 d | < .0011 | ||
| No | 6135 (99.11%) | 81 (93.10%) | |
| Yes | 55 (0.89%) | 6 (6.90%) | |
| Stillbirth, GA 37 wks 0 d to 38 wks 6 d | .0011 | ||
| No | 26667 (99.87%) | 302 (98.69%) | |
| Yes | 35 (0.13%) | 4 (1.31%) | |
| Stillbirth, GA 39 wks 0 d to 40 wks 6 d | .3551 | ||
| No | 48461 (99.91%) | 496 (99.80%) | |
| Yes | 42 (0.09%) | 1 (0.20%) | |
| Stillbirth, GA 41 wks 0 d to 41 wks 6 d | 1.001 | ||
| No | 6209 (99.95%) | 95 (100.0%) | |
| Yes | 3 (0.05%) | 0 | |
| Stillbirth, GA 42 wks 0 d and above | 1.001 | ||
| No | 563 (99.82%) | 5 (100.0%) | |
| Yes | 1 (0.18%) | 0 | |
| Duration of first stage of labor, hc | 6.9 (2.1, 17.1) | 495 (1.8, 24.7) | < .001 |
| Duration of second stage of labor, minc | 29 (6, 124) | 35 (7, 160) | < .001 |
| Duration of third stage of labor, minc | 5 (2,11) | 52 (32, 1443) | < .001 |
| Hospital type | < .001 | ||
| University-affiliated teaching hospital | 27,748 (30.91%) | 444 (42.41%) | |
| Community teaching hospital | 50,498 (56.26%) | 546 (52.15%) | |
| Community nonteaching hospital | 11,519 (12.83%) | 57 (5.44%) | |
| Intrauterine growth restrictiond | .183 | ||
| No | 88,270 (98.33%) | 1495 (97.80%) | |
| Yes | 1495 (1.67%) | 23 (2.20%) | |
| Large for gestational agee | .726 | ||
| No | 88672 (98.78%) | 1033 (98.66%) | |
| Yes | 1093 (1.22%) | 14 (1.34%) | |
| Use of epidural | < .001 | ||
| No | 20,211 (22.52%) | 294 (28.08%) | |
| Yes | 69,554 (77.48%) | 753 (71.92%) | |
| Chorioamnionitis | <.001 | ||
| No | 88,558 (98.66%) | 1019 (97.33%) | |
| Yes | 1207 (1.34%) | 28 (2.67%) | |
| History of cesarean delivery | .803 | ||
| No | 86,887 (96.79%) | 1012 (96.66%) | |
| Yes | 2878 (3.21%) | 35 (3.34%) | |
| Oxytocin exposure | .044 | ||
| No | 28,668 (31.94%) | 365 (34.86%) | |
| Yes | 61,097 (68.06%) | 682 (65.14%) | |
| Duration from first oxytocin use to delivery, mina | 298.8 (353.9) | 386.6 (559.5) | < .001 |
BMI, body mass index; GA, gestational age; ICD-9, International Classification of Disease, 9th revision.
Other variables analyzed include history of abortion, episiotomy, induction of labor agent (misoprostol, dinoprostone, artificial rupture of membranes, or oxytocin), group B streptococcal status, duration of rupture of membranes, and episiotomy; Results are presented as number of observations (percentage) with χ2 test unless noted as follows
Results are presented as mean (SD) with a Student t test
Results are presented as median (10th percentile, 90th percentile) with Wilcoxon rank sum test
Intrauterine growth restriction (ICD-9)
Large for gestational age (ICD-9).
They were also more likely to have a stillbirth (3.0% vs 0.3%; P < .001), chorioamnionitis (2.7% vs 1.3%; P < .001), and a longer length of first and second stages of labor (P < .001). Women with retained placenta had a significantly higher rate of postpartum hemorrhage than women without retained placenta (11.56% vs 3.13%; P < .001). However, no significant difference was found in the rate of postpartum blood transfusion between the women with a retained placenta and the women without a retained placenta (7.02% vs 5.32%; P = .092).
