Abstract
Objectives:
We aimed to determine the optimal testing strategy to identify children with perinatally acquired hepatitis C virus (HCV) infection.
Study Design:
We used a decision-tree framework with a Markov disease progression model to conduct an economic analysis of four strategies, based on combinations of type and timing of test: Anti-HCV with reflex to HCV RNA at 18 months among children known to be perinatally exposed (ie, baseline comparison strategy); HCV RNA testing at 2–6 months among infants known to be perinatally exposed (Test Strategy 1); universal anti-HCV with reflex to HCV RNA at 18 months among all children (Test Strategy 2); universal HCV RNA testing at 2–6 months among all infants (Test Strategy 3). We estimated total cost, quality-adjusted life years (QALYs), and disease sequalae for each strategy.
Results:
Each of the three alternative testing strategies resulted in an increased number of children tested and improved health outcomes. HCV RNA testing at 2–6 months (Test Strategy 1) was cost-saving and resulted in a population-level difference in cost of $469,671. The two universal testing strategies resulted in an increase in QALYs and an increase in total costs.
Conclusions:
Testing of perinatally exposed infants at age 2–6 months with a single HCV RNA test will reduce costs and improve health outcomes, preventing morbidity and mortality associated with complications from perinatal HCV infections.
Keywords: hepatitis C virus, diagnosis, economic evaluation, maternal antibodies
Introduction
The incidence of hepatitis C virus (HCV) infection more than quadrupled during 2010 through 2020 in the United States, with the highest rates and increases among persons 20–39 years of age1. Increasing HCV infections among reproductive age adults has resulted in higher numbers of infants with perinatal HCV exposure1,2. studies estimate that 5.8% (95% CI, 4.2%–7.8%) of infants born to HCV-infected persons acquire hepatitis C perinatally and approximately 80% of those infants develop chronic HCV infection3,4
Curative hepatitis C treatment is approved for use in children beginning at 3 years of age7,8. The identification of children with perinatally acquired HCV is the first step. CDC and USPSTF hepatitis C screening recommendations among adults, updated in 2020 and followed with similar recommendations from the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine in 2021, call for hepatitis C screening of all pregnant persons during every pregnancy. Testing recommendations for infants with perinatal exposure to HCV have been developed by medical organizations with variability in timing and type of test recommended7-10. Recommendations include HCV antibody testing at age 15 to 18 months, allowing time for passively acquired maternal antibodies to clear, followed by HCV RNA testing for children with reactive antibody results or HCV RNA detection either once or twice in the first year of life starting at 2 months of age7-12. Differences in recommendations can contribute to inconsistent implementation by providers.
Recognition of perinatal exposure requires maternal HCV screening and communication of positive results to the child’s health provider. Universal pediatric HCV screening could overcome these barriers, but the cost-effectiveness of this approach has not been evaluated. Optimal timing of pediatric evaluation is also unclear. Attendance at recommended well-child visits is highest immediately after birth, including among low-income populations with limited access to care13. HCV testing among exposed children at age 18 months or older does not usually exceed 50%, and various assessments have found that only 10–30% receive testing 14-18. Current testing algorithms call for anti-HCV testing at or after 18 months followed by an HCV RNA test when antibody tests are reactive. Accelerating pediatric HCV evaluation among exposed children from age 18 months or older to age 2–6 months using an HCV RNA test with reliable results, could increase the number of infected children identified by providing testing while more children are engaged in routine care. While HCV RNA testing is more expensive than anti-HCV testing, the cost-effectiveness of an HCV RNA-only testing strategy has not previously been evaluated and must be weighed against high loss to follow-up when testing is delayed.
This modeling study aimed to identify the optimal strategy for identifying children with perinatally acquired HCV infection by assessing the impact and cost-effectiveness of several strategies: a single HCV RNA test during age 2–6 months of age compared with anti-HCV with reflex to HCV RNA at age 18 months or later; each testing approach among children with known perinatal HCV exposure was compared with universal screening among all children.
Methods
We used a decision analysis framework to compare testing strategies for a cohort of US infants born in 2021. Primary considerations for defining each strategy were timing of testing and type of test used. We defined four testing strategies based on combinations of the type of test, including anti-HCV, HCV RNA, and anti-HCV with reflex to HCV RNA, and timing of test at either age 2–6 months or age 18 months or older. Estimates for sensitivity and specificity of each type of test came from published studies19,21. Although some previous recommendations suggest the use of two HCV RNA tests in order to capture transient viremia, we modeled the use of a single HCV RNA test because assays currently used in the United States have greatly improved and now routinely detect HCV viral loads of 15 I.U./ml or less22. We modeled these strategies: Anti-HCV with reflex to HCV RNA at 18 months among children known to be perinatally exposed (i.e., baseline comparison strategy); HCV RNA testing at 2–6 months among children known to be perinatally exposed (Test Strategy 1); universal anti-HCV with reflex to HCV RNA at 18 months among all children (Test Strategy 2); universal HCV RNA testing at 2–6 months among all infants (Test Strategy 3).
Epidemiologic Inputs
Epidemiologic inputs used to define each strategy are in Table 1. The distinction between universal testing strategies and testing strategies among children with known perinatal exposure is the proportion of HCV-infected pregnant persons that are screened during pregnancy. We conducted a systematic review of studies from 2001 to 2021 to identify the prevalence of HCV testing in pregnancy, and rates of testing, transmission, and loss to follow-up among perinatally exposed children. In base case analyses, we utilized data from that review and assumed 0.64% of births occur among persons infected with HCV and that 44.7% of those pregnant persons are screened for HCV infection23. Considering this estimate is changing because of a recent recommendation to test all pregnant persons for HCV infection, we conducted several sensitivity analyses to account for expected increases in testing (described below). Additionally, we modeled two points for potential loss to follow-up. First, not all children born to HCV-infected persons receive HCV testing and this differs based on the age of child at which testing is conducted. Second, the opportunity for testing differs between children born to HCV-infected persons and those born to persons without HCV infection because probability of attendance at well childcare visits for the children will vary during age 2–6 months and age 18 months or older. For children with perinatal exposure to HCV, probability of a child being tested was defined as 73.5% for children tested at age 2–6 months and as 43.0% for children tested at age 18 months or older24. For children without perinatal exposure to HCV, probability of child testing was defined as 85.0% for children tested at age 2–6 months and as 75.0% for children tested at age 18 months or older25.
