Abstract

The primary objective of this research was to identify and explore the most potent and efficacious cyclooxygenase inhibitors, utilizing indole acetic acid drugs as a lead molecule. To achieve this objective, various derivatives (2a–2c and 2e–2g) of the selected lead molecule, indomethacin, were synthesized using a reflux condensation process, targeting the hydroxyl group. The synthesized analogues were subjected to different spectroscopic procedures to determine their structure and confirm their analogues. These derivatives were further screened for acute toxicity and anti-nociceptive and anti-inflammatory activity using established protocols. Docking analysis was performed to evaluate the possible protein–ligand interaction. The test compounds were found to be safe at doses of 50, 75, 100, and 200 mg/kg, i.p. The pharmacological screening revealed that test compounds 2a–2f had a superior peripheral analgesic effect at a dose of 10 mg/kg, in comparison to the parent drug indomethacin, while compound 2g exhibited slightly lower activity at the same dose. The hot plate results showed lower central analgesic activity of the test compounds compared to the standard Tramal, but it was still significant. Anti-inflammatory results were significant, comparable to Diclofenac sodium and indomethacin, except for compounds 2b, 2c, and 2e at a dose of 10 mg/kg body weight. Molecular docking analysis demonstrated that the derived compounds had augmented negative binding energies (−149.39, −146.72, −160.85, −159.34, −140.03, and −150.91 KJ/mol) compared to the parent drugs (−141.07), which supported the research’s theme of producing stronger derivatives of standard drugs with significant anti-nociceptive and anti-inflammatory potential. The derived compounds exhibited significant analgesic and anti-inflammatory activities and, therefore, have the potential to be studied further as new drug candidates for pain and inflammation.
1. Introduction
Cyclooxygenase (COX) inhibitors are commonly prescribed for their analgesic, antipyretic, and anti-inflammatory properties. Nonsteroidal anti-inflammatory drugs (NSAIDs), including indole acetic acids, arylpropionic acids, β-ketones, and arylacetic acids, are known to inhibit COX enzymes.1 The choice of NSAID is determined by the patient’s clinical condition, the selectivity of COX1 and COX2 enzymes, and the risk of cardiovascular and gastric ulcerative2 complications. There is ongoing research to improve the efficacy, potency, safety, and selectivity of existing therapeutic compounds, with a focus on analogue-based drug design.3
Despite the extensive and costly process of synthesizing new drugs, there is still uncertainty about their therapeutic potential and the risk of clinical failure. To address this, researchers are now turning to analogue-based drug design to improve the effectiveness, potency, and safety of lead compounds.4,5 Computer-aided drug design has emerged as a useful tool for designing, synthesizing, and predicting the virtual profiles of chemical entities or analogues of existing lead compounds using molecular and quantum mechanics.6
By synthesizing derivatives of well-established NSAID lead molecules, researchers can take advantage of predictable pharmacological activities, duration of action, doses, and effects. Pharmacological screening of such analogues through in vitro, in vivo, and computer docking can provide data for further optimization of the existing lead molecule, leading to greater efficacy and fewer side effects. The literature reports various stories related to computer-aided drug discoveries to date.7
Indomethacin, a classical NSAID belonging to the indole acetic acid class of drugs, is an effective painkiller used to treat migraines and headaches. It inhibits both isoforms of the COX enzyme responsible for prostaglandin biosynthesis8 and modulates a wide range of receptors and enzymes.9,10 Efforts have been made to synthesize indomethacin analogues of improved potency, efficacy, and lower toxicity,11 with promising results reported for 3-[(2-imidazolyl)alkyl] indole analogues and other derivatives.12−16 By substituting the carboxylic group of NSAIDs, researchers can improve their pharmacological properties while retaining structural stability. As such, new ester analogues of indomethacin (2a–2g) have been synthesized based on supporting studies.
2. Results
2.1. Synthesis
Indomethacin was subjected to a chemical reaction in the presence of various alkyl halides, K2CO3 as a base and tetrahydrofuran (THF) as a solvent. This resulted in the formation of several ester derivatives (2a, 2b, 2c, 2e, 2f, and 2g) in good yield. The reaction mechanism involved K2CO3 capturing the proton of the carboxylic acid of indomethacin to form a carboxylate ion, which then attacked the partial positive charge carbon of the alkyl halide to form the ester derivative. This process is known as functional group interconversion and is illustrated in Scheme 1. The structure, molecular weight, and molecular formula of the synthesized analogues were confirmed using various spectroscopic techniques such as 1H NMR, electron impact mass spectrometry (EIMS), and Fourier transform infrared (FTIR) and are presented in Table 1.
Scheme 1. General Reaction for the Synthesis of Indomethacin Analogues.
Table 1. Ester Derivatives of Indomethacin (2a, 2b, 2c, 2e, 2f, and 2g).

2.1.1. Acetoxymethyl 2-(1-(4-Chloro benzoyl)-5-methoxy-2-methyl-1H-indol-3-yl) Acetate (2a)
Yellowish powder; mp 99–102 °C; yield = 84%; 1H NMR (400 MHz, CDC13): δ 7.64 (d, J2′,3′/6′,5′ = 8.5 Hz, 2H, H-2′, H-6′), 7.44 (d, J3′,2′/5′,6′ = 8.5 Hz, 2H, H-3′, H-5′), 6.97 (d, J5,7 = 2.5 Hz, 1H, H-5), 6.86 (d, J8,7 = 9.0 Hz, 1H, H-8), 6.65 (dd, J7,8 = 8.5 Hz, J7,5 = 2.5 Hz, 1H, H-7), 4.62 (s, 2H, H-1″), 3.82 (s, 3H, H-11), 3.79 (s, 2H, H-13), 3.72 (s, 3H, H-10), 2.36 (s, 3H, H-3″). EI-MS: 429.1 [MS (EI) m/z (% relative abundance), 429.1 (M+, 95), 312 (51), 138.9 (100), 111 (34)]; FTIR: 3032.3 and 2962.6 cm–1 (Ar–H and C–H stretching vibration), strong band at 1742.0 cm–1 (C=O ester stretching), 1691.5 cm–1 (C=O amide stretching), 1469.7 cm–1 (C=C stretching vibration), 1369.4 cm–1 (C–O), and 1037.7 cm–1 (C–Cl).