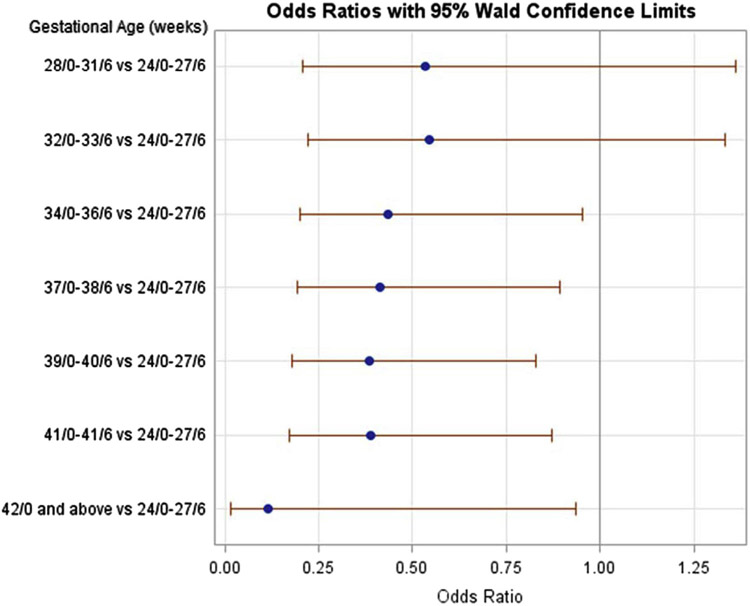
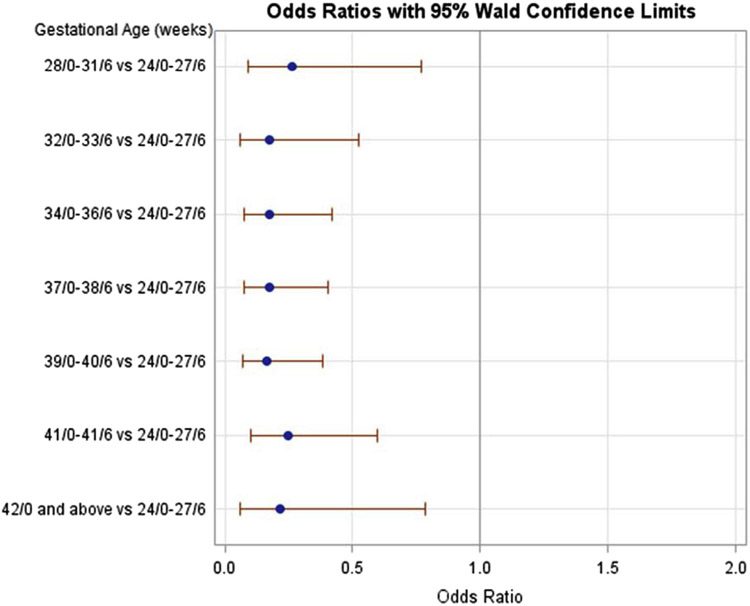
After stratifying by parity and adjusting for confounding factors, multiple significant risk factors for retained placenta in both nulliparous and multiparous women were identified (Tables 2 and 3, respectively). Regardless of parity, risk factors included increasing maternal age >30 years, early preterm delivery <27 6/7 weeks compared with 34 0/7 weeks or later (Figures 2 and 3), and stillbirth and delivery in a university-affiliated or community teaching hospital. Maternal BMI, chorioamnionitis, and IUGR had no association with retained placenta.
TABLE 2.
