Abstract
Lewy body dementia is the third most common and costliest type of dementia. It is an umbrella term for dementia with Lewy bodies and Parkinson’s disease dementia, both of which place a substantial burden on the person and society. Recent findings outline ethnoracial differences in dementia risk. Delayed and misdiagnosis across ethnoracial groups contribute to higher levels of burden. In this context, we aimed to summarize current knowledge, gaps, and unmet needs relating to race and ethnicity in Lewy body dementia. In this narrative review, we provide an overview of studies on Lewy body dementia focusing on differences across ethnoracial groups and outline several recommendations for future studies. The majority of the findings comparing different ethnoracial groups were from North American sites. There were no differences in clinical prevalence and progression across ethnoracial groups. Compared to people identifying as non-Hispanic White, co-pathologies were more common and clinical diagnostic accuracy was lower for people identifying as Black. Co-morbidities (e.g., diabetes, hypertension) were more common and medication use rates (e.g., antidepressants, antiparkinsonian agents) were lower for people identifying as Black or Hispanic compared to people identifying as White. More than 90% of clinical trial participants identified as non-Hispanic White. Despite increasing efforts to overcome disparities in Alzheimer’s disease and related dementias, inclusion of individuals from minoritized communities in Lewy body dementia studies continues to be limited and the findings are inconclusive. Representation of diverse populations is crucial to improve the diagnostic and therapeutic efforts in Lewy body dementia.
Keywords: Alzheimer’s disease, dementia, diversity, equity, ethnicity, inclusion, Lewy body disease, racial groups
INTRODUCTION
Lewy body (LB) dementia, an umbrella term for Parkinson’s disease dementia (PDD) and dementia with Lewy bodies (DLB), is the second most common neurodegenerative dementia following Alzheimer’s disease (AD) [1]. DLB accounts for 4.2% of all dementia in community-based dementia populations and 7.5% of dementia cases in clinical populations [2]; PDD accounts for 3.6% of all dementia cases with a point prevalence of 25–30% in people with Parkinson’s disease [3, 4]. Heterogeneity of clinical presentations in LB dementia contributes to the mis- and under-diagnosis, with potentially more than half of cases missed [5]. Currently, diagnosis relies on clinical features and there are no diagnostic biomarkers [6, 7]. Recent advances in biomarkers (e.g., seeded aggregation assays for alpha-synuclein and immunohistochemical detection of pathologic alpha-synuclein) are promising [8], although lack of diversity in study cohorts limits the generalizability of the findings.
LB dementia poses a significant burden on affected individuals, their care partners, and broader support systems, with substantial negative economic effects both personally and societally [9–13]. In fact, despite being the third most common overall dementia type following AD and vascular dementia, LB dementia is the costliest form of dementia in the United States (US) [14]. Compared to the US average health care spending of $11,462 per person in 2019, medical costs were $18,309 one year before the diagnosis and $29,174 one year after the diagnosis per person with LB dementia [9]. Motor and non-motor symptoms in LB dementia such as falls, urinary incontinence or infection, depression, anxiety, dehydration, and delirium were associated with these high costs [14]. Available treatments are only symptomatic and provide limited benefit. There is an urgent need to develop effective management strategies, but it is challenging to identify large and diverse samples of LB dementia with adequate power for longitudinal studies and clinical trials [15].
Race and ethnicity in dementia
Prevalence of dementia and AD is often reported to be higher in underrepresented racial and ethnic groups compared to people identifying as White [16]. These differences are largely explained by variations in health conditions (e.g., diabetes, cardiovascular disease), socioeconomic risk factors, health-related behaviors, and life experiences [16, 17]. Socioeconomic risk factors commonly relate to disparities resulting from structural racism, including higher rates of poverty, less access to quality education, increased exposure to environmental hazards, and greater exposure to discrimination and adversity in minoritized populations [16]. Cumulative burden of chronic stress and life events is associated with cognitive decline and contributes to racial disparities [18,19]. Other factors include cultural norms and attitudes regarding aging and dementia, lack of trust in healthcare, health literacy, and implicit and explicit bias [20, 21].
Individuals in the US with AD and dementia identifying as Asian, Black, or Hispanic are more likely to live in urban areas and areas with higher poverty and less access to education, timely care, mental health care, and dementia specialists. They also have more difficulty communicating with their healthcare professionals than people identifying as White [22–24]. People identifying as Black or Hispanic with dementia are less likely to receive and more likely to discontinue antidementia drugs, have higher hospital mortality rates, and are less likely to have advanced care planning, more likely to reside in nursing homes with lower quality of care, less likely to enroll in hospice, and have markedly higher end-of-life inpatient expenditures compared to people identifying as White with dementia [25].
So far, anxiety, depression, stroke, hypertension, diabetes, and hyperlipidemia have been associated with a higher risk of LB dementia (Table 1) [26–31]. Prevalence of these medical conditions, health care access, and outcomes for these conditions seem to differ across ethnoracial groups [32]. Within AD, clinical profile, progression rate, risk factors, and prevalence of biomarkers differ across ethnoracial groups [33, 34]. Whether such differences exist in LB dementia needs investigation. For example, research is needed on the degree to which educational attainment, vascular comorbidities, and mood disorders may contribute to potential differences in LB presentations between groups [24, 26, 35]. Environmental factors likely also contribute to the risk and manifestation of LB dementia [26, 36] and may be different between ethnoracial groups. Geography (rather than race) may be a driver; people identifying as Black or African-American in the US had a higher prevalence of Parkinson’s disease than people identifying as Black or African in sub-Saharan Africa [37, 38]. This likely relates to various considerations including diagnostic rates and life expectancy. However, Parkinson’s disease prevalence can differ across regions within a single country or rural and urban areas within a single region [39, 40], which may interact with race and ethnicity [24]. The effect of urban or rural living has not been studied extensively in LB dementia and should be considered when assessing ethnoracial differences for prevalence.
Table 1.
Summary of risk factors reported for Lewy body dementia
| Dementia with Lewy bodies (DLB) [26,27,31,57] | Parkinson’s disease dementia (PDD) [28–30] |
|---|---|
| Male sex | Older age |
| Low caffeine intake | Male sex |
| Family history of Parkinson’s disease | Low education level |
| Family history of dementia | Smoking |
| Hypertension | Hypertension |
| Hyperlipidemia | Longer Parkinson’s disease duration |
| Diabetes | More severe motor impairment |
| Stroke | Akinetic-rigid phenotype |
| Anxiety | Postural instability |
| Depression | Hallucinations |
| Genetic factors (GBA, BIN1, TMEM175, SNCA, APOE) | REM sleep behavior disorder |
| Genetic factors (GBA1, COMT, MAPT, APOE) |
Education is suggested to be a protective factor against PDD and cardiovascular diseases, which are associated with higher LB dementia risk [28, 41]. Compared to people identifying as White, people identifying as Black or Hispanic can have lower educational attainment and lower quality of education [42]. A recent review showed that disparities between people identifying as Black and White with dementia can be reduced by about half by equalizing the education quality [43]. This supports that potential ethnoracial disparities for LB dementia can be overcome if determined and addressed.
Race and ethnicity terminology
When investigating ways to improve diagnosis and treatment in LB dementia, considering race and ethnicity can reveal important disparities in care and inform generalizability of findings to all affected people. Race, ethnicity, and ancestry are often used synonymously, although they have different meanings. Race is defined as “a group of people connected by common descent or origin”, and ethnicity is defined as “membership of a group regarded as ultimately of common descent or having a common national or cultural tradition” [44, 45]. They are social constructs without biological meanings. The terms ancestry (referencing a person’s lineage or region of origin) or genetic admixture (describing a person’s genetic background from different ancestries) likely better reflect disease risk than race and ethnicity, but race and ethnicity still have important social meanings and implications for health research [26]. Recent publications highlight the importance of precise terminology for race and ethnicity in healthcare research [26]. Use and classification of race and ethnicity should be consistent and self-reported rather than assigned by an observer [46]. Categories should not be viewed as absolute as people may identify with more than one race or ethnicity. The Inclusive Language section of the AMA Manual of Style can be used for guidance and clarification about the correct up to date terminology [26].
Association of ethnicity and race with overall health and disease states is due to complex relationships between ancestry, heritage, demographic, socioeconomic, cultural, structural, institutional, and other factors [47–50]. Therefore, race and ethnicity should not be reported in isolation but instead accompanied by sociodemographic factors and social determinants of health. Although definitions and categories can change over time, place, and context, lack of information on race and ethnicity can lead to neglection of social stratification, injustices, inequities, and health [47, 48]. As social factors play an important role in cognitive decline and dementia [51], inclusion of diverse populations and a closer look at underrepresented ethnoracial groups in dementia research can help advance diagnostic and therapeutic efforts.
Approach to the narrative review
The majority of the studies in LB dementia come from Japan, the United Kingdom, and the US; 79% of the authors for the most recent DLB diagnostic criteria were working in these countries [15]. This challenges the applicability of current literature across different populations. As the European population is expected to shrink and the sub-Saharan African population is expected to double in the coming decades [52], generalizability of the available data on dementia will decrease even further.
In this review, we aimed to underscore the current knowledge and gaps to raise awareness and outlined recommendations for future research in LB dementia to advance efforts for diversity, equity, and inclusivity. We present and discuss the available findings for differences across ethnoracial groups in LB dementia, stratifying for DLB and PDD when possible, with a focus on risk, prevalence, progression, clinical and neuropathological features, treatment options, and clinical trials. In the US, the National Institutes of Health (NIH) standard for classification of race includes the options of “American Indian or Alaska Native”, “Asian”, “Black or African American”, “Native Hawaiian or Other Pacific Islander”, “White”, “More than one race”, or “Unknown”. Classification of ethnicity includes the options of “Hispanic or Latino”, “Not Hispanic or Latino”, or “Unknown” [53]. Recent studies from US tend to follow these standards. However, these categories reflect US-based populations and not ethnic and racial groups globally. Therefore, we outline the racial and ethnic groups as reported in the included studies or report the country of origin when such data are not disclosed.
