Abstract
The negative regulator of splicing (NRS) from Rous sarcoma virus suppresses viral RNA splicing and is one of several cis elements that account for the accumulation of large amounts of unspliced RNA for use as gag-pol mRNA and progeny virion genomic RNA. The NRS can also inhibit splicing of heterologous introns in vivo and in vitro. Previous data showed that the splicing factors SF2/ASF and U1, U2, and U11 small nuclear ribonucleoproteins (snRNPs) bind the NRS, and a correlation was established between SF2/ASF and U11 binding and activity, suggesting that these factors are important for function. These observations, and the finding that a large spliceosome-like complex (NRS-C) assembles on NRS RNA in nuclear extract, led to the proposal that the NRS is recognized as a minor-class 5′ splice site. One model to explain NRS splicing inhibition holds that the NRS interacts nonproductively with and sequesters U2-dependent 3′ splice sites. In this study, we provide evidence that the NRS interacts with an adenovirus 3′ splice site. The interaction was dependent on the integrity of the branch point and pyrimidine tract of the 3′ splice site, and it was sensitive to a mutation that was previously shown to abolish U11 snRNP binding and NRS function. However, further mutational analyses of NRS sequences have identified a U1 binding site that overlaps the U11 site, and the interaction with the 3′ splice site correlated with U1, not U11, binding. These results show that the NRS can interact with a 3′ splice site and suggest that U1 is of primary importance for NRS splicing inhibition.
Expression of genes in simple retroviruses like Rous sarcoma virus (RSV) is completely dependent on the host cell transcriptional and RNA processing machineries. The primary transcript must undergo RNA splicing to generate, in the simplest cases, the envelope mRNA. In contrast to host cell messages which are usually spliced to completion, a substantial portion of the retroviral RNA remains unspliced and is transported to the cytoplasm, where it serves as gag-pol mRNA and genomic RNA for new virions. Thus, in a cellular environment which favors complete splicing, the virus must subvert the splicing apparatus to allow accumulation of sufficient full-length RNA to fulfill the cytoplasmic roles. One mechanism for regulating RSV splicing involves a cis-acting sequence that represses viral RNA splicing, the negative regulator of splicing (NRS), that is located in the gag region of the viral genome (2, 29). The NRS is novel in that it is a bipartite element that lies ∼400 nucleotides (nt) downstream of the viral 5′ splice site (5′ ss) and over 4,000 nt upstream of the nearest 3′ ss, and so is not associated with the splice sites (2). The NRS can also inhibit splicing of heterologous introns in vivo (2, 21), and the finding that in vitro splicing of model pre-mRNAs is blocked in an aberrantly large spliceosome-like complex suggested that the inhibition was direct and at the spliceosome level (12).
The spliceosome is a macromolecular complex in which the catalytic reactions of pre-mRNA splicing occur (17, 24). The spliceosome assembles in a stepwise fashion through the sequential binding of U1, U2, U5, and U4:U6 small nuclear ribonucleoprotein particles (snRNPs) and a large number of non-snRNP splicing factors. Early studies of metazoan spliceosome assembly by native gel analysis revealed the ATP-dependent prespliceosomal A complex and mature spliceosomal B complex (15, 16). Subsequently, it was shown that the earliest detectable complex in assembly is the ATP-independent early (E) complex, in which pre-mRNA becomes committed to the splicing pathway (22, 26). The E complex, which is detected by gel filtration chromatography but not by native gel analysis, is comprised of U1 snRNP, U2AF, mammalian branch point binding protein (mBBP)/SF1, and a large number of spliceosome-associated proteins (5, 23). The 5′ and 3′ ss can independently assemble ATP-independent complexes, called E5′ and E3′, respectively, but the E complex assembles more efficiently on pre-mRNAs containing both splice sites (23). The 5′ and 3′ ss are recognized and become functionally associated in the E complex in a process that is promoted by SR protein splicing factors (23, 28). SR proteins are thought to bridge the 5′ ss-3′ ss association through interactions with RS domains in U2AF35 and the U1 70K protein (11, 25, 31), which suggests that these factors play a role in splice site selection at an early step. An interaction of the NRS with a 3′ ss would presumably take place between factors that normally interact with the 3′ ss and NRS binding factors.
It was previously shown that U1 and U2 snRNPs of the major splicing pathway and the minor-pathway U11 snRNP bind the NRS. There is also strong evidence that binding of the SR protein SF2/ASF is required for inhibition (12, 19). These observations suggested that the NRS may be recognized as a 5′ ss; in support of this proposal, we recently showed that the NRS assembles an ATP-independent complex, called NRS-C, that resembles the U1 E5′ complex (7). NRS-C assembly requires ASF/SF2, U1 snRNP, and the U11 binding site, and based on a correlation between U11 binding and function (12), we speculated that NRS-C may represent a U11 E5′ complex (8). However, the accompanying article (20) challenges a functional role for U11 and strongly suggests that U1 binding correlates best with inhibition in vivo. It has been proposed that the NRS blocks splicing through nonproductive interactions with U2-dependent 3′ ss, perhaps through interactions analogous to those in E complex assembly, and thereby sequesters the 3′ ss from interacting with the normal 5′ ss (12, 19).
To determine if the NRS can interact with a U2-dependent 3′ ss and, if it can, to establish if U1 and/or U11 snRNP is involved, we used Sephacryl S500 chromatography to detect early interactions between the NRS and an adenovirus (Ad) 3′ ss (Ad3′). We found that an NRS-Ad3′ chimeric substrate assembled into a specific complex more efficiently than either the NRS or Ad3′ substrate alone. This is also the case for pre-mRNA 5′ and 3′ ss (23), which suggests that the NRS and Ad3′ interact. Chimeric substrates that contained an NRS mutation that abolishes U1 but not U11 binding (20), or mutations in the branch point or pyrimidine tract of Ad3′, were impaired for complex assembly. A specific U11 mutation was without effect. Consistent with the suggestion that U1 and 3′ ss factors mediated the interaction, substrate competition experiments showed that the wild-type substrate was present in specific complexes at higher levels than substrates containing the NRS or Ad3′ mutations. In agreement with results in the accompanying article (20), our data for chimeras also indicate that a U1 binding site overlaps the U11 site. Thus, these results suggest that the NRS may function by interacting nonproductively with a 3′ ss and, contrary to previous suggestions of an important role for U11, that splicing inhibition by the NRS likely involves U1 snRNP.
