Abstract
Background:
Maternal adaptations may vary by fetal sex. Whether male infants influence long-term mortality in mothers remains uncertain.
Objective:
To examine if male infants increase the risk of maternal mortality.
Methods:
This study included pregnant women enrolled at 12 U.S. sites from 1959–1966 in the Collaborative Perinatal Project (CPP). CPP records were linked to the National Death Index and the Social Security Master Death File to ascertain deaths until 2016. Fetal sex was determined by infant sex at birth, defined as the total number of male or female infants in pregnancies prior to or during enrollment in the CPP. In secondary analyses, exposure was defined as infant sex at the last CPP delivery. Outcomes included all-cause and underlying cause of mortality. We used Cox proportional hazards models weighted by the number of prior livebirths and stratified our models by parity and race/ethnicity.
Results:
Among 48,188 women, 50.8% had a male infant at their last registered CPP pregnancy and 39.0% had a recorded death after a mean follow-up of 47.8 years (SD 10.5). No linear association was found between the number of liveborn males and all-cause mortality (Primipara women: HR 1.02, 95% CI 0.95, 1.09, Multipara women, 1 prior livebirth: HR 0.96, 95% CI 0.89, 1.03, Multipara women, ≥2 prior livebirths: HR 0.97, 95% CI 0.85, 1.11). A similar trend was noted for cardiovascular- and cancer-related mortality. At the last delivery, women with a male infant did not have an increased risk of all-cause or cause-specific mortality compared to women with a female infant. These findings were consistent across racial/ethnic groups.
Conclusions:
Women who give birth to male infants, regardless of number, are not at increased risk of all-cause and cause-specific mortality. These findings suggest that giving birth to male infants may not independently influence the long-term health of women.
Keywords: infant sex, male infant, maternal mortality, race/ethnicity
BACKGROUND
Pregnancy involves immunological and physiological changes in the maternal system to adapt to the demands of the developing fetus. Maternal adaptations to pregnancy may differ by fetal sex due to the influence of genetic and hormonal factors on the maternal-fetal system.1 Male fetuses may also induce a heightened proinflammatory state in mothers, above the naturally occurring immunological changes during pregnancy.2,3 Testosterone levels have also been shown to be elevated at conception among women who give birth to male infants.4 The higher birthweights in male compared to female fetuses may provide an added stress to the maternal system.5–8 However, mothers who carry a male fetus to term, despite the consistently higher rates of pregnancy loss for male fetuses throughout gestation,9 suggests that these women may be better adapted for pregnancy and minimally impacted by the biological effects of carrying a male infant. Despite these acute changes during pregnancy, the impact of sex differences on long-term maternal mortality is not well understood.
Fetal sex may play a role in long-term maternal health through acute exposures to inflammation, stress, and hormonal factors during pregnancy. Elevated testosterone levels during pregnancy may impact long-term survival by increasing the risk of type 2 diabetes and polycystic ovary syndrome10 and subsequent development of cardiometabolic conditions and female malignancies.11,12 Additionally, chronic systemic inflammation is an established risk factor for the development of chronic conditions including coronary heart disease and cardiovascular-related mortality.13,14 Given that no prior studies have investigated the influence of infant sex at birth on long-term survival there is a need to better understand how carrying male fetuses may influence long-term maternal health. Moreover, the complexities of studying infant sex across multiple pregnancies has not been previously explored. There is also a need to investigate whether this relationship differs by race/ethnicity, a proxy for societal and contextual factors, due to the differential risk of chronic diseases and subsequent mortality across racial/ethnic groups in the U.S.15,16 We aimed to examine the association between male fetuses and long-term mortality in women and whether this association varies by race/ethnicity, in a diverse cohort of U.S. pregnant women.
METHODS
Cohort Selection
The current study included women enrolled in the Collaborative Perinatal Project (CPP) and the CPP Mortality Linkage Study that have been described in detail elsewhere.17,18 The CPP is a geographically and racially diverse, historical cohort of pregnant women, enrolled from 1959–1966 across 12 clinical sites in the U.S. (n=48,197). Information on sociodemographic, medical, and obstetrical characteristics of women in the CPP were collected at the first and all subsequent study pregnancies. Women were enrolled at their first prenatal visit with a physician and returned for follow-up visits throughout pregnancy. A fifth of women (n=8,772, 18.2%) had more than one registered pregnancy in the CPP.
The CPP Mortality Linkage Study linked records of women enrolled in the CPP to the National Death Index (NDI) and the Social Security Master Death File (SSMDF) to ascertain date of death of women until December 31st, 2016. The NDI is a national registry of deaths in the U.S. occurring from 1979. Records in the NDI are linked to individuals using a probabilistic method and include the underlying cause of death based on the World Health Organization International Classification of Diseases (ICD) 9th and 10th revisions.19,20 Records in the CPP were linked to the NDI based on combinations of demographic characteristics of women, including first and last name, social security number, date of birth, and sex. 21 To minimize missing vital status in women who died prior to 1979, CPP records were also linked to the SSMDF using exact matches on name or social security number and date of birth.18 The CPP Mortality Linkage study found 80% overall agreement between the NDI and the SSMDF.
Exposure
Our exposure was infant sex at the time of delivery, defined as the cumulative number of male or female infants, prior to or during enrollment in the CPP. For this analysis, we compared women with a history of only liveborn male infants to women with a history of only female born infants. In secondary analyses, exposure was defined as infant sex at the last registered pregnancy in the CPP, with female infants as the reference. Women with a multifetal gestation were classified as having a male infant if at least one of the infants was male. This analysis explored the acute effect of exposure to a male infant accounting for the number of prior liveborn males.