Table 1.
Epidemiologic inputs, annual transition rates, health state utility values and health state costs used in cost-effectiveness analysis of testing approaches for infants perinatally exposed to hepatitis C virus, United States, 2022
| Base Case |
Lower-Upper | Source | ||
|---|---|---|---|---|
| Strategies: Anti-HCV with reflex to HCV RNA at 18 | ||||
| P HCV RNA+ among pregnant persons (%) | 0.64 | 0.32 – 1.28 | 22 | |
| P of HCV RNA+ pregnant persons screened (%) | 44.7 | 25.0 – 65.0 | 23 | |
| P of infant being tested (no perinatal exposure; %) | 75.0 | 65.0 – 85.0 | 25 | |
| P of infant being tested (perinatal exposure; %) | 43.0 | 35.0 – 52.0 | 24 | |
| P of HCV infection among infants perinatally exposed (%) | 5.8 | 4.2 - 7.8 | 3 | |
| Anti-HCV sensitivity (18 mo; %) | 98.1 | 92.6 – 99.7 | 21 | |
| Anti-HCV specificity (18 mo; %) | 99.8 | 99.2 – 99.9 | 21 | |
| P of maternal antibodies (18 mo; %) | 1.6 | 0.5 – 4.5 | 12 | |
| Reflex HCV RNA sensitivity (18 mo; %) | 100 | 87.5 – 100 | 19 | |
| Reflex HCV RNA specificity (18 mo; %) | 100 | 98.3 −100 | 19 | |
| P of Spontaneous clearance (%) | 20.0 | 15.0 −25.0 | 28 | |
| P of SVR among treated (%) | 99 | 92.4 −100 | 27 | |
| Strategies: HCV RNA test at 2–6 months | ||||
| P HCV RNA+ among pregnant persons (%) | 0.64 | 0.32 – 1.28 | 23 | |
| P of HCV RNA+ pregnant persons screened (%) | 44.7 | 25.0 – 65.0 | 23 | |
| P of infant being tested (no perinatal exposure; %) | 85.0 | 75.0 – 95.0 | 25 | |
| P of infant being tested (perinatal exposure; %) | 73.5 | 68.0 - 81.0 | 24 | |
| P of HCV infection among infants perinatally exposed (%) | 5.8 | 4.2 – 7.8 | 3 | |
| RNA test sensitivity (2–6 mo; %) | 100 | 87.5 – 100 | 19 | |
| RNA test specificity (2–6 mo; %) | 100 | 98.3 – 100 | 19 | |
| P of Spontaneous clearance (%) | 20.0 | 15.0 – 25.0 | 28 | |
| P of SVR among treated (%) | 99.0 | 92.4 – 100 | 27 | |
| Testing Cost Inputs (in 2021 USD) | ||||
| Anti-HCV test (CPT: 86803) | 14.27 | 10.8 – 17.8 | 40 | |
| HCV RNA quantitative PCR (CPT: 87522) | 42.84 | 32.1 – 53.6 | 40 | |
| Annual Health State Transition Rates | ||||
| From: | To: | |||
| Chronic HCV (<18 years) | Compensated cirrhosis | 0.0018 | 0.001-0.110 | 31 |
| Chronic HCV (18+ years) | Compensated cirrhosis | 0.110 | 0.075-0.133 | 31 |
| Compensated cirrhosis | Decompensated cirrhosis | 0.035 | 0.027-0.043 | 27 |
| Compensated cirrhosis | HCC | 0.024 | 0.018-0.031 | 27 |
| Decompensated cirrhosis | HCC | 0.068 | 0.030-0.083 | 27 |
| Decompensated cirrhosis | Liver transplant | 0.033 | 0.017-0.049 | 27 |
| Decompensated cirrhosis | Liver-related death | 0.216 | 0.162-0.270 | 27 |
| HCC | Liver transplant | 0.033 | 0.017-0.049 | 27 |
| HCC | Liver-related death | 0.411 | 0.310-0.510 | 27 |
| Liver transplant | Post liver transplant | 0.857 | 0.841-0.876 | 27 |
| Liver transplant | Liver-related death | 0.143 | 0.124-0.159 | 27 |
| Post liver transplant | Liver-related death | 0.034 | 0.024-0.043 | 27 |
| Health State Utility Values | ||||
| Chronic HCV | 0.806 | 0.767-0.845 | 27 | |
| Compensated cirrhosis | 0.726 | 0.680-0.772 | 27 | |
| Decompensated cirrhosis | 0.657 | 0.602-0.711 | 27 | |
| Hepatocellular carcinoma | 0.717 | 0.647-0.788 | 27 | |
| Liver transplant | 0.500 | 0.720-0.840 | 31 | |
| Post liver transplant | 0.712 | 0.657-0.767 | 27 | |
| HCV cured (SVR) | 0.841 | 0.801-0.880 | 27 | |
| Annual Health State Costs (2021 USD) | ||||
| Chronic HCV | 882 | 682-1,138 | 31,32 | |
| Compensated cirrhosis | 1,678 | 1,299-2,166 | 31,32 | |
| Decompensated cirrhosis | 22,619 | 1,350-39,176 | 31,32 | |
| Hepatocellular carcinoma | 47,612 | 27,488-82,467 | 31,32 | |
| Liver transplant | 222,824 | 128,649-385,940 | 31,32 | |
| Post liver transplant | 40,243 | 23,234-69,702 | 31,32 | |
| Cost of treatment | 70,200 | 7,338-94,500 | 34 | |
Abbreviations: HCV, hepatitis C virus; anti-HCV, hepatitis c virus antibody; P, probability; SVR, sustained virologic response; USD, United States Dollars; CPT, Current Procedural Terminology; CMS CLFS, Centers for Medicare and Medicaid Services Clinical Laboratory Fee Schedule; mo, months; HCC, hepatocellular carcinoma; SVR, sustained virologic response. Notes: Annual health state costs include all costs associated with HCV-related medical care.
