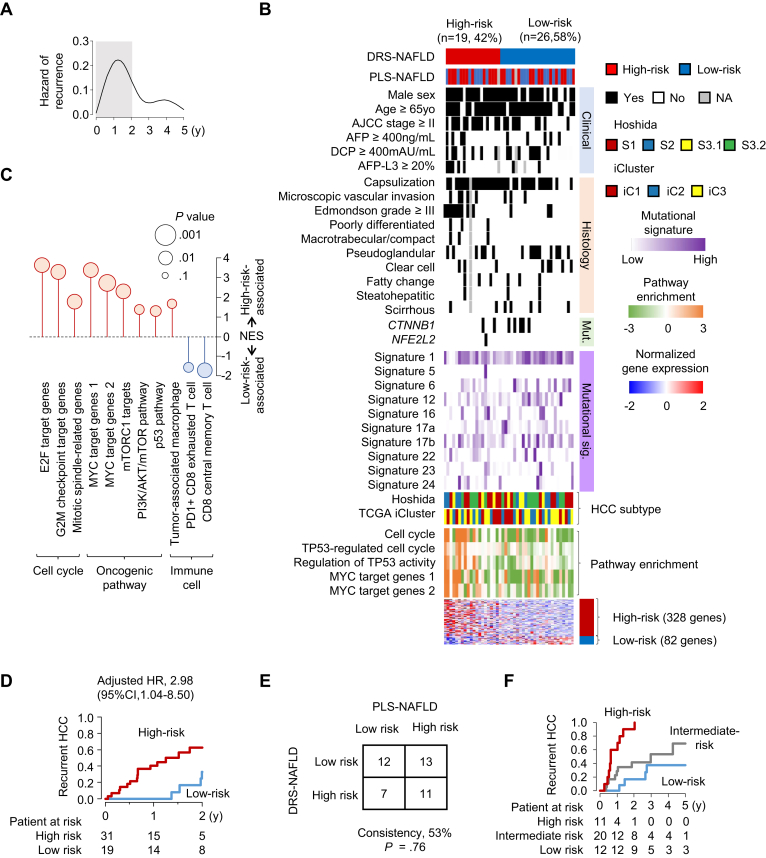
Management of recurrent hepatocellular carcinoma (HCC) from nonalcoholic fatty liver disease (NAFLD), the fastest rising HCC etiology,1 is critical given the extremely high recurrence rate even after surgical therapies with curative intent (70% within 5 years of treatment).2 Prediction of HCC recurrence is expected to guide decision of neo/adjuvant therapies currently under active development.3 For more precise recurrence prediction to enable rational interventions with clinically available molecular targeted and/or immune-based agents, it is important to predict both of the following 2 distinct types of recurrence, that is, disseminative recurrence originating from microscopic dissemination of HCC cells from surgically resected tumor, and de novo recurrence attributable to independent tumor clones newly arisen from NAFLD-affected liver.2 Clinically, the former is observed within 1–2 years after surgical resection as early recurrence, whereas the latter is often observed thereafter as late recurrence, recognized as 2 peaks in recurrence hazard plot over time after the surgery.2
We previously identified a hepatic transcriptome signature, prognostic liver signature (PLS)-NAFLD, that stratified NAFLD patients according to future HCC risk.4 In HCC-naïve NAFLD patients, PLS-NAFLD identified low-risk individuals who were HCC-free over 15 years of longitudinal follow-up. These low-risk NAFLD patients therefore could be spared from the guideline-recommended semiannual HCC screening to mitigate the burden on the screening program, which is already overwhelmed with the vast size of the NAFLD patient population.5 In contrast, in surgically treated NAFLD-related HCC patients, PLS-NAFLD successfully predicted postsurgical HCC recurrence (adjusted hazard ratio [aHR], 2.28; 95% confidence interval [CI], 1.02–5.07), but the residual risk of HCC recurrence in the low-risk patients was substantial (3-year recurrence rate of 40% mostly occurred with 2 years).4 This observation suggested that the nontumor liver-derived PLS-NAFLD does not capture the risk of disseminative recurrence. In fact, the recurrence hazard plot showed a sharp peak of early recurrence within 2 years after surgery (Figure A).