Incidence and adjusted odds ratios of risk factors for retained placenta in nulliparous women
| Nulliparous | Retained placenta | ||
|---|---|---|---|
| Risk factora | No, n (%) | Yes, n (%) | AORb (95% CI) |
| Age, y | |||
| <20 | 8273 (21.1) | 88 (17.4) | 0.87 (0.64–1.17) |
| 20–24 | 14,896 (38.1) | 166 (32.8) | 0.98 (0.77–1.25) |
| 25–29 | 9511 (24.3) | 109 (21.5) | Reference |
| 30–34 | 4705 (12.0) | 88 (17.4) | 1.50 (1.12–2.01) |
| 35–39 | 1479 (3.8) | 41 (8.1) | 2.02 (1.39–2.95) |
| ≥40 | 267 (0.68) | 14 (2.8) | 3.12 (1.73–5.67) |
| Gestational age, wks | |||
| 24/0–27/6 | 144 (0.37) | 11 (2.2) | Reference |
| 28/0–31/6 | 262 (0.67) | 10 (2.0) | 0.53 (0.21–1.36) |
| 32/0–33/6 | 439 (1.1) | 12 (2.37) | 0.54 (0.22–1.33) |
| 34/0–36/6 | 2789 (7.1) | 45 (8.9) | 0.44 (0.20–0.95) |
| 37/0–38/6 | 10747 (27.5) | 137 (27.1) | 0.41 (0.19–0.89) |
| 39/0–40/6 | 21139 (54.0) | 243 (48.02) | 0.39 (0.18–0.82) |
| 41–41/6 | 3365 (8.6) | 47 (9.3) | 0.39 (0.17–0.87) |
| ≥42/0 | 246 (0.63) | 1 (0.20) | 0.11 (0.01–0.93) |
| BMI at admission | |||
| <18.5 | 36 (0.1) | 1 (0.2) | 1.65 (0.21–13.12) |
| 18.5–24.9 | 6030 (15.4) | 74 (14.6) | Reference |
| 25–29.9 | 13,744 (35.1) | 175 (34.6) | 1.0 (0.76–1.32) |
| 30–34.9 | 7906 (20.2) | 99 (19.6) | 0.88 (0.65–1.20) |
| 35–39.9 | 2959 (7.6) | 40 (7.9) | 0.93 (0.62–1.38) |
| ≥40 | 1531 (9.1) | 32 (6.3) | 1.34 (0.87–2.07) |
| Missing | 6925 (17.7) | 85 (16.8) | 0.87 (0.61–1.23) |
| Race | |||
| Non-Hispanic black | 6889 (17.6) | 71 (14.0) | 0.63 (0.47–0.86) |
| Non-Hispanic white | 20,966 (53.6) | 287 (56.7) | Reference |
| Hispanic | 6161 (15.7) | 93 (18.4) | 1.11 (0.84–1.46) |
| Asian/Pacific Islander | 1668 (4.3) | 16 (3.2) | 0.80 (0.47–1.35) |
| Stillbirth | 142 (0.36) | 18 (3.6) | 5.85 (3.22–10.63) |
| Hospital type | |||
| University affiliated teaching | 12,405 (31.7) | 193 (38.1) | 2.50 (1.2–5.19) |
| Community teaching | 22,612 (57.8) | 293 (57.9) | 2.47 (1.52–4.00) |
| Community nonteaching | 4114 (10.5) | 20 (4.0) | Reference |
| Duration first stage, hc | 8.72 (3.27, 20.4) | 11.29 (4.07, 30.27) | 1.01 (1.01–1.02) |
| Duration second stage, hc | 0.98 (0.27, 2.7) | 1.23 (0.27, 3.25) | 1.06 (1.02–1.10) |
| IUGRd | 813 (2.1) | 14 (2.8) | 1.11 (0.63–1.94) |
| Chorioamnionitis | 902 (2.3) | 22 (4.35) | 1.36 (0.87–2.14) |
| Epidural | 31,961 (81.7) | 387 (76.5) | 0.70 (0.56–0.90) |
AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; GA, gestational age; ICD-9, International Classification of Disease, 9th revision; IUGR, intrauterine fetal growth restriction.
Other risk factors analyzed but not listed above include the following: gravidity, other race, history of abortion, large for gestational age, intrauterine growth restriction, duration of rupture of membranes, method of induction, oxytocin use, and antepartum group B streptococcal status
AOR
Results are presented as median (10th percentile, 90th percentile) with Wilcoxon rank sum test
IUGR (ICD-9).
TABLE 3.
