Abstract
Atrial fibrillation disrupts contraction of the atria, leading to stroke and heart failure. We deciphered how immune and stromal cells contribute to atrial fibrillation. Single-cell transcriptomes from human atria documented inflammatory monocyte and SPP1+ macrophage expansion in atrial fibrillation. Combining hypertension, obesity, and mitral valve regurgitation (HOMER) in mice elicited enlarged, fibrosed, and fibrillation-prone atria. Single-cell transcriptomes from HOMER mouse atria recapitulated cell composition and transcriptome changes observed in patients. Inhibiting monocyte migration reduced arrhythmia in Ccr2−/− HOMER mice. Cell-cell interaction analysis identified SPP1 as a pleiotropic signal that promotes atrial fibrillation through cross-talk with local immune and stromal cells. Deleting Spp1 reduced atrial fibrillation in HOMER mice. These results identify SPP1+ macrophages as targets for immunotherapy in atrial fibrillation.
One-Sentence Summary
Using scRNA-seq, we identify a macrophage culprit subset in atrial fibrillation, the world’s most common rhythm disorder.
In healthy atria, excitations arising in the sinus node coordinate atrial contractions that help fill the ventricles in diastole. Cardiomyocytes constitute 30% of atrial cells (1). The remaining 70% of atrial cells are fibroblasts, endothelial cells, and resident immune cells. Together, these provide cardiomyocytes with housekeeping functions, including matrix scaffold, blood supply, waste removal, and immunosurveillance (2, 3). Atrial fibrillation (AFib) is the most common arrhythmia (4). During AFib, the lack of coordinated atrial excitation and contraction slows blood flow into the ventricles. This sluggishness can diminish cardiac performance and give rise to atrial blood clots that cause thromboembolic stroke.
Inflammation may contribute to AFib through electrical remodeling (5-7), during which altered ion handling perturbs the action potential (8). Fibrosis, another consequence of inflammation, induces heterogeneous atrial depolarization, which provides an opportunity for electrical conduction reentry and subsequent AFib (8, 9). To examine how immune and stromal cells contribute to AFib, we performed single-cell RNA-sequencing (scRNA-seq) of freshly isolated normal and diseased human atrial tissue.
Macrophages expand in human atrial disease
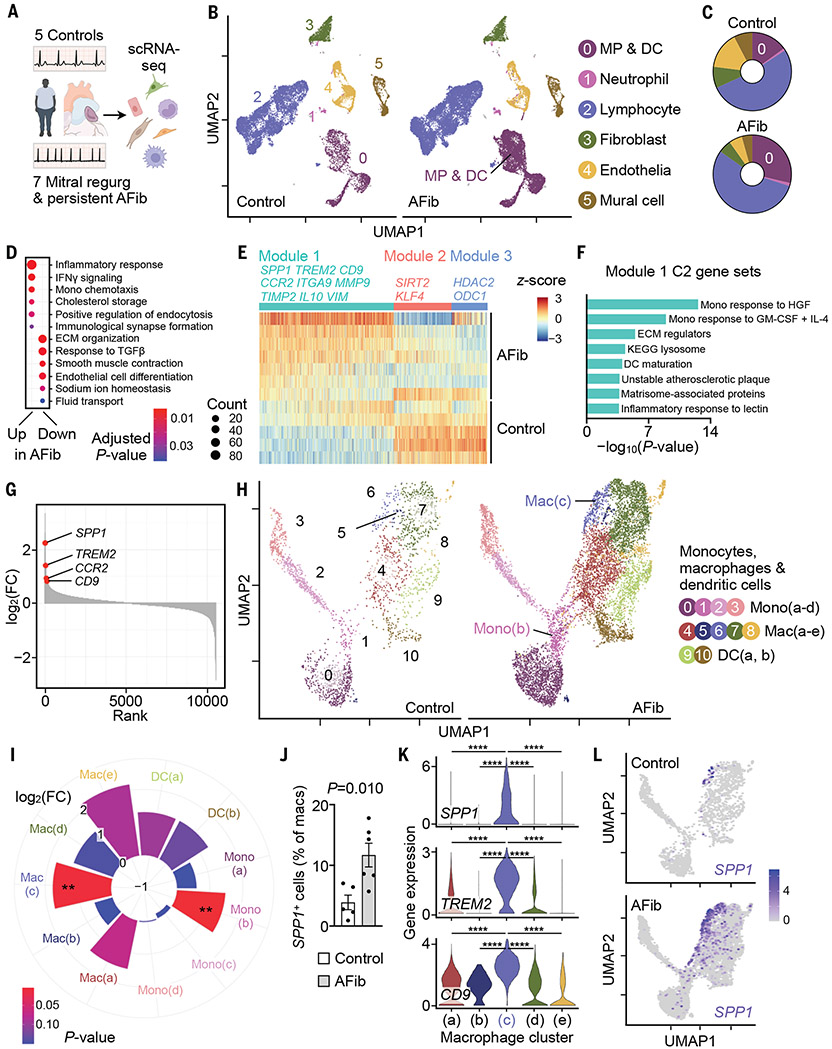
We collected left atrial tissue from five control participants and seven patients with persistent AFib undergoing heart surgery (table S1) and performed scRNA-seq on the 10X Genomics Chromium platform (Fig. 1A and fig. S1A). A total of 41,609 viable, single cells from both patients with AFib and controls were partitioned into clusters (fig. S1B), which we annotated using known lineage markers (fig. S1C). We identified six major noncardiomyocyte cell types, which included (in order of decreasing frequency) lymphocytes, mononuclear phagocytes and dendritic cells (MP/DCs), endothelial cells, fibroblasts, mural cells, and neutrophils (Fig. 1, B and C; fig. S2; and table S2).
Fig. 1. Single-cell atlas of human atrial disease.
