Abstract
Introduction:
Diagnosis of interstitial lung disease (ILD) is based on multidisciplinary team discussion (MDD) with incorporation of clinical, radiographical, and histopathological information if available. We aim to evaluate the diagnostic yield and safety outcomes of transbronchial lung cryobiopsy (TBLC) in the diagnosis of ILD.
Methodology and study design:
We conducted a meta-analysis by comprehensive literature search to include all studies that evaluated the diagnostic yields and/or adverse events with TBLC in patients with ILD. We calculated the pooled event rates and their 95% confidence intervals (CI) for the diagnostic yield by MDD, histopathological diagnostic yield, and various clinical adverse events.
Results:
We included 68 articles (44 full texts and 24 abstracts) totaling 6,386 patients with a mean age of 60.7±14.1 years and 56% men. The overall diagnostic yield of TBLC to achieve a definite or high-confidence diagnosis based on MDD was 82.3% (95% CI 78.9–85.2) and histopathological diagnosis of 72.5% (95% CI 67.7–76.9). The overall rate of pneumothorax was 9.6% (95% CI 7.9–11), while the rate of pneumothorax requiring drainage by a thoracostomy tube was 5.3% (95% CI 4.1–6.9%). The rate of moderate bleeding was 11.7% (95% CI 9.1–14.9%) while the rate of severe bleeding was 1.9% (95% CI 1.4%−2.6%). The risk of mortality attributed to the procedure was 0.9% (95% CI 0.7–1.3%)
Conclusion:
Among patients with undiagnosed or unclassified ILD requiring tissue biopsy for diagnosis, transbronchial cryobiopsy represents a reliable alternative to surgical lung biopsy with decreased incidence of various clinical adverse events.
Keywords: Transbronchial cryobiopsy, interstitial lung disease, multidisciplinary discussion
Introduction
Interstitial lung disease (ILD) represents a heterogenous spectrum of diseases that is characterized by inflammatory and fibrotic changes of the lung. The specific diagnosis is made in multidisciplinary team discussion (MDD) with incorporation of clinical, radiographical, and histopathological data before establishing a final diagnosis. In approximately 30% of patients, lung biopsy is needed to reach a final diagnosis 1,2,3.
Surgical lung biopsy (SLB) is considered the gold standard method for obtaining lung tissue for diagnosis in patients with ILD. However, it is associated with risks of infection, prolonged air leak, prolonged hospital stay, and mortality 4,5. On the other hand, transbronchial forceps biopsy (TBFB) is often non-diagnostic given the small biopsy size which is usually insufficient for detailed pathological evaluation 6,7.
Transbronchial lung cryobiopsy (TBLC) has emerged as a new tool to obtain lung biopsy for the diagnosis of ILD. In a recent study, TBLC was concordant with SLB in achieving a final definite or high-confidence MDD diagnosis 2. However, another study has shown poor concordance between both approaches 8. Previous meta-analyses reported a diagnostic yield of TBLC of around 80% 9–11. Nevertheless, the reported diagnostic yield varies significantly among published studies ranging between 50–90%. As the use of cryobiopsy has been more common, additional studies have been published with larger patient populations, especially in those with multiple comorbidities who are at an increased risk of perioperative complications with SLB.
We aimed to perform a comprehensive literature search and meta-analysis with meta-regression analyses to evaluate the efficacy and safety of the TBLC in ILD patients and to determine whether specific factors are associated with an increased diagnostic yield of this procedure.
Methodology
Study design and study selection
Our study is a systematic review and meta-analysis performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) 2015 Statement 12. We performed a comprehensive literature search utilizing major electronic databases, PubMed and Embase, from inception until May 1st, 2021. Articles were first screened by titles and abstracts; then full text of eligible articles were reviewed before final inclusion or exclusion based on predefined criteria. In addition, references of relevant articles and manual web search was also performed. Literature search was conducted separately and independently by two reviewers (Y.Z and M.B.). Any discrepancy was solved by a consensus with a third reviewer (H.M.). The study was registered at the National Institute of Health Research, International Prospective Register of Systematic reviews (PROSPERO; CRD42021251444).
Inclusion and exclusion criteria
We included all studies that evaluated the diagnostic yield and/or safety of TBLC in the evaluation of ILD as a single arm or with a comparator arm. Case series, retrospective, and prospective studies, as well as randomized controlled trials (RCTs) were considered for inclusion. We excluded studies that evaluated patients with acute hypoxemic respiratory failure in the intensive care unit, lung transplant patients, or those with lung nodules or malignancy. In order to avoid duplication of the patient population, we only included one study from each center if there was an overlap in the timeline from which patients were enrolled or retrospectively reviewed. Either the last published study or the study with the largest patient population was included. Case reports and review papers were excluded.
Outcome definitions
We aimed to determine the pooled efficacy and safety of TBLC. Efficacy outcomes included the diagnostic yield of cryobiospy based on MDD or histopathological diagnosis. An MDD diagnosis is defined as a diagnosis of a specific ILD that was established based on combining clinical, radiographical data, and tissue pathology obtained from cryobiopsy to establish a definite or confident (≥90%) or provisional diagnosis with high confidence (70–89%) without need for repeat biopsy based on the most recent guideline recommendations 3. Pathological diagnosis is defined as a specific histopathological pattern that is sufficient to make a clinical diagnosis in concordance with clinical and radiographical data. We also assessed the rate of performing surgical lung biopsy in undiagnosed patients.
Safety outcomes included occurrences of pneumothorax, pneumothorax that required drainage with thoracostomy tube, moderate bleeding (defined as a bronchial bleeding that did not resolve with endoscopic aspiration and needed further intervention like installation of epinephrine or cold saline, bronchial occlusion, or other interventions but the procedure was completed and not terminated), severe bleeding (defined as a bleeding thar required upgrade of level of care, hemodynamic instability, blood transfusion, or surgical intervention for control of bleeding), and mortality that was attributed secondary to the cryobiospy procedure
Data extraction
In addition to the extraction of the predefined outcomes, we extracted baseline patient characteristics including age, sex, smoking status, forced vital capacity percentage of predicted (FVC%), and diffusion capacity of carbon monoxide percentage of predicted (DLCO%). Specific study characteristics were also extracted and included: number of patients, type of study and if a comparator arm existed, study year, the study center, country of each study, specific procedure technique, number of biopsies, size of the biopsy, the number of segments biopsied, number of lobes biopsied.
Quality Assessment
We evaluated the quality of included studies based on the Quality assessment, Data Abstraction and Synthesis-2 (QUADAS-2) tool with assessment of four main domains including patient selection, index test, reference standard, and flow and timing using signaling questions to determine the risk of bias to be low, high, or unclear. As well, we assessed for applicability by evaluating patient selection, index test, and reference standard.13 Two reviewers independently performed the quality assessment separately and independently (Y.Z., M.B.)
Statistical analysis
We calculated the pooled diagnostic yields and events rates and their corresponding 95% confidence intervals (CI) using the random effects model. We used the Cochrane test for assessing heterogeneity with I2 <25%, 25–50%, and >50% represent low, moderate, and high heterogeneity, respectively. To further examine the source of heterogeneity and if there are specific factors that might affect the diagnostic yields or safety outcomes, we performed meta regression analyses based on study level covariates. These included prospective vs retrospective studies, full text articles vs abstracts, the type of bronchoscopy (flexible, rigid, or two scopes techniques), size of the probe (1.9 mm, 2.4 mm, or studies that used different size among different patients), number of lobes (1, >1, or variable between patients in the same study), number of segments (1, >1, or variable between patients in the same study), number of biopsies, the diameter of the biopsy specimen, baseline FVC% of predicted, and baseline DLCO% of predicted. We used Comprehensive Meta-analysis software V3 for analysis.