Incidence and adjusted odds ratios of risk factors for retained placenta in multiparous women
| Multiparousa | Retained placenta | ||
|---|---|---|---|
| Risk factor | No, n (%) | Yes, n (%) | AOR (95% CI) |
| Age, y | |||
| <20 | 1362 (2.7) | 14 (2.6) | 0.92 (0.52–1.61) |
| 20–24 | 10,668 (21.1) | 128 (23.7) | 1.22 (0.95–1.56) |
| 25–29 | 17,501 (34.6) | 146 (27.0) | Reference |
| 30–34 | 13,561 (26.8) | 140 (25.9) | 1.28 (1.01–1.62) |
| 35–39 | 6207 (12.3) | 85 (15.7) | 1.59 (1.20–2.10) |
| ≥40 | 1335 (2.6) | 28 (5.2) | 2.44 (1.59–3.75) |
| Gestational age, weeks | |||
| 24/0–27/6 | 95 (0.19) | 12 (2.3) | Reference |
| 28/0–31/6 | 238 (0.5) | 6 (1.1) | 0.26 (0.09–0.77) |
| 32/0–33/6 | 416 (0.8) | 6 (1.1) | 0.18 (0.06–0.53) |
| 34/0–36/6 | 3401 (6.7) | 42 (7.8) | 0.18 (0.70–0.42) |
| 37/0–38/6 | 15,955 (31.5) | 169 (31.2) | 0.17 (0.08–0.41) |
| 39–40/6 | 27,364 (54.0) | 254 (47.0) | 0.16 (0.07–0.38) |
| 41/0–41/6 | 2847 (5.6) | 48 (8.9) | 0.25 (0.10–0.60) |
| ≥42/0 | 318 (0.6) | 4 (0.7) | 0.21 (0.06–0.79) |
| BMI at admission, kg/m2 | |||
| <18.5 | 19 (0.1) | 0 (0) | |
| 18.5–24.9 | 6293 (12.4) | 64 (11.8) | Reference |
| 25–29.9 | 16853 (33.3) | 182 (33.6) | 1.08 (0.81–1.45) |
| 30–34.9 | 11522 (22.8) | 128 (23.7) | 1.02 (0.78–1.39) |
| 35–39.9 | 4835 (9.6) | 54 (10.0) | 1.00 (0.69–1.46) |
| ≥40 | 2794 (5.5) | 37 (6.8) | 1.16 (0.76–1.77) |
| Missing | 8318 (16.4) | 76 (14.1) | 1.04 (0.72–1.51) |
| Race | |||
| Non-Hispanic black | 9198 (18.2) | 122 (22.6) | 0.952 (0.73–1.24) |
| Non-Hispanic white | 27882 (55.1) | 265 (49.0) | Reference |
| Hispanic | 8575 (16.9) | 111 (20.5) | 1.1 (0.82–1.38) |
| Asian/Pacific Islander | 1535 (3.0) | 9 (1.7) | 0.79 (0.40–1.54) |
| Stillbirth | 112 (0.22) | 13 (2.40) | 4.47 (2.06–9.68) |
| Hospital type | |||
| University-affiliated teaching | 15343 (30.3) | 241 (46.4) | 2.22 (1.03–4.80) |
| Community teaching | 27886 (55.1) | 253 (46.8) | 1.93 (1.31–2.76) |
| Community nonteaching | 7405 (14.6) | 37 (6.8) | Reference |
| Duration first stage, hb | 5.78 (1.55, 13.8) | 6.18 (1.22, 17.3) | 1.00 (0.99–1.01) |
| Duration second stage, hb | 0.28 (0.08, 1.08) | 0.3 (0.08, 1.33) | 1.01 (0.97–1.05) |
| IUGRc | 682 (1.4) | 9 (1.7) | 1.25 (0.63–2.48) |
| Chorioamnionitis | 305 (0.6) | 6 (1.1) | 0.99 (0.41–2.43) |
| Epidural | 37,593 (74.2) | 366 (67.7) | 0.86 (0.70–1.05) |
| History of cesarean delivery | 2861 (5.7) | 35 (6.5) | 0.92 (0.65–1.32) |
AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; ICD-9, International Classification of Disease, 9th revision; IUGR, intrauterine fetal growth restriction.
