Abstract
Multimodality cancer therapy has led to remarkable improvements in survival of childhood and young adult cancer, with survival rates exceeding 85%. Such remission rates come with their own adverse sequelea or ‘late effects’. Although the cause of these late effects is multi-factorial, radiation-related adverse effects are one of the most prevalent. Hypopituitarism is a recognised complication of irradiation of brain tumours distant to the hypothalamo-pituitary (HP) axis when the axis is included within the exposed field. Much of the data concerning the development of hypopituitarism, however, relate to early forms of photon-based radiotherapy. In this narrative review, we discuss advances in individual radiotherapy techniques currently used in treating brain tumours and their theoretical benefits based primarily on dosimetric studies. Increasingly precise radiation techniques, including advances in the delivery of photons (i.e. intensity-modulated radiotherapy) and proton beam therapy, are now available options. The premise behind these newer techniques is to reduce the dose and volume of normal tissue irradiated whilst maintaining an effective radiation dose to target tissue. When treating brain tumours distant to the HP axis the expectation, based upon dosimetric studies, is that newer forms of radiotherapy will less frequently involve the HP axis in the exposed field, and where incorporated within the field it will be exposed to a lower radiotherapy dosage. Intuitively the dosimetric studies should translate into significant reductions in the prevalence of HP dysfunction. These data are promising; however, to date there are minimal robust clinical data to determine if the theoretical benefits of these newer techniques on HP dysfunction is to be realised.
Key Words: radiotherapy, hypopituitarism, photon, proton, hypothalamo-pituitary dysfunction
Background
Improvements in childhood and young adult (CAYA; 0–24 years) cancer therapy have led to dramatic improvements in survival in the last few decades of the 20th century; however, recent improvements in survival rates have been more measured. Overall, 5-year survival of CAYA cancer survivors now exceeds 85% (1). Currently, there are estimated to be around half a million CAYA cancer survivors in Europe (2) who have undergone multimodality therapy including combinations of surgery, radiotherapy (RT), chemotherapy, and immunotherapy. Although potentially lifesaving, these interventions often come with their own adverse sequelae that occur both acutely and after a variable duration of time, in some cases decades, following completion of treatment. Over 40% of the population of CAYA cancer survivors suffer from severe, disabling, or life-threatening complications in the 30 years following diagnosis (3). Although the cause of these complications is multi-factorial, radiation-induced adverse effects are considered as one of the most important factors and not infrequently occur many decades after the original cancer diagnosis (4). As such, CAYA cancer survivors necessitate regular long-term follow-up and monitoring to allow early identification and intervention to prevent treatment-associated morbidity.
Over the last few decades, chemotherapy regimens have enabled delaying and de-intensification of RT schedules in terms of delivered dose and/or volume of tissue exposed to minimise impact on young developing brains, particularly in children under the age of 3 years. Exposure of healthy brain tissue to radiation, however, continues to occur during treatment of most central nervous system tumours, skull base, parameningeal, selective head and neck tumours, acute leukaemia with CNS involvement, as well as during total body irradiation (TBI) utilised as preconditioning before bone or stem cell transplantation. Cranial RT treatment volumes may be localised to the tumour's original site (focal irradiation), or the entire cranial contents may be intentionally irradiated, such as in whole-brain RT, craniospinal RT (CSRT), or TBI.
Early side effects of irradiation to the CNS are in part the consequence of cerebral oedema, which can lead to acute neurological symptoms such as weakness, speech, and sensory disturbance. In more severe cases, this can result in nausea, vomiting, and additional sequelae of raised intracranial pressure including seizures. Additional early sequelae of cranial RT can include hair loss, fatigue, skin, and scalp changes. Patients not infrequently develop somnolence syndrome in the intermediate weeks after completion of cranial irradiation. In the long-term, CNS irradiation is associated with hearing loss, cataracts, endocrinopathies, stroke, neurocognitive impairment, secondary tumours, worse socioeconomic outcomes, and excess mortality (5, 6). The excess mortality of CAYA cancer survivors predominantly relates to recurrence or progression of the primary disease; however, an increase in cardiovascular mortality (5) and risk of early- and late-occurring stroke (6, 7) are also observed. As a consequence, survivors are more likely to be hospitalised compared with the general population.
Effects of conventional radiotherapy on hypothalamo-pituitary axis function
It is estimated that around 40–50% of CAYA cancer survivors develop an endocrinopathy requiring long-term surveillance and follow-up (8). Growth disorders, hypopituitarism, central precocious puberty, primary hypothyroidism, primary gonadal insufficiency, hyperparathyroidism, and low bone mass occur at increased prevalence in CAYA cancer survivors. Hypothalamo-pituitary (HP) dysfunction is one of the most frequent endocrinopathies resulting directly from tumours in the hypothalamus and suprasellar cistern, surgery in the region of the HP axis, RT and putatively from raised intracranial pressure from hydrocephalus. Hypopituitarism is however most commonly a sequelae of irradiation therapy when the HP axis lies within the exposed field. Robust data to support the occurrence of hypopituitarism following the use of chemotherapy in CAYA cancer survivors remain elusive (9). The pathological mechanism leading to radiation-induced pituitary hormone deficiencies is not fully understood but may result from a combination of damage to hypothalamic nuclei, portal vasculopathy, pituitary fibrosis, or the development of pituitary atrophy secondary to hypothalamic damage.
HP dysfunction can present as central precocious puberty (CPP) and/or pituitary hormone deficiencies. CPP is believed to result from the release of the pre-pubertal inhibitory effects of higher centres on the gonadotrophin-releasing hormone pump, leading to the onset of gonadotrophin secretion. Pituitary hormone deficits can involve one or multiple anterior pituitary hormone axes. The risk of developing HP dysfunction is dependent on a number of variables, including total radiation dose at the HP axis, dose per fraction, volume of the HP axis exposed to radiation, time since irradiation, and age at radiation. It is unsurprising therefore that the development of radiation-induced HP dysfunction, following radiation delivered during childhood, can present either during childhood itself or following the completion of growth and puberty. Higher radiation doses lead to greater damage to the HP axes, with both earlier onset and a greater number of pituitary hormone deficits (10).
