Abstract

An organic acid, salicylic acid, and its derivatives are constituents of various natural products possessing remarkable bioactivity. O-Acetyl salicylate (aspirin) is a well-known life-saving drug. Its peptide derivative salicylamide has also been explored in the designing of peptide-based therapeutic drugs. An organic base, picolylamine has been recently explored for designing diagnostic probes. However, both the acid and base have common features as metal chelating with coordinating metals. Thus, these scaffolds could be used for designing inhibitors of various metalloenzymes. Their characteristic properties encourage us to design peptides containing both scaffolds (salicylic acid and picolylamine) at opposite terminals. So far there is no report available on such conjugated peptides. This report describes the synthesis, conformational analysis, and biochemical assessment of rationally designed N-salicyl-AAn-picolamide peptides. Pleasantly, we have obtained the crystal structures of representative peptides that confirm their roles in conformational changes. Our biological assessment as quorum sensing inhibitors has revealed that their di/tripeptides inhibit quorum sensing of the pathogenic bacterium PA14 strain. Hence, these peptides have promising foldameric and therapeutic values.
Introduction
Salicylic acid and its derivatives are constituents of various natural products comprising the phenolic group with numerous therapeutic effects.1 The simplest derivative, O-acetyl salicylate, also known as aspirin, is a nonsteroidal anti-inflammatory drug (NSAID).2 Salicylic acid-conjugated amino acids/peptides are also explored in the development of novel drug candidates. The amide derivatives of salicylic acid, salicylamide (SAM), possess analgesic and antipyretic properties.3 Salicylamide is also considered as a potential scaffold for designing therapeutic peptide drugs by altering the peptide structure and conformation.4,5 Deng has synthesized novel salicylamide derivatives which show selective Ache inhibition, antioxidant features, Ab-aggregation, anti-neuroinflammation, and BBB permeability, a potential drug of Alzheimer’s disease.6 The N-salicylic-dipeptides inhibit human platelet aggregation, which is induced by collagen, ADP, and adrenaline.7 However, RGD analogues containing salicylic acid derivatives show antiplatelet activities.8 De Grado has used salicylic acid for novel self-assembling foldamers that inhibit heparin–protein interactions.9 Zeng has synthesized foldable aromatic oligoamides comprising a salicylic acid moiety which form helical structures through continuous hydrogen bonding.10,11 Imramovsky has synthesized benzamide derivatives of N-salicylate amino acid molecules that induce apoptosis in cancer cell lines.12 A very few picolylamine containing peptides are reported and employed for the chelation with metal ions.13−16 In the literature, salicylic acid and its derivatives exhibit antibacterial properties by inhibiting the quorum sensing (QS) system of pathogenic bacteria.17 QS is a bacterial cell–cell communication process that involves the production, detection, and response to extracellular signaling molecules.18 In general, Gram-positive and Gram-negative bacteria use QS communication circuits to regulate their various physiological activities such as symbiosis, virulence, competence, conjugation, antibiotic production, motility, sporulation, and biofilm formation.19 To control bacterial infections, drug-design strategies have been advanced by aiming the inhibition of the virulence factors against designing the complete bactericidal drugs (QS inhibitors). Nowadays, antibiotic drug-design strategies have been changed to find a sustainable solution to counteract the multidrug resistance issue.20 A multidrug-resistant and highly adaptable bacterium is responsible for nosocomial infections. The pathogenic bacterium Pseudomonas aeruginosa (PA) is also a multidrug-resistant and highly adaptable bacterium which is responsible for nosocomial infections21 and a prime concern for hospital-acquired infections in European countries.22 The persistent use of antibiotics has significantly reduced mortality but, at the same time, become the leading cause for the emergence of antibiotic-resistant bacterial pathogenic strains.23 Recently, our group has also explored nonbenzenoid derivatives, aminotroponyl sulfones, as anti-QS inhibitors.24 In the repertoire of salicylic acid–picolylamin-conjugated peptides, we have rationally designed novel peptides N-salicyl-AAn-picolylamine to evaluate their role in the structural folding and therapeutic values. This report describes their synthesis, conformational analyses, and QS inhibition studies in PA14.
Results and Discussion
We derivatized salicylic acid (1) into N-salicyl-amino acid ester derivatives, Sa-AA-OMe (2), under amide bond coupling reaction conditions (Scheme 1). At this stage, the nonpolar amino acids ester derivatives (glycine, alanine, valine, and leucine) were employed to prepare the respective Sa-AA-OMe (2a–2d). These derivatives were conjugated with picolylamine in two steps (hydrolysis of ester and amide coupling), which produced rationally designed picolylamine-conjugated dipeptides, Sa-AA-Pico (3a–3d). We also synthesized salicyl dipeptides, Sa-AA2-OMe (4a–4d), and salicyl tripeptides, Sa-AA3-OMe (6a–6d), to use as a spacer between picolylamine and salicylic acid. Subsequently, these peptides were conjugated with picolylamine and converted into the desired peptides Sa-(AA)n-Pico (5 and 7) under amide coupling conditions. The characterization data (NMR and HRMS) of all new products are provided in the Supporting Information (SI).
Scheme 1. Synthesis of Salicylic Acid and Picolylamine-Conjugated Peptides.
For structural and conformational studies, we attempted to crystallize those peptides and obtained the single crystals of 3a, 3b, 3d, and 5 in DCM/MeOH (1:1). Their solved data are submitted to the Cambridge Crystallographic Data Centre (CCDC). Their reference numbers are 2253679–2253684, which also describe concerning compounds. Their structural refinement parameters are provided in the SI. Carefully, we prepared their packing diagram from the respective crystal X-ray data and explored the role of salicylic acid/picolylamine scaffolds in the structural organization of novel peptides through noncovalent interactions. The peptide Sa-Gly-Pico (3a) forms a three-dimensional cross-linked supramolecular structure through the intermolecular H-bonding with the bond distance of 2.1 Å, owing to the amide groups in both fashions, parallel horizontally and antiparallel vertically (Figure 1A). In addition, its salicylic residue has intramolecular hydrogen bonding. The peptide Sa-Ala-Pico (3b) forms a novel supramolecular helical structure through hydrogen bonding between amide groups at a distance of 2.0 Å (Figure 1B). The peptide Sa-Leu-Pico (3d) forms a ladder-type supramolecular structure because of intermolecular hydrogen bonding between two antiparallel molecules with a distance of 2.3 Å (Figure 1C). Its salicylic acid residue also has intramolecular hydrogen bonding. The peptide Sa-Gly-Ala-Pico (5) has folded structure and forms unique supramolecular helical structures owing to the intermolecular hydrogen bonding through amide groups. Two consecutive helices are also joined by another set of hydrogen bonding with a bond distance of 2.0 Å (Figure 1D). In addition, its salicylic acids also exhibit intramolecular hydrogen bonding.