PREVALENCE
International
There are several systematic reviews focusing on the prevalence of DLB and PDD [2, 3, 54, 55]. Studies from broad geographic areas, including North and South America, Europe, and Asia, are represented in these systematic reviews; however, data across studies were averaged to overall prevalence rates of 5% for DLB and 3.5% for PDD of all dementia cases. Differences across ethnoracial groups within included countries were not evaluated in these studies. In a separate study, higher age-standardized Parkinson’s disease prevalence rates were reported in North America with lower rates in Africa [56]. Although there are currently no studies investigating ethnoracial differences for LB dementia prevalence on a global scale, there are efforts to combine LB dementia cohorts from different regions [15]. Such initiatives will enable future efforts to compare prevalence and incidence of LB dementia based on ancestry, ethnicity, and race.
Geographical differences can be important for LB dementia and contribute to potential ethnoracial differences. Prevalence and clinical correlates of genetic factors (e.g., GBA, LRRK2), social determinants of health (e.g., education), medical conditions (e.g., cardiovascular disease), and exposure to environmental factors (e.g., smoking) are associated with different risk for LB dementia and these can differ across regions [26–28, 38, 57].
Immigration status represents another social determinant of health that can interact with ethnicity and race [58]. A study in Belgium compared DLB prevalence across their clinical cohort including people born in Belgium and first-generation immigrants (including people born in North Africa, Sub-Saharan Africa, Europe, and Latin America) [59]. DLB prevalence was significantly higher in people born in North Africa (6.3% out of all born in North Africa, total n = 205) and Latin America (19.0% out of all born in Latin America, total n = 21) compared to people born in Belgium (5.2% out of all born in Belgium, total n = 2,011). However, the number of first-generation immigrants was much smaller than people born in Belgium.
US-based
For the general demographics of the US population 65 years and older (total estimate = 52,888,621), American Community Survey 5-year estimates from 2021 note 0.5% of people identify as American Indian and Alaska Native, 4.8% as Asian, 9.2% as Black, 0.1% as Native Hawaiian and Other Pacific Islander, 79.8% as White, 3.0% as two or more races; 8.6% Hispanic and 75.7% as non-Hispanic White [60].
Despite the fact that most LB dementia studies reflect primarily non-Hispanic White populations, the frequency of DLB-related diagnosis codes was similar across ethnoracial groups amongst Medicare fee-for-service beneficiaries over the age of 68 as of December 31, 2013 in the US (total n = 21,624,228) [61]. Among all beneficiaries with a claim for dementia (n = 3,110,654), 4.0% of people identifying as non-Hispanic Black, 4.4% of people identifying as American Indian or Alaska Native, 5.4% of people identifying as Asian or Pacific Islander, 5.5% of people identifying as non-Hispanic White and 6.1% of people identifying as Hispanic were diagnosed with DLB [61]. DLB prevalence was lower in people living in rural (4.5%) compared to urban areas (5.2–5.5%). Although rural living has been associated with increased Parkinson’s disease risk, potentially as a result of agricultural pesticide or other environmental factors [62], higher Parkinson’s disease prevalence in urban areas is also reported [40]. Higher DLB prevalence in urban areas may reflect bias of more diagnoses made among populations living closer to academic/specialized care centers or areas with higher density of clinicians trained in diagnosing these disorders.
DIAGNOSIS AND CLINICAL FEATURES
Clinical diagnostic accuracy is limited in DLB, with pathological confirmation required for the definite diagnosis of both DLB and PDD [6, 7, 63]. In the US-based National Alzheimer Coordinating Center (NACC) database including standardized clinical and neuropathological research data from centers across the US [64–66], age of onset for cognitive decline and functional status were similar across people identifying as Hispanic (n = 122), non-Hispanic Black (n = 130), and non-Hispanic White (n = 1,782) clinically diagnosed with mild cognitive impairment (MCI) or dementia associated with a LB disorder [67]. Compared to people identifying as non-Hispanic White, people identifying as non-Hispanic Black or Hispanic were more likely to be female, single, and living alone; had fewer years of education; and were more likely to have a history of diabetes and hypertension. Depression frequency and severity were higher for people identifying as Hispanic compared to people identifying as non-Hispanic Black or non-Hispanic White. People identifying as Hispanic were more impaired on attention and language tests; people identifying as Black were more impaired on executive function, attention, and language tests compared to people identifying as White after adjusting for sex, education, hypertension, diabetes, and cognitive severity. Although these findings suggest an ethnoracial difference for risk factors, cognitive profile, and clinical features, the need for culturally-sensitive test batteries was also emphasized. Caution is also needed given that NACC is not population-based and recruitment biases could affect results.
The majority of the literature guiding the diagnostic criteria for DLB came from research in people identifying as White or studies from Asia, Europe, and North America [15]. It is uncertain whether this might affect diagnostic accuracy in other populations. In individuals with limbic or neocortical LB pathology in NACC (including 61% with intermediate/high likelihood of a DLB phenotype), only 28.6% had a clinical diagnosis of LB disease at their last visit (n = 1,555) [68]. While every group had a lower rate of clinical diagnosis than pathological diagnosis, the gap was significantly wider for people identifying as Black. A clinical LB disease diagnosis was less common for people identifying as Black (15% of all identifying as Black, total n = 60 versus 24.1% of all identifying as Hispanic, total n = 54 and 30.4% of all identifying as White, total n = 1,441) despite 70% of people identifying as Black having an intermediate or high likelihood of a DLB phenotype based on pathology (versus 40.8% of all identifying as Hispanic and 61.4% of all identifying as White). In a separate study of individuals with LB and/or AD neuropathology in NACC, as dementia worsened over time, clinical diagnostic accuracy increased in people identifying as White, whereas accuracy declined in people identifying as Black [69]. Reasons for this are uncertain and may reflect performance of the clinical diagnostic criteria in different ethnic and racial groups, clinician or center biases in diagnosis (e.g., many centers participating in NACC are focused on AD), or other factors.
PROGRESSION
Overall, LB dementia is associated with over three-times higher mortality rates than the general population. DLB is associated with a faster cognitive and functional decline as well as shorter survival compared to AD [70–74]. Studies suggest similar rates of cognitive decline and mortality for PDD and DLB [73, 75, 76]. However, Parkinson’s disease mortality can differ across ethnoracial groups. Across 138,728 US Medicare beneficiaries with Parkinson’s disease identified in 2002 and followed through 2008, compared to people identifying as White (total n = 125,660; 64.6%), the death rate was higher for people identifying as Black (total n = 8,527; 66.4%) and lower for people identifying as Hispanic (total n = 3,036; 55.4%) or Asian (total n = 1,505; 50.8%) [77]. Dementia was also associated with higher risk of death and PDD was diagnosed more often in people identifying as Black (78.2%) and Hispanic (73.1%) compared to people identifying as White (69.0%) or Asian (66.8%) [77].
Neuropathological studies suggest that neocortical LB pathology (versus limbic) and AD co-pathology are associated with shorter survival in LB diseases [78–81]. Across 41 participants with AD identifying as Black and 81 participants with AD identifying as White in the Rush Alzheimer’s Disease Clinical Core, mixed LB and AD pathology was more likely in people identifying as Black [82], which may suggest a higher risk for shorter survival. Nevertheless, this higher likelihood of mixed LB and AD pathologies in people identifying as Black was not replicated in another cohort of people with limbic or neocortical LB pathology in the NACC [68]. In this NACC study, people identifying as Hispanic (n = 54) had longer unadjusted survival compared to people identifying as Black (n = 60) or White (n = 1,441). However, this significant difference did not survive adjustment for age at symptom onset, sex, education, LB pathology type (limbic versus neocortical), and Braak neurofibrillary tangle stage. Thus, the limited available data in the literature are inconclusive in terms of progression and mortality rates across US-based ethnoracial groups in LB dementia.
NEUROPATHOLOGY
Autopsy confirmation of LB pathology remains the gold standard for diagnosis of both DLB and PDD [6, 7]. Level of substantia nigra neuron loss and LB pathology as well as the prevalence and level of co-pathologies (e.g., AD and cerebrovascular pathologies) are associated with the clinical phenotype and can differ for PDD and DLB [83, 84]. In US-based cohorts, studies examining LB pathology tend to show higher rates of co-pathology in minoritized populations. In the Rush AD Clinical Core, people with clinical diagnoses of AD identifying as Black (n = 41) had a higher likelihood of LB pathology and/or more severe vascular pathology accompanying AD pathology when compared to a matched sample of individuals identifying as White (n = 81) [82]. In a NACC study of individuals with a clinical diagnosis of dementia at last visit, people identifying as Black (n = 110) had more frequent co-pathologies, including LB pathology, AD pathology, frontotemporal lobar degeneration with tau and TDP-43 inclusions, and various vascular findings compared to people identifying as White (n = 2,500) [85]. Compared to people identifying as White, people identifying as Black were more likely to have LB pathology as a contributing neuropathological diagnosis associated with their dementia. In a NACC study focused on individuals with limbic or neocortical LB pathology, people identifying as Black (n = 60), Hispanic (n = 54), or White (n = 1,441) who died at similar ages had similar levels of Braak tau staging. However, the Black cohort had more women, hypertension, neocortical LB pathology, and infarcts/lacunes. The Hispanic cohort had a higher ratio of people with diabetes, limbic LB pathology and hemorrhages/microbleeds [68].
While most studies report more co-pathology in minoritized populations, one study utilizing multiple US-based aging cohorts found that the frequency of LB pathology and co-morbid degenerative or vascular pathology was largely similar between individuals identifying as Black (n = 81) and a matched sample of individuals identifying as White (n = 154) [86]. This sample included individuals with normal cognition, MCI, and dementia. Clinical diagnoses of DLB (one person identifying as Black, one person identifying as White) and Parkinson’s disease (four people identifying as Black, seven people identifying as White) were uncommon, and PDD was not mentioned [86]. Across brain regions, the LB count only differed in the anterior cingulate cortex (higher count for people identifying as Black). The association between LB pathology and cognition and olfaction was similar across racial groups, despite a lower smell test score for people identifying as Black. People identifying as Black had a stronger association between LB pathology and parkinsonism.