Following exposure of the HP axis to RT pituitary hormone deficits generally occur in a relatively predictable order. The growth hormone axis is almost exclusively the first to be impaired, followed by gonadotrophins, adrenocorticotrophic hormone (ACTH), and lastly thyroid-stimulating hormone (TSH). With increasing recognition that TSH deficiency can be reflected by a fall in free thyroxine levels of >20% (11), it is becoming clear that TSH deficiency likely occurs earlier and in parallel with the development of gonadotrophin and ACTH deficiency. A causative effect of irradiation to impair posterior pituitary function is yet to be shown. The prevalence of pituitary hormone deficits in a large single-centre cohort of childhood cancer survivors followed for a median of 24 years and in whom the HP axis was exposed to RT, estimated the prevalence of deficiencies to be 40.2% for growth hormone, 11.1% for TSH, 10.6% for luteinizing hormone (LH)/follicle-stimulating hormone, and 3.2% for ACTH. CPP was present in 0.9% (12). The largest study performed in adult survivors of non-pituitary brain tumours showed a comparatively higher prevalence of pituitary dysfunction of 88.8% after a median follow-up of 8 years. For individual axes, growth hormone deficiency (GHD) was the most frequent (86.9%), followed by lutenising hormone/follicle stimulating hormone deficiency (LH/FSHD) (34.6%), adrenocorticotrophic hormone deficiency (ACTHD) (23.4%), and thyroid stimulating hormone deficiency (TSHD) (11.2%), with hyperprolactinemia reported in 15% of patients (13). The cohort was however, in part, selected for surveillance based on the dose and distribution of the RT delivered (13).
In childhood cancer survivors, a radiation dose threshold below which deficiency of an individual axis rarely occurs has been described. A dose of ≥18 Gy to the HP axis is required to increase the risk of GHD and CPP, but higher doses of ≥30 Gy significantly increase the risk of LH/FSHD, ACTHD, and TSHD (14). It has however been suggested that radiation doses of <30 Gy may cause LH/FSHD and TSHD but at a much later time period, indicating the importance of ongoing surveillance (12). Not dissimilar data regarding dose thresholds for individual anterior pituitary hormone deficits have been reported for adult survivors of gliomas (15). In CAYA cancer survivors, the time from RT exposure to HP dysfunction varies for each individual hormone axis, with GHD occurring earliest at <1.0–4.4 years, followed by TSHD 1.8–5.1 years, ACTHD 2.5–7.0 years, LH/FSHD 4.5–10.2 years; and 3.1–3.8 years for CPP (16).
Clinically, the presentation of hypopituitarism occurring following HP axis irradiation is not different from other causes of HP disease, though it may be more insidious and difficult to recognise symptomatically within the milieu of symptoms relating directly to the previous tumour, treatment thereof, and additional late sequelae. Growth hormone deficiency presents in childhood with reduced height velocity and in adults with adult growth hormone deficiency syndrome. When considering growth, other treatment-related factors including the direct impact of previous CSRT and chemotherapy on vertebral growth plates need to be considered. Gonadotrophin dysfunction during childhood presents either with CPP or alternately with failure of pubertal progression and in adults with symptoms of sex steroid deficiency and subfertility. ACTH deficiency presents similarly in children and adults, though hypoglycaemia generally only occurs in children. The presentation of TSH deficiency is indistinguishable from primary hypothyroidism.
Without intervention, deficiencies in these hormones during childhood can contribute to a reduction in final adult height, failure of pubertal development, gynaecomastia, osteoporosis, subfertility, adverse body composition and vascular risk profile, and impaired quality of life (QoL). Importantly, untreated ACTH deficiency can result in life-threatening adrenal crises. Diabetes insipidus when present is unrelated to irradiation and should stimulate investigation for an alternate cause of posterior pituitary dysfunction. CAYA cancer survivors with one or more endocrine or metabolic disorders are more likely to report poorer health-related QoL and sub-optimal physical activity levels (17).
Evolution of conventional XRT
The paediatric population, due to their growth and development potential, is particularly sensitive to radiation-induced side effects. Competing objectives to maximise cure and minimise toxicity including late effects are pivotal in younger vulnerable patients. This has led to innumerable efforts in the past few decades for the development of increasingly precise radiation techniques to reduce the dose and volume of normal tissue irradiated (18, 19). The use of modern imaging equipment for planning; co-registration of planning CT and diagnostic MRI sequences to optimise accurate RT targets and organs at risk (OAR) delineation; evolution of RT from conventional to intensity-modulated RT (IMRT) and volumetric-modulated arc therapy (VMAT) to further improve conformality; and modern irradiation techniques like proton beam therapy (PBT) and stereotactic RT (SRT) have enabled better sparing of normal tissues in this population. These in turn have resulted in reduced long-term side effects, improved QoL, and have allowed dose escalation in radio-resistant tumours (20).
Two- and three-dimensional radiotherapy
The interaction of high-energy electromagnetic waves with the tumour cells is the basis of photon-based RT. Photons, while traversing through the body, interact with the electrons and deposit energy, thereby causing DNA damage. Due to their unique properties, maximum dose deposition occurs shortly after entering the body and thereafter continuously decreases until they exit. As such, conventional RT is not the ideal modality to treat tumours situated at a depth, especially paediatric tumours, where the intervening and surrounding developing normal tissues will receive a significant RT dose, thereby increasing side effects.
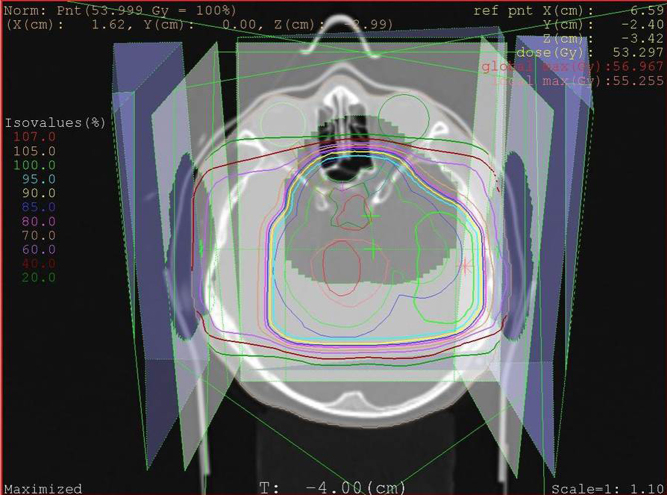
Historically, RT treatment comprised 2D techniques relying on bony anatomy and hand-drawn shielding blocks for treatment planning. The earliest use of RT in paediatric malignancies dates back to 1919 when Harvey Cushing innovated its use in the treatment of medulloblastoma (21). In the 1930s, orthovoltage and cobalt therapy were the main RT modalities in treating childhood tumours (22). During the 2D era, the adjustment of fields to the actual tumour volume was difficult. By the end of the last millennium, sophisticated 3D imaging was incorporated into planning software, which along with the introduction of multileaf collimation, allowed accurate delivery of radiation to mirror the shape of the tumour (conformal; Fig. 1). As a consequence of these advancements during the last few decades, two 2D RT has been superseded by 3D conformal techniques and, more latterly, by IMRT (23, 24).