Outcomes
The primary outcome was all-cause mortality ascertained in either the NDI or SSMDF. In women with deaths recorded in both, the date of death in the NDI was used as the SSMDF does not have information on cause of death. Secondary outcomes were the underlying cause of death including cardiovascular disease (CVD), cancer, diabetes, dementia, liver, renal, respiratory, and suicide defined using ICD 9/10 codes listed in Online Table 1.
Statistical Analyses
Sociodemographic, medical, and reproductive history prior to the index pregnancy were included as covariates in our models based on their association with infant sex and mortality as per our directed acyclic graph (DAG; Online Figure 1). Covariates included age at the last registered delivery, race/ethnicity (White [reference], Black, Other [Asian, Puerto Rican, Other]), marital status (married/common-law [reference], divorced/separated, single/widow), family income (increments of $2,000 with $4,000 – 5,999 as the reference), number of years of education, pre-pregnancy body mass index (BMI), smoking status (never [reference], ever/former, current), pre-pregnancy hypertension (yes/no), pre-pregnancy diabetes (yes/no), history of a diagnosis of infertility (yes/no), prior gynecological infections (yes/no; including vaginitis, pelvic inflammatory disease), age at menarche (categorical; <12 years, 12–15 [reference], ≥ 16), and year of last observed pregnancy in the CPP (1-year increments; 1963 as the reference).
For all analyses, with the exception of models stratified by parity, we used a Cox proportional hazards model weighted by the total number of prior livebirths. The models were weighted to account for variations in the risk of mortality with increasing age and the potential for selection bias resulting from over 70% of women reporting ≥2 prior livebirths. The underlying time scale for our models was maternal age (1-year increments) with time zero defined as the age at the last observed pregnancy. We used regression calibration to account for the adjustment of proxy measures of inflammation as outlined in the DAG. We were concerned with inflammation that occurs prior to pregnancy and influences infant sex at birth through the primary sex ratio (as per the DAG). Based on our prior work, male fetuses are more susceptible to an inflammatory environment and more likely to experience a pregnancy loss.21 To account for the potential for misclassification due to the use of proxy measures of inflammation and to account for differences due to the historical nature of the CPP, we adjusted our covariate values for BMI, smoking, and diabetes using the regression coefficients from the National Health and Nutrition Examination Survey cohort in the paper by Sjaarda et al. that examined predictors of low-grade inflammation in women of reproductive age (Online Table 2).22 Although these factors may not fully account for the influence of inflammation, we attempted to minimize the potential for residual confounding using regression calibration. Our models were stratified by race/ethnicity for all-cause, CVD, and cancer mortality since these underlying causes account for the largest proportion of deaths among women in the U.S.23 Relative and absolute estimates of risk are presented as hazard ratios (HR) and risk differences (RD) and corresponding 95% confidence intervals (CI). RD estimates were calculated as the cumulative risk of mortality at age 80 with covariates set to their reference values (as outlined previously) and are reported as the number of excess deaths per 100 women. Age 80 was used to estimate absolute measures of risk of death since the average age of women at the last delivery was 24 and the follow-up ranged from 47–57 years.
Missing Data
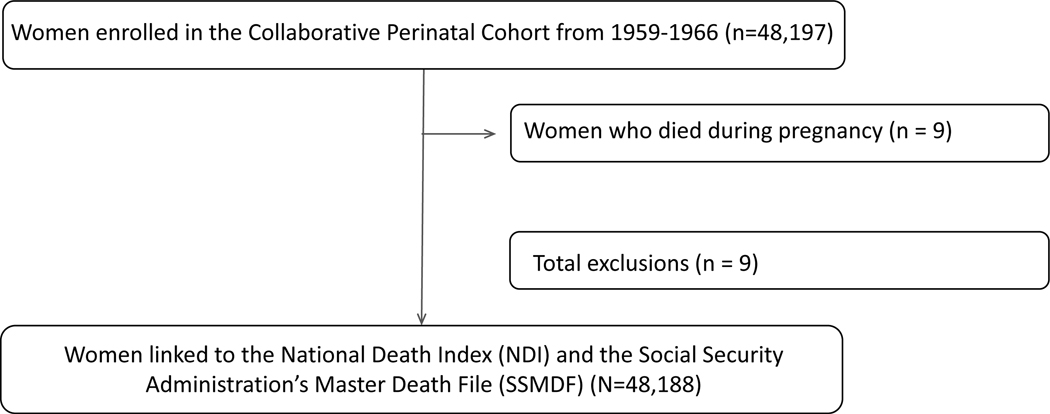
Missing data on exposure (n=3,124, 6.5%), covariates, and outcome (n=1,637, 3.4%; Figure 1) were imputed using multivariable imputation by the chained equations method in 10 imputed datasets. Information on infant sex was imputed for women with unknown infant sex (n=3,124 [6.5%] of births), including women with pregnancy losses, that occurred prior to last registered delivery in the CPP, with unknown infant sex. Imputation models included exposure, covariates, vital status, follow-up time, and variables that may inform missingness. For 1,637 (3.4%) women with insufficient information to link to the NDI or SSMDF, vital status was imputed using the discriminant function and cause of death was imputed using predictive mean matching. For these models, vital status or follow-up time were not included. We pooled estimates and corresponding 95% CIs across the imputed datasets using Rubin’s rule.24
Figure 1. Women in the Collaborative Perinatal Project Mortality Linkage Study.
Flow diagram of women in the Collaborative Perinatal Project (CPP) and CPP Mortality Linkage Study by infant sex at the last registered delivery in the CPP. The final sample size frequencies represent infant sex at the last registered pregnancy in CPP.