Analytic Model
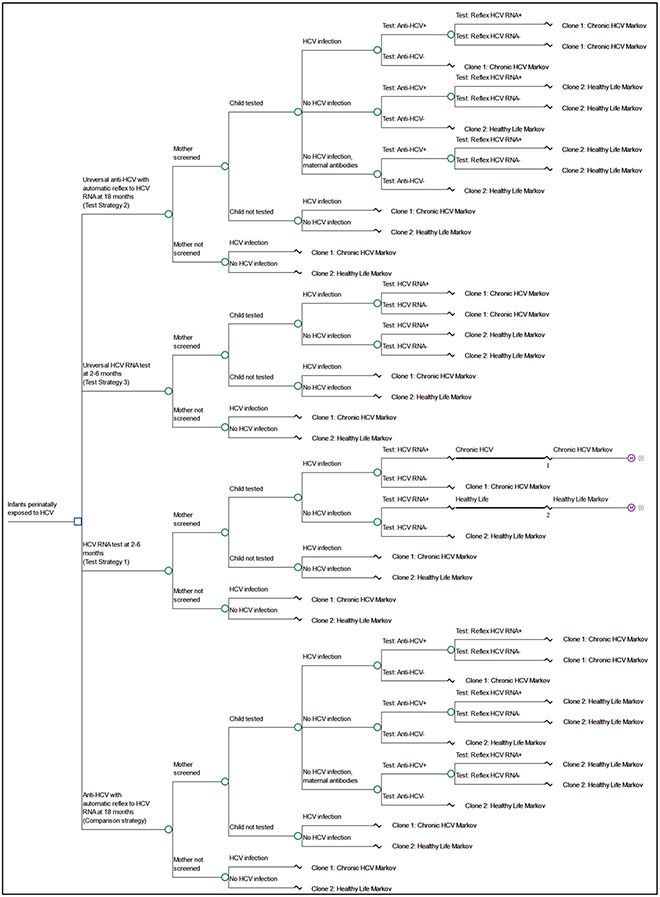
We analyzed the costs and health outcomes of all strategies using a decision tree model (Figure 2; online) with a Markov model of hepatitis C disease progression in TreeAge Pro 2022. We utilized microsimulation to model trials that represent individual children and each trial resulted in either having a documented HCV infection or healthy life (mutually exclusive) prior to entering the Markov stages. Trials that entered a healthy life process were assumed to never acquire an HCV infection and experienced age-specific annual probability of death as defined by the National Vital Statistics System 2018 US Life Tables26. All trials that experienced a documented HCV infection entered a Markov process that was adapted from a previously published model of HCV progression among persons infected as infants27 which included health states that represent acute and chronic HCV infection, potential treatment and advanced liver disease states (Table 1). We assumed 20% of children with a perinatally acquired infection experienced spontaneous clearance28 and that all children with infections that were identified in each testing strategy were subsequently linked to treatment at 3 years of age. Trials that had perinatally acquired infections that were not tested and identified in the respective strategy were assumed to not be diagnosed or treated later in life.
Figure 2.
Decision Tree used in cost-utility analysis of testing approaches for children perinatally exposed to hepatitis C virus, United States, 2022
The time step for the Markov processes was 1 year and trials accumulated costs and quality-adjusted life years (QALYs) for the lifetime of the cohort. Additionally, we modeled several epidemiologic outcomes among children with perinatally acquired infections, including number of diagnosed HCV infections, treated/cured HCV infections, hepatocellular carcinoma cases, liver transplants and liver-related deaths. We used a limited societal perspective29,30 that included costs associated with HCV testing and all direct medical costs related to HCV infection and resulting sequelae in 2021 US Dollars (USDs; Table 1). The cost of an anti-HCV test (CPT: 86803) was $14.27 (range: $10.80 to $17.80) and the cost of an HCV RNA test (CPT: 87522) was $42.84 (range: $32.10 to $53.60). All annual health state costs were from a previously published model31 and converted to 2021 USD using the Medical Care Consumer Price Index (CPI)32,33. We estimated cost of treatment to be $70,200 per regimen (range: $7,338 to $94,500), which was the average commercial prices of the three most commonly used treatment regimens (Glecaprevir-pibrentasvir, Sofosbuvir-velpatasvir, and Ledipasvir-sofosbuvir)34. Effectiveness was quantified using QALYs, which were estimated by using previously published utility weights27. All costs and utilities were discounted at 3% per year and the analytic horizon was the lifetime of the cohort.
Analysis
We estimated average cost and average QALYs per trial for each strategy and compared strategies by calculating incremental cost-effectives ratios (ICERs) and cost per additional diagnosed pediatric infection. Additionally, assuming an annual total of 3.6 million births35 with 0.64% occurring among pregnant persons with an HCV infection23, we estimated population-level results for a cohort of 23,040 perinatally exposed children. The base case analysis included the most plausible point estimate for each model input.
sensitivity analyses
we conducted a one -way interval sensitivity analysis on the proportion of pregnant persons that are screened for HCV infection, which is the distinguishing input between the universal testing strategies and the comparable strategies that test infants with known exposure. Base case value for this input (44.7%) is informed by a systematic review of available data23, but this value is widely expected to increase as uptake improves for the 2020 recommendation for HCV screening 36. we conducted a probabilistic sensitivity analysis to evaluate the combined uncertainty of all model inputs. Finally, we conducted a scenario analysis that utilizes recently published data on vertical transmission of HCV infection and timing of clearance among children with perinatally-acquired infection5,6. These studies, which were published after our analytic work began, report data from three prospective European cohorts. We conducted a scenario sensitivity analysis that assumed 7.2% of perinatally-exposed infants acquired HCV infection6 and 20.9% of children with HCV infections that were treated at three years of age would have spontaneously cleared their infection without treatment5.
Results
Compared with the baseline comparison strategy each of the three alternative testing strategies resulted in an increased number of children tested and improved health outcomes (Table 2). Additionally, Test Strategy 1, representing the testing of known HCV perinatally exposed children at age 2–6 months with an HCV RNA test, was cost-saving compared with the baseline comparison strategy, with a population-level difference in cost of $469,671. Compared with the baseline comparison strategy, each of the two universal testing strategies resulted in an increase in QALYs and an increase in total costs. Test Strategy 2, representing universal testing of children at age 18 months or older with anti-HCV and reflex to HCV RNA test, increased population-level costs by over $38 million (ICER=26,105) and Test Strategy 3, representing universal testing of children at age 2–6 months with HCV RNA test, increased population-level costs by over $129 million (ICER=35,887), compared with the baseline comparison strategy.
Table 2.