Results
After reviewing 1,003 studies, 68 studies (44 full texts 2,14,23–32,15,33–42,16,43–52,17,53–56,18–22 and 24 abstracts57,58,67–76,59,77–80,60–66) met our inclusion criteria and were included in the final analysis with a total of 6,386 patients. The mean age of the group was 60.7±14.1 years and 56% were male. All studies included patients with ILD with no definite or confident diagnosis based on clinical and radiographical data and a tissue biopsy was needed to aid in the diagnosis. Figure 1 shows the flow diagram of the search process. Publication bias was assessed by visual inspection of the funnel plot which suggests low risk of publication bias (Figure 2). Supplementary Table 1 and Table 2 describe the characteristics of included studies while the baseline characteristics of included patients are shown in Supplementary Table 3.
Figure 1:
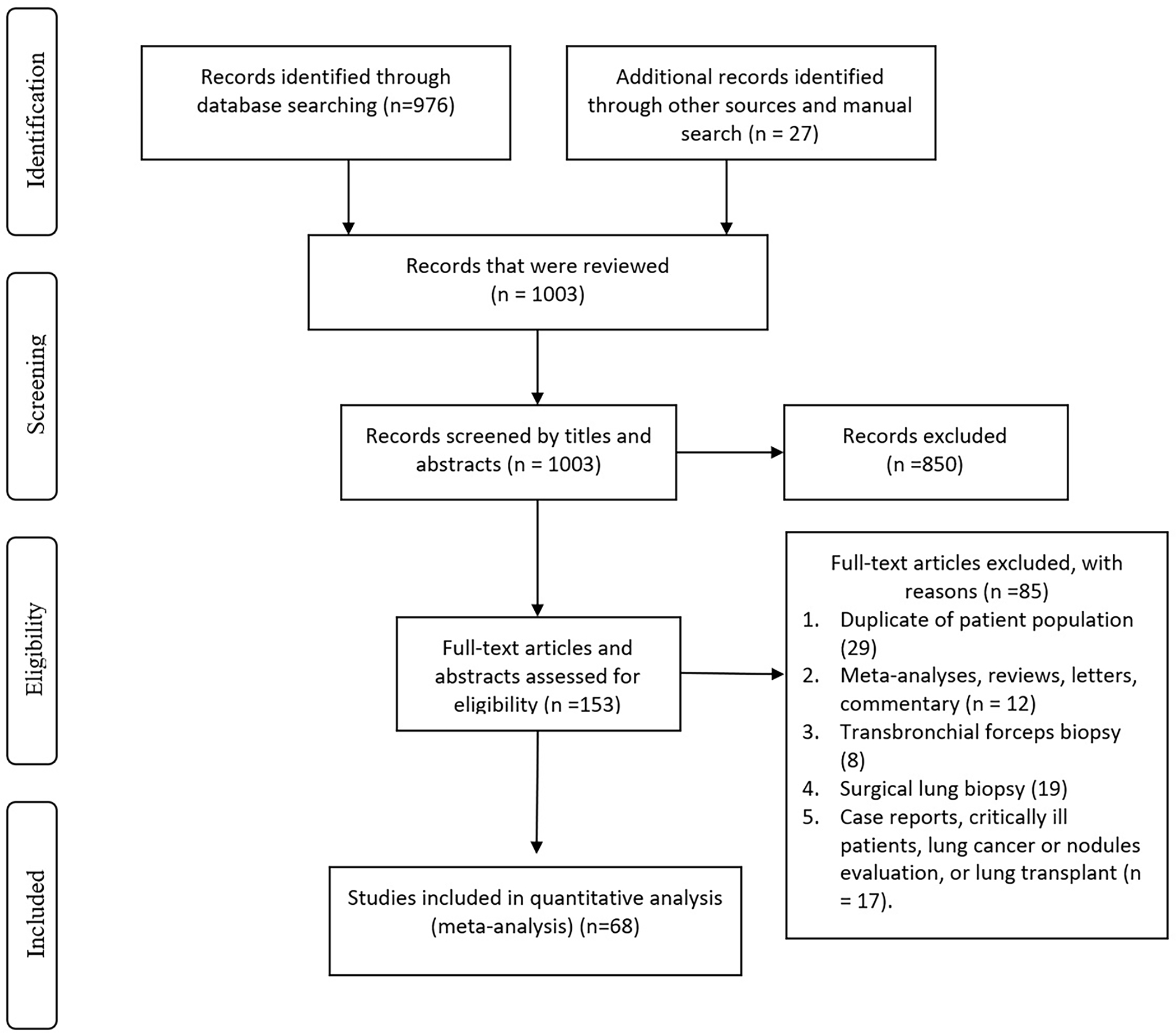
Flow chart of literature search and study selection
Figure 2:
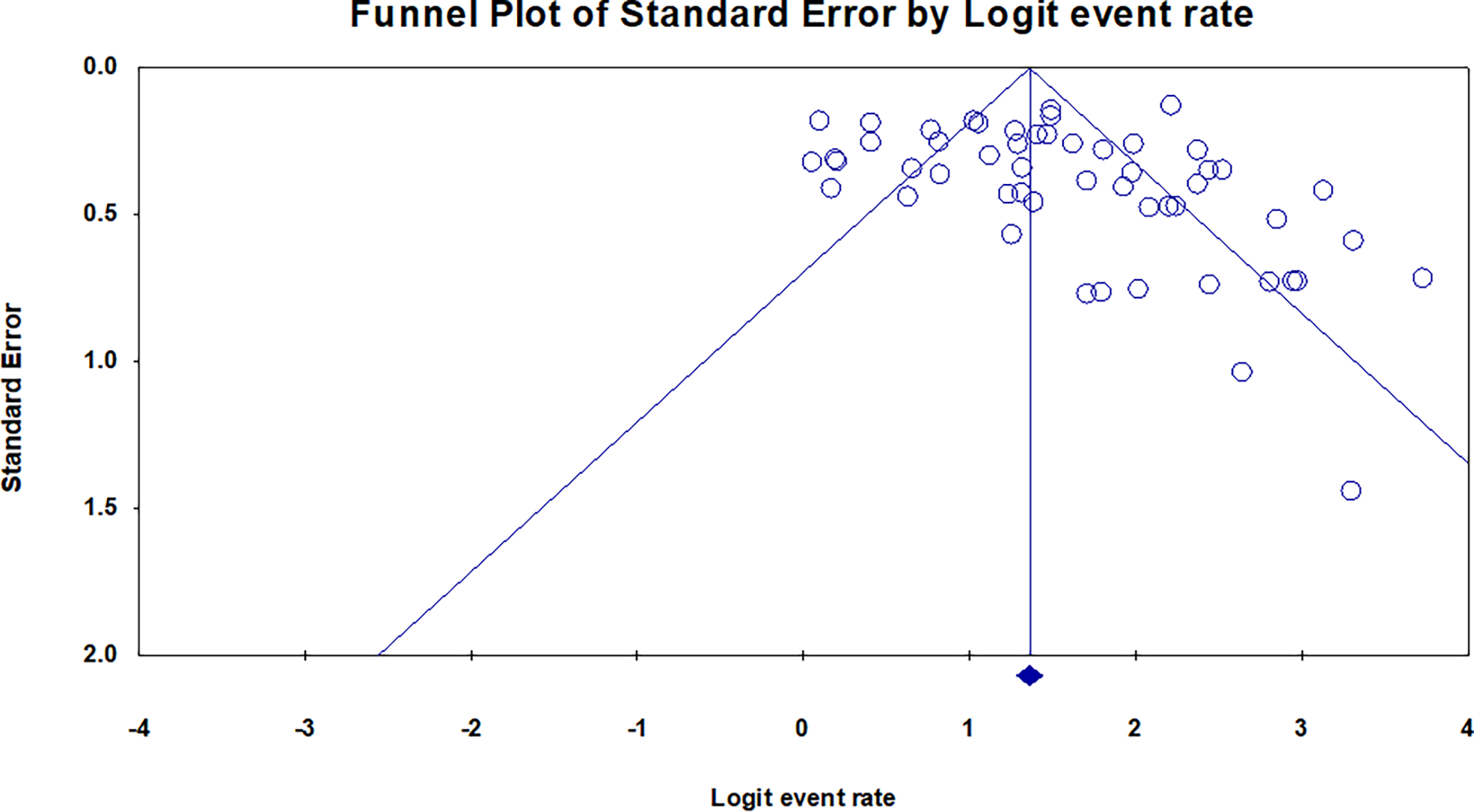
Funnel plot for publication bias showed low risk for publication bias
Most of the included studies were retrospective or prospective single arm cohort studies. Four cohort studies compared TBLC to TBFB and two studies, one cohort and one randomized trial, compared SLB to TBLC. Most studies were performed under general anesthesia using a flexible bronchoscope. Prophylactic use of endobronchial blockers to prevent bleeding was used more commonly in studies in the last few years in comparison to older studies. Although most of the included studies have low risk of bias in patient selection and index test domain, almost all the studies lack the comparison to a SLB as reference test which makes these studies at a moderate to high risk of bias. The quality assessment for each study is detailed in Supplementary Table 4.