Sensitivity Analyses
In sensitivity analyses, we assessed the influence of infant sex at the first registered delivery in the CPP since women with preexisting conditions may not go on to have a subsequent pregnancy or may be more likely to experience a pregnancy loss. Additionally, our primary models were stratified by parity to account for the differential risk of exposure to male infants with higher order births. We also examined the effect of exposure to multiple male fetuses in women with multifetal gestations. Given the small number of women with multifetal gestations, this analysis was exploratory in nature.
Ethics Approval
The Linkage Study obtained institutional review board approval from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Emmes Corporation where abstraction and linkage were performed.
RESULTS
In their last CPP pregnancy, 50.8% of women delivered a male infant. The average age of women was 24.5 years and approximately half of the cohort self-identified as Black (Table 1). A small proportion of women reported having diabetes (5.7%) prior to enrollment in the CPP. Three quarters of women had a history of a prior pregnancy and most women reported a time to pregnancy of <6 months, with less than 1.0% of women having a prior diagnosis for infertility. Half of women had no prior liveborn male infants and a quarter of women reported having 2 or more prior male infants. No important differences were noted between women with a male or female infant at their last registered delivery.
Table 1.
Characteristics of Women in the Collaborative Perinatal Project by infant sex at the last registered pregnancy.
| Characteristicsa | Overall N=48,188 | Male N=24,473 | Female N=23,715 |
|---|---|---|---|
| Sociodemographic | |||
| Age at index pregnancy, years, mean (SD) | 24.5 (6.2) | 24.5 (6.2) | 24.5 (6.2) |
| Age at death, years, median (SD) | 72.4 (11.0) | 72.4 (11.0) | 72.3 (11.1) |
| Time from last registered pregnancy to end of follow-up, years, mean (SD) | 47.8 (10.5) | 47.9 (10.4) | 47.8 (10.5) |
| Race/Ethnicity, n (%) | |||
| White | 22,147 (46.0) | 11,412 (46.6) | 10,735 (45.3) |
| Black | 22,079 (45.8) | 11,041 (45.1) | 11,038 (46.5) |
| Otherb | 3,962 (8.2) | 2,020 (8.3) | 1,942 (8.2) |
| No. years of education, mean (SD) | 10.7 (2.6) | 10.7 (2.6) | 10.6 (2.6) |
| Missing | 2,505 (5.2) | 1,265 (5.2) | 1,240 (5.2) |
| Family income (≥ $4,000), n (%) | |||
| No income | 335 (0.7) | 167 (0.7) | 168 (0.7) |
| ≤ $2,000 – 3,999 | 27,662 (57.4) | 13,917 (56.9) | 1.3,745 (59.3) |
| $4,000 – 5,999 | 11,874 (24.6) | 6,075 (24.8) | 5,799 (24.5) |
| $6,000 – 7,999 | 5,313 (11.0) | 2,771 (11.3) | 2,542 (10.7) |
| $8,000 - ≥ $10,000 | 3,004 (6.2) | 1,542 (6.3) | 1,461 (6.2) |
| Missing | 4,944 (10.3) | 2,509 (10.2) | 2,435 (10.3) |
| Marital status, n (%) | |||
| Married/common law | 36,824 (76.4) | 18,777 (76.7) | 18,046 (76.1) |
| Divorced/separated/widowed | 4,201 (8.7) | 2,075 (8.5) | 2,126 (9.0) |
| Single | 7,163 (14.9) | 3,620 (14.8) | 3,543 (14.9) |
| Missing | 5 (0) | 3 (0) | 2 (0) |
| Clinical | |||
| Pre-pregnancy body mass index, kg/m2, mean (SD) | 25.0 (4.8) | 25.1 (4.9) | 25.0 (4.8) |
| Missing | 29,943 (62.1) | 15,181 (62.1) | 14,763 (62.3) |
| Smoking status, n (%) | |||
| Current | 22,317 (46.3) | 11,371 (46.5) | 10,946 (46.2) |
| Former/Ever | 7,014 (14.6) | 3,551 (14.5) | 3,463 (14.6) |
| Never | 18,857 (39.1) | 9,551 (39.0) | 9,307 (39.2) |
| Missing | 1,872 (3.9) | 950 (3.9) | 922 (3.9) |
| Pre-pregnancy hypertension, n (%) | 1,958 (4.1) | 993 (4.1) | 965 (3.9) |
| Missing | 665 (1.4) | 348 (1.4) | 318 (1.3) |
| Pre-pregnancy diabetes, n (%) | 781 (1.6) | 393 (1.6) | 388 (1.6) |
| Missing | 675 (1.4) | 350 (1.4) | 325 (1.4) |
| Pre-pregnancy cardiovascular diseaseb, n (%) | 5,670 (11.8) | 2,892 (11.8) | 2,778 (11.7) |
| Missing | 733 (1.5) | 379 (1.6) | 354 (1.5) |
| Pre-pregnancy cancer, n (%) | 1,853 (3.8) | 950 (3.9) | 903 (3.8) |
| Missing | 733 (1.5) | 379 (1.6) | 354 (1.5) |
| Pregnancy History | |||
| Parity, n (%) | |||
| Primipara | 13,235 (27.5) | 6,766 (27.6) | 6,469 (27.3) |
| Multipara | 34,953 (72.5) | 17,707 (72.4) | 17,246 (72.7) |
| Missing | 1,405 (2.9) | 732 (3.0) | 673 (2.8) |
| Reproductive History | |||
| Age at menarche, n (%) | |||
| < 12 years | 24,817 (51.5) | 12,627 (51.6) | 12,190 (51.4) |
| 12 – 15 years | 15,410 (32.0) | 7,840 (32.0) | 7,570 (31.9) |
| > 15 years | 7,961 (16.5) | 4,006 (16.4) | 3,955 (16.7) |
| Missing | 34,046 (70.7) | 17,283 (70.6) | 16,763 (70.7) |
| Time to pregnancy, n (%) | |||
| < 6 months | 45,483 (94.4) | 23,128 (94.5) | 22,355 (94.3) |
| 6–12 months | 1,073 (2.2) | 524 (2.1) | 549 (2.3) |
| > 12 months | 1,227 (2.6) | 630 (2.6) | 597 (2.5) |
| Missing | 405 (0.8) | 192 (0.8) | 213 (0.9) |
| Diagnosis of infertility, n (%) | 328 (0.7) | 198 (0.8) | 175 (0.7) |
| Missing | 788 (1.6) | 411 (1.7) | 377 (1.6) |
| Prior pregnancies with male liveborn, n (%) | |||
| 0 | 23,438 (48.6) | 11,955 (48.8) | 11,483 (48.4) |
| 1 | 12,476 (25.9) | 6,349 (25.9) | 6,127 (25.8) |
| ≥ 2 | 12,274 (25.5) | 6,169 (25.2) | 6,105 (25.7) |
Abbreviations: SD: standard deviation.