Base case population-level results1 for HCV testing strategies among a cohort of infants, United States, 2022
| Testing among known exposed2 |
Universal testing of all infants |
|||
|---|---|---|---|---|
| Outcome | Anti-HCV with reflex to HCV RNA at 18 months3 (Comparison) |
HCV RNA test at 2–6 months4 (Test Strategy 1) |
Anti-HCV with reflex to HCV RNA at 18 months3,5 (Test Strategy 2) |
HCV RNA test at 2–6 months4,6 (Test Strategy 3) |
| Total costs (2021 USD) | 77,621,446 | 77,151,775 | 116,433,899 | 207,170,025 |
| Testing outcomes | ||||
| Tested infants | 4,478 | 7,588 | 2,709,916 | 3,076,918 |
| Anti-HCV tests | 4,478 | 0 | 2,709,916 | 0 |
| HCV RNA tests | 330 | 7,588 | 6,120 | 3,076,918 |
| Epidemiologic outcomes among perinatally exposed infants | ||||
| Diagnosed HCV infections | 254 | 443 | 553 | 971 |
| Hepatocellular carcinoma cases | 419 | 342 | 316 | 151 |
| Decompensated cirrhosis cases | 463 | 388 | 334 | 157 |
| Liver transplants | 74 | 62 | 58 | 28 |
| HCV Liver-related deaths | 758 | 626 | 550 | 259 |
| Treated/cured HCV infections | 196 | 347 | 438 | 775 |
| Total QALYs | 693,059 | 694,026 | 694,546 | 696,669 |
| Total Life-years | 1,807,109 | 1,812,187 | 1,814,939 | 1,826,061 |
Assumes 3.6 million births with 0.64% of births occurring among persons who are HCV RNA+.
Assumes 44.7% of all pregnant persons are screened for HCV infection.
Assumes 43.0% of infants born to HCV RNA+ pregnant persons attend an 18-month visit.
Assumes 73.5% of infants born to HCV RNA+ pregnant persons attend a 2–-6 month visit.
Assumes 75.0% of infants born to pregnant persons without HCV attend an 18-month visit.
Assumes 85.0% of infants born to pregnant persons without HCV attend a 2–6 month visit.
Abbreviations: HCV, hepatitis C virus; anti-HCV, hepatitis C virus antibody; USD, United States Dollars; QALYs, quality-adjusted life-years.
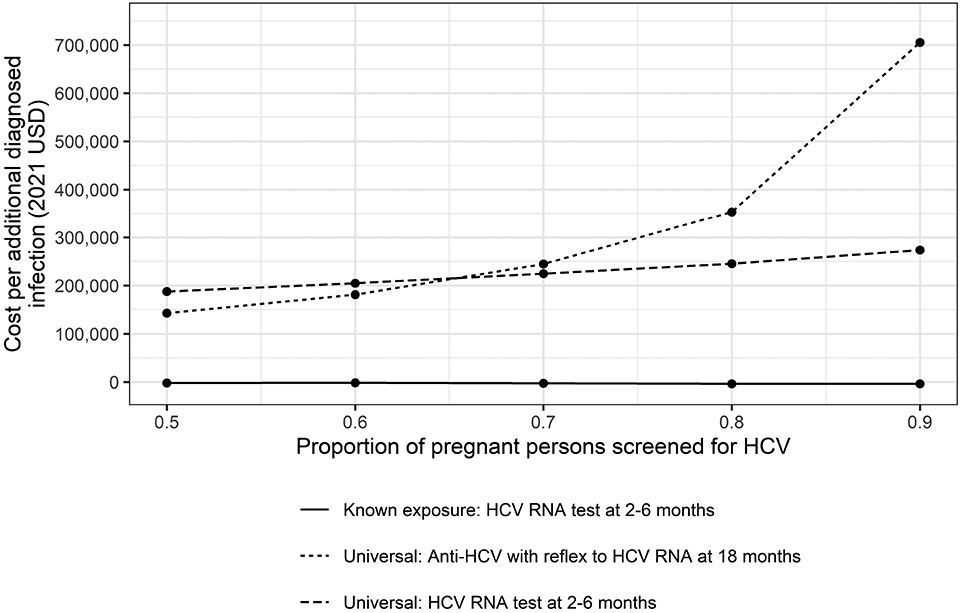
One-way interval sensitivity analysis on the proportion of pregnant persons screened for HCV infection indicated that Test Strategy 1, testing of known HCV perinatally exposed children at age 2–6 months with an HCV RNA test, was cost-saving and resulted in improved health outcomes in all scenarios. Additionally, as a higher proportion of pregnant persons were screened, the cost per unit of beneficial health outcomes increased in the two universal testing scenarios (Figure). Population-level results for two scenarios that assume 65% and 90% of pregnant persons are screened for HCV infection are presented in Table 4 (online). A comparison of the two universal testing strategies (Test Strategy 2 and Test Strategy 3) against Test Strategy 1 at different assumptions of the percent of persons screened for HCV infection is presented in Table 3. Compared with Test Strategy 1, universal testing of children with anti-HCV tests at 18 months results in higher costs and fewer QALYs in all scenarios in which at least 60% of pregnant persons are screened for HCV infection. Results from the probabilistic sensitivity analysis on all model inputs are presented in Table 5 (online). Notably, across all model runs, Test Strategy 1 was dominant over the baseline comparison strategy, resulting in lower cost and improved health outcomes. Population-level results from the scenario sensitivity analysis assuming higher rates of vertical transmission and clearance are presented in Table 6 (online). In this scenario analysis, Test Strategy 1 identified 76% more documented HCV infections and had an ICER of $455, compared with the baseline comparison strategy.
Sensitivity analysis results: Cost (in 2021 USD) per additional diagnosed infection, by strategy and proportion of pregnant persons screened for HCV, compared with anti-HCV with reflex to HCV RNA testing at 18 months among infants with known exposure, United States, 2022. As a higher proportion of pregnant persons were screened, the cost per additional diagnosed infection increased in the two universal testing scenarios.
Table 4.