Figure 1.
Crystal structure and packing diagram of peptides: (A) structure of 3a (a) and its packing diagram (b), (B) structure of peptide 3b (a) and its packing diagram (b–c), (C) structure of peptide 3d (a) and its packing diagram (b–c), and (D) structure of peptide 5 (a) and its packing diagram (b–d).
We also obtained the crystal structures of salicyl peptides without containing picolylamine (4a/4c) in CDCl3. Their structure and packing diagrams are depicted in Figure 2, while other X-ray parameters are provided in the Supporting Information. The crystal structure of the Sa-α-peptide (4a) forms a unique cylindrical supramolecular structure with a diameter of ∼4.5 Å through intermolecular hydrogen bonding (∼2.1 Å) owing to the amide groups such as N–H···O=C in the antiparallel orientation (Figure 2A). The Sa-α-γ-peptide (4c) forms a supramolecular β-sheet-type structure owing to the intermolecular hydrogen bonding in its antiparallel amide groups with a distance of 2.0 Å. In addition, salicylic phenolic residue also forms intermolecular H-bonds (1.8 Å) with parallel amide carbonyl molecules (Figure 2B).
Figure 2.
Crystal structure and packing diagram of peptides: (A) structure of 4a (a) and its packing diagram (b–c) and (B) structure of peptide 4c (a) and its packing diagram (b).
Herein, we attempted to find the conformation of peptides (3/5/7) in the solution phase by 1D/2D-NMR techniques. We recorded 2D-NMR of peptides 3a–3d in CDCl3 and of peptides 5/7a–7d in DMSO-d6. We assigned their chemical shifts from respective 2D-COSY spectra and then extracted their NOE connectivity from respective 2D-NOESY spectra (see the Supporting Information). We noticed strong NOE peaks including the aromatic ring/amide protons that helped to propose the possible conformations as a unique foldamer (Figures S41–S58). Next, we performed 1H NMR DMSO-d6 titration experiment for finding the intramolecular hydrogen bonding owing to amide N–H’s of the chloroform (CDCl3)-soluble peptides (3a–3d) and generated their titration profiles (δ (ppm) vs VDMSO-d6 in μL) (Figures 3A and S59–S62). In peptide 3a, we noticed marginal downfield shifts (in ppm) of picolylamide N–H (∼0.5 in 3a, ∼0.5 in 3b, and ∼0.2 in 3c/3d) and amino acid amide N–H (∼1.0 in 3a, ∼0.5 3b, ∼0.3 in 3c, and ∼0.2 in 3d). In the literature,25,26 the lower value of chemical shift change indicates the stronger intramolecular hydrogen bonding in CDCl3. Our result supports that amino acid residues have significant roles in the intramolecular hydrogen bonding ability of their amide N–H. Hence, Sa-AA-Pico peptides (3a–3d) form a stable folded structure in CDCl3 owing to the intramolecular hydrogen bonding.
Figure 3.
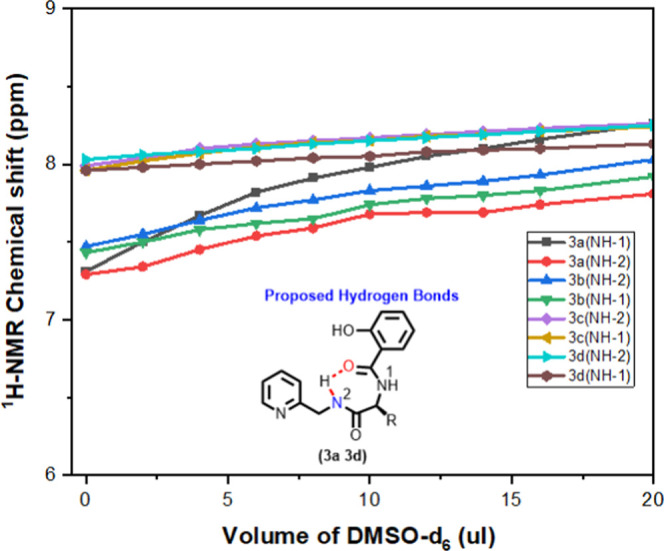
DMSO-d6 titration NMR profile of peptides 3a–3d.
We noticed that tetrapeptides 7a–7d are soluble only in the DMSO solvent. Thus, we attempted to optimize their structure theoretically using MMFF94 and to find energetically favorable conformation by GMMX. Their energy plots show various possible conformations, which are provided in the SI (Figures 4 and S75–S78). Peptide 7a shows intramolecular hydrogen bonding (N–H···O=C; O···H–O=C) and π–π interactions between picolylamide and salicylate aromatic rings at 3.9 Å (Figure 4A). Contrastingly, peptide 7b exhibits only intramolecular hydrogen bonding, no such π–π interactions owing to the bulky amino acid residue phenyl alanine. However, the pyridine ring of 7b exhibit hydrogen bonding with salicylic O–H as N–H···O (Figure 4B). The lowest energy conformer of peptides 7c and 7d also exhibits intramolecular hydrogen bonding and π–π interactions though their hydrogen donors and acceptors are partially altered. Hence salicyl-picolamide-conjugated peptides have a strong ability to form unique foldamers.
Figure 4.
Conformation of the theoretically minimized structure using MMFF94 and GMMX of peptides (A) 7a and (B) 7b.
The swarming motility and biofilm formation of PA14 reduces the efficacy of drug penetrating ability into the cell walls. These virulent factors and their capacity to conquer antibiotics are controlled by the complex cell to cell chemical signaling system, also known as quorum sensing (QS).27,28 We treated bacterium PA14 with peptides (3a–3d/5/7a–7d) and examined swarming of bacterial cells. In comparison to control studies, only peptides 3c and 5 significantly reduced swarming, despite affecting the bacteria’s growing ability even with 100 μM (Figures 5A and S69). Thus, peptides 3c/5 are potential antiquorum sensing agents. The QS system is also the key regulator of biofilm formation in the bacterium PA14 strain. We performed an initial screening of those peptides (3a–3d/5/7a–7d) at a higher concentration (100 μM) to evaluate the biofilm formation activity in the PA14 strain and compared it with the control. Importantly, only two peptides 3c/5 remarkably suppressed the biofilm formation in PA14. Then, we performed concentration-dependent biofilm formation in the same bacterial strain with those two peptides (3c/5). Their biofilm activities are provided as a bar diagram (Figure 5B). Our experimental results show that peptides 3c and 5 suppressed the biofilm formation most effectively at a low concentration of ∼40 μM. In the QS system, pyocyanin is a redox-active secondary metabolite phenazine compound that has been considered as both a virulence factor and a quorum sensing signaling molecule in bacterium P. aeruginosa.29 The production of pyocyanin indicates the pathogenicity and sustaining fitness of PA14 in a competitive environment. Thus, we examined the impact of synthetic peptide derivatives (3a–3d/5/7a–7d) on pyocyanin production in the PA14 strain. However, only two peptides (3c/5) exhibit a significant reduction of the pyocyanin synthesis (p < 0.001) at 100 μM. Next, we performed a pyocyanin production assay in a dose-dependent manner and summarized in a bar diagram (Figure 5C). Our result shows that peptide 5 has inhibited the pyocyanin synthesis by 39.18% at 40 μM while 60% at 100 μM.