Figure 1.
Conventional radiotherapy planning scan for a low-grade glioma showing isodose lines representative of percentage of the tumoural dose.
Parallel to these improvements in photon-based techniques, during the period 1970–2000, emerging concerns regarding radiation-induced life altering, often irreversible and at times devastating late effects, dampened enthusiasm in the use of RT in the management of paediatric brain tumours (23). The interest in systemic chemotherapy use surged to defer or minimise the use of RT, especially in extremely young children with cancer. However, RT remains an essential component of current contemporary multimodality treatment regimes for CAYA cancers.
IMRT, VMAT, and IGRT
A landmark development in the evolution of RT techniques is the introduction of IMRT, which is an advanced method of delivering conventional RT. The availability of better imaging modalities, modern linear accelerators, and advanced treatment planning software has made IMRT more clinically relevant. This has enabled the optimisation of RT fields to the actual tumour volume and clear delineation of normal tissues that need to be protected from radiation (OAR). IMRT has allowed dose escalation to the target tissue and dose constraints to OAR. IMRT techniques are now the standard of care for the majority of paediatric photon treatments (23, 24, 25). Dosimetric studies comparing conformality and dose homogeneity have established the superiority of IMRT over conventional RT (26).
The techniques for photon therapy have further evolved over the past few decades with the introduction of image-guided RT (IGRT) and arc-based therapies like VMAT (Fig. 2) and helical tomotherapy. In IGRT, imaging techniques like CT and MRI are incorporated to increase precision in planning and daily treatment. Arc therapy allows patients to be treated from a full 360° beam angle thereby reducing treatment time. Tomotherapy (slice therapy) machines are a combination of a CT scanner and linear accelerator, where radiation is delivered in a fan-shaped distribution with the help of a continuous rotating gantry. This innovative technique provides daily 3D imaging of the tumour, achieving precision and decreased toxicity (27).
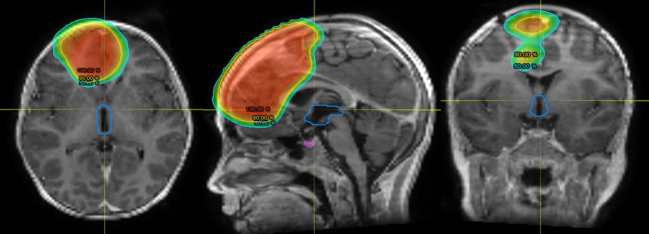
Figure 2.
Planning dosimetric scans for delivery of craniospinal radiotherapy using volumetric-modulated arc therapy (VMAT) for a metastatic ependymoma. Isodose The colour scale represents the percentage of the tumoural dose.
There are however potential disadvantages of IMRT. IMRT could expand the volume of non-target tissue receiving low-dose radiation while trying to limit the volume exposed to high-dose irradiation. This increase in the total region exposed to low-dose radiation may increase the risk of secondary cancers developing through a higher ‘integral dose’. However, the true incidence of secondary cancers can be quantified only through long-term follow-up data, which is as yet unavailable. When compared to conventional planning, IMRT and VMAT involve more complex treatment planning (Fig. 2) and are more labour-intensive for the RT multidisciplinary team (28).
Newer forms of radiotherapy
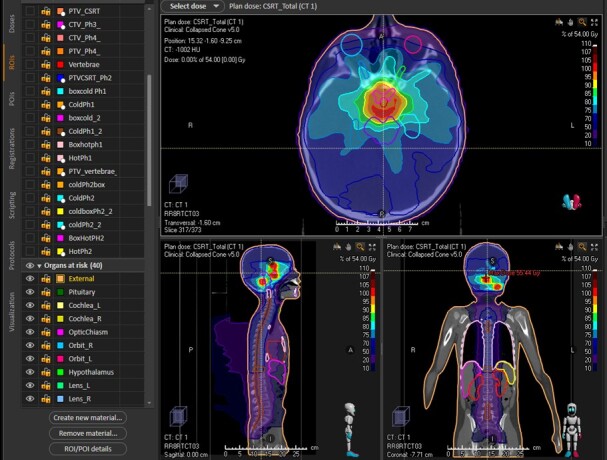
Much of the data suggesting advantages of newer forms of radiation are derived from a comparison of modelled dose distributions which take into account properties of the RT (i.e. photons or protons); delivery of the radiation (i.e. beams, arc therapy), and target volume which allow prediction of both effects on the tumour and OAR. These dosimetric models estimate the distribution of the RT and dosage delivered to the tumour, surrounding tissue, and OAR (Figs. 1, 2 and 3).
Figure 3.
Dosimetric planning study for delivery of proton therapy to a high-grade glioma showing 100, 80, and 50% isodose lines. The hypothalamus is outlined in blue and the pituitary in purple.
Proton beam therapy
PBT involves the delivery of proton particles in place of X-rays (photons) for radiation treatment. The potential therapeutic benefit of protons was first reported as early as 1946 by physicist Robert Wilson (29), with the first patient being treated at the University of California in 1954 (30). Protons travel through tissue in a straight line with increasing energy loss and decreasing pace, resulting in a steep dose fall off (Bragg peak) distal to the target tissue. Due to these unique physical properties, protons can deliver a characteristic dose distribution of minimal dose to surrounding uninvolved organs, while maintaining dose and efficacy against the tumour (Fig. 3). Comparison of modelled dose distributions of PBT with advanced photon-based techniques demonstrates that the dose received by the surrounding normal tissues is lower with PBT (31, 32). Intuitively therefore PBT offers similar chances of cure with a theoretical reduction in long-term side effects. Based on the dosimetry, PBT, when used to treat brain tumours distant to the HP axis, can potentially reduce the risks of growth and developmental disorders, endocrine dysfunction, and incidence of second cancers, while maintaining equivalent efficacy to photons (33). Side effects and toxicities still ensue with PBT, as tissues within and in close proximity to the target treatment volume receive a comparable high dose of radiation. Variance in the radiobiologic effect between photon and proton therapy on brain parenchyma and vasculature may give rise to different spectrums of toxicities, for example brain stem toxicity.