Data are reported based on imputed values;
Including Asian, Puerto Rican, and other;
Including rhematic fever and hypertension.
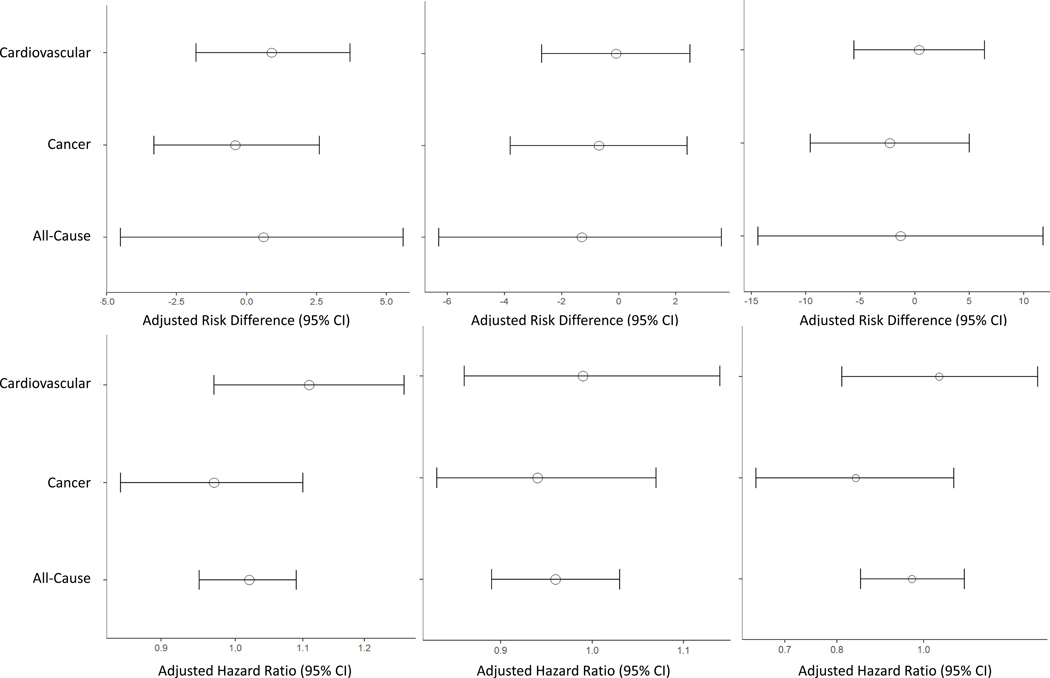
At a mean of 47.8 years (SD 10.5) after the last delivery, 39.0% of women died, which did not differ by infant sex (male: 9,558 [39.1%] versus female: 9,250 [39.0%]). A linear relationship was seen in the absolute risk of all-cause, CVD, and cancer mortality and number of prior livebirths with the highest proportion of deaths among women with ≥2 prior livebirths, which is likely due to the older age of women in this group (Table 2). Despite this, no differences in mortality between women with female versus male infants were found across any of the strata, suggesting that that the number of male-born infants does not confer an excess risk of mortality in multipara women (Figures 2a–c; Primipara women: HR 1.02, 95% CI 0.95, 1.09, multipara women, 1 prior livebirth: HR 0.96, 95% CI 0.89, 1.03, multipara women, ≥2 prior livebirths: HR 0.97, 95% CI 0.85, 1.11).
Table 2.
All-cause and cause-specific mortality by infant sex at birth by number of prior livebirths in women in the Collaborative Perinatal Project Mortality Linkage Study.a
| Only Female Infant(s) | Only Male Infant(s) | |
|---|---|---|
| No. Deaths, (%) | No. Deaths, (%) | |
| Women with no prior livebirths (n=12,843) | ||
| N=6,283 | N=6,560 | |
| All-cause | 1,690 (26.9) | 1,789 (27.3) |
| CVD | 439 (7.0) | 505 (7.7) |
| Cancer | 612 (9.7) | 621 (9.5) |
| Women with 1 prior livebirth (N=9,904) | ||
| N=4,870 | N=5,034 | |
| All-cause | 1,627 (33.4) | 1,622 (32.2) |
| CVD | 443 (9.1) | 453 (9.0) |
| Cancer | 571 (11.7) | 565 (11.2) |
| Women with ≥ 2 prior livebirth (N=2,313) | ||
| N=1,053 | N=1,260 | |
| All-cause | 439 (41.7) | 520 (41.3) |
| CVD | 128 (12.2) | 159 (12.6) |
| Cancer | 140 (13.3) | 142 (11.3) |
Abbreviations: CVD: cardiovascular disease.