(A) scRNA-seq was performed in five controls and seven patients with AFib. (B) UMAP delineates six major cardiac cell types. (C) Distribution of cell populations. Color code corresponds to (B). (D) Gene ontology biological process (GOBP) gene sets (48, 49) up- or down-regulated in AFib from GSEA of MP/DC cluster. Circle size denotes number of enriched genes. ECM, extracellular matrix. (E) Gene expression in MP/DC cluster represented by z-scores of the log-transformed normalized counts. Rows, samples grouped by disease state; columns, genes in the three gene modules significantly associated with disease state; gene coexpression modules and exemplary genes are labeled. (F) Select MSigDB C2 gene sets (50) overrepresented in module 1 described in (E). GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; IL-4, interleukin-4; KEGG, Kyoto Encyclopedia of Genes and Genomes. (G) Genes in MP/DC cluster ranked according to log2-fold changes [log2(FC)] between seven patients with AFib and five controls. (H) Subclustering of 10,555 MP/DCs from five controls and seven patients with AFib. (I) Difference in abundance of cells belonging to MP/DC subclusters between seven patients with AFib and five control patients. Pie size indicates log2(FC); color (blue, high; red, low) indicates the P value (two-tailed Student’s t test). **P < 0.01. (J) Fraction of SPP1+ macrophages in controls and patients with AFib. Bar graph shows mean ± SEM, n = 5 to 6 per group, two-tailed Student’s t test. (K) Gene expression levels (represented by log-transformed normalized counts) across macrophage clusters; Kruskal–Wallis (P < 2.2 × 10−16) followed by two-tailed Wilcoxon rank sum test. ****Adjusted P = 2 × 10−16. (L) SPP1 expression in MP/DCs from controls and patients with AFib.
In diseased atria, the MP/DC cluster expanded twofold, whereas endothelia and mural cells decreased in frequency. The other cell classes remained stable (Fig. 1C and table S2). Because the MP/DC cluster increased considerably, we scrutinized the transcriptomes of these cells more closely. We identified genes that were differentially expressed in MP/DCs from AFib and control atria with a pseudo-bulk approach that aggregated cells from the same individual to determine differential gene expression between the two patient groups (10). We then performed gene set enrichment analysis (GSEA) (11) on the resulting list of differentially expressed genes to define the pathways and functional gene groups that were altered in MP/DCs from patients with AFib. In this disease setting, MP/DCs up-regulated gene sets related to the inflammatory response, interferon-γ (IFN-γ) signaling, monocyte recruitment, and cholesterol metabolism (Fig. 1D and data S1). Thus, atrial MP/DCs undergo substantial immunometabolic remodeling during their expansion in patients with AFib.
To gain a broader understanding of the transcriptional programs that are activated or repressed in MP/DCs, we used weighted correlation network analysis to study groups of correlated genes that were differentially expressed between patients and controls (12). We identified 24 modules of correlated genes across all participants, three of which were significantly correlated with disease state. Module 1 was up-regulated in AFib and contained inflammatory and profibrotic genes such as CCR2, IL10, ITGA9, MMP9, SPP1, TIMP2, and VIM (Fig. 1E and data S2) (13-15).
Overrepresentation analysis confirmed that inflammatory and extracellular matrix-related gene sets characterized module 1 (Fig. 1F and data S2). Module 1 genes SPP1 and CCR2 and the cell surface markers TREM2 and CD9 were among the top up-regulated genes in MP/DCs from patients with AFib (Fig. 1G). SPP1 encodes the matricellular protein osteopontin, which participates in bone remodeling and fibrosis by promoting cell survival, adhesion, and migration as well as inflammatory cell activation (13). The chemokine receptor CCR2 mediates monocyte egress from the bone marrow (16) and is essential for monocyte recruitment (14). Gene modules 2 and 3, whose expression was down-regulated in MP/DCs from patients with AFib, included genes involved in protecting against cardiac disease [SIRT2 (17)], cardiac resident macrophage proliferation [KLF4 (18)], and epigenetic constraint of cytokine production [HDAC2 (19) and ODC1 (20)] (Fig. 1E and data S2). These data imply that the atria of patients with AFib have elevated numbers of myeloid cells that assume a more inflammatory phenotype, implicating macrophages in the tissue remodeling that disrupts coordinated excitation of atrial cardiomyocytes. Other leukocytes and stromal cells also altered their gene expression (fig. S3 and data S3 and S4). Fibroblasts, for example, responded by up-regulating inflammatory pathways and matrix production (fig. S3C). In endothelial and mural cells, we observed up-regulation of genes indicative of ischemia and vascular remodeling (fig. S4).
Depending on their origin and microenvironment, macrophages and their subsets take on phenotypes that enable a range of diverging and even opposing functions (3, 21). To identify cell types that contribute to atrial remodeling, we examined atrial myeloid cells in detail. We reclustered MP/DCs, choosing a resolution that maximized the number of differentially expressed genes between subclusters (fig. S5, A and B). Reclustering separated three monocyte subsets, an established number in human blood (21), and a fourth inflammatory monocyte cluster whose expression profile (fig. S5C and data S5) and uniform manifold approximation and projection (UMAP) position adjacent to macrophages (Fig. 1H) suggested an intermediate phenotype during monocyte-to-macrophage differentiation. At this resolution, we could distinguish five macrophage clusters (Fig. 1H, figs. S5C and S6, and data S6) and two DC clusters (Fig. 1H and fig. S5C). The inflammatory mono(b) cluster and the SPP1+ mac(c) cluster expanded 2.8- and 3.4-fold, respectively, in diseased atria (Fig. 1I). The frequency of SPP1+ atrial cells increased in patients with AFib when compared with non-AFib controls (Fig. 1J). The expanding mac(c) cluster was the dominant SPP1 source in atria of patients with AFib (Fig. 1, K and L); other immune and stromal cells did not express SPP1 at high levels (fig. S7). TREM2 and CD9, which encode proteins that can be detected by cell surface staining (22), further distinguished the SPP1+ mac(c) cluster from other cells (Fig. 1K and fig. S7). RNA in situ hybridization confirmed that macrophages express SPP1 in atria of patients with AFib (fig. S8, A and B). Once secreted by macrophages, SPP1 associates with extracellular matrix (13). Accordingly, immunofluorescence microscopy identified expanding colocalization of SPP1 protein with collagen in patients with AFib (fig. S8, C and D).
To explore atrial macrophage expansion in a larger cohort, we used immunofluorescence histology on left atrial appendages of 108 patients with AFib and/or mitral regurgitation and 41 controls in sinus rhythm (table S3). Atrial macrophages and their monocyte-derived CCR2+ subset expanded most in patients with AFib and mitral regurgitation (fig. S8, E and F). In patients who had mitral regurgitation without AFib or AFib without mitral regurgitation, macrophages expanded to a lesser degree (fig. S8, E and F). In patients who had AFib without mitral regurgitation, AFib chronicity was less pronounced (table S3), which may explain why we observed fewer atrial macrophages in this cohort.