Diagnostic Yield
The overall diagnostic yield of TBLC to achieve a definite or high-confident diagnosis based on MDD, combining clinical, radiographical, and histopathological examination of the cryobiopsy, was 82.3% (95% CI 78.9–85.2%; I2=85%; 53 studies; 4,744 patients) (Figure 3). The overall diagnostic yield to achieve a histopathological diagnosis with TBLC was 72.5% (95% CI 67.7–76.9%; I2=88%; 3,750 patients; 38 studies) (Supplementary Figure 1).
Figure 3:
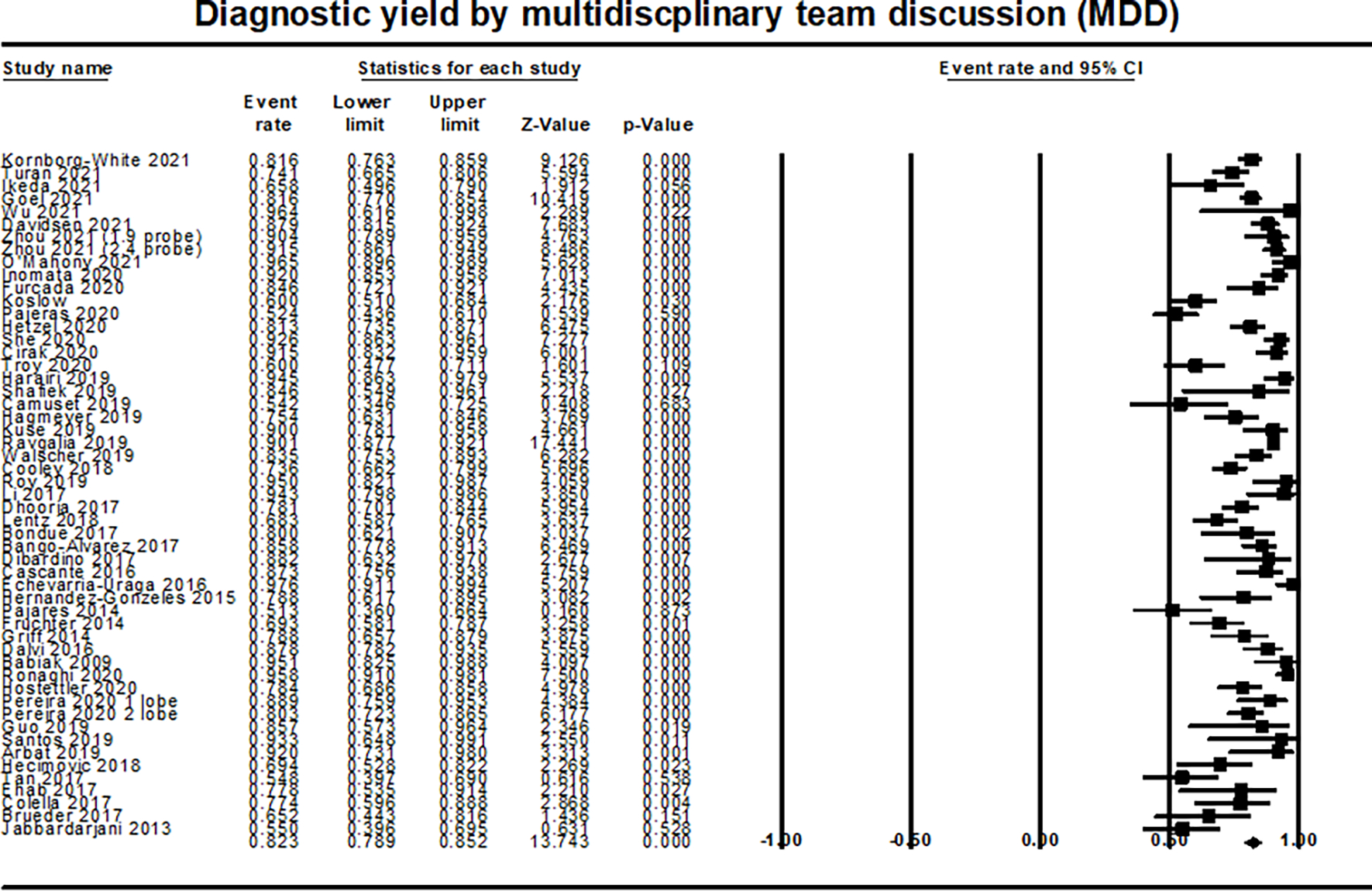
Forest plot of diagnostic yield by multidisciplinary team discussion (MDD)
Based on meta-regression analysis, there was a higher diagnostic yield by MDD in patients with higher FVC (P=0.005). However, this association was not found for histopathological diagnostic yield (Supplementary Figure 2). There was no significant effect on the diagnostic yield by other study-level covariates including prospective vs retrospective studies, full text articles vs abstracts, sample size, bronchoscopy type, probe size, number of lobes or segments biopsied, number of biopsies obtained, size of the biopsy, and baseline percent predicted DLCO.
Safety outcomes
The overall rate of pneumothorax was 9.6% (95% CI 7.9–11.7; I2= 81%; 61 studies; 6,123 patients) (Figure 4). The overall rate of pneumothorax requiring drainage by a thoracostomy tube was 5.3% (95% CI 4.1–6.9%, I2=72%; 47 studies; 4673 patients) (Supplementary Figure 3). Based on meta regression analysis, there was an increased risk of developing pneumothorax with higher number of biopsies (P=0.043; Supplementary Figure 4). This association remained consistent for pneumothorax requiring chest tube placement. Unfortunately, we were not able to detect an exact cut off number where the risk of pneumothorax increased because the data were reported as a continuous variable. There was also a trend toward an increased risk of developing pneumothorax in patients with lower percent predicted DLCO, however this difference did not reach a statistical significance (P=0.051).