PBT is internationally considered as the treatment modality of choice for the CAYA population in view of its dosimetric advantage over conventional photon RT, resulting in significantly less radiation exposure to vulnerable healthy developing tissues, in turn translating into a lower risk of developing some RT-related acute and long-term toxicities. An additional benefit is anticipated in radio-resistant tumours where higher doses are required to optimise the chance of cure. In the absence of robust clinical outcome data, both Holland and Denmark have adopted the dosimetric model-based approach as the basis for their proton programmes (34). Similarly, based on a review of the dosimetry modelling and early treatment outcomes, NHS England has published a commissioning policy for the use of PBT in CAYA with malignant and non-malignant tumours (Table 1) (35). When compared with other RT modalities, the availability and distribution of proton centres are currently limited; however, the last decade has witnessed a global expansion in PBT centres, which is likely to continue into the future.
Table 1.
NHS England criteria for commissioning proton beam therapy (PBT) for children, teenagers, and young adults in the treatment of malignant and non-malignant tumours.
| (1) A clear indication for radiotherapy; defined as curable; and with a reasonable disease-specific 5-year survival expectation. |
| (2) Confirmation by a Children’s Principal Treatment Centre (PTC) or TYA PTC multi-disciplinary team (MDT) that treatment with PBT is an option. |
| (3) Age from birth up to about 25 years of age. |
| (4) No evidence of distant metastases, with the exception of tumours which remain curable when metastatic (i.e. metastatic intracranial germinomas). |
| (5) Adequate performance status and medically sufficiently stable to undergo PBT without delay, which may lead to increased risk of recurrence or compromise cure rates. |
| (6) Exclusion of patients requiring radiotherapy for indications where there is no dosimetric advantage for protons over photons (i.e. TBI, WBRT). |
| (7) Shared Decision-Making Tool: To be utilised as a framework for discussing radiotherapy with patients and their guardians, including knowns and unknowns regarding benefits and risks for PRT vs PBT. |
PRT, photon radiotherapy; TBI, total body irradiation; WBRT, whole-brain radiotherapy.
Despite the clear dosimetric advantages of proton therapy when treating brain tumours, there are as yet few robust studies comparing the clinical outcome, short- and long-term adverse effects of PBT vs photon-based therapies. The results of available studies are as yet inconclusive, due to biases or incorrect analysis (35). The sparsity of comparative evidence, particularly with the most advanced photon techniques, remains a challenge in quantifying the efficacy and putative safety advantages of PBT over photons. Furthermore, the difference in relative biological effectiveness also ideally needs to be taken into consideration. A particularly difficult challenge is the ethical and social concerns of carrying out RCTs in paediatric populations given the theoretical benefits of PBT.
Clinical data regarding efficacy and adverse effects are increasingly becoming available as PBT is utilised for a more expansive range of CAYA brain tumours including medulloblastomas, ependymomas, germinomas, low-grade gliomas, selective meningiomas, and CNS embryonal tumours, craniopharyngiomas, and head and neck tumours. Accepting that current data are limited due to a small sample size, limited follow-up duration, and lack of randomisation, the available data are in keeping with the dose distribution data that adverse sequelae of radiation are less with PBT compared with photon-based RT. Compared with photon-based RT, PBT has been shown to maintain a higher level of QoL (36) in survivors of childhood-onset brain tumours; similar rates of ototoxicity in those treated for medulloblastomas (37) and equivalence in progression-free and overall survival in children treated for standard-risk medulloblastomas and ependymomas (38, 39, 40). In contrast to the data in childhood-onset tumours, recent data have demonstrated a benefit in overall survival for proton-based vs photon-based RT in adult-onset low-grade gliomas; however, selection bias may have contributed to this outcome (41). Alongside this, cognitive function and QoL have been shown to be preserved 5 years out from treatment of adult-onset grade 2 gliomas (42). These results are promising but require more detailed mapping with hippocampal and temporal lobe RT dose-volume histogram parameters to inform whether there is a benefit over those who have received photon treatments.
Acute toxicity from PBT when treating childhood brain tumours is generally low grade; predominantly fatigue, alopecia, and dermatitis; and manageable with supportive care (43). In a prospective study of 174 paediatric low-grade gliomas, only 22 experienced nausea or vomiting requiring anti-emetics during treatment with PBT, and only two required glucocorticoids (44). After a median follow-up of 4.4 years, 4% experienced severe toxicity (brain stem necrosis, symptomatic vasculopathy, epilepsy, retinopathy) (44). In a further study, CAYA who received proton therapy >50.4 Gy involving the brain stem, the cumulative incidence of brain stem toxicity at two years was 3.8%, with the incidence of grade 3+ toxicity (severe interference with activities of daily life through to grade 5, death) of 2.1% (45).
The extensive evidence supporting the dosimetric advantages of PBT with lesser dose to normal tissue and the theoretical advantage of the reduction in long-term side effects leads to PBT to be considered the treatment of choice internationally. However, taking into consideration the sparsity of robust comparative evidence, clinicians should discuss the pros and cons of the different treatment modalities with patients and their parents before deciding on the best treatment for an individual as per the NHSE Clinical Commissioning Policy (35).
Stereotactic radiotherapy (SRS or SRT)
Precise immobilisation technique, CT/MRI, and use of multiple intersecting beams are the central features of stereotactic radiosurgery (SRS). This has the potential to deliver a single large dose of radiation to a very discrete tumour volume to ablate it, thereby reducing the dose to particularly sensitive developing brain tissues (46). The fact that treatment can be delivered in a single visit or fewer number of visits makes it favourable in the paediatric age group. But on the reverse, SRT often involves invasive immobilisation techniques such as frames fixed to the skull with metal pins. To overcome this hurdle, frameless options have evolved utilising a mask-based approach. Children often require sedation for this procedure.