A mouse model of atrial disease
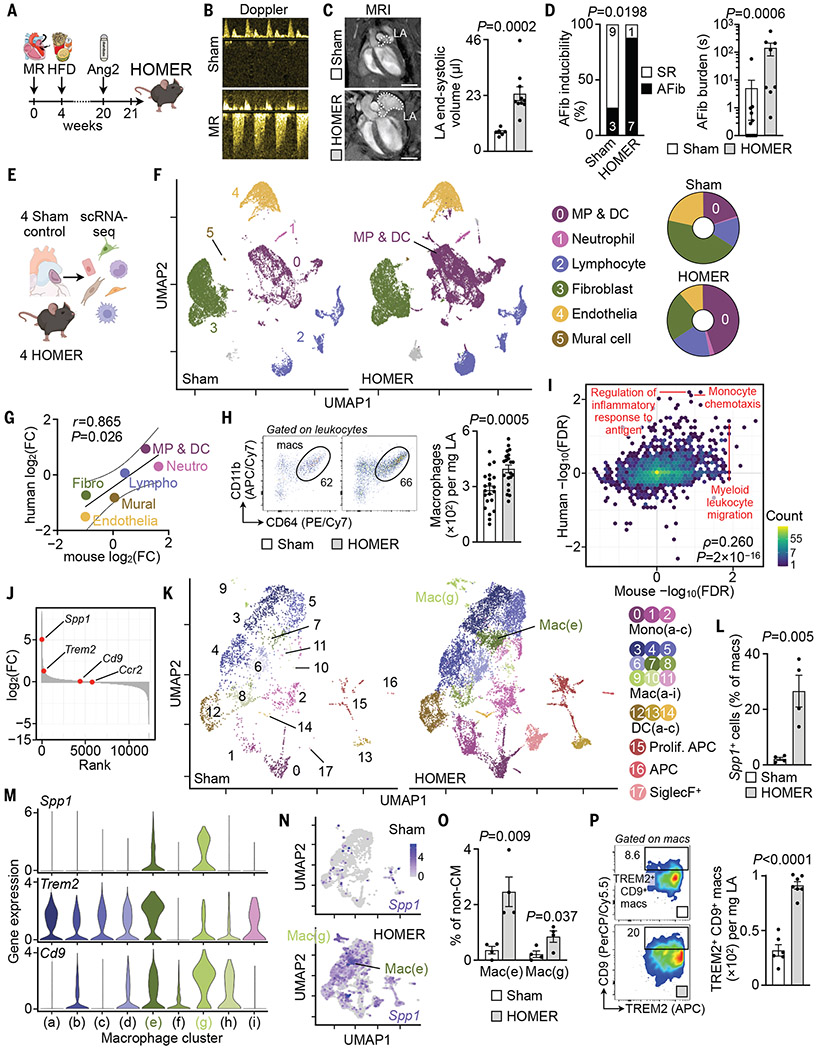
To dissect immune pathways involved in human AFib mechanistically, we used a mouse model that combined hypertension, obesity, and mitral valve regurgitation (HOMER) (Fig. 2A). Snaring the mitral valve to the ventricular wall produced mitral valve regurgitation (Fig. 2B). Mice consumed an obesogenic diet for 4 months, mirroring the risk that obesity engenders for AFib (23). Hypertension, also a risk factor for AFib, was induced with angiotensin 2 (fig. S9). The HOMER model elicited left atrial enlargement (Fig. 2C) commonly found in patients with AFib (24). HOMER mice developed increased AFib inducibility and burden compared with those of sham-operated normotensive control mice that consumed standard chow (hereafter referred to as controls) (Fig. 2D and fig. S10). Among individual HOMER components, obesity elicited the highest AFib inducibility, whereas mitral valve regurgitation enlarged atria the most (fig. S11). Spontaneous AFib episodes, which were monitored with electrocardiogram telemeters, were absent in HOMER mice. In HOMER hearts, left ventricles hypertrophied and dilated mildly, and left ventricular systolic function was slightly reduced (fig. S12A and table S4). Blood leukocyte counts, including Ly6Chi monocytes, rose in HOMER mice (fig. S12, B and C). Histological analysis revealed more numerous macrophages, fibrosis, and collagen deposition in HOMER atria compared with those in controls (fig. S12, D and E), results that align with the fibrosis observed in patients with AFib (25) and the effects of hypertension and metabolic stress on the ventricles (15, 26, 27). Hypertension and mitral valve regurgitation most clearly increased atrial macrophage numbers and fibrosis (fig. S13).
Fig. 2. Single-cell atlas of mouse atria.
(A) HOMER mice are exposed to mitral valve regurgitation (MR), high-fat diet (HFD), and angiotensin 2 (Ang2). (B) Doppler of MR. (C) (Left) MRI of left atrium (LA). Scale bars, 3 mm. (Right) LA end-systolic volume, n = 6 to 10 per group from two independent experiments, two-tailed Mann–Whitney test. (D) AFib inducibility and burden. n = 8 to 12 per group from two independent experiments. (Left) Two-sided Fisher’s exact test. (Right) Two-tailed Mann–Whitney test. Other parameters are shown in table S7. SR, sinus rhythm. (E) scRNA-seq was performed in four sham and four HOMER mice. (F) (Left) UMAP. (Right) Cell population distributions. (G) Log2(FC) of cell abundance in mouse and human atria. (H) Flow cytometry of sham and HOMER atria. n = 21 per group from five independent experiments, two-tailed Student’s t test. APC/Cy7, allophycocyanin/cyanine 7; PE/Cy7, phycoerythrin/cyanine 7. (I) Scatterplot showing C5 GOBP GSEA species concordance. GSEA is performed on lists of genes ranked according to differential expression in the disease state. −log10(FDR) is plotted for mouse (x axis) and human (y axis). −log10(FDR) are negative if the gene set is down-regulated. Yellow indicates high and blue indicates low gene set density. (J) Genes in MP/DC cluster ranked according to log2(FC) between four HOMER and four sham mice. The y axis was broken at −5 for displaying the top down-regulated genes. (K) Subclustering of MP/DCs from four sham and four HOMER mice. APC, antigen-presenting cell. (L) Atrial Spp1+ macrophages by scRNA-seq. n = 4 per group, two-tailed Student’s t test. (M) Gene expression levels (represented by log-transformed normalized counts) across macrophage clusters. (N) Spp1 expression in MP/DCs from sham and HOMER mice. (O) Spp1+ subpopulations in sham and HOMER mice. n = 4 per group, two-tailed Student’s t test. non-CM, noncardiomyocyte. (P) Flow cytometry of atrial macrophages in sham and HOMER mice. n = 6 to 7 per group from two independent experiments, two-tailed Student’s t test. PerCP/Cy5.5, peridinin-chlorophyll-protein/cyanine 5.5. All bar graph data are mean ± SEM with individual values for data distribution.