TREATMENT AND CLINICAL TRIALS
Available treatment options for DLB and PDD offer limited symptomatic relief and there is an urgent need for effective disease-modifying strategies. For a cohort of people with LB dementia or MCI in NACC, people identifying as White (n = 1,782) were significantly more likely to use an antiparkinsonian drug at the time of the visit compared to people identifying as Hispanic (n = 122) or non-Hispanic Black (n = 130) despite similar reported levels of parkinsonism [67]. Report of antidepressant and AD medication use at the time of the visit also differed across the ethnoracial groups, with lowest rates seen in people identifying as non-Hispanic Black and highest rates in people identifying as White, although these differences did not persist after multiple comparison correction.
Centers in Japan, US, and Europe have been the main drivers of clinical trials in LB dementia [15]. As of February 7, 2023, ClinicalTrials.gov listed 14 completed clinical trials with a total of 1,676 participants with DLB, PDD, or LB dementia across studies (Table 2). Four studies were conducted in Asia (n = 415, 24.8% of total participants in all trials), seven in North America (n = 718, 42.8%) and three had sites in North America and Europe (n = 543, 32.4%). Of the seven studies reporting detailed race data (n = 873, four in North America, three in North America and Europe), 96.1% of the participants identified as White (Fig. 1). Of the five studies reporting detailed ethnicity data (total n = 819), 92.8% of the participants identified as non-Hispanic with only 6.5% identifying as Hispanic (two in North America, three in North America and Europe).
Table 2.
Summary of completed studies with available results on ClinicalTrials.gov (n=14)
| ClinicalTrials.gov Identifier | Intervention | Phase | Study population | Completion date | Location | Ethnicity and race of study population, % |
|---|---|---|---|---|---|---|
| NCT00294554 | Memantine | PDD, n=20 | December 2008 | Maryland (US) |
Race: White- 100% |
|
| NCT00543855[125] | Donepezil hydrochloride | 2 | Probable DLB, n=139 | February 2010 | Japan | Not reported |
| NCT00623103[126] | Rivastigmine | 3 | PDD, n=359 | November 2010 | Alabama, Arizona, California, Florida, Georgia, Idaho, Illinois, Kansas, New York, Ohio, Pennsylvania, Texas, Utah, Washington (US); Argentina; Australia; Austria; Belgium; Canada; France; Germany; Italy; Netherlands; Spain; Turkey; United Kingdom | Not reported |
| NCT02708186[127] | Nelotanserin | 2 | Lewy body dementia with REM sleep behavior disorder, n=34 | November 2010 | Alabama, Arizona, Arkansas, Colorado, Florida, Georgia, Indiana, Kansas, Nebraska, North Carolina, North Dakota, Ohio, Rhode Island, Tennessee, Texas (US) |
Race: Asian- 2.9% White- 97.1% |
| NCT00598650[128] | Donepezil hydrochloride | 2 | Probable DLB, n=108 | March 2011 | Japan | Not reported |
| NCT00988117 | Rivastigmine | 4 | PD-MCI, n=6 PDD, n=6 Controls, n=15 |
April 2011 | California (US) | Not reported |
| NCT01023672 | Armodafinil | 4 | Probable DLB, n=17 | March 2012 | Minnesota (US) | Not reported |
| NCT01785628[129] | Sarcosine | PDD, n=30 | July 2012 | Taiwan | Not reported | |
| NCT01278407[130] | Donepezil | 3 | Probable DLB, n=138 | April 2013 | Japan | Not reported |
| NCT02258152 | Landipirdine | 2 | PDD, n=82 | October 2017 | Alabama, Arizona, California, Florida, Georgia, Illinois, Iowa, Kansas, Maryland, Massachusetts, Minnesota, New Jersey, North Carolina, Pennsylvania, South Carolina, Texas (US) |
Ethnicity: Non-Hispanic- 98.8% Unknown- 1.2% |
| NCT02669433[131] | Intepirdine | 2 | Probable DLB, n=268 | December 2017 | Arizona, California, Colorado, District of Columbia, Florida, Georgia, Illinois, Indiana, Kentucky, Massachusetts, Minnesota, New York, North Carolina, Ohio, Oregon, Pennsylvania, Texas, Virginia (US); Canada; France; Italy; Netherlands; Spain; United Kingdom |
Ethnicity: Hispanic- 3.7% Non-Hispanic- 95.1% Unknown- 1.1% |
| NCT04001517[132] | Neflamapimod | 2 | Probable DLB, n=91 | June 2020 | California, Colorado, Florida, Kansas, Massachusetts, Michigan, Minnesota, Nevada, New York, North Carolina, Ohio, Oregon, Pennsylvania, Virginia, Washington (US); Netherlands |
Ethnicity: Hispanic- 7.7% Non-Hispanic- 92.3% |
| NCT03305809[133] | Mevidalen | 2 | Lewy body dementia, n=344 | July 2020 | Alabama, Arizona, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Illinois, Indiana, Kansas, Maine, Massachusetts, Michigan, Missouri, Nevada, New Hampshire, New Jersey, Ney York, North Carolina, Pennsylvania, South Carolina, Texas, Virginia, Washington, Wisconsin (US); Canada; Puerto Rico |
Ethnicity: Hispanic- 9.6% Non-Hispanic- 89.8% Unknown- 0.6% |
| NCT04764669 | Irsenontrine | 2 | Probable DLB, n=21 Probable PDD, n=13 |
January 2022 | Arizona, Florida, Kentucky, New Jersey, New York, Ohio, Oregon (US); Canada |
Ethnicity: Hispanic- 8.8% Non-Hispanic- 91.2% |
PD-MCI: Parkinson’s disease with mild cognitive impairment; PDD: Parkinson’s disease dementia; DLB: dementia with Lewy bodies. Individual states are listed for United States, reporting of one country other than United States does not imply a single site study. Ethnicity and race data is presented as reported in ClinicalTrials.gov. For those without available data on ClinicalTrials.gov, ethnicity or race data were also not available on cited publications.
Fig. 1.
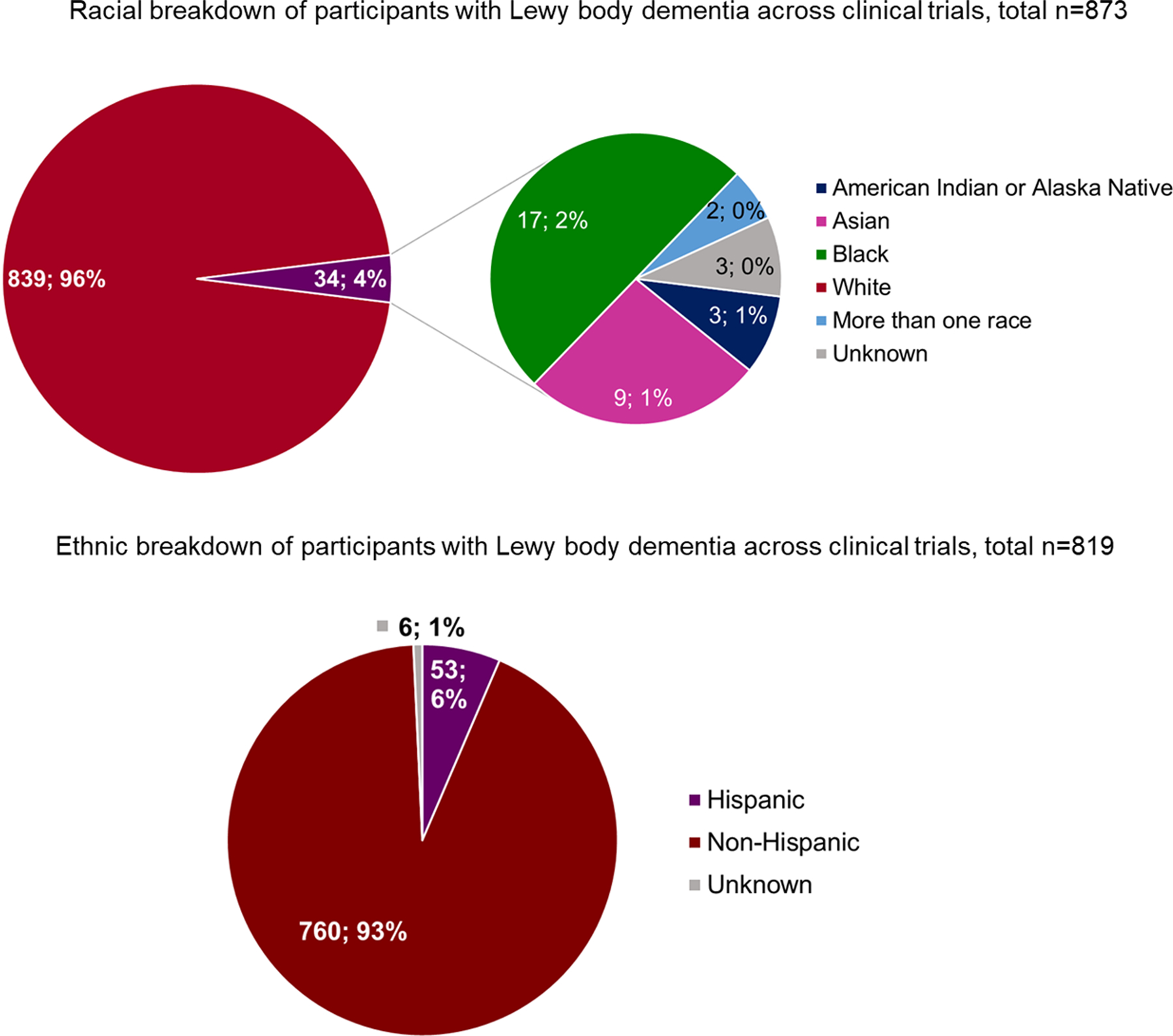
Race and ethnic breakdown of participants with Lewy body dementia in clinical trials that reported detailed race and ethnicity data.
DISCUSSION
In this review, we outlined available findings for diagnosis, progression, clinical profile, neuropathology, and management for LB dementia across ethnoracial groups (Table 3). Despite the high prevalence and burden reported in LB dementia, this review identified a paucity of research investigating racial, ethnic, or ancestral considerations in the epidemiology, clinical presentation, diagnosis, experience, progression, pathology, or treatment of LB dementia. This underscores the importance of health disparities as a research priority as identified by the AD and Related Dementias Summits, hosted most recently in 2022 [87].
Table 3.