Figure 5.

(A) Swarming images of PA14 in the DMSO control and the presence of compounds (3c and 5) at 100 μM concentration, (B) dose-dependent biofilm formation for control and peptides (3c and 5), and (C) dose-dependent pyocyanin production of peptides 3c and 5.
In the literature, the lasI/R QS circuit regulates the expression of virulent factors.30 lasR is a transcriptional activator, while lasI regulates the production of the autoinducer N-(3-oxododecanoyl) homoserine lactone (PAI-1).31 lasR and PAI-1 are required for the induction of lasB (encoding elastase) and other virulence genes. Elastase formation, one of the most virulent characteristics of PA, is the primary cause of PA-mediated death in hospitals. Elastase hydrolyzes internal peptide bonds found on the amino part of hydrophobic amino acid residues, allowing it to cleave a diverse spectrum of proteinaceous substrates. Thus, we conducted a qRT-PCR experiment to assess the effectiveness of synthesized peptide derivatives 3c and 5 at the gene level. The transcript levels of the 16S RNA gene were similar in control (cells grown LB medium) or in LB medium supplemented with the mentioned peptide, hence it was used for normalization. The compound 5 downregulated lasI and lasR significantly by 98.8% at 40 μM concentration (Figure 6A,B). Interestingly, compound 3c had no impact on both targeted genes (lasI and lasR), while significantly reducing biofilm formation, pyocyanin production, and swarming motility. The same expression pattern was observed in lasB in the presence of compound 5 (SI, Figure S72). Next, we performed a cytotoxicity experiment in HEK239T cell lines for ensuring the safe usage of synthesized peptides as anti-QS drugs to combat PA14 infection in the human body (SI, Figure S73). We could not notice any significant cytotoxicity at optimal concentration, significantly greater than their anti-QS concentration. Further, to examine the virulence effect of P. aeruginosa in cells, another cytotoxicity assay was performed using our synthesized peptide derivative as a therapeutic agent against PA14. PA14 was found to be cytotoxic against the HEK293T cell line, significantly reducing viability when compared to the DMSO control. Their data are summarized in a bar diagram (Figure 6C). This result indicates that compound 5 significantly reduced PA14 virulence-mediated cytotoxicity toward HEK293T cells and dramatically increased viability. Around 72.9% (p < 0.0001) of PA14-infected cells survived in the presence of the compound. In the literature, the phase contrast microscopic studies have been explored to visualize morphology (the shape and size) of bacteria which are directly related to their membrane.32 Thus, we planned to study the impact of peptide 5 in the bacteria membrane by phase contrast microscopic techniques and compared with the control study (without peptide 5). Their images are provided in the SI (Figure S74). We notice that the shape and size of the bacterium are unaffected with peptide 5. These studies further support that bacterial membranes are unaffected with peptide 5. As a result of these findings, compound 5 (40 μM) may be useful as a biofilm, swarming, pyocyanin, and virulence inhibitor in acute PA infections.
Figure 6.

Effect of peptide 5 on expression of (A) lasR and (B) lasI genes and (C) % of viable cells in HEK293T cells in the presence of peptides 3c and 5.
We successfully synthesized the rationally designed salicylic and polyamine-conjugated peptides. Their structures and confirmations in the solution phase are demonstrated by NMR, while their solid-state structures are confirmed by single-crystal X-ray studies. A few of them form a supramolecular unique self-assembly structure. Interestingly, their packing diagram exhibit unique structures such as helix, β turn, and cross-linking. Our biochemical studies reveal that one of conjugated peptides is a quorum sensing inhibitor.
Experimental Section
Materials and Instrumentation
Except as otherwise specified, all commercial reagents were used without further purification. Natural and unnatural amino acids, salicylic acid, picolylamine, DIPA, NMM, EDC.HCl, and HOBt were purchased from Spectrochem and Sigma-Aldrich. Anhydrous DMF was purchased from Merck. Reactions were carefully monitored by thin-layer chromatography (TLC) and visualized under UV or by performing a ninhydrin test. Silica gel column chromatography was carried out on Merck silica gel 100–200 mesh. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker 400 MHz spectrometer operating at 400 and 101 MHz for 1H and 13C acquisitions, respectively. Chemical shifts for 1H and 13C are reported in ppm downfield from tetramethyl silane as an internal standard. Data are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quartet, quin = quintet, m = multiplate, and br = broad. All HRMS data were recorded with a Bruker MicroTOF-Q II spectrometer
General Procedure for Peptide Synthesis
Synthesis of Salicylic Acid-Derivatized Peptides
Salicylic acid/salicylic acid-derivatized l-amino acid (1 equiv) was dissolved in commercially purchased anhydrous DMF, followed by addition of NMM (3 equiv). Resulting solution was cooled to 0 °C, and EDC.HCl (1.2 equiv) was added, followed by addition of HOBt (1.2 equiv) and l-methylated amino acid. Then, the reaction mixture is removed from the ice bath and placed in a preheated heating bath at 55 °C for 8 h. After completion, the reaction mixture is concentrated under reduced pressure and extracted three times in water and ethyl acetate. The organic layer is dried over sodium sulfate and concentrated. The concentrated crude mixture is purified by column chromatography with organic solvent system EtOAc and hexane and characterized by 1H, 13C NMR and ESI-HRMS techniques. Hydrolysis of salicylic acid-derivatized l-amino acid methyl ester is performed by 1N LiOH over 8 h.