Other risk factors analyzed but not listed above include the following: gravidity, other race, history of abortion, large for gestational age, intrauterine growth restriction, duration of rupture of membranes, method of induction, oxytocin use, and antepartum group B Strep status
Results are presented as median (10th percentile, 90th percentile) with Wilcoxon rank sum test
IUGR (ICD-9).
FIGURE 2. Retained placenta in nulliparous women by gestational age compared to 24 0/7 to 27 6/7.
The adjusted odds ratios were adjusted for parity, maternal age, gestational age, admission BMI, race, history of abortion, history of cesarean delivery, large for gestational age (defined at >90% fetal weight), intrauterine growth restriction (defined as <5% fetal weight), duration of first and second stages of labor, duration of rupture of membranes to delivery, labor induction agent, group B streptococcal status, chorioamnionitis, use of epidural, episiotomy, stillbirth, hospital type, and duration of exposure to oxytocin.
BMI, body mass index.
FIGURE 3. Retained placenta in multiparous women by gestational age compared to 24 0/7 to 27 6/7.
The adjusted odds ratios were adjusted for parity, maternal age, gestational age, admission BMI, race, history of abortion, history of cesarean delivery, large for gestational age (defined at >90% fetal weight), intrauterine growth restriction (defined as <5% fetal weight), duration of first and second stages of labor, duration of rupture of membranes to delivery, labor induction agent, group B streptococcal status, chorioamnionitis, use of epidural, episiotomy, stillbirth, hospital type, and duration of exposure to oxytocin.
BMI, body mass index.
Additional risk factors in nulliparous women (Table 2) included an increased duration of first and second stages of labor. After adjusting for other risk factors, non-Hispanic black race compared with non-Hispanic white race was associated with a decreased risk of retained placenta, and there was no association with other races. The use of an epidural was associated with a decreased odds of retained placenta. Among the multiparous women (Table 3), there was no association with maternal race or with duration of the first and second stages of labor. Prior cesarean delivery was also not a risk factor for retained placenta.
Comment
To our knowledge, this is a novel study identifying stillbirth, maternal age >30 years, delivery between 24 0/7 and 27 6/7 compared with delivery after 34 0/7 weeks, and delivery in a teaching hospital as risk factors for retained placenta in a US obstetrical population.
A few studies have evaluated risk factors for retained placenta in populations outside of the United States.7-10 In our population, we found the 1.1% incidence of retained placenta to be concordant with a previously reported incidence of 0.5–3%.2,7-10 Multiple risk factors for retained placenta identified in previous studies include increasing maternal age and preterm birth5,7,10,11,13 were confirmed in our study.
Previous studies have suggested maternal age >35 years was an independent risk factor for retained placenta10; however, our study suggests age >30 years is a risk factor. Furthermore, as maternal age increased, the odds of a retained placenta increased. It is unknown whether advanced maternal age is associated with a decreased quality of placentation or a difference in angiogenesis that may be responsible for the increased risk of a retained placenta. This is a subject that warrants future investigation.
Most strikingly, we identified a strong association between stillbirth and a retained placenta. Only one other study has reported this association. Endler et al9 noted a 1.71-fold risk of retained placenta in a primiparous, Swedish population between 37 and 41 weeks of gestation. In comparison, our study, which stratified by parity and accounted for multiple confounders, found an increased risk of retained placenta more than double than that described in the Swedish population. Unlike the study by Endler et al, however; our study did not find an association between IUGR and retained placenta. The differences may be due to different definitions because Endler et al used birthweight less than 2 SD from the mean for gestational age and sex as a proxy for IUGR, whereas we used an intrauterine definition.
IUGR has a diverse set of etiologies that may not involve the placenta such as fetal chromosomal abnormalities, congenital anomalies, fetal-maternal hemorrhage, and malnutrition.14 Whereas IUGR may lead to stillbirth, there are separate etiologies for both outcomes so they are not always related.14 It is feasible that a different placental mechanism exists that leads to the development of IUGR, and it is separate from the mechanism responsible for stillbirth and retained placenta, or it may just be that IUGR itself is not associated with a retained placenta in the absence of stillbirth.