2.1.2. Hexyl 2-(1-(4-Chloro benzoyl)-5-methoxy-2-methyl-1H-indol-3-yl) Acetate (2b)
Yellowish powder; mp 97–102.5 °C; yield = 64%; Rf (Hex/EA, 8:2), 1H NMR (400 MHz, CDCl3): δ 7.66 (d, J2′,3′/6′,5′ = 8.5 Hz, 2H, H-2′, H-6′), 7.44 (d, J3′,2′/5′,6′ = 8.5 Hz, 2H, H-3′, H-5′), 6.94 (d, J5,7 = 2.5 Hz, 1H, H-5), 6.84 (d, J8,7 = 9 Hz, 1H, H-8), 6.64 (dd, J7,8 = 8.5 Hz, J7,5 = 2.5 Hz, 1H, H-7), 4.07 (d, 2H, H-1″), 3.85 (s, 3H, H-11), 3.63 (s, 2H, H-13), 3.72 (s, 3H, H-10), 1.60 (m, 2H, H-2″), 1.25 (m, 6H, H-3″, H-4″- H-5″), 0.83 (t, 3H, H-6″). EI-MS: 441.3 [MS (EI) m/z (% relative abundance), 441.3 (M+, 84), 312 (50), 139 (100), 83.1 (65), 49.2 (51.1)]; FTIR: 3031.2 and 2951.1, 2895.5 cm–1 (Ar–H and C–H stretching vibration), 1746.5 cm–1 (C=O ester stretching), 1694.5 cm–1 (C=O amide stretching), 1463.6 cm–1 (C=C stretching vibration), 1353.7 cm–1 (C–O), and 1029.7 cm–1 (C–Cl).
2.1.3. 2-(.tert-Butoxy)-2-oxoethyl-2-(1-(4-chloro benzoyl)-5-methoxy-2-methyl-1H-indol-3-yl) Acetate (2c)
Yellowish powder; mp 97–102.5 °C; yield = 54%; Rf (Hex/EA, 8:2), 1H NMR (500 MHz, CDC13): δ 7.64 (d, J2′,3′/6′,5′ = 8.5 Hz, 2H, H-2′, H-6′), 7.45 (d, J3′,2′/5′,6′ = 8.5 Hz, 2H, H-3′, H-5′), 6.98 (d, J5,7 = 2.5 Hz, 1H, H-5), 6.86 (d, J8,7 = 9.0 Hz, 1H, H-8), 6.65 (dd, J7,8 = 8.5 Hz, J7,5 = 2.5 Hz, 1H, H-7), 4.50 (s, 2H, H-1″), 3.82 (s, 3H, H-11), 3.75 (s, 2H, H-13), 3.36 (s, 3H, H-10), 1.43 (s, 9H, 4″-H, 5″-H , 6″-H). EI-MS: 471.2 [MS (EI) m/z (% relative abundance) 471.2 (M+, 88), 312.1 (44), 139 (100), 111 (22)]; FTIR: 3001.2 and 2931.1, 2885.5 cm–1 (Ar–H and C–H stretching vibration), strong band at 1755.5 cm–1 (C=O ester stretching), 1689.5 cm–1 (C=O amide stretching), 1473.6 cm–1 (C=C stretching vibration), 1334.7 cm–1 (C–O), and 1037.7 cm–1 (C–Cl).
2.1.4. 2-Oxo-2-phenylethyl2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3yl) (Acetate) 2e
0.35 g as white crystalline powder (% yield), mp 97–102.5 °C, Rf (Hex/EA, 8:2), 1H NMR (500 MHz, CDC13): δ 7.85 (d, J4′,5′/8′,7′ = 9.0 Hz, 2H, H-4″, H-8″), 7.67 (d, J2′,3′/6′,5′ = 8.5 Hz, 2H, H-2′, H-6′), 7.58 (t, 1H, J6″,5″ = 7.0 Hz, J6″,7″ = 7.5 Hz, H-6″), 7.45 (d, J3′,2′/5′,6′ = 8.5 Hz, 2H, H-3′, H-5′), 7.43 (dd, 2H, J5″,4″/7″,8″, = 7.5 Hz, J5″,6″/7″,6″, = 5.0 Hz, H-5″, H-7″), 7.04 (d, J5,7 = 2.5 Hz, 1H, H-5), 6.88 (d, J8,7 = 9.0 Hz, 1H, H-8), 6.66 (dd, J7,8 = 8.5 Hz, J7,5 = 2.5 Hz, 1H, H-7), 5.43 (s, 2H, H-1″), 3.81 (s, 3H, H-11), 3.59 (s, 2H, H-13), 3.26 (s, 3H, H-10). EI-MS: 475.3 [MS (EI) m/z (% relative abundance) 475.3 (M+, 82), 312.1 (35), 139 (100), 111 (30)]; FTIR: 3055.2 and 2924.1, 2831.5 cm–1 (Ar–H and C–H stretching vibration), 1743.6 cm–1 (C=O ester stretching), 1698.7 cm–1 (C=O amide stretching), 1454.3 cm–1 (C=C stretching vibration), 1319.3 cm–1 (C–O), and 1087.8 cm–1 (C–Cl).