SRS has been established in the treatment of both primary and metastatic adult brain tumours. Equivalent data with respect to the use of SRT for the treatment of paediatric brain tumours have not been well studied to date. Currently, its role in paediatric tumours is limited to palliative scenarios after fractionated photon treatments have been thoroughly explored (47). Within the adult population, SRT has been used in a variety of clinical scenarios; for example, in high-risk ependymoma patients treated with adjuvant RT, SRS can be incorporated as a boost to the tumour site. The use of single-fraction high-dose SRT in palliative approaches to ablate oligometastatic disease has been tried with promising results (48). Recurrent or residual disease is another area where SRS can be utilised. As this population might have received radiation in the primary setting, risks and complications related to re-radiation like radiation necrosis are higher (49). SRS may be helpful in these cases as it causes minimal re-radiation of critical normal structures that have previously been exposed to radiation. This is however dependent on the OAR, as after prior RT, SRS would not be suitable if the recurrence was considered too proximate to the optic chiasm or brainstem.
SRS is a technically feasible method of treatment delivery with minimal adverse effects to date. However, multicentric prospective trials are needed to examine the impact of SRS on late endocrine effects and to assess the risks/benefits with minimal bias. In the majority of the retrospective studies, previous irradiation is a significant confounding factor.
Evolution of hypopituitarism with newer forms of radiation
The radiation dose received at the HP axis is arguably the most important determinant of HP dysfunction. RT technological advances aim to limit the dose of radiation to surrounding critical normal tissues, including the HP axis. Newer techniques achieve this through a significant reduction of the volume of normal tissue within the field such that the HP axis is not exposed, or alternatively, where the axis is within the field, ensuring it is exposed to a significantly lower radiation dosage. It can therefore be theorised that when treating brain tumours distant to the HP axis, modern RT modalities and techniques will reduce the incidence of subsequent hypopituitarism.
Data comparing contemporary RT with conventional options are beginning to emerge; however, there remains a lack of robust data. Proton RT has the potential for reducing radiation exposure of the HP region during irradiation of tumours distant to the axis as a consequence of the more rapid fall-off of radiation with distance from the tumour (Bragg peak effect). These properties, based on dosimetry studies, may in turn limit the occurrence of late-onset endocrinopathies. Standard radiation schedules for medulloblastoma place the child at significant long-term risk of developing hypopituitarism (50). A number of small studies have shown that the use of proton therapy for the treatment of medulloblastoma has reduced the incidence of primary hypothyroidism, sex hormone deficiency, and the need for any hormone replacement therapy (51, 52). One study reported that those treated with protons had improved height outcomes; however, this may primarily reflect the lesser impact of protons on the growth plates in the spine due to the Bragg peak effect, which minimises exposure of the vertebral bodies (51). Differences in the incidence of GHD, central hypothyroidism, and adrenal insufficiency have not been conclusively shown (51, 53), and not all studies have shown a clear reduction in overall neuroendocrine deficits (39). The benefit, in terms of reduced endocrine late effects, has not been at the expense of treatment efficacy thus far (38) and seems to be in keeping with expected outcomes from dosimetric and toxicity modelling in childhood-onset medulloblastoma patients (54). In a study of 70 patients treated with PBT for paediatric CNS ependymoma and followed for 42 months, 1 of 32 developed central hypothyroidism, 2 of 25 tested had developed GHD, with a further 7 patients having a new low insulin-like growth factor 1 value but had not received a diagnosis of GHD (55). Height data were available in 57 patients after a median of 41 months from PBT and showed a decline in median height from the 54th percentile at baseline to the 36th percentile (55). In a cohort of adult-onset grade 2 glioma, new endocrine dysfunction was detected in 6 of 20 patients treated with PBT after 5.1 years of surveillance (42).
There are however significant limitations to the current data, which are based on relatively small numbers of patients, limited duration of follow-up, absence of control groups, lack of randomised prospective studies, and data directly comparing proton therapy with modern photon techniques such as IMRT.
Comparison of stereotactic conformal RT with conventional photon RT in CAYA with residual or progressive low-grade brain tumours has been undertaken in a randomised clinical trial (56). The study showed SRT to achieve superior neurocognitive and neuroendocrine functional outcomes at 5 years. Pituitary hormone deficits were recorded in only 29% of the individuals who received SRT vs 52% in the group who received conventional RT while maintaining comparable overall survival rates (56). Findings in adults can be conferred from prospective data using fractionated stereotactic radiation therapy for craniopharyngiomas, resulting in no new endocrinopathy in 16 patients, while providing good tumour control (57).
Summary
Survival of CAYA cancer survivors has improved markedly over the last few decades, with 5-year survival now exceeding 85%. The long-term sequelae of multimodality cancer therapy however remains a concern. Although RT is recognised as central to many of the adverse long-term sequelae experienced by CAYA cancer survivors, it remains an essential part of the treatment regimes for a number of brain tumours, head and neck soft tissue sarcomas, and bone tumours. This is unlikely to change in the foreseeable future given how effective radiation therapy is for certain tumour subtypes. Over the last decade, it has also become clear that individuals with adult-onset brain tumours experience similar levels of pituitary hormone deficits following irradiation compared with CAYA brain tumour survivors. If we are to use radiation therapy safely and avoid the adverse late sequelae associated with this modality, a reduction in exposure of normal tissue to radiation is required.
When considering the data characterising the adverse late effects from RT that we are observing currently in our patients with multiple organ systems, it has to be recognised that many of these are a reflection of the conventional RT in use 20–30 years ago. RT techniques have been transformed over the last few decades to improve the targeting of the tumoural tissue with greater sparing of the normal healthy tissue from radiation exposure. As such, it is hoped that we will start to observe fewer radiation-related late effects in both children and adults. Based on dosimetric modelling, state-of-the-art photon systems such as IMRT, in addition to PBT, should bring this closer to reality. As a consequence of the sharp fall-off in radiation dose around the target organ with PBT and the susceptibility of growing brain tissue to radiation-induced damage, PBT is likely to become the treatment of choice for many childhood cancers requiring radiation therapy to induce remission. Current data support at least the equivalence of PBT to photon-based therapies in terms of recurrence-free survival and overall survival.