Figure 4:
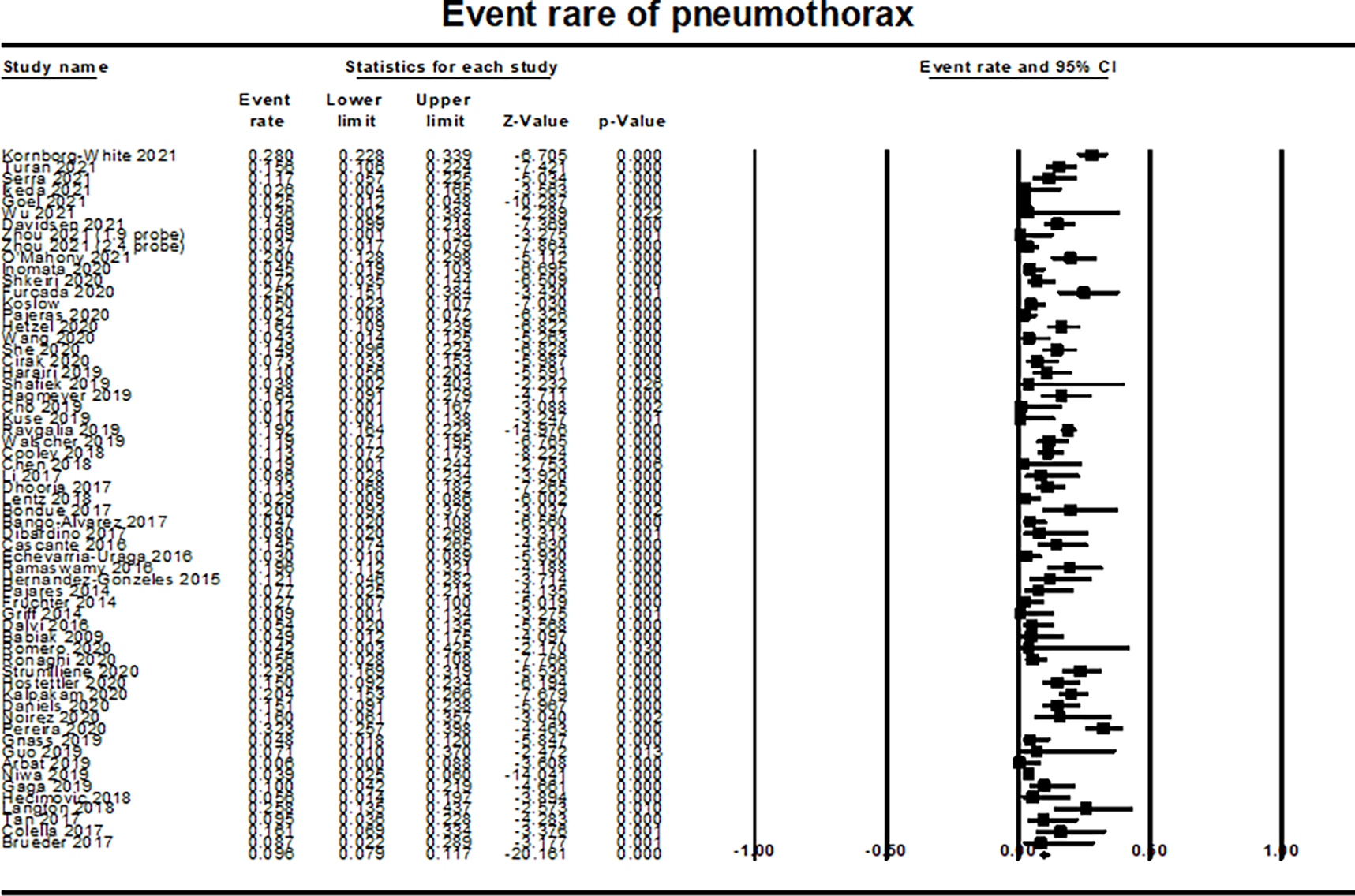
Forest plot for event rate of pneumothorax showing event rate for pneumothorax
The overall rate of moderate bleeding was 11.7% (95% CI 9.1–14.9%, I2=86%, 47 studies; 4360 patients) (Supplementary Figure 5), while the rate of severe bleeding was 1.9% (95% CI 1.4%−2.6%, I2=33%; 49 studies; 4,477 patients) (Figure 5). There was an increased risk of moderate bleeding in patients who underwent biopsies from more than one segment (P=0.042; Supplementary Figure 6). There were no other significant differences based on other study-level covariates including the use of endobronchial blockers as prophylaxis to prevent bleeding.
Figure 5:
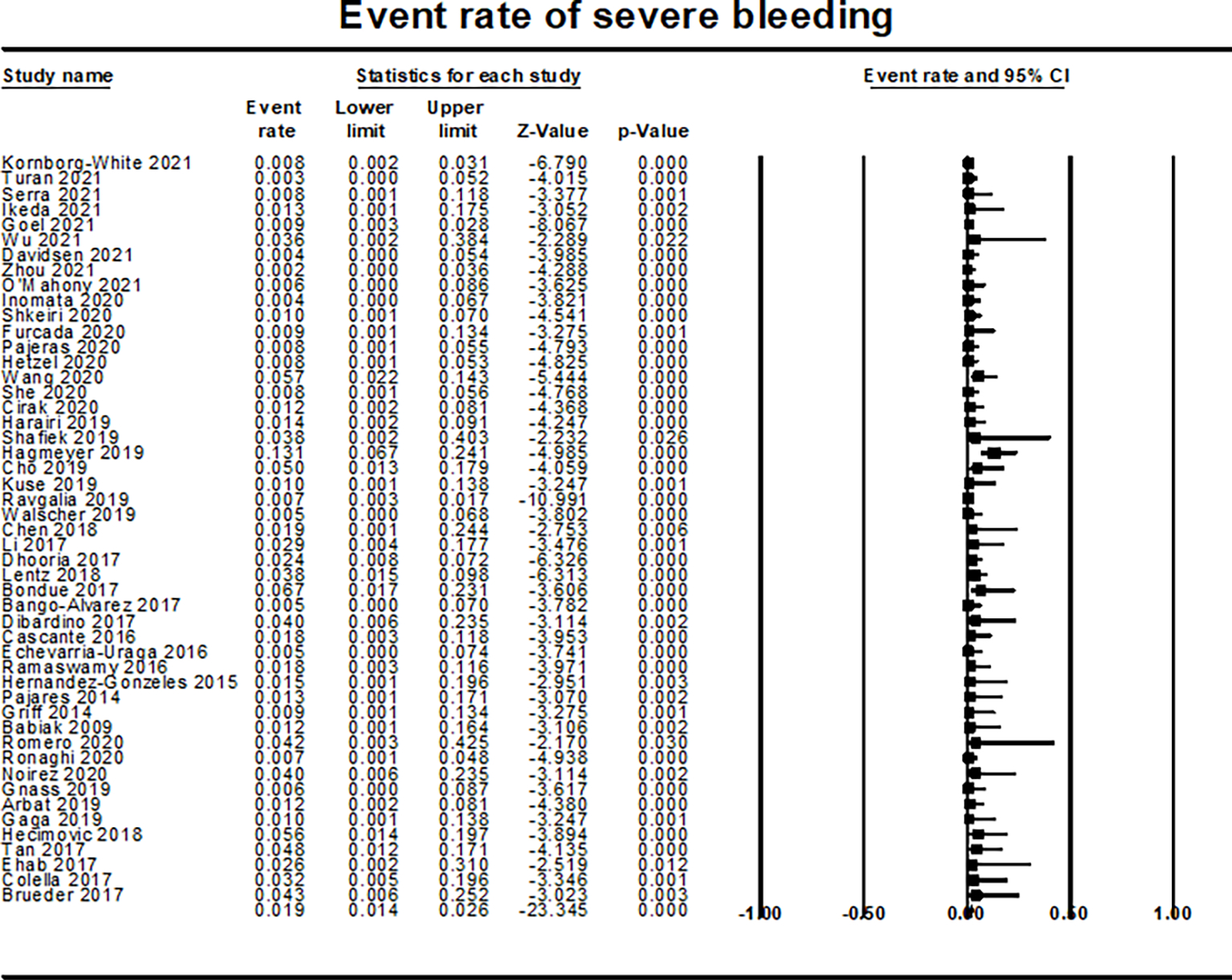
Forest plot for event rate of severe bleeding showing rate of severe bleeding
Regarding the mortality attributed to the procedure, the risk of death was 0.9% (95% CI 0.7–1.3%; I2 =0%, 49 studies; 5,144 patients) (Supplementary Figure 7). The most common cause of death was respiratory failure and acute exacerbation of ILD (9 patients). Other reported causes included thrombotic neoplastic microangiopathy/carcinomatous lymphangitis, massive pulmonary embolism, and pneumothorax leading to respiratory failure and mechanical ventilation. No deaths were attributed to severe bleeding. In the total patient population, the rate of undergoing surgical lung biopsy for further evaluation for unclassified disease was 7.6% (95% CI 5.5–10.5%; I2=71%; 22 studies; 2,170 patients) (Supplementary Figure 8).