Coupling of Picolylamine
Salicylic acid-derivatized l-amino acid (1 equiv) was dissolved in commercially purchased anhydrous, DMF followed by addition of di-isopropyl amine (DIPA) (1.5 equiv). Resulting solution was cooled to 0 °C, and EDC.HCl (1.2 equiv) was added, followed by addition of HOBt (1.2 equiv) and picolylamine (1.2 equiv). The reaction mixture is stirred overnight at 55 °C. After completion, the reaction mixture is concentrated under reduced pressure and extracted three times in ethyl acetate and water. The organic layer is dried over sodium sulfate and concentrated. The concentrated crude mixture is purified by column chromatography with organic solvent system EtOAC and MeOH and characterized by 1H, 13C NMR and ESI-HRMS techniques.
Methyl (2-Hydroxybenzyol) Glycinate (2a)
Compound 2a was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (12:88) as a colorless gummy compound (86% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 12.01 (s, 1H), 7.46 (d, J = 8.0 Hz, 1H), 7.40 (t, J = 7.7 Hz, 1H), 7.06 (s, 1H), 6.96 (d, J = 12.0 Hz, 1H), 6.85 (t, J = 7.5 Hz, 1H), 4.22 (d, J = 4.0 Hz, 2H), 3.82(s,3H). 13C NMR (101 MHz, CDCl3) δ (ppm) 170.4, 170.1, 161.4, 134.6, 125.8, 118.9, 118.6, 113.7, 52.7, 41.2. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C10H11NO4Na 232.0586, observed 232.0600.
Methyl (2-Hydroxybenzoyl)-l-alaninate (2b)
Compound 2b was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (15:85) as a white solid (84% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 12.09 (s, 1H), 7.44 (d, J = 8.0 Hz, 1H), 7.38 (t, J = 7.7 Hz, 1H), 7.10 (s, 1H), 6.94 (d, J = 8.3 Hz, 1H), 6.83 (t, J = 7.6 Hz, 1H), 4.78 (dd, J = 14.3, 7.1 Hz, 1H), 3.80 (s, 3H), 1.52 (d, J = 4.0 Hz, 3H). 13 C NMR (101 MHz, CDCl3) δ (ppm) 173.5, 169.5, 161.5, 134.5, 125.8, 118.8, 118.5, 113.8, 52.8, 48.2, 18.3. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C11H13NO4Na 246.0737, observed 246.0733.
Methyl (2-Hydroxybenzoyl)-l-valinate (2c)
Compound 2c was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (12:88) as a white solid (83% yield). 1H NMR (700 MHz, CDCl3) δ (ppm) 12.05 (s, 1H), 7.51–7.47 (m, 1H), 7.42–7.37 (m, 1H), 6.93–6.91 (m, 1H), 6.89–6.84 (m, 2H), 4.74 (dd, J = 8.4, 4.9 Hz, 1H), 3.79 (s, 3H), 2.29 (dd, J = 6.8, 5.0 Hz, 1H), 1.03 (dd, J = 6.8, 3.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ (ppm) 172.3, 169.8, 161.5, 134.5, 125.7, 118.8, 118.6, 113.9, 57.1, 52.5, 31.6, 18.9, 18.1. HRMS (ESI-TOF) m/z: [M + H]+ calcd. For C13H18NO4 252.1236, observed 252.1223.
Methyl (2-Hydroxybenzoyl)-l-leucinate (2d)
Compound 2d was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (10:90) as a white solid (81% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 12.07 (s, 1H), 7.45 (dd, J = 8.0, 1.4 Hz, 1H), 7.38–7.31 (m, 1H), 7.12 (d, J = 8.0 Hz, 1H), 6.93–6.89 (m, 1H), 6.84–6.77 (m, 1H), 4.90–4.80 (m, 1H), 3.79 (s, 3H), 1.81–1.64 (m, 3H), 0.98 (d, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ (ppm) 173.9, 169.9, 161.4, 134.5, 125.8, 118.7, 118.5, 113.7, 52.6, 50.8, 41.3, 24.9, 22.8, 21.8. HRMS (ESI-TOF) m/z: [M + H]+ calcd. For C14H20NO4 266.1392, observed 266.1367.
2-Hydroxy-N-(2-oxo-2-((pyridine-2-ylmethyl)amino)ethyl)benzamide (3a)
Compound 3a was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/methanol (98:2) as a white solid (79% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 12.13 (s, 1H), 8.54 (d, J = 4.6 Hz, 1H), 7.69 (t, J = 7.6 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.44 (s, 1H), 7.40 (t, J = 7.9 Hz, 1H), 7.28 (d, J = 10.5 Hz, 3H), 7.25–7.20 (m, 1H), 6.97 (d, J = 8.4 Hz, 1H), 6.86 (t, J = 7.6 Hz, 1H), 4.63 (d, J = 4.9 Hz, 2H), 4.21(d, J = 4.7 Hz, 2H). 13C NMR (100.06 MHz, CDCl3) δ (ppm) 170.1, 168.2, 161.5, 155.4, 149.1, 137.0, 134.5, 126.0, 122.7, 122.1, 118.9, 118.5, 113.9, 44.4, 42.8. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C15H15N3O3Na 308.1006, observed 308.1003.
2-Hydroxy-N-(1-oxo-1((pyridine-2-ylmethyl)amino)propan-2-yl)benzamide (3b)
Compound 3b was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/methanol (97:3) as a white solid (77% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 12.19 (s, 1H), 8.52 (d, J = 4.0 Hz, 1H), 7.67 (t, J = 4.0 Hz, 1H), 7.53 (d, J = 8.0 Hz, 2H), 7.46 (s, 1H), 7.37 (t, J = 8.0 Hz, 1H), 7.28–7.24 (d, J = 4.0 Hz, 1H), 7.23–7.17 (t, J = 8.0 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 6.82 (t, J = 8.0 Hz, 1H), 4.78 (m, 1H), 4.59 (t, J = 4.0 Hz, 2H), 1.53 (d, J = 8.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ (ppm) 172.2, 169.5, 161.3, 155.8, 149.0, 137.0, 134.3, 126.3, 122.6, 122.1, 118.8, 118.3, 114.2, 48.9, 44.5, 18.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd. For C16H18N3O3 300.1348, observed 300.1355.