Population-level results1 for HCV testing strategies among a cohort of children, by proportion of pregnant persons screened for HCV infection, United States, 2022.
| Universal testing of all infants |
Testing known exposed |
|||||||
|---|---|---|---|---|---|---|---|---|
| 44.7% of pregnant persons screened (base case) |
65% of pregnant persons screened |
90% of pregnant persons screened |
||||||
| Outcome | Anti-HCV with reflex to HCV RNA at 18 months2,4 (Comparison) |
HCV RNA test at 2-6 months3,5 (Test Strategy 1) |
Anti-HCV with reflex to HCV RNA at 18 months2 (Test Strategy 2) |
HCV RNA test at 2-6 months3 (Test Strategy 3) |
Anti-HCV with reflex to HCV RNA at 18 months2 (Test Strategy 2) |
HCV RNA test at 2-6 months3 (Test Strategy 3) |
Anti-HCV with reflex to HCV RNA at 18 months2 (Test Strategy 2) |
HCV RNA test at 2-6 months3 (Test Strategy 3) |
| Total costs (2021 USD) | 116,433,899 | 207,170,025 | 77,621,446 | 77,151,775 | 77,383,912 | 76,635,250 | 77,590,443 | 76,244,415 |
| Testing outcomes | ||||||||
| Tested infants | 2,709,916 | 3,076,918 | 4,478 | 7,588 | 6,466 | 10,993 | 8,918 | 15,221 |
| Anti-HCV tests | 2,709,916 | 0 | 4,478 | 0 | 6,466 | 0 | 8,918 | 0 |
| HCV RNA tests | 6,120 | 3,076,918 | 330 | 7,588 | 470 | 10,993 | 649 | 15,221 |
| Epidemiologic outcomes among perinatally exposed infants | ||||||||
| Diagnosed HCV infections | 553 | 971 | 254 | 443 | 362 | 632 | 498 | 875 |
| HCC cases | 316 | 151 | 419 | 342 | 382 | 275 | 334 | 185 |
| DCC cases | 334 | 157 | 463 | 388 | 416 | 305 | 356 | 197 |
| Liver transplants | 58 | 28 | 74 | 62 | 68 | 50 | 62 | 34 |
| HCV Liver-related deaths | 550 | 259 | 758 | 626 | 684 | 496 | 588 | 327 |
| Treated/cured HCV infections | 438 | 775 | 196 | 347 | 284 | 501 | 393 | 698 |
| Total QALYs | 694,546 | 696,669 | 693,059 | 694,026 | 693,590 | 694,967 | 694,319 | 696,221 |
| Total Life-years | 1,814,939 | 1,826,061 | 1,807,109 | 1,812,187 | 1,809,846 | 1,817,052 | 1,813,710 | 1,823,653 |
Assumes 3.6 million births with 0.64% of births occurring among pregnant persons who are HCV RNA+.
Assumes 43.0% of children born to HCV RNA+ pregnant persons attend an 18-month visit.
Assumes 73.5% of children born to HCV RNA+ pregnant persons attend a 2-6 month visit.
Assumes 75.0% of children born to pregnant persons without HCV attend an 18-month visit.
Assumes 85.0% of children born to pregnant persons without HCV attend a 2-6 month visit.
Abbreviations: HCV, hepatitis C virus; anti-HCV, hepatitis C virus antibody; USD, United States Dollars; HCC, hepatocellular carcinoma; DCC, decompensated cirrhosis; QALYs, quality-adjusted life-years.
Table 3.
Sensitivity analysis results: Incremental cost-effectiveness ratios for HCV infant testing strategies versus HCV RNA testing at 2–6 months for infants with known exposure, by proportion of pregnant persons that are screened for HCV infection, United States, 2022
| Strategy (reference=Known exposure: HCV RNA test at 2–6 months) | |||
|---|---|---|---|
| Proportion of pregnant persons screened for HCV |
Known exposure: Anti-HCV with reflex to HCV RNA at 18 months (Test Strategy 1) |
Universal testing: Anti-HCV with reflex to HCV RNA at 18 months (Test Strategy 2) |
Universal testing: HCV RNA test at 2–6 months (Test Strategy 3) |
| 0.50 | More expensive, fewer QALYS | 129,476 | 53,665 |
| 0.55 | More expensive, fewer QALYS | 569,548 | 59,532 |
| 0.60 | More expensive, fewer QALYS | More expensive, fewer QALYS | 67,746 |
| 0.65 | More expensive, fewer QALYS | More expensive, fewer QALYS | 76,727 |
| 0.70 | More expensive, fewer QALYS | More expensive, fewer QALYS | 92,427 |
| 0.75 | More expensive, fewer QALYS | More expensive, fewer QALYS | 113,463 |
| 0.80 | More expensive, fewer QALYS | More expensive, fewer QALYS | 139,075 |
| 0.85 | More expensive, fewer QALYS | More expensive, fewer QALYS | 197,018 |
| 0.90 | More expensive, fewer QALYS | More expensive, fewer QALYS | 292,229 |
| 0.95 | More expensive, fewer QALYS | More expensive, fewer QALYS | 661,659 |
Notes: The reference strategy is HCV RNA testing at 2–6 months among infants with known exposure. Assumes 43.0% of infants born to HCV RNA+ pregnant persons attend an 18-month visit. Assumes 73.5% of infants born to HCV RNA+ pregnant persons attend a 2–6 month visit. Assumes 75.0% of infants born to pregnant persons without HCV attend an 18-month visit. Assumes 85.0% of infants born to pregnant persons without HCV attend a 2–6 month visit.
Table 5.