Replicating our human scRNA-seq pipeline by using left atrial tissues dissected from four HOMER mice and four control animals (Fig. 2E and fig. S14A), we successfully obtained 40,112 viable single cells, among which we identified the same cell types as in human atria (Fig. 2F; fig. S14, B and C; and table S5). The cellular composition changes in diseased atria correlated strongly between both species [Pearson’s correlation coefficient (r) = 0.87] (Fig. 2G). We observed a twofold expansion of MP/DCs in HOMER mice similar to that in the human disease setting (Fig. 2F and table S5). Flow cytometry confirmed the HOMER-induced changes in the abundance of macrophages (Fig. 2H and fig. S15, A to C). Cardiac macrophage subsets can be distinguished by expression of CCR2 and major histocompatibility complex II (MHCII) (28, 29). In atria of HOMER mice, the number of CCR2+ macrophages and monocytes rose (fig. S15, D and E).
We next examined the gene expression changes that occurred in MP/DCs of HOMER mice in relation to our human data. As before, we first performed GSEA on differentially expressed genes from a pseudo-bulk analysis comparing MP/DCs isolated from atria of HOMER or control mice. We then plotted the significance and direction of change for all Molecular Signatures Database (MSigDB) C5 (Gene Ontology) gene sets against the corresponding values in humans and found significant concordance between the two GSEA data sets [Spearman’s rank correlation coefficient (ρ) = 0.26, P < 2 × 10−16] (Fig. 2I and data S7), indicating that the HOMER model recapitulated the pathway-level gene expression changes observed in patients. As in humans, Spp1 was among the top up-regulated genes in MP/DCs of HOMER atria (Fig. 2J). We then reclustered MP/DCs to obtain a higher-resolution view of these cells, choosing a resolution that separated three monocyte clusters [a number previously described in mouse blood (30)], nine macrophage clusters, three DC clusters, two antigen-presenting cell clusters, and one SiglecF+ cell cluster (Fig. 2K). Spp1+ cell frequencies increased in HOMER atria (Fig. 2L), a rise driven by the macrophage subpopulations mac(e) and mac(g) and not by other immune or stromal cells (Fig. 2, M and N, and fig. S16, A and B). In close concordance with our human findings, Spp1+ mac(e) and mac(g) clusters coexpressed Trem2 and Cd9 (Fig. 2M) and expanded in HOMER mice (Fig. 2, O and P, and fig. S16, C and D). Comparing MP/DC clusters across the two species (using the top 100 genes that distinguished each subcluster), we found that each human MP/DC cluster significantly matched at least one mouse cluster and that the human SPP1+ cluster mac(c) was similar to the mouse Spp1+ cluster mac(e) (fig. S17). This strong interspecies correlation is likely caused by similar risk factors in human patients and HOMER mice.
We next assayed contributions of individual HOMER components. Ccr2 was expressed highest in HOMER atria but also increased in hypertensive mice (fig. S18A). Atrial expression of Csf1, chemokines, adhesion molecules, inflammatory markers, and matrix increased the most in HOMER mice (fig. S18, B to F). Spp1 expression increased in HOMER mice but not after exposure to single components (fig. S18G). Thus, HOMER components have additive (for example, Ccr2 expression) and some synergistic effects (for example, Spp1 expression). Two of three HOMER components elicited intermediate atrial remodeling and Spp1 expression (fig. S19). Although combining all HOMER components reflected the risk factors present in our patient cohort most faithfully, further studies of single conditions (for example, metabolic consequences of high-fat diet on conducting cells) are warranted.
We then contrasted left atrial gene expression changes to the left ventricular myocardium. Unlike the atria, we observed no significant expansion of ventricular leukocyte frequencies in HOMER mice (fig. S20, A to C, and table S6). The decline of fibroblast frequencies that occurred in HOMER atria was absent in the ventricles (fig. S20, A to C, and table S6). Compared with ventricular cells, more genes changed in atrial myeloid cells and fibroblasts. For example, there were 1982 atrial but only 19 ventricular differentially expressed genes [with false discovery rate (FDR) < 0.05] in MP/DCs (with nine differentially regulated genes shared between the atria and ventricles) (fig. S20, D and E, and data S8). This pattern was inversed for endothelial cells, which had more differentially expressed genes in the ventricles than in the atria (fig. S20, D and E, and data S8). A principal component analysis revealed that in controls, atrial MP/DCs were located at a large distance from ventricular MP/DCs. The distance between atrial and ventricular MP/DCs was less pronounced in HOMER mice (fig. S20F). We hypothesize that HOMER-induced activation of inflammatory programs equalizes some of the differences normally imprinted by the atrial versus the ventricular environment. That the HOMER model affects left ventricular leukocytes to a lesser degree than in the left atria corresponds with the more severe atrial than ventricular dilation observed with magnetic resonance imaging (MRI) (Fig. 2C and fig. S12A). The ventricular data reveal that hypertension, high-fat diet, and mitral valve disease affect not only atria but the entire heart, a notion reflected by the clinical association of AFib with heart failure (31).
The generation of the HOMER mouse and its scRNA-seq validation against patients with AFib provided us with a tool to assess whether immune cell expansion merely associates with—or alternatively, contributes to—AFib. We used the HOMER mouse for two follow-up studies that tested causal implications for key observations in patients with AFib: (i) expansion of inflammatory atrial macrophages and (ii) increased atrial SPP1 expression by those macrophages.