Figure.
Disseminative Recurrence Signature for NAFLD (DRS-NAFLD). (A) Hazard of HCC recurrence over time after surgical resection in the derivation cohort. The first 2 years is shaded with gray color. (B) Expression pattern of DRS-NAFLD in the derivation cohort. The signature-based risk prediction as well as clinical, histological, and molecular characteristics are attached. (C) Molecular pathways enriched in patients with high- or low-risk DRS-NAFLD prediction in the derivation cohort. (D) HCC recurrence in the validation cohort stratified by DRS-NAFLD. (E) Concordance of risk prediction between tumor-derived DRS-NAFLD and liver-derived PLS-NAFLD. (F) HCC recurrence stratified by combination of PLS-NAFLD and DRS-NAFLD. PLS, prognostic liver signature; NAFLD, nonalcoholic fatty liver disease; DRS, Disseminative Recurrence Signature; HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee of Cancer; AFP, alpha-fetoprotein; DCP, Des-gamma-carboxy-prothrombin; AFP-L3, AFP L3 fraction; Mut., somatic mutation; Mutational sig., mutational signature.
To refine the recurrence prediction in patients with NAFLD-related HCC, we defined a tumor-derived transcriptomic signature associated with the recurrence in the derivation cohort of 45 patients who underwent surgical HCC resection, among which 25 (56%) experienced recurrence (Table A1), by using genome-wide transcriptome profiles generated with RNA-Seq (see Supplementary Methods). We identified a 410 gene signature, consisting of 328 high- and 82 low-risk genes (Figure B, Table A2) as Disseminative Recurrence Signature for NAFLD-related HCC (DRS-NAFLD). DRS-NAFLD was associated with tumor stage and histological nuclear atypia. Among the high-risk genes, SPP1 (encoding osteopontin) is known to promote tumor progression, metastasis, and escape from antitumor immunity via induction of suppressive immune checkpoint proteins.6 CD36 encodes a fatty acid receptor and promotes HCC progression by regulating aerobic glycolysis through Src/PI3K/AKT axis.7 Among the low-risk genes, ACVR2A was reported as a potential tumor suppressor and its somatic mutation is more frequently observed in NAFLD-related HCC.8 Molecular pathway analyses revealed that DRS-NAFLD is highly associated with cell proliferation, especially TP53- and Myc-mediated cell cycle-related genes (Figure C), consistent with the high prevalence of a nuclear atypia. Myc is also known to induce evasion from antitumor immunity.9 Interestingly, enrichment of dysfunctional antitumor immunity such as PD1-positive exhausted CD8 T cells was less in the tumors with high-risk DRS compared to the rest, suggesting that the signature may inform response to immune checkpoint blockades (eg, suboptimal response of high-risk DRS tumors to anti-PD1 due to scarcity of the target cell population).
Subsequently, DRS-NAFLD in tumor tissues was evaluated in an independent validation cohort of 50 NAFLD-related HCC patients who underwent surgical resection for early-stage HCC (American Joint Committee of Cancer stage I-II) (Table A1). DRS-NAFLD identified 31 (62%) high-risk and 19 (38%) low-risk patients, which was associated with recurrence-free survival within 2 years (aHR, 2.98; 95% CI, 1.04–8.50) with incidence rates at 2 years of 67% and 37% in high- and low-risk HCC, respectively (Figure D). It is noteworthy that the recurrence risk predictions by the tumor-derived DRS-NAFLD and liver-derived PLS-NAFLD were independent of each other (Figure B and E), suggesting that these signatures capture complementary risk information, corresponding to disseminative and de novo recurrence, respectively. The complementary risk signatures were combined to define high-, intermediate-, or low-risk groups as high-risk prediction by both, either, or none of the 2 signatures, respectively. This integrative prediction identified 11 (26%) high-, 20 (47%) intermediate-, and 12 (28%) low-risk patients with further improved risk stratification (aHR, 9.24 [95% CI, 2.48–34.4] for high-risk compared to low-risk patients; aHR, 2.81 [95% CI, 0.86–9.21] for intermediate-risk compared to low-risk patients) (Figure F). The recurrence rates in the high-, intermediate- and low-risk patients at 2 years were 90%, 42%, and 17%, respectively.