MATERIALS AND METHODS
DNA constructs and in vitro transcription.
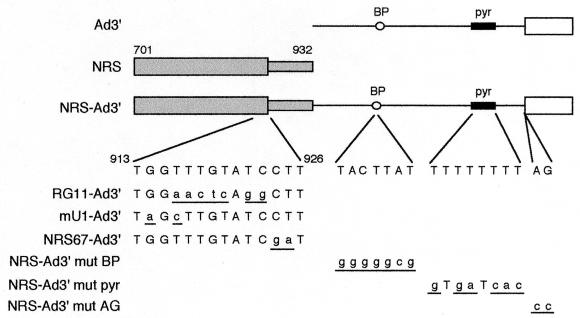
The salient features of the constructs used are shown in Fig. 1. To generate pRG11-Ad3′ and pNRS67-Ad3′, PCR fragments from RSV DNA (nt 703 to 930 [RSV numbering system of Schwartz et al. [27]) containing the RG11 12) or CT67GA (nt 924 and 925 changed from CT to GA [20]) mutation were inserted into the BstEII intron site of pAdHS (7) (KpnI and XbaI sites were appended to the ends of the PCR fragment). All primer sequences are available upon request. The resulting constructs (pAdKXRG11 and pAdKXBB67) and pAdKXBB (8), which contains the wild-type sequence, were cleaved with HindIII and KpnI to remove the 5′ ss, blunted, and religated to make pRG11-Ad3′, pNRS67-Ad3′, and pNRS-Ad3′, respectively. pNRS-Ad3′mutBP, in which the branch point was changed from TACTTAT to GGGGGCG (branch point adenosine is underlined), and pNRS-Ad3′mutAG, in which the terminal AG at the 3′ ss was changed to CC, were made by site-directed mutagenesis of pAdKXBB, using a Morph mutagenesis kit (5 Prime-3 Prime, Inc.). The mutant constructs were then cleaved with HindIII and KpnI to remove the 5′ ss, blunted, and religated. pNRS-Ad3′mutPyr, in which the pyrimidine tract was changed from TTTTTTTT to GTGATCAC, and pmU1-Ad3′, in which nt 914 and 916 of the NRS were changed from G and T to A and C, were made with a U.S.E. (unique site elimination) mutagenesis kit (Pharmacia Biotech) and pAdKXBB as the template. The resulting constructs were then cleaved with HindIII and KpnI to remove the 5′ ss, blunted, and religated.
FIG. 1.
Schematic representation of substrates used in this study. For Ad3′, Ad major late sequences representing the last 80 nt of the first intron (line) and first 48 nt of the second exon (open box), the branch point (BP), and the pyrimidine tract (pyr) are shown. For the NRS, 701 and 932 indicate the endpoints of the RSV sequence. NRS-Ad3′ is a chimeric substrate in which the NRS is linked to Ad3′. The relevant wild-type RSV (nt 913 to 926) and Ad sequences are shown below this construct. Mutations in the NRS-Ad3′ derivatives are shown below the wild-type sequence, with mutations in lowercase and underlined. RG11-Ad3′, mU1-Ad3′, and NRS67-Ad3′ are chimeric substrates containing the indicated mutations in the NRS. NRS-Ad3′mutBP, NRS-Ad3′mutPyr, and NRS-Ad3′mutAG are chimeric substrates containing the indicated mutations in the branch point, pyrimidine tract, and terminal AG of Ad3′, respectively. The diagram is not drawn to scale.
A different set of plasmids was made for use in the substrate competition assay such that long and short versions of each RNA could be produced. All of the above constructs except pNRS-Ad3′mutAG were cleaved with SacI, blunted, and recut with AflIII; the 754-nt fragment was then inserted into pGEM-4Z that was cut with XbaI, blunted, and recut with AflIII. pAd3′ was made by cutting pAdHS with HindIII and BstEII to remove exon 1 and part of the intron, blunting, and religating. p3ZBB, used to generate NRS RNA, was described previously (7). pAdmutPyr, in which the pyrimidine tract is changed from TTTTTTTT to GTGATCAC, was made by U.S.E. mutagenesis (Pharmacia Biotech) of pAdHS. The longer version of Ad (pAd-long) for use in the substrate competition assay was made by cutting pAd2HB (10) with ScaI and HindIII and inserting the 209-nt fragment into pGEM-4Z that had been cut with HincII and HindIII. All plasmids were linearized with EcoRI except p3ZBB, which was linearized with BamHI. RNA was produced in vitro with T7 RNA polymerase as described elsewhere (7). In vitro transcription of biotinylated RNAs was done as previously described (8) except that biotin-11-UTP constituted 20% of the total UTP.
Gel filtration.
Nuclear extracts were prepared by standard methods except that buffer D contained 20 mM Tris-HCl (pH 8.0) rather than HEPES (9). Nuclear extract was preincubated for 20 min at room temperature to deplete endogenous ATP. Complexes were assembled at 30°C for 20 min in 60% nuclear extract under in vitro splicing conditions (100 μl; 17 mM Tris-HCl [pH 7.8], 60 mM KCl) except that no ATP, creatine phosphate, or MgCl2 was added. The amounts of RNA incubated in the assembly reactions are indicated in the figure legends. Gel filtration was performed as described elsewhere (7) at a flow rate of 6 ml/h. For gel filtration, samples were applied to a 1.5- by 50-cm Sephacryl S500 column equilibrated in FSP buffer (20 mM Tris-HCl [pH 7.8], 0.1% Triton X-100, 60 mM KCl, 2.5 mM EDTA), the column was developed at 6 ml/h, 1-ml fractions were collected, and 50 μl was counted in a Packard microwell plate scintillation counter.