Women with a history of livebirths with female and male infants were not included in these analyses.
Figure 2. The relationship between the cumulative number of male infants at the last registered delivery in the CPP and overall and cause-specific mortality stratified by the number of prior livebirths.
Plots represent the relative and absolute effect estimates and confidence intervals (CI) for all-cause and cardiovascular- and cancer-specific mortality within strata of women with no prior livebirths (Primipara) , women with 1 prior livebirth , and women with 2 or more prior livebirths.
When examining this relationship based on infant sex at the last registered pregnancy, there was no suggestion of an increased risk of all-cause mortality associated with a male infant on either the relative or absolute scales (HR 1.00, 95% CI 0.97, 1.03; RD 0.1 95% CI −2.4, 2.6; Table 3). The results were similar for CVD- and cancer-related mortality. Given the potential link between male sex and inflammation, diabetes, and progression to cardiometabolic diseases, we examined the association with other causes of death, primarily infection, diabetes, and renal disease. In our cause-specific analyses, male sex was not associated with mortality from other causes (Online Table 3).
Table 3.
All-cause and cause-specific mortality by infant sex at the last registered delivery both overall and stratified by race/ethnicity in the Collaborative Perinatal Project Mortality Linkage Study(N=48,188).
| Female infant | Male Infant | Female Infant | Male Infant | |||||
|---|---|---|---|---|---|---|---|---|
| Deaths No, (%) | Adjusted HR | Deaths No, (%) | Unadjusted HR (95% CI) |
Adjusted HRa (95% CI) |
Adjusted RD | Unadjusted RD (95% CI) | Adjusted RDa (95% CI) |
|
| Overall | ||||||||
| N=23,715 | N=24,473 | |||||||
| All-cause | 9,250 (39.0) | 1.00 (Reference) | 9,558 (39.1) | 1.00 (0.97, 1.03) | 1.00 (0.97, 1.03) | 0.00 (Reference) | −0.0 (−1.7, 1.7) | 0.1 (−2.4, 2.6) |
| CVD | 2,758 (11.6) | 1.00 (Reference) | 2,888 (11.8) | 1.01 (0.96, 1.07) | 1.04 (0.88, 1.22) | 0.00 (Reference) | 0.2 (−0.8, 1.2) | 0.2 (−1.0, 1.4) |
| Cancer | 3,004 (12.7) | 1.00 (Reference) | 2,988 (12.2) | 0.96 (0.91, 1.02) | 0.96 (0.91, 1.01) | 0.00 (Reference) | −0.7 (−1.6, 0.3) | −0.5 (−1.9, 1.0) |
| White | ||||||||
| N=10,735 | N=11,412 | |||||||
| All-cause | 4,025 (37.5) | 1.00 (Reference) | 4,235 (37.1) | 1.02 (0.93, 1.11) | 0.98 (0.94, 1.02) | 0.00 (Reference) | −0.7 (−3.1, 1.7) | −0.7 (−3.9, 2.5) |
| CVD | 1,075 (10.0) | 1.00 (Reference) | 1,166 (10.2) | 1.01 (0.95, 1.07) | 1.01 (0.92, 1.10) | 0.00 (Reference) | −0.2 (−1.0, 1.5) | 0.0 (−1.6, 1.7) |
| Cancer | 1,437 (13.4) | 1.00 (Reference) | 1,419 (12.4) | 0.93 (0.86, 1.00) | 0.92 (0.85, 0.99) | 0.00 (Reference) | −1.3 (−2.7, 0.2) | −0.9 (−2.8, 1.0) |
| Black | ||||||||
| N=11,041 | N=11,038 | |||||||
| All-cause | 4,580 (41.5) | 1.00 (Reference) | 4,571 (41.1) | 1.00 (0.93, 1.08) | 1.00 (0.96, 1.05) | 0.00 (Reference) | −0.0 (−2.8, 2.8) | 0.1 (−4.0, 4.2) |
| CVD | 1,513 (13.7) | 1.00 (Reference) | 1,512 (13.7) | 1.01 (0.94, 1.07) | 1.01 (0.93, 1.09) | 0.00 (Reference) | 0.0 (−1.6, 1.7) | 0.0 (−2.2, 2.4) |
| Cancer | 1,412 (12.8) | 1.00 (Reference) | 1,376 (12.5) | 0.97 (0.90, 1.06) | 0.97 (0.90, 1.06) | 0.00 (Reference) | −0.5 (−2.1, 1.1) | −0.4 (−2.8, 2.0) |
| Other | ||||||||
| N=1,942 | N=2,020 | |||||||
| All-cause | 646 (33.3) | 1.00 (Reference) | 752 (45.6) | 1.15 (0.94, 1.42) | 1.12 (1.00, 1.25) | 0.00 (Reference) | 5.4 (−0.0, 10.9) | 4.7 (−3.1, 12.5) |
| CVD | 204 (43.8) | 1.00 (Reference) | 245 (12.1) | 0.98 (0.83, 1.15) | 1.14 (0.93, 1.41) | 0.00 (Reference) | 2.1 (−1.1, 5.4) | 1.6 (−2.5, 5.8) |
| Cancer | 164 (8.5) | 1.00 (Reference) | 204 (10.1) | 1.20 (0.97, 1.49) | 1.21 (0.97, 1.50) | 0.00 (Reference) | 2.2 (−0.5, 4.8) | 2.0 (−1.8, 5.8) |
Abbreviations: CI: confidence interval; CVD: cardiovascular disease; HR: hazard ratio; RD: risk difference.