CCR2-dependent macrophage recruitment promotes AFib
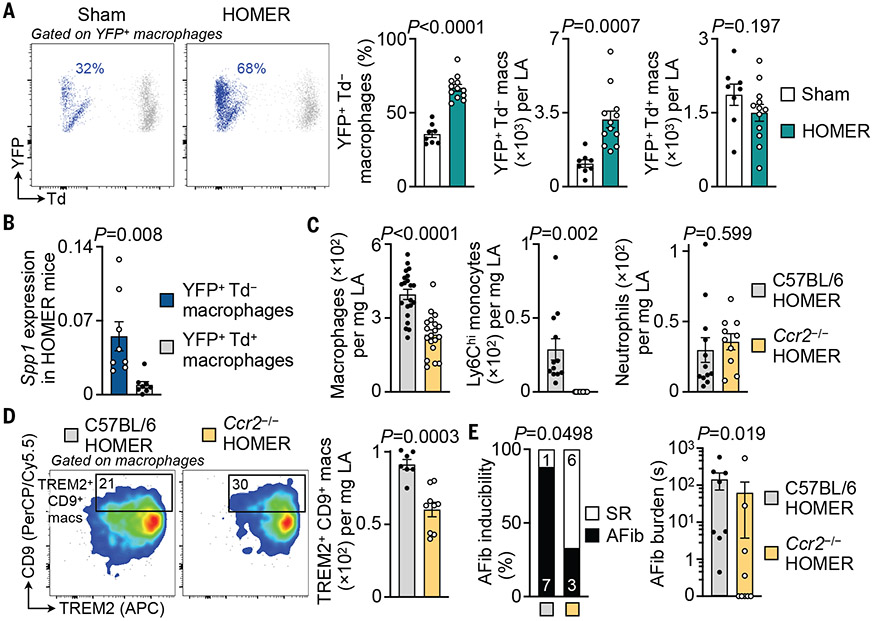
Macrophage ontogeny often dictates function (32). For example, steady-state resident macrophages in the heart and brain are distinctly noninflammatory (33), whereas recruited infarct or stroke macrophages are inflammatory and damage tissues (34). We consequently sought to investigate macrophage origins in normal and diseased atria. Because atrial macrophages expand in diseased atria of HOMER mice (Fig. 2, F and H, and table S5) and enrich for the chemokine receptor CCR2 (fig. S15D), which recruited macrophages express (34), we combined the HOMER procedure with genetic fate mapping in Cx3cr1CreER/+Ai9fl/+ mice (fig. S21A). Although atrial macrophages were more likely to be derived from circulating monocytes than ventricular macrophages, most atrial macrophages were not sourced from monocytes in steady state (fig. S21B). In HOMER mice, recruitment expanded so that 67% of atrial macrophages derived from monocytes, a significant increase from the steady state (36%) (Fig. 3A). In line with increased atrial expression of Csf1 (fig. S18B) and up-regulation of the “Leukocyte proliferation” gene set by MP/DCs (fig. S21C), macrophage proliferation increased in HOMER atria (fig S21, D and E). Recruited macrophages in atria of HOMER mice were the primary source of Spp1, whereas locally sourced macrophages did not express this gene at high levels (Fig. 3B).
Fig. 3. CCR2-dependent monocyte recruitment contributes to left atrial macrophage expansion and atrial disease.
(A) Flow cytometric quantification of recruited (YFP+ Td−) and locally sourced (YFP+ Td+) left atrial macrophages in Cx3cr1CreER/+ Ai9fl/+ sham and HOMER mice. n = 8 to 12 per group from two independent experiments, two-tailed Student’s t test. Td, tdTomato; YFP, yellow fluorescent protein. (B) Relative Spp1 expression levels by qPCR in recruited (YFP+ Td−) and locally sourced (YFP+ Td+) left atrial macrophages in Cx3cr1CreER/+ Ai9fl/+ HOMER mice. n = 8 per group from two independent experiments, two-tailed Wilcoxon matched-pairs signed rank test. (C) Flow cytometric quantification of myeloid cell populations in left atrial tissues from C57BL/6 and Ccr2−/− HOMER mice. Macrophages: 21 C57BL/6 and 19 Ccr2−/− HOMER mice from five independent experiments; monocytes and neutrophils: 12 C57BL/6 and 10 Ccr2−/− HOMER mice from three independent experiments; two-tailed Student’s t test. (D) Flow cytometric quantification of TREM2+ CD9+ left atrial macrophages in C57BL/6 and Ccr2−/− HOMER mice. n = 7 to 9 per group from two independent experiments, two-tailed Student’s t test. (E) AFib inducibility and burden in C57BL/6 and Ccr2−/− HOMER mice by means of electrophysiological (EP) study. n = 8 to 9 per group from two independent experiments. (Left) Two-sided Fisher’s exact test. (Right) Two-tailed Mann–Whitney test. Other EP parameters are shown in table S8. All bar graph data are mean ± SEM with individual values for data distribution.
We used the HOMER procedure in Ccr2−/− mice to test the relevance of expanded macrophage recruitment for AFib. Blocking monocyte migration in Ccr2−/− HOMER mice decreased blood monocyte, atrial Ly6Chi monocyte, and macrophage expansion when compared with C57BL/6 HOMER mice, whereas other cell densities and blood pressure were unaffected (Fig. 3C and fig. S22, A to D). TREM2+ CD9+ macrophages were also lower in Ccr2−/− HOMER mice (Fig. 3D). Cardiac MRI revealed smaller left atrial volumes in Ccr2−/− HOMER mice (fig. S22E). Ccr2−/− HOMER mice exhibited lower AFib inducibility and AFib burden than those of C57BL/6 HOMER mice (Fig. 3E). Thus, macrophage recruitment expands during atrial remodeling, recruited macrophages highly express Spp1, and inhibiting macrophage recruitment reduces AFib.
To test whether CCR2 inhibition could alleviate established disease, we treated mice with the CCR2 antagonist CCL2-Fc (35) for 4 weeks after the last HOMER treatment component (fig. S23A). CCR2 inhibition reduced fibrosis in atria of HOMER mice (fig. S23, B and C). AFib inducibility and burden were lower in the treatment group, although this effect was not statistically significant (fig. S23D).