Substrate competition assay.
Assembly reactions were as described above except that equal moles (as indicated in the figure legends) of two competing RNA substrates were incubated together in the reaction. To aid in distinguishing each RNA, longer substrates contained an additional 21 nt of vector-derived sequence. After gel filtration, equal counts per minute from the specific and nonspecific peaks were extracted with phenol-chloroform-isoamyl alcohol and ethanol precipitated, and RNA was separated by electrophoresis in a denaturing 8 M urea–4% polyacrylamide gel. Bands were visualized by autoradiography and quantitated with a Molecular Dynamics Storm 860 PhosphorImager.
Affinity selection.
Biotinylated or nonbiotinylated, 32P-labeled RNA (990 fmol) was incubated in ATP-depleted nuclear extract for 30 min at 30°C in a 25-μl reaction mixture under splicing conditions except that no ATP, creatine phosphate, or MgCl2 was added. Samples were put on ice, and an equal volume of SB buffer (10 mM Tris-HCl [pH 7.8], 3 mM MgCl2, 1 mM dithiothreitol, 540 mM KCl) was added to raise the KCl concentration to 300 mM. A 50:50 slurry of streptavidin-agarose beads (20 μl) was added and mixed at 4°C for 1 h, followed by three washes in 1 ml of NET-300 buffer (50 mM Tris-HCl [pH 7.8], 0.05% Nonidet P-40, 0.5 mM dithiothreitol, 300 mM KCl) for 10 min. Bound material was released by incubating the beads for 15 min at 37°C in 200 μl of proteinase K buffer (10 mM Tris-HCl, [pH 7.8], 5 mM EDTA, 0.5% sodium dodecyl sulfate) containing 0.5 mg of proteinase K per ml. The eluted RNAs were then extracted with phenol-chloroform-isoamyl alcohol, ethanol precipitated, separated by electrophoresis in a denaturing 8 M urea–8% polyacrylamide gel, and electrophoretically transferred to a ZetaProbe GT (Bio-Rad) membrane. The blot was hybridized overnight at 50 to 55°C with U1 and U11 riboprobes as described elsewhere (8).
RESULTS
RNP assembly on chimeric NRS-Ad3′ substrates.
Michaud and Reed (23) previously showed by gel filtration that the 5′ and 3′ ss of an Ad pre-mRNA interact in vitro in the E complex and that E complex assembly is more efficient on an intact substrate than on the isolated 5′ or 3′ ss. It has been proposed that the NRS blocks splicing by interacting with downstream 3′ ss, precluding the normal 5′ ss-3′ ss interaction. To test this idea, we used Sephacryl S500 gel filtration chromatography to detect possible early interactions between the NRS and Ad3′, the latter chosen because it was earlier demonstrated that the NRS blocks Ad pre-mRNA splicing in vitro (12). Radiolabeled RNA (Fig. 1) was incubated in nuclear extract under conditions that promote E complex assembly, complexes were applied to Sephacryl S500 columns, and column fractions were collected and counted. Since Ad3′ RNA alone makes an ATP-independent complex (E3′ [23]), we empirically determined the moles of Ad3′ RNA that gave a 1:1 ratio of E3′ to nonspecific (H) complex (Fig. 2A) and used the same moles of RNA to assay complex formation on the isolated NRS (nt 701 to 932) and the chimeric substrates containing the NRS and Ad3′ in cis. The ratio of NRS to H complex with NRS RNA was 1:2 (Fig. 2B), indicating that NRS-C assembly is less efficient than E3′ complex formation. When the chimeric NRS-Ad3′ substrate was used, a specific H complex ratio of 3:1 was observed (Fig. 2C), indicating that assembly is more efficient on NRS-Ad3′ than on the individual substrates. Since the nature of this complex is unknown, we shall refer to it here simply as the specific (S) complex. When the isolated NRS and Ad3′ RNAs were incubated together in trans in nuclear extract and then subjected to gel filtration, the S/H complex ratio was ∼1:1 (Fig. 2D), which verified that the 3:1 ratio on NRS-Ad3′ was not simply the sum of the NRS and E3′ complexes. Thus, when the NRS and Ad3′ are in cis, the specific complex assembles much more efficiently than on the individual substrates. One interpretation of these results is that the NRS and Ad3′ interact, consistent with results obtained using authentic 5′ and 3′ ss (23).
FIG. 2.
Gel filtration of complexes formed on Ad3′, the NRS, and NRS-Ad3′ chimeric substrates suggests an interaction between the NRS and Ad3′. The indicated radiolabeled RNA (600 fmol) was incubated under splicing conditions in nuclear extract in the absence of ATP for 20 min at 30°C. Samples were then applied to Sephacryl S500 columns, fractions were collected, and aliquots were counted in a scintillation counter. Peaks eluting in fractions 30 to 40 and 70 to 80 contain the void volume and degraded RNA, respectively. In panel D, 600 fmol each of NRS and Ad3′ RNAs was added to nuclear extract in trans, and the sample was applied to the Sephacryl S500 column. Peaks representing the E3′, H (nonspecific), NRS, and S (specific) complexes are indicated.
To determine if the interaction was dependent on the U1 and U11 sites, a chimeric substrate (RG11-Ad3′) containing the RG11 NRS mutation (12), which abolishes both U11 and U1 snRNP binding and splicing inhibition (20), was assayed for complex assembly. The more efficient assembly characteristics of NRS-Ad3′ were lost with RG11-Ad3′, which, like Ad3′ alone, yielded a 1:1 ratio of S to H complex (Fig. 2E). These data indicate that the sequences mutated in RG11 are required for the interaction with Ad3′ and imply that U1 and/or U11 is involved (see below).