Discussion
In our pooled analysis that involved more than 6300 patients with ILD who needed a tissue biopsy for final diagnosis, TBLC has a diagnostic yield for MDD diagnosis or histopathological diagnosis of 82.3% and 72.5%, respectively with 7.6% requiring surgical lung biopsy for further classification of unclassified or undiagnosed disease. The rates of complications were low, with the risk of pneumothorax 9.6%, moderate bleeding 11.7%, and severe bleeding 1.9%. Importantly, the rate of mortality attributed to the procedure was much lower (0.9%) compared to surgical lung biopsy (2.4%) 4.
These findings are similar to the previous meta-analyses that showed a similar diagnostic yield 10,11. However, our meta-analysis has included the more recently published studies with a larger patient population to include a total of 68 full articles and abstracts and more than 6,300 patients. We included abstracts in our study to avoid publication bias as larger studies from larger academic centers are more likely to be published than smaller studies. Furthermore, we were able to conduct a meta-regression analysis based on several study level covariates to determine if that will affect the overall diagnostic yield or various safety outcomes.
In our meta-regression analyses based on variable study-level covariates, patients with higher FVC were more likely to obtain MDD diagnosis. While this finding needs to be verified further, the reason for this association is not obvious. Some potential explanations could be that more sufficient tissue could be obtained given that they could tolerate the procedure better. Another possible explanation could be that they potentially had less fibrosis and more active disease leading to a more definitive diagnosis on tissue samples obtained. Our meta-regression analysis also found that there was an increased risk of developing pneumothorax with higher number of biopsies. In addition, there was an increased risk of developing moderate bleeding when more than one segment is biopsied.
There is a significant difference between the different techniques used in obtaining cryobiopsy which might be reflected in the variable diagnostic yield of 50–90% among the included studies. These differences in techniques and equipment including the size of the probe, among other variables, can potentially affect the size and the quality of the obtained tissue that allow for a sufficient histopathological examination of microscopic features that will assist in obtaining a final confident diagnosis. Although the optimum size of the cryobiopsy is yet to be determined, a biopsy size of 5 × 5 mm is considered sufficient for diagnosis in majority of cases. Our analysis did not find significant difference in the diagnostic yield based on the size of the probe, number of biopsies, number of segments or lobes biopsied, or the type of bronchoscopy. Furthermore, the diameter of the biopsy piece obtained did not change the diagnostic yield in our study. Notably, only 20 out of 68 studies reported tissue sample size which might affect our conclusion and most of these studies obtained a biopsy with a diameter more than 5mm.
SLB is the gold standard to obtain a tissue for diagnostic purposes with a reported pooled diagnostic yield of 92% in one meta-analysis. However, it is associated with increased risk of mortality and adverse outcomes 11. Furthermore, its role in assisting in the diagnosis of undiagnosed or unclassified disease after TBLC remains unclear. In a recent study among patients who had unclassified disease after TBLC, SLB only reclassified 23% of patients 2. Similarly, in another study performing SLB after non-diagnostic TBLC, histopathological diagnosis was changed in three out of twelve patients while the histopathological pattern remained unchanged and confirmed the diagnosis in five patient, and four patients out of the twelve continued to have unclassified disease 36. This raises a concern that SLB may predispose patients to more complications without aiding in diagnosis for majority of patients who have unclassified disease after TBLC. Two recent prospective studies have conducted a direct comparison between SLB and TBLC. The first study conducted sequential TBLC and SLB, but the concordance rate to obtain a final MDD diagnosis was only 38% 8. Few factors limit the interpretation of these findings as the interobserver level of agreement between the two pathologists was very low (57%) and the level of agreement between pathological diagnosis after SLB and MDD was 62% which is considered very low for a gold- standard method. However, this was not significantly different from the agreement between TBLC and MDD (48%). Furthermore, the SLB and TBLC were discussed simultaneously which could have introduced bias 8,81. More recently the COLDICE trial (Cryobiopsy versus Open Lung Biopsy in the Diagnosis of Interstitial Lung Disease) has shown 76.9% agreement to establish a diagnosis by MDD between SLB and TBLC, which demonstrates that TBLC is a reliable alternative for the gold standard method 2.
Our meta-analysis of all available literature to date on TBLC draws conclusions which for the most part are in concordance with the most recent American College of Chest Physicians (ACCP) guidelines on transbronchial cryobiopsy for diagnosis of ILD except that our study demonstrates that biopsy of more than one segment does not increase diagnostic yield, a finding that is limited by the fact that only two studies performed biopsies from single segments while all other studies performed biopsies from multiple segments. In addition, there may be a limited role of SLB after a non-diagnostic TBLC 82.
In light of this information from our analysis, efforts should be directed from reporting a diagnostic yield and safety outcomes, into performing more controlled randomized controlled trials to compare clinical outcomes between different techniques and to determine those more tolerated by the patients and associated with higher diagnostic yields. Furthermore, studies should also compare the long-term outcomes between patients undergoing TBLC and SLB to determine the accuracy of the labeled diagnosis by assessing response to therapy, or if the diagnosis will change based on more clinical information that can appear as the disease manifests itself more with time.
Limitations
Our study has several limitations. First, we included studies of different designs and techniques in performing the procedure as well as different definitions of the severity of bleeding. Second, our subgroup and meta-regression analyses were limited by the high heterogeneity of the procedure among different studies and the low number of studies provided data about each variable. Third, most studies were largely confined to a relatively small, but increasing, number of specialized centers with established experience, which may limit their external validity with variable levels of experience of the pathologist and the bronchoscopist. Fourth, we were not able to examine the performance of TBLC based on the type of ILD (nonspecific interstitial pneumonia, idiopathic pulmonary fibrosis, hypersensitivity pneumonitis, etc.) due to lack of information from the included studies. Fifth, in most of the studies, patients did not undergo the gold standard test to assess the accuracy of the diagnostic yield of TBLC. Lastly, we were not able to perform a direct comparison between SLB and TBLC because of the limited number of studies that conducted head-to-head comparison with different designs among those studies.
Conclusion
Among patients with ILD who needed a biopsy for diagnosis, TBLC yielded an MDD and histopathological diagnosis in 82% and 72% respectively. The incidence of complications is relatively low. TBLC represents a safe and reliable alternative to surgical lung biopsy in the diagnosis of ILD. Further studies are needed to determine the most appropriate technique that may provide an accurate diagnostic yield with lowest incidence of adverse events.
Supplementary Material
Supplementary Figure 2: Meta-regression analysis for diagnostic yield by MDD based on the forced vital capacity percent of predicted (FVC%) showing increased diagnostic yield with higher FVC% (P=0.005)
Supplementary Figure 1: Forest plot of diagnostic yield for a histopathological diagnosis
Supplementary Figure 3: Forest plot for event rate of pneumothorax requiring drainage by a thoracostomy tube
Supplementary Figure 4 Meta-regression analysis for pneumothorax based on the number of biopsies for each procedure shows an increased risk of pneumothorax with the increased number of biopsies (P=0.043).
Supplementary Figure 5: Forest plot for event rate of moderate bleeding
Supplementary Figure 6: Meta-regression analysis for moderate bleeding shows an increased risk of bleeding when more than one segment was biopsied. here was an increased risk of moderate bleeding (P=0.042).
Supplementary Figure 7: Forest plot for event rate of mortality attributed to the procedure
Supplementary Figure 8: Forest plot for event rate of performing surgical lung biopsy for undiagnosed disease.