It has been hypothesized that retained placenta results from uterine atony because of ineffective myometrial contractility15-17 or an intrinsic placental abnormality.18,19 Perhaps this intrinsic placental dysfunction is responsible for the association of stillbirth with retained placenta. Kidron et al,20 studied 120 stillbirths and placentas. It was concluded that 88% of all stillbirths were extrinsic to the fetus including placental, cord, or chorioamnionitis. In a larger study examining 310 stillbirths, Horn et al21 found 62% of stillbirths were caused by placenta pathology, whereas 2.2% were due to intrauterine infection.
Although both of these studies support a placental pathology as the culprit for stillbirth, neither discuss the outcome of retained placenta. Pinar et al22 found that the placentas of stillbirths harbored more abnormal histopathological findings than live births, but the lesions varied among gestational ages of both live-born and stillborn infants, without one dominant histopathology leading to stillbirth. It may be that the more frequently detected multiple histpathological lesions associated with stillbirth leads to the later association with retained placenta.
Unique to our study was the analysis of race and BMI. We found a decreased risk of retained placenta among non-Hispanic black compared with non-Hispanic white women after taking other risk factors into account but only in nulliparous women. Extremes of BMI have been linked to many obstetric complications.23-25 A large Swedish cohort found that women with a BMI >40 kg/m2 had a higher risk of postpartum hemorrhage compared with those with a BMI 18.5–24.9 kg/m2.26 In the same population, no association was found between postpartum hemorrhage or maternal obesity with retained placenta.26
Our study, similarly, did not find a statistically significant association between BMI and retained placenta Women with increased BMI are prone to increased oxidative stress, but whether that could lead to changes in placental physiology that affect retained placenta is unknown.27 However, our findings did not support our hypothesis.
According to the Centers for Disease Control and Prevention, the cesarean rate in 2012 in the United States was 32.8%.28 Even though some suggest the increased cesarean delivery rate is contributing to an increased incidence of placenta accreta,29 we did not find an increased risk for retained placenta in patients with a previous cesarean delivery.
Our data conflict with the results from previous studies.13,30,31 A large Swedish study that found a 1.45-fold risk of retained placenta in pregnancies delivered after the first cesarean delivery.30 An additional large study in Israel found a 1.71-fold risk of retained placenta delivered after a history of one previous cesarean delivery.13 The lack of association in our current study may be explained by a low rate of successful vaginal birth after cesarean delivery in the study population.
Using the Consortium on Safe Labor database, we had the unique ability to study the hospital type for a given outcome. Regardless of parity, we found an increased risk of retained placenta in both university-affiliated and community teaching hospitals compared with nonteaching community hospitals. It is known that teaching hospitals care for a greater proportion of high-risk patients who may have probable confounding risk factors for retained placenta.
The strengths of this study include a large, diverse population from multiple geographic centers around the United States. We were able to extrapolate multiple demographics including race and BMI that had not been previously studied. Additionally, stratifying by parity excluded the nulliparous population from any previous obstetric history that could introduce bias into the analysis.
Although there are multiple strengths to this study, the Consortium on Safe Labor database also has its limitations. There is the potential for provider bias when documenting retained placenta. It is unclear from the Consortium on Safe Labor database how each case of retained placenta was diagnosed or what maneuvers were used to deliver the placenta. The Consortium on Safe Labor database also had a low incidence of successful vaginal birth after cesarean delivery. In future studies, it may be helpful to study risk factors for retained placenta in a large cohort of patients with a history of cesarean deliveries.
In summary, many of the risk factors identified for retained placenta are minimally modifiable. However, it is clinically important to identify these risk factors. Early identification of risk factors allows the team to counsel patients about realistic expectations, risks of possible complications like retained placenta and postpartum hemorrhage, and additional interventions. Additionally, physicians and team members can anticipate and prepare for the possibility of a retained placenta that may require additional interventions. In the future, further examination is needed to more clearly elucidate the underlying pathophysiology between the retained placenta and outcomes such as stillbirth.