Adjusted for age at delivery (continuous), BMI (continuous), race/ethnicity (ref: white), education (in years, continuous), family income (categorical; ref: $4,000–5,999), smoking status (ref: never smoked), marital status (ref: married/common-law), history of a diagnosis for infertility, pre-pregnancy hypertension, pre-pregnancy diabetes, history of infection (including vaginitis and pelvic inflammatory disease), age at menarche (ref: 12–15), number of prior male infants, year of delivery. All models were weighted by the total number of pregnancies and covariates for BMI, diabetes, and smoking were adjusted by regression calibration using the coefficients from the NHANES sample of women in the Sjaarda et al. paper.22
When stratified by race/ethnicity, the absolute risk of death was higher among Black women (41.4%) compared to White women (37.3%) or women of other races/ethnicities (35.3%). A higher absolute risk for Black women was also seen for deaths due to CVD (13.7%) compared to White women (10.1%) and women of other race/ethnicities (11.3%). Giving birth to a male infant was not associated with an increased risk of all-cause mortality in White or Black women (Table 2). A similar trend was seen for deaths due to CVD and cancer. For women who were Puerto Rican, Asian, or other race/ethnicity, there was a suggestion that giving birth to a male compared to a female infant may be associated with an increased risk of all-cause (HR 1.12, 95% CI 1.00, 1.25, RD: 4.7, 95% CI −3.1, 12.5), CVD (HR 1.14, 95% CI 0.93, 1.41, RD 1.6, 95% CI −2.5, 5.8), and cancer mortality (HR 1.21, 95% CI 0.96, 1.50, RD 2.0, 95% CI −1.8, 5.8). However, due to the small number of events and heterogeneous nature of this group, the wide CIs preclude any definitive conclusions. In secondary analyses, no important differences within strata of race/ethnicity were found in women at the last registered delivery (Online Table 4).
We further examined the cumulative effect of male infants in women with multifetal gestations, with the underlying hypothesis that women exposed to two male infants would be at increased risk of mortality compared to women exposed to two female infants. In our adjusted models, we found that women with two male infants had a higher risk of CVD (HR 1.33, 95% CI 0.69, 2.55) compared to women with two female infants (Table 4). However, the small number of events and the wide CIs suggests this finding warrants further investigation. Additionally, women with mixed sex multifetal gestation were found to have a 23.0% higher risk of all-cause mortality (HR 1.23, 95% CI 0.90, 1.68) and a 41% increased risk of CVD mortality (HR 1.41, 95% CI 0.76, 2.61) compared to women delivering only female infants (Table 4). However, these findings need to be replicated in studies with larger number of women with multifetal gestations.
Table 4.
All-cause and cause-specific mortality by infant sex at birth among women with multifetal gestation in the Collaborative Perinatal Project (N=524).
| Multiple birth - Female infants (N=179) | Multiple birth - Female and male infant (N=172) | Multiple birth - Male infants (N=173) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. Deaths, (%) | Adjusted HR* (95% CI) | No. Deaths, (%) | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) | No. Deaths, (%) | Unadjusted HR (95% CI) | Adjusted Ha (95% CI) | |
| All-cause | 75 (42.0) | 1.00 (Reference) | 91 (52.6) | 1.23 (0.90, 1.68) | 1.22 (0.88, 1.70) | 65 (37.7) | 0.96 (0.69, 1.35) | 0.97 (0.69, 1.38) |
| CVD | 21 (11.8) | 1.00 (Reference) | 29 (16.8) | 1.41 (0.76, 2.61) | 1.36 (0.72, 2.59) | 24 (13.9) | 1.28 (0.68, 2.40) | 1.33 (0.69, 2.55) |
| Cancer | 28 (15.7) | 1.00 (Reference) | 25 (14.2) | 0.91 (0.51, 1.61) | 0.88 (0.48, 1.61) | 17 (24.2) | 0.65 (0.35, 1.20) | 0.71 (0.37, 1.36) |
Abbreviations: CI: confidence interval; CVD: cardiovascular disease; HR: hazard ratio.
Adjusted for age at delivery, BMI (continuous), race/ethnicity (ref: white), education (in years, continuous), family income (categorical; ref: $4,000–5,999), BMI (continuous), smoking status (ref: never smoked), marital status (ref: married/common-law), history of a diagnosis for infertility, pre-pregnancy hypertension, pre-pregnancy diabetes, history of infection (including vaginitis and pelvic inflammatory disease), age at menarche (ref: 12–15), number of prior male infants, year of delivery.
In our sensitivity analysis examining the association between infant sex and mortality at the first registered pregnancy to assess the potential impact of depletion of susceptibles due to women not having subsequent pregnancies, our findings were similar to our primary analyses (Online Table 5).
COMMENTS
Principal Findings
In a large diverse cohort of pregnant women in the U.S., we found that the number of male infants that a woman gives birth to was not associated with an increased risk of all-cause and cause-specific mortality. This association did not differ by infant sex at the last registered pregnancy in the CPP and were consistent between Black and White women. These findings suggest that giving birth to male infants alone may not independently influence the long-term health of women.
Strengths of the Study
Our study has several strengths. First, the CPP is one of the largest geographically and racially diverse cohorts of women in the U.S. with detailed reproductive and obstetrical history. Second, the use of a historical cohort allowed for the long-term follow-up of women. Third, the CPP included well-documented pregnancy characteristics that allowed for the adjustment of important confounders.