Abbreviations
- ILD
interstitial lung disease
- TBFB
transbronchial forceps biopsy
- TBLC
transbronchial lung cryobiopsy
- SLB
surgical lung biopsy
- MDD
multidisciplinary discussion
- RCTs
randomized control trials
- DLCO%
and diffusion capacity of carbon monoxide percentage of predicted
- FVC%
forced vital capacity percent of predicted
References
- 1.Guenther A, Krauss E, Tello S, et al. The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res. 2018;19(1):141. doi: 10.1186/s12931-018-0845-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med. 2020;8(2):171–181. doi: 10.1016/S2213-2600(19)30342-X [DOI] [PubMed] [Google Scholar]
- 3.Ryerson CJ, Corte TJ, Lee JS, et al. A Standardized Diagnostic Ontology for Fibrotic Interstitial Lung Disease. An International Working Group Perspective. Am J Respir Crit Care Med. 2017;196(10):1249–1254. doi: 10.1164/rccm.201702-0400PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hariri LP, Roden AC, Chung JH, et al. The Role of Surgical Lung Biopsy in the Diagnosis of Fibrotic Interstitial Lung Disease: Perspective from the Pulmonary Fibrosis Foundation. Ann Am Thorac Soc. Published online May 18, 2021:AnnalsATS.202009–1179FR. doi: 10.1513/AnnalsATS.202009-1179FR [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med. 2016;193(10):1161–1167. doi: 10.1164/rccm.201508-1632OC [DOI] [PubMed] [Google Scholar]
- 6.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 7.Sharp C, McCabe M, Adamali H, Medford AR. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease—a systematic review and cost analysis. QJM. Published online August 13, 2016:hcw142. doi: 10.1093/qjmed/hcw142 [DOI] [PubMed] [Google Scholar]
- 8.Romagnoli M, Colby TV., Berthet J-P, et al. Poor Concordance between Sequential Transbronchial Lung Cryobiopsy and Surgical Lung Biopsy in the Diagnosis of Diffuse Interstitial Lung Diseases. Am J Respir Crit Care Med. 2019;199(10):1249–1256. doi: 10.1164/rccm.201810-1947OC [DOI] [PubMed] [Google Scholar]
- 9.Ravaglia C, Bonifazi M, Wells AU, et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration. 2016;91(3):215–227. doi: 10.1159/000444089 [DOI] [PubMed] [Google Scholar]
- 10.Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Diagnostic Yield and Complications of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease: A Systematic Review and Meta-analysis. Ann Am Thorac Soc. Published online July 28, 2016:AnnalsATS.201606–461SR. doi: 10.1513/AnnalsATS.201606-461SR [DOI] [PubMed] [Google Scholar]
- 11.Iftikhar IH, Alghothani L, Sardi A, Berkowitz D, Musani AI. Transbronchial Lung Cryobiopsy and Video-Assisted Thoracoscopic Lung Biopsy in the Diagnosis of Diffuse Parenchymal Lung Disease: A Meta-analysis of Diagnostic Test Accuracy. Ann Am Thorac Soc. Published online April 11, 2017:AnnalsATS.201701–086SR. doi: 10.1513/AnnalsATS.201701-086SR [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 14.Dhooria S, Mehta RM, Srinivasan A, et al. The safety and efficacy of different methods for obtaining transbronchial lung cryobiopsy in diffuse lung diseases. Clin Respir J. 2018;12(4):1711–1720. doi: 10.1111/crj.12734 [DOI] [PubMed] [Google Scholar]
- 15.Kronborg-White S, Sritharan SS, Madsen LB, et al. Integration of cryobiopsies for interstitial lung disease diagnosis is a valid and safe diagnostic strategy-experiences based on 250 biopsy procedures. J Thorac Dis. 2021;13(3):1455–1465. doi: 10.21037/jtd-20-2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turan D, Gonca E, Koç AS, et al. Transbronchial cryobiopsy for diagnosing parenchymal lung diseases : real-life experience from a tertiary referral center. 2021;38(1):1–10. doi: 10.36141/svdld.v38i1.11029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra C, Torrego A, Pajares V, et al. Bronchoalveolar lavage complements transbronchial cryobiopsy diagnosis in diffuse interstitial lung diseases. J Bronchol Interv Pulmonol. 2021;00(00). doi: 10.1097/LBR.0000000000000770 [DOI] [PubMed] [Google Scholar]
- 18.Goel MK, Kumar A, Maitra G, et al. Safety and diagnostic yield of transbronchial lung cryobiopsy by flexible bronchoscopy using laryngeal mask airway in diffuse and localized peripheral lung diseases: A single-center retrospective analysis of 326 cases. Lung India. 2021;38(2):109–116. doi: 10.4103/lungindia.lungindia_220_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda T, Nakao A, Igata F, et al. Feasibility, utility, and safety of transbronchial cryobiopsy for interstitial lung diseases in Japan. Multidiscip Respir Med. 2021;16(February 2018):16–19. doi: 10.4081/MRM.2021.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu W jue, Mo HY, Liu H. Transbronchial lung cryobiopsy for connective tissue disease–related interstitial lung disease and interstitial pneumonia with autoimmune features: a single center retrospective case series. Clin Rheumatol. Published online 2021. doi: 10.1007/s10067-021-05671-1 [DOI] [PubMed] [Google Scholar]
- 21.Davidsen JR, Skov IR, Louw IG, Laursen CB. Implementation of transbronchial lung cryobiopsy in a tertiary referral center for interstitial lung diseases: a cohort study on diagnostic yield, complications, and learning curves. BMC Pulm Med. 2021;21(1):1–10. doi: 10.1186/s12890-021-01438-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou G, Ren Y, Li J, et al. The effect of 1.9-mm versus 2.4-mm probes in transbronchial cryobiopsies for interstitial lung diseases: a prospective analysis. Ann Transl Med. 2021;9(1):20–20. doi: 10.21037/atm-20-4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Mahony AM, Burke L, Cavazza A, Maher MM, Kennedy MP, Henry MT. Transbronchial lung cryobiopsy (TBLC) in the diagnosis of interstitial lung disease: experience of first 100 cases performed under conscious sedation with flexible bronchoscope. Ir J Med Sci. Published online 2021. doi: 10.1007/s11845-020-02453-7 [DOI] [PubMed] [Google Scholar]
- 24.Inomata M, Kuse N, Awano N, et al. Prospective multicentre study on the safety and utility of transbronchial lung cryobiopsy with endobronchial balloon. ERJ Open Res. 2020;6(2):00008–02020. doi: 10.1183/23120541.00008-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shkeiri R, Schneer S, Avarmovich A, Adir Y. Transbronchial Cryobiopsy in Diffuse Parenchymal Lung Diseases in a Community Medical Center. Isr Med Assoc J. 2020;22(12):781–783. [PubMed] [Google Scholar]
- 26.Koslow M, Edell ES, Midthun DE, et al. Bronchoscopic Cryobiopsy and Forceps Biopsy for the Diagnostic Evaluation of Diffuse Parenchymal Lung Disease in Clinical Practice. Mayo Clin Proc Innov Qual Outcomes. 2020;4(5):565–574. doi: 10.1016/j.mayocpiqo.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pajares V, Núñez-Delgado M, Bonet G, et al. Transbronchial biopsy results according to diffuse interstitial lung disease classification. Cryobiopsy versus forceps: MULTICRIO study. PLoS One. 2020;15(9 September):1–13. doi: 10.1371/journal.pone.0239114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maritano Furcada J, Castro R, Precerutti JA, et al. [Cryobiopsy in interstitial lung disease: a real-life experience from a single center in Argentina]. Medicina (B Aires). 2020;80(6):663–669. [PubMed] [Google Scholar]