The interaction of the 5′ ss and 3′ ss in Ad pre-mRNAs was sensitive to mutations in the pyrimidine tract, whereas the branch point and terminal AG mutations had no effect (6, 23). To determine which elements comprising Ad3′ were required for the NRS interaction, the same mutations in the branch point, pyrimidine tract, or terminal dinucleotides of the 3′ ss were incorporated into the chimeric substrates (Fig. 1). Mutation of the terminal dinucleotides of Ad3′ did not affect the enhanced assembly efficiency (ratio of 3:1), indicating that the terminal dinucleotides are not required for the interaction of Ad3′ with the NRS (Fig. 2F). However, the mutant branch point and pyrimidine tract substrates showed diminished amounts of the S complex; the column profiles showed S/H complex ratios of 1.6:1 and 1.3:1, respectively (Fig. 2G and H), which are slightly better than the 1:1 ratio obtained with Ad3′ alone but not as good as the 3:1 ratio obtained with the wild-type NRS-Ad3′ substrate. Thus, the results suggest that the branch point and pyrimidine tract contribute to the interaction of Ad3′ with the NRS in an early spliceosome-like complex.
Wild-type chimeric substrates exhibit a competitive advantage in early complex assembly.
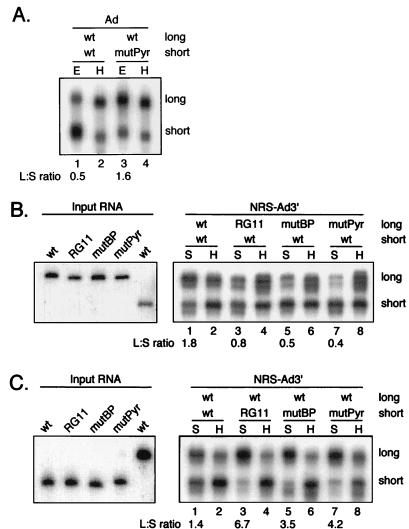
The experiments described above showed that specific sequences within the NRS and Ad3′ were required for the interaction between the NRS and Ad3′. To independently verify this result, we used a substrate competition assay that was used previously to show that the 5′ ss and 3′ ss of a pre-mRNA functionally interact in the E complex (23). NRS-Ad3′ was mixed with equal moles of chimeric RNA containing mutations in the NRS, or in the Ad3′ branch point or pyrimidine tract, and the RNAs were added to nuclear extract. The complexes were separated by gel filtration, and the distribution of the wild-type and mutant RNAs in specific and nonspecific complexes was determined by denaturing gel electrophoresis. To aid in distinguishing the competing RNAs, one of the substrates contained an additional 21 vector-derived nt. One would predict that wild-type substrates would have a competitive advantage over mutant RNAs in specific complex assembly, and therefore wild-type RNA should be enriched and the mutant RNA should be underrepresented in the specific peak.
To determine the potential magnitude of the competitive effect, Ad pre-mRNAs were used as positive controls to demonstrate the competitive advantage of wild-type RNA in E complex assembly compared to a pre-mRNA containing a specific mutation in the pyrimidine tract, as first shown by Michaud and Reed (23). When longer and shorter versions of wild-type Ad pre-mRNA were incubated together in nuclear extract and separated by gel filtration, the E complex peak contained more shorter RNA than longer RNA (Fig. 3A, lane 1). The ratio of long to short Ad pre-mRNA was 0.5, indicating that the extra nucleotides in the longer substrate slightly inhibited E complex formation. However, when a shorter pre-mRNA containing a mutant pyrimidine tract was competed against the longer wild-type pre-mRNA, the wild-type RNA was abundant in the E complex peak (ratio of 1.6 [Fig. 3A, lane 3]). These results confirmed that this experimental approach could be used to detect interactions between the NRS and Ad3′.
FIG. 3.
Substrate competition assay provides support for an NRS interaction with Ad3′. (A) Ad pre-mRNAs (Ad) were used as positive controls to demonstrate that the wild-type substrate has a competitive advantage over a pyrimidine tract mutant in complex assembly. One of the competing RNAs (long) contained an additional 21 nt of vector-derived sequence to distinguish it from the short RNA (short) in the gel. Equal amounts (1,800 fmol) of two Ad pre-mRNAs, either wild type (wt) or a pyrimidine tract mutant (mutPyr), were incubated together in nuclear extract, and the samples were applied to Sephacryl S500 columns. RNA was extracted from fractions containing the specific E complex (E; lanes 1 and 3) and nonspecific H complex (H; lanes 2 and 4) and separated in a denaturing 8 M urea–8% polyacrylamide gel, and autoradiography was performed. (B) The indicated NRS-Ad3′ chimeric substrates (1,800 fmol) were used in the substrate competition assay, and the RNAs in the specific (S) and nonspecific (H) complexes were identified by autoradiography as for panel A. The shorter RNA (short) in each competition was wild type (wt), while the longer substrate (long) was wild type (lanes 1 and 2), contained the NRS RG11 mutation (RG11; lanes 3 and 4), or contained the branch point mutation (mutBP; lanes 5 and 6) or pyrimidine tract mutation (mutPyr; lanes 7 and 8) in Ad3′. The left part of the panel shows the input RNAs. (C) Same as panel B except that the longer RNA was wild type while the shorter RNAs were as indicated. The left part of the panel shows the input RNAs. Samples obtained from the specific (S) and nonspecific (H) complexes are indicated above the lanes; the ratios of long to short RNA (L:S) in the S complexes are indicated below the lanes.
The substrate competition assay was next used with the chimeric substrates. When two wild-type substrates of different lengths were used, the longer substrate was 1.8-fold more abundant than the shorter substrate in the specific peak (Fig. 3B, lane 1), indicating that the longer RNA had an advantage in S complex assembly. However, when either the NRS RG11 mutation or the branch point or pyrimidine tract mutation of Ad3′ was incorporated into the longer substrates, the longer mutant RNAs were less abundant than the wild-type RNAs in the S complex (Fig. 3B, lanes 3, 5, and 7). The ratios of long to short RNA changed from 1.8 for the wild-type competition to 0.8, 0.5, and 0.4 when the RG11, branch point, and pyrimidine tract mutants, respectively, were used. These data are in support of the results obtained by gel filtration.