Summary of research studies on ethnoracial differences in LB dementia
| Category | Summary of current research on ethnic and racial differences |
|---|---|
| Prevalence | DLB prevalence higher for immigrants from North Africa and Latin America than natives in Belgium [59], but not different across ethnoracial groups in the United States [61]. |
| Diagnosis and clinical features | In the NACC, clinical diagnostic accuracy is lower in people identifying as Black compared to people identifying as White [69]. Despite more advanced LB pathology stage, diagnosis of LB disease is less common for people identifying as Black compared to people identifying as Hispanic or White [68]. Compared to people identifying as White, higher rates of diabetes, hypertension, depression, female sex, single status, living alone, as well as lower education levels and worse performance on various cognitive tests were observed in people identifying as Black or Hispanic that were clinically diagnosed with MCI or dementia due to a LB disorder [67]. |
| Progression | No significant differences for progression and mortality after accounting for age at onset, sex, education and the level of underlying LB and AD pathology [68]. |
| Neuropathology | Compared to people identifying as White, co-pathology rates (e.g. vascular, AD, frontotemporal lobar degeneration) may be higher in people not identifying as White [82] [85]. Regional pathology burden and clinicopathological correlations can differ; higher LB count in the anterior cingulate cortex, stronger association between LB pathology and parkinsonism in people identifying as Black compared to people identifying as White [86]. |
| Treatment and clinical trials | Antiparkinsonian, antidepressant and Alzheimer’s drug use may be higher in people identifying as White compared to people identifying as Black non-Hispanic or Hispanic [67]. More than 9 out of 10 people participating in clinical trials identify as non-Hispanic White (see Table 2). |
Racial, ethnic, and ancestral considerations in LB dementia clinical care
Clinical diagnosis of LB dementia is challenging regardless of the person’s background. Medicare and NACC data in this review were mixed on whether race and ethnicity affect identification of LB dementia [61, 67, 68, 77]. Risk of LB dementia misdiagnosis can be higher in a primary care setting than a tertiary care facility [63, 88]. In the US, access to timely care and specialists is significantly lower for people identifying as Asian, Black, or Hispanic in comparison to people identifying as White [24]. Different or delayed diagnosis is common in LB dementia, and leads to uncertainty, fear, worry and concern about the future for both people with LB dementia and their families [10, 89]. Mis- or delayed diagnosis interferes with timely support and treatment, which are top research priorities for people with LB dementia and caregivers [90]. Misdiagnosis can also to inappropriate treatments, such as risky antipsychotic use [91].
Minoritized populations with dementia may be at a higher risk for receiving delayed or insufficient healthcare with less access to new treatment options [92, 93]. Disparities in healthcare access and obtaining accurate diagnoses expand to disparities in access to appropriate treatments, as suggested by different rates of medication use across people with LB dementia or MCI identifying as Black, Hispanic, or White [67].
Racial and ethnic considerations in LB dementia research
While a few studies suggest potential ethnoracial differences in LB pathology and co-pathology, this research is severely limited given small numbers of individuals from minoritized groups, likely reflecting a combination of under-diagnosis and concerns regarding research participation and brain donation [94]. More representation is needed across LB studies, including neuropathological studies but also observational studies and clinical trials. Of clinical trials reporting detailed ethnicity and race data, over 90% of participants with identified as White and non-Hispanic. This likely reflects a complex interplay of barriers to accessing healthcare and historically rooted distrust of clinical research [33, 95]. Although many of these studies are conducted in the US and Europe, the ratio of research participants identifying as White continues to be higher than the ratio of people identifying as White in the population.
Increasing participation of underrepresented groups in clinical trials requires efforts from individuals, institutions and funding agencies involved with research. Research suggests that minoritized people are interested in participating in trials [96] and are more likely to be recruited from internal sources such as referrals by healthcare providers, community outreach and local research registries [34, 97]. However, healthcare professionals may assume lack of interest or other barriers and not provide information [96]. This can significantly limit the participation of diverse populations in trials. Diversifying the research team and including professionals that can speak the native language of the targeted populations will allow people to feel more comfortable and overcome potential mistrust.
In addition to recruitment challenges, trial inclusion and exclusion criteria may affect enrollment of racial and ethnic minorities. The fact that individuals from underrepresented groups may have more co-morbidities and are more likely to be single (and thus potentially lack convenient study partners) may influence the likelihood of fulfilling trial eligibility criteria [67]. Trials may also use cognitive tools developed and validated in cohorts mostly including people identifying as White to determine eligibility. For instance, people identifying as Black without cognitive impairment may have scores below average or low-average, whereas people identifying as White may have high-average or superior scores, leading to misdiagnosis of cognitive impairment in these groups [98]. Applying ethnically and culturally appropriate tests or test norms can help interpret the level of impairment more reliably to determine eligibility. Additionally, views regarding dementia may vary by culture [99] and this may affect study partner responses to commonly used tools such as the Clinical Dementia Rating Scale [34].
Additional suggested strategies for improving recruitment of individuals from minoritized communities can include providing resources to cover costs associated with participation, expanding research sites to community settings, and incentives from funding agencies to the research teams to encourage recruitment of underrepresented groups [97, 100, 101].
There are established consortia and emerging collaborative initiatives to diversify and expand LB dementia cohorts [15, 102]. Supporting these efforts to reach more diverse populations and harmonization of data across different consortia studies can allow analysis of geographic, social, and other factors driving the ethnoracial differences more reliably. More global research is still needed to incorporate both additional countries and representation of different groups within formal borders.
Recommendations for future research in LB dementia
Improving representation in LB dementia research starts with improving recognition and diagnosis in underrepresented groups (Table 4). This has critical implications for accurate prevalence estimates and for connecting individuals with LB dementia to both clinical care and research. Research into barriers affecting diagnosis and access to dementia care is critical. Diagnosis of LB dementia relies heavily on informant report for core clinical features including symptoms of dementia, REM sleep behavior disorder, visual hallucinations, and cognitive fluctuations [6]. Research outside LB dementia suggests that informant reporting may vary by background in terms of cognitive symptoms [103, 104] and level of functioning [105, 106]. Additionally, the type of caregiver available (e.g., spouse versus child) and whether that caregiver lives with the older adult may vary among racial-ethnic groups [107], which has implications for assessing functionality and symptoms like dream enactment [106]. Whether LB dementia diagnostic criteria perform well across different people groups also requires further study.
Table 4.
Recommendations for research in Lewy body dementia relating to race, ethnicity, and ancestral considerations
| Category | Considerations |
|---|---|
| Prevalence | Determine prevalence by geographic reasons and different people groups |
| Diagnosis | Identify barriers to symptom recognition or reporting Identify and address barriers to accessing healthcare for dementia Determine whether diagnostic criteria perform equally well across groups Develop and implement culturally sensitive neuropsychological test batteries Interpret cognitive testing with consideration of language, social determinants of health |
| Risk factors | Assess risk factors (e.g. environmental exposures) that may vary by geographic location (e.g. rural/urban) or background (e.g. vascular risk factors) |
| Observational studies | Include diverse cohorts Collect data on social determinants of health Assess biomarker validity across groups Increase diversity in brain bank studies |
| Clinical trials | Recruit diverse populations Identify ways to increase access to clinical trials for underrepresented groups (e.g. with regard to travel arrangements, stipends) Use measures with validated versions across different languages Employ culturally sensitive neuropsychological test batteries as outcome measures |
Studies should use culturally sensitive batteries and neuropsychological tests adapted and validated across populations and languages. Prior US-based research, for example, has found that neuropsychological tests were less specific and had higher false-positive error rates for people identifying as Black or Hispanic [108]. Implications relating to primary language and social determinants of health/disparities need to be incorporated into assessments of impairment based on neuropsychological testing, in addition to classically assessed factors such as age, gender, and education [109].
Longitudinal studies should assess whether risk factors (e.g., environmental exposures, comorbid vascular conditions) vary between different people groups and how this might affect both diagnosis and progression. Consistent and systematic collection of data for structural and social determinants of health is also critical, ideally using approaches that will allow harmonization of data and findings from different cohorts [110]. Research in AD is mixed regarding whether biomarkers (e.g., cerebrospinal fluid, plasma biomarkers) perform differently based on participant background and medical comorbidities [111–113], suggesting that biomarkers should be validated across diverse LB dementia populations.
There is a critical need to assess whether there are any pathologic differences by background, which will necessitate greater diversity in studies including pathology. Religious beliefs and cultural attitudes, which may vary along with race, ethnicity, and ancestry, play an important role in the decision of brain donation [114]. In one US-based study, however, people identifying as Black were as likely as people identifying as White to be willing to donate their brains once approached by healthcare professionals [115]. From a global standpoint, each country’s own legislation and the attitudes of the ethnic, racial, or religious group in the majority can impact the approach towards brain donation [116, 117]. Barriers to brain donation include lack of knowledge and the approach of healthcare teams when providing education [116–118].
As demonstrated in this review, increased diversity in LB dementia clinical trials is a critical unmet need. Diverse populations are necessary to provide general-izable findings in clinical trials as pharmacokinetics and pharmacodynamics can differ across ethnoracial groups, with implications for both safety and efficacy [119–121]. Barriers to diverse clinical trial participation include LB dementia-specific considerations, such as under-diagnosis, and systemic barriers such as mistrust. A comprehensive review of approaches to improve clinical trial diversity is outside of the scope of this review, but such approaches include engaging diverse research staff (in terms of background and language), study team training regarding communication with diverse communities, designing culturally- and linguistically-appropriate documents using accessible literacy levels, using study materials available in multiple languages, choosing inclusion/exclusion criteria that allow for a broad range of backgrounds (e.g., in terms of language, education, study partner requirements), and sponsor require ments for enrollment of diverse populations [96, 100, 122].
Strengths and limitations of review
This is the first known review focusing on ethnicity and race in LB dementia and it identifies numerous gaps in the existing literature. Limitations include the fact that this is a non-systematic review. Narrative reviews are prone to selection bias compared to systematic reviews. A systematic review with a clearly defined hypothesis, search method using different search engines, predefined selection criteria, data extraction and analysis based on the hypothesis can provide a better summary of existing data than a narrative review [123, 124]. A narrative review, on the other hand, can provide a broad overview of the studies with more emphasis on interpretation and critique [123, 124]. As our primary focus was to discuss current knowledge in order to outline future directions to advance the LB dementia field, we used a narrative review approach. Many of the identified studies are US-based and the majority use NACC, which is not population-based and which has an under-representation of racial and ethnic minorities. It is also uncertain whether the NACC battery is ideally suited to assess individuals from different racial-ethnic and linguistic backgrounds. Furthermore, available US-based studies are limited to groups identifying as Black, Hispanic, or White, with almost no data for other groups including Asian, Pacific Islander, and Native/Indigenous populations.