Macrophage-derived SPP1 is a pleiotropic AFib catalyst
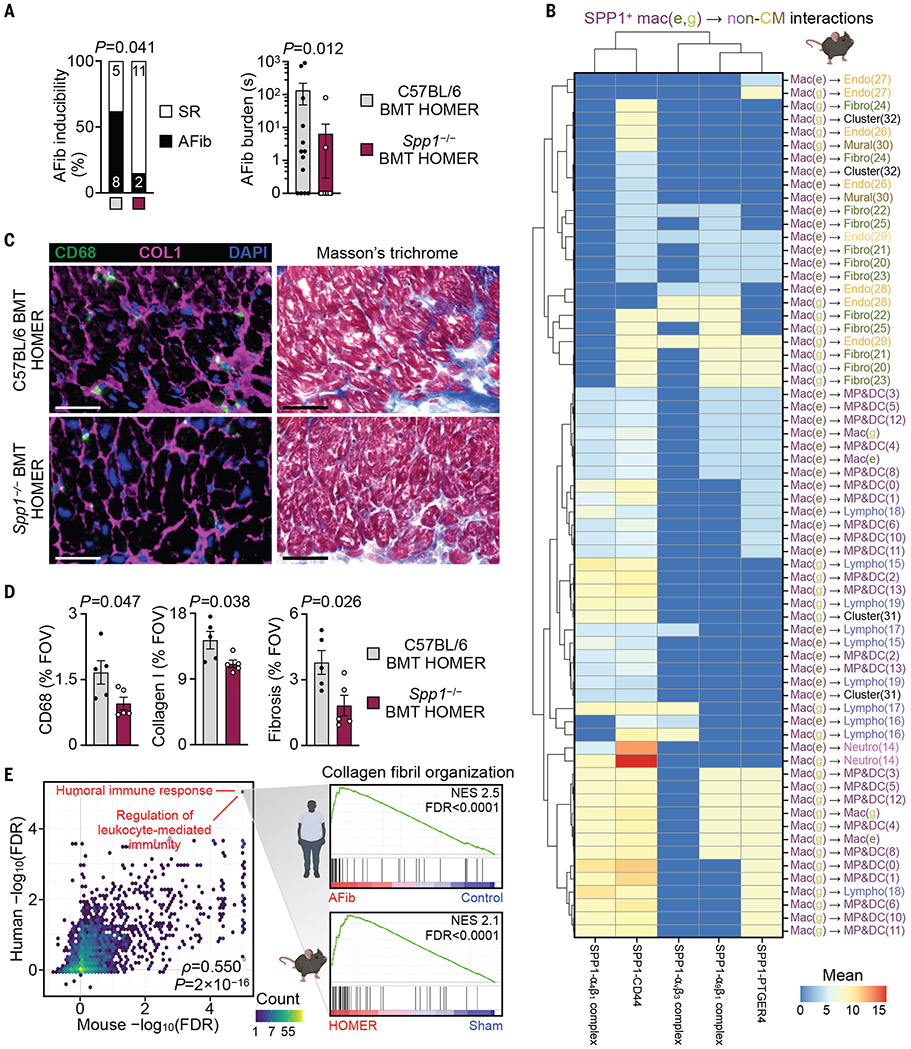
Given that Spp1 is among the most up-regulated macrophage genes in AFib, we examined the causal role of this matricellular protein. We transplanted bone marrow from Spp1−/− mice into wild-type recipients (Spp1−/− BMT HOMER) to delete Spp1 in recruited macrophages (fig. S24, A and B). Wild-type recipients that received wild-type bone marrow and then underwent HOMER (C57BL/6 BMT HOMER) served as controls. Blood pressure and leukocyte counts were similar in both cohorts (fig. S24, C to E). Left atrial and ventricular volumes were preserved and left ventricular hypertrophy was lessened in mice in which Spp1 was deleted (fig. S24F). Deleting Spp1 in bone marrow-derived cells reduced AFib inducibility and AFib burden (Fig 4A).
Fig. 4. Macrophage-derived SPP1 promotes atrial disease.
(A) EP study of AFib inducibility and burden in C57BL/6 BMT HOMER and Spp1−/− BMT HOMER mice. n = 13 per group from three independent experiments. (Left) Two-sided Fisher’s exact test. (Right) Two-tailed Mann–Whitney test. Other EP parameters are shown in table S9. (B) Heatmap of SPP1–receptor interactions between SPP1+ macrophages [mac(e) and mac(g)] and other non-CMs in four HOMER mice. SPP1 and cognate receptors are shown on the x axis. Cell populations that express Spp1 and the receptor are shown on the y axis and are color-coded according to the major cardiac cell types identified in Fig. 2F. Color of scale bar (red, high; blue, low) indicates average Spp1 and receptor expression levels in their respective interacting subpopulations (represented by mean normalized counts) if the enrichment of an interacting SPP1–receptor pair in the given interacting subpopulations was statistically significant (P < 0.05). Non-significant interactions were assigned a value of 0. (C) Representative immunofluorescent staining of macrophages (CD68, green), collagen deposition (COL1, magenta), and nuclei [4′,6-diamidino-2-phenylindole (DAPI), blue] and histological staining of fibrosis (Masson’s trichrome staining) in left atrial tissue from C57BL/6 BMT HOMER and Spp1−/− BMT HOMER mice. Scale bars, 50 μm. (D) Quantification of CD68+, COL1+, and fibrotic area in left atrial tissue from C57BL/6 BMT HOMER and Spp1−/− BMT HOMER mice. Bar graphs show percentage of positive staining per field of view (FOV); n = 5 per group from two independent experiments, two-tailed Student’s t test. (E) Scatterplot showing concordance between C5 GOBP GSEA results in human and mouse fibroblasts in AFib and HOMER (as in Fig. 2I). (Inset) Detailed GSEA results for gene set “GOBP collagen fibril organization” in human and mouse fibroblast clusters. NES, normalized enrichment score. All bar graph data are mean ± SEM with individual values for data distribution.
To outline mechanisms by which osteopontin exacerbates AFib, we investigated the crosstalk between Spp1-expressing macrophages and other atrial cells, using CellPhoneDB (36). In HOMER mice and patients with AFib, paracrine and autocrine interactions were found between macrophage-derived SPP1 and cognate SPP1 receptors expressed by immune and stromal cells (Fig. 4B and fig. S25). In HOMER mice, CellPhoneDB analysis implied SPP1+ macrophage interactions with client cells via three integrins (α4β1, αvβ3, and α9β1), CD44, and prostaglandin E2 receptor 4 (EP4/PTGER4). These interactions, which included signaling to various macrophage and fibroblast clusters, suggested that SPP1 acts as a pleiotropic amplifier for atrial inflammation and fibrosis, which in turn contribute to an AFib substrate (37).