2.1.5. Propyl 2-(1-(4-Chloro benzoyl)-5-methoxy-2-methyl-1H-indol-3yl) Acetate (2f)
Yellowish powder; mp 99–102.5 °C; yield = 84%; 1H NMR (400 MHz, CDC13): δ 7.64 (d, J2′,3′/6′,5′ = 8.5 Hz, 2H, H-2′, H-6′), 7.44 (d, J3′,2′/5′,6′ = 8.5 Hz, 2H, H-3′, H-5′), 6.94 (d, J5,7 = 2.5 Hz, 1H, H-5), 6.84 (d, J8,7 = 9.0 Hz, 1H, H-8), 6.64 (dd, J7,8 = 8.5 Hz, J7,5 = 2.5 Hz, 1H, H-7), 4.04 (d, 2H, H-1″), 3.85 (s, 3H, H-11), 3.63 (s, 2H, H-13), 3.72 (s, 3H, H-10), 1.63 (m, 2H, H-2″), 0.89 (s, 3H, H-7″). EI-MS: 399.1 [MS (EI) m/z (% relative abundance), 399.1 (M+, 75), 312 (71), 138.9 (100), 111 (39)]; FTIR: 3045.2 and 2944.1, 2861.5 cm–1 (Ar–H and C–H stretching vibration), 1753.6 cm–1 (C=O ester stretching), 1696.7 cm–1 (C=O amide stretching), 1448.3 cm–1 (C=C stretching vibration), 1329.6 cm–1 (C–O), and 1077.8 cm–1 (C–Cl).
2.1.6. Heptyl 2-(1-(4-Chloro benzoyl)-5-methoxy-2-methyl-1H-indol-3-yl) Acetate (2g)
Yellowish powder; mp 99–102.5 °C; yield = 84%; 1H NMR (400 MHz, CDC13): δ 7.64 (d, J2′,3′/6′,5′ = 8.5 Hz, 2H, H-2′, H-6′), 7.44 (d, J3′,2′/5′,6′ = 8.5 Hz, 2H, H-3′, H-5′), 6.94 (d, J5,7 = 2.5 Hz, 1H, H-5), 6.84 (d, J8,7 = 9.0 Hz, 1H, H-8), 6.64 (dd, J7,8 = 8.5 Hz, J7,5 = 2.5 Hz, 1H, H-7), 4.04 (d, 2H, H-1″), 3.86 (s, 3H, H-11), 3.63 (s, 2H, H-13), 3.72 (s, 3H, H-10), 1.61 (m, 2H, H-6″), 1.29–1.19 (m, 8H, H-5″, H-4″, H-3″, H-2″), 0.84 (d, 3H, H-3″). EI-MS: 455.4 [MS (EI) m/z (% relative abundance), 455.4 (M+, 82), 312 (69), 139 (100), 111 (19)]; FTIR: 3042.2 and 2924.1, 2869.8 cm–1 (Ar–H and C–H stretching vibration), 1742.6 cm–1 (C=O ester stretching), 1691.4 cm–1 (C=O amide stretching), 1450.2 cm–1 (C=C stretching vibration), 1321.8 cm–1 (C–O), and 1067.1 cm–1 (C–Cl) (Table 1).
2.2. Acute Toxicity Study
At doses of 50, 75, 100, and 200 mg/kg i.p. after 24 h, no death was noted in the acute toxicity study.
2.3. Analgesic Assay
2.3.1. Acetic-Induced Assay
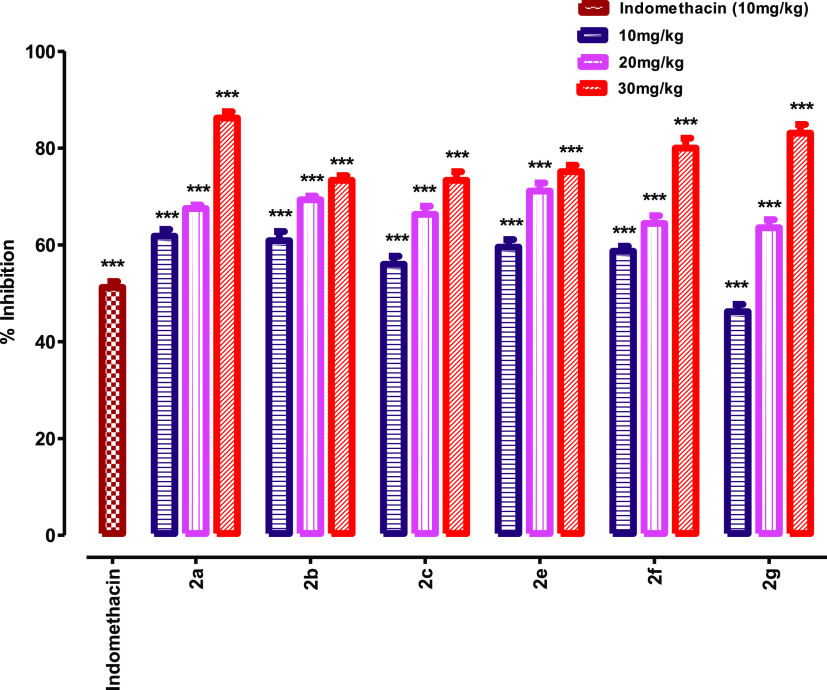
Figure 1 clearly shows that percent inhibition increases in dose-dependent manner for each analogue of the lead compound. The extent to which the test compound 2a inhibits writhing at doses of 10, 20, and 30 mg/kg i.p. is 61.7, 67.5, and 86.2%, compound 2b showed 60.8, 69.3, and 73.3%, compound 2c showed 56, 66.3, and 73.3%, compound 2e showed 59.5, 71.1, and 75.1%, compound 2f showed 58.6, 64.4, and 80%, and compound 2g showed percent inhibitions of 46.2, 63.5, and 83.1% as compared to the negative control group (Table 2). Results confirmed that at a dose of 10 mg/kg, all the analogues except 2g showed greater percent inhibition than the standard indomethacin (lead compound) which is 51.23%. Analogue compounds showed lesser activity in the hot plate analgesic assay than the standard tramadol. Hence, it is concluded that the analogue compounds have much better peripheral analgesic activity than the parent lead compound with no or very less central analgesic activity.
Figure 1.
Antinociceptive activity of indomethacin derivatives using the acetic acid-induced abdominal constriction assay protocol. Analysis of variance (ANOVA) was applied before Dennett’s post hoc analysis. ***p < 0.001, **p < 0.01, and *p < 0.05 compared to the negative control group, n = 6.