Since the longer wild-type chimeric substrate had an advantage over the short one in complex assembly, the converse experiment was performed to show that the results were independent of the vector-derived nucleotides at the 3′ end of the longer substrate. Therefore, the longer substrate was made the wild-type RNA whereas the shorter substrates contained the mutations. Again, when two wild-type substrates were competed, the longer substrate had a slight (1.4-fold) advantage in specific complex assembly over the shorter wild-type substrate (Fig. 3C, lane 1). When competed against the mutant substrates, the wild-type RNA again had a distinct advantage in specific complex assembly. The long/short RNA ratio changed from 1.4 with the wild-type competition to 6.7, 3.5, and 4.2 with the RG11, branch point, and pyrimidine tract mutants, respectively (Fig. 3C, lanes 3, 5, and 7), consistent with the previous results. These results provide independent support for an interaction between the NRS and Ad3′ at an early stage of complex assembly. The interaction is likely mediated by snRNPs bound to the NRS and factors that normally interact with the 3′ ss elements.
U1 and U11 snRNP binding to chimeric RNAs.
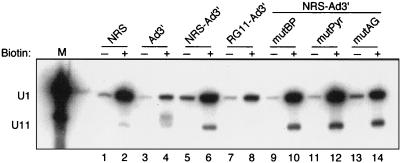
In light of the finding that the RG11 mutation reduced binding of both U1 and U11 to the free NRS (20), it remained unknown which snRNP was responsible for the interaction observed above. To address this question directly, U1 and U11 binding to the NRS, Ad3′, or various chimeras was assessed. Complexes were assembled on biotinylated or nonbiotinylated control RNAs and were affinity selected from nuclear extract with streptavidin-agarose beads, and snRNA components of the bound snRNPs were identified by Northern analysis with U1 and U11 riboprobes. As expected with the 701–932 NRS substrate, the results showed modest U11 snRNP binding to the isolated NRS (Fig. 4, lane 2) but not to Ad3′ (lane 4). Binding of U11 to NRS-Ad3′ (lane 6) was also evident and was repeatedly ∼3-fold higher than with the isolated NRS (lane 2). This increase in U11 snRNP binding is consistent with the more efficient assembly observed above with NRS-Ad3′. RG11-Ad3′, which does not support more efficient complex assembly (Fig. 2E), did not bind U11 snRNP (Fig. 4, lane 8). While these results supported a possible role for U11 in the interaction, this conclusion was compromised by the fact that RG11 also affects U1 binding (20).
FIG. 4.
The NRS RG11 mutation disrupts both U11 and U1 snRNP binding. The indicated biotinylated (+) or nonbiotinylated (−) RNA (990 fmol) was incubated in nuclear extract and affinity selected with streptavidin-agarose beads (see Materials and Methods), and the snRNA components of bound snRNPs were extracted and identified by Northern analysis with U1 and U11 antisense riboprobes. The band of slightly slower migration than the U11 band in lane 4 is the Ad3′ substrate. U1 and U11 snRNA markers (M) were extracted from nuclear extract. The positions of U1 and U11 snRNA are indicated at the left. RNAs are designated as in Fig. 1.
The results with the U1 probe showed that U1 snRNP binding was well above background for the isolated NRS (Fig. 4, lanes 1 and 2) and was much lower but easily detected with the isolated Ad3′ (lane 4). It was previously shown that U1 is present at low levels in E3′ assembled on Ad3′ (23). Binding of U1 to NRS-Ad3′ was also strong (lane 6), but the amount of U1 selected by RG11-Ad3′ (lane 8) was repeatedly about threefold lower. Thus, as with the isolated NRS (20), the RG11 mutation affects both U1 and U11 binding to the chimeras. These results made it formally possible that the interaction of the NRS with Ad3′ was through U11 and U1 snRNPs, or either one alone (addressed below).
We also determined if U1 and/or U11 binding to chimeric substrates was affected by the Ad3′ mutations. The data showed that binding of both snRNPs was unaffected by mutations in the branch point, pyrimidine tract, or terminal AG of Ad3′ (Fig. 4, lanes 10, 12, and 14), indicating that no single mutation in the Ad3′ elements affected U1 or U11 binding to the NRS. These results (i) are consistent with the NRS and Ad3′ regions being recognized independently in the chimeric RNA and (ii) suggest that the interaction between them is through snRNPs bound to the NRS and factors that bind the Ad3′ region.
U1 snRNP and the NRS interaction with Ad3′ in vitro.
Because U11 binding to RG11-Ad3′ was eliminated and U1 binding was repeatedly ∼3-fold lower than with NRS-Ad3′, it was possible that either or both snRNPs were involved in the NRS/Ad3′ interaction. To address this, NRS mutations which were shown to selectively disrupt U1 or U11 binding to the free NRS (Fig. 5A and reference 20) were incorporated into chimeric RNAs to appraise their effect on the interaction. The mU1 mutation changes nt 914 and 916 from G and T to A and C, respectively, and disrupts the U1 site but not the U11 site. The NRS67 mutation, meanwhile, changes nt 924 and 925 of the U11 consensus from GTATCCTT to GTATCGAT, a mutation that abrogated function of an authentic U11 5′ ss (14) and eliminated U11 but not U1 binding to the free NRS (20). The ability of the mutants to bind U1 and U11 snRNPs was determined by the affinity selection assay. Levels of U11 binding were similar with mU1-Ad3′ and NRS-Ad3′, but U1 binding to mU1-Ad3′ was decreased fourfold (Fig. 5B; compare lanes 6 and 2). This diminution in U1 binding is similar to that observed with RG11-Ad3′ (lane 4). Thus, as expected, the mU1 mutation reduced U1 snRNP binding but did not affect U11 snRNP binding. Likewise, U1 snRNP binding was slightly greater with NRS67-Ad3′ than with NRS-Ad3′ (compare lanes 8 and 2), but NRS67-Ad3′ did not bind U11 snRNP (lane 8), indicating the specificity of the NRS67 mutation. The increased U1 snRNP binding observed when the U11 site was mutated suggests that U1 and U11 snRNPs compete for binding to this region in the chimera, as was true for the free NRS (20). Thus, the snRNP-specific chimeras could be used in gel filtration to determine which snRNP was involved in the interaction.