Probabilistic sensitivity analysis1 population-level results2 for HCV testing strategies among a cohort of children, United States, 2022
| Testing known exposed children |
Universal testing of all children |
|||
|---|---|---|---|---|
| Anti-HCV with reflex to HCV RNA at 18 months (Comparison) |
HCV RNA test at 2-6 months (Test Strategy 1) |
Anti-HCV with reflex to HCV RNA at 18 months (Test Strategy 2) |
HCV RNA test at 2-6 months (Test Strategy 3) |
|
| Outcome | median (95% CI) | median (95% CI) | median (95% CI) | median (95% CI) |
| Total costs (2021 USD) | 90,963,369 (66,954,031 – 132,947,567) | 87,229,844 (61,499,748 – 126,163,535) | 118,280,437 (87,224,385 – 169,656,366) | 191,266,412 (130,885,944 – 295,828,495) |
| Testing outcomes | ||||
| Tested infants | 4,602 (3,046 – 6,151) | 7,787 (5,338 – 10,585) | 2,477,119 (1,517,732 – 4,385,218) | 2,742,208 (1,672,632 – 5,055,815) |
| Anti-HCV tests | 4,602 (3,046 – 6,151) | 0 (0 - 0) | 2,477,119 (1,517,732 – 4,385,218) | 0 (0 - 0) |
| HCV RNA tests | 358 (222 - 523) | 7,851 (5,371 – 10,680) | 8,652 (3,775 – 21,498) | 2742208 (1,672,632 – 5,055,815) |
| Epidemiologic Outcomes among infants born to HCV-infected persons | ||||
| Diagnosed HCV infections | 248 (154 - 340) | 439 (289 - 630) | 526 (416 - 701) | 939 (754 - 1159) |
| HCC cases | 434 (320 - 554) | 359 (255 - 500) | 330 (250 - 426) | 178 (119 - 252) |
| DCC cases | 493 (377 - 635) | 408 (290 - 537) | 358 (275 - 481) | 183 (122 - 260) |
| Liver transplants | 85 (57 - 127) | 71 (47 - 110) | 63 (41 - 94) | 33 (17 - 51) |
| Liver-related deaths | 815 (627 - 992) | 668 (486 - 886) | 606 (463 - 765) | 311 (213 - 423) |
| Treated/cured HCV infections | 196 (120 - 269) | 348 (233 - 501) | 423 (326 - 557) | 758 (598 - 925) |
| Total QALYs | 691,004 (686,437 – 694,672) | 692,111 (688,277 – 695,554) | 692,862 (689,224 – 696,127) | 695,510 (692,364 – 698,273) |
| Total Life-years | 1,799,819 (1,787,793 – 1,811,646) | 1,806,173 (1,793,694 – 1,815,681) | 1,809,147 (1,800,278 – 1,818,163) | 1,821,715 (1,814,756 – 1,827,927) |
A triangle distribution was defined for each input, with the base case value set as the distribution mean and the upper and lower limits set as the bounds of the distribution. We sampled 100 input parameter sets and ran 100,000 microsimulation trials with each parameter set. For all outcomes, we report the median and 95% interval (2.5th and 97.5th percentile) from the probabilistic sensitivity analysis results.
Assumes 3.6 million births.
Abbreviations: HCV, hepatitis C virus; anti-HCV, hepatitis C virus antibody; HCC, hepatocellular carcinoma; DCC, decompensated cirrhosis; USD, United States Dollars; QALYs, quality-adjusted life-years.
Table 6.
Population-level results1 from a scenario sensitivity analysis using data from Ades et al2 for HCV testing strategies among a cohort of children, United States, 2022.
| Testing known exposed children3 |
Universal testing of all children |
|||
|---|---|---|---|---|
| Outcome | Anti-HCV with reflex to HCV RNA at 18 months4 (Comparison) |
HCV RNA test at 2-6 months5 (Test Strategy 1) |
Anti-HCV with reflex to HCV RNA at 18 months4,6 (Test Strategy 2) |
HCV RNA test at 2-6 months5,7 (Test Strategy 3) |
| Total costs (2021 USD) | 80,058,982 | 82,265,899 | 122,475,587 | 219,378,669 |
| Testing outcomes | ||||
| Tested children | 4,478 | 7,588 | 2,709,916 | 3,076,918 |
| Anti-HCV tests | 4,478 | 0 | 2,709,916 | 0 |
| HCV RNA tests | 391 | 7,588 | 6,254 | 3,076,918 |
| Epidemiologic outcomes among perinatally exposed children | ||||
| Diagnosed HCV infections | 315 | 555 | 690 | 1,214 |
| HCC cases | 410 | 335 | 306 | 146 |
| DCC cases | 449 | 375 | 322 | 151 |
| Liver transplants | 75 | 62 | 55 | 27 |
| HCV Liver-related deaths | 736 | 607 | 533 | 251 |
| Treated/cured HCV infections | 489 | 637 | 724 | 1,052 |
| Total QALYs | 691,536 | 692,465 | 692,998 | 695,033 |
| Total Life-years | 1,807,966 | 1,812,814 | 1,815,616 | 1,826,263 |
Assumes 3.6 million births with 0.64% of births occurring among persons who are HCV RNA+.
Assuems prevalence of HCV infection among children perinatally exposed is 7.2% and 20.9% of children that are treated at age 3 would have spontaneous cleared infection without treatment.
References:
Ades AE, Gordon F, Scott K, Collins IJ, Thorne C, Pembrey L, Chappell E, Marine-Barjoan E, Butler K, Indolfi G, Gibb DM, Judd A. Overall vertical transmission of HCV, transmission net of clearance, and timing of transmission. Clin Infect Dis. 2022 Apr 11:ciac270. doi: 10.1093/cid/ciac270. Epub ahead of print. PMID: 35403676
Ades AE, Gordon F, Scott K, Collins IJ, Thorne C, Pembrey L, Chappell E, Marine-Barjoan E, Butler K, Indolfi G, Gibb DM, Judd A. Spontaneous Clearance Of Vertically Acquired Hepatitis C Infection: Implications For Testing And Treatment. Clin Infect Dis. 2022 Apr 9:ciac255. doi: 10.1093/cid/ciac255. Epub ahead of print. PMID: 35396848.
Assumes 44.7% of all pregnant persons are screened for HCV infection.
Assumes 43.0% of children born to HCV RNA+ pregnant persons attend an 18-month visit.
Assumes 73.5% of children born to HCV RNA+ pregnant persons attend a 2-6 month visit.
Assumes 75.0% of children born to pregnant persons without HCV attend an 18-month visit.
Assumes 85.0% of children born to pregnant persons without HCV attend a 2-6 month visit.
Abbreviations: HCV, hepatitis C virus; anti-HCV, hepatitis C virus antibody; HCC, hepatocellular carcinoma; DCC, decompensated cirrhosis; USD, United States Dollars; QALYs, quality-adjusted life-years.
Discussion
The objective of this modeling study was to identify the optimal testing strategy that should be used to diagnose perinatally acquired HCV infections. All three comparison strategies resulted in more children tested and better population-level health outcomes including fewer cases of hepatocellular carcinoma, decompensated cirrhosis, liver transplants, and HCV-related deaths, compared with the currently accepted standard of anti-HCV testing at age 18 months or older. Testing of known perinatally exposed children at age 2–6 months with an HCV RNA test compared with the baseline strategy resulted in population-level cost-savings of $469,671. As expected, more testing in each of the universal comparison strategies resulted in increased QALYs, but also over $38 million in increased population-level costs for anti-HCV with reflex to HCV RNA at age 18 months or older and over $129 million in increased population-level costs for testing of all children at age 2–6 months with an HCV RNA test. As uptake of testing recommendations for HCV infection during pregnancy continues to increase, the difference in the number of infections identified between universal testing strategies and testing children with known exposure will continue to decline.