In support of this idea, the histology of atria collected from Spp1−/− BMT HOMER mice showed fewer CD68+ macrophages when compared with that of C57BL/6 BMT HOMER mice (Fig. 4, C and D), indicating that SPP1 may influence atrial macrophage supply. Atrial monocyte numbers were unchanged in Spp1−/− BMT HOMER mice (fig. S26A), making a role of SPP1 in monocyte recruitment unlikely. TUNEL (terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling) and CD68 staining of atria indicated that macrophage death was also unchanged in the cohort in which Spp1 was deleted (fig. S26B). However, macrophage proliferation, which is up-regulated by HOMER conditions (fig. S21, D and E), declined in Spp1−/− BMT HOMER compared with that in C57BL/6 BMT HOMER mice (fig. S26, C and D). Thus, SPP1 augments atrial inflammation through increased macrophage proliferation.
Matrix production by fibroblasts increased in HOMER mice (S26, E and F), and immunoreactive α smooth muscle actin (αSMA) staining of HOMER atria documented an expansion of activated fibroblasts, which were reduced in Spp1−/− BMT HOMER mice (fig. S26, G to J). Atrial fibroblasts isolated from Spp1−/− BMT HOMER mice expressed less collagen than those from C57BL/6 BMT HOMER (fig. S26K). Furthermore, we detected reductions in collagen I deposition and Masson’s trichrome staining in atria of Spp1−/− BMT HOMER mice (Fig. 4, C and D), which points to reduced fibroblast matrix production if these cells do not sense SPP1. This interaction may be particularly relevant because integrins—which, according to our CellPhoneDB analysis, fibroblasts use to sense SPP1—regulate fibroblast migration, proliferation, and activation, processes that precede increased tissue fibrosis (38, 39).
Comparing left atrial cells from Spp1−/− BMT HOMER with those from C57BL/6 BMT HOMER mice by means of scRNA-seq (fig. S27, A and B), we identified fibroblast clusters that expressed collagens at high levels (fig. S27, C to E). For these clusters, we pursued GSEA to examine through which pathways SPP1 activates atrial fibroblasts (fig. S27F). Fibroblast clusters from C57BL/6 BMT HOMER mice up-regulated Tgfb1 gene sets. Inflammatory activation of fibroblasts by SPP1 was supported by the up-regulation of inflammatory gene sets in fibroblasts from C57BL/6 BMT HOMER when compared with Spp1−/− BMT HOMER mice (fig. S27F). Thus, SPP1 participates in inflammatory fibroblast activation partially through transforming growth factor-β (TGF-β) pathways. In atrial tissue from AFib patients, SPP1 colocalization with collagen 1A1 expanded (fig. S8, C and D), lending further credence to SPP1 promotion of AFib through enhanced fibrosis.
To ascertain whether the transcriptional changes that occurred in atrial fibroblasts of HOMER mice are representative of alterations in humans, we compared human and mouse fibroblast transcriptomes. We first performed GSEA (again using all C5 ontology MSigDB gene sets) on differentially expressed genes from a pseudo-bulk analysis comparing all fibroblast clusters isolated from atria of patients with AFib versus humans without AFib, as well as HOMER mice versus control mice. We then plotted the significance and direction of change for all gene sets for both species. Both GSEA data sets correlated strongly (Spearman’s ρ = 0.55, P < 2 × 10−16) with gene sets related to immune response and collagen organization enriched in diseased atria (Fig. 4E and data S9). This pathway-level interspecies correlation further bolstered the physiological relevance of the HOMER model while also underscoring inflammation and fibrosis as central processes in AFib.
Discussion
In this study, we comprehensively assayed atria from patients and mice and documented large-scale shifts in the cellular frequencies and phenotypes during AFib. Macrophages expand more than any other cell type in AFib. Experiments in mice, in which either Ccr2 or Spp1 was deleted, demonstrated that targeting macrophages strengthens resilience against AFib (fig. S28).
In human and mouse atria, SPP1 was among the top up-regulated macrophage genes. This matricellular protein, which increases in the blood of patients with AFib (40), stabilizes collagen (41, 42) and promotes fibrosis in hypertension (43, 44). We speculate that macrophage-derived SPP1 (45) augments atrial tissue heterogeneity and hinders conduction between cardiomyocytes. SPP1 signals to most other atrial cell classes, enhancing inflammation and fibrosis through interaction with various cell surface receptors. In the ventricular myocardium and in other organs, such signaling promotes myeloid cell activation and migration as well as extracellular matrix production by fibroblasts (13, 15, 43, 44). Thus, targeting SPP1 may have several distinctive effects that together preserve atrial conduction. The expanding numbers and altered phenotypes of atrial macrophages may have other disease-promoting consequences. For example, a loss of resident macrophages’ relationships with cardiomyocytes (46, 47) may compromise atrial conduction.
Going forward, therapeutic strategies could target recruitment of inflammatory macrophages (CCR2) or macrophage-derived signals (SPP1). Macrophage-targeted therapy for atrial cardiomyopathy is most likely to succeed with concomitant normalization of risk factors—for example, through surgical valve repair, weight loss, and blood pressure management. Our data centrally position macrophages as targets for immunotherapy in patients with AFib.
Supplementary Material
Acknowledgments
We thank A. Bard and N. Higgins for pathology assistance and K. Joyes for editing the manuscript. The authors also thank the HSCI-CRM Flow Cytometry Core for assistance with cell sorting and the BWH Specialized Histopathology Services Core for histology support. We acknowledge Servier Medical Art (https://smart.servier.com) and BioRender (IH23GLWC6I) for cartoon components.
Funding
This work was supported by National Institutes of Health grants DK040561 (M.H.), HL155097 (M.H.), HL149647 (M.H.), HL142494 (K.N. and M.N.), HL139598 (M.N.), CA225655 (K.N.), HL007604 (A.B.), HL158040 (F.E.P.), HL092577 (P.T.E.), HL157635 (P.T.E.), and HL139731 (P.T.E.); American Heart Association grants 19CDA34490005 (M.H.) and 18SFRN34110082 (P.T.E.); Deutsche Forschungsgemeinschaft (DFG) #453989101 SFB 1525 Mercator fellow (M.N.); DFG (M.J.S. and S.S.); British Heart Foundation (A.L. and B.C.); NIHR Oxford Biomedical Research Centre (A.L. and B.C.); European Union MAESTRIA 965286 (P.T.E.).