- 29.Hetzel J, Wells AU, Costabel U, et al. Transbronchial cryobiopsy increases diagnostic confidence in interstitial lung disease: A prospective multicentre trial. Eur Respir J. 2020;56(6). doi: 10.1183/13993003.01520-2019 [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Xu J, Liu C, et al. The significance of multidisciplinary classifications based on transbronchial pathology in possible idiopathic interstitial pneumonias. Medicine (Baltimore). 2020;99(28):e20930. doi: 10.1097/MD.0000000000020930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.She S, Steinfort DP, Ing AJ, et al. Transbronchial Cryobiopsy in Interstitial Lung Disease: Safety of a Standardized Procedure. J Bronchol Interv Pulmonol. 2020;27(1):36–41. doi: 10.1097/LBR.0000000000000633 [DOI] [PubMed] [Google Scholar]
- 32.Çirak AK, Katgi N, Erer OF, Çimen P, Tuksavul FF, Hakoğlu B. Diagnostic approach in parenchymal lung diseases: Transbronchial lung biopsy or cryobiopsy? Turkish J Med Sci. 2020;50(6):1535–1539. doi: 10.3906/sag-1910-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shafiek H, Elbialy S, El Achy SN, Gad AYS. Transbronchial cryobiopsy validity in diagnosing diffuse parenchymal lung diseases in Egyptian population. J Multidiscip Healthc. 2019;12:719–726. doi: 10.2147/JMDH.S208824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harari S, Cereda F, Pane F, et al. Lung cryobiopsy for the diagnosis of interstitial lung diseases: A series contribution to a debated procedure. Med. 2019;55(9). doi: 10.3390/medicina55090606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camuset J, Naccache JM, Dhalluin X, et al. Cryobiopsies trans-bronchiques au cours des pneumopathies interstielles diffuses – expériences préliminaires. Rev Mal Respir. 2019;36(4):455–460. doi: 10.1016/j.rmr.2018.10.618 [DOI] [PubMed] [Google Scholar]
- 36.Hagmeyer L, Theegarten D, Wohlschläger J, et al. Transbronchial cryobiopsy in fibrosing interstitial lung disease: Modifications of the procedure lead to risk reduction. Thorax. 2019;74(7):711–714. doi: 10.1136/thoraxjnl-2018-212095 [DOI] [PubMed] [Google Scholar]
- 37.Kuse N, Inomata M, Awano N, et al. Management and utility of transbronchial lung cryobiopsy in Japan. Respir Investig. 2019;57(3):245–251. doi: 10.1016/j.resinv.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 38.Wälscher J, Gro B, Eberhardt R, et al. Transbronchial Cryobiopsies for Diagnosing Interstitial Lung Disease: Real-Life Experience from a Tertiary Referral Center for Interstitial Lung Disease. Respiration. 2019;97(4):348–354. doi: 10.1159/000493428 [DOI] [PubMed] [Google Scholar]
- 39.Ravaglia C, Wells AU, Tomassetti S, et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: A large cohort of 699 patients. BMC Pulm Med. 2019;19(1):1–10. doi: 10.1186/s12890-019-0780-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen XB, Luo Q, Chen Y, et al. [The efficacy and safety of transbronchial lung cryobiopsy in interstitial lung disease: a prospective study]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese J Tuberc Respir Dis. 2018;41(6):467–471. doi: 10.3760/cma.j.issn.1001-0939.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 41.Lentz RJ, Taylor TM, Kropski JA, et al. Utility of Flexible Bronchoscopic Cryobiopsy for Diagnosis of Diffuse Parenchymal Lung Diseases. J Bronchol Interv Pulmonol. 2018;25(2):88–96. doi: 10.1097/LBR.0000000000000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooley J, Balestra R, Aragaki-Nakahodo AA, et al. Safety of performing transbronchial lung cryobiopsy on hospitalized patients with interstitial lung disease. Respir Med. 2018;140:71–76. doi: 10.1016/j.rmed.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 43.Cho R, Zamora F, Gibson H, Dincer HE. Transbronchial Lung Cryobiopsy in the Diagnosis of Interstitial Lung Disease. J Bronchology Interv Pulmonol. 2019;26(1):15–21. doi: 10.1097/LBR.0000000000000514 [DOI] [PubMed] [Google Scholar]
- 44.Li YS, Guo SL, Yi XH, et al. [Efficacy and safety of transbronchial cryobiopsy in the etiologic diagnosis of diffuse lung disease]. Zhonghua Yi Xue Za Zhi. 2017;97(46):3617–3623. doi: 10.3760/cma.j.issn.0376-2491.2017.46.004 [DOI] [PubMed] [Google Scholar]
- 45.Bondue B, Pieters T, Alexander P, et al. Role of transbronchial lung cryobiopsies in diffuse parenchymal lung diseases: Interest of a sequential approach. Pulm Med. 2017;2017. doi: 10.1155/2017/6794343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bango-Álvarez A, Ariza-Prota M, Torres-Rivas H, et al. Transbronchial cryobiopsy in interstitial lung disease: Experience in 106 cases –how to do it. ERS Monogr. 2017;3(1). doi: 10.1183/23120541.00148-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiBardino DM, Haas AR, Lanfranco AR, Litzky LA, Sterman D, Bessich JL. High complication rate after introduction of transbronchial cryobiopsy into clinical practice at an academic medical center. Ann Am Thorac Soc. 2017;14(6):851–857. doi: 10.1513/AnnalsATS.201610-829OC [DOI] [PubMed] [Google Scholar]
- 48.Cascante JA, Cebollero P, Herrero S, et al. Transbronchial cryobiopsy in interstitial lung disease are we on the right path? J Bronchol Interv Pulmonol. 2016;23(3):204–209. doi: 10.1097/LBR.0000000000000292 [DOI] [PubMed] [Google Scholar]
- 49.Echevarria-Uraga JJ, Pérez-Izquierdo J, García-Garai N, et al. Usefulness of an angioplasty balloon as selective bronchial blockade device after transbronchial cryobiopsy. Respirology. 2016;21(6):1094–1099. doi: 10.1111/resp.12827 [DOI] [PubMed] [Google Scholar]
- 50.Ramaswamy A, Homer R, Killam J, et al. Comparison of Transbronchial and Cryobiopsies in Evaluation of Diffuse Parenchymal Lung Disease. J Bronchology Interv Pulmonol. 2016;23(1):14–21. doi: 10.1097/LBR.0000000000000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pajares V, Puzo C, Castillo D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: A randomized trial. Respirology. 2014;19(6):900–906. doi: 10.1111/resp.12322 [DOI] [PubMed] [Google Scholar]
- 52.Fruchter O, Fridel L, El Raouf BA, Abdel-Rahman N, Rosengarten D, Kramer MR. Histological diagnosis of interstitial lung diseases by cryo-transbronchial biopsy. Respirology. 2014;19(5):683–688. doi: 10.1111/resp.12296 [DOI] [PubMed] [Google Scholar]
- 53.Griff S, Schonfeld N, Ammenwerth W, et al. Diagnostic yield of transbronchial cryobiopsy in non-neoplastic lung disease: A retrospective case series. BMC Pulm Med. 2014;14(1):1–6. doi: 10.1186/1471-2466-14-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalvi P, Kretz G, Clum S, et al. Transbronchial Lung Cryobiopsy (TBLC) at a Tertiary Referral Hospital: A Two-Year Experience. Pulm Crit Care Med. 2016;2(1):1–4. doi: 10.15761/pccm.1000122 [DOI] [Google Scholar]
- 55.Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: A new tool for lung biopsies. Respiration. 2009;78(2):203–208. doi: 10.1159/000203987 [DOI] [PubMed] [Google Scholar]
- 56.Hernández-González F, Lucena CM, Ramírez J, et al. Cryobiopsy in the Diagnosis of Diffuse Interstitial Lung Disease: Yield and Cost-Effectiveness Analysis. Arch Bronconeumol. 2015;51(6):261–267. doi: 10.1016/j.arbr.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 57.Asadi Gharabaghi M, Idani E, Mohammadi F, et al. Should we perform surgical lung biopsy when transbronchial cryobiopsy provides unclassifiable interstitial pneumonitis in a patient with diffuse interstitial lung disease? Published online 2020:1577. doi: 10.1183/13993003.congress-2020.1577 [DOI]