2-Hydroxy-N-(3-methyl-1-oxo-1-((pyridine-2-ylmethyl)amino)butan-2-yl)benzamide (3c)
Compound 3c was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/methanol (97:3) as a white solid (71% yield). .1H NMR (400 MHz, CDCl3) δ (ppm) 12.20 (s, 1H), 8.50 (d, J = 1.8 Hz, 1H), 7.87 (s, 2H), 7.63 (m, 2H), 7.36–7.31 (m, 1H), 7.30–7.27 (m, 1H), 7.18 (d, J = 1.9 Hz, 1H), 6.94 (m, 1H), 6.77 (m, 1H), 4.59 (d, J = 5.8 Hz, 3H), 2.22 (m, 1H), 1.25–0.77 (d, J = 8.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ (ppm) 171.5, 169.5, 160.7, 156.2, 148.9, 136.9, 134.1, 126.9, 122.5, 122.1, 118.8, 118.1, 114.7, 58.77, 44.56, 31.29, 19.3, 18.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd. For C18H22N3O3 328.1661, observed 328.1681.
2-Hydroxy-N-(4-methyl-1-oxo-1-((pyridin-2-ylmethylamino)pentan-2-yl)benzamide (3d)
Compound 3d was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/methanol (97:3) as a white solid (70% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 12.21 (s, 1H), 8.47 (d, J = 4.4 Hz, 1H), 8.19 (d, J = 8.0 Hz, 1H), 8.10 (q, J = 16.0 Hz, 1H), 7.66 (dd, J = 8.0, 1.3 Hz, 1H), 7.60 (t, J = 7.7 Hz, 1H), 7.33–7.24 (m, 2H), 7.15 (dd, J = 7.3, 5.1 Hz, 1H), 6.89 (d, J = 8.4 Hz, 1H), 6.70 (t, J = 7.6 Hz, 1H), 4.85 (m, 1H), 4.56 (d, J = 5.4 Hz, 2H), 1.82–1.54 (m, 3H), 0.95–0.83 (m, 6H). 13C NMR (101 MHz, CDCl3) δ (ppm) 172.9, 169.74, 160.73, 156.54, 148.9, 137.0, 134.1, 127.1, 122.5, 122.0, 118.8, 118.0, 114.7, 52.0, 44.7, 41.1, 24.9, 22.9, 21.9. HRMS (ESI-TOF) m/z: [M + H]+ calcd. For C19H24N3O3 342.1818, observed 342.1793.
Methyl (2-Hydroxybenzoyl)glycyl-l-alaninate (4a)
Compound 4a was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (60:40) as a white solid (79% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 12.03 (s, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.43–7.34 (m, 2H), 6.98 (d, J = 8.3 Hz, 1H), 6.86 (t, J = 7.6 Hz, 1H), 6.65 (s, 1H), 4.63 (m, 1H), 4.15 (d, J = 4.8 Hz, 2H), 3.77 (s, 3H), 1.47 (d, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ (ppm) 173.1, 170.2, 168.0, 161.5, 134.6, 126.0, 118.9, 118.5, 113.7, 52.7, 48.4, 42.8, 18.3. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C13H16N2O5Na 303.0951, observed 303.0952.
Methyl 3-(2-(2-Hydroxybenzamido)acetamido)propanoate (4b)
Compound 4b was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (65:35) as a white solid (73% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 12.01 (s, 1H), 7.68 (s, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.37 (t, J = 7.8 Hz, 1H), 6.94 (d, J = 8.3 Hz, 1H), 6.83 (t, J = 7.5 Hz, 2H), 4.07 (d, J = 5.0 Hz, 2H), 3.68 (s, 3H), 3.57 (q, J = 6.0 Hz, 2H), 2.58 (t, J = 6.0 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ (ppm) 172.3, 170.3, 168.8, 161.3, 134.5, 126.2, 118.9, 118.4, 113.9, 51.9, 43.1, 35.2, 33.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd. For C13H17N2O5 281.1137, observed 281.1131.
Methyl 4-(2-(2-Hydroxybenzamido)acetamido)butanoate (4c)
Compound 4c was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (65:35) as a white solid (69% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 12.02 (s, 1H),8.05 (m, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.36 (t, J = 7.7 Hz, 1H), 7.00 (m, 1H), 6.94 (d, J = 8.3 Hz, 1H), 6.83 (t, J = 7.6 Hz, 1H), 4.09 (d, J = 5.2 Hz, 2H), 3.64 (s, 3H), 3.33 (dd, J = 12.7, 6.5 Hz, 2H), 2.37 (t, J = 7.1 Hz, 2H), 1.91–1.79 (m, 2H). 13C NMR (101 MHz, CDCl3) δ (ppm) 174.0, 170.4, 169.4, 161.1, 134.5, 126.6, 119.0, 118.2, 114.1, 51.8, 43.2, 39.2, 31.4, 24.3. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C14H18N2O5Na 317.1108, observed 317.1103.
2-Hydroxy-N-(2-oxo-2-((pyridine-2-ylmethyl)amino)propan-2-yl)amino)ethyl)benzamide (5)
Compound 5 was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/methanol (96:4) as a light gray solid (yield 73%). 1H NMR (400 MHz, DMSO) δ (ppm) 12.14 (s, 1H), 9.05 (s, 1H), 8.49 (d, J = 4.0 Hz, 2H), 8.32 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.73 (t, J = 8.0 Hz, 1H), 7.40 (t, J = 8.0 Hz, 1H), 7.25 (m, 2H), 6.91 (m, 2H), 4.37 (d, J = 4.0 Hz, 3H), 3.99 (d, J = 4.0 Hz, 2H), 1.28 (d, J = 4.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ (ppm) 172.8, 168.9, 168.7, 159.7, 159.0, 149.3, 137.1, 134.1, 128.9, 122.5, 121.1, 119.2, 117.7, 116.2, 48.9, 44.6, 42.8, 18.6. HRMS (ESI-TOF) m/z: [M + H]+ calcd. For C18H21N4O4 357.1563, observed 357.1540.
Methyl (2-Hydroxybenzoyl)glycyl-l-alanyl Glycinate (6a)
Compound 6a was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (75:25) as a white solid (72% yield). 1H NMR (400 MHz, MeOD4) δ (ppm) 7.82 (d, J = 8.1 Hz, 1H), 7.41 (t, J = 7.7 Hz, 1H), 6.92 (m, 2H), 4.48 (d, J = 7.2 Hz, 1H), 4.11 (s, 2H), 3.97 (s, 2H), 3.73 (s, 3H), 3.33 (s, 1H), 1.42 (d, J = 7.2 Hz, 3H). 13C NMR (101 MHz, MeOD4) δ (ppm) 174.0, 170.2, 170.1, 169.8, 159.5, 133.6, 128.1, 118.8, 116.9, 115.6, 51.23, 48.9, 42.4, 40.4, 16.5. HRMS (ESI-TOF) m/z: [M + H]+ calcd. For C15H20N3O6 338.1352, observed 338.1337.