CONCLUSION
The majority of the published studies in LB dementia do not report race, ethnicity, and ancestry of participants or take social factors into account. Countries with diverse racial and ethnic populations need to increase efforts for inclusion and engagement in research. A more globally inclusive approach should also be implemented to support efforts from countries underrepresented in LB dementia research. There is an urgent need for further investigation on ethnoracial, cultural, social, and environmental differences in DLB and PDD to better identify, understand and adequately serve all communities affected by LB dementia. Approaches will likely need to include education for clinicians to improve cultural competence and a variety of efforts to improve research diversity. Social factors must be considered with ethnicity and race for a better understanding of the directional ity and interplay of risk. Once factors contributing to LB dementia risk, progression, and response to treatment are identified, strategies to overcome barriers in access and care have the potential to significantly improve diagnostic and therapeutic efforts in LB dementia.
ACKNOWLEDGMENTS
This project was conceived and executed by the Community Engagement Working Group of the Lewy Body Dementia Association Research Centers of Excellence program. Lewy Body Dementia Association Community Engagement Working Group includes Angela Lunde, Angela Taylor, Bhavana Patel, Bradley Boeve, Brandi Hackett, Chris Schwilk, Ece Bayram, Henry Paulson, Holly Shill, Jori Fleisher, Julia Wood, Keith Fargo, Kelly Stacy, Leah Forsberg, Melissa Armstrong, Natalie Hernandez, Renee Gadwa, Samantha Holden, and Susan Erlichman. Dr. Bayram receives research support from the National Institutes of Health (K99AG073453, U01NS119562). Dr. Holden receives research support from the NIH (R21AG072153) and as the local PI of a Lewy Body Dementia Association Research Center of Excellence. Dr. Fullard receives research support from the Michael J. Fox Foundation, Davis Phinney Foundation and Lorna G. Moore Faculty Launch Award. Dr. Armstrong receives research support from the NIH (R01AG068128, P30AG047266, R01NS121099, R44AG062072), the Florida Department of Health (grant 20A08), and as the local PI of a Lewy Body Dementia Association Research Center of Excellence. The views and opinions expressed in this publication represent those of the authors and do not necessarily reflect the official views of the Lewy Body Dementia Association or funding mechanisms.
FUNDING
Co-authors on this review received support from the National Institutes of Health (K99AG073453, R01AG068128, P30AG047266), Lewy Body Dementia Association, Michael J. Fox Foundation, Davis Phinney Foundation, and Lorna G. Moore Faculty Launch Award, which in part supported this work.
Footnotes
CONFLICT OF INTEREST
Dr. Armstrong serves on the Data and Safety Monitoring Boards for the Alzheimer’s Therapeutic Research Institute/Alzheimer’s Clinical Trial Consortium and the Alzheimer’s Disease Cooperative Study. She has provided educational content for Medscape. The authors have no other conflict of interest to report.
REFERENCES
- [1].Donaghy PC, McKeith IG (2014) The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther 6, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vann Jones SA, O’Brien JT (2014) The prevalence and incidence of dementia with Lewy bodies: A systematic review of population and clinical studies. Psychol Med 44, 673–683. [DOI] [PubMed] [Google Scholar]
- [3].Aarsland D, Zaccai J, Brayne C (2005) A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord 20, 1255–1263. [DOI] [PubMed] [Google Scholar]
- [4].Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, Ballard C (2017) Cognitive decline in Parkinson disease. Nat Rev Neurol 13, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Palmqvist S, Hansson O, Minthon L, Londos E (2009) Practical suggestions on how to differentiate dementia with Lewy bodies from Alzheimer’s disease with common cognitive tests. Int J Geriatr Psychiatry 24, 1405–1412. [DOI] [PubMed] [Google Scholar]
- [6].McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies. Neurology 89, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22, 1689–1707. [DOI] [PubMed] [Google Scholar]
- [8].Scott GD, Arnold MR, Beach TG, Gibbons CH, Kanthasamy AG, Lebovitz RM, Lemstra AW, Shaw LM, Teunissen CE, Zetterberg H, Taylor AS, Graham TC, Boeve BF, Gomperts SN, Graff-Radford NR, Moussa C, Poston KL, Rosenthal LS, Sabbagh MN, Walsh RR, Weber MT, Armstrong MJ, Bang JA, Bozoki AC, Domoto-Reilly K, Duda JE, Fleisher JE, Galasko DR, Galvin JE, Goldman JG, Holden SK, Honig LS, Huddleston DE, Leverenz JB, Litvan I, Manning CA, Marder KS, Pantelyat AY, Pelak VS, Scharre DW, Sha SJ, Shill HA, Mari Z, Quinn JF, Irwin DJ (2022) Fluid and tissue biomarkers of Lewy body dementia: Report of an LBDA symposium. Front Neurol 12, 805135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Desai U, Chandler J, Kirson N, Georgieva M, Cheung HC, Westermeyer B, Lane H, Biglan K (2022) Epidemiology and economic burden of Lewy body dementia in the United States. Curr Med Res Opin 38, 1177–1188. [DOI] [PubMed] [Google Scholar]
- [10].Galvin JE, Duda JE, Kaufer DI, Lippa CF, Taylor A, Zarit SH (2010) Lewy body dementia: Caregiver burden and unmet needs. Alzheimer Dis Assoc Disord 24, 177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goldman JG, Forsberg LK, Boeve BF, Armstrong MJ, Irwin DJ, Ferman TJ, Galasko D, Galvin JE, Kaufer D, Leverenz J, Lippa CF, Marder K, Abler V, Biglan K, Irizarry M, Keller B, Munsie L, Nakagawa M, Taylor A, Graham T (2020) Challenges and opportunities for improving the landscape for Lewy body dementia clinical trials. Alzheimers Res Ther 12, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taylor JP, McKeith IG, Burn DJ, Boeve BF, Weintraub D, Bamford C, Allan LM, Thomas AJ, O’Brien JT (2020) New evidence on the management of Lewy body dementia. Lancet Neurol 19, 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Savica R, Boeve BF, Logroscino G (2016) Epidemiology of alpha-synucleinopathies: From Parkinson disease to dementia with Lewy bodies. Handb Clin Neurol 138, 153–158. [DOI] [PubMed] [Google Scholar]
- [14].Chen Y, Wilson L, Kornak J, Dudley RA, Merrilees J, Bonasera SJ, Byrne CM, Lee K, Chiong W, Miller BL, Possin KL (2019) The costs of dementia subtypes to California Medicare fee-for-service, 2015. Alzheimers Dement 15, 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].D’Antonio F, Kane JPM, Ibañez A, Lewis SJG, Camicioli R, Wang H, Yu Y, Zhang J, Ji Y, Borda MG, Kandadai RM, Babiloni C, Bonanni L, Ikeda M, Boeve BF, Leverenz JB, Aarsland D; ISTAART Lewy body dementias Consortia Working Group (2021) Dementia with Lewy bodies research consortia: A global perspective from the ISTAART Lewy Body Dementias Professional Interest Area working group. Alzheimers Dement (Amst) 13, e12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].(2021)2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17, 327–406. [DOI] [PubMed] [Google Scholar]
- [17].Kornblith E, Bahorik A, Boscardin WJ, Xia F, Barnes DE, Yaffe K (2022) Association of race and ethnicity with incidence of dementia among older adults. JAMA 327, 1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zuelsdorff M, Okonkwo OC, Norton D, Barnes LL, Graham KL, Clark LR, Wyman MF, Benton SF, Gee A, Lambrou N, Johnson SC, Gleason CE (2020) Stressful life events and racial disparities in cognition among middle-aged and older adults. J Alzheimers Dis 73, 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Letang SK, Lin SS, Parmelee PA, McDonough IM (2021) Ethnoracial disparities in cognition are associated with multiple socioeconomic status-stress pathways. Cogn Res Princ Implic 6, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee S, Kim D, Lee H (2022) Examine race/ethnicity disparities in perception, intention, and screening of dementia in a community setting: Scoping review. Int J Environ Res Public Health 19, 8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Duran-Kiraç G, Uysal-Bozkir Ö, Uittenbroek R, van Hout H, Broese van Groenou MI (2022) Accessibility of health care experienced by persons with dementia from ethnic minority groups and formal and informal caregivers: A scoping review of European literature. Dementia 21, 677–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tsamakis K, Gadelrab R, Wilson M, Bonnici-Mallia AM, Hussain L, Perera G, Rizos E, Das-Munshi J, Stewart R, Mueller C (2021) Dementia in people from ethnic minority backgrounds: Disability, functioning, and pharmacotherapy at the time of diagnosis. J Am Med Dir Assoc 22, 446–452. [DOI] [PubMed] [Google Scholar]
- [23].Lin PJ, Zhu Y, Olchanski N, Cohen JT, Neumann PJ, Faul JD, Fillit HM, Freund KM (2022) Racial and ethnic differences in hospice use and hospitalizations at end-of-life among medicare beneficiaries with dementia. JAMA Netw Open 5, e2216260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Albaroudi A, Chen J (2022) Consumer assessment of healthcare providers and systems among racial and ethnic minority patients with Alzheimer disease and related dementias. JAMA Netw Open 5, e2233436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aranda MP, Kremer IN, Hinton L, Zissimopoulos J, Whit-mer RA, Hummel CH, Trejo L, Fabius C (2021) Impact of dementia: Health disparities, population trends, care interventions, and economic costs. J Am Geriatr Soc 69, 1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Boot BP, Orr CF, Ahlskog JE, Ferman TJ, Roberts R, Pankratz VS, Dickson DW, Parisi J, Aakre JA, Geda YE, Knopman DS, Petersen RC, Boeve BF (2013) Risk factors for dementia with Lewy bodies: A case-control study. Neurology 81, 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cheng CK, Tsao YC, Su YC, Sung FC, Tai HC, Kung WM (2018) Metabolic risk factors of Alzheimer’s disease, dementia with Lewy bodies, and normal elderly: A population-based study. Behav Neurol 2018, 8312346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xu Y, Yang J, Shang H (2016) Meta-analysis of risk factors for Parkinson’s disease dementia. Transl Neurodegener 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vasconcellos LF, Pereira JS (2015) Parkinson’s disease dementia: Diagnostic criteria and risk factor review. J Clin Exp Neuropsychol 37, 988–993. [DOI] [PubMed] [Google Scholar]