ACKNOWLEDGMENTS
Institutions involved in the Consortium on Safe Labor database include, in alphabetical order, the following: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; the Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, UT; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH; the Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville MD (data coordinating center); the University of Illinois at Chicago, Chicago, IL; the University of Miami, Miami, FL; and the University of Texas Health Science Center at Houston, Houston, TX.
This study was supported by the Graduate Medical Education program, MedStar Washington Hospital Center, and supported in part by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development. The data included in this paper were obtained from the Consortium on Safe Labor, supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through contract HHSN267200603425C.
Footnotes
Institutions involved in the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health Consortium on Safe Labor are listed in the Acknowledgments.
The authors report no conflict of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Presented in poster format at the 35th annual meeting of the Society for Maternal-Fetal Medicine, San Diego, CA, Feb. 3-8, 2015.
Contributor Information
Elizabeth M. Coviello, Department of Obstetrics and Gynecology, MedStar Washington Hospital Center; Department of Obstetrics and Gynecology, MedStar Georgetown University Hospital, Washington, DC.
Katherine L. Grantz, Department of Obstetrics and Gynecology, MedStar Georgetown University Hospital, Washington, DC.
Chun-Chih Huang, Department of Biostatistics and Bioinformatics, MedStar Health Research Institute, Hyattsville, MD.
Tara E. Kelly, Department of Obstetrics and Gynecology, MedStar Georgetown University Hospital, Washington, DC.
Helain J. Landy, Department of Obstetrics and Gynecology, MedStar Georgetown University Hospital, Washington, DC; Division of Maternal Fetal Medicine, MedStar Georgetown University Hospital, Washington, DC.
REFERENCES
- 1.World Health Organization. WHO Recommendations for the prevention and treatment of postpartum haemorrhage. Geneva (Switzerland): World Health Organization; 2012. p.3. [PubMed] [Google Scholar]
- 2.Callahan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol 2010;202:353. [DOI] [PubMed] [Google Scholar]
- 3.National Collaborating Centre for Women’s and Children’s Health (NCCWCH). Intrapartum Care. Care of healthy women and their babies during childbirth. London (United Kingdom): RCOG Press; 2007. [PubMed] [Google Scholar]
- 4.World Health Organization. Pregnancy, childbirth, postpartum and newborn care: a guide for essential practice, 2nd ed. Geneva (Switzerland): World Health Organization; 2006. B11. [PubMed] [Google Scholar]
- 5.Combs CA, Laros RK. Prolonged third stage of labor: morbidity and risk factors. Obstet Gynecol 1991;77:863–7. [PubMed] [Google Scholar]
- 6.Combs CA, Murphy EL, Laros RK. Factors associated with postpartum hemorrhage with vaginal birth. Obstet Gynecol 1991;77:69–76. [PubMed] [Google Scholar]
- 7.Adelusi B, Soltan M, Chowdury N, Kangave D. Risk of retained placenta: a multivariate approach. Acta Obstet Gynecol Scand 1997;76:414–8. [DOI] [PubMed] [Google Scholar]
- 8.Endler M, Grunewalk C, Saltvedt S. Epidemiology of retained placenta. Oxytocin as an independent risk factor. Obstet Gynecol 2014;119:801–8. [DOI] [PubMed] [Google Scholar]
- 9.Endler M, Saltvedt S, Cnattingius S, Stephansson O, Wilkstrom A-K. Retained placenta is associated with pre-eclampsia, stillbirth, giving birth to a small-for-gestational-age infant and spontaneous preterm birth: a national register-based study. BJOG 2014;121:1462–70. [DOI] [PubMed] [Google Scholar]