Limitations of the Data
Our study has several potential limitations. First, we were not able to ascertain infant sex for women with a spontaneous or induced abortion. Similar to previous studies examining infant sex, ascertainment of infant sex for women who experience a pregnancy loss is an inherent limitation since many losses occur prior to the time at which the sex of the infant can be identified. To overcome this limitation, we imputed infant sex for these women with the assumption that these data were missing at random. Second, we did not have the full reproductive history of women since women were not followed beyond their last registered pregnancy in the CPP. Therefore, information on pregnancies that occurred after the study period would not be captured in our study. Third, other factors that may occur over several decades and contribute to long-term mortality, including psychosocial factors (e.g., stress and socioeconomic impact of raising children) were not available. However, these factors would be considered intermediates of the relationship of interest and therefore would not be adjusted for in the analyses Fourth, measured levels of pre-pregnancy inflammation were also not available, and regression calibration may not have fully accounted for residual confounding. Finally, the use of a historical cohort may not reflect changes in obstetrical practice and preconception health characteristics over the last several decades. However, as previously mentioned a historical cohort is needed to answer the study question.
Interpretation
Early demographic studies have shown conflicting results for the relationship between maternal survival and the number of liveborn males. A study by Helle et al. using data from Finland from 1640 –1870 found an inverse association between the number of liveborn sons and number of surviving sons and long-term survival in mothers.25 A more recent study using demographic records from women giving birth between 1966–1982 in rural villages in Bangladesh, found an inverse relationship between the number of surviving sons and maternal mortality but no association with the number of liveborn sons.26 However, three studies found conflicting results. Two studies using preindustrial demographic data from Germany (1720–1874), Canada (1608–1874), and Sweden (1658–1831) found no association between the cumulative number of liveborn sons and maternal survival.27,28 A subsequent study in Poland using postindustrial demographic data found that women bearing children had lower survival rates compared to women with no children and that survival rates did not differ by infant sex.29 Although these studies had a sufficiently long follow-up period, they are prone to confounding bias since they relied solely on birth and death records. Additionally, since the majority of these studies used data prior to the 20th century and/or included women who gave birth in rural villages their findings may not be generalizable to contemporary populations of women of reproductive age in the U.S. The current study may also be limited by the historical nature of the cohort. A study comparing women enrolled in a contemporary cohort (Consortium on Safe Labor) to those in the CPP, found that women in the CPP were younger and less likely to receive an epidural and to have a cesarean delivery.30 They also had shorter first stages of labor, which the authors suggest may be a result of changes in obstetrical practice over time. However, our study is the first to examine the influence of infant sex on long-term mortality. which is only possible due to the historical nature of the cohort with long-term follow-up and information on factors that may influence this relationship.
The relationship between infant sex and maternal mortality may originate from the interactions between the maternal-fetal system. Hormone levels, primarily testosterone levels, are found to increase by over 70% during pregnancy31 and are affected by genetic and lifestyle factors including age and BMI.32 Additionally, women carrying male fetuses are found to have higher levels of circulating testosterone levels compared to women carrying female fetuses with a doubling of testosterone levels in the third trimester.33,34 However, testosterone levels are found to return to pre-pregnancy levels and to be similar between women who delivered male and female fetuses at 6 weeks post-partum.34 Despite the rise in testotestrone levels in women who carry male fetuses, our findings suggest that this acute exposure to elevated levels of testosterone during pregnancy does not contribute to disease progression and long-term mortality in women.
Many chronic conditions, such as diabetes, hypertension, CVD, and cancer are characterized by inflammation and a heightened immune response that contribute to disease-specific phenotypes.35 The potential heightened inflammatory state from male fetuses due to differential gene expression and hormonal factors may exacerbate inflammation among women with preexisting conditions (e.g., type 1 diabetes, lupus).36 An inflammatory state that may result from exposure to male fetuses could also be exacerbated by preexisting risk factors among mothers that can contribute to the progression of chronic conditions. Despite the plausible mechanisms underlying the relationship between infant sex and disease progression, our findings suggest that the acute exposure to males fetuses may not result in a level of inflammation that contributes to long-term maternal health.
Our analyses in women with multifetal gestations was performed to explore the potential biological effects of exposure to two male infants. We found that women carrying two male infants or mixed sex infants may be at increased risk of CVD mortality. Multifetal gestations place an added stress to the maternal system, primarily the cardiovascular system, due to increased cardiac outputs to support the development of multiple fetuses.37 Women with multifetal gestations are also at increased risk of pregnancy complications, including preeclampsia and gestational diabetes.38,39 Given the complex relationships that heighten the stress and demands to the maternal system in with multifetal gestations, these findings warrant further investigation in studies with larger samples of women with multifetal pregnancies.
CONCLUSIONS
Giving birth to one or more liveborn male infants is not associated with an increased risk of all-cause and cause-specific mortality in a cohort of reproductive women in the U.S. The risk did not differ by race/ethnicity. These findings suggest that infant sex may not independently influence the long-term health of mothers.
Supplementary Material
SYNOPOSIS.
Study question:
Does infant sex at birth influence long-term mortality risk in mothers.
What’s already known:
Prior studies have suggested potential sex differences in maternal adaptations to pregnancy.
No previous studies have examined the influence of infant sex at birth and maternal long-term mortality.
What this study adds:
In a diverse cohort of pregnant women in the U.S., we found that the number of male infants that a woman gives birth to was not associated with an increased risk of all-cause and cause-specific mortality
No differences were found for all-cause and cause-specific mortality across racial/ethnic groups.
Infant sex at the time of delivery does not influence the long-term health of women.
ACKNOWLEDGEMENTS
Funding Source:
Supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; contracts HHSN275200800002I/27500013)
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Social media quote: Women who give birth to male infants are not at increased risk of all-cause and cause-specific mortality.
Data Availability:
Data will be made accessible in NICHD repositories and electronic archives after completion of the study’s analytic phases.