- [30].Moore SF, Barker RA (2014) Predictors of Parkinson’s disease dementia: Towards targeted therapies for a heterogeneous disease. Parkinsonism Relat Disord 20, S104–S107. [DOI] [PubMed] [Google Scholar]
- [31].Woodruff BK, Graff-Radford NR, Ferman TJ, Dickson DW, DeLucia MW, Crook JE, Arvanitakis Z, Brassler S, Waters C, Barker W, Duara R (2006) Family history of dementia is a risk factor for Lewy body disease. Neurology 66, 1949–1950. [DOI] [PubMed] [Google Scholar]
- [32].Rooks RN, Simonsick EM, Klesges LM, Newman AB, Ayonayon HN, Harris TB (2008) Racial disparities in health care access and cardiovascular disease indicators in Black and White older adults in the Health ABC Study. J Aging Health 20, 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chin AL, Negash S, Hamilton R (2011) Diversity and disparity in dementia: The impact of ethnoracial differences in Alzheimer’s disease. Alzheimer Dis Assoc Disord 25, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Raman R, Quiroz YT, Langford O, Choi J, Ritchie M, Baumgartner M, Rentz D, Aggarwal NT, Aisen P, Sperling R, Grill JD (2021) Disparities by race and ethnicity among adults recruited for a preclinical Alzheimer disease trial. JAMA Netw Open 4, e2114364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vilsaint CL, NeMoyer A, Fillbrunn M, Sadikova E, Kessler RC, Sampson NA, Alvarez K, Green JG, McLaughlin KA, Chen R, Williams DR, Jackson JS, Alegría M (2019) Racial/ethnic differences in 12-month prevalence and persistence of mood, anxiety, and substance use disorders: Variation by nativity and socioeconomic status. Compr Psychiatry 89, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gonzalez-Latapi P, Bayram E, Litvan I, Marras C (2021) Cognitive impairment in Parkinson’s disease: Epidemiol ogy, clinical profile, protective and risk factors. Behav Sci (Basel) 11, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dotchin C, Msuya O, Kissima J, Massawe J, Mhina A, Moshy A, Aris E, Jusabani A, Whiting D, Masuki G, Walker R (2008) The prevalence of Parkinson’s disease in rural Tanzania. Mov Disord 23, 1567–1672. [DOI] [PubMed] [Google Scholar]
- [38].Ben-Joseph A, Marshall CR, Lees AJ, Noyce AJ (2020) Ethnic variation in the manifestation of Parkinson’s disease: A narrative review. J Parkinsons Dis 10, 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Llibre-Guerra JJ, Prina M, Sosa AL, Acosta D, Jimenez-Velazquez IZ, Guerra M, Salas A, Llibre-Guerra JC, Valvuerdi A, Peeters G, Ziegemeier E, Acosta I, Tanner C, Juncos J, Llibre Rodriguez JJ (2022) Prevalence of parkinsonism and Parkinson disease in urban and rural populations from Latin America: A community based study. Lancet Reg Health Am 7, 100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen CC, Chen TF, Hwang YC, Wen YR, Chiu YH, Wu CY, Chen RC, Tai JJ, Chen THH, Liou HH (2009) Different prevalence rates of Parkinson’s disease in urban and rural areas: A population-based study in Taiwan. Neuroepidemiology 33, 350–357. [DOI] [PubMed] [Google Scholar]
- [41].He J, Zhu Z, Bundy JD, Dorans KS, Chen J, Hamm LL (2021) Trends in cardiovascular risk factors in US adults by race and ethnicity and socioeconomic status, 1999–2018. JAMA 326, 1286–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rosselli M, Uribe IV, Ahne E, Shihadeh L (2022) Culture, ethnicity, and level of education in Alzheimer’s disease. Neurotherapeutics 19, 26–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Liu C, Murchland AR, VanderWeele TJ, Blacker D (2022) Eliminating racial disparities in dementia risk by equalizing education quality: A sensitivity analysis. Soc Sci Med 312, 115347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Race - Oxford Reference. http://www.oxfordreference.com
- [45].Ethnicity - Oxford Reference. http://www.oxfordreference.com
- [46].Lu C, Ahmed R, Lamri A, Anand SS (2022) Use of race, ethnicity, and ancestry data in health research. PLOS Glob Public Health 2, e0001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AHB, Bibbins-Domingo K, Rodríguez-Santana JR, Lenoir MA, Gavin JR, Kittles RA, Zaitlen NA, Wilkes DS, Powe NR, Ziv E, Burchard EG (2021) Race and genetic ancestry in medicine — a time for reckoning with racism. N Engl J Med 384, 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ioannidis JPA, Powe NR, Yancy C (2021) Recalibrating the use of race in medical research. JAMA 325, 623–624. [DOI] [PubMed] [Google Scholar]
- [49].Flanagin A, Frey T, Christiansen SL (2021) Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA 326, 621–627. [DOI] [PubMed] [Google Scholar]
- [50].Bauchner H (2015) Race, poverty, and medicine in the United States. JAMA 313, 1423. [DOI] [PubMed] [Google Scholar]
- [51].Röhr S, Pabst A, Baber R, Engel C, Glaesmer H, Hinz A, Schroeter ML, Witte AV, Zeynalova S, Villringer A, Löffler M, Riedel-Heller SG (2022) Social determinants and lifestyle factors for brain health: Implications for risk reduction of cognitive decline and dementia. Sci Rep 12, 12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].United Nations, Shifting Demographics. https://www.un.org/en/un75/shifting-demographics
- [53].NOT-OD-15–089: Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes. https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html
- [54].Zaccai J, McCracken C, Brayne C (2005) A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing 34, 561–566. [DOI] [PubMed] [Google Scholar]
- [55].Hogan DB, Fiest KM, Roberts JI, Maxwell CJ, Dykeman J, Pringsheim T, Steeves T, Smith EE, Pearson D, Jetté N (2016) The prevalence and incidence of dementia with Lewy bodies: A systematic review. Can J Neurol Sci 43, S83–S95. [DOI] [PubMed] [Google Scholar]
- [56].GBD 2016 Parkinson’s Disease Collaborators (2018) Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chia R, Sabir MS, Bandres-Ciga S, Saez-Atienzar S, Reynolds RH, Gustavsson E, Walton RL, Ahmed S, Viollet C, Ding J, Makarious MB, Diez-Fairen M, Portley MK, Shah Z, Abramzon Y, Hernandez DG, Blauwendraat C, Stone DJ, Eicher J, Parkkinen L, Ansorge O, Clark L, Honig LS, Marder K, Lemstra A, St George-Hyslop P, Londos E, Morgan K, Lashley T, Warner TT, Jaunmuktane Z, Galasko D, Santana I, Tienari PJ, Myllykangas L, Oinas M, Cairns NJ, Morris JC, Halliday GM, Van Deerlin VM, Trojanowski JQ, Grassano M, Calvo A, Mora G, Canosa A, Floris G, Bohannan RC, Brett F, Gan-Or Z, Geiger JT, Moore A, May P, Krüger R, Goldstein DS, Lopez G, Tayebi N, Sidransky E, Sotis AR, Sukumar G, Alba C, Lott N, Martinez EMG, Tuck M, Singh J, Bacikova D, Zhang X, Hupalo DN, Adeleye A, Wilkerson MD, Pollard HB, Norcliffe-Kaufmann L, Palma JA, Kaufmann H, Shakkottai VG, Perkins M, Newell KL, Gasser T, Schulte C, Landi F, Salvi E, Cusi D, Masliah E, Kim RC, Caraway CA, Monuki ES, Brunetti M, Dawson TM, Rosenthal LS, Albert MS, Pletnikova O, Troncoso JC, Flanagan ME, Mao Q, Bigio EH, Rodríguez-Rodríguez E, Infante J, Lage C, González-Aramburu I, Sanchez-Juan P, Ghetti B, Keith J, Black SE, Masellis M, Rogaeva E, Duyckaerts C, Brice A, Lesage S, Xiromerisiou G, Barrett MJ, Tilley BS, Gentleman S, Logroscino G, Serrano GE, Beach TG, McKeith IG, Thomas AJ, Attems J, Morris CM, Palmer L, Love S, Troakes C, Al-Sarraj S, Hodges AK, Aarsland D, Klein G, Kaiser SM, Woltjer R, Pastor P, Bekris LM, Leverenz JB, Besser LM, Kuzma A, Renton AE, Goate A, Bennett DA, Scherzer CR, Morris HR, Ferrari R, Albani D, Pickering-Brown S, Faber K, Kukull WA, Morenas-Rodriguez E, Lleó A, Fortea J, Alcolea D, Clarimon J, Nalls MA, Ferrucci L, Resnick SM, Tanaka T, Foroud TM, Graff-Radford NR, Wszolek ZK, Ferman T, Boeve BF, Hardy JA, Topol EJ, Torkamani A, Singleton AB, Ryten M, Dickson DW, Chiò A, Ross OA, Gibbs JR, Dalgard CL, Traynor BJ, Scholz SW (2021) Genome sequencing analysis identifies new loci associated with Lewy body dementia and provides insights into its genetic architecture. Nat Genet 53, 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Moon H, Badana ANS, Hwang SY, Sears JS, Haley WE (2019) Dementia prevalence in older adults: Variation by race/ethnicity and immigrant status. Am J Geriatr Psychiatry 27, 241–250. [DOI] [PubMed] [Google Scholar]
- [59].Segers K, Benoit F, Meyts JM, Glibert G, Levy S, Surquin M (2020) Dementia with Lewy bodies in first-generation immigrants in a European memory clinic. Acta Neurol Belgica 121, 219–223. [DOI] [PubMed] [Google Scholar]
- [60].American Community Survey, S0103 Population 65 Years and over in the United States 2021: ACS 5-Year Estimates Subject Tables. https://data.census.gov/table?q=S0103&tid=ACSST5Y2021.S0103
- [61].Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM (2017) Prevalence of dementia subtypes in U.S. Medicare fee-for-service beneficiaries, 2011–2013. Alzheimers Dement 13, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Isotalo J, Vahlberg T, Kaasinen V (2017) Unchanged long-term rural-to-urban incidence ratio of Parkinson’s disease. Mov Disord 32, 474–475. [DOI] [PubMed] [Google Scholar]