These results support that the integrative use of the tumor-derived DRS-NAFLD and the liver-derived PLS-NAFLD enables more precise prediction of HCC recurrence after surgical resection. Molecular pathways of dysfunctional antitumor immunity associated with the high-risk DRS-NAFLD and PLS-NAFLD suggest their clinical utility in informing indication of neo/adjuvant therapies with immune-targeted agents. These assays, already implemented in an Food and Drug Administration-approved diagnostic platform, are readily applicable in daily clinical practice by using waste surgical tissues (only one five-micron-thick formalin-fixed paraffin-embedded tissue section) without obtaining any additional specimen. Such refined recurrence risk stratification can also inform the strategy of postsurgical patient follow-up. For example, the use of sensitive but costly screening tests such as circulating methylated cell-free DNA assay10 may be justified for patients with a high-risk prediction for cost-effective early detection of HCC recurrence followed by rational salvage treatment. In conclusion, the tumor-derived DRS-NAFLD improves HCC recurrence prediction by the liver-derived PLS-NAFLD and will refine clinical management of patients with NAFLD-related HCC and contribute to improvement of the poor patient survival.
Acknowledgments
Authors' Contributions:
Naoto Fujiwara: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing - original draft, Visualization. Naoto Kubota: Data curation. Shijia Zhu: Formal analysis. Shigeki Nakagawa: Resources, Data curation. Hideo Baba: Resources, Data curation. Yujin Hoshida: Conceptualization, Methodology, Validation, Writing - review and editing, Funding acquisition.
Footnotes
Conflicts of Interest: These authors disclose the following: Y.H. serves as an advisory board member for Helio Genomics, Espervita Therapeutics, and Roche Diagnostics, a shareholder for Alentis Therapeutics and Espervita Therapeutics, and receive research support from Allergan/AbbVie, Kyowa Kirin, and Morphic Therapeutics. The remaining authors disclose no conflicts.
Funding: This work was supported by US NIH (DK099558, CA233794, CA222900, CA230694, CA255621), European Commission (ERC-2014-AdG-671231, ERC-AdG-2020-101021417), Cancer Prevention and Research Institute of Texas (RR180016, RP200554) to Y.H.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: All data are publicly available at the NCBI GEO (accession number, GSE193080, GSE214435). The research team will provide an email address for communication once the information sharing is approved. The proposal should include detailed aims, statistical plan, and other information/materials to guarantee the rationality of requirement and the security of the data. The related patient data will be shared after review and approval of the submitted proposal and any related requested materials. Of note, data with patient names and other identifiers cannot be shared.
Reporting Guidelines: Helsinki Declaration.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2023.03.021.
Contributor Information
N. Fujiwara, Email: naoto-fujiwara@med.mie-u.ac.jp.
Y. Hoshida, Email: Yujin.Hoshida@UTSouthwestern.edu.
Supplementary materials
References
- 1.Huang D.Q., et al. Cell Metab. 2022;34:969–977.e2. doi: 10.1016/j.cmet.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujiwara N., et al. J Hepatol. 2018;68:526–549. doi: 10.1016/j.jhep.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marron T.U., et al. J Hepatocell Carcinoma. 2022;9:571–581. doi: 10.2147/JHC.S340935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujiwara N., et al. Sci Transl Med. 2022;14:eabo4474. doi: 10.1126/scitranslmed.abo4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y.T., et al. Hepatology. 2022 doi: 10.1002/hep.32779. [DOI] [Google Scholar]
- 6.Song Z., et al. Hepatology. 2021;73:1594–1608. doi: 10.1002/hep.31582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara N., et al. Gut. 2018;67(8):1493–1504. doi: 10.1136/gutjnl-2017-315193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S.K., et al. Int J Cancer. 2016;139:2512–2518. doi: 10.1002/ijc.30379. [DOI] [PubMed] [Google Scholar]
- 9.Dhanasekaran R., et al. Nat Rev Clin Oncol. 2022;19:23–36. doi: 10.1038/s41571-021-00549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin N., et al. Hepatol Commun. 2022;6:1753–1763. doi: 10.1002/hep4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.