Although the dosimetric studies are reassuring, as clinicians we wish to see robust clinical data that show the theoretical advantages of state-of-the-art photon and PBT have translated into better long-term health outcomes for our patients, particularly late effects. Currently, this is lacking. Specifically, in relation to HP dysfunction, irradiation of tumours distant to the HP axis with either state-of-the-art photon and PBT intuitively should result in fewer pituitary hormone deficits. To date, however, robust clinical outcome data regarding the reduction in late effects of PBT are not available. Studies have been small, of short duration, and often compare protons to older photons rather than state-of-the-art techniques. There may also be unconscious bias of the patients included, as the proton is more likely to be utilised if the target tissue is adjacent to critical structures such as the HP axis. Both larger observational and randomised studies are required; however, given the difficulties of undertaking randomised studies in children, data are likely to be derived from the adult population and large databases of ‘real-world’ data. We await the outcome of these studies with trepidation and in the hope that newer techniques will significantly reduce the risk of the observed late effects associated with conventional 2D and 3D RT.
Declaration of interest
RDM receives research funding from Ipsen, Pfizer, and Sandoz Pharma. DM, ADPR, NK, & MKW have no conflicts of interest.
Funding
This article has not received any funding.
Data availability
The article does not contain new data.
Acknowledgements
We would like to thank Danny Indelicato, MD, Department of Radiation Oncology, University of Florida, for providing the proton therapy planning images.
References
- 1.Public Health England. Children, teenagers and young adults. UK cancer statistics report 2021. London, UK: National Cancer Registration and Analysis Service for England, 2021. (available at: http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/cancer_in_children_teenagers_and_young_adults/) [Google Scholar]
- 2.Vassal G, Schrappe M, Pritchard-Jones K, Arnold F, Basset L, Biondi A, Bode G, Eggert A, Hjorth L, Kameric L, et al. The SIOPE strategic plan: a European cancer plan for children and adolescents. Journal of Cancer Policy 2016817–32. ( 10.1016/j.jcpo.2016.03.007) [DOI] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, et al. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine 20063551572–1582. ( 10.1056/NEJMsa060185) [DOI] [PubMed] [Google Scholar]
- 4.Mertens AC Liu Q Neglia JP Wasilewski K Leisenring W Armstrong GT Robison LL & Yasui Y. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. Journal of the National Cancer Institute 20081001368–1379. ( 10.1093/jnci/djn310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, Hudson MM, Donaldson SS, King AA, Stovall M, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. Journal of the National Cancer Institute 2009101946–958. ( 10.1093/jnci/djp148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, Stovall M, Yasui Y, Nicholson HS, Wolden S, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: childhood Cancer Survivor Study. Cancer 200397663–673. ( 10.1002/cncr.11095) [DOI] [PubMed] [Google Scholar]
- 7.Bowers DC Liu Y Leisenring W McNeil E Stovall M Gurney JG Robison LL Packer RJ & Oeffinger KC. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology 2006245277–5282. ( 10.1200/JCO.2006.07.2884) [DOI] [PubMed] [Google Scholar]
- 8.Wallace WH, Blacklay A, Eiser C, Davies H, Hawkins M, Levitt GA, Jenney ME. & Late Effects Committee of the United Kingdom Children's Cancer Study Group (UKCCSG). Developing strategies for long term follow up of survivors of childhood cancer. BMJ 2001323271–274. ( 10.1136/bmj.323.7307.271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seejore K Kyriakakis N & Murray RD. Is chemotherapy implicated in the development of hypopituitarism in childhood cancer survivors? Journal of Clinical Endocrinology and Metabolism 2020105. ( 10.1210/clinem/dgz132) [DOI] [PubMed] [Google Scholar]
- 10.Schmiegelow M Lassen S Poulsen HS Feldt-Rasmussen U Schmiegelow K Hertz H & Muller J. Cranial radiotherapy of childhood brain tumours: growth hormone deficiency and its relation to the biological effective dose of irradiation in a large population based study. Clinical Endocrinology 200053191–197. ( 10.1046/j.1365-2265.2000.01079.x) [DOI] [PubMed] [Google Scholar]
- 11.Fleseriu M Hashim IA Karavitaki N Melmed S Murad MH Salvatori R & Samuels MH. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 20161013888–3921. ( 10.1210/jc.2016-2118) [DOI] [PubMed] [Google Scholar]
- 12.van Lersel L, Li Z, Srivastava DK, Brinkman TM, Bjornard KL, Wilson CL, Green DM, Merchant TE, Pui CH, Howell RM, et al. Hypothalamic-pituitary disorders in childhood cancer survivors: prevalence, risk factors and long-term health outcomes. Journal of Clinical Endocrinology and Metabolism 20191046101–6115. ( 10.1210/jc.2019-00834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyriakakis N Lynch J Orme SM Gerrard G Hatfield P Loughrey C Short SC & Murray RD. Pituitary dysfunction following cranial radiotherapy for adult-onset nonpituitary brain tumours. Clinical Endocrinology 201684372–379. ( 10.1111/cen.12969) [DOI] [PubMed] [Google Scholar]
- 14.Constine LS Woolf PD Cann D Mick G McCormick K Raubertas RF & Rubin P. Hypothalamic-pituitary dysfunction after radiation for brain tumors. New England Journal of Medicine 199332887–94. ( 10.1056/NEJM199301143280203) [DOI] [PubMed] [Google Scholar]
- 15.Kyriakakis N Lynch J Orme SM Gerrard G Hatfield P Short SC Loughrey C & Murray RD. Hypothalamic-pituitary axis irradiation dose thresholds for the development of hypopituitarism in adult-onset gliomas. Clinical Endocrinology 201991131–140. ( 10.1111/cen.13971) [DOI] [PubMed] [Google Scholar]