- [63].Rizzo G, Arcuti S, Copetti M, Alessandria M, Savica R, Fontana A, Liguori R, Logroscino G (2018) Accuracy of clinical diagnosis of dementia with Lewy bodies: A systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 89, 358–366. [DOI] [PubMed] [Google Scholar]
- [64].Besser L, Kukull W, Knopman DS, Chui H, Galasko D, Weintraub S, Jicha G, Carlsson C, Burns J, Quinn J, Sweet RA, Rascovsky K, Teylan M, Beekly D, Thomas G, Bollenbeck M, Monsell S, Mock C, Zhou XH, Thomas N, Robichaud E, Dean M, Hubbard J, Jacka M, Schwabe-Fry K, Wu J, Phelps C, Morris JC; Neuropsychology Work Group, Directors, and Clinical Core leaders of the National Institute on Aging-funded US Alzheimer’s Disease Centers (2018) Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord 32, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Besser LM, Kukull WA, Teylan MA, Bigio EH, Cairns NJ, Kofler JK, Montine TJ, Schneider JA, Nelson PT (2018) The revised National Alzheimer’s Coordinating Center’s Neuropathology form—available data and new analyses. J Neuropathol Exp Neurol 77, 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Beekly DL, Ramos EM, Lee WW, Deitrich WD, Jacka ME, Wu J, Hubbard JL, Koepsell TD, Morris JC, Kukull WA (2007) The National Alzheimer’s Coordinating Center (NACC) Database: The uniform data set. Alzheimer Dis Assoc Disord 21, 249–258. [DOI] [PubMed] [Google Scholar]
- [67].Kurasz AM, Smith GE, McFarland MG, Armstrong MJ, O’Bryant S (2020) Ethnoracial differences in Lewy body diseases with cognitive impairment. J Alzheimers Dis 77, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kurasz AM, De Wit L, Smith GE, Armstrong MJ (2022) Neuropathological and clinical correlates of Lewy body disease survival by race and ethnicity in the National Alzheimer’s Coordinating Center. J Alzheimers Dis 89, 1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wei H, Masurkar AV, Razavian N (2021) On gaps of clinical diagnosis of dementia subtypes: A study of Alzheimer’s disease and Lewy body disease. Alzheimers Dement 17, e057714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mueller C, Soysal P, Rongve A, Isik AT, Thompson T, Maggi S, Smith L, Basso C, Stewart R, Ballard C, O’Brien JT, Aarsland D, Stubbs B, Veronese N (2019) Survival time and differences between dementia with Lewy bodies and Alzheimer’s disease following diagnosis: A meta-analysis of longitudinal studies. Ageing Res Rev 50, 72–80. [DOI] [PubMed] [Google Scholar]
- [71].Gu Y, Kociolek A, Fernandez KK, Cosentino SA, Zhu CW, Jin Z, Leverenz JB, Stern YB (2022) Clinical trajectories at the end of life in autopsy-confirmed dementia patients With Alzheimer disease and Lewy bodies pathologies. Neurology 98, e2140–e2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Price A, Farooq R, Yuan JM, Menon VB, Cardinal RN, O’Brien JT (2017) Mortality in dementia with Lewy bodies compared with Alzheimer’s dementia: A retrospective naturalistic cohort study. BMJ Open 7, e017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Larsson V, Torisson G, Londos E (2018) Relative survival in patients with dementia with Lewy bodies and Parkinson’s disease dementia. PLoS One 13, e0202044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Shea YF, Lee SC, Shum ACK, Chiu PKC, Chu LW, Chan HWF (2020) Chinese patients with Lewy body dementia had shorter survival and developed complications earlier than those with Alzheimer’s disease. Singapore Med J 61, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fereshtehnejad SM, Lökk J, Wimo A, Eriksdotter M (2018) No significant difference in cognitive decline and mortality between Parkinson’s Disease dementia and dementia with Lewy bodies: Naturalistic longitudinal data from the Swedish dementia registry. J Parkinsons Dis 8, 553–561. [DOI] [PubMed] [Google Scholar]
- [76].Matar E, White SR, Taylor JP, Thomas A, McKeith IG, Kane JPM, Surendranathan A, Halliday GM, Lewis SJG, O’Brien JT (2021) Progression of clinical features in Lewy body dementia can be detected over 6 months. Neurology 97, e1031–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA (2012) Predictors of survival in patients with Parkinson disease. Arch Neurol 69, 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, Lee EB, Van Deerlin VM, Lopez OL, Kofler JK, Nelson PT, Jicha GA, Woltjer R, Quinn JF, Kaye J, Leverenz JB, Tsuang D, Longfellow K, Yearout D, Kukull W, Keene CD, Montine TJ, Zabetian CP, Trojanowski JQ (2017) Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol 16, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T (2003) Age-associated prevalence and risk factors of Lewy body pathology in a general population: The Hisayama study. Acta Neuropathol 106, 374–382. [DOI] [PubMed] [Google Scholar]
- [80].Graff-Radford J, Aakre J, Savica R, Boeve B, Kremers WK, Ferman TJ, Jones DT, Kantarci K, Knopman DS, Dickson DW, Kukull WA, Petersen RC (2017) Duration and pathologic correlates of Lewy body disease. JAMA Neurol 74, 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Blanc F, Mahmoudi R, Jonveaux T, Galmiche J, Chopard G, Cretin B, Demuynck C, Martin-Hunyadi C, Philippi N, Sellal F, Michel JM, Tio G, Stackfleth M, Vandel P, Magnin E, Novella JL, Kaltenbach G, Benetos A, Sauleau EA (2017) Long-term cognitive outcome of Alzheimer’s disease and dementia with Lewy bodies: Dual disease is worse. Alzheimers Res Ther 9, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, Buchman AS, Bennett DA, Schneider JA (2015) Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 85, 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Coughlin DG, Xie SX, Liang M, Williams A, Peterson C, Weintraub D, McMillan CT, Wolk DA, Akhtar RS, Hurtig H, Coslett HB, Hamilton R, Siderowf A, Duda JE, Rascovsky K, Lee EB, Lee VMY, Grossman M, Trojanowski JQ, Irwin DJ (2019) Cognitive and pathological influences of tau pathology in Lewy body disorders. Ann Neurol 85, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jellinger KA (2022) Are there morphological differences between Parkinson’s disease-dementia and dementia with Lewy bodies? Parkinsonism Relat Disord 100, 24–32. [DOI] [PubMed] [Google Scholar]
- [85].Graff-Radford NR, Besser LM, Crook JE, Kukull WA, Dickson DW (2016) Neuropathological differences by race from the National Alzheimer’s Coordinating Center. Alzheimers Dement 12, 669–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nag S, Barnes LL, Yu L, Buchman AS, Bennett DA, Schneider JA, Wilson RS (2021) Association of Lewy bodies with age-related clinical characteristics in Black and White decedents. Neurology 97, e825–e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].ADRD Summit 2022 Report. https://www.ninds.nih.gov/sites/default/files/documents/ADRD%20Summit%202022%20Report%20to%20NINDS%20Council%20FINALˍ508C.pdf
- [88].Mok W, Chow TW, Zheng L, Mack WJ, Miller C (2004) Clinicopathological concordance of dementia diagnoses by community versus tertiary care clinicians. Am J Alzheimers Dis Other Demen 19, 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bentley A, Morgan T, Salifu Y, Walshe C (2021) Exploring the experiences of living with Lewy body dementia: An integrative review. J Adv Nurs 77, 4632–4645. [DOI] [PubMed] [Google Scholar]
- [90].Holden SK, Bedenfield N, Taylor AS, Bayram E, Schwilk C, Fleisher J, Duda J, Shill H, Paulson HL, Stacy K, Wood J, Corsentino P, Sha SJ, Litvan I, Irwin DJ, Quinn JF, Goldman JG, Amodeo K, Taylor JP, Boeve BF, Armstrong MJ (2023) Research priorities of individuals and caregivers with Lewy body dementia. Alzheimer Dis Assoc Disord 37, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Armstrong MJ, Weintraub D (2017) The case for antipsychotics in dementia with Lewy bodies. Mov Disord Clin Pract 4, 32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Balls-Berry JJE, Babulal GM (2022) Health disparities in dementia. Continuum (Minneap Minn) 28, 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cooper C, Tandy AR, Balamurali TB, Livingston G (2010) A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry 18, 193–203. [DOI] [PubMed] [Google Scholar]
- [94].Boise L, Hinton L, Rosen HJ, Ruhl MC, Dodge H, Mattek N, Albert M, Denny A, Grill JD, Hughes T, Lingler JH, Morhardt D, Parfitt F, Peterson-Hazan S, Pop V, Rose T, Shah RC (2017) Willingness to be a brain donor. Alzheimer Dis Assoc Disord 31, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Milani SA, Swain M, Otufowora A, Cottler LB, Striley CW (2021) Willingness to participate in health research among community-dwelling middle-aged and older adults: Does race/ethnicity matter? J Racial Ethn Heal Disparities 8, 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Damron L, Litvan I, Bayram E, Berk S, Siddiqi B, Shill H (2021) Hispanic perspectives on Parkinson’s disease care and research participation. J Alzheimers Dis 81, 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Manly JJ, Gilmore-Bykovskyi A, Deters KD (2021) Inclusion of underrepresented groups in preclinical Alzheimer disease trials—opportunities abound. JAMA Netw Open 4, e2114606. [DOI] [PubMed] [Google Scholar]
- [98].Werry AE, Daniel M, Bergström B (2019) Group differences in normal neuropsychological test performance for older non-Hispanic White and Black/African American adults. Neuropsychology 33, 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sayegh P, Knight BG (2013) Cross-cultural differences in dementia: The Sociocultural Health Belief Model. Int Psychogeriatr 25, 517–530. [DOI] [PubMed] [Google Scholar]