- 58.Kalpakam H, Bhat R, Bansal S, Bajaj P, Mehta R. Transbronchial Lung Cryobiopsy (TBLC) for Interstitial Lung Disease (ILD) can be safely done in patients with multiple medical comorbidities. 2020;(Ild):1580. doi: 10.1183/13993003.congress-2020.1580 [DOI] [Google Scholar]
- 59.Romero A, Ivanick N, Lum M, Krishna G. Cryobiopsy With Cone Beam Computerized Tomography for Diffuse Parenchymal Lung Disease. Chest. 2020;158(4):A1938. doi: 10.1016/j.chest.2020.08.1677 [DOI] [Google Scholar]
- 60.Ronaghi R, He T, Oberg C, et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in the Diagnosis of Interstitial Lung Disease. Chest. 2020;158(4):A1941–A1942. doi: 10.1016/j.chest.2020.08.1680 [DOI] [Google Scholar]
- 61.Noirez L, Von Garnier C, Lovis A. Transbronchial lung cryobiopsies: A single-center interventional pulmonology experience with first 25 cases. Respiration. 2020;99(8):725. 10.1159/000508318 [DOI] [Google Scholar]
- 62.Hostettler KE, Savic S, Bubendorf L, et al. Diagnostic value of cryobiopsy in patients with interstitial lung diseases integrating bronchoalveolar lavage, radiologic and clinical data. Published online 2020:789. doi: 10.1183/13993003.congress-2020.789 [DOI]
- 63.Daniels JMA, Nijman SFM, Ruiter G, Nossent EJ. Transbronchial lung cryobiopsy in interstitial lung disease: safety & learning curve. Published online 2020:1190. doi: 10.1183/13993003.congress-2020.1190 [DOI]
- 64.Strumiliene E, Gruslys V. Complications - based learning curve of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases. Published online 2020:1191. doi: 10.1183/13993003.congress-2020.1191 [DOI] [Google Scholar]
- 65.Niwa T, Baba T, Murotani K, et al. Safety and learning curve for Transbronchial Lung Cryobiopsy. Published online 2019:PA1323. doi: 10.1183/13993003.congress-2019.pa1323 [DOI]
- 66.Fonseca Ferreira Santos GS, Gomes R, Lopes M, Grumete H, Soares J, Duarte J. Transbronchial lung cryobiopsy (TBLC) and bronchoalveolar lavage (BAL) in diagnosis of Intersticial Lung Diseases (ILD). 2019;(Ild):PA3104. doi: 10.1183/13993003.congress-2019.pa3104 [DOI] [Google Scholar]
- 67.Arbat S, Arbat A, Bakamwar S, Deshpande P, Agrawal B, Niranjane V. Diagnostic yield and safety profile of cryobiopsy in various lung pathologies. Published online 2019:PA3108. doi: 10.1183/13993003.congress-2019.pa3108 [DOI] [Google Scholar]
- 68.Gaga M, Samitas K, Kolilekas L, Vamvakaris I, Gkogkou C, Filippousis P ZE. Introducing transbronchial cryobiopsies in diagnosing diffuse parenchymal lung diseases in Greece: Implementing training into clinical practice. Am J Respir Crit Care Med. 2019;199:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gnass M, Czyzewski D, Filarecka A, Orzechowski S, Bartczak A SJ, Szotkowska M, Langfort RSA. Transbronchial lung cryobiopsy under mini-probe EBUS guidance for interstitial lung diseases-multicenter Polish study. Eur Respir J. 2019;54 Supplem:3–4. [Google Scholar]
- 70.Guo S, Ao Z, Zeng W, et al. Transbronchial cryobiopsy in the multidisciplinary diagnosis of pneumoconiosis. Published online 2019:PA3106. doi: 10.1183/13993003.congress-2019.pa3106 [DOI] [Google Scholar]
- 71.Pereira NFTC, Machado D, Sucena I, Coutinho D, Nogueira C MI, Sanches A, Oliveira A, Almeida J, Campainha S NS. Should we biopsy one or two lobes?-diagnostic yield and risks of transbronchial lung cryobiopsy. Eur Respir J. 2019;54 Supplem:2021. [Google Scholar]
- 72.Vlacic G, Kern I RA. Transbronchial cryobiopsy : A single-center experience. Virchows Arch. 2018;473 Supple:1–2. [Google Scholar]
- 73.Hećimović A, Badovinac S, Roglić M, et al. Single centre experience in safety and diagnostic yield of cryobiopsy. Published online 2018:PA3381. doi: 10.1183/13993003.congress-2018.pa3381 [DOI]
- 74.C. L, J. D, B. D, D. L, R. M, A. R. Transbronchial lung cryobiopsy (TBLC) in the diagnosis of interstitial lung disease: Diagnostic yield, complication rate and cost-effectiveness. Am J Respir Crit Care Med. 2018;197(MeetingAbstracts):30–31. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L622968103 [Google Scholar]
- 75.Fernandes LSCS, Antunes C, Machado A, Brysch E, Abreu T, Mota L, Semedo J, Ferreira L, Ribeiro A BC. Transbronchial cryobiopsy protocol and diagnostic yield in diffuse parenchymal lung disease: A retrospective study. Eur Respir J. 2018;52(Supplement 62). [Google Scholar]
- 76.Colella S, Massaccesi C, Fioretti F, et al. Transbronchial Lung cryobiopsy in lung diseases: diagnostic yield and safety. Published online 2017:PA3025. doi: 10.1183/1393003.congress-2017.pa3025 [DOI]
- 77.Tan M, Hnin K, Grosser D, Smith B. Diagnostic yield and safety of transbronchial lung cryobiopsy for diagnosis of interstitial lung disease. Published online 2017:PA3020. doi: 10.1183/1393003.congress-2017.pa3020 [DOI]
- 78.Ehab A, El-Badrawy MK, Moawad AA, Abo-Shehata ME. Efficacy and safety of transbronchial cryobiopsy in interstitial lung diseases. Published online 2017:PA3021. doi: 10.1183/1393003.congress-2017.pa3021 [DOI]
- 79.A. B, R. R, D.W. K, et al. Transbronchial cryobiopsy for the diagnosis of interstitial lung diseases: Diagnostic yield, outcomes and risk factors for complications. Am J Respir Crit Care Med. 2017;195:5–6. 10.1164/ajrccm-conference.2017.A2428035851 [DOI] [Google Scholar]
- 80.Jabbardarjani H, Kiani A, Karimi M FM. Cryobiopsy shows better safety profile and diagnostic yield as compared to conventional forceps for interstitial lung disease. Eur Respir J. 2013;42(Suppl 57):P2301. [Google Scholar]
- 81.Bourdin A, Suehs CM, Colby TV., Vachier I, Molinari N, Romagnoli M. Reply to Wand et al. : Role of Transbronchial Cryobiopsy in Interstitial Lung Diseases: An Ongoing Tale. Am J Respir Crit Care Med. 2020;201(2):260–261. doi: 10.1164/rccm.201909-1736LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maldonado F, Danoff SK, Wells AU, et al. Transbronchial Cryobiopsy for the Diagnosis of Interstitial Lung Diseases: CHEST Guideline and Expert Panel Report. Chest. 2020;157(4):1030–1042. doi: 10.1016/j.chest.2019.10.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 2: Meta-regression analysis for diagnostic yield by MDD based on the forced vital capacity percent of predicted (FVC%) showing increased diagnostic yield with higher FVC% (P=0.005)
Supplementary Figure 1: Forest plot of diagnostic yield for a histopathological diagnosis
Supplementary Figure 3: Forest plot for event rate of pneumothorax requiring drainage by a thoracostomy tube
Supplementary Figure 4 Meta-regression analysis for pneumothorax based on the number of biopsies for each procedure shows an increased risk of pneumothorax with the increased number of biopsies (P=0.043).
Supplementary Figure 5: Forest plot for event rate of moderate bleeding
Supplementary Figure 6: Meta-regression analysis for moderate bleeding shows an increased risk of bleeding when more than one segment was biopsied. here was an increased risk of moderate bleeding (P=0.042).
Supplementary Figure 7: Forest plot for event rate of mortality attributed to the procedure
Supplementary Figure 8: Forest plot for event rate of performing surgical lung biopsy for undiagnosed disease.