Table 2. Analgesic Activity (Acetic Acid-Induced Writhing) of Indomethacin Derivatives.
| treated group | dose (mg/kg i.p.) | no. of writhing (20 min) | percent Analgesia (%) |
|---|---|---|---|
| normal saline | 10 | 37.50 ± 1.25 | |
| indomethacin | 10 | 17.04 ± 1.22*** | 51.23 |
| test compound 2a | 10 | 14.33 ± 1.43*** | 61.79 |
| 20 | 12.17 ± 0.70*** | 67.55 | |
| 30 | 5.17 ± 1.33*** | 86.22 | |
| test compound 2b | 10 | 14.67 ± 1.89*** | 60.88 |
| 20 | 11.50 ± 0.76*** | 69.33 | |
| 30 | 10.00 ± 1.00*** | 73.33 | |
| test compound 2c | 10 | 16.50 ± 1.65*** | 56.00 |
| 20 | 12.50 ± 1.67*** | 66.33 | |
| 30 | 10.00 ± 1.79*** | 73.33 | |
| test compound 2e | 10 | 15.17 ± 1.54*** | 59.55 |
| 20 | 10.83 ± 1.68*** | 71.12 | |
| 30 | 9.33 ± 1.33*** | 75.12 | |
| test compound 2f | 10 | 15.50 ± 1.06*** | 58.67 |
| 20 | 13.33 ± 1.58*** | 64.46 | |
| 30 | 7.50 ± 2.04*** | 80.00 | |
| test compound 2g | 10 | 20.17 ± 1.49*** | 46.21 |
| 20 | 13.67 ± 1.67*** | 63.55 | |
| 30 | 6.33 ± 1.78*** | 83.11 |
2.3.2. Hot Plate Assay
Table 3 clearly shows the latency of nociceptive response in seconds in a dose-dependent manner for each analogue of the lead compound. Analogue compounds showed lesser activity in the hot plate analgesic assay than the standard tramadol. Hence, it is concluded that the analogue compounds have much better peripheral analgesic activity than the parent lead compound with no or very less central analgesic activity.
Table 3. Hot Plate-Induced Analgesic Assaya.
| treatment groups | dose (mg/kg) | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|---|
| saline | 10 | 9.03 ± 0.64 | 9.28 ± 0.58 | 8.27 ± 1.29 | 9.17 ± 0.71 | 9.02 ± 0.71 |
| tramal | 10 | 10.32 ± 0.57 | 26.22 ± 1.07 | 25.45 ± 2.52*** | 20.37 ± 0.88*** | 20.07 ± 1.66*** |
| 2a | 50 | 9.02 ± 0.44 | 14.88 ± 1.43 | 15.90 ± 0.87** | 16.00 ± 0.54*** | 15.33 ± 1.30** |
| 75 | 10.82 ± 0.59 | 15.68 ± 1.24 | 16.15 ± 1.71** | 16.25 ± 1.40*** | 15.88 ± 1.07** | |
| 100 | 9.53 ± 0.38 | 17.43 ± 0.86 | 17.55 ± 0.66*** | 17.60 ± 1.13*** | 16.82 ± 1.05*** | |
| 2b | 50 | 9.54 ± 0.32 | 13.68 ± 1.37 | 13.95 ± 1.32* | 15.10 ± 1.27** | 15.72 ± 1.31** |
| 75 | 9.58 ± 0.46 | 14.37 ± 1.29 | 15.23 ± 0.38** | 15.62 ± 1.51*** | 16.27 ± 1.01*** | |
| 100 | 10.08 ± 0.38 | 15.98 ± 0.85 | 16.40 ± 0.66*** | 16.42 ± 0.68*** | 16.78 ± 0.54*** | |
| 2c | 50 | 9.55 ± 0.48 | 14.30 ± 1.24 | 15.80 ± 0.66** | 13.95 ± 1.11** | 13.65 ± 0.97* |
| 75 | 10.03 ± 0.75 | 14.82 ± 0.53 | 16.78 ± 2.11** | 14.30 ± 0.75** | 14.02 ± 1.04* | |
| 100 | 10.80 ± 1.08 | 16.02 ± 1.02 | 18.12 ± 1.02*** | 16.88 ± 1.40*** | 16.63 ± 1.34*** | |
| 2e | 50 | 9.06 ± 0.88 | 13.60 ± 1.23 | 14.08 ± 1.00* | 16.37 ± 1.11*** | 14.77 ± 1.41** |
| 75 | 9.64 ± 0.66 | 13.95 ± 1.32 | 14.68 ± 1.11** | 16.65 ± 1.44*** | 15.47 ± 1.39** | |
| 100 | 10.82 ± 0.58 | 14.55 ± 0.92 | 15.65 ± 0.35** | 16.68 ± 1.16*** | 16.38 ± 0.56*** | |
| 2f | 50 | 10.46 ± 0.28 | 13.95 ± 1.28 | 15.68 ± 0.70** | 14.83 ± 0.76** | 14.70 ± 1.44* |
| 75 | 10.42 ± 0.36 | 17.23 ± 1.71 | 17.38 ± 1.02*** | 15.25 ± 1.11** | 15.23 ± 1.31** | |
| 100 | 9.78 ± 0.33 | 17.43 ± 0.86 | 17.78 ± 0.96*** | 15.70 ± 1.54*** | 15.58 ± 0.93** | |
| 2g | 50 | 10.12 ± 0.80 | 15.18 ± 1.49 | 15.40 ± 1.09** | 15.45 ± 0.83*** | 14.88 ± 0.52*** |
| 75 | 10.46 ± 0.68 | 16.05 ± 1.25 | 16.45 ± 0.74*** | 16.57 ± 1.13*** | 16.50 ± 1.14*** | |
| 100 | 10.10 ± 1.02 | 16.57 ± 0.56 | 16.75 ± 0.71*** | 16.78 ± 1.20*** | 16.70 ± 1.20*** |
For group (n = 6), these values are presented as mean ± SEM. The data were analyzed using ANOVA through Dunnett’s test. Compared to negative control, ***p < 0.001, **p < 0.01, and *p < 0.05.