- [100].Di Luca DG, Macklin EA, Hodgeman K, Lopez G, Pothier L, Callahan KF, Lowell J, Chan J, Videnovic A, Lungu C, Lang AE, Litvan I, Schwarzschild MA, Simuni T (2023) Enrollment of participants from marginalized racial and ethnic groups. Neurol Clin Pract 13, e200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Schneider MG, Swearingen CJ, Shulman LM, Ye J, Baumgarten M, Tilley BC (2009) Minority enrollment in Parkinson’s disease clinical trials. Parkinsonism Relat Disord 15, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Peterson B, Armstrong M, Galasko D, Galvin JE, Goldman J, Irwin D, Paulson H, Kaufer D, Leverenz J, Lunde A, McKeith IG, Siderowf A, Taylor A, Amodeo K, Barrett M, Domoto-Reilly K, Duda J, Gomperts S, Graff-Radford N, Holden S, Honig L, Huddleston D, Lippa C, Litvan I, Manning C, Marder K, Moussa C, Onyike C, Pagan F, Pantelyat A, Pelak V, Poston K, Quinn J, Richard I, Rosenthal LS, Sabbagh M, Scharre D, Sha S, Shill H, Torres-Yaghi Y, Christie T, Graham T, Richards I, Koehler M, Boeve B (2019) Lewy Body Dementia Association’s Research Centers of Excellence Program: Inaugural meeting proceedings. Alzheimers Res Ther 11, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Potter GG, Plassman BL, Burke JR, Kabeto MU, Langa KM, Llewellyn DJ, Rogers MAM, Steffens DC (2009) Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement 5, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Morrell L, Camic PM, Genis M (2019) Factors associated with informant-reported cognitive decline in older adults: A systemised literature review. Dementia 18, 2760–2784. [DOI] [PubMed] [Google Scholar]
- [105].Lin PJ, Daly AT, Olchanski N, Cohen JT, Neumann PJ, Faul JD, Fillit HM, Freund KM (2021) Dementia diagnosis disparities by race and ethnicity. Med Care 59, 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hackett K, Mis R, Drabick DAG, Giovannetti T (2020) Informant reporting in mild cognitive impairment: Sources of discrepancy on the functional activities questionnaire. J Int Neuropsychol Soc 26, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Choi H, Heisler M, Norton EC, Langa KM, Cho TC, Con-nell CM (2021) Family care availability and implications for informal and formal care used by adults with dementia in the US. Health Aff (Millwood) 40, 1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Rivera Mindt M, Byrd D, Saez P, Manly J (2010) Increasing culturally competent neuropsychological services for ethnic minority populations: A call to action. Clin Neuropsychol 24, 429–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Heaton RK, Ryan L, Grant I (2009) Demographic influences and use of demographically corrected norms in neuropsychological assessment. In Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders, 3rd ed. Oxford University Press, New York, NY, US, pp. 127–155. [Google Scholar]
- [110].Stites SD, Midgett S, Mechanic-Hamilton D, Zuelsdorff M, Glover CM, Marquez DX, Balls-Berry JE, Streitz ML, Babulal G, Trani JF, Henderson JN, Barnes LL, Karlawish J, Wolk DA (2022) Establishing a framework for gathering structural and social determinants of health in Alzheimer’s disease research centers. Gerontologist 62, 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hajjar I, Yang Z, Okafor M, Liu C, Waligorska T, Goldstein FC, Shaw LM (2022) Association of plasma and cerebrospinal fluid Alzheimer disease biomarkers with race and the role of genetic ancestry, vascular comorbidities, and neighborhood factors. JAMA Netw Open 5, e2235068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].O’Bryant SE, Petersen M, Hall J, Johnson LA (2023) Medical comorbidities and ethnicity impact plasma Alzheimer’s disease biomarkers: Important considerations for clinical trials and practice. Alzheimers Dement 19, 36–43. [DOI] [PubMed] [Google Scholar]
- [113].Windon C, Iaccarino L, Mundada N, Allen I, Boxer AL, Byrd D, Rivera-Mindt M, Rabinovici GD (2022) Comparison of plasma and CSF biomarkers across ethnoracial groups in the ADNI. Alzheimers Dement (Amst) 14, e12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Boise L, Hinton L, Rosen HJ, Ruhl M (2016) Will my soul go to heaven if they take my brain? Beliefs and worries about brain donation among four ethnic groups. Gerontologist 57, 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Deep-Soboslay A, Mighdoll MI, Jaffe AE, Thomas SB, Herman MM, Sirovatka J, King JP, Fowler DR, Zulauf D, DiAngelo C, Hyde TM, Kleinman JE (2019) African-American and Caucasian participation in postmortem human brain donation for neuropsychiatric research. PLoS One 14, e0222565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Al-Hashim AH, Al-Busaidi M (2015) Understanding the concept of brain death in the Middle East. Oman Med J 30, 75–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Singh A, Arulogun O, Akinyemi J, Nichols M, Calys-Tagoe B, Ojebuyi B, Jenkins C, Obiako R, Akpalu A, Sarfo F, Wahab K, Sunday A, Owolabi LF, Adigun M, Afolami I, Olorunsogbon O, Ogunronbi M, Melikam ES, Laryea R, Asibey S, Oguike W, Melikam L, Sule A, Titiloye MA, Yahaya IS, Bello A, Kalaria RN, Jegede A, Owolabi M, Ovbiagele B, Akinyemi R (2022) Biological sample donation and informed consent for neurobiobanking: Evidence from a community survey in Ghana and Nigeria. PLoS One 17, e0267705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lin MP, Jowsey T, Curtis MA (2019) Why people donate their brain to science: A systematic review. Cell Tissue Bank 20, 447–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Franzen S, Smith JE, van den Berg E, Rivera Mindt M, van Bruchem-Visser RL, Abner EL, Schneider LS, Prins ND, Babulal GM, Papma JM (2022) Diversity in Alzheimer’s disease drug trials: The importance of eligibility criteria. Alzheimers Dement 18, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Goldstein DB, Tate SK, Sisodiya SM (2003) Pharmacogenetics goes genomic. Nat Rev Genet 4, 937–947. [DOI] [PubMed] [Google Scholar]
- [121].Bjornsson TD, Wagner JA, Donahue SR, Harper D, Karim A, Khouri MS, Murphy WR, Roman K, Schneck D, Sonnichsen DS, Stalker DJ, Wise SD, Dombey S, Loew C (2003) A review and assessment of potential sources of ethnic differences in drug responsiveness. J Clin Pharmacol 43, 943–967. [DOI] [PubMed] [Google Scholar]
- [122].Grill JD, Sperling RA, Raman R (2022) What should the goals be for diverse recruitment in Alzheimer clinical trials? JAMA Neurol 79, 1097–1098. [DOI] [PubMed] [Google Scholar]
- [123].Greenhalgh T, Thorne S, Malterud K (2018) Time to challenge the spurious hierarchy of systematic over narrative reviews? Eur J Clin Invest 48, e12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Pae CU (2015) Why systematic review rather than narrative review? Psychiatry Investig 12, 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Mori E, Ikeda M, Kosaka K; Donepezil-DLB Study Investigators (2012) Donepezil for dementia with Lewy bodies: A randomized, placebo-controlled trial. Ann Neurol 72, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Emre M, Poewe W, De Deyn PP, Barone P, Kulisevsky J, Pourcher E, Van Laar T, Storch A, Micheli F, Burn D, Durif F, Pahwa R, Callegari F, Tenenbaum N, Strohmaier C (2014) Long-term safety of rivastigmine in Parkinson disease dementia: An open-label, randomized study. Clin Neuropharmacol 37, 9–16. [DOI] [PubMed] [Google Scholar]
- [127].Stefani A, Santamaria J, Iranzo A, Hackner H, Schenck CH, Högl B (2021) Nelotanserin as symptomatic treatment for rapid eye movement sleep behavior disorder: A double-blind randomized study using video analysis in patients with dementia with Lewy bodies or Parkinson’s disease dementia. Sleep Med 81, 180–187. [DOI] [PubMed] [Google Scholar]
- [128].Ikeda M, Mori E, Kosaka K, Iseki E, Hashimoto M, Matsukawa N, Matsuo K, Nakagawa M; Donepezil-DLB Study Investigators (2013) Long-term safety and efficacy of Donepezil in patients with dementia with Lewy bodies: Results from a 52-week, open-label, multicenter extension study. Dement Geriatr Cogn Disord 36, 229–241. [DOI] [PubMed] [Google Scholar]
- [129].Tsai CH, Huang HC, Liu BL, Li CI, Lu MK, Chen X, Tsai MC, Yang YW, Lane HY (2014) Activation of N-methyl-D-aspartate receptor glycine site temporally ameliorates neuropsychiatric symptoms of Parkinson’s disease with dementia. Psychiatry Clin Neurosci 68, 692–700. [DOI] [PubMed] [Google Scholar]
- [130].Ikeda M, Mori E, Matsuo K, Nakagawa M, Kosaka K (2015) Donepezil for dementia with Lewy bodies: A randomized, placebo-controlled, confirmatory phase III trial. Alzheimers Res Ther 7, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lang FM, Kwon DY, Aarsland D, Boeve B, Tousi B, Harnett M, Mo Y, Noel Sabbagh M (2021) An international, randomized, placebo-controlled, phase 2b clinical trial of intepirdine for dementia with Lewy bodies (HEADWAY-DLB). Alzheimers Dement (N Y) 7, e12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Jiang Y, Alam JJ, Gomperts SN, Maruff P, Lemstra AW, Germann UA, Stavrides PH, Darji S, Malampati S, Peddy J, Bleiwas C, Pawlik M, Pensalfini A, Yang D-S, Subbanna S, Basavarajappa BS, Smiley JF, Gardner A, Blackburn K, Chu HM, Prins ND, Teunissen CE, Harrison JE, Scheltens P, Nixon RA (2022) Preclinical and randomized clinical evaluation of the p38[H9251] kinase inhibitor neflamapimod for basal forebrain cholinergic degeneration. Nat Commun 13, 5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Biglan K, Munsie L, Svensson KA, Ardayfio P, Pugh M, Sims J, Brys M (2022) Safety and efficacy of mevidalen in Lewy body dementia: A phase 2, randomized, placebo-controlled trial. Mov Disord 37, 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]