Footnotes
Competing interests: M.H., M.J.S, I.L., N.M., K.N., and M.N. are inventors on a patent application related to this work (US patent application no. 63/501,286). J.C.A. serves on the scientific advisory board of Cellestia, and is a consultant to Remix Therapeutics and Ayala Pharmaceuticals. P.T.E. receives funds or material research support from Bayer AG, IBM Research, Bristol Myers Squibb, Pfizer, MyoKardia and Novartis. M.N. has received funds or material research support from Alnylam, Biotronik, CSL Behring, GlycoMimetics, GSK, Medtronic, Novartis and Pfizer, as well as consulting fees from Biogen, Gimv, IFM Therapeutics, Molecular Imaging, Sigilon, Verseau Therapeutics and Bitterroot. The other authors declare no competing interests.
Data and materials availability: All data are available in the main text, supplementary materials, or online storage. Raw and processed mouse scRNA-seq data as well as processed human scRNA-seq data are available at the NCBI’s Gene Expression Omnibus database under accession no. GSE224959. Raw human scRNA-seq data are deposited in the controlled access repository Data Use Oversight System (DUOS; https://duos.broadinstitute.org) under accession no. DUOS-000150 under the restrictions listed by this system.
References and Notes
- 1.Litvinukova M et al. , Cells of the adult human heart. Nature 588, 466–472 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolás-Ávila JA, Hidalgo A, Ballesteros I, Specialized functions of resident macrophages in brain and heart. J Leukoc Biol 104, 743–756 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Forte E, Furtado MB, Rosenthal N, The interstitium in cardiac repair: role of the immune-stromal cell interplay. Nat Rev Cardiol 15, 601–616 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Benjamin EJ et al. , Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 139, e56–e528 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Yamashita T et al. , Recruitment of immune cells across atrial endocardium in human atrial fibrillation. Circ J 74, 262–270 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Smorodinova N et al. , Analysis of immune cell populations in atrial myocardium of patients with atrial fibrillation or sinus rhythm. PLoS One 12, e0172691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao C et al. , Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 138, 2227–2242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki YK, Nishida K, Kato T, Nattel S, Atrial fibrillation pathophysiology: implications for management. Circulation 124, 2264–2274 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Rudolph V et al. , Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med 16, 470–474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson MD, McCarthy DJ, Smyth GK, edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian A et al. , Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langfelder P, Horvath S, WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frangogiannis NG, Matricellular proteins in cardiac adaptation and disease. Physiol Rev 92, 635–688 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charo IF, Ransohoff RM, The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354, 610–621 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Hulsmans M et al. , Cardiac macrophages promote diastolic dysfunction. J Exp Med 215, 423–440 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serbina NV, Pamer EG, Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7, 311–317 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Tang X et al. , SIRT2 Acts as a Cardioprotective Deacetylase in Pathological Cardiac Hypertrophy. Circulation 136, 2051–2067 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao X et al. , Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci U S A 115, E4661–E4669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q et al. , Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardbower DM et al. , Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci U S A 114, E751–E760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koltsova EK, Hedrick CC, Ley K, Myeloid cells in atherosclerosis: a delicate balance of anti-inflammatory and proinflammatory mechanisms. Curr Opin Lipidol 24, 371–380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramachandran P et al. , Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huxley RR et al. , Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 123, 1501–1508 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsang TS et al. , Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc 76, 467–475 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Xintarakou A, Tzeis S, Psarras S, Asvestas D, Vardas P, Atrial fibrosis as a dominant factor for the development of atrial fibrillation: facts and gaps. Europace 22, 342–351 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Schilling JD, Machkovech HM, Kim AH, Schwendener R, Schaffer JE, Macrophages modulate cardiac function in lipotoxic cardiomyopathy. Am J Physiol Heart Circ Physiol 303, H1366–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L et al. , CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J 39, 1818–1831 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Epelman S et al. , Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bajpai G et al. , The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 24, 1234–1245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegler-Heitbrock L et al. , Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–80 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Gopinathannair R et al. , Managing Atrial Fibrillation in Patients With Heart Failure and Reduced Ejection Fraction: A Scientific Statement From the American Heart Association. Circ Arrhythm Electrophysiol 14, HAE0000000000000078 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Blériot C, Chakarov S, Ginhoux F, Determinants of Resident Tissue Macrophage Identity and Function. Immunity 52, 957–970 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Hoyer FF et al. , Tissue-Specific Macrophage Responses to Remote Injury Impact the Outcome of Subsequent Local Immune Challenge. Immunity 51, 899–914.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epelman S, Lavine KJ, Randolph GJ, Origin and functions of tissue macrophages. Immunity 41, 21–35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paavola CD et al. , Monomeric monocyte chemoattractant protein-1 (MCP-1) binds and activates the MCP-1 receptor CCR2B. J Biol Chem 273, 33157–33165 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Efremova M, Vento-Tormo M, Teichmann SA, Vento-Tormo R, CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat Protoc 15, 1484–1506 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Harada M, Nattel S, Implications of Inflammation and Fibrosis in Atrial Fibrillation Pathophysiology. Card Electrophysiol Clin 13, 25–35 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Graf K et al. , Angiotensin II and alpha(v)beta(3) integrin expression in rat neonatal cardiac fibroblasts. Hypertension 35, 978–984 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Sarrazy V et al. , Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc Res 102, 407–417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molvin J et al. , Exploration of pathophysiological pathways for incident atrial fibrillation using a multiplex proteomic chip. Open Heart 7, e001190 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liaw L et al. , Altered wound healing in mice lacking a functional osteopontin gene (spp1). J Clin Invest 101, 1468–1478 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Künzel SR et al. , Diminished PLK2 Induces Cardiac Fibrosis and Promotes Atrial Fibrillation. Circ Res 129, 804–820 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins AR et al. , Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. J Am Coll Cardiol 43, 1698–1705 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Matsui Y et al. , Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension 43, 1195–1201 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Lopez B et al. , Osteopontin-mediated myocardial fibrosis in heart failure: a role for lysyl oxidase. Cardiovasc Res 99, 111–120 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Nicolas-Avila JA et al. , A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 183, 94–109.e23 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Hulsmans M et al. , Macrophages Facilitate Electrical Conduction in the Heart. Cell 169, 510–522.e20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashburner M et al. , Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25, 25–29 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carbon S, The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res 49, D325–D334 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liberzon A et al. , The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1, 417–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.