Methyl (2-Hydroxybenzoyl)glycyl-l-alanyl Phenylalaninate (6b)
Compound 6b was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (70:30) as a white solid (69% yield). 1H NMR (400 MHz, CDCl3) δ (ppm) 8.08 (s, 1H), 7.74 (s, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.42 (s, 1H), 7.33 (t, J = 8.0 Hz, 1H), 7.22–7.16 (m, 4H), 7.07 (d, J = 8.0 Hz, 2H), 6.93 (d, J = 12.0 Hz, 1H), 6.79 (t, J = 8.0 Hz, 1H), 4.89–4.80 (m, 1H), 4.62–4.54 (m, 1H), 4.06 (d, J = 4.0 Hz, 2H), 3.67 (s, 3H), 3.15–3.0 (m,2H), 1.32 (d, J = 4.0 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ (ppm) 172.5, 171.9, 170.3, 169.3, 160.9, 135.1, 134.4, 129.2, 128.5, 127.1, 126.7, 118.9, 118.2, 116.5, 114.2, 111.6, 53.5, 52.5, 49.2, 42.9, 37.7, 18.1. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C22H25N3O6Na 450.1636, observed 450.1633.
Methyl (3-(2-(2-Hydroxybenzamido)acetamido)propanoyl)-l-alaninate (6c)
Compound 6c was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (75:25) as a white solid (67% yield). 1H NMR (400 MHz, DMSO) δ (ppm) 12.22 (s, 1H), 9.04 (t, J = 8.0 Hz, 1H), 8.32 (d, J = 8.0 Hz, 1H), 8.03 (t, J = 4.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.40 (t, J = 8.0 Hz, 1H), 6.91 (m, 2H), 4.26 (m, 1H), 3.88 (d, J = 4.0 Hz, 2H), 3.61 (s, 3H), 3.28 (q, J = 12.0 Hz, 2H), 2.31 (t, J = 8.0 Hz, 2H), 1.26 (d, J = 8.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ (ppm) 173.7, 170.7, 169.0, 168.8, 159.9, 134.1, 128.9, 119.2, 117.7, 116.1, 52.3, 48.0, 42.8, 35.6, 35.3, 17.4. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C16H21N3O6Na 374.1323, observed 374.1304.
Methyl (4-(2-(2-Hydroxybenzamido)butanoyl-l-alanate (6d)
Compound 6d was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/hexane (75:25) as a white solid (63% yield). 1H NMR (400 MHz, DMSO) δ (ppm) 12.24 (s, 1H), 9.05 (t, J = 5.5 Hz, 1H), 8.25 (d, J = 6.9 Hz, 1H), 8.03 (t, J = 5.4 Hz, 1H), 7.87 (d, J = 7.8 Hz, 1H), 7.40 (t, J = 7.7 Hz, 1H), 6.91 (m, 2H), 4.24 (m, 1H), 3.89 (d, J = 5.5 Hz, 2H), 3.61 (s, 3H), 3.08 (q, J = 16.0 Hz, 2H), 2.12 (t, J = 7.4 Hz, 2H), 1.64 (p, J = 7.2 Hz, 2H), 1.25 (d, J = 7.3 Hz, 3H). 13C NMR (101 MHz, DMSO) δ (ppm) 173.7, 172.3, 169.0, 168.7, 159.9, 134.1, 128.9, 119.2, 117.7, 116.2, 52.3, 47.9, 42.8, 38.7, 32.9, 25.7, 17.4. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C17H23N3O6Na 388.1479, observed 388.1480.
2-Hydroxy-N-(2-oxo-2-((1-oxo-1-((2-oxo-2-((pyridin-2-ylmethyl)amino)ethyl)amino)propan-2-yl)amino)ethyl)benzamide (7a)
Compound 7a was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/methanol (95:5) as an off-white solid (69% yield). 1H NMR (400 MHz, DMSO) δ (ppm) 12.12 (s, 1H), 9.02 (t, J = 8.0 Hz, 1H), 8.46 (d, J = 4.0 Hz, 1H), 8.32 (d, J = 8.0 Hz, 2H), 8.27 (t, J = 8.0 Hz, 1H), 7.85 (d, J = 8.0 Hz, 1H), 7.70 (t, J = 8.0 Hz, 1H), 7.40 (t, J = 8.0 Hz, 1H), 7.29–7.20 (m, 2H), 6.90 (m, 2H), 4.40–4.29 (m, 3H), 3.97 (d, J = 8.0 Hz, 2H), 3.77 (t, J = 8.0 Hz, 2H), 1.24 (d, J = 4.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ (ppm) 173.0, 169.4, 168.9, 168.8, 159.7, 158.8, 149.2, 137.1, 134.1, 128.9, 122.5, 121.3, 119.2, 117.7, 116.3, 48.9, 44.5, 42.6, 18.5. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C20H23N5O5Na 436.1597, observed 436.0931.
2-Hydroxy-N-(2-oxo-2-(((2S)-1-oxo-1-((1-oxo-3-phenyl-1-((pyridine-2-ylmethyl)amino)propan-2-yl)amino)propan-3-yl)amino)ethyl)benzamide (7b)
Compound 7b was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/MeOH (96:4) as white solid (68% yield). 1H NMR (400 MHz, DMSO) δ (ppm) 12.15 (s, 1H), 9.06 (t, J = 4.0 Hz, 1H), 8.50 (t, J = 8.0 Hz, 1H), 8.46 (d, J = 8.0 Hz, 1H), 8.28 (d, J = 4.0 Hz, 1H), 8.15 (d, J = 8.0 Hz, 1H), 7.87 (d, J = 8.0 Hz, 1H), 7.64 (t, J = 8.0 Hz, 1H), 7.41 (t, J = 8.0 Hz, 1H), 7.32–7.16 (m, 7H), 7.01 (d, J = 8.0 Hz, 1H), 6.92 (m, 2H), 4.55 (m, 1H), 4.41–4.25 (m, 3H), 3.95 (d, J = 4.0 Hz, 2H), 3.07 (dd, J = 12.0, 8.0 Hz, 1H), 2.89 (dd, J = 12.0, 8.0 Hz, 1H), 1.17 (d, J = 4.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ (ppm) 172.5, 171.3, 168.9, 168.8, 159.6, 158.8, 149.2, 138.1, 137.1, 134.1, 129.7, 129.0, 128.6, 126.8, 122.5, 121.0, 119.3, 117.7, 116.3, 54.7, 48.8, 44.5, 42.8, 42.6, 37.8, 18.6. HRMS (ESI-TOF) m/z: [M + Na]+ calcd. For C27H29N5O5Na 526.2067, observed 526.2086.