2.4. Anti-Inflammatory Activity
It is clear from the results obtained statistically (Table 4) that all derivatives of indomethacin showed significant anti-inflammatory activity except 2b, 2c, and 2e at 10 mg/kg dose comparable to tramadol and indomethacin administered in the same dose during 1st hour but at 2, 3, 4, and 5 h, the results were significant (***p < 0.001). All the derivatives show significant results at 20 and 30 mg/kg i.p. dose. However, there is variation among different derivatives in terms of its activity. This is due to the attachment of different carbon-length alkyl halides.
Table 4. Carrageenan-Induced Paw Edema Modela.
| treatment | dose (mg/kg) | 1 h | 2 h | 3 h | 4 h | 5 h |
|---|---|---|---|---|---|---|
| saline | 10 | 3.408 ± 0.131 | 3.605 ± 0.065 | 3.402 ± 0.234 | 3.509 ± 0.242 | 3.305 ± 0.228 |
| standard (diclofenac–Na) | 10 | 2.283 ± 0.201*** | 2.142 ± 0.105*** | 2.004 ± 0.224*** | 2.010 ± 0.185*** | 2.019 ± 0.022*** |
| indomethacin | 10 | 2.462 ± 0.032*** | 2.207 ± 0.218*** | 2.100 ± 0.122*** | 2.128 ± 0.370*** | 2.184 ± 0.632*** |
| test compound 2a | 10 | 2.417 ± 0.102*** | 2.404 ± 0.056*** | 2.285 ± 0.456*** | 2.293 ± 0.208*** | 2.308 ± 0.215*** |
| 20 | 2.403 ± 0.098*** | 2.322 ± 0.049*** | 2.142 ± 0.045*** | 2.143 ± 0.124*** | 2.170 ± 0.123*** | |
| 30 | 2.350 ± 0.280*** | 2.125 ± 0.140*** | 2.074 ± 0.268*** | 2.100 ± 0.327*** | 2.112 ± 0.099*** | |
| test compound 2b | 10 | 2.722 ± 0.193* | 2.604 ± 0.096** | 2.196 ± 0.426*** | 2.198 ± 0.244*** | 2.200 ± 0.364*** |
| 20 | 2.467 ± 0.148** | 2.433 ± 0.282*** | 2.046 ± 0.154*** | 2.047 ± 0.083*** | 2.060 ± 0.152*** | |
| 30 | 2.225 ± 0.274*** | 2.105 ± 0.364*** | 2.026 ± 0.148*** | 2.048 ± 0.063*** | 2.053 ± 0.053*** | |
| test compound 2c | 10 | 2.717 ± 0.074* | 2.458 ± 0.237*** | 2.142 ± 0.082*** | 2.148 ± 0.654*** | 2.160 ± 0.064*** |
| 20 | 2.433 ± 0.080*** | 2.254 ± 0.246*** | 2.078 ± 0.095*** | 2.087 ± 0.933*** | 2.099 ± 0.822*** | |
| 30 | 2.233 ± 0.240*** | 2.106 ± 0.122*** | 2.052 ± 0.082*** | 2.058 ± 0.783*** | 2.073 ± 0.753*** | |
| test compound 2e | 10 | 2.700 ± 0.175* | 2.205 ± 0.238*** | 2.186 ± 0.128*** | 2.198 ± 0.024*** | 2.200 ± 0.644*** |
| 20 | 2.517 ± 0.147** | 2.108 ± 0.302*** | 2.090 ± 0.254*** | 2.097 ± 0.042*** | 2.120 ± 0.422*** | |
| 30 | 2.205 ± 0.222*** | 2.104 ± 0.264*** | 2.005 ± 0.522*** | 2.008 ± 0.085*** | 2.023 ± 0.363*** | |
| test compound 2f | 10 | 2.467 ± 0.126*** | 2.433 ± 0.063*** | 2.343 ± 0.008*** | 2.348 ± 0.165*** | 2.400 ± 0.424*** |
| 20 | 2.417 ± 0.125*** | 2.362 ± 0.252*** | 2.120 ± 0.016*** | 2.127 ± 0.296*** | 2.180 ± 0.252*** | |
| 30 | 2.217 ± 0.265*** | 2.208 ± 0.368*** | 2.026 ± 0.032*** | 2.028 ± 0.044*** | 2.053 ± 0.683*** | |
| test compound 2g | 10 | 2.692 ± 0.127** | 2.646 ± 0.255** | 2.389 ± 0.026*** | 2.398 ± 0.038*** | 2.440 ± 0.054*** |
| 20 | 2.650 ± 0.126** | 2.425 ± 0.258*** | 2.038 ± 0.264*** | 2.047 ± 0.032*** | 2.180 ± 0.082*** | |
| 30 | 2.433 ± 0.297*** | 2.218 ± 0.182*** | 2.006 ± 0.528*** | 2.008 ± 0.046*** | 2.023 ± 0.023*** |
For a group of six animals, results are presented as mean ± S.E.M. ANOVA and Dunnett’s test were used to evaluate data. Asterisks from the control represent statistically significant values, ***p < 0.001, **p < 0.01, and *p < 0.05.
2.5. Molecular Docking Study
The indomethacin derivatives termed 2a, 2b, 2c, 2e, 2f, and 2g were also docked inside the naproxen-COX2 [PDB ID: 3PGH] complex for virtual target identification and binding affinity assessment. The indole nucleus and chlorobenzoyl portion of the parent drug are shared by all the corresponding derivatives with variations made only to the acetic acid hydroxyl of indomethacin. This means that major variations in the binding energies of the derived compounds will be due to the different side chains.