- 16.van Lersel L, Mulder RL, Denzer C, Cohen LE, Spoudeas HA, Meacham LR, Sugden E, Schouten-van Meeteren AYN, Hoving EW, Packer RJ, et al. Hypothalamic-pituitary and other endocrine surveillance among childhood cancer survivors. Endocrine Reviews 202243794–823. ( 10.1210/endrev/bnab040) [DOI] [PubMed] [Google Scholar]
- 17.Pradhan KR, Chen Y, Moustoufi-Moab S, Krull K, Oeffinger KC, Sklar C, Armstrong GT, Ness KK, Robison L, Yasui Y, et al. Endocrine and metabolic disorders in survivors of childhood cancers and health-related quality of life and physical activity. Journal of Clinical Endocrinology and Metabolism 20191045183–5194. ( 10.1210/jc.2019-00627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meadows AT Friedman DL Neglia JP Mertens AC Donaldson SS Stovall M Hammond S Yasui Y & Inskip PD. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. Journal of Clinical Oncology 2009272356–2362. ( 10.1200/JCO.2008.21.1920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida Y, Sakamoto N, Kamibeppu K, Kakee N, Iwai T, Ozono S, Maeda N, Okamura J, Asami K, Inada H, et al. Late effects and quality of life of childhood cancer survivors: Part 2. Impact of radiotherapy. International Journal of Hematology 20109295–104. ( 10.1007/s12185-010-0611-z) [DOI] [PubMed] [Google Scholar]
- 20.Claude L Todisco L Leseur J Laprie A Alapetite C & Bernier V. New radiation techniques in paediatric cancers. Bulletin du Cancer 201198571–580. ( 10.1684/bdc.2011.1350) [DOI] [PubMed] [Google Scholar]
- 21.Kunschner LJ. Harvey Cushing and medulloblastoma. Archives of Neurology 200259642–645. ( 10.1001/archneur.59.4.642) [DOI] [PubMed] [Google Scholar]
- 22.Ajithkumar T Price S Horan G Burke A & Jefferies S. Prevention of radiotherapy-induced neurocognitive dysfunction in survivors of paediatric brain tumours: the potential role of modern imaging and radiotherapy techniques. Lancet. Oncology 201718e91–e100. ( 10.1016/S1470-2045(1730030-X) [DOI] [PubMed] [Google Scholar]
- 23.Jairam V Roberts KB & Yu JB. Historical trends in the use of radiation therapy for pediatric cancers: 1973–2008. International Journal of Radiation Oncology, Biology, Physics 201385e151–e155. ( 10.1016/j.ijrobp.2012.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor A & Powell ME. Intensity-modulated radiotherapy--what is it? Cancer Imaging 2004468–73. ( 10.1102/1470-7330.2004.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernier J Hall EJ & Giaccia A. Radiation oncology: a century of achievements. Nature Reviews. Cancer 20044737–747. ( 10.1038/nrc1451) [DOI] [PubMed] [Google Scholar]
- 26.Hua C Gray JM Merchant TE Kun LE & Krasin MJ. Treatment planning and delivery of external beam radiotherapy for pediatric sarcoma: the St. Jude Children's Research Hospital experience. International Journal of Radiation Oncology, Biology, Physics 2008701598–1606. ( 10.1016/j.ijrobp.2007.12.013) [DOI] [PubMed] [Google Scholar]
- 27.Teoh M Clark CH Wood K Whitaker S & Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. British Journal of Radiology 201184967–996. ( 10.1259/bjr/22373346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filippi AR Vanoni V Meduri B Cozzi L Scorsetti M Ricardi U & Lohr F. Intensity modulated radiation therapy and second cancer risk in adults. International Journal of Radiation Oncology, Biology, Physics 201810017–20. ( 10.1016/j.ijrobp.2017.09.039) [DOI] [PubMed] [Google Scholar]
- 29.Wilson RR. Radiological use of fast protons. Radiology 194647487–491. ( 10.1148/47.5.487) [DOI] [PubMed] [Google Scholar]
- 30.Lawrence JH Tobias CA Born JL McCombs RK Roberts JE Anger HO Low-Beer BV & Huggins CB. Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer Research 195818121–134. [PubMed] [Google Scholar]
- 31.Lin R Hug EB Schaefer RA Miller DW Slater JM & Slater JD. Conformal proton radiation therapy of the posterior fossa: a study comparing protons with three-dimensional planned photons in limiting dose to auditory structures. International Journal of Radiation Oncology, Biology, Physics 2000481219–1226. ( 10.1016/s0360-3016(0000741-0) [DOI] [PubMed] [Google Scholar]
- 32.Moeller BJ Chintagumpala M Philip JJ Grosshans DR McAleer MF Woo SY Gidley PW Vats TS & Mahajan A. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiation Oncology 2011658. ( 10.1186/1748-717X-6-58) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas H & Timmermann B. Paediatric proton therapy. British Journal of Radiology 20209320190601. ( 10.1259/bjr.20190601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langendijk JA Lambin P De Ruysscher D Widder J Bos M & Verheij M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiotherapy and Oncology 2013107267–273. ( 10.1016/j.radonc.2013.05.007) [DOI] [PubMed] [Google Scholar]
- 35.NHS England. Clinical Commissioning Policy: Proton Beam Therapy for Children, Teenagers and Young Adults in the Treatment of Malignant and Non-malignant Tumours. Leeds, UK: NHS England; 2020. (available at: https://www.england.nhs.uk/publication/proton-beam-therapy-for-children-teenagers-and-young-adults-in-the-treatment-of-malignant-and-non-malignant-tumours/) [Google Scholar]
- 36.Yock TI, Bhat S, Szymonifka J, Yeap BY, Delahaye J, Donaldson SS, MacDonald SM, Pulsifer MB, Hill KS, DeLaney TF, et al. Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiotherapy and Oncology 201411389–94. ( 10.1016/j.radonc.2014.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulino AC Mahajan A Ye R Grosshans DR Fatih Okcu M Su J McAleer MF McGovern S Mangona VA & Chintagumpala M. Ototoxicity and cochlear sparing in children with medulloblastoma: proton vs. photon radiotherapy. Radiotherapy and Oncology 2018128128–132. ( 10.1016/j.radonc.2018.01.002) [DOI] [PubMed] [Google Scholar]