2-Hydroxy-N-(2-oxo-2-((4-oxo-4-((1-oxo-1-((pyridine-2-ylmethyl)amino)propan-2-yl)amino)butyl)amino)ethyl)benzamide (7c)
Compound 7c was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/MeOH (95:5) as a gray solid (61% yield). 1H NMR (400 MHz, DMSO) δ (ppm) 12.22 (s, 1H), 9.05 (t, J = 5.5 Hz, 1H), 8.47 (m, 2H), 8.16 (d, J = 7.3 Hz, 1H), 8.05 (t, J = 5.5 Hz, 1H), 7.87 (d, J = 7.9 Hz, 1H), 7.75 (t, J = 7.6 Hz, 1H), 7.40 (t, J = 7.7 Hz, 1H), 7.25 (m, 2H), 7.00–6.84 (m, 2H), 4.36 (d, J = 5.8 Hz, 2H), 4.34–4.29 (m, 1H), 3.88 (d, J = 5.5 Hz, 2H), 3.33–3.28 (m, 2H), 2.34 (m, 2H), 1.25 (d, J = 7.2 Hz, 3H). 13C NMR (101 MHz, DMSO) δ (ppm) 173.1, 170.7, 168.9, 168.8, 159.9, 159.0, 149.1, 137.2, 134.1, 128.9, 122.5, 121.1, 119.2, 117.7, 116.1, 48.9, 44.5, 42.8, 35.8, 35.6, 18.5. ESI-HRMS m/z: [M + Na]+ calcd. For C21H25N5O5Na 450.1748, observed 450.1942.
2-Hydroxy-N-(2-oxo-2-((4-oxo-4-((1-oxo-1-((pyridine-2-ylmethyl)amino)propan-2-yl)amino)butyl)amino)ethyl)benzamide (7d)
Compound 7d was synthesized by the abovementioned procedure and purified by column chromatography with solvent system ethyl acetate/MeOH (95:5) as a white solid (58% yield). 1H NMR (400 MHz, DMSO) δ (ppm) 12.22 (s, 1H), 9.04 (t, J = 5.4 Hz, 1H), 8.46 (m, 2H), 8.04 (m, 2H), 7.87 (d, J = 8.0 Hz, 1H), 7.73 (t, J = 8.0 Hz, 1H), 7.40 (t, J = 8.0 Hz, 1H), 7.24 (m, 2H), 6.90 (t, J = 8.0 Hz, 2H), 4.35 (d, J = 8.0 Hz, 2H), 4.30 (t, J = 8.0 Hz, 1H), 3.89 (d, J = 8.0 Hz, 2H), 3.20–2.98 (m, 2H), 2.15 (t, J = 7.3 Hz, 2H), 1.64 (m, 2H), 1.24 (d, J = 4.0 Hz, 3H). 13C NMR (101 MHz, DMSO) δ (ppm) 173.2, 172.2, 168.7, 159.9, 159.1, 149.2, 137.1, 134.1, 128.9, 122.5, 121.1, 119.2, 117.7, 48.8, 44.5, 42.8, 33.0, 25.8, 18.6, 0.6. HRMS (ESI-TOF) m/z: [M + Na + H]+ calcd. For C22H29N5O5Na 465.1988, observed 465.1974.
Acknowledgments
The authors thank SERB-New Delhi (Govt. of India, New Delhi) for the CRG grant with number CRG/2020/001028.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c03404.
1H/13C NMR, HRMS, and 2D-NMR (COSY and NOESY) spectra; crystal structure of peptides; DMSO-d6 titration experiment spectra; and X-ray data of peptide derivatives (3a, 3b, 3d, 4a, 4c, and 5) (PDF)
Crystallographic data 1 (CIF)
Crystallographic data 2 (CIF)
Crystallographic data 3 (CIF)
Crystallographic data 4 (CIF)
Crystallographic data 5 (CIF)
Crystallographic data 6 (CIF)
The authors declare no competing financial interest.
Supplementary Material
References
- Raskin I. Role of salicylic acid in plants. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1992, 43, 439–463. 10.1146/annurev.pp.43.060192.002255. [DOI] [Google Scholar]
- Bindu S.; Mazumder S.; Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babhair S. A.; Al-Badr A. A.; Aboul-Enein H. Y.. Salicylamide. In Analytical Profiles of Drug Substances; Elsevier, 1984; Vol. 13, pp 521–551. [Google Scholar]
- Nishiya T.; Yamauchi S.; Hirota N.; Baba M.; Hanazaki I. Fluorescence studies of intramolecularly hydrogen-bonded o-hydroxyacetophenone, salicylamide, and related molecules. J. Phys. Chem. A 1986, 90, 5730–5735. 10.1021/j100280a053. [DOI] [Google Scholar]
- Catalan J.; Toribio F.; Acuna A. Intramolecular hydrogen bonding and fluorescence of salicylaldehyde, salicylamide, and o-hydroxyacetophenone in gas and condensed phase. J. Phys. Chem. A 1982, 86, 303–306. 10.1021/j100391a034. [DOI] [Google Scholar]
- Song Q.; Li Y.; Cao Z.; Qiang X.; Tan Z.; Deng Y. Novel salicylamide derivatives as potent multifunctional agents for the treatment of Alzheimer’s disease: Design, synthesis and biological evaluation. Bioorg. Chem. 2019, 84, 137–149. 10.1016/j.bioorg.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Stavropoulos G.; Magafa V.; Liakopoulou-Kyriakides M.; Sinakos Z.; Aaberg A. Synthesis of salicyl-peptides and their effect on human platelet aggregationin vitro. Amino Acids 1997, 13, 171. 10.1007/BF01373215. [DOI] [Google Scholar]
- Sarigiannis Y. M.; Stavropoulos G. P.; Liakopoulou-Kyriakides M. T.; Makris P. E. Novel synthetic RGD analogs incorporating salicylic acid derivatives show antiplatelet activity in vitro. Lett. Pept. Sci 2002, 9, 101–109. 10.1023/A:1024101218318. [DOI] [Google Scholar]
- Montalvo G. L.; Zhang Y.; Young T. M.; Costanzo M. J.; Freeman K. B.; Wang J.; Clements D. J.; Magavern E.; Kavash R. W.; Scott R. W.; Liu D.; DeGrado W. F. De Novo Design of Self-Assembling Foldamers That Inhibit Heparin–Protein Interactions. ACS Chem. Biol. 2014, 9, 967–975. 10.1021/cb500026x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.; Qin B.; Shu Y.; Chen X.; Yip Y. K.; Zhang D.; Su H.; Zeng H. Helical organization in foldable aromatic oligoamides by a continuous hydrogen-bonding network. Org. Lett. 2009, 11, 1201–1204. 10.1021/ol802679p. [DOI] [PubMed] [Google Scholar]
- Montalvo G. L.Foldamers: Design, synthesis, and characterization of non-natural peptides with diverse backbones, 2012.