FIG. 5.
The mU1 and NRS67 mutations specifically disrupt binding of U1 and U11 snRNP, respectively, to chimeric substrates. (A) The wild-type (wt) NRS sequence (nt 913 to 926) is shown along with the corresponding changes (lowercase) in the RG11, mU1, and NRS67 mutants. Dashes represent unchanged nucleotides. The U11 site is overlined, and the U1 site is underlined. (B) Affinity selection of the indicated biotinylated (+) or nonbiotinylated (−) chimeric RNA (990 fmol) was performed as for Fig. 4, and snRNA components of bound snRNPs were identified by Northern analysis. U1 and U11 snRNA markers (M) were extracted from nuclear extract. The positions of U1 and U11 snRNA are indicated at the left.
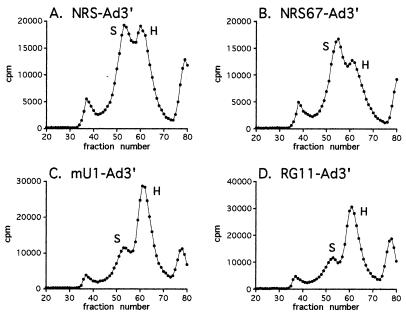
For gel filtration, we used an empirically determined amount of NRS-Ad3′ RNA that resulted in a 1:1 ratio of S to H complex (Fig. 6A). The column profile generated by NRS67-Ad3′, which binds U1 snRNP but not U11 snRNP, was similar to that seen with NRS-Ad3′ (S/H ratio of 1.2 [Fig. 6B]). Thus, assembly of the specific complex on NRS67-Ad3′ was slightly more efficient than on the wild-type NRS-Ad3′ substrate and correlated with the slight increase in U1 snRNP binding (Fig. 5, lane 8). This result suggests that U11 snRNP does not play a major role in the interaction between the NRS and Ad3′ in vitro. In contrast, mU1-Ad3′, which exhibits normal U11 binding but decreased U1 binding, formed much less specific complex (S/H ratio of ∼1:2.5 [Fig. 6C]). The column profile for RG11-Ad3′, which exhibited decreased U1 snRNP binding and does not bind U11 snRNP, also resulted in an S/H ratio of ∼1:2.5 (Fig. 6D). Thus, both mU1-Ad3′ and RG11-Ad3′ bind U1 snRNP poorly and do not form the specific complex efficiently.
FIG. 6.
U1 snRNP, but not U11, participates in the in vitro interaction of the NRS with Ad3′. The amount of NRS-Ad3′ RNA (3950 fmol) that resulted in a 1:1 ratio of S to H complex was used to assay complex formation on the substrates indicated in each panel. The indicated radiolabeled RNAs were incubated in nuclear extract in the absence of ATP for 20 min at 30°C, the samples were applied to Sephacryl S500 columns, and fractions were collected and counted in a scintillation counter. Peaks eluting in fractions 30 to 40 and 70 to 80 contain the void volume and degraded RNA, respectively. The specific (S) and nonspecific (H) peaks are indicated.
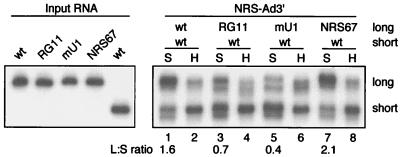
The above results were confirmed with the substrate competition assay (Fig. 7). As before, the longer wild-type NRS-Ad3′ substrate had an advantage (1.6-fold) over the shorter wild-type substrate (Fig. 7, lane 1), and RG11-Ad3′ was a poor competitor against NRS-Ad3′ (ratio of 0.7 [lane 3]). The mU1-Ad3′ substrate, which binds U1 inefficiently, was also a poor competitor, as NRS-Ad3′ was enriched in the specific peak (ratio 0.4, lane 5). However, the NRS67-Ad3′ substrate, which does not bind U11 snRNP, was a very efficient competitor against NRS-Ad3′ (ratio of 2.1 [lane 7]), even better than the wild-type RNA. This is also consistent with the increased U1 binding shown by this substrate. Thus, the substrate competition results provide further support that U1 snRNP, but not U11, plays a major role in the interaction between the NRS and Ad3′ in vitro; collectively, the data implicate U1 snRNP in NRS splicing inhibition.
FIG. 7.
Substrate competition assay provides support for a role of U1 snRNP in the NRS-Ad3′ interaction. NRS-Ad3′ chimeric substrates (2,850 fmol) were used in the substrate competition assay as for Fig. 3. The shorter RNA (short) in each competition was wild type (wt), while the longer substrate (long) was wild type (lanes 1 and 2) or contained the NRS RG11 (lanes 3 and 4), mU1 (lanes 5 and 6), or NRS67 (lanes 7 and 8) mutation. Samples from the specific (S) and nonspecific (H) complexes are indicated above the lanes; ratios of long to short RNA (L:S) in the S complexes are indicated below the lanes.
DISCUSSION
The maintenance of a large pool of unspliced RNA is an important aspect of retroviral replication. In RSV, this is accomplished in part by the NRS, an RNA element which binds components of the splicing machinery including snRNPs and SR proteins. Models to explain NRS splicing inhibition have centered on U11 since its binding was originally thought to correlate with activity and because evidence of functional roles for U1 and U2 has been lacking. However, results in the accompanying paper challenge this view and suggest a role for U1 snRNP (20). It has been proposed that splicing inhibition might be elicited through a nonproductive interaction between the NRS and 3′ ss which these new data imply would involve U1. This report provides evidence that such an interaction indeed occurs between the NRS and Ad3′ which is dependent on the Ad3′ branch point/pyrimidine tract and correlates with binding of U1 to the NRS rather than U11.
Sephacryl S500 gel filtration chromatography has been used extensively to identify and study components of the multiple complexes in the spliceosome assembly pathway (E, A, B, and C) (3, 13, 22, 23), complexes that assemble on RNA splicing enhancers (28, 30), and the cap binding complex (18). Our use of this approach also revealed a complex that assembles on the isolated NRS (NRS-C) that has several characteristics in common with the E complex and particularly the E5′ complex that assembles on isolated 5′ ss (7, 8). First, the NRS-C is detected by gel filtration chromatography but not native gel analysis. Second, assembly is ATP independent, and preformed NRS-C is dissociated by ATP. Third, factors required for NRS-C assembly are limiting in nuclear extract. Fourth, SR proteins and U1 snRNP are required for NRS-C assembly, and each is a component of the complex. Given the evidence that the NRS is recognized as a 5′ ss and to provide some support for the idea that splicing inhibition involves an interaction between the NRS and 3′ ss, we sought to demonstrate an association between the NRS and the Ad 3′ ss. Much like the results of Michaud and Reed (23) which indicated that the 5′ and 3′ ss of an Ad splicing substrate associate in E complex, the gel filtration and substrate competition experiments provide two independent lines of evidence that an interaction occurs between the NRS and Ad3′.
Consistent with the findings in the accompanying paper of a U1 site that overlaps the U11 site and that U1 binding strongly correlates with function (20), it was shown here that U1 and U11 binding to the NRS-Ad3′ chimeras can be separated and that the interaction was related to U1 rather than U11. Our previous result that debilitating U1 in nuclear extract has a dramatic effect on NRS-C (7, 8), which was curious at the time but not easily explained given the strength of the U11 data, was the first suggestion of a functional role for U1. The earlier results are now reconciled with the finding that NRS-C is also sensitive to the mU1 mutation but not to the U11-specific mutation (data not shown) and are compatible with the in vitro interaction of the NRS with Ad3′ being primarily mediated by U1 and not U11.
With pre-mRNA, it was found that the pyrimidine tract but not the branch point is required for the interaction of 5′ and 3′ ss (6, 23). In the present study, we found that both the pyrimidine tract and the branch point sequences were required for the NRS/Ad3′ interaction. While this may mean that the interactions in the two cases differ in nature, we also found that the 5′ ss-3′ ss interaction with our Ad pre-mRNA was sensitive to the branch point mutation. The discrepancy between this finding and earlier data (6, 23) may stem from subtle sequence differences between the two Ad pre-mRNA constructs in the branch point region (data not shown). The dependence of the interaction on the pyrimidine tract suggests that the NRS/Ad3′ interaction may require a protein that binds the pyrimidine tract, U2AF65 (32), and a smaller associated protein, U2AF35 (33). SR proteins can interact with the RS domain in U2AF35 and the U1 70K protein (31). Thus, the NRS bridging interactions could be mediated by these proteins in a manner similar to that proposed for pre-mRNAs (25). Recently, Berglund et al. (4) showed that mBBP/SF1 and U2AF65 interact to promote cooperative binding to the branch point/pyrimidine tract region. The dependence of the NRS/Ad3′ interaction in our constructs on the branch point sequence may be due to a lack of branch point recognition by mBBP/SF1 and, as a consequence, less efficient U2AF65 binding. It is also possible that the NRS/Ad3′ interaction is between U1 and mBBP/SF1, since this has been suggested as a possible intron bridging mechanism for pre-mRNAs (1). While the details of the interactions remain to be determined, it is likely that the major features of the NRS interaction, which ultimately block splicing, resemble natural 5′ ss-3′ ss interactions.
The data presented here and in the accompanying paper (20) provide in vitro and in vivo support for a primary role of U1 snRNP in NRS-mediated splicing inhibition. The observation that common splicing factors can be subverted to block splicing suggests that the NRS mechanism may be used by the cell for splicing control. How does U1 binding to the NRS result in splicing inhibition, and does splicing inhibition result from this or subsequent steps? Further analysis of the in vitro interaction between the NRS and Ad3′ are in progress to address these questions.
ACKNOWLEDGMENTS
We thank members of the McNally lab for critical reviews of the manuscript.
This research was supported by Public Health Service grant R29 CA63348 from the National Cancer Institute.
REFERENCES
- 1.Abovich N, Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell. 1997;89:403–412. doi: 10.1016/s0092-8674(00)80221-4. [DOI] [PubMed] [Google Scholar]
- 2.Arrigo S, Beemon K. Regulation of Rous sarcoma virus RNA splicing and stability. Mol Cell Biol. 1988;8:4858–4867. doi: 10.1128/mcb.8.11.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett M, Michaud S, Kingston J, Reed R. Protein components specifically associated with prespliceosome and spliceosome complexes. Genes Dev. 1992;6:1986–2000. doi: 10.1101/gad.6.10.1986. [DOI] [PubMed] [Google Scholar]
- 4.Berglund J A, Abovich N, Rosbash M. A cooperative interaction between U2AF65 and mBBP/SF1 facilitates branchpoint region recognition. Genes Dev. 1998;12:858–867. doi: 10.1101/gad.12.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berglund J A, Chua K, Abovich N, Reed R, Rosbash M. The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Cell. 1997;89:781–787. doi: 10.1016/s0092-8674(00)80261-5. [DOI] [PubMed] [Google Scholar]
- 6.Champion-Arnaud P, Gozani O, Palandjian L, Reed R. Accumulation of a novel spliceosomal complex on pre-mRNAs containing branch site mutations. Mol Cell Biol. 1995;15:5750–5756. doi: 10.1128/mcb.15.10.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook C R, McNally M T. Characterization of an RNP complex that assembles on the Rous sarcoma virus negative regulator of splicing element. Nucleic Acids Res. 1996;24:4962–4968. doi: 10.1093/nar/24.24.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook C R, McNally M T. SR protein and snRNP requirements for assembly of the Rous sarcoma virus negative regulator of splicing complex in vitro. Virology. 1998;242:211–220. doi: 10.1006/viro.1997.8983. [DOI] [PubMed] [Google Scholar]
- 9.Dignam J D, Lebovitz R M, Roeder R D. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frendaway D, Keller W. Stepwise assembly of a pre-mRNA splicing complex requires U-snRNPs and specific intron sequences. Cell. 1985;42:355–367. doi: 10.1016/s0092-8674(85)80131-8. [DOI] [PubMed] [Google Scholar]
- 11.Fu X D. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 12.Gontarek R R, McNally M T, Beemon K. Mutation of an RSV intronic element abolishes both U11/U12 snRNP binding and negative regulation of splicing. Genes Dev. 1993;7:1926–1936. doi: 10.1101/gad.7.10.1926. [DOI] [PubMed] [Google Scholar]
- 13.Gozani O, Patton J G, Reed R. A novel set of spliceosome-associated proteins and the essential splicing factor PSF bind stably to pre-mRNA prior to catalytic step II of the splicing reaction. EMBO J. 1994;13:3356–3367. doi: 10.1002/j.1460-2075.1994.tb06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolossova I, Padgett R A. U11 snRNA interacts in vivo with the 5′ splice site of U12-dependent (AU-AC) pre-mRNA introns. RNA. 1997;3:227–233. [PMC free article] [PubMed] [Google Scholar]
- 15.Konarska M M, Sharp P A. Electrophoretic separation of complexes involved in the splicing of precursors to mRNA. Cell. 1986;46:845–855. doi: 10.1016/0092-8674(86)90066-8. [DOI] [PubMed] [Google Scholar]
- 16.Konarska M M, Sharp P A. Interactions between small nuclear ribonucleoprotein particles in the formation of spliceosomes. Cell. 1987;49:763–744. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- 17.Kramer A. The structure and function of proteins involved in mammalian pre-mRNA splicing. Annu Rev Biochem. 1996;65:367–409. doi: 10.1146/annurev.bi.65.070196.002055. [DOI] [PubMed] [Google Scholar]
- 18.Lewis J D, Izaurralde E, Jarmolowski A, McGuigan C, Mattaj I W. A nuclear cap-binding complex facilitates association of U1 snRNP with the cap-proximal 5′ splice site. Genes Dev. 1996;10:1683–1698. doi: 10.1101/gad.10.13.1683. [DOI] [PubMed] [Google Scholar]
- 19.McNally L M, McNally M T. An RNA splicing enhancer-like sequence is a component of a splicing inhibitor element from Rous sarcoma virus. Mol Cell Biol. 1998;18:3103–3111. doi: 10.1128/mcb.18.6.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNally L M, McNally M T. U1 small nuclear ribonucleoprotein and splicing inhibition by the Rous sarcoma virus negative regulator of splicing element. J Virol. 1999;73:2385–2393. doi: 10.1128/jvi.73.3.2385-2393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNally M T, Gontarek R R, Beemon K. Characterization of Rous sarcoma virus intronic sequences that negatively regulate splicing. Virology. 1991;185:99–108. doi: 10.1016/0042-6822(91)90758-4. [DOI] [PubMed] [Google Scholar]
- 22.Michaud S, Reed R. An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes Dev. 1991;5:2534–2546. doi: 10.1101/gad.5.12b.2534. [DOI] [PubMed] [Google Scholar]
- 23.Michaud S, Reed R. A functional association between the 5′ and 3′ splice site is established in the earliest prespliceosome complex (E) in mammals. Genes Dev. 1993;7:1008–1020. doi: 10.1101/gad.7.6.1008. [DOI] [PubMed] [Google Scholar]
- 24.Moore M J, Query C C, Sharp P A. Splicing of precursors to mRNA by the spliceosome. In: Gesteland R, Atkins J, editors. The RNA world. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 303–357. [Google Scholar]
- 25.Reed R. Initial splice-site recognition and pairing during pre-mRNA splicing. Curr Opin Genet Dev. 1996;6:215–220. doi: 10.1016/s0959-437x(96)80053-0. [DOI] [PubMed] [Google Scholar]
- 26.Reed R. Protein composition of mammalian spliceosomes assembled in vitro. Proc Natl Acad Sci USA. 1990;87:8031–8035. doi: 10.1073/pnas.87.20.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz D E, Tizard R, Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983;32:853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- 28.Staknis D, Reed R. SR proteins promote the first specific recognition of pre-mRNA and are present together with the U1 small nuclear ribonucleoprotein particle in a general splicing enhancer complex. Mol Cell Biol. 1994;14:7670–7682. doi: 10.1128/mcb.14.11.7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoltzfus C M, Fogarty S J. Multiple regions in the Rous sarcoma virus src gene intron act in cis to affect the accumulation of unspliced RNA. J Virol. 1989;63:1669–1676. doi: 10.1128/jvi.63.4.1669-1676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian M, Maniatis T. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell. 1993;74:105–114. doi: 10.1016/0092-8674(93)90298-5. [DOI] [PubMed] [Google Scholar]
- 31.Wu J Y, Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- 32.Zamore P D, Patton J G, Green M R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992;355:609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Zamore P D, Carmo-Fonseca M, Lamond A I, Green M R. Cloning and intracellular localization of the U2 small nuclear ribonucleoprotein auxiliary factor small subunit. Proc Natl Acad Sci USA. 1992;89:8769–8773. doi: 10.1073/pnas.89.18.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]