- 38.Eaton BR, Esiashvili N, Kim S, Weyman EA, Thornton LT, Mazewski C, MacDonald T, Ebb D, MacDonald SM, Tarbell NJ, et al. Clinical outcomes among children with standard-risk medulloblastoma treated with proton and photon radiation therapy: a comparison of disease control and overall survival. International Journal of Radiation Oncology, Biology, Physics 201694133–138. ( 10.1016/j.ijrobp.2015.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, Jones RM, MacDonald SM, Pulsifer MB, Lavally B, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet. Oncology 201617287–298. ( 10.1016/S1470-2045(1500167-9) [DOI] [PubMed] [Google Scholar]
- 40.Sato M, Gunther JR, Mahajan A, Jo E, Paulino AC, Adesina AM, Jones JY, Ketonen LM, Su JM, Okcu MF, et al. Progression-free survival of children with localized ependymoma treated with intensity-modulated radiation therapy or proton-beam radiation therapy. Cancer 20171232570–2578. ( 10.1002/cncr.30623) [DOI] [PubMed] [Google Scholar]
- 41.Jhaveri J, Cheng E, Tian S, Buchwald Z, Chowdhary M, Liu Y, Gillespie TW, Olson JJ, Diaz AZ, Voloschin A, et al. Proton vs. photon radiation therapy for Primary gliomas: an Analysis of the National Cancer Data Base. Frontiers in Oncology 20188440. ( 10.3389/fonc.2018.00440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shih HA, Sherman JC, Nachtigall LB, Colvin MK, Fullerton BC, Daartz J, Winrich BK, Batchelor TT, Thornton LT, Mancuso SM, et al. Proton therapy for low-grade gliomas: results from a prospective trial. Cancer 20151211712–1719. ( 10.1002/cncr.29237) [DOI] [PubMed] [Google Scholar]
- 43.Suneja G Poorvu PD Hill-Kayser C & Lustig RA. Acute toxicity of proton beam radiation for pediatric central nervous system malignancies. Pediatric Blood and Cancer 2013601431–1436. ( 10.1002/pbc.24554) [DOI] [PubMed] [Google Scholar]
- 44.Indelicato DJ Rotondo RL Uezono H Sandler ES Aldana PR Ranalli NJ Beier AD Morris CG & Bradley JA. Outcomes following proton therapy for pediatric low-grade glioma. International Journal of Radiation Oncology, Biology, Physics 2019104149–156. ( 10.1016/j.ijrobp.2019.01.078) [DOI] [PubMed] [Google Scholar]
- 45.Indelicato DJ Flampouri S Rotondo RL Bradley JA Morris CG Aldana PR Sandler E & Mendenhall NP. Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncologica 2014531298–1304. ( 10.3109/0284186X.2014.957414) [DOI] [PubMed] [Google Scholar]
- 46.Hodgson DC Goumnerova LC Loeffler JS Dutton S Black PM Alexander E Xu R Kooy H Silver B & Tarbell NJ. Radiosurgery in the management of pediatric brain tumors. International Journal of Radiation Oncology, Biology, Physics 200150929–935. ( 10.1016/s0360-3016(0101518-8) [DOI] [PubMed] [Google Scholar]
- 47.NHS England Radiotherapy Clinical Reference Group. Stereora diosurgery and Steroeotactic Radiotherapy (Intracranial) (All Ages). London, UK: NHS England, 2019. (available at: https://www.england.nhs.uk/wp-content/uploads/2019/04/SRS-SRT-Intracranial.pdf) [Google Scholar]
- 48.Ludmir EB Grosshans DR & Woodhouse KD. Radiotherapy advances in pediatric neuro-oncology. Bioengineering (Basel) 20185. ( 10.3390/bioengineering5040097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aggarwal R Yeung D Kumar P Muhlbauer M & Kun LE. Efficacy and feasibility of stereotactic radiosurgery in the primary management of unfavorable pediatric ependymoma. Radiotherapy and Oncology 199743269–273. ( 10.1016/s0167-8140(9701926-9) [DOI] [PubMed] [Google Scholar]
- 50.Uday S Murray RD Picton S Chumas P Raju M Chandwani M & Alvi S. Endocrine sequelae beyond 10 years in survivors of medulloblastoma. Clinical Endocrinology 201583663–670. ( 10.1111/cen.12815) [DOI] [PubMed] [Google Scholar]
- 51.Eaton BR, Esiashvili N, Kim S, Patterson B, Weyman EA, Thornton LT, Mazewski C, MacDonald TJ, Ebb D, MacDonald SM, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro-Oncology 201618881–887. ( 10.1093/neuonc/nov302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aldrich KD Horne VE Bielamowicz K Sonabend RY Scheurer ME Paulino AC Mahajan A Chintagumpala M Okcu MF & Brown AL. Comparison of hypothyroidism, growth hormone deficiency, and adrenal insufficiency following proton and photon radiotherapy in children with medulloblastoma. Journal of Neuro-Oncology 202115593–100. ( 10.1007/s11060-021-03847-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bielamowicz K, Okcu MF, Sonabend R, Paulino AC, Hilsenbeck SG, Dreyer Z, Suzawa H, Bryant R, Adesina A, Dauser R, et al. Hypothyroidism after craniospinal irradiation with proton or photon therapy in patients with medulloblastoma. Pediatric Hematology and Oncology 201835257–267. ( 10.1080/08880018.2018.1471111) [DOI] [PubMed] [Google Scholar]
- 54.Ho ESQ Barrett SA & Mullaney LM. A review of dosimetric and toxicity modeling of proton versus photon craniospinal irradiation for pediatrics medulloblastoma. Acta Oncologica 2017561031–1042. ( 10.1080/0284186X.2017.1324207) [DOI] [PubMed] [Google Scholar]
- 55.Macdonald SM, Sethi R, Lavally B, Yeap BY, Marcus KJ, Caruso P, Pulsifer M, Huang M, Ebb D, Tarbell NJ, et al. Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro-Oncology 2013151552–1559. ( 10.1093/neuonc/not121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalali R, Gupta T, Goda JS, Goswami S, Shah N, Dutta D, Krishna U, Deodhar J, Menon P, Kannan S, et al. Efficacy of stereotactic conformal radiotherapy vs conventional radiotherapy on benign and low-grade brain tumors: a randomized clinical trial. JAMA Oncology 201731368–1376. ( 10.1001/jamaoncol.2017.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Astradsson A, Munck Af Rosenschöld P, Feldt-Rasmussen U, Poulsgaard L, Wiencke AK, Ohlhues L, Engelholm SA, Broholm H, Hansen Møller E, Klose M, et al. Visual outcome, endocrine function and tumor control after fractionated stereotactic radiation therapy of craniopharyngiomas in adults: findings in a prospective cohort. Acta Oncologica 201756415–421. ( 10.1080/0284186X.2016.1270466) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The article does not contain new data.



 This work is licensed under a
This work is licensed under a