REFERENCES
- 1.Al-Qaraghouli M, Fang YMV. Effect of Fetal Sex on Maternal and Obstetric Outcomes. Front Pediatr 2017;5:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghidini A, Salafia CM. Gender differences of placental dysfunction in severe prematurity. BJOG 2005;112:140–144. [DOI] [PubMed] [Google Scholar]
- 3.Enninga EA, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol 2015;73:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James WH. Evidence that mammalian sex ratios at birth are partially controlled by parental hormone levels at the time of conception. J Theor Biol 1996;180:271–286. [DOI] [PubMed] [Google Scholar]
- 5.Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol 2015;213:449 e1- e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS Med 2017;14:e1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiserud T, Piaggio G, Carroli G, et al. Correction: The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS Med 2017;14:e1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papageorghiou AT, Ohuma EO, Altman DG, et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet 2014;384:869–879. [DOI] [PubMed] [Google Scholar]
- 9.Orzack SH, Stubblefield JW, Akmaev VR, et al. The human sex ratio from conception to birth. Proc Natl Acad Sci U S A 2015;112:E2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruth KS, Day FR, Tyrrell J, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med 2020;26:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faulkner JL, Belin de Chantemele EJ. Sex hormones, aging and cardiometabolic syndrome. Biol Sex Differ 2019;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamimi RM, Hankinson SE, Chen WY, Rosner B, Colditz GA. Combined estrogen and testosterone use and risk of breast cancer in postmenopausal women. Arch Intern Med 2006;166:1483–1489. [DOI] [PubMed] [Google Scholar]
- 13.Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 16.Bornstein E, Eliner Y, Chervenak FA, Grunebaum A. Racial Disparity in Pregnancy Risks and Complications in the US: Temporal Changes during 2007–2018. J Clin Med 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niswander KR GM. The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The Women and Their Pregnancies. Philadelphia: W.B. Saunders Company; 1972. [Google Scholar]
- 18.Pollack AZ, Hinkle SN, Liu D, et al. Vital Status Ascertainment for a Historic Diverse Cohort of U.S. Women. Epidemiology 2020;31:310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Manual of the International Classification of Diseases, Injuries, and Causes of Death, based on recommendations of the Ninth Revision Conference, 1975. 1977; Geneva. [Google Scholar]
- 20.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. Geneva; 1992. [PubMed] [Google Scholar]
- 21.Radin RG, Mumford SL, Silver RM, et al. Sex ratio following preconception low-dose aspirin in women with prior pregnancy loss. J Clin Invest 2015;125:3619–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjaarda LA, Radin RG, Swanson C, et al. Prevalence and Contributors to Low-grade Inflammation in Three U.S. Populations of Reproductive Age Women. Paediatr Perinat Epidemiol 2018;32:55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Leading Causes of Death - Females - All races and origins - United States, 2017. Accessed on August 15, 2020 at https://www.cdc.gov/women/lcod/2017/all-races-origins/index.htm.
- 24.Rubin DB. Multiple Imputation After 18+ Years. J Am Stat Assoc 1996:473–89. [Google Scholar]
- 25.Helle S, Lummaa V, Jokela J. Sons reduced maternal longevity in preindustrial humans. Science 2002;296:1085. [DOI] [PubMed] [Google Scholar]
- 26.Hurt LS, Ronsmans C, Quigley M. Does the number of sons born affect long-term mortality of parents? A cohort study in rural Bangladesh. Proc Biol Sci 2006;273:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cesarini D, Lindqvist E, Wallace B. Maternal longevity and the sex of offspring in pre-industrial Sweden. Ann Hum Biol 2007;34:535–546. [DOI] [PubMed] [Google Scholar]
- 28.Beise J, Voland E. Effect of producing sons on maternal longevity in premodern populations. Science 2002;298:317; author reply [DOI] [PubMed] [Google Scholar]
- 29.Jasienska G, Nenko I, Jasienski M. Daughters increase longevity of fathers, but daughters and sons equally reduce longevity of mothers. Am J Hum Biol 2006;18:422–425. [DOI] [PubMed] [Google Scholar]
- 30.Laughon SK, Branch DW, Beaver J, Zhang J. Changes in labor patterns over 50 years. Am J Obstet Gynecol 2012;206:419 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem 1991;37:667–672. [PubMed] [Google Scholar]
- 32.Toriola AT, Vaarasmaki M, Lehtinen M, et al. Determinants of maternal sex steroids during the first half of pregnancy. Obstet Gynecol 2011;118:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehouse AJ, Mattes E, Maybery MT, et al. Sex-specific associations between umbilical cord blood testosterone levels and language delay in early childhood. J Child Psychol Psychiatry 2012;53:726–734. [DOI] [PubMed] [Google Scholar]
- 34.Meulenberg PM, Hofman JA. Maternal testosterone and fetal sex. J Steroid Biochem Mol Biol 1991;39:51–54. [DOI] [PubMed] [Google Scholar]
- 35.Duan L, Rao X, Sigdel KR. Regulation of Inflammation in Autoimmune Disease. J Immunol Res 2019;2019:7403796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol 2019;15:9–17. [DOI] [PubMed] [Google Scholar]
- 37.Kametas NA, McAuliffe F, Krampl E, Chambers J, Nicolaides KH. Maternal cardiac function in twin pregnancy. Obstet Gynecol 2003;102:806–815. [DOI] [PubMed] [Google Scholar]
- 38.Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol 1998;147:1062–1070. [DOI] [PubMed] [Google Scholar]
- 39.Pare E, Parry S, McElrath TF, Pucci D, Newton A, Lim KH. Clinical risk factors for preeclampsia in the 21st century. Obstet Gynecol 2014;124:763–770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made accessible in NICHD repositories and electronic archives after completion of the study’s analytic phases.