Three different orientations of indomethacin with mild variations in the binding energies may be visualized inside the COX2-binding site. It may either be having the methoxy end of the indole nucleus or chlorobenzoyl appendage or acetate tail deep toward the aromatic amino acid residues, i.e., Tyr 385 and Phe 381. However, most of the derivatives portray the third stereo-chemical alignment with more favorable binding energies that signify ligand–protein stability. The chlorobenzoyl moiety is directed toward the important amino acid in the COX-2 active site, i.e., Arg120 and Tyr355 (Figure 2). The methoxy end of the indole nucleus is attracted toward Val523. The acetoxymethyl end of compound 2a is involved in a hydrogen bond with Tyr385 and Arg120 (Figure 3 and Figure 4). Compound 2b represents almost similar coordinates except for a hydrogen bond of the acetate end with Ser530 (∼2.99 Å). The compound 2c derivative possesses extra hydrogen-bond acceptors that contribute to having an extra hydrogen bond with Tyr385 (∼2.98 Å). Compound 2e possesses an aromatic ring in its tail/extension, which in addition to having a hydrogen-bonding interaction with Ser530, also holds aromatic contact with Phe 518. As compared to the rest of the derivatives, compounds 2f and 2g establish weak hydrogen bonds with Ala 527 and Gly 526, respectively. Compound 2g in comparison to 2f marks a lower Rerank score, which is due to extra energy dissipated by folding the comparatively longer tail and adjusting it correspondingly to its surroundings. The interaction energies of binding poses of the indomethacin derivatives in comparison with the parent drug are provided in Table 5.
Figure 2.

Molecular overlay of the virtual binding modes revealing molecular contacts between ligands and the COX-2 protein.
Figure 3.

Molecular binding mode of 2a inside the active site of COX-2.
Figure 4.

Closer view of molecular interactions between indomethacin and amino acid residues surrounding the active site of COX-2. Dotted lines indicate hydrogen-bonding interactions.
Table 5. Molecular Docking Study of Indomethacin Derivatives.
| interaction
energies |
||||
|---|---|---|---|---|
| s. no. | ligand | MolDock score (kJ/ mol) | rerank score (kJ/ mol) | H-bond (kJ/ mol) |
| 1 | indomethacin | –141.07 | –109.34 | –1.89 |
| 2 | 2a | –149.39 | –103.77 | –0.31 |
| 3 | 2b | –146.72 | –94.01 | –2.19 |
| 4 | 2c | –160.85 | –69.19 | –2.50 |
| 5 | 2e | –159.34 | –97.86 | –1.50 |
| 6 | 2f | –140.03 | –100.20 | –0.31 |
| 7 | 2g | –150.91 | –93.82 | –0 |
3. Discussion
Despite significant advances in medicinal chemistry, inflammatory disorders continue to pose a significant challenge for symptomatic and therapeutic treatment.17 Our research work aimed to develop new and effective COX inhibitors that are safer for patients. We focused on replacing the hydroxyl group at C-14 in the indomethacin structure with bulkier groups to enhance its anti-inflammatory, analgesic, and antipyretic activities. All the analogues were synthesized according to the planned protocol and confirmed through spectroscopic analysis. Our in vivo experiments demonstrated that, except for compounds 2b, 2c, and 2e, all the analogues showed significant anti-inflammatory effects at a dose of 10 mg/kg body weight in mice, comparable to diclofenac sodium (positive control), while at a dose of 20 and 30 mg/kg, almost all were significant. This may be attributed to the length of the carbon chain in alkyl halides that replaced the OH group. Our initial structure–activity relationship data also suggests that all analogues possessed marked peripheral analgesic activities, as confirmed through in vivo screening, except for 2g at a dose of 10 mg/kg.
Our docking study revealed that the indole nucleus and chlorobenzoyl portion of the parent drug are shared by all the corresponding derivatives, with variations made only to the acetic acid hydroxyl of indomethacin. Therefore, the significant differences in the binding energies of the derived compounds are due to the different side chains. This virtual molecular insight supports the highly significant anti-inflammatory potential of indomethacin derivatives compared to the parent drug. Additionally, the enhanced negative binding energies of the derived compounds as compared to those of the parent drug support our research theme of producing stronger derivatives of standard drugs with potent analgesic and antipyretic properties.
4. Materials and Methods
4.1. General
The thin-layer chromatography (TLC) plate used was precoated with Kieselgel 60 F25 silica gel (Merck, Germany) for reaction progress and clarity of synthesized analogues; melting point was determined using the Bicot melting point apparatus (Biby Scientific Limited. UK), 1H NMR spectra were recorded using Burker AVANCE Neo 400 and 500 MHz with chemical shift (δ) described in parts per million, EIMS was performed on JEOL MS Route (JMS 600 H), FTIR was conducted using Prestige IR-21 of Shimadzu, Japan, UV spectroscopy was performed using PerkinElmer Lambda 25 UV/VIS spectrometer, and CHN analysis was recorded using “Carlo Erba Strumentazione” (Mod-1106), Italy.
Research-grade indomethacin was obtained from Abbot Laboratories Pakistan Limited. All chemicals (THF, potassium carbonate, n-hexane, and ethyl acetate) were acquired from Sigma-Aldrich, USA, and of analytical grade. Normal saline (Otsuka Japan) was used for the preparation of a solution of synthesized analogue to administer to animals to observe in vivo response.
4.2. General Procedure for the Synthesis of Indomethacin Analogues
All analogues of indomethacin were synthesized according to Scheme 1. Lead molecules (indomethacin) were dissolved in high-performance liquid chromatography-grade THF, and potassium carbonate (2 mol equiv) was added to a round-bottom flask, fitted with a reflux condenser. It was stirred for 30 min at 50 °C, and then various alkyl halides were added and allowed for reflux condensation at 50–60 °C. The reaction was observed through TLC, while after completion, a workup was performed by adding water and then the product was extracted by ethyl acetate using a separating funnel. Extraction was repeated three times. The purification of the crude product was performed through silica gel chromatography to afford the desired product (2a–2g).