These analyses indicate the optimal timing and most efficient test is a single HCV RNA during age 2–6 months among children perinatally exposed to HCV. Factors driving these results include pediatric loss to follow up, , high attendance at well-child visits in the first 6 months of life, and highly sensitive nucleic acid testing with reliable results starting at age two months. Testing further from birth is associated with loss to follow up and may reduce the likelihood that perinatal exposure will be documented in the child’s medical record (e.g., change of providers, loss of custody etc.). This is especially true as HCV-exposed children are disproportionately publicly insured, and infants born to HCV-infected mothers are more likely to be involved with child services and foster care17,37,38. One study assessing >150,000 children at two health networks spanning 20 states, determined children rarely missed 2-month, 4-month, and 6-month well-care visits, while 15-month and 18-month visits were attended by less than half of publicly insured children13. With advancements in analytical sensitivity allowing detection of low-level viremia, nucleic acid testing for diagnosis of perinatally acquired HCV infection in early infancy provides excellent diagnostic performance19,22.
. Among exposed children, the increased number of children diagnosed with a single HCV RNA at younger ages with the 2–6 month strategy was cost-saving and had corresponding improved health outcomes, compared with the widely recommended 18 month strategy using anti-HCV with reflex to HCV RNA. The success of these strategies relies heavily on widespread testing during pregnancy and transfer of information to providers caring for infants. Updated CDC hepatitis C screening guidelines call for testing of all pregnant people during each pregnancy but testing currently remains below 50%39. At current levels of HCV testing among pregnant persons, a universal approach using anti-HCV testing at 18 months or older costs an additional $142,764 per infection diagnosed, and HCV RNA testing at 2–6 months an additional $187,561 per infection diagnosed, compared with testing known exposed children with an HCV RNA test at age 2–6 months. As the proportion of pregnant persons tested approaches 90%, universal testing strategies result in diminishing return with costs per additional diagnosed infection increasing to $705,403 for anti-HCV testing, and $273,813 for HCV RNA testing. Cost per additional diagnosed pediatric infection for either universal strategy become prohibitive as the proportion of pregnant persons screened for HCV infection exceeds 70%.
Results of this analysis should be interpreted with a few limitations. First, there are limited data on the proportion of infants with perinatal exposure that would be tested at 2-6 months and 18 months. Inputs were based on a single study of infant follow-up at each time period and may not be generalizable to all settings. Second, we only quantified costs and health outcomes among children. We did not quantify any potential costs or benefits that would be gained by the resulting diagnosis and treatment of a birthing parent because of identifying an HCV infection in their child. Additionally, we assumed perinatally-acquired infections undiagnosed in childhood would not be diagnosed and treated later in life, which may result in an overestimate of poor outcomes among persons with undiagnosed perinatal infections. Third, to align with current recommendations7,10 we assumed treatment occurs at or after age 3 years in all strategies and there was no difference in linkage to treatment or costs associated follow-up testing that providers may recommend prior to 3 years of age. Evaluating approaches in which treatment was initiated at different ages was outside of the scope of this study, which focused on testing strategies. Fourth, the model of HCV disease progression does not include coinfections with HIV, hepatitis B, or other infections, which could alter quality (and length) of life, progression of HCV infection, and associated medical costs. Finally, for universal testing strategies, we did not model program implementation costs that would likely be incurred in such a large scale up of HCV testing, which results in an underestimate of total costs for the universal strategies.
. Identifying optimal strategies for diagnosis of perinatally infected children that are cost-effective are essential for preventing morbidity and mortality associated with complications from perinatal HCV infection, improving individual and population health outcomes, and achieving national hepatitis C elimination goals.
Acknowledgements:
The authors acknowledge Taiwo Abimbola and Monica Trigg for their contributions to the development and organization of this paper.
Funding/Support:
This work was supported by the National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention, as part of the NCHHSTP Epidemiologic and Economic Modeling Cooperative Agreement (grant number: 5U38PS004650).
Abbreviations:
- HCV
hepatitis C virus (HCV)
- RNA
Ribonucleic acid
- anti-HCV
hepatitis C virus antibodies
- ICER
incremental cost-effectiveness ratio
- USD
United States Dollar
- QALY
quality-adjusted life years
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures:
Author EWH reports receiving consulting fees from Merck & Co. for work unrelated to this research. All other authors have no conflicts of interest to report. LP, NN, CW, and ALS report salaries as employees at the US Centers for Disease Control and Prevention.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the authors affiliated institutions.
There are no prior publications or submissions with any overlapping information, including studies and patients.