- 10.Owolabi AT, Dare FO, Oguniola IO, Kuti O, Bisiriyu LA. Risk Factors for retained placenta in southwestern Nigeria. Singapore Med J 2008;49:532–7. [PubMed] [Google Scholar]
- 11.Romero R, Hsu YC, Athanassiadis AP, et al. Preterm delivery: a risk factor for retained placenta. Am J Obstet Gynecol 1990;193:823–5. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol 2010;203:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashwal E, Melamed N, Hiersch L, Wiznitzer A, Yogev Y, Peled Y. The incidence and risk factors for retained placenta after vaginal delivery–a single center experience. J Matern Fetal Neonatal Med 2014;27:1897–900. [DOI] [PubMed] [Google Scholar]
- 14.Reddy UM, Goldenberg R, Silver R, et al. Sillbirth classification–developing an international consensus for research: executive summary of a Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop. Obstet Gynecol 2009;114:901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman A, Weinraub Z, Bukovsky I, Arieli S, Zabow P, Caspi E. Dynamic ultrasonographic imaging of the third stage of labor: new perspectives into third-stage mechanisms. Am J Obstet Gynecol 1993;168:1496. [DOI] [PubMed] [Google Scholar]
- 16.Krapp M, Baschat AA, Hankeln M, Gembruch U. Gray scale and color Doppler sonography in the third state of labor for early detection of failed placental separation. Ultrasound Obstet Gynecol 2000;15:138–42. [DOI] [PubMed] [Google Scholar]
- 17.Weeks AD. Placental influence on the rate of labour progression: a pilot study. Eur J Obstet Gynecol Reprod Biol 2003;106:158. [DOI] [PubMed] [Google Scholar]
- 18.Heazell AE, Worton SA, Higgins LE, et al. Recognition of placental failure is key in saving babies’ lives. Placenta 2015;36:S20–8. [DOI] [PubMed] [Google Scholar]
- 19.Ptacek I, Sebire NJ, Man JA, Brownbill P, Heazell AE. Systemic review of placental pathology reports in association with stillbirth. Placenta 2014;35:552–62. [DOI] [PubMed] [Google Scholar]
- 20.Kidron D, Bernheim J, Avriam R. Placental findings contributing to fetal death, a study of 120 stillbirths between 23 and 40 weeks gestation. Placenta 2009;30:700–4. [DOI] [PubMed] [Google Scholar]
- 21.Horn LC, Langner A, Stiehl P, Wittekind C, Faber R. Identification of the causes of intrauterine death during 310 consecutive autopsies. Placenta 2004;113:134–8. [DOI] [PubMed] [Google Scholar]
- 22.Pinar H, Goldenberg RL, Koch MA, et al. Placental findings in singleton stillbirths. Obstet Gynecol 2014;123:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol 2004;103:219–24. [DOI] [PubMed] [Google Scholar]
- 24.Ehrenberg HM, Dierker L, Milluzzi C, Mercer BM. Low maternal weight, failure to thrive in pregnancy and adverse pregnancy outcomes. Am J Obstet Gynecol 2003;189:1726–30. [DOI] [PubMed] [Google Scholar]
- 25.Robinson HE, O’Connell CM, Joseph KS, McLeod NL. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol 2005;106:1357–64. [DOI] [PubMed] [Google Scholar]
- 26.Blomberg M. Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol 2011;118:561–8. [DOI] [PubMed] [Google Scholar]
- 27.Malti N, Merzouk H, Merzouk SamLoukidi B, Karaouzene N, Malti A, Narce M. Oxidative stress and maternal obesity: feto-placental unit interaction. Placenta 2014;35:411–6. [DOI] [PubMed] [Google Scholar]
- 28.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data 2012. Natl Vital Stat Rep 2013;62:8. [PubMed] [Google Scholar]
- 29.Oyelese Y, Smulian JC. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol 2006;107:927–41. [DOI] [PubMed] [Google Scholar]
- 30.Belachew J, Cnattingius S, Mulic-Lutvica A, Eurenius K, Axelsson O, Wilstrom AK. Risk of retained placenta in women previously delivered by caesarean section: a population-based cohort study. BJOG 2014;121:224–9. [DOI] [PubMed] [Google Scholar]
- 31.Sarit A, Sokolov A, Many A. Is epidural analgesia during labor related to retained placenta? J Perinat Med 2015:e1–5. [DOI] [PubMed] [Google Scholar]