4.3. Experimental Animals
Animals (BALB-c mice) for pharmacological screening were arranged/purchased from the animal house of the Pharmacy department, University of Peshawar KPK, Pakistan. All animals were kept in an animal house under standard lab conditions. 12 h of light/dark cycle with temperature 25 ± 2 °C was maintained. The ethical committee granted permission for the experiment to be carried out under reference no. 04/EC/F.LIFE-2020. During the experiment, all animals were handled and treated according to approved standard ethical procedures.
4.4. Acute Toxicity
An acute toxicity study was performed using BALB-c mice (n = 6), treated with different test compound doses (50, 75, 100, and 200 mg/kg, i.p.); normal saline was administered to the control group (10 mL/kg) for the purpose to detect any probable toxicity. All experimental animals were carefully monitored for full 24 h for any major effects or mortality.18
4.5. Anti-Inflammatory Activity
A total of 30 mice were randomly assigned to five groups with six mice in each group.19−21 To induce inflammation, 0.1 mL of 1% carrageenan solution was injected into the plantar surface of the mouse’s hind paw. Test groups I, II, and III were administered with 10, 20, and 30 mg/kg of the test compound, respectively. Group IV, which served as the control group, was treated with normal saline (1 mL/kg), while group V was treated with diclofenac (10 mg/kg) as the reference standard, 30 min before carrageenan injection. The paw volume was measured at 0, 1, 2, 3, 4, and 5 h using a digital plethysmometer, and the degree of edema was quantified as the percentage inhibition using following equation.
| 1 |
where α and β represent the increase in paw volume of control- and drug-treated animals, respectively.
4.6. Analgesic Assay
4.6.1. Acetic Acid-Induced Writhing Assay
During this study, mice (BALB-c) of either sex were used weighing 18–22 g. Mice were kept on fasting for 2 h before the experiment under standard laboratory conditions. Mice were distributed into five (05) groups. The mice in group I received normal saline (10 mL/kg), while mice in group II were injected with standard drug indomethacin (10 mg/kg i.p.). Similarly, groups III–V were injected test doses of indomethacin analogues (10, 20, and 30 mg/kg i.p.), respectively. After 30 min of N. saline, standard, and test dose administration, all mice were injected 1% v/v (10 mL/kg, i.p.) acetic acid. The writhing reflex of each mouse was keenly observed and counted after 5 min of AA administration.22 The percent analgesic effect was determined as
4.6.2. Hot Plate Assay
During this study, mice (BALB-c) of either sex were used weighing 18–22 g. 1 h before the assay, animals were acclimatized to the working environment and provided with standard lab food and water ad-libitum. Pretest latency time of all mice was noted using the Harvard apparatus maintained at a temperature of 54 ± 0.1 °C. All those animals showing pretesting latency times above 15 s were excluded from the experiment.23 All mice were divided into five groups. After 30 min of pretesting, group I received normal saline (10 mL/kg), while group II received tramadol (20 mg/kg). Similarly, groups III–V received test doses of indomethacin analogue (10, 20, and 30 mg/kg i.p.) compounds, respectively. Latency times at 30, 60, 90, and 120 min were recorded at Harvard apparatus. A cutoff time of 30 s was obligatory for the purpose to avoid any tissue damage. The percent analgesic effect was determined as
4.7. Statistical Analysis
Graph Pad Prism ver. 5.01 was applied to evaluate the data. ANOVA was used to assess the results of pharmacological screening, and then the post hoc Dunnet’s test was performed. P values between 0.05 and 0.001 were significant and very significant, respectively. Whole data is stated as mean ± S.E.M for a group of six (06) animals (mice).
5. Conclusions
The currently designed study illustrated an effective synthesis of new indomethacin analogues starting from indomethacin. The test analogues 2a, 2b, 2c, 2e, and 2f showed a pronounced peripheral analgesic effect, while 2g exhibited a slightly lesser analgesic effect at a dose comparable to the parent lead compound. However, lesser but significant central analgesic effects compared to standard tramadol were perceived in the hot plate assay for the test analogues. Similarly, the entire derivatives showed significant anti-inflammatory results at the doses of 20 and 30 mg/kg body weight. Further detailed studies were suggested from our findings about these compounds to determine their clinical efficacy.
Acknowledgments
Authors are thankful to the researchers supporting project number (RSP2023R491), King Saud University, Riyadh, Saudi Arabia.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c02033.
1H NMR spectra of compound 2a, 1H NMR spectra of compound 2b, 1H NMR spectra of compound 2C, 1H NMR spectra of compound 2e, 1H NMR spectra of compound 2f, and 1H NMR spectra of compound 2g (PDF)
Author Contributions
Conceptualization, A.A.A, K.H., M.R.S., and S.M.A.H; methodology, F.R. and Z.A.; I.K software, F.R and A.R., A.A., M.A.; writing—original draft preparation, H.A.R.S. and Z.A.; supervision, I.U. and M.R.S.; The manuscript’s published version has been read and approved by all authors.
Researchers Supporting Project number (RSP2023R491), King Saud University, Riyadh, Saudi Arabia.
The authors declare no competing financial interest.
Notes
Ethical approval was granted from the ethical committee, Department of Pharmacy, University of Peshawar (04/EC/F.LIFE-2020; 20.01.2020). All animals used in the experiment were handled and treated by ethical standards that had been authorized by an ethical committee.