References
- [1].CDC. Viral hepatitis surveillance—United States, 2020. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2022. [Google Scholar]
- [2].Patrick SW, Dupont WD, McNeer E, McPheeters M, Cooper WO, Aronoff DM, et al. Association of Individual and Community Factors With Hepatitis C Infections Among Pregnant People and Newborns. JAMA Health Forum. 2021;2:e213470–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tovo PA, Pembrey LJ, Newell ML. Persistence rate and progression of vertically acquired hepatitis C infection. European Paediatric Hepatitis C Virus Infection. J Infect Dis. 2000;181:419–24. [DOI] [PubMed] [Google Scholar]
- [5].Ades AE, Gordon F, Scott K, Collins IJ, Thorne C, Pembrey L, et al. Spontaneous Clearance Of Vertically Acquired Hepatitis C Infection: Implications For Testing And Treatment. Clin Infect Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ades AE, Gordon F, Scott K, Collins IJ, Thorne C, Pembrey L, et al. Overall vertical transmission of HCV, transmission net of clearance, and timing of transmission. Clin Infect Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leung DH, Squires JE, Jhaveri R, Kerkar N, Lin CH, Mohan P, et al. Hepatitis C in 2020: A North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper. J Pediatr Gastroenterol Nutr. 2020;71:407–17. [DOI] [PubMed] [Google Scholar]
- [8].Committee on Infectious Diseases AAoP, Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH. Hepatitis C. Red Book: 2021–2024 Report of the Committee on Infectious Diseases: American Academy of Pediatrics; 2021. p. 0. [Google Scholar]
- [9].Lam NC, Gotsch PB, Langan RC. Caring for pregnant women and newborns with hepatitis B or C. Am Fam Physician. 2010;82:1225–9. [PubMed] [Google Scholar]
- [10].AASLD-IDSA. Unique Populations: HCV in Children. Recommendations for HCV testing of perinatally exposed children and siblings of children with HCV infection. . AASLD-IDSA; 2021. p. AASLD-IDSA Guidance. [Google Scholar]
- [11].England K, Pembrey L, Tovo PA, Newell ML. Excluding hepatitis C virus (HCV) infection by serology in young infants of HCV-infected mothers. Acta Paediatr. 2005;94:444–50. [DOI] [PubMed] [Google Scholar]
- [12].Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192:1880–9. [DOI] [PubMed] [Google Scholar]
- [13].Wolf ER, Hochheimer CJ, Sabo RT, DeVoe J, Wasserman R, Geissal E, et al. Gaps in Well-Child Care Attendance Among Primary Care Clinics Serving Low-Income Families. Pediatrics. 2018;142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lopata SM, McNeer E, Dudley JA, Wester C, Cooper WO, Carlucci JG, et al. Hepatitis C Testing Among Perinatally Exposed Infants. Pediatrics. 2020;145:03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bell R, Wolfe I, Cox D, Thakarar K, Lucas L, Craig A. Hepatitis C Screening in Mothers and Infants Exposed to Opioids. Hosp. 2019;9:639–42. [DOI] [PubMed] [Google Scholar]
- [16].Chappell CA, Hillier SL, Crowe D, Meyn LA, Bogen DL, Krans EE. Hepatitis C Virus Screening Among Children Exposed During Pregnancy. Pediatrics. 2018;141:06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Watts T, Stockman L, Martin J, Guilfoyle S, Vergeront JM. Increased Risk for Mother-to-Infant Transmission of Hepatitis C Virus Among Medicaid Recipients - Wisconsin, 2011-2015. MMWR - Morbidity & Mortality Weekly Report. 2017;66:1136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kuncio DE, Newbern EC, Johnson CC, Viner KM. Failure to Test and Identify Perinatally Infected Children Born to Hepatitis C Virus-Infected Women. Clin Infect Dis. 2016;62:980–5. [DOI] [PubMed] [Google Scholar]
- [19].Gowda C, Smith S, Crim L, Moyer K, Sanchez PJ, Honegger JR. Nucleic Acid Testing for Diagnosis of Perinatally-Acquired Hepatitis C Virus Infection in Early Infancy. Clinical Infectious Diseases. 2020;08:08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Epstein RL, Sabharwal V, Wachman EM, Saia KA, Vellozzi C, Hariri S, et al. Perinatal Transmission of Hepatitis C Virus: Defining the Cascade of Care. J Pediatr. 2018;203:34–40.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abdel-Hamid M, El-Daly M, El-Kafrawy S, Mikhail N, Strickland GT, Fix AD. Comparison of second- and third-generation enzyme immunoassays for detecting antibodies to hepatitis C virus. J Clin Microbiol. 2002;40:1656–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Association of Public Health Laboratories. Interpretation of Hepatitis C Virus Test Results: Guidance for Laboratories. 2019. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2019Jan-HCV-Test-Result-Interpretation-Guide.pdf
- [23].Centers for Disease Control and Prevention. Unpublished data. 2022.
- [24].Towers CV, Fortner KB. Infant follow-up postdelivery from a hepatitis C viral load positive mother. J Matern Fetal Neonatal Med. 2019;32:3303–5. [DOI] [PubMed] [Google Scholar]
- [25].Goyal NK, Rohde JF, Short V, Patrick SW, Abatemarco D, Chung EK. Well-Child Care Adherence After Intrauterine Opioid Exposure. Pediatrics. 2020;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Arias E, Xu J. United States Life Tables, 2018. Natl Vital Stat Rep. 2020;69:1–45. [PubMed] [Google Scholar]
- [27].Greenaway E, Haines A, Ling SC, Krahn M. Treatment of Chronic Hepatitis C in Young Children Reduces Adverse Outcomes and Is Cost-Effective Compared with Deferring Treatment to Adulthood. J Pediatr. 2021;230:38–45.e2. [DOI] [PubMed] [Google Scholar]
- [28].Three broad modalities in the natural history of vertically acquired hepatitis C virus infection. Clin Infect Dis. 2005;41:45–51. [DOI] [PubMed] [Google Scholar]
- [29].Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-effectiveness in health and medicine: Oxford University Press; 2016. [Google Scholar]
- [30].Kim DD, Silver MC, Kunst N, Cohen JT, Ollendorf DA, Neumann PJ. Perspective and Costing in Cost-Effectiveness Analysis, 1974–2018. Pharmacoeconomics. 2020;38:1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nguyen J, Barritt ASt, Jhaveri R. Cost Effectiveness of Early Treatment with Direct-Acting Antiviral Therapy in Adolescent Patients with Hepatitis C Virus Infection. J Pediatr. 2019;207:90–6. [DOI] [PubMed] [Google Scholar]
- [32].Bureau of Labor Statistics (BLS). Consumer Price Index for All Urban Consumers (CPI-U): US city average, by expenditure category, December 2021. 2022.
- [33].Dunn A, Grosse SD, Zuvekas SH. Adjusting Health Expenditures for Inflation: A Review of Measures for Health Services Research in the United States. Health Serv Res. 2018;53:175–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Myhre J, Sifris D. FDA-Approved Hepatitis C Drugs. In: VeryWellHealth, editor.2020. [Google Scholar]
- [35].Osterman M, Hamilton B, Martin JA, Driscoll AK, Valenzuela CP. Births: Final Data for 2020. Natl Vital Stat Rep. 2021;70:1–50. [PubMed] [Google Scholar]
- [36].Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC Recommendations for Hepatitis C Screening Among Adults - United States, 2020. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2020;69:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Espinosa C, Myers J, Smith M. Patterns of testing in children exposed perinatally exposed to hepatitis c. Open Forum Infectious Diseases 2017. p. S197. [Google Scholar]
- [38].Lairmore S, Stone KE, Huang R, McLeigh J. Infectious disease screening in a dedicated primary care clinic for children in foster care. Child Abuse Negl. 2021;117:105074. [DOI] [PubMed] [Google Scholar]
- [39].Kaufman HW, Osinubi A, Meyer WAI, Khan M, Huang X, Panagiotakopoulos L, et al. Hepatitis C Virus Testing During Pregnancy After Universal Screening Recommendations. Obstet Gynecol. 2022: 10.1097/AOG.0000000000004822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Center for Medicare & Medicaid Services (CMS). Clinical diagnostic laboratory fee (CLAB). 2022.