- Imramovský A.; Jorda R.; Pauk K.; Řezníčková E.; Dušek J.; Hanusek J.; Kryštof V. Substituted 2-hydroxy-N-(arylalkyl) benzamides induce apoptosis in cancer cell lines. Eur. J. Med. Chem. 2013, 68, 253–259. 10.1016/j.ejmech.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Kirin S. I.; Dübon P.; Weyhermüller T.; Bill E.; Metzler-Nolte N. Amino acid and peptide bioconjugates of copper (II) and zinc (II) complexes with a modified N, N-bis (2-picolyl) amine ligand. Inorg. Chem. 2005, 44, 5405–5415. 10.1021/ic048343b. [DOI] [PubMed] [Google Scholar]
- Pantalon Juraj N.; Muratović S.; Perić B.; Šijaković Vujičić Na.; Vianello R.; Žilić D.; Jagličić Z.; Kirin S. k. I. Structural Variety of Isopropyl-bis (2-picolyl) amine Complexes with Zinc (II) and Copper (II). Cryst. Growth Des. 2020, 20, 2440–2453. 10.1021/acs.cgd.9b01625. [DOI] [Google Scholar]
- Darshani T.; Thushara N.; Weerasuriya P.; Fronczek F. R.; Perera I. C.; Perera T. Fluorescent di-(2-picolyl) amine based drug-like ligands and their Re (CO) 3 complexes towards biological applications. Polyhedron 2020, 185, 114592 10.1016/j.poly.2020.114592. [DOI] [Google Scholar]
- Cui S.; Liu G.; Pu S.; Chen B. A highly selective fluorescent probe for Zn2+ based on a new photochromic diarylethene with a di-2-picolylamine unit. Dyes Pigm. 2013, 99, 950–956. 10.1016/j.dyepig.2013.07.038. [DOI] [Google Scholar]
- Dotto C.; Lombarte Serrat A.; Ledesma M.; Vay C.; Ehling-Schulz M.; Sordelli D. O.; Grunert T.; Buzzola F. Salicylic acid stabilizes Staphylococcus aureus biofilm by impairing the agr quorum-sensing system. Sci. Rep. 2021, 11, 2953 10.1038/s41598-021-82308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. B.; Bassler B. L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- Alonso B.; Fernández-Barat L.; Di Domenico E. G.; Marín M.; Cercenado E.; Merino I.; de Pablos M.; Muñoz P.; Guembe M. Characterization of the virulence of Pseudomonas aeruginosa strains causing ventilator-associated pneumonia. BMC Infect. Dis. 2020, 20, 1–8. 10.1186/s12879-020-05691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalsky T. M.; Soohoo A. M.; Wang J.; Walsh C. T.; Wright G. D.; Gordon E. M.; Gray N. S.; Khosla C. Prospects for antibacterial discovery and development. J. Am. Chem. Soc. 2021, 143, 21127–21142. 10.1021/jacs.1c10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal V. D.; Al-Abdely H. M.; El-Kholy A. A.; AlKhawaja S. A. A.; Leblebicioglu H.; Mehta Y.; Rai V.; Hung N. V.; Kanj S. S.; Salama M. F.; et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010-2015: Device-associated module. Am. J. Infect. Control 2016, 44, 1495–1504. 10.1016/j.ajic.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Lambert M.-L.; Suetens C.; Savey A.; Palomar M.; Hiesmayr M.; Morales I.; Agodi A.; Frank U.; Mertens K.; Schumacher M.; Wolkewitz M. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect. Dis. 2011, 11, 30–38. 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- Choi H.; Ham S.-Y.; Cha E.; Shin Y.; Kim H.-S.; Bang J. K.; Son S.-H.; Park H.-D.; Byun Y. Structure–activity relationships of 6-and 8-gingerol analogs as anti-biofilm agents. J. Med. Chem. 2017, 60, 9821–9837. 10.1021/acs.jmedchem.7b01426. [DOI] [PubMed] [Google Scholar]
- Meher S.; Kumari S.; Dixit M.; Sharma N. K. Cu-Catalyzed Synthesis of Alkylaminotroponyl Sulfones as Pseudomonas Aeruginosa Quorum Sensing Inhibitors Targeting lasI/R QS Circuitry. Chem. - Asian J. 2022, 17, e202200866 10.1002/asia.202200866. [DOI] [PubMed] [Google Scholar]
- Dalabehera N. R.; Meher S.; Bhusana Palai B.; Sharma N. K. Instability of amide bond with trifluoroacetic acid (20%): synthesis, conformational analysis, and mechanistic insights into cleavable amide bond comprising β-troponylhydrazino acid. ACS omega 2020, 5, 26141–26152. 10.1021/acsomega.0c03729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M. K.; Jena C. K.; Balachandra C.; Sharma N. K. Unusual Pseudopeptides: Syntheses and Structural Analyses of Ethylenediprolyl Peptides and Their Metal Complexes with Cu (II) Ion. J. Org. Chem. 2021, 86, 16327–16336. 10.1021/acs.joc.1c01676. [DOI] [PubMed] [Google Scholar]
- Kearns D. B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang S. K.; Vrla G. D.; Fröhlich K. S.; Gitai Z. Surface association sensitizes Pseudomonas aeruginosa to quorum sensing. Nat. Commun 2019, 10, 4118 10.1038/s41467-019-12153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich L. E. P.; Price-Whelan A.; Petersen A.; Whiteley M.; Newman D. K. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 2006, 61, 1308–1321. 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- Hurley M. N.; Cámara M.; Smyth A. R. Novel approaches to the treatment of Pseudomonas aeruginosa infections in cystic fibrosis. Eur. Respir. J. 2012, 40, 1014–1023. 10.1183/09031936.00042012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaseelan S.; Ramaswamy D.; Dharmaraj S. Pyocyanin: production, applications, challenges and new insights. World J. Microbiol. Biotechnol. 2014, 30, 1159–1168. 10.1007/s11274-013-1552-5. [DOI] [PubMed] [Google Scholar]
- Reshes G.; Vanounou S.; Fishov I.; Feingold M. Cell shape dynamics in Escherichia coli. Biophys. J. 2008, 94, 251–264. 10.1529/biophysj.107.104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