Supplementary Material
References
- Ray W. A.; Varas-Lorenzo C.; Chung C. P.; Castellsague J.; Murray K. T.; Stein C. M.; Daugherty J. R.; Arbogast P. G.; García-Rodríguez L. A. Cardiovascular risks of nonsteroidal antiinflammatory drugs in patients after hospitalization for serious coronary heart disease. Circ. Cardiovas. Qual. Outcomes 2009, 2, 155–163. 10.1161/circoutcomes.108.805689. [DOI] [PubMed] [Google Scholar]
- Van Hecken A.; Schwartz J. I.; Depré M.; De Lepeleire I.; Dallob A.; Tanaka W.; Wynants K.; Buntinx A.; Arnout J.; Wong P. H.; et al. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J. Clin. Pharmacol. 2000, 40, 1109–1120. 10.1177/009127000004001005. [DOI] [PubMed] [Google Scholar]
- Wlodawer A. Rational approach to AIDS drug design through structural biology. Annu. Rev. Med. 2002, 53, 595–614. 10.1146/annurev.med.53.052901.131947. [DOI] [PubMed] [Google Scholar]
- Fischer J.; Ganellin C. R. Analogue-based drug discovery. Chem. Int. Newsmag. IUPAC 2010, 32, 12–15. 10.1515/ci.2010.32.4.12. [DOI] [Google Scholar]
- Schneider G.; Fechner U. Computer-based de novo design of drug-like molecules. Nat. Rev. Drug Discovery 2005, 4, 649–663. 10.1038/nrd1799. [DOI] [PubMed] [Google Scholar]
- Lu P.; Bevan D. R.; Leber A.; Hontecillas R.; Tubau-Juni N.; Bassaganya-Riera J.. Computer-aided drug discovery. In Accelerated Path to Cures; Springer, 2018, pp 7–24. [Google Scholar]
- Kubinyi H. Success stories of computer-aided design. Comp. Appl. Pharm. Res. Dev. 2006, 2, 377. 10.1002/0470037237. [DOI] [Google Scholar]
- Dannhardt G.; Kiefer W. Cyclooxygenase inhibitors – current status and future prospects. Eur. J. Med. Chem. 2001, 36, 109–126. 10.1016/s0223-5234(01)01197-7. [DOI] [PubMed] [Google Scholar]
- Hla T.; Neilson K. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. U.S.A. 1992, 89, 7384–7388. 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J. M.; Lenhard J. M.; Oliver B. B.; Ringold G. M.; Kliewer S. A. Peroxisome Proliferator-activated Receptors α and γ Are Activated by Indomethacin and Other Non-steroidal Anti-inflammatory Drugs. J. Biol. Chem. 1997, 272, 3406–3410. 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- Amir M.; Kumar H.; Javed S. A. Synthesis and pharmacological evaluation of condensed heterocyclic 6-substituted-1, 2, 4-triazolo [3, 4-b]-1, 3, 4-thiadiazole derivatives of naproxen. Bioorg. Med. Chem. Lett. 2007, 17, 4504–4508. 10.1016/j.bmcl.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Verma M.; Tripathi M.; Saxena A.; Shanker K. Antiinflammatory activity of novel indole derivatives. Eur. J. Med. Chem. 1994, 29, 941–946. 10.1016/0223-5234(94)90193-7. [DOI] [Google Scholar]
- Amir M.; Kumar S. anti-inflammatory and gastro sparing activity of some new indomethacin derivatives. Int. J. Pharm. Med. Chem. 2005, 338, 24–31. 10.1002/ardp.200400891. [DOI] [PubMed] [Google Scholar]
- Kalgutkar A. S.; Marnett A. B.; Crews B. C.; Remmel R. P.; Marnett L. J. Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J. Med. Chem. 2000, 43, 2860–2870. 10.1021/jm000004e. [DOI] [PubMed] [Google Scholar]
- da Silva Guerra A. S. H.; do Nascimento Malta D. J.; Laranjeira L. P. M.; Maia M. B. S.; Colaço N. C.; do Carmo Alves de Lima M.; Galdino S. L.; da Rocha Pitta I.; Gonçalves-Silva T. Anti-inflammatory and antinociceptive activities of indole–imidazolidine derivatives. Int. Immunopharmacol. 2011, 11, 1816–1822. 10.1016/j.intimp.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Chennamaneni S.; Zhong B.; Lama R.; Su B. COX inhibitors Indomethacin and Sulindac derivatives as antiproliferative agents: synthesis, biological evaluation, and mechanism investigation. Eur. J. Med. Chem. 2012, 56, 17–29. 10.1016/j.ejmech.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Shi C.; Zhou Z.; Chi X.; Xiu S.; Yi C.; Jiang Z.; Chen R.; Zhang L.; Liu Z. Recent advances in gout drugs. Eur. J. Med. Chem. 2023, 245, 114890. 10.1016/j.ejmech.2022.114890. [DOI] [PubMed] [Google Scholar]
- Khan H.; Saeed M.; Gilani A. u. H.; Khan M. A.; Khan I.; Ashraf N. Antinociceptive activity of aerial parts of Polygonatum verticillatum: attenuation of both peripheral and central pain mediators. Phytother Res. 2011, 25, 1024–1030. 10.1002/ptr.3369. [DOI] [PubMed] [Google Scholar]
- Khan I.; Nisar M.; Shah M. R.; Shah H.; Gilani S. N.; Gul F.; Abdullah S. M.; Ismail M.; Khan N.; Kaleem W. A.; et al. Anti-inflammatory activities of Taxusabietane A isolated from Taxus wallichiana Zucc. Fitoterapia 2011, 82, 1003–1007. 10.1016/j.fitote.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Alblihed M. A. Astragalin attenuates oxidative stress and acute inflammatory responses in carrageenan-induced paw edema in mice. Mol. Biol. Rep. 2020, 47, 6611–6620. 10.1007/s11033-020-05712-z. [DOI] [PubMed] [Google Scholar]
- Rai U.; Rawal A.; Singh S. Evaluation of the anti-inflammatory effect of an anti-platelet agent crinumin on carrageenan-induced paw oedema and granuloma tissue formation in rats. Inflammopharmacology 2018, 26, 769–778. 10.1007/s10787-017-0411-7. [DOI] [PubMed] [Google Scholar]
- Miranda H. F.; Puig M. M.; Prieto J. C.; Pinardi G. Synergism between paracetamol and nonsteroidal anti-inflammatory drugs in experimental acute pain. Pain 2006, 121, 22–28. 10.1016/j.pain.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Eddy N. B.; Leimbach D. Synthetic analgesics. II. Dithienylbutenyl-and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 385–393. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.