Abstract
Adenocarcinoma that occurs at the ileostomy site after proctocolectomy (TPC) with an end ileostomy for ulcerative colitis (UC) and/or familial adenomatous polyposis (FAP) is a late and uncommon complication. To ascertain the rate of adenocarcinoma at the empirical ileostomy site following TPC, a review of the literature was conducted. PubMed, MEDLINE, the Cumulative Index of Nursing and Allied Health Literature, EMBASE, Google search engine, and the Cochrane Database were investigated for research published between January 1975 and December 2016. Search criteria included English language and human-only publications; broad search terms related to UC, FAP, ileostomy procedures, and dysplasias were used. Abstracts were eliminated if they were foreign language and nonhuman studies; editorials also were excluded. Secondary and hand/manual searches of reference lists, other studies cross-indexed by authors, reviews, commentaries, books, and meeting abstracts also were performed. Data extracted included age at diagnosis, operation technique, interval to ileostomy cancer, age when cancer was diagnosed, histology for both UC and FAP patients, and subsequent treatment. Papers were included on the basis of available evidence for each specific point of interest. Final and conclusive agreement was assessed with the k statistics during the title review and abstract review. Studies that did not report original data also were excluded. A total of 5753 publications were identified; 5697 publications did not conform to inclusion criteria and were eliminated. Among the reviewed publications (all case studies), 57 patients were diagnosed with ileostomy adenocarcinoma after TPC; 42 had UC, and 15 had FAP. The interval between TPC operation and ileostomy cancer diagnosis ranged from 3 to 51 years for UC and from 9 to 40 years for FAP, with a mean interval of 30 and 26 years, respectively. Biopsies were performed of all polypoid lesions found at the stoma site. Patients were treated with wide excision and refashioning (diversion) of the stoma. While adenocarcinoma arising at the mucocutaneous junction at the ileostomy site with adjacent skin invasion after TPC for UC and FAP appears to be rare, patients and clinicians need to be aware of this potential complication even years after surgery and regular screening is recommended.
Keywords: literature review, malignant neoplasm, ileostomy, ulcerative colitis, familial adenomatous polyposis
Background
A permanent end ileostomy is recommended/indicated for patients who are not eligible for an ileal pouch, those who suffer from ileal pouch failure and/or poor baseline continence, and those who are dissatisfied with a temporary end ileostomy. Proctocolectomy intervention involves the removal of the entire colon and rectum while preserving bowel continuity, evacuation, continence/deferral, discrimination, and fertility. Some of these patients will subsequently require pouch excision with a creation of a permanent end ileostomy due to pouch failure.1-5
According to standard surgical treatment procedure, a total proctocolectomy (TPC) is performed in patients with drug-refractory fulminant ulcerative colitis (UC)1-3 and/or familial adenomatous polyposis (FAP) because these persons carry the adenomatous polyposis coli gene.2 In addition, according to prospectively maintained database studies,4,5 TPC is performed in carefully selected patients with Crohn’s disease of the colon (Crohn’s colitis), although it is not a recommended first-line treatment. Further, according to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) 2012 Guidelines for the treatment of colorectal cancer,2,6 the TPC procedure is indicated as a second-line treatment option for hereditary nonpolyposis colorectal cancer,6 synchronous colorectal cancer,7 and severe colorectal constipation refractory to conservative drug treatment.2 According to the JSCCR, TPC with an ileal pouch provides the opportunity to avoid a permanent ileostomy.8 According to meta-analyses, one third of patients with UC1 and almost all patients with FAP9 will eventually require surgery to create a temporary stoma. In this regard, prospective observational studies have shown 3 types of surgeries are recommended: conventional TPC with permanent ileostomy,10,11 restorative proctocolectomy (RPC) with ileal pouch anal anastomosis (IPAA), and total abdominal colectomy with ileorectal anastomosis (IRA).8,9 According to cross-sectional studies, RPC is now the standard procedure for treating UC12 and FAP.13 The emphasis for each procedure is different, with conventional proctocolectomy indicated and the surgical options commonly including colectomy with IRA and RPC.13
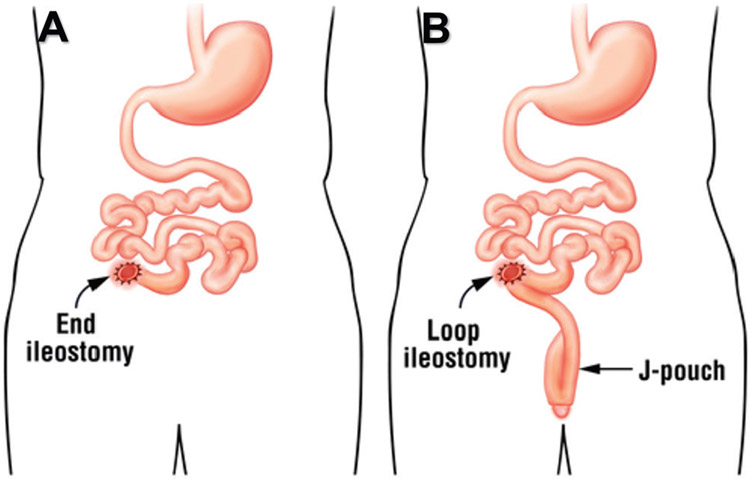
Brooke14 introduced the total colectomy procedure with eversion/diversion ileostomy in 1952.15-19 According to standard/recommended surgical operation procedure,8 2 types of ileostomy commonly are performed for UC and/or FAP patients (see Figure 1): end/terminal ileostomy after subcolectomy (see Figure 1A) and diverting loop ileostomy after proctocolectomy and ileal pouch-anal anastomosis (see Figure 1B). Since the introduction of total colectomy, a number of complications associated with the procedure have been observed.14-20 These complications include skin excoriations, retraction or prolapsed of the stoma, stenosis, intestinal obstruction, abscess, fistula, ileitis, and inflammatory polyps.14,16 The development of adenocarcinoma with invasion into adjacent skin20,21 at the ileostomy meatus20,21 or at the mucocutaneous junction of the ileostomy site is readily apparent, with changes easily recognized, although case reports and series have found it to be uncommon (see Figure 2).22-24 However, case control studies have shown metaplastic cell growth at the ileostomy site can result in adenocarcinoma with invasion into adjacent skin.25
Figure 1.
Two (2) types of ileostomy following colectomy advocated for ulcerative colitis and/or familial adenomatous polyposis: A) end/terminal ileostomy and B) diverting loop ileostomy. From Karimuddin A, Gilles G. Surgery for ulcerative colitis. Available at: Trustedtherapies.com. Adapted with permission.
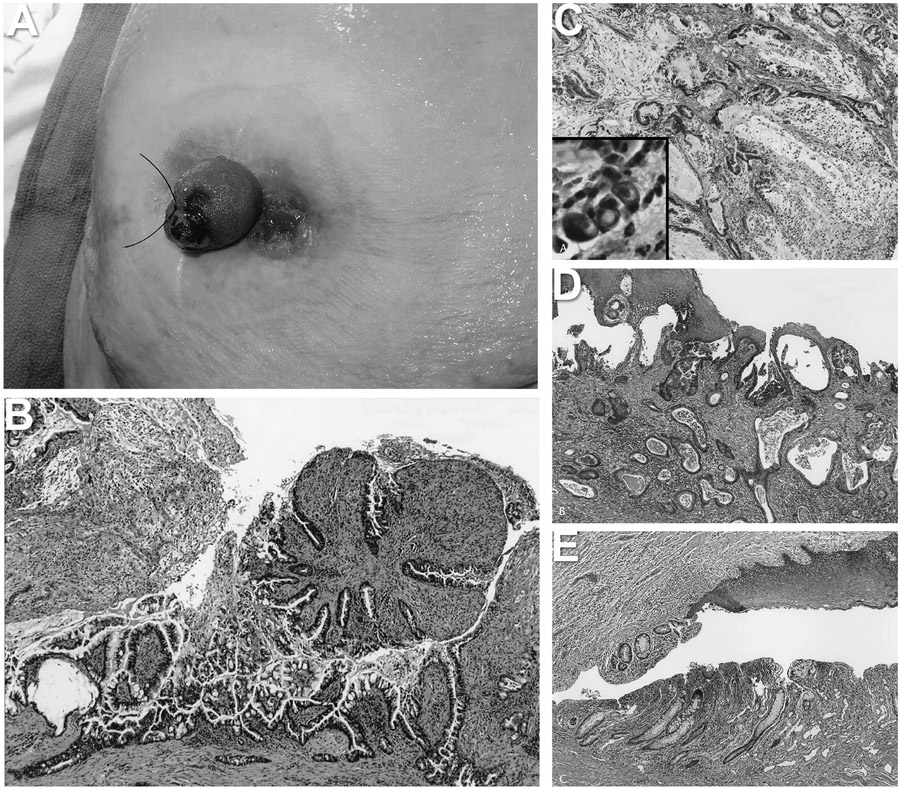
Figure 2.
Ileostomy cancer samples. A) ileostomy in situ image showing ulceration and adjacent ulcerated mass with granulation tissue; B) initial biopsy from the granulation tissue revealing proliferating epithelium with moderate cytologic atypia, thought to represent dysplasia; C) low magnification image of the tumor showing moderately differentiated carcinoma with abundant extracellular mucin pools typical of mucinous adenocarcinoma. High magnification inset showing scattered signet-ring cells infiltrating the stoma; D) section from the enterocutaneous junction showing adenocarcinoma extending underneath the squamous epithelium of the epidermis; and E) section from the enterocutaneous junction showing colonic metaplasia of the small bowel mucosa.
A review of the literature was conducted to critically assess and evaluate research studies that address the rate of adenocarcinoma arising at the ileostomy site following TPC surgery for UC and/or FAP.
Methods
A review of the literature was conducted for research regarding adenocarcinoma arising at the ileostomy site in patients who had undergone ileostomy following proctocolectomy for UC and FAP published between January 1975 and December 2016 using PubMed, Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica database (EMBASE), Current Nursing and Allied Health Literature (CINAHL), the Cochrane library, Web of Science, and the Google search engine. The following search terms were used: familial adenomatous polyposis, colectomy, total proctocolectomy, ileorectal anastomosis, Kock pouch, continent ileostomy, restorative proctocolectomy, ileal pouch-anal anastomosis, ileostomy, villous adenomas, adenocarcinoma, dysplasia, ileostomy neoplasia, ileostomy cancer, and mucosectomy. Secondary and hand/manual searches of reference lists, other studies cross-indexed by authors, reviews, commentaries, books, and meeting abstracts also were performed. All publications describing subsequent ileostomy site adenocarcinoma and management after TPC in humans were reviewed by the authors, and any queries or questions on inclusion were decided by the senior author. Studies that were published in languages other than English and did not use human subjects and editorials were excluded. Papers were included on the basis of available evidence for each specific point of interest. Final and conclusive agreement was determined using k statistics during the title review and abstract review. Data extracted from the publications included study type, age at diagnosis, operation technique, interval from surgery to ileostomy cancer diagnosis, age when ileostomy cancer was diagnosed, histology, and bowel pathology adjacent to tumor for both UC and FAP patients.
Data collection and analysis.
The data were first collected to a spreadsheet based on the most recent available evidence for each specific point of interest. Final and conclusive agreement was assessed with the k statistic during the title review and abstract review. If the value was >0.6, the titles were reviewed and divided into 2 sets; each was reviewed by 1 of the 2 researchers. If the value was <0.6, reviewers discussed discrepancies, followed by other assessments of agreement.
Results
Of the total of 5753 publications identified, 5697 did not conform to inclusion criteria: 977 were duplicate and 3799 were not relevant to the topic, leaving 921 full-text articles that were reviewed. Out of 921 reviewed articles, 865 were further excluded because they were not English-language publications and/or nonhuman studies. Ultimately, 56 qualified for inclusion in this review. All were case reports; no prospective studies, randomized controlled trials, or meta-analyses were identified. As a result, neither incidence nor prevalence could be calculated.
Of the 57 reported cases of ileostomy orifice (stomal site) adenocarcinoma, 42 occurred in TPC-UC patients15-22,25-53 and 15 in TPC-FAP patients53-65 (see Table 1 and Table 2). Ileostomy cancer with adjacent skin invasion was reported in 1 TPC-UC patient.21
Table 1.
Reported ileostomy neoplasia following total proctocolectomy for ulcerative colitis (UC)
| Author, year (reference number) |
Age at UC, diagnosis, (years) |
Interval from surgery to ICa (years) |
Age at ICa (years) |
Histology | Lesion pathology |
|---|---|---|---|---|---|
| Sigler, 1969 (26) | 40 | 19 | 59 | ACa | Adenocarcinoma of an ileal stoma |
| Cuesta, 1976 (27) | 29 | 31 | 60 | ACa | Glandular hyperplasia |
| Johnson, 1980 (28) | 39 | 3 | 45 | ACa | Villous adenoma |
| Baciewicz, 1983 (29) | 13 | 32 | 45 | ACa | Colonic metaplasia and chronic inflammation |
| Stryker, 1983 (30) | 41 | 25 | 66 | ACa | Moderate to severe dysplasia |
| Suarez, 1985 (15) | 25 | 4 | 51 | ACa | Adenocarcinoma |
| Bedetti, 1986 (31) | 22 | 23 | 45 | ACa | Colonic metaplasia and epithelial dysplasia |
| Vasilevsky, 1986 (32) | 22 | 38 | 60 | ACa | Adenocarcinoma at the ileocutaneous junction |
| Longo, 1986 (33) | 45 | 28 | 73 | ACa | Adenocarcinoma arising in an ileostomyorifice |
| Suarez, 1986 (15) | 29 | 4 | 40 | ACa | Ileal carcinoma adjacent to the mucocutaneous junction of an ileostomy |
| Suarez, 1987 (15) | 30 | 4 | 70 | ACa | Adenocarcinoma adjacent to the mucocutaneous junction of an ileostomy |
| O’Connell, 1987 (34) | 34 | 36 | 70 | SCC | Intestinal and squamous metaplasia |
| Carter, 1988 (17) | 36 | 43 | 79 | ACa | Severe chronic inflammation, dysplasia and metaplasia |
| Suarez, 1988 (15) | 35 | 4 | 60 | ACa | Adenocarcinoma adjacent to the mucocutaneous junction of an ileostomy |
| Smart, 1988 (18) | 32 | 28 | 60 | ACa | Colonic metaplasia and severe dysplasia |
| Coen, 1988 (35) | 39 | 3 | 42 | ACa | Adenocarcinoma of the ileocutaneous junction |
| Ewing, 1989 (36) | 28 | 30 | 58 | ACa | Primary adenocarcinoma |
| Roberts, 1989 (22) | 33 | 25 | 58 | ACa | Synchronous ileal adenocarcinoma |
| Roberts, 1989 (22) | 23 | 31 | 54 | ACa | Ileal adenocarcinoma |
| Berman, 1989 (25) | 27 | 34 | 61 | ACa | Colonic metaplasia |
| Pellissier, 1990 (37) | 28 | 33 | 61 | ACa | Severe dysplasia |
| Ferrandez Rivera, 1990 (38) | 36 | 23 | 59 | ACa | Adenocarcinoma at an ileostomy site |
| Gadacz, 1990 (39) | 26 | 34 | 60 | ACa | Colonic metaplasia and focal dysplasia |
| Carey, 1993 (40) | 28 | 36 | 64 | ACa | Adenomatous changes, dysplasia and chronic inflammation |
| Starke, 1993 (19) | 27 | 35 | 62 | ACa | Adenocarcinoma |
| Listinsky, 1994 (41) | 51 | 11 | 62 | ACa | Neuroendocrine carcinoma |
| Attanoos, 1995 (16) | 33 | 36 | 69 | ACa | Polypoid mucinous adenocarcinoma |
| Attanoos, 1995 (16) | 37 | 27 | 64 | ACa | Polypoid invasive adenocarcinoma |
| Cox, 1997 (42) | 22 | 17 | 39 | ACa | Adenocarcinoma involving the intussusception valve |
| Reissman, 1997 (43) | 16 | 48 | 64 | ACa | Colonic metaplasia |
| Carne, 2001 (44) | 25 | 41 | 66 | ACa | Squamous cell carcinoma in an ileostomy stoma |
| Pranesh, 2002 (45) | 20 | 58 | 78 | ACa | B-cell non-Hodgkins lymphoma of the ileum |
| Ramanujam, 2002 (46) | 25 | 51 | 76 | ACa | Squamous cell carcinoma at the stomal site |
| Abela, 2004 (20) | 18 | 30 | 48 | ACa | Colonic-type glands invading submucosa and infiltrate the adjacent skin |
| Achneck, 2005 (47) | 39 | 46 | 85 | ACa | Moderately differentiated invasive adenocarcinoma with positive margins |
| Agibiti, 2005 (48) | 31 | ACa | Moderately differentiated adenocarcinoma of the mucocutaneous ileostomy junction | ||
| Azem, 2005 (49) | 19 | 29 | 69 | ACa | Adenocarcinoma |
| Quah, 2005 (50) | 43 | 35 | 78 | Peristomal | Adenocarcinoma |
| Annam, 2008 (21) | 30 | 35 | 65 | MEC | Mucin-secreting adenocarcinoma |
| Mohandas, 2010 (51) | 13 | 48 | 61 | ACa | Adenocarcinoma |
| Chang, 2014 (52) | 29 | 21 | 63 | ACa | Well-differentiated squemous cell carcinoma |
| Procaccino, 2015 (53) | 35 | 50 | 85 | ACa | Well-differentiated adenocarcinoma |
ICa=ileostomy cancer; ACa=adenocarcinoma; PTC=proctocolectomy; SCC=subcutanous cancer; MEC=malignant epithelial cells
Table 2.
Reported ileostomy neoplasia following total proctocolectomy for familal adenomatous polyposis
| Author, year (reference number) |
Age at FAP diagnosis |
Interval from surgery to ICa (years) |
Age at Ica (years) |
Histology | Lesion pathology |
|---|---|---|---|---|---|
| Roth, 1982 (54) | 35 | 9 | 44 | ACa | Adenomatous polyps |
| Ross, 1987 (55) | 24 | 32 | 56 | ACa | Adenocarcinoma |
| Suarez, 1988 (15) | 11 | 29 | 40 | ACa | Adenomatous changes with mild dysplasia |
| Primrose, 1988 (56) | 47 | 25 | 72 | ACa | Adenomatous changes with mild dysplasia |
| Lopez, 1991(57) | 23 | 27 | 50 | ACa | Adenomatous polyps |
| Gilson, 1992 (58) | 29 | 40 | 69 | ACa | Adenomatous polyps |
| Lux, 1993 (59) | 47 | 25 | 72 | ACa | Adenomatous polyps |
| Johnson, 1993 (23) | 40 | 25 | 65 | ACa | Colonic metaplasia |
| Wu, 1998 (60) | 25 | 42 | 67 | ACa and SCC | Adenomatous polyposis gene |
| Mumira, 1999 (61) | 33 | 21 | 54 | ACa | Well-differentiated adenocarcinoma with submucosainvasion |
| Iizuki, 2002 (62) | 55 | 14 | 69 | ACa | Not reported |
| Hata, 2003 (63) | 25 | 32 | 57 | ACa | Adenomatous polyps |
| Shenoy, 2009 (64) | 25 | 10 | 60 | ACa | Tubulovilous adenocarcinoma |
| Huntington, 2017 (65) | 17 | 24 | 41 | ACa | Tubulovilous adenocarcinoma |
| Procaccino, 2015 (53) | 48 | 30 | 78 | ACa | Moderate differentiated adenocarcinoma |
TPC=total proctocolectomy; FAP=familial adenomatous poplyposis; ACa=adenocarcinoma; ICa=ileostomy cancer
Polypoid adenocarcinomas tended to arise decades after ileostomy creation21,23,42; the reported interval between TPC operation and ileostomy cancer diagnosis ranged from 3 to 51 years for UC and from 9 to 40 years for FAP,23,26,58 with a mean interval of 30 and 26 years, respectively. The occurrence of this complication was not limited to the traditional Brooke ileostomy.42
Management of these tumors was uniform across studies. Upon histological diagnosis of intramucosal well- to moderately differentiated adenocarcinoma, patients underwent a wide local resection of the tumor and the surrounding skin with adequately wide margins, as well as lymph node dissection and reconstruction of the ileostomy site.
The prognoses regarding functionality and patient quality of life were generally good after surgery, allowing for individual considerations of age, past medical history, presentation, and staging of the disease.
Ileostomies also were associated with a number of other complications in 11 patients and included skin excoriation, stenosis, parastomal herniation, intestinal obstruction, retraction or prolapse of the stoma, abscess, fistula formation, and ileitis40 (see Figure 3 and Figure 4).
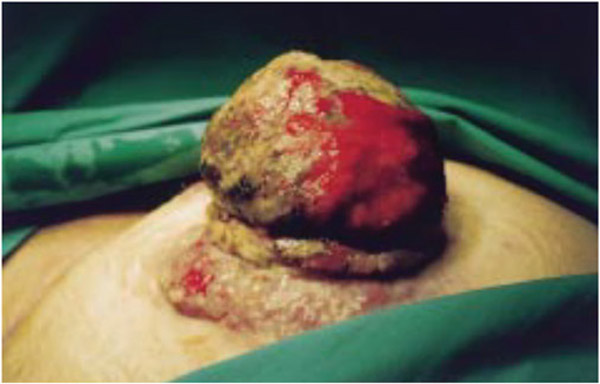
Figure 3.
Image showing the site of a long-standing end/terminal ileostomy at the right iliac fossa in a 78-year-old man. The ileostomy site was created 20 years earlier after subtotal colectomy for fulminant ulcerative colitis. The patient had a 6.4 cm in diameter functional but prolapsed stoma that extended approximately 10 cm from the skin surface that was very hard and swollen. Its tip appeared bruised and black, stenosed, and hemorrhagic. Examination confirmed a swollen, necrotic, stenosed stoma with 2 skin deposits adjacent to the stoma and a large parastomal hernia. A clinical diagnosis of a malignant change was made; standard staging tests found the tumor to be localized. B-cell non-Hodgkins lymphoma of the ileum was detected by histology. The tumor appeared to have been completely excised.45 (Used with permission from the author.)
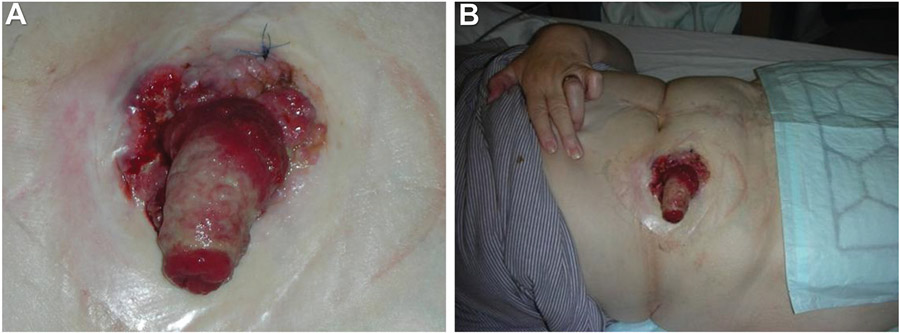
Figure 4.
A-61-year-old woman presented at the colorectal clinic with a 3-month history of decreased stoma output, weight loss, and general malaise. She also had a history of panproctocolectomy and ileostomy for ulcerative colitis at the age of 13. She had been admitted with small bowel obstruction 4 months prior to the current visit and treated conservatively. A follow-up small bowel barium follow-through did not show any small bowel obstruction. Further examination of the abdomen revealed an ulceroproliferative growth involving the mucocutaneous junction and ileostomy site extending from the 9 to 6 o’clock position (A and B). The sprout of the ileostomy site was thickened and stenosed. Biopsy from the lesion revealed an adenocarcinoma. Blood test showed a carcinoembryonic antigen level of 9, Ca 19–9 of 228, and Ca 125 of 21.6. A computerized tomography scan of her abdomen and pelvis did not show any evidence of distant metastasis. The patient underwent a wide local excision at the ileostomy site and the adjacent anterior abdominal wall with a 2-cm margin and resiting of the stoma.51 (Used with permission from the author.)
Discussion
This overview found 56 case studies of patients diagnosed with stoma site adenocarcinoma — 42 cases described TPC-UC and 14 described TPC-FAP patients. Ileostomy cancer (Ica) with adjacent skin invasion was reported in 2 TPC-UC patients.21 No case series, prevalence, or incidence studies were identified.
Currently, no drugs can cure UC or FAP. Conservative therapies for symptomatic relief for UC include the use of immunomodulatory drugs and human anti-tumor necrosis factor alpha monoclonal antibodies. These options are not curative. Long-term use of these agents suppresses the immune system, leading to severe side effects, and/or causes the disease to become refractory to the drugs. In these instances, patients will eventually require TPC (and ileal pouch-anal anastomosis with temporary ileostomy) to control their symptoms.8,13 Patients with FAP are generally healthy, are not taking immunosuppressive medications, and have a normal bowel except for the presence of adenomas. Virtually all surgically untreated patients with FAP inevitably develop cancer in their lifetime because they carry the adenomatous polyposis coli gene; thus, prophylactic TPC is indicated.66 Patients with a long-standing ileostomy following TPC for UC and FAP appear to be at a greater risk of developing adenocarcinoma at the ileostomy site than persons with an end ileostomy due to other non-UC and/or no FAP disease.15,16,22,30,39,40,48 Two (2) etiologies of ileostomy cancer can develop. First, long-duration ileostomies can result in several chronic pathologic conditions, including repetitive peristomal exposure, trauma caused by ill-fitting devices, and chronic mechanical and chemical irritation of the mucocutaneous junction associated with changes in the bacterial flora and milieu in and around the stoma.64 Over time, these environmental risk factors may lead to metaplasia, dysplasia, and ultimately carcinoma.61 The second theory refers to the inherent predisposition of FAP patients to bowel adenomas after ileostomy. A case report63 showed samples from these cancer patients were positive for K-ras mutation as well as immunostaining for β-catenin and p53, suggesting the presence of genetic alterations that predisposed them to ileostomy adenocarcinoma.
Most of the cases of adenocarcinoma at the ileostomy site have been reported within the past 5 years, suggesting a rise in disease incidence. The timing of this observed rise corresponds to end of a biologic latency period for the Brooke ileostomy.14 Therefore, it seems reasonable to postulate that the number of cases of adenocarcinoma at the ileostomy site may increase in an aging population with patients who have undergone the Brooke ileostomy, although at this time, data to support this hypothesis are lacking.
Conventional proctocolectomy often leads to permanent ileostomy.10,11 Adenocarcinoma arising in the abnormally placed small bowel mucosa (ie, mucosa not in its anatomically correct location or the stoma was not created correctly) may occur years after the surgery and is beginning to garner attention. The cause is not clear, but physical trauma and chemical irritation may predispose the ileal mucosa to colonic metaplasia, dysplasia, and adenoma, which ultimately result in malignant changes15,17 (ie, adenocarcinoma40). Practicioners will need to perform biopsies on all polyps arising at the mucocutaneous junction and nonprolapse associated polyps that have appeared elsewhere on the stoma after an ileostomy in order to screen for ileostomy cancers.16
UC.
Primary adenocarcinoma arising at the ileostomy site is a rare and infrequently reported complication after TPC for UC.43 The earliest case of adenocarcinoma arising at the ileostomy site after TPC for UC was documented in 1969.26 The current literature search of English-language publications yielded 42 case reports on patients diagnosed with Ica after TPC for UC to date. ICa with adjacent skin invasion also has been reported in 2 post TPC-UC patients. Barclay et al67 reported the incidence of small intestinal malignancy in the general population to be 0.7 per 100 000. Suarez et al15 estimated the incidence of ICa in the UK to be 0.2 to 0.4 per 100 000 patients who had undergone ileostomy creation. The findings of the current review yielded case studies only. While not sufficient to make reliable conclusions about the rate of ICa, it may suggest that although rare, ICa may occur more frequently than small intestinal carcinoma. Incidence was not part of the analysis because the studies were not TPC/ICa-specific to either UC or FAP.
The pathogenesis of ICa is unclear.68,69 Some studies16,22,43,62 suggest the generative epithelial hyperproliferation brought about by chronic irritation at the mucocutaneous junction is a factor. Additionally, physical trauma and/or irritation from chemical agents such as those used as stoma adhesives also may cause colonic metaplasia, adenoma, and ultimately carcinoma.70 Changes in the bacterial flora also have been reported to be associated with cancer occurrence,71 as well as an association between ileitis or backwash ileitis and mucosal dysplasia and cancer transformation.72 It appears the sequence starts with chronic inflammation and ends with colonic epithelial metaplasia.25,67 Cytological atypia and architectural abnormalities are thought to possibly ensue in dysplasia, which ultimately leads to carcinoma. In contrast, while examining their patient with ileostomy adenocarcinoma, Metzger et al24 found no signs of inflammation at the ileostomy site and no evidence of Crohn’s disease in the terminal ileum.
Most ileostomy carcinomas reported in the literature appear to be slow-growing, mucin-secreting adenocarcinoma.16,45 However, the vast majority of reported adenocarcinoma cases seemed to occur in patients who had undergone colectomy for UC,16 compared to a fewer number of cases in patients who had undergone colectomy for FAP.23,58,62 This may be because FAP patients are healthy without inflammation except for the presence of adenomas.66
FAP.
Epidemiological studies64 have shown FAP patients are highly vulnerable to extracolonic gastrointestinal cancers, with an incidence of 0.7 per 100 000 patients. Ileal adenomas associated with FAP are a common finding, and approximately 20% of FAP patients have adenomatous polyps in the ileum.73 Recent meta-analyses following TPC for FAP have confirmed the presence of multiple ileal adenomas and an increase in ileal mucosal proliferation. The earliest case on carcinoma arising at the ileostomy site after TPC for FAP was documented in 1982.54 Ileal polyps and adenocarcinoma at the end ileostomy site, although considered rare, frequently manifest themselves subsequent to TPC in FAP patients.23,54,58,61,73 This review of the literature revealed 14 cases of ICa after TPC for FAP with an average interval between TPA and an ICa diagnosis of 26 years. The incidence of ICa after TPC, which is a recommended prophylactic surgery for FAP, is greater than its incidence in the general population.54,62 The case report by Hamilton et al74 noted the presence of ileal polyps (benign adenomas) in 9 FAP-postcolectomy patients.
A case report and histologic mucin study from University of Tokyo proposed 4 possible hypotheses for the etiology of ICa,61 supported by case-controlled studies from other institutions.22-24,61,64,71,75 First, chronic mechanical or chemical irritation at the stoma mucocutaneous junction may result in cancer growth over time. Second, backwash ileitis in TPC patients may result in malignant growth, as illustrated in the case report by Roberts et al.22 Third, colorectal mucosal migration or retention also may result in malignant growth, as reported in 2 publications included in this overview.23,64 Finally, colonic metaplasia within the ileal mucosa at the ileostomy site can progress to cancer.24,61,70,75
Genetic changes also can contribute to the development of Ica following TPC for FAP. In their investigation of genetic alterations in ileostomy adenoma and carcinoma, Herring et al76 and Hata et al63 reported the presence of K-ras mutation and the loss of heterozygosity (LOH) at chromosome 17p (p53) in their study samples. K-ras mutation was detected in samples from ileostomy polyps using the 2-step polymerase chain reaction-restriction fragment length polymorphism (RFLP), while no mutations were detected in normal ileum. The mutation in codon 12 of the K-ras oncogene altered it from GGT to GAT (Asp). LOH at p53 was detected in ileostomy polyps. Immunostaining for β-catenin in humans, a dual function protein involved in regulation and coordination of cell-to-cell adhesion and gene transcription, was detected in the adenomatous and carcinomatous portions but was not detected in normal ileum. Immunostaining for p53 was focally positive in the carcinomatous portions and negative for normal ileum.63
Clinical and exploration genotype-phenotype correlation studies60,77 have demonstrated that symptoms of FAP, including extracolonic manifestations, are correlated with the genotype or mutations of the adenomatous polyposis coli gene. The severity of the polyps depends on the mutation site. Mutations between codon 1309 and 1328 are associated with a more severe disease, while those between codon 1020 and 1169 as well as those located downstream of the mRNA (3’) end of the gene are associated with an attenuated form of FAP.78,79
ICa patients may present with a variety of symptoms, including bowel obstruction and complaints such as ileostomy site irritation, pain, and bleeding.20,21 Any suspicious signs or symptoms such as bleeding, pain, or polyp-like lesions at the ileostomy site should prompt a biopsy followed by histological examination, because neoplastic features may be overshadowed by inflammatory changes.21 Early detection of malignant lesions may increase the success of surgical treatment.21 The appropriate recommended treatment for ICa is a wide stoma site excision with ileostomy site reconstruction.21,62 Adjuvant therapy, especially for patients with nodal disease, may have additional benefits.62 Patient education is important to encourage early disease detection as the lesions typically appear late (on average, 27 years after ileostomy) based on the case report studies identified in this and other studies.21,53,65 Proper cancer care is necessary because these tumors have significant recurrence and metastatic potential.26,33 Lymph node metastasis is reported to occur in 19% of patients, with a survival rate of at least 85%.24
Characteristics of parastomal malignancy.
Patients typically present with a parastomal mass, reducible parastomal hernia, fungating mass at the ileostomy site accompanied by chronic skin irritation, lethargy, dehydration, small bowel obstruction, and difficulty with proper placement of the stoma appliance.53 Common differential diagnoses for parastomal lesions include contact dermatitis, psoriasis, and pyoderma gangrenosum due to constant contact of the surrounding skin with feces. This chronic irritation more commonly causes a dermatological condition rather than a malignancy. Malignancy must be validated histologically. Biopsy of the mass is essential to distinguish it from other more common differentials. Computed tomography scan is often used to visualize the extent of the mass.50,53 Treatment may involve surgical excision and relocation of the stoma or a laparotomy with resection of the terminal small bowel, ileostomy, and abdominal wall skin, and creation of a new terminal ileostomy.
Conclusion
This overview of the literature, based on 56 articles reporting adenocarcinoma cases arising at ileostomy site, found 42 cases of TPC-operated for UC and 15 patients of TPC-operated for FAP. Patients who have a long-standing ileostomy may be at risk for ileostomy adenocarcinoma at the stoma with potential of invasion into the adjacent skin. While direct causality has not been established, repetitive peristomal exposure to chronic mechanical and chemical irritation at the mucocutaneous junction may play a role. Additionally, changes in the bacterial flora and milieu in and around the stoma orifice can contribute to cancer development. The cases presented suggest patients with ICa usually present first with peristomal skin changes that are unresponsive to conservative treatment measures. Therefore, yearly ileostomy site surveillance by medical personnel such as ostomy care nurses, attending physicians, colorectal surgeons, and gastroenterologists is highly recommended, supplemented by frequent examination by the patients themselves. Suspicious symptoms and lesions should prompt a thorough examination followed by a biopsy, careful histologic examination and, if needed, excision or wide resection of the anterior abdominal wall and reconstruction of the stoma. The case studies identified highlight the importance of regular screening, especially in persons who have had their ileostomy for many years.
Key Points.
Although rare, adenocarcinoma at the ileostomy site following total proctocolectomy surgery for ulcerative colitis and familial adenomatous polyposis has been reported.
The authors conducted a review of the literature published between 1975 and 2016 and identified 56 case studies describing this complication.
Based on the cases reported, the average time between surgery adenocarcinoma development was between 26 and 30 years.
Ongoing follow-up and screening are important, regardless of how many years have passed since the time of surgery.
Acknowledgment
The authors thank the Meharry Office for Scientific Editing and Publications for scientific editing support (NIH/S21MD000104).
Potential Conflicts of Interest:
This research was supported by Meharry Schools of Medicine and Graduate Studies and Research; National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases-R21DK095186; Vanderbilt University Institute for Clinical and Translational Research (VICTR-CTSA)-1UL1RR024975-01; NIH/National Center for Advancing Translational Sciences VICTR-2UL1TR000445-06; NIH/National Cancer Institute (NCI)-3U54CA091408–09S1; and NIH/NCI-3U54CA091408–09S2.
Contributor Information
Samuel D. James, Department of Pathology, Meharry Medical College School of Medicine, Nashville General Hospital, Nashville, TN; Department of Pathology, Microbiology, and Immunology, Tennessee Valley Health Systems VA Medical Center, Vanderbilt University Medical Center, Nashville, TN..
Alexander T. Hawkins, Department of Surgery, Vanderbilt University School of Medicine..
Amosy E. M’Koma, Department of Biochemistry, Cancer Biology, Neuroscience and Pharmacology, Meharry Medical College School of Medicine; Department of Surgery, Vanderbilt University School of Medicine..
References
- 1.Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103(5):14–51. [DOI] [PubMed] [Google Scholar]
- 2.Furst A [Differential indications for ileoanal pouch anastomosis: ulcerative colitis, familial adenomatous polyposis, synchronous colorectal cancer — Crohn’s disease, constipation]. Chirurg. 2017;88(7):555–558. [DOI] [PubMed] [Google Scholar]
- 3.Lightner AL, Mathis KL, Dozois EJ, et al. Results at up to 30 years after ileal pouch-anal anastomosis for chronic ulcerative colitis. Inflamm Bowel Dis. 2017;23(5):781–790. [DOI] [PubMed] [Google Scholar]
- 4.Le Q, Melmed G, Dubinsky M, et al. Surgical outcome of ileal pouch-anal anastomosis when used intentionally for well-defined Crohn’s disease. Inflamm Bowel Dis. 2013;19(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen B, Patel S, Lian L. Natural history of Crohn’s disease in patients who underwent intentional restorative proctocolectomy with ileal pouch-anal anastomosis. Aliment Pharmacol Ther. 2010;31(7):745–753. [DOI] [PubMed] [Google Scholar]
- 6.Ishida H, Iwama T, Tomita N, et al. [Diagnosis and management of hereditary colorectal cancer according to the JSCCR Guidelines 2012 for the Clinical Practice of Hereditary Colorectal Cancer]. Nihon Rinsho. 2014;72(1):143–149. [PubMed] [Google Scholar]
- 7.Lee BC, Yu CS, Kim J, et al. Clinicopathological features and surgical options for synchronous colorectal cancer. Medicine (Baltimore). 2017;96(9):e6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M’Koma AE, Wise PE, Muldoon RL, Schwartz DA, Washington MK, Herline AJ. Evolution of the restorative proctocolectomy and its effects on gastrointestinal hormones. Int J Colorectal Dis. 2007;22(10):1143–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulow S, Bulow C, Vasen H, Jarvinen H, Bjork J, Christensen IJ. Colectomy and ileorectal anastomosis is still an option for selected patients with familial adenomatous polyposis. Dis Colon Rectum. 2008;51(9):1318–1323. [DOI] [PubMed] [Google Scholar]
- 10.Kilic E, Taycan O, Belli AK, Ozmen M. [The effect of permanent ostomy on body image, self-esteem, marital adjustment, and sexual functioning]. Turk Psikiyatri Derg. 2007;18(4):302–310. [PubMed] [Google Scholar]
- 11.Das P, Smith JJ, Tekkis PP, Heriot AG, Antropoli M, Nicholls RJ. Quality of life after indefinite diversion/pouch excision in ileal pouch failure patients. Colorectal Dis. 2007;9(8):718–724. [DOI] [PubMed] [Google Scholar]
- 12.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2(6130):85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLaughlin SD, Clark SK, Tekkis PP, Ciclitira PJ, Nicholls RJ. Review article: restorative proctocolectomy, indications, management of complications and follow-up--a guide for gastroenterologists. Aliment Pharmacol Ther. 2008;27(10):895–909. [DOI] [PubMed] [Google Scholar]
- 14.Brooke BN. The management of an ileostomy, including its complications. Lancet. 1952;2(6725):102–104. [DOI] [PubMed] [Google Scholar]
- 15.Suarez V, Alexander-Williams J, O’Connor HJ, et al. Carcinoma developing in ileostomies after 25 or more years. Gastroenterology. 1988;95(1):205–208. [DOI] [PubMed] [Google Scholar]
- 16.Attanoos R, Billings PJ, Hughes LE, Williams GT. Ileostomy polyps, adenomas, and adenocarcinomas. Gut. 1995;37(6):840–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter D, Choi H, Otterson M, Telford GL. Primary adenocarcinoma of the ileostomy after colectomy for ulcerative colitis. Dig Dis Sci. 1988;33(4):509–512. [DOI] [PubMed] [Google Scholar]
- 18.Smart PJ, Sastry S, Wells S. Primary mucinous adenocarcinoma developing in an ileostomy stoma. Gut. 1988;29(11):1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starke J, Rodriguez-Bigas M, Marshall W, Sohrabi A, Petrelli NJ. Primary adenocarcinoma arising in an ileostomy. Surgery. 1993;114(1):125–128. [PubMed] [Google Scholar]
- 20.Abela J Ileostomy Adenocarcinoma. A case report. MMJ. 2004;16(4):42. [Google Scholar]
- 21.Annam V, Panduranga C, Kodandaswamy C, Suresh DR. Primary mucinous adenocarcinoma in an ileostomy with adjacent skin invasion: a late complication of surgery for ulcerative colitis. J Gastrointest Cancer. 2008;39(1–4):138–140. [DOI] [PubMed] [Google Scholar]
- 22.Roberts PL, Veidenheimer MC, Cassidy S, Silverman ML. Adenocarcinoma arising in an ileostomy. Report of two cases and review of the literature. Arch Surg. 1989;124(4):497–499. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JA 3rd, Talton DS, Poole GV. Adenocarcinoma of a Brooke ileostomy for adenomatous polyposis coli. Am J Gastroenterol. 1993;88(7):1122–1124. [PubMed] [Google Scholar]
- 24.Metzger PP, Slappy AL, Chua HK, Menke DM. Adenocarcinoma developing at an ileostomy: report of a case and review of the literature. Dis Colon Rectum. 2008;51(5):604–609. [DOI] [PubMed] [Google Scholar]
- 25.Berman JJ, Ullah A. Colonic metaplasia of ileostomies. Biological significance for ulcerative colitis patients following total colectomy. Am J Surg Pathol. 1989;13(11):955–960. [PubMed] [Google Scholar]
- 26.Sigler L, Jedd FL. Adenocarcinoma of the ileostomy occurring after colectomy for ulcerative colitis: report of a case. Dis Colon Rectum. 1969;12(1):45–48. [DOI] [PubMed] [Google Scholar]
- 27.Cuesta MA, Donner R. Adenocarcinoma arising at an ileostomy site: report of a case. Cancer. 1976;37(2):949–952. [DOI] [PubMed] [Google Scholar]
- 28.Johnson WR, McDermott FT, Pihl E, Hughes ESR. Adenocarcinoma of an ileostomy in a patient with ulcerative colitis. Dis Colon Rectum. 1980;23(5):351–352. [DOI] [PubMed] [Google Scholar]
- 29.Baciewicz F, Sparberg M, Lawrence JB, Poticha SM. Adenocarcinoma of an ileostomy site with skin invasion: a case report. Gastroenterology. 1983;84(1):168–170. [PubMed] [Google Scholar]
- 30.Stryker SJ. Primary stomal adenocarcinoma. An unusual complication of ileostomy. Dis Colon Rectum. 1983;26(1):47–49. [DOI] [PubMed] [Google Scholar]
- 31.Bedetti CD, DeRisio VJ. Primary adenocarcinoma arising at an ileostomy site. An unusual complication after colectomy for ulcerative colitis. Dis Colon Rectum. 1986;29(9):572–575. [DOI] [PubMed] [Google Scholar]
- 32.Vasilevsky CA, Gordon PH. Adenocarcinoma arising at the ileocutaneous junction occurring after proctocolectomy for ulcerative colitis. Br J Surg. 1986;73(5):378. [DOI] [PubMed] [Google Scholar]
- 33.Longo WE, Stephan RN, True LD, August DA, McCullough WB. Recurrent ileal mucinous adenocarcinoma in an ileostomy. J Ciin Gastroenterol. 1986;8(2):192–194. [DOI] [PubMed] [Google Scholar]
- 34.O’Connell PR, Dozois RR, Irons GB, Scheithauer BW. Squamous cell carcinoma occurring in a skin-grafted ileostomy stoma. Report of a case. Dis Coion Rectum. 1987;30(6):475–478. [DOI] [PubMed] [Google Scholar]
- 35.Coen LD, Lambert WG, Gray PB. Adenocarcinoma at the ileo-cutaneous junction following subtotal colectomy for ulcerative colitis. Case report. Acta Chir Scand. 1988;154(11-12):685–686. [PubMed] [Google Scholar]
- 36.Ewing HP, Manolas SG. Primary adenocarcinoma arising at an ileostomy site: an unusual cause of bowel obstruction. Aust N Z J Surg. 1989;59(11):898–899. [DOI] [PubMed] [Google Scholar]
- 37.Pellissier PE, David A, Coppéré H, et al. [Primary adenocarcinoma of the ileostomy after total proctocolectomy for ulcerative colitis]. Gastroenterol Clin Biol. 1990;14(8–9):672–674. [PubMed] [Google Scholar]
- 38.Ferrandez Rivera M, Baltar Boileve J, et al. [Adenocarcinoma at an ileostomy site after colectomy for ulcerative colitis] J Chi (Paris: ). 1990;127(8-9):412–415. [PubMed] [Google Scholar]
- 39.Gadacz TR, McFadden DW, Gabrielson EW, Ullah A, Berman JJ. Adenocarcinoma of the ileostomy: the latent risk of cancer after colectomy for ulcerative colitis and familial polyposis. Surgery. 1990;107(6):698–703. [PubMed] [Google Scholar]
- 40.Carey PD, Suvarna SK, Baloch KG, Guillou PJ, Monson JR. Primary adenocarcinoma in an ileostomy: a late complication of surgery for ulcerative colitis. Surgery. 1993;113(6):712–715. [PubMed] [Google Scholar]
- 41.Listinsky CM, Halpern NB, Workman RB, Herrera GA. Ultrastructural and immunocytochemical features of a case of neuroendocrine carcinoma developing in a prior ileostomy site. Ultrastruct Pathol. 1994;18(5):503–509. [DOI] [PubMed] [Google Scholar]
- 42.Cox CL, Butts DR, Roberts MP, Wessels RA, Bailey HR. Development of invasive adenocarcinoma in a long-standing Kock continent ileostomy: report of a case. Dis Colon Rectum. 1997;40(4):500–503. [DOI] [PubMed] [Google Scholar]
- 43.Reissman P, Avroutis O, Cohen DY, Shiloni E. Ileostomy-site colonic metaplasia with adenocarcinoma after proctocolectomy for ulcerative colitis. Am J Gastroenterol. 1997;92(10):1932–1933. [PubMed] [Google Scholar]
- 44.Carne PW, Farmer KC. Squamous-cell carcinoma developing in an ileostomy stoma: report of a case. Dis Coion Rectum. 2001;44(4):594. [DOI] [PubMed] [Google Scholar]
- 45.Pranesh N Lymphoma in an ileostomy. Postgrad Med J. 2002;78(920):368–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramanujam P, Venkatesh KS. An unusual case of squamous cell carcinoma arising at the stomal site: case report and review of the literature. J Gastrointest Surg. 2002;6(4):630–631. [DOI] [PubMed] [Google Scholar]
- 47.Achneck HE, Wong IY, Kim PJ, Fernandes MA, et al. Ileostomy adenocarcinomas in the setting of ulcerative colitis. J Clin Gastroenterol. 2005;39(5):396–400. [DOI] [PubMed] [Google Scholar]
- 48.Agabiti E, Loganathan A, Rimmer M, Eames RA, Cullen PT. Cancer of ileostomy: a late complication of colectomy for ulcerative colitis. Acta Chir Belg. 2005;105(1):99–101. [PubMed] [Google Scholar]
- 49.Azem J, López-Cano M, Ponseti JM, et al. Ileostomy-site adenocarcinoma after proctocolectomy for ulcerative colitis. Int J Colorectal Dis. 2005;20(6):549–550. [DOI] [PubMed] [Google Scholar]
- 50.Quah HM, Samad A, Maw A. Ileostomy carcinomas a review: the latent risk after colectomy for ulcerative colitis and familial adenomatous polyposis. Colorectal Dis. 2005;7(6):538–544. [DOI] [PubMed] [Google Scholar]
- 51.Mohandas S, Lake S. Primary adenocarcinoma of ileostomy: case report with review of the literature. Case Rep Med. 2010;2010:921328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang A, Davis B, Snyder J, Pulskamp S, et al. Considerations for diagnosis and management of ileostomy-related malignancy: a report of two cases. Ostomy Wound Manage. 2014;60(5):38–43. [PubMed] [Google Scholar]
- 53.Procaccino L, Rehman S, Abdurakhmanov A, McWhorter P, et al. Adenocarcinoma arising at ileostomy sites: two cases and a review of the literature. World J Gastrointest Surg. 2015;7(6):94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roth JA, Logio T. Carcinoma arising in an ileostomy stoma: an unusual complication of adenomatous polyposis coli. Cancer. 1982;49(10):2180–2184. [DOI] [PubMed] [Google Scholar]
- 55.Ross DS, Bussing R, Dietrich J. Carcinoma arising in an ileostomy. IMJ Ill Med J. 1987;172(3):163–166. [PubMed] [Google Scholar]
- 56.Primrose JN, Quirke P, Johnston D. Carcinoma of the ileostomy in a patient with familial adenomatous polyposis. Br J Surg. 1988;75(4):384. [DOI] [PubMed] [Google Scholar]
- 57.Lopez JF, Granger P, Dupre A. [Malignant degeneration on ileostomy for rectocolonic polyposis]. J Chir (Paris: ). 1991;128(4):201–203. [PubMed] [Google Scholar]
- 58.Gilson TP, Sollenberger LL. Adenocarcinoma of an ileostomy in a patient with familial adenomatous polyposis. Report of a case. Dis Colon Rectum. 1992;35(3):261–265. [DOI] [PubMed] [Google Scholar]
- 59.Lux N, Wedell J, Busch M, van Calker H. [Adenocarcinoma of the ileostomy after total proctocolectomy in familial polyposis. A case report and synthesis of previously published cases]. Chirurg. 1993;64(5):416–418. [PubMed] [Google Scholar]
- 60.Wu JS, McGannon EA, Church JM. Incidence of neoplastic polyps in the ileal pouch of patients with familial adenomatous polyposis after restorative proctocolectomy. Dis Colon Rectum. 1998;41(5):552–556. [DOI] [PubMed] [Google Scholar]
- 61.Mimura T, Kuramoto S, Yamasaki K, Kaminishi M. Familial adenomatous polyposis: a case report and histologic mucin study. J Clin Gastroenterol. 1999;28(4):372–376. [DOI] [PubMed] [Google Scholar]
- 62.Iizuka T, Sawada T, Hayakawa K, Hashimoto M, Udagawa H, Watanabe G. Successful local excision of ileostomy adenocarcinoma after colectomy for familial adenomatous polyposis: report of a case. Surg Today. 2002;32(7):638–641. [DOI] [PubMed] [Google Scholar]
- 63.Hata K, Watanabe T, Kawamura YJ, et al. K-ras mutation and loss of heterozygosity at 17p with beta-catenin accumulation in intramucosal carcinoma of the ileostomy in familial adenomatous polyposis: a case report. Dig Dis Sci. 2003;48(12):2310–2314. [DOI] [PubMed] [Google Scholar]
- 64.Shenoy S, Cassim R. Ileostomy adenocarcinoma associated with familial adenomatous polyposis (FAP): new problem in old disease. Int J Colorectal Dis. 2009;24(12):1475–1476. [DOI] [PubMed] [Google Scholar]
- 65.Huntington JT, Stanich PP, Harzman AE. Adenocarcinoma arising from an end ileostomy in a patient with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2017;15(4):A27–A28. [DOI] [PubMed] [Google Scholar]
- 66.Smith JC, Schäffer MW, Ballard BR, et al. Adenocarcinomas after prophylactic surgery for familial adenomatous polyposis. J Cancer Ther. 2013;4(1):260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barclay TH, Schapira DV. Malignant tumors of the small intestine. Cancer. 1983;51(5):878–881. [DOI] [PubMed] [Google Scholar]
- 68.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14(2):145–153. [DOI] [PubMed] [Google Scholar]
- 69.Levi GS, Harpaz N. Intestinal low-grade tubuloglandular adenocarcinoma in inflammatory bowel disease. Am J Surgical Pathol. 2006;30(8):1022–1029. [DOI] [PubMed] [Google Scholar]
- 70.Deutsch AA, McLeod RS, Cullen J, Cohen Z. Results of the pelvicpouch procedure in patients with Crohn’s disease. Dis Colon Rectum. 1991;34(6):475–477. [DOI] [PubMed] [Google Scholar]
- 71.Gorbach SL, Nahas L, Weinstein L. Studies of intestinal microflora: a unique microbial ecology. Gastroenterology. 1967;53(6):875–880. [PubMed] [Google Scholar]
- 72.Schlippert W, Mitros F, Schulze K. Multiple adenocarcinomas and pre-malignant changes in “backwash” ileitis. Am J Med. 1979;66(5):879–882. [DOI] [PubMed] [Google Scholar]
- 73.Parc YR, Olschwang S, Desaint B, Schmitt G, Parc RG, Tiret E. Familial adenomatous polyposis: prevalence of adenomas in the ileal pouch after restorative proctocolectomy. Ann Surg. 2001;233(3):360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamilton SR, Bussey HJ, Mendelsohn G, et al. Ileal adenomas after colectomy in nine patients with adenomatous polyposis coli/Gardner’s syndrome. Gastroenterology. 1979;77(6):1252–1257. [PubMed] [Google Scholar]
- 75.Hartley JE, Fazio VW, Remzi FH, et al. Analysis of the outcome of ileal pouch-anal anastomosis in patients with Crohn’s disease. Dis Colon Rectum. 2004;47(11):1808–1815. [DOI] [PubMed] [Google Scholar]
- 76.Herring JA, Hall CC, Johnson JA, et al. K-ras mutation in a tubular adenoma originating at an ileostomy in a familial adenomatous polyposis patient. Am J Gastroenterol. 1996;91(3):587–591. [PubMed] [Google Scholar]
- 77.Bertario L, Russo A, Sala P, et al. Multiple approach to the exploration of genotype-phenotype correlations in familial adenomatous polyposis. J Clin Oncol. 2003;21(9):1698–1707. [DOI] [PubMed] [Google Scholar]
- 78.Crabtree MD, Tomlinson IP, Hodgson SV, Neale K, Phillips RK, Houlston RS. Explaining variation in familial adenomatous polyposis: relationship between genotype and phenotype and evidence for modifier genes. Gut. 2002;51(3):420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedl W, Meuschel S, Caspari R, et al. Attenuated familial adenomatous polyposis due to a mutation in the 3’ part of the APC gene. A clue for understanding the function of the APC protein. Hum Genet. 1996;97(5):579–584. [DOI] [PubMed] [